Abstract
Objective
Some crush-resistant tablet formulations (CRTs) reduce prescription opioid abuse by nonoral routes of administration (ROAs), especially insufflation and injection, while oral abuse increases. Oral abuse involving product manipulation vs swallowing whole for CRTs and comparators was examined.
Methods
Abuse by oral modes of administration (e.g., swallowing whole, chewing, dissolving in the mouth), was examined using the ASI-MV, a computerized, clinical interview for adults in substance abuse treatment from January 2009 to March 2015. CRTs (reformulated oxycodone extended-release [ER], reformulated oxymorphone ER, and tapentadol ER) were compared with non-CRT versions, morphine ER, and oxycodone immediate-release single entity. Analyses employed descriptive statistics and logistic regression.
Results
Among 364,329 unique assessments, 18,135 patients reported oral abuse of the CRTs and comparators examined. CRTs had a higher prevalence of oral abuse involving product manipulation than comparators (P < 0.0001) among all abusers of product. Oral abuse involving product manipulation for CRTs was greater among the subset of patients reporting oral abuse and significantly higher than comparators (P < 0.003). CRTs were significantly less likely than comparators to be swallowed whole (P < 0.0001) and significantly more likely to be chewed (P < 0.003). CRTs were more likely to be dissolved in the mouth than most comparators.
Conclusions
Results suggest the need for abuse-deterrent formulations designed to reduce abuse by oral administration with product manipulation, such as chewing. Advances in this area may reduce the overall abuse of prescription opioids and interrupt the progression from abuse by swallowing whole to oral administration involving product manipulation and other ROAs.
Keywords: Crush-Resistant, Abuse-Deterrent, Route of Administration, Oral Route, Chew, NAVIPPRO
Introduction
While opioid analgesics are generally considered an important treatment option for patients with chronic, noncancer pain [1], it is widely recognized that as prescriptions for these medications have increased over the last 15 years, so have levels of abuse, addiction, diversion, and overdose [2]. Despite these risks, treatment of chronic pain remains a significant challenge, and undertreatment of pain continues to be a public health problem [3]. Over the past decade, a variety of efforts have been initiated to help achieve a balance between appropriate use of prescription opioids for pain management, while at the same time, reducing, preventing, or eliminating the risks associated with abuse (and by extension addiction, diversion, and many cases of overdose). These initiatives include efforts to encourage ongoing risk screening and monitoring for risk [4,5], continuing educational programs for prescribers [6], increased implementation of prescription drug monitoring programs (PDMPs) [7,8], expansion of access to buprenorphine and methadone maintenance treatment [9], and development of prescription opioid formulations with abuse-deterrent properties [10].
Current versions of products intended to deter abuse attempt to impede abuse by reducing a product’s abuse potential, which has long been presumed to depend on its pharmacokinetic properties through specific routes of administration (ROAs) [11]. That is, individuals who abuse prescription opioids attempt to manipulate the original formulation (i.e., tablet, capsule, patch, or film) [12] in order to achieve a combination of rapid release of the active pharmaceutical ingredient (API) and use by a route that provides a high blood concentration of the opioid (high Cmax) in the shortest time possible (short Tmax) for a potent and rapid “high” (i.e., euphoric effect) [13]. A recent review of the impact of several currently marketed products that aim to be abuse deterrent [14] suggests that extended-release (ER) formulations tend to be abused more often by routes that require tampering (such as chewing, insufflation, and injection) than the intended route (i.e., swallowing the tablet or capsule whole) [14] and, further, that tampering may lead to greater morbidity associated with injection and insufflation of prescription opioid products [13]. As a result of such findings, the first generation of products formulated to deter abuse focused on formulations intended to reduce the prevalence of insufflation and injection, several of which were crush-resistant tablets intended to be hard to crush and to gel on exposure to water.
To date, there are several published postmarketing studies of crush-resistant tablets and the impact on abuse by unintended ROAs [14]. Probably the most comprehensively studied of these is crush-resistant oxycodone ER (OxyContin; Purdue Pharma L.P., Stamford, CT, USA). Prior to the reformulation, oxycodone ER tended to have relatively greater prevalence of insufflation and injection, although the most commonly reported route for abuse was oral [12]. In postmarketing studies comparing the original and reformulated oxycodone ER, the crush-resistant version of oxycodone ER did not eliminate abuse by insufflation and injection but did show significant reduction of abuse by these routes among individuals who continue to abuse the reformulated product [15–17]. Evidence further suggests that the number of individuals abusing oxycodone ER has decreased significantly since its reformulation [17]. Such results have led some reviewers to conclude that the observed reduction in abuse of crush-resistant tablets, especially reductions in riskier, nonoral abuse routes “appears to represent an important step toward curbing the epidemic of prescription opioid analgesic abuse and diversion, while ensuring the availability of effective pain medications for patients with legitimate medical need” [14]. Despite this notion, published and presented data on crush-resistant oxycodone ER [15–17] and oxymorphone ER [18], as well as the OROS formulation of hydromorphone ER [19], clearly show that formulations intended to deter abuse continue to be abused, including abuse by insufflation and injection. Moreover, for at least crush-resistant oxycodone ER, the prevalence of oral abuse has increased among those who continue to abuse that product [15,16].
Oral abuse of prescription opioids is clearly a major contributor to the opioid abuse epidemic. Furthermore, there is some evidence of a progression that starts with oral ingestion, proceeds to insufflation (snorting), and finally to injection by the more experienced abusers [13]. Despite the importance of this issue, we were only able to locate two studies from a research group in Kentucky. Chart review studies of opioid-dependent individuals [20,21] found that individuals’ initial abuse of oxycodone ER was overwhelmingly oral (83%; 86/104 [20] and 93/112 [21]). Initial use by injection in this sample was rare (1%), with only about 16% reporting snorting during their initial use. However, at the time of admission to treatment—presumably after sufficient progress in severity of opioid abuse and dependence occurred to require substance abuse treatment—this group of individuals was more likely to report snorting (62%) and injection (26%), with only 14% reporting oral abuse. A limitation of these studies was that “oral” abuse did not differentiate swallowing the tablet whole vs efforts to tamper with or manipulate the product before swallowing by methods such as chewing, dissolving in the mouth (i.e., sucking on the tablet), or dissolving in a liquid and drinking [13]. Because oral ingestion appears to be the preferred route of novices/initiates, Katz et al. [13] proposed the concept of an “abuse trajectory,” where abuse “starts by swallowing whole products and evolves to chewing, to snorting, to injecting” [13]. However, a lack of longitudinal data inhibits a full understanding of the causal pathways in this trajectory of abuse.
To date, postmarketing studies of existing crush-resistant tablet products have focused on insufflation and injection [15–18]. No epidemiological study has yet examined the impact of current CRTs on oral ingestion preceded by manipulation of the product by chewing or dissolving. To begin to address this omission, reports of oral modes of administration (MOAs) of currently marketed crush-resistant tablets and comparators were evaluated in a large sample of individuals evaluated for substance use problems to determine the trends in oral abuse of crush-resistant tablets and comparators.
Methods
Data Source Description: ASI-MV Substance Abuse Treatment Data
The National Addictions Vigilance Intervention and Prevention Program (NAVIPPRO; Inflexxion, Inc. Waltham, MA, USA) surveillance system provides real-time monitoring of patterns and trends of prescription medication use and abuse for pharmaceutical companies and other public health stakeholders. NAVIPPRO offers ongoing surveillance and epidemiological studies for US Food and Drug Administration (FDA)–regulated products that are addictive and pose health risks. NAVIPPRO data streams provide product-specific and ROA-specific data that allow evaluation of the risk profile of a given opioid analgesic product in the context of abuse patterns of comparator products.
