Abstract
Background
Chronic pain is prevalent, costly, and disabling among older adults. Although mobility decline is inevitable with aging, it is clear, from current evidence, that older adults with chronic pain experience a greater rate of functional mobility decline than their pain-free peers. Past studies suggest that pain expedites the age-related decline in functional mobility; however, the pathways through which pain affects mobility remain unclear. Gerontological experts hypothesize that the age-related decline in mobility may be driven by alterations in energy expenditure; these concepts are outlined in a model known as the Energetic Pathway of Mobility Loss. Pain may play a critical role in this process through a pathway of energetic inefficiency, physical inactivity, and decreased capacity.
Purpose
The purposes of this article are to 1) summarize the current literature that supports the Energetic Pathway of Mobility Loss model and 2) propose a new framework, known as the Pain Energy Model, to clarify how the disablement process may be amplified among older adults with painful conditions.
Conclusion
This new framework is designed to generate new clinical research and to suggest new clinical implications for older adults with painful conditions by identifying key steps and potential treatment targets in the pathway to functional mobility decline.
Keywords: Geriatric, Chronic Pain, Energy Expenditure, Conceptual Model, Mobility, Disability
Introduction
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential damage, or described in terms of such damage” [1]. Pain is a multifactorial, complex process that places a massive burden on society. Experts estimate that chronic pain conditions account for more than $600 billion annually in direct and indirect health care costs [2]. The prevalence of chronic pain rises with age, primarily due to the development of musculoskeletal conditions and neurodegenerative processes [3]. Furthermore, pain-related health care costs continue to rise in the aging population [4]. Medicare claims data from 1991 to 2002 reveal a 300% increase in charges related to low back pain [4], a common chronic condition among older adults [5]. In addition, older adults with chronic pain are at a higher risk for disability and a reduced quality of life [6–10].
Decreases in walking speed, which are a hallmark sign of the disablement process [11,12], are commonly seen among older adults with chronically painful conditions (e.g., symptomatic knee osteoarthritis and chronic low back pain). Walking is arguably the most important aspect of functional mobility, as walking speed alone is a strong predictor of adverse outcomes, such as disability [12–14] and mortality [15]. Current evidence suggests that older adults with chronic pain walk slower than their pain-free peers [7,10,16–20]; however, the primary drivers of this deterioration in mobility remain unclear. To develop effective interventions, we must first have a better understanding of the mechanisms through which disability develops in these geriatric patient populations.
Among older adults with pain, there are many conceivable pathways through which limitations in mobility may occur. Perhaps the most commonly held belief is that some individuals with pain avoid certain activities for fear of pain or injury (i.e., fear-avoidant behavior) [21]. Theoretically, as people become more fearful of exacerbating their pain, they engage in less physical activity, resulting in physical deconditioning and a perpetuation of the pain and disability cycle [21,22]. Indeed, physical deconditioning has been linked to increased risk of disability [23,24] and death [25,26], regardless of the presence of pain. Yet fear avoidance models largely ignore the physiological underpinning of pain that may contribute to this process, instead focusing heavily on the psychosocial factors of pain, which may be highly subjective and variable between individuals. A new model that explores the impact of pain on physiological processes, such as energy metabolism, could generate new research that would enhance our understanding of how painful conditions lead to functional mobility deficits among older adults.
In 2010, Schrack et al. proposed a convincing conceptual framework, known as the Energetic Pathway of Mobility Loss, to explain the potential physiological mechanisms behind age-related walking speed decline (i.e., functional mobility decline) [27–29]. Pain may play an important role in this process, but its role has not been investigated beyond the broad categorization of “lower extremity arthritis pain.” In this article, we aim to 1) summarize the current literature supporting the Energetic Pathway of Mobility Loss and 2) propose a model ancillary to that of Schrack et al., the Pain Energy Model, to help clarify the physiological aspects of the disablement process among older adults experiencing pain.
Aging and Energetics
In the Energetic Pathway of Mobility Loss framework, Schrack et al. explain how mobility limitations may be the result of age-related changes in the following energy constructs:
Energy capacity: the upper limit of energy expenditure per minute available to perform vigorous activities. Often, this is measured by maximal or peak oxygen consumption during sustained, vigorous activity (VO2 max or VO2 peak, respectively) [27].
Energy cost of mobility: the energy cost of walking and other mobility-related tasks (e.g., sit-to-stand transitions, stair climbing, etc.). Energy expenditure is commonly measured by analyzing the amount of oxygen one consumes during aerobic activity. Because walking is a good surrogate measure for functional mobility [12,15], this construct is measured by quantifying the amount of oxygen consumed during walking at a fixed slow pace or at self-selected pace. If measured at a self-selected pace, oxygen consumption is often standardized to walking speed to give walking economy (i.e., the energy required to walk one meter) [30].