The Addiction Severity Index–Multimedia Version (ASI-MV) is the NAVIPPRO data stream that captures data on individuals assessed for substance use problems for clinical treatment planning and triage purposes [22]. The ASI-MV is a structured, self-administered, computerized interview that measures the severity of a range of problem areas typically associated with drug and alcohol abuse. This electronic assessment is based on the Addiction Severity Index (ASI), a standard clinical assessment designed for use on admission to drug and alcohol treatment [23]. The ASI has well-established reliability and validity [24–26]. When a patient indicates use of a prescription analgesic, the ASI-MV captures data related to past 30-day use and abuse for more than 60 name brand and generic prescription opioid products, including information on routes of administration used and sources of procurement for each product selected. When a respondent has completed the assessment locally at the treatment site for clinical purposes, individual-level data are de-identified and electronically uploaded to a central server where they are available for analysis [22]. This data stream has been employed widely to evaluate the relative abuse potential of various products and drug compounds as well as comparing ROA profiles among specific products and compounds [12,15,17,27–29]. The ASI-MV data are collected primarily for clinical purposes; therefore, analyses of de-identified aggregate data for research purposes have been determined to be exempt from institutional review board review by the New England Institutional Review Board (NEIRB).
Data for the study were obtained from clinical assessments of unique adult (age 18 years and older) respondents evaluated by the ASI-MV between January 1, 2009, and March 31, 2015. While individuals can be administered the ASI-MV multiple times, only the first assessment of any individual during the time period was retained. Further, respondents were selected based on having reported abuse of at least one of the products included in six product categories: a composite of crush-resistant tablets, the non-CRT (i.e., non-crush-resistant) versions of those CRT products, and four other comparators—original or generic oxycodone ER, any morphine ER (excluding EMBEDA), original or generic oxymorphone ER, and IR oxycodone single entity (SE). The individual product options selected by the respondents were grouped into product categories as presented in Table 1.
Table 1.
Target products (CRT) and comparator group products
| Target Product/Category | ASI-MV Product Selection Options |
|---|---|
| CRT ER opioid category | OxyContin (reformulated; Purdue Pharma L.P., Stamford, CT, USA) |
| OxyNEO (reformulated; Purdue Pharma Canada, Pickering, ON, Canada) | |
| Opana ER (reformulated; Endo Pharmaceuticals Inc., Malvern, PA, USA) | |
| Nucynta ER (Depomed, Inc. Newark, CA, USA) | |
| Non-CRT versions of CRT category | Original or generic oxycodone ER |
| Original or generic oxymorphone ER | |
| Nucynta IR (Depomed, Inc. Newark, CA, USA) | |
| Original or generic oxycodone ER | Original OxyContin (Purdue Pharma L.P., Stamford, CT, USA) |
| Xartemis XR (Mallinckrodt Pharmaceuticals, Inc., Hazelwood, MO, USA) | |
| Apo-oxycodone CR (Apotex Inc., Ontario, Canada) | |
| Co-oxycodone CR (Actavis [formerly Cobalt Pharmaceuticals Company], Mississauga, Canada) | |
| All morphine ER (excluding EMBEDA) | KADIAN (Actavis Pharma, Inc. Parsippany, NJ, USA) |
| AVINZA (Pfizer Inc., New York, NY, USA) | |
| Oramorph SR (Roxane Laboratories, Inc., Columbus, OH, USA) | |
| MS Contin (Purdue Pharma L.P., Stamford, CT, USA) | |
| Other morphine ER products | |
| Original or generic oxymorphone ER | Opana ER (original formulation; Endo Pharmaceuticals Inc., Malvern, PA, USA) |
| Generic ER oxymorphone | |
| Oxycodone IR SE | Roxicodone (Mallinckrodt Pharmaceuticals, Damastown, Mulhuddart, Dublin 15, Ireland) |
| Generic oxycodone IR SE |
CRT = crush-resistant tablet; ER = extended-release; IR = immediate-release; SE = single entity; SR = sustained-release.
Definition of Abuse and Abuse by Route of Administration
Self-reported abuse of a product is captured in the ASI-MV by individuals who indicated which product(s) they have used in the past 30 days (using pictures, audio, and text with slang or street names). Responses to a series of questions regarding use via alternate ROAs, source of the product, and use of the product not as prescribed for pain established the individual as having engaged in nonmedical use that was considered to be abuse [12,15,22]. Respondents who abuse any prescription opioid are presented all prescription analgesic screens and may select any product they have used in the past 30 days.
Once a product is selected as having been used in the past 30 days, a subsequent question asks, “How have you usually used <product name>. Select all that apply.” The intended route (usually oral—swallowing a tablet or capsule whole) is included, along with alternate oral MOAs, for example, “chewed it, and then swallowed it,” “dissolved in mouth,” and “drank it after it dissolved in liquid.” ROAs other than oral are also captured as “snorted it,” “smoked it,” “injected it with a needle into my vein,” “injected it with a needle into my skin or muscle,” and “other route.” As respondents are permitted to select all routes that apply, the route categories are not mutually exclusive. For this study, the focus was on an examination of reported oral MOAs, especially swallowed whole vs oral preceded by some effort to tamper with the formulation, namely by chewing, dissolving in the mouth, or dissolving in liquid and drinking (collectively referred to as “alternate oral MOAs”).
Data Analytic Strategy
Descriptive statistics were used to characterize ASI-MV respondents for the entire data set of unique individuals contributing data (“total sample”), the subset of those who reported any past 30-day prescription opioid abuse (“any Rx opioid abuse”), and the sample of individuals who reported abuse of at least one of the products or compounds being evaluated by any oral MOA (“oral abusers of the target products”) (see Table 1 and Figure 1). Analyses comparing the various oral MOAs for crush-resistant tablets and comparators consisted of employing logistic regression models among those who reported past 30-day abuse of a prescription opioid via an oral route. Specifically, the probabilities and 95% confidence intervals (CIs) of abusing a CRT, non-CRT, and other select comparators were estimated and contrasted for 1) any oral MOA that involved manipulation (i.e., any oral ingestion other than by swallowing whole) among those who abused any of the target products (see Figure 1), 2) any oral MOA that involved manipulation among any oral abusers of the target products, and 3) each oral MOA separately (i.e., swallowing whole, chewing, dissolving in the mouth, or dissolving in liquid and drinking) among any oral abusers of the target products. Note that the study compares groups of products rather than individual products (see Table 1). Within each group, there are more cases of abuse of some products than others, largely due to some products having greater market presence (e.g., OxyContin versus Opana ER or Nucynta ER, or MS Contin vs other morphine ER products). For the model to account for this discrepancy in contribution of route-specific data, comparator group values were weighted proportional to the number of abuse cases associated with each of the products within that comparator group. This approach ensured that products with a greater number of abuse cases would be weighted proportionally more than products with fewer abuse cases in the estimation of the weighted probability of the group to which the products belonged (CRT, non-CRT group, other comparator groups) [30]. The logistic regression models were employed using the NLMIXED procedure in SAS 9.4/STAT 14.1 (SAS Institute Inc., Cary, NC, USA) due to its capability of fitting such models while simultaneously estimating, constructing confidence intervals, and contrasting the parameters of interest; that is, the weighted probabilities [31]. The level of significance was set at an α of 0.05 for all tests.
Figure 1.
Sample and subsample descriptions and Ns. *Target products = crush-resistant tablets (reformulated oxycodone extended-release [ER], reformulated oxymorphone ER, and tapentadol ER), non–crush-resistant tablet formulation versions of these products, original/generic versions of oxycodone ER and oxymorphone ER, morphine ER, and oxycodone immediate-release single entity. CRT = crush-resistant tablet; MOA = mode of administration.
Results
Participant Characteristics
Between January 1, 2009, and March 31, 2015, 432,180 ASI-MV assessments were collected from 1,008 treatment centers in 44 US states and the District of Columbia. Among these, 364,329 were unique adult (age 18 years or older) respondents and 76,108 (20.9%) reported past 30-day abuse of any prescription opioid. Table 2 presents the demographic characteristics of these respondents for the entire sample of respondents collected during the study period, those reporting any past 30-day prescription opioid abuse, and those reporting oral abuse of the target products.
Table 2.
Demographics
| Demographics | Total Sample (N = 364,329) | Percent | Any Rx Opioid Abuse (N = 76,108) | Percent | Oral Abusers of Target Products (N = 18,135) | Percent |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 236,804 | 65.0 | 41,386 | 54.4 | 10,215 | 56.3 |
| Female | 127,505 | 35.0 | 34,722 | 45.6 | 7,920 | 43.7 |
| Missing | 20 | 0 | 0 | — | 0 | — |
| Age, y | ||||||
| 18–24 | 88,962 | 24.4 | 19,357 | 25.4 | 4,481 | 24.7 |
| 25–34 | 124,774 | 34.2 | 29,924 | 39.3 | 7,129 | 39.3 |
| 35–44 | 75,829 | 20.8 | 14,815 | 19.5 | 3,634 | 20.0 |
| 45+ | 74,764 | 20.5 | 12,012 | 15.8 | 2,891 | 15.9 |
| Missing | 0 | — | 0 | — | 0 | — |
| Race | ||||||
| Caucasian | 213,885 | 58.7 | 55,300 | 72.7 | 13,010 | 71.7 |
| Black | 71,797 | 19.7 | 8,568 | 11.3 | 2,041 | 11.3 |
| Hispanic | 56,500 | 15.5 | 9,242 | 12.1 | 2,366 | 13.0 |
| Other | 22,130 | 6.1 | 2,996 | 3.9 | 718 | 4.0 |
| Missing | 17 | 0 | 2 | 0 | 0 | — |
| US Region | ||||||
| Northeast | 16,811 | 4.6 | 5,989 | 7.9 | 1,163 | 6.4 |
| South | 169,265 | 46.5 | 36,440 | 47.9 | 9,143 | 50.4 |
| West | 107,233 | 29.4 | 17,127 | 22.5 | 4,346 | 24.0 |
| Midwest | 71,017 | 19.5 | 16,552 | 21.8 | 3,483 | 19.2 |
| Missing | 3 | 0 | 0 | — | 0 | — |
As can be seen in Table 2, the analyzed sample for this project consisted of 18,135 adults, age 18 years and older, assessed using the ASI-MV [22], who self-reported past 30-day abuse of at least one of the target products by the oral route. These assessments were collected at 675 sites in 39 US states. The sample was mostly male (56%), between 21 and 34 years of age (64%), and predominately white (72%). Geographically, most individuals were assessed at centers located in the South (50%), followed by the West (24%), the Midwest (19%), and the Northeast (6%).
Oral Abuse Route that Involved Product Manipulation Among Any Abusers of the Target Products
In this analysis, oral abuse that involved product manipulation (i.e., chewing, dissolving in the mouth, or dissolving in a liquid and drinking) was significantly greater (that is, larger weighted probabilities) for CRT products than for all comparators among any individuals who abused the target products listed in Table 1. CRTs were abused by an alternate oral MOA 1.40 times more often than non-CRTs (relative risk [RR] = 0.72), 1.27 times more often than non-CRT oxycodone ER (RR = 0.78), 3.45 times more often than morphine ER (RR = 0.29), 4.67 times more often than non-CRT oxymorphone ER (RR = 0.21), and 1.80 times more often than oxycodone immediate-release (IR) SE (RR = 0.55; all P < 0.0001) (see Table 3).
Table 3.
Weighted prevalence of CRT abuse by alternative oral methods of administration among abusers of the product category by any route
| Product | Weighted Abuse Prevalence* | Prevalence 95% CI | Relative Risk | P | |
|---|---|---|---|---|---|
| CRT | 26.25 | 25.25–27.28 | Reference | ||
| Comparators | Non-CRT versions† | 18.82 | 18.33–19.32 | 0.72‡ | <0.0001 |
| Non-CRT oxycodone ER | 20.63 | 20.09–21.18 | 0.78 | <0.0001 | |
| Morphine ER | 7.62 | 6.15–9.40 | 0.29 | <0.0001 | |
| Non-CRT Oxymorphone ER | 5.63 | 4.59–6.88 | 0.21 | <0.0001 | |
| Oxycodone IR SE | 14.58 | 13.85–15.34 | 0.55 | <0.0001 | |
CI = confidence interval; CRT = crush-resistant tablet formulations.
The denominator is the number of abusers of the product category by any route; the prevalence estimate is the cases per 100 abusers of product by any route.
See Table 1 for specific products within each category.
Note that the relative risk values below 1 indicate that the comparator estimate is smaller than the reference product although the difference may or may not be significant. Relative risk values greater than 1 imply that the comparator estimate is greater than the reference.
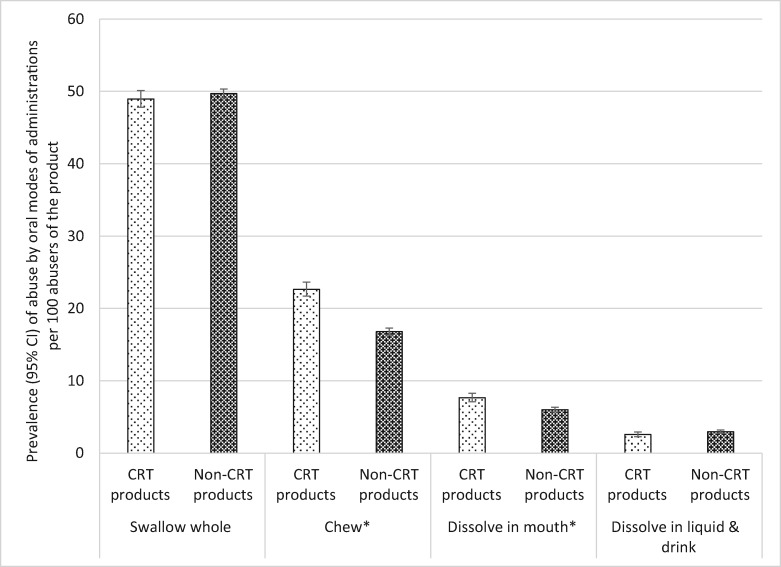
A more in-depth look at the oral mode of administration profiles of the CRT products relative to their non-CRT comparators among any abusers of the CRT and non-CRT products (i.e., regardless of other routes that may have been used) reveals interesting findings presented in Table 4 and Figure 2. CRT abuse by swallowing whole was not different from the non-CRT version. However, abuse of CRT products by chewing was reported 1.35 times more often than the non-CRT formulations (RR = 0.74). Similarly, the CRT formulations were dissolved in the mouth 1.28 times more than the non-CRT formulation (RR = 0.78). On the other hand, the CRT and non-CRT products were not significantly different with respect to dissolving the product in liquid (see Table 4 and Figure 2).
Table 4.
Oral MOA profiles of the CRT products relative to their non-CRT comparators among oral abusers of the product category
| MOA | Product Category | Weighted Abuse Prevalence* | 95% CI | Relative Risk | 95% CI | P |
|---|---|---|---|---|---|---|
| Swallowing whole | CRT | 48.96 | 47.80–50.12 | Reference | ||
| Non-CRT | 49.70 | 49.08–50.32 | 1.01 | 0.99–1.04 | 0.2734 | |
| Chewing | CRT | 22.65 | 21.70–23.64 | Reference | ||
| Non-CRT | 16.79 | 16.32–17.27 | 0.74 | 0.70–0.78 | <0.0001 | |
| Dissolving in the mouth | CRT | 7.66 | 7.09–8.28 | Reference | ||
| Non-CRT | 6.00 | 5.68–6.33 | 0.78 | 0.71–0.86 | <0.0001 | |
| Dissolving in liquid | CRT | 2.58 | 2.26–2.94 | Reference | ||
| Non-CRT | 2.96 | 2.74–3.20 | 1.01 | 0.99–1.34 | 0.8705 | |
CI = confidence interval; CRT = crush-resistant tablet formulations; MOA = mode of administration.