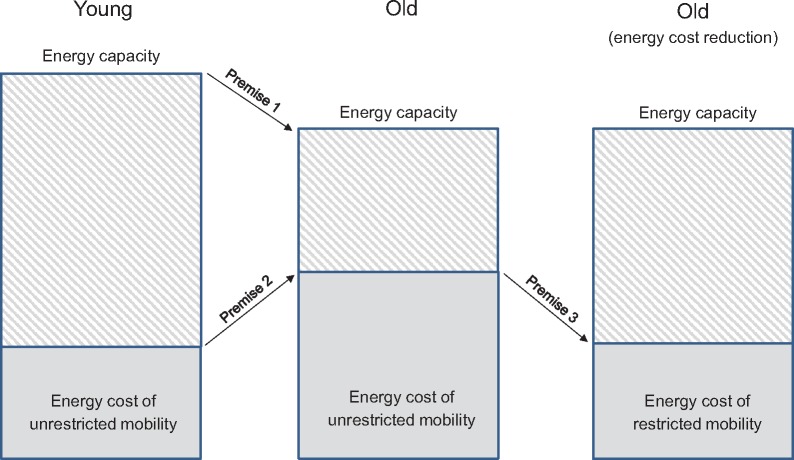
These and other key terms are summarized in Table 1. In general, the Energetic Pathway to Mobility Loss framework can be summarized in three simple premises, which are illustrated in Figure 1. Citations: Schrack et al. propose that with age, 1) energy capacity decreases, 2) the energy cost of mobility increases, and 3) mobility limitations develop when the energy cost of mobility approaches the maximum level of energy that a person is capable of expending (i.e., energy capacity) [27–29]. In the following sections, supporting literature is reviewed for each premise.
Table 1.
Glossary of key terms
| Term | Definition |
|---|---|
| Mobility limitation | Mobility limitations are deficits in the ability to move or change body positions or location, which includes walking.* |
| Energy capacity | The upper limit of energy expenditure per minute available to perform vigorous activities. Often, this is measured by maximal or peak oxygen consumption during sustained, vigorous activity (VO2 max or VO2 peak, respectively). |
| Energy cost of mobility | The energy cost of walking and other mobility-related tasks (e.g., sit-to-stand transitions, stair climbing, etc.). Energy expenditure is commonly measured by analyzing the amount of oxygen one consumes during aerobic activity. |
| VO2 | The volume of oxygen consumed during submaximal or maximal (i.e., VO2 max or peak) aerobic activity, also known as indirect calorimetry. It is quantified as the amount of oxygen consumed in milliliters per kilogram of bodyweight per minute (mL/kg/min). |
| Daily physical activity | Any bodily movement produced by skeletal muscles that requires energy expenditure.* |
From the World Health Organization website [accessed on 5 May 2017]
Figure 1.
Illustration of the Energetic Pathway of Mobility by Schrack et al. with premises noted. With age: 1) energy capacity declines while 2) the energy cost of unrestricted mobility increases. Mobility limitations occur when the energy cost of unrestricted mobility approaches the maximum level of energy a person is capable of expending (i.e., energy capacity). Mobility restrictions are a compensatory strategy for the age-related increase in the energy cost of unrestricted mobility.
Premise 1: Energy Capacity Decreases with Age
Oxygen consumption is an indirect measure of energy expenditure, known as indirect calorimetry, and it is quantified as the amount of oxygen consumed in milliliters per kilogram of bodyweight per minute (mL/kg/min). Maximal oxygen consumption, the upper limit of energy available to perform vigorous activities, is often measured as VO2 max or VO2 peak. VO2 max begins to decline during the third decade of life and exponentially deteriorates with each passing decade [31]. For example, previous research suggests that between ages 30 and 39 years, VO2 peak (a surrogate measure for VO2 max) declines an average of 0.9 mL/kg/min, whereas between ages 60 and 69 years this decline is accelerated, averaging 6.6 mL/kg/min [31]. This nonlinear trend continues well into older age, with a reduction in VO2 max values of 12 to 16 mL/kg/min occurring between the ages of 80 and 90 years [32]. Experts have hypothesized that once VO2 max falls below a certain threshold (approximately 18 mL/kg/min), functional limitations are more likely to occur [23].
Age-related changes in the musculoskeletal system, as well as in the central and peripheral cardiovascular system, combine to contribute to reduced energy capacity [31,33,34]. Sarcopenia, the reduction in lean body mass with age, can contribute to the age-related deterioration of VO2 max [31]. Furthermore, reductions in heart and blood vessel function have been shown to be closely related to declines in VO2 max [31]. With age, stroke volume (i.e., the amount of blood ejected from the heart during each beat) decreases [31,34], and the ability for oxygen to perfuse through the capillary vessel walls is attenuated [31]. The combined effect of these physiological impairments drives the age-related decline of energy capacity [31,33,34].