The denominator is the number of abusers of the product by oral route only; the prevalence estimate is the cases per 100 oral abusers of the product category.
Figure 2.
Prevalence of abuse of crush-resistant tablets (CRTs) and non-CRTs for all oral modes of administration (MOAs) among any respondents who abuse the product. *Difference between CRT and non-CRT significant at P < 0.0001. Note: The denominator for each drug category is all individuals who abused at least one of the products included in the category (see Table 1 and Figure 1). The numerator is individuals who abused the product by the MOA. CI = confidence interval; CRT = crush-resistant tablet.
Oral Abuse Route that Involved Product Manipulation Among Oral Abusers
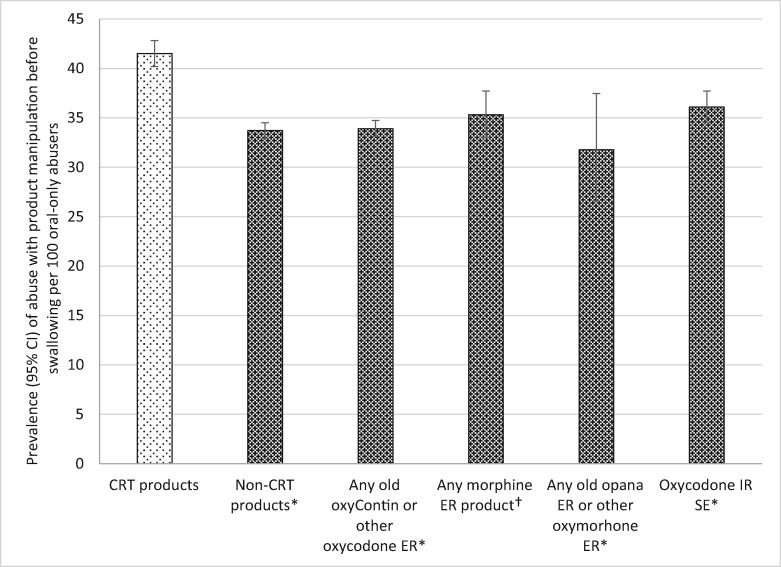
Among the subgroup of those who abuse opioids orally (N = 18,135), CRTs were significantly more likely to be abused through the oral route after product manipulation than all other comparators (see Table 5 and Figure 3). Specifically, CRTs were abused by alternate MOAs (i.e., manipulated before swallowing) 1.23 times more often than non-CRTs (RR = 0.81), 1.22 times more often than non-CRT oxycodone ER (RR = 0.82), 1.18 times more often than morphine ER (RR = 0.85), 1.31 times more often than non-CRT oxymorphone ER (RR = 0.77), and 1.15 times more often than oxycodone IR SE (RR = 0.87).
Table 5.
Prevalence of CRT and comparator abuse by various oral methods of administration among oral abusers of the product category
| Weighted Abuse Prevalence* | Prevalence 95% CI | Relative Risk | P | ||
|---|---|---|---|---|---|
| Abuse by any alternative oral method requiring tampering | |||||
| CRT | 41.51 | 40.20–42.83 | Reference | ||
| Comparators | Non-CRT versions† | 33.72 | 32.92–34.52 | 0.81‡ | <0.0001 |
| Non-CRT oxycodone ER | 33.91 | 33.11–34.73 | 0.82 | <0.0001 | |
| Morphine ER | 35.33 | 33.01–37.71 | 0.85 | <0.0001 | |
| Non-CRT oxymorphone ER | 31.79 | 26.60–37.47 | 0.77 | 0.0027 | |
| Oxycodone IR SE | 36.10 | 34.51–37.72 | 0.87 | <0.0001 | |
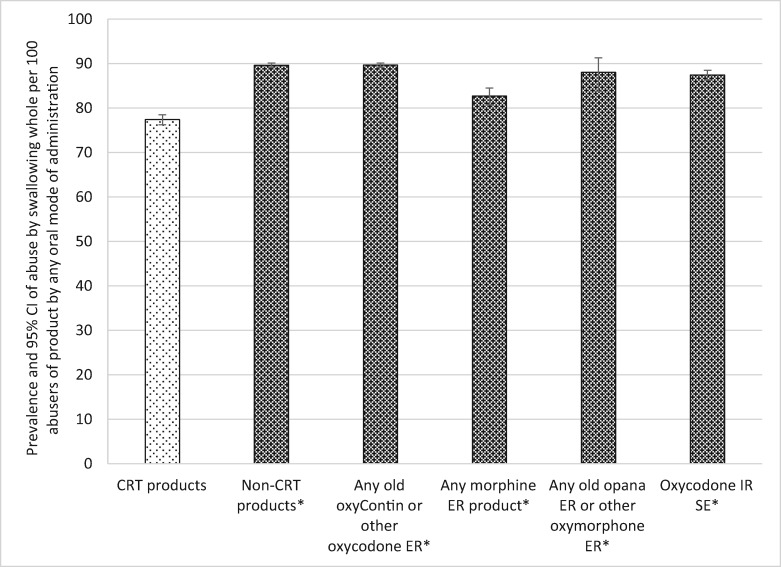
| Abuse by swallowing whole (no tampering) | |||||
| CRT | 77.39 | 76.25–78.48 | Reference | ||
| Comparators | Non-CRT versions | 89.62 | 89.10–90.12 | 1.16 | <0.0001 |
| Non-CRT oxycodone ER | 89.64 | 89.11–90.14 | 1.16 | <0.0001 | |
| Morphine ER | 82.69 | 80.74–84.49 | 1.07 | <0.0001 | |
| Non-CRT oxymorphone ER | 88.01 | 83.76–91.27 | 1.14 | <0.0001 | |
| Oxycodone IR SE | 87.43 | 86.31–88.47 | 1.13 | <0.0001 | |
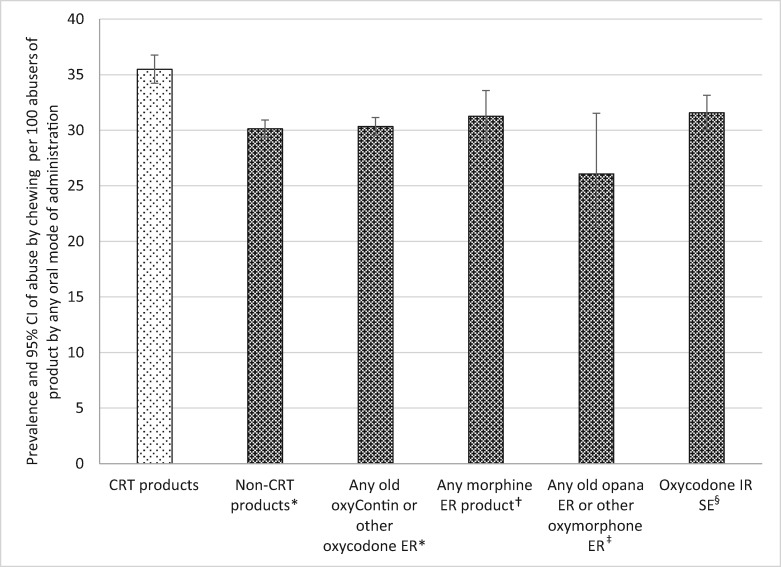
| Abuse by chewing | |||||
| CRT | 35.48 | 34.22–36.77 | Reference | ||
| Comparators | Non-CRT versions | 30.12 | 29-35–30.91 | 0.85 | <0.0001 |
| Non-CRT oxycodone ER | 30.34 | 29-56–31.14 | 0.86 | <0.0001 | |
| Morphine ER | 31.26 | 29.05–33.57 | 0.88 | 0.0004 | |
| Non-CRT oxymorphone ER | 26.07 | 21.26–31.53 | 0.73 | 0.0026 | |
| Oxycodone IR SE | 31.57 | 30.04–33.14 | 0.89 | 0.0002 | |
| Abuse by dissolving in the mouth (sucking) | |||||
| CRT | 12.39 | 11.53–13.29 | Reference | ||
| Comparators | Non-CRT versions | 10.51 | 9.98–11.07 | 0.85 | 0.0003 |
| Non-CRT oxycodone ER | 10.61 | 10.07–11.18 | 0.86 | 0.0006 | |
| Morphine ER | 7.90 | 6.11–9.51 | 0.64 | 0.0001 | |
| Non-CRT oxymorphone ER | 8.93 | 6.11–12.88 | 0.72 | 0.0920 | |
| Oxycodone IR SE | 12.14 | 11.09–13.27 | 0.98 | 0.7282 | |
| Abuse by dissolving in liquid and drinking | |||||
| CRT | 4.30 | 3.79–4.87 | Reference | ||
| Comparators | Non-CRT versions | 5.26 | 4.87–5.67 | 1.22 | 0.0066 |
| Non-CRT oxycodone ER | 5.31 | 4.92–5.74 | 1.23 | 0.0047 | |
| Morphine ER | 4.99 | 3.93–6.30 | 1.16 | 0.2751 | |
| Non-CRT oxymorphone ER | 4.29 | 2.45–7.39 | 1.00 | 0.9933 | |
| Oxycodone IR SE | 4.99 | 4.32–5.77 | 1.16 | 0.1256 | |
CI = confidence interval; CRT = crush-resistant tablet formulations.
The denominator is the number of abusers of the product by oral route only; the prevalence estimate is the cases per 100 oral abusers of the product category.
See Table 1 for specific products within each category.
Relative risk values below 1 indicate that the comparator estimate is smaller than the reference. Relative risk values greater than 1 imply that the comparator estimate is greater than the reference.
Figure 3.
Prevalence of oral abuse that involved product manipulation prior to oral ingestion among oral abusers of target products. *Crush-resistant tablet (CRT) products significantly greater than comparator products at P < 0.0001. †CRT products significantly greater than comparator products at P = 0.0027. Note: The denominator for each drug category is individuals who report any oral abuse of at least one of the products included in the category (see Table 1). The numerator is individuals who abused the product by an oral mode that involved product manipulation. CI = confidence interval; CRT = crush-resistant tablet; ER = extended-release; IR = immediate-release; MOA = mode of administration; SE = single entity.
Abuse by Swallowing Whole Among Oral Abusers
Among those respondents who reported abuse by the oral route, swallowing whole was by far the most common oral MOA for the opioid groupings examined, ranging from a low of 77.4 cases of swallowing per 100 oral abusers for the CRTs to a high of 89.64 cases per 100 oral abusers for non-CRT oxycodone ER (see Table 5 and Figure 4). As ASI-MV respondents can select all routes or modes of administration that apply, these high levels obtained for swallowing whole reinforce the finding that swallowing the tablet whole is observed in individuals who also use other oral MOAs. Among those who abuse the products orally, CRTs were significantly less likely to be abused by swallowing whole than all other comparators. CRTs were abused by swallowing whole less often than non-CRTs (RR = 1.16), less often than non-CRT oxycodone ER (RR = 1.16), less often than morphine ER (RR = 1.07), less often than oxymorphone ER (RR = 1.14), and less often than oxycodone IR SE (RR = 1.13). All comparisons were significant at a P value of less than 0.0001 (Table 5 and Figure 4).
Figure 4.
Prevalence of abuse of crush-resistant tablets (CRTs) and comparator products by swallowing whole among oral abusers (no tampering). *CRT products significantly less than comparator products at P < 0.0001. Note: The denominator for each drug category is individuals who report any oral abuse of at least one of the products included in the category (see Table 1). The numerator is individuals who abused at least one of the products in the category by swallowing the tablet or capsule whole (i.e., intended mode of administration). CI = confidence interval; CRT = crush-resistant tablet; ER = extended-release; IR = immediate-release; SE = single entity.
Abuse by Chewing Among Oral Abusers
Chewing the product was the second most common oral MOA reported by oral abusers; between about 26 and 35 cases per 100 oral abusers. Among those who abuse the products orally, CRTs were significantly more likely to be abused by chewing than ER non-CRTs, non-CRT oxycodone ER, morphine ER, non-CRT oxymorphone ER, and oxycodone IR SE (see Table 5 and Figure 5).
Figure 5.
Prevalence of tampering with an abuse-deterrent formulation and comparator products by chewing among oral abusers. *Crush-resistant tablet (CRT) products significantly greater than comparator products at P < 0.0001. †CRT products significantly greater than comparator products at P = 0.0004. ‡CRT products significantly greater than comparator products at P = 0.0026. §CRT products significantly greater than comparator products at P = 0.0002. Note: The denominator for each drug category is individuals who report any oral abuse of at least one of the products included in the category (see Table 1). The numerator is individuals who abused at least one of the products in the category by chewing. CI = confidence interval; CRT = crush-resistant tablet; ER = extended-release; IR = immediate-release; SE = single entity.
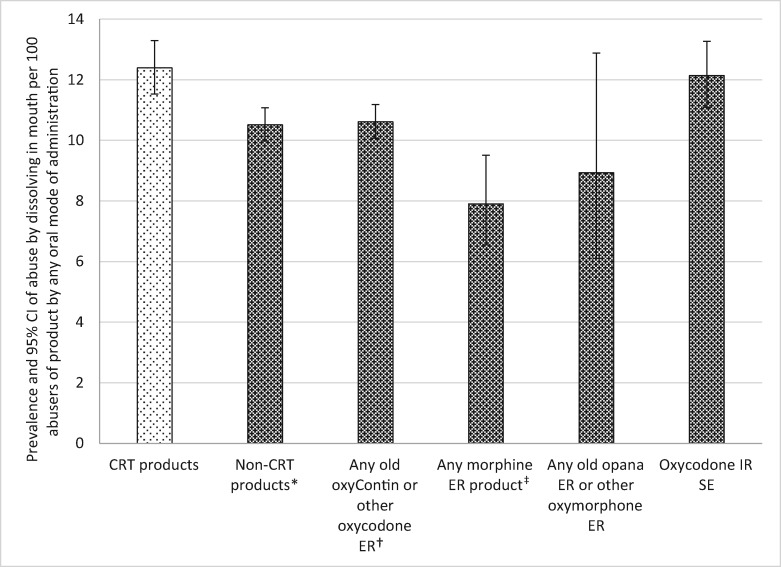
Abuse by Dissolving in the Mouth Among Oral Abusers
CRTs were significantly more likely to be abused by dissolving in the mouth (i.e., sucking on the tablet) than most of the comparators (see Table 5 and Figure 6). Thus, CRTs were abused by sucking on the tablet 1.18 times more often than non-CRTs (RR = 0.85), 1.17 times more often than non-CRT oxycodone ER (RR = 0.86), and 1.58 times more often than morphine ER (RR = 0.64). Two comparator groups did not reach significance. Abuse by dissolving in the mouth was reported for non-CRT oxymorphone ER less often than in the CRT group; however, due to the wide confidence interval for non-CRT oxymorphone ER, this difference was not significant. Abuse by dissolving in the mouth was not different for oxycodone IR SE compared with the CRTs.
Figure 6.
Prevalence of tampering with an abuse-deterrent formulation and comparator products by dissolving in the mouth among oral abusers. *Crush-resistant tablet (CRT) products significantly greater than comparator products at P = 0.0003. †CRT products significantly greater than comparator products at P = 0.0006. ‡CRT products significantly greater than comparator products at P < 0.0001. Note: The denominator for each drug category is individuals who report any oral abuse of at least one of the products included in the category (see Table 1). The numerator is individuals who abused at least one of the products in the category by dissolving in the mouth (e.g., by sucking on tablet). CI = confidence interval; CRT = crush-resistant tablet; ER = extended-release; IR = immediate-release; SE = single entity.
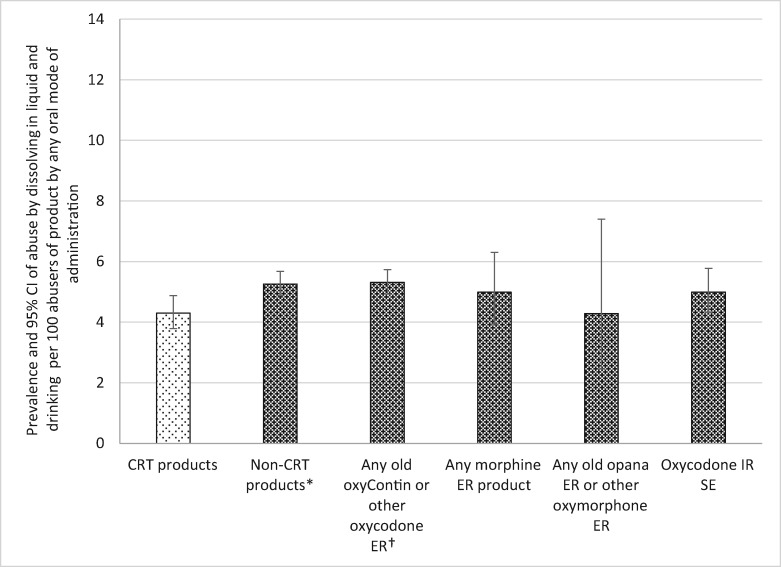
Abuse by Dissolving in Liquid and Drinking Among Oral Abusers
Abuse by dissolving in a beverage and then drinking was reported for all product categories less than 6% of the time. In this analysis, about 4.3% of oral abusers reported using CRTs by drinking after dissolving in liquid, which was significantly fewer cases reporting abuse by this MOA than the non-CRTs and non-CRT oxycodone ER. Reports of abuse of CRTs by this MOA were not different from morphine ER, non-CRT oxymorphone ER, or oxycodone IR SE (see Table 5 and Figure 7).
Figure 7.
Prevalence of tampering with an abuse-deterrent formulation and comparator products by dissolving in liquid and drinking among oral abusers. *Crush-resistant tablet (CRT) products significantly less than comparator products at P = 0.0066. †CRT products significantly less than comparator products at P = 0.0047. Note: The denominator for each drug category is individuals who report any oral abuse of at least one of the products included in the category (see Table 1). The numerator is individuals who abused at least one of the products in the category by dissolving in a liquid and drinking. CI = confidence interval; CRT = crush-resistant tablet; ER = extended-release; IR = immediate-release; SE = single entity.
Discussion
This observational study investigated the relative use of various oral MOAs of currently marketed crush-resistant tablets formulated to have abuse-deterrent properties. The primary findings in this study were that, among those abusers who continue to abuse the crush-resistant tablets, ASI-MV respondents were more likely to report manipulation prior to oral abuse of CRTs compared with non-CRT comparators. More specifically, among those who abuse the opioid analgesics orally, CRTs were less likely than non-CRT comparators to be swallowed whole and more likely to be chewed. CRTs were also more likely than most comparators to be dissolved in the mouth (i.e., sucked on).
Prior studies suggest that some CRTs intended to deter abuse show promise for reducing nonoral abuse when compared with the non-CRT versions [14,15–17]. While achieving the goal of reducing nonoral abuse is consistent with the FDA guidance on evaluating abuse deterrence [32], the guidance is clear that such formulations may not prevent oral abuse. The same point is made in the recently published CDC guideline for prescribing opioids for chronic noncancer pain, which states that 1) while abuse-deterrent technologies may be expected to make manipulation of opioids more difficult or less rewarding, they do not prevent opioid abuse through oral intake, which is the most common route of opioid abuse, 2) CRTs formulated to deter abuse can still be abused by nonoral routes, and 3) an abuse-deterrent label does not guarantee that there is no risk for abuse [33].
The literature on CRTs thus far appears to assume that a CRT that effectively reduces use by nonoral routes will, more or less inevitably, result in a shift to oral abuse of the products [16]. So far, however, the existing literature does not distinguish between oral, “as-intended” administration (swallowing a tablet whole) and other oral MOAs that involve product manipulation before swallowing. There is a general consensus that chewing in particular has been a popular and effective way for individuals to extract the API from ER formulations in order to achieve a more potent and rapid rewarding effect [13,16,34]. By examining the different MOAs used by oral abusers, the present study sheds light on this question, suggesting that when a CRT is marketed, at least part of the response by abusers to greater difficulty insufflating or injecting is to use the product by tampering prior to oral abuse.
Little is known about the oral abuse of prescription opioids. While it is generally true that oral intake is the most common route for abusing opioids [33], more detailed examination of routes and products reveals variability in ROA patterns across products and populations. In one study of individuals in substance abuse treatment [12], only about 30% of abusers of hydromorphone IR and morphine IR products used the tablet forms orally, while about 55% injected. For morphine ER abusers, oral abuse was reported by 39% of respondents, while injection was reported by 45%. Thus, not all ER products have a high prevalence of oral abuse. In another study, Butler et al. [15] documented that among those who abused non-CRT oxymorphone ER, between 30% and 38% used this product orally, compared with snorting, which was observed in 62% to 69% of the respondents. Likewise, morphine ER products in that study were taken orally at about the same rate as injected (around 45%). Such across-product differences have been observed in other studies with different populations, such as individuals who post on online drug-abuse forums [35]. ROA patterns vary across age groups, drug use experience, gender, and geographic location (see [14] for a full review). Thus, oral abuse may represent a more complex phenomenon than is sometimes assumed.
Most authorities agree that abuse by oral routes is associated with fewer complications than insufflation and injection with respect to major adverse societal and health impacts [36]. Well-known risks associated with injection include serious infection (e.g., sepsis, endocarditis, cellulitis, and osteomyelitis), disease transmission (e.g., HIV, hepatitis A, B, and C), and pulmonary complications, while insufflation has been associated with nasal pain, tissue necrosis, septal and palatal perforation, and fungal infections [14]. Katz et al. [13] examined poison control data and found that 8.6% of oral ingestions, 10.2% of insufflations, and 16.5% of injections were associated with major adverse effects or death. However, examination of death cases found that 96% of deaths were associated with oral ingestion, a reflection that oral administration is the most frequently reported method of abuse in the poison control data [13]. Also, it should be noted that many of those who inject and snort prescription opioids also report oral use [12]. At the same time, the role of oral use in general and alternate MOAs, such as chewing in particular, are routinely implicated in the progression from initial use to abuse and addiction. Several authors have suggested that a major goal of CRTs would be to interrupt this progression from abusing a prescription opioid orally to use by more dangerous ROAs or switching to heroin [13,16,36]. The current crush-resistant tablets may discourage insufflation and injection, but individuals who initiate abuse of prescription opioids may still “progress” to alternate oral MOAs, especially chewing. Cicero and Ellis [16] have recently predicted that as the ability to manipulate prescription opioids is reduced, some individuals will be discouraged from nonmedical use entirely.
Limitations of the ASI-MV have been enumerated in detail elsewhere [12,15,37]. The ASI-MV data are a convenience sample of individuals assessed at treatment facilities that are part of the ASI-MV network, and therefore analyses of these data do not yield nationally representative estimates of abuse prevalence. The ASI-MV data also do not represent individuals who misuse or abuse prescription opioids but are not in treatment. Findings cannot be generalized to individuals in treatment at facilities not included in the ASI-MV network. ASI-MV data are self-reported, which carries some biases, although self-report is the only way to collect data on specific products and routes used. Some authors have emphasized the limitations of all national-level data streams that currently provide postmarketing data for evaluation of abuse potential, including the ASI-MV [38]. We concur that, like the other data streams, the ASI-MV has the important limitations mentioned above. Nevertheless, one should not lose sight of the fact that the ASI-MV sample is large and draws from treatment sites across the country and that abuse data are obtained using consistent methods across time, sites, patients, and drug compounds/formulations. Furthermore, the ASI-MV data stream, used in this paper, was also used as one data stream in an evaluation of the abuse-deterrent properties of OxyContin. That investigation involved 10 different studies and multiple data streams. In addition to the ASI-MV’s substance abuse treatment center data, data were utilized from poison control centers, other drug treatment data, diversion data, data on overdose, prescription data, and surveys of abusers [17]. In this large study, the ASI-MV data yielded results for the target product and comparators that were in concordance with the direction and magnitude of the findings from all of these studies, suggesting that ASI-MV data reflect national patterns of abuse of prescription opioids.
Another limitation of this study is that route of administration is a self-reported behavior, not an effect. Chewing, dissolving in the mouth (i.e., sucking), dissolving in liquid and drinking, and snorting and injecting are reported behaviors that may or may not be effective in extracting the API. This is a limitation of ASI-MV data because the assessment does not ask about the quality of the resulting euphoric effect (i.e., high). In vitro and in vivo studies of crushed, reformulated OxyContin for insufflation found that API extraction by crushing reduces the rate and extent of nasal API absorption when compared with the non-CRT version [39,40]. Subjective reports of the effects of insufflation of reformulated OxyContin compared with the non-CRT version showed decreased liking [40] and increased nasal discomfort [39]. Nevertheless, crushing the CRT version did result in some API extraction and greater overall drug liking, take the drug again VAS, and subjective ratings of drug value compared to “OxyContin (OC) placebo” [40]. Another study found that after oral administration, mean peak plasma oxycodone concentrations for crushed reformulated oxycodone ER were significantly higher than intact reformulated oxycodone ER and bioequivalent to IR oxycodone, with a median Tmax for crushed reformulated oxycodone ER that was the same as for IR oxycodone [41]. If we extend such findings to chewing, for instance, it is possible that those in this study who chewed the product may have experienced a euphoric benefit beyond swallowing the opioid intact. The validity of such an assumption would require human abuse liability studies that directly evaluate drug liking of the CRT product when chewed compared with the non-CRT version or other comparator.
A final limitation is the use of a CRT category of products, rather than comparing individual products with their non-CRT versions (e.g., oxycodone ER and oxymorphone ER). The primary aim of this work was examination of the crush-resistant formulations that these products share, although differences in oral bioavailability of the APIs may impact oral abuse patterns of these products. Post hoc examination of product-specific CRT vs non-CRT differences for oxycodone ER and oxymorphone ER suggests that in both cases swallowing whole was less prevalent for the CRT than for the non-CRT formulation. That is, among oral abusers of these products, the CRT version of each product was less likely to be swallowed whole than the non-CRT version, confirming the categorical findings. Evidence of greater oral use by methods requiring tampering (i.e., chewing, dissolving in the mouth, or dissolving and drinking) for the CRT version for oxycodone ER was similar to the findings using the CRT categories. There were relatively fewer oral abusers of oxymorphone ER, possibly due to its lower oral bioavailability, and differences for the MOAs that require tampering were not significant, likely due to power issues when examined among oral abusers of oxymorphone ER only.
Conclusions and Clinical Implications
The main finding of this study is that oral abuse involving tampering/manipulation (e.g., chewing, dissolving in the mouth) is more commonly reported in abuse of CRT opioids than non-CRT opioids. While initial studies of CRT opioid analgesic products suggest that these products may reduce abuse by nonoral ROAs, for those who continue to abuse these products, the prevalence of abuse by oral routes generally increases. Oral abuse is not well understood, particularly for ER opioids, and data presented here illustrate that oral abuse often involves product manipulation. Formulations that can better resist manipulation are important because they can potentially interrupt the progression from initial use by swallowing whole to chewing/crushing, to insufflation, and eventually to injection. These findings may also be relevant when prescribing for pain patients with dysphagia, odynophagia, or difficulty swallowing [41]. Even nonabuse (i.e., misuse)-related “tampering” of a crush-resistant product by a patient or caregiver could disrupt the extended-release mechanism, which is a safety concern.
Taken together, these results suggest the need for abuse-deterrent formulations designed to reduce oral abuse, including by MOAs that involve tampering, such as chewing. The overall goal of CRTs can be viewed as discouraging abuse by all nonintended routes (i.e., routes other than swallowing whole), thereby reducing the attractiveness or desirability of these products to abusers [42,43]. In any event, prescribers should remember that prescribing an abuse-deterrent or crush-resistant opioid does not fully address concerns about abuse, misuse, or diversion. Prescribers should document in the treatment plan assessments performed, rationale for the prescription decision, and approaches taken to address the risk of oral abuse and product manipulation.
Funding sources: Funding for this research was provided by Collegium Pharmaceutical, Inc. (Canton, MA, USA) to Inflexxion, Inc. (Waltham, MA, USA) for support in the writing and editing of this article.
Disclosure and conflicts of interest: SFB is an employee and stockholder of Inflexxion, Inc. RAB is an employee of Nova Southeastern University, Center for Psychological Studies, and works part-time for Inflexxion. ABF is an employee of Collegium Pharmaceutical, Inc. Inflexxion has contracts for various projects with companies that have interests in some of the products that were included in the drugs evaluated for this article. The sponsor was involved in reviewing the analyses and the content of this manuscript; all data collection, analysis, and ultimate data interpretation were made by the authors independently.
References
- 1. Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain 2009;102:113–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: A systematic review for a National Institutes of Health pathways to prevention workshop. Ann Intern Med 2015;1624:276–86. [DOI] [PubMed] [Google Scholar]
- 3. Levy B, Paulozzi L, Mack KA, Jones CM.. Trends in opioid analgesic—prescribing rates by specialty, U.S., 2007–2012. Am J Prev Med 2015;493:409–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Butler SF, Zacharoff K, Charity S, Lawler K, Jamison RN.. Electronic opioid risk assessment program for chronic pain patients: Barriers and benefits of implementation. Pain Pract 2014;143:E98–105. [DOI] [PubMed] [Google Scholar]
- 5. Butler SF, Zacharoff KL, Charity S, et al. Impact of an electronic pain and opioid risk assessment program: Are there improvements in patient encounters and clinic notes? Pain Med 2016;1711:2047–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. FDA. Introduction for the FDA blueprint for prescriber education for extended-release and long-acting opioid analgesics [Internet]. 2014. Available at: http://www.fda.gov/downloads/drugs/drugsafety/informationbydrugclass/ucm277916.pdf (accessed January 1, 2016).
- 7. Paulozzi LJ, Kilbourne EM, Desai HA.. Prescription drug monitoring programs and death rates from drug overdose. Pain Med 2011;12:747–54. [DOI] [PubMed] [Google Scholar]
- 8. Griggs C, Weiner S, Feldman J.. Prescription drug monitoring programs: Examining limitations and future approaches. West J Emerg Med 2015;161:67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Thomas CP, Fullerton CA, Kim M, et al. Medication-assisted treatment with buprenorphine: Assessing the evidence. Psychiatr Serv 2014;652:158–70. [DOI] [PubMed] [Google Scholar]
- 10. Compton WM, Boyle M, Wargo E.. Prescription opioid abuse: Problems and responses. Prev Med (Baltim) 2015;80:5–9. [DOI] [PubMed] [Google Scholar]
- 11. Farré M, Camí J.. Pharmacokinetic considerations in abuse liability evaluation. Br J Addict 1991;8612:1601–6. [DOI] [PubMed] [Google Scholar]
- 12. Butler SF, Black RA, Cassidy TA, Dailey TM, Budman SH.. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J 2011;81:29–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Katz NP, Dart RC, Bailey E, et al. Tampering with prescription opioids: Nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse 2011;374:205–17. [DOI] [PubMed] [Google Scholar]
- 14. Gasior M, Bond M, Malamut R.. Routes of abuse of prescription opioid analgesics: A review and assessment of the potential impact of abuse-deterrent formulations. Postgrad Med 2016;1281:85–96. [DOI] [PubMed] [Google Scholar]
- 15. Butler SF, Cassidy TA, Chilcoat H, et al. Abuse rates and routes of administration of reformulated extended-release oxycodone: Initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. J Pain 2013;144:351–8. [DOI] [PubMed] [Google Scholar]
- 16. Cicero TJ, Ellis MS.. Abuse-deterrent formulations and the prescription opioid abuse epidemic in the United States. JAMA Psychiatry 2015;631105:1–6. [DOI] [PubMed] [Google Scholar]
- 17. Coplan PM, Chilcoat HD, Butler SF, et al. The effect of an abuse-deterrent opioid formulation (OxyContin) on opioid abuse-related outcomes in the postmarketing setting. Clin Pharmacol Ther 2016;1003:275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cassidy TA, Budman SH, Butler SF. Evaluation of an ADF product’s route of administration profile. 76th Annual CPDD Meeting (June 14–19). San Juan, Puerto Rico; 2014.
- 19. Butler SF, McNaughton EC, Black RA, Cassidy TA. Evaluation of the relative abuse of an OROS extended-release hydromorphone HCI product: Results from three post-market surveillance studies. 2016; under review. [DOI] [PubMed]
- 20. Hays LR, Kirsh KL, Passik SD.. Seeking drug treatment for OxyContin abuse: A chart review of consecutive admissions to a substance abuse treatment facility in Kentucky. J Natl Compr Canc Netw 2003;13:423–8. [DOI] [PubMed] [Google Scholar]
- 21. Hays LR. A profile of OxyContin addiction. J Addict Dis 2004;234:1–9. [DOI] [PubMed] [Google Scholar]
- 22. Butler SF, Budman SH, Licari A, et al. National addictions vigilance intervention and prevention program (NAVIPPRO): A real-time, product-specific, public health surveillance system for monitoring prescription drug abuse. Pharmacoepidemiol Drug Saf 2008;1712:1142–54. [DOI] [PubMed] [Google Scholar]
- 23. Butler SF, Budman SH, Goldman RJ, et al. Initial validation of a computer-administered addiction severity index: The ASI–MV. Psychol Addict Behav 2001;151:4–12. [DOI] [PubMed] [Google Scholar]
- 24. Hendricks V, Kaplan C, VanLimbeek J, Geerlings P.. The addiction severity index: Reliability and validity in a Dutch addict population. J Subst Abuse Treat 1989;6:133–41. [DOI] [PubMed] [Google Scholar]
- 25. Kosten T, Rounsaville B, Kleber H.. Concurrent validity of the addiction severity index. J Nerv Ment Disord 1983;17110:606–10. [DOI] [PubMed] [Google Scholar]
- 26. McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat 1992;93:199–213. [DOI] [PubMed] [Google Scholar]
- 27. Cassidy TA, McNaughton EC, Varughese S, et al. Nonmedical use of prescription ADHD stimulant medications among adults in a substance abuse treatment population: Early findings from the NAVIPPRO surveillance system. J Atten Disord 2015;194:275–83. [DOI] [PubMed] [Google Scholar]
- 28. Chilcoat H, Bartelson BB, Severtson SG, et al. Trends in abuse and diversion in multiple surveillance systems three years after introduction of reformulated OxyContin. Drug Alcohol Depend 2015;146:e246. [Google Scholar]
- 29. Cassidy TA, DasMahapatra P, Black RA, Wieman MS, Butler SF.. Changes in prevalence of prescription opioid abuse after introduction of an abuse-deterrent opioid formulation. Pain Med 2014;153:440–51. [DOI] [PubMed] [Google Scholar]
- 30. Myers JL, Well AD, Lorch RF.. Research Design and Statistical Analysis, 3rd edition Mahwah, NJ: Erlbaum; 2010. [Google Scholar]
- 31. SAS Institute Inc. SAS/STAT 14.1 User’s Guide. Cary, NC: SAS Institute Inc; 2015. [Google Scholar]
- 32.Food and Drug Administration. Guidance for Industry: Abuse-deterrent opioids—evaluation and labeling [Internet]. 2015. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM334743.pdf (accessed January 12, 2015).
- 33. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR. 2016;65(No. RR-1):1–49. [DOI] [PubMed] [Google Scholar]
- 34. Cone EJ. Ephemeral profiles of prescription drug and formulation tampering: Evolving pseudoscience on the Internet. Drug Alcohol Depend 2006;83(suppl 1):S31–9. [DOI] [PubMed] [Google Scholar]
- 35. Katz N, Fernandez K, Chang A, Benoit C, Butler SF.. Internet-based survey of nonmedical prescription opioid use in the United States. Clin J Pain 2008;246:528–35. [DOI] [PubMed] [Google Scholar]
- 36. Budman SH, Grimes Serrano JM, Butler SF.. Can abuse deterrent formulations make a difference? Expectation and speculation. Harm Reduct J 2009;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Butler SF, McNaughton EC, Black RA.. Tapentadol abuse potential: A postmarketing evaluation using a sample of individuals evaluated for substance abuse treatment. Pain Med 2015;161:119–30. [DOI] [PubMed] [Google Scholar]
- 38. Secora AM, Dormitzer CM, Staffa JA, Dal Pan GJ.. Measures to quantify the abuse of prescription opioids: A review of data sources and metrics. Pharmacoepidemiol Drug Saf 2014;2312:1227–37. [DOI] [PubMed] [Google Scholar]
- 39. Perrino PJ, Colucci SV, Apseloff G, Harris SC.. Pharmacokinetics, tolerability, and safety of intranasal administration of reformulated OxyContin tablets compared with original OxyContin tablets in healthy adults. Clin Drug Investig 2013;336:441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harris SC, Perrino PJ, Smith I, et al. Abuse potential, pharmacokinetics, pharmacodynamics, and safety of intranasally administered crushed oxycodone HCl abuse-deterrent controlled-release tablets in recreational opioid users. J Clin Pharmacol 2014;544:468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gudin J, Levy-Cooperman N, Kopecky EA, Fleming AB.. Comparing the effect of tampering on the oral pharmacokinetic profiles of two extended-release oxycodone formulations with abuse-deterrent properties. Pain Med 2015;1611:2142–51. [DOI] [PubMed] [Google Scholar]
- 42. Butler SF, Fernandez KC, Chang A, et al. Measuring attractiveness for abuse of prescription opioids. Pain Med 2010;111:67–80. [DOI] [PubMed] [Google Scholar]
- 43. Butler SF, Black R, Grimes Serrano JM, et al. Estimating attractiveness for abuse of a not-yet-marketed “abuse-deterrent” prescription opioid formulation. Pain Med 2010;111:81–91. [DOI] [PubMed] [Google Scholar]