Premise 2: The Energy Cost of Mobility Increases with Age (i.e., Energy Inefficiency)
Walking is an essential aspect of most daily activities; thus, it serves as a good marker of functional mobility. Walking becomes less efficient with age [35]. Schrack et al. have shown that, regardless of age, people will naturally select a walking speed with an energy cost of approximately 13.0 mL/kg/min; for older adults, the walking speed needed to achieve this rate is much slower than that of younger and middle-aged adults [28], highlighting the age-related changes in energetic efficiency. It is important to note that increases in energy cost of activities are not limited to only walking; Knaggs et al. found that the energy cost of a number of daily activities increases with age when the speed at which each is performed is taken into account [36].
The mechanisms by which age leads to energy inefficiencies are less clear and may be person dependent. One mechanism that has been postulated is the age-related increase in chronic disease burden. Chronic disease can cause homeostatic imbalance, which can result in higher levels of resting energy expenditure in order to maintain homeostasis [37,38]. Furthermore, age-related changes in gait mechanics have an impact on energy expenditure as the severity of gait impairments has been shown to be directly related to the level of energy cost that walking requires among older adults [39]. As one ages, variability in gait mechanics becomes more prominent, resulting in more frequent and larger deviations from optimal biomechanics [40]; these additional movements may be extraneous to the task of walking, increasing the overall energy cost [41] and thereby resulting in energy inefficiency.
Premise 3: Mobility Limitations Develop when the Energy Cost of Mobility Approaches Energy Capacity
The age-related decline in the quantity and speed of movement is not a human-specific phenomenon, but rather a core physiologic strategy that is universal across species [27]. For example, Carter et al. found that rats walk slower as they age, and slower-walking rats have a higher risk of mortality [42]. As noted above, energy capacity declines with age [31]. Consistently performing near the maximal level of energy capacity is dangerous to sustaining life because of the risk of homeostatic collapse [43]. Priede et al. demonstrated that when fish swim at near-maximal energy capacity they have a higher risk of mortality [44]. As a strategy, animals will decrease their quantity and speed of movement near the end of life (i.e., advanced age) [42,45], presumably when the energy capacity to perform activities is low and the energy cost of movement is high.
This theory is also consistent with findings in humans. Recently, experts have hypothesized that low energy capacity and high energy cost of mobility work in tandem with one another yield mobility limitations. Schrack et al. have shown that higher energy cost of walking is predictive of walking speed decline in older adults but not in middle-aged and younger adults [46]. This indicates that higher energy cost of activities is important in the deterioration of mobility, but only in the presence of low energy capacity. In support, prior work in middle-aged and older adults has shown that the energy cost of walking is strongly related to mobility levels, but only when energy capacity falls below a certain threshold (approximately 18 mL/kg/min) [29]. Taken together, these findings suggest that limitations in mobility, such as decreased walking speed, are more likely to occur when the energy cost of mobility approximates energy capacity.
Pain Energy Model: A Novel Conceptual Approach
The findings that older adults with chronic musculoskeletal pain conditions walk slower [7,10,16,18,20] and experience greater reductions in walking speed over time, compared with those without pain [17,19], support the notion that these conditions expedite the age-related decline of functional mobility. In our physiological framework, the Pain Energy Model, we hypothesize that the following factors are important in the acceleration of mobility decline among older adults with painful conditions:
Pain experience: an unpleasant sensory and emotional experience associated with actual or potential damage, or described in terms of damage [1]. Pain can arise from injury or insult to tissues, which can result in unpleasant sensations. These sensations can be augmented by psychological (e.g., anxiety, fear, etc.) and social (e.g., rejection, attachment, etc.) factors, resulting in not only emotional distress, but also in neurobiological changes [47].
Energy cost of mobility: previously defined (Energetic Pathway of Mobility Loss Energy Constructs).
Daily physical activity: The World Health Organization defines physical activity as any bodily movement produced by skeletal muscles that requires energy expenditure [48]. Physical inactivity has been linked to a host of adverse health outcomes, including mortality [49]. Physical activity has been measured through self-report questionnaires and step activity monitors.
Energy capacity: previously defined (Energetic Pathway of Mobility Loss Energy Constructs).
The Pain Energy Model consists of three separate premises, as shown graphically in Figure 2. We propose that among older adults with acute pain, A) the pain experience increases the energy cost of functional mobility and B) an increase in the energy cost of mobility leads to a restriction in physical activity. As pain persists, we hypothesize that C) long-term physical activity restrictions result in further reductions of energy capacity. We hypothesize that when pain is acute, the pain experience drives physical inactivity, in part, by energetic inefficiency without causing clinically relevant levels of functional mobility limitation; however, as pain and physical inactivity persist, energy capacity decreases to the point that it approaches energy cost of mobility, and functional mobility limitations develop. These limitations, in turn, may contribute to increased energy cost of mobility, further driving this disability cycle. Furthermore, exacerbations of chronically painful conditions (i.e., acute-on-chronic episodes) may also expedite this process.
Figure 2.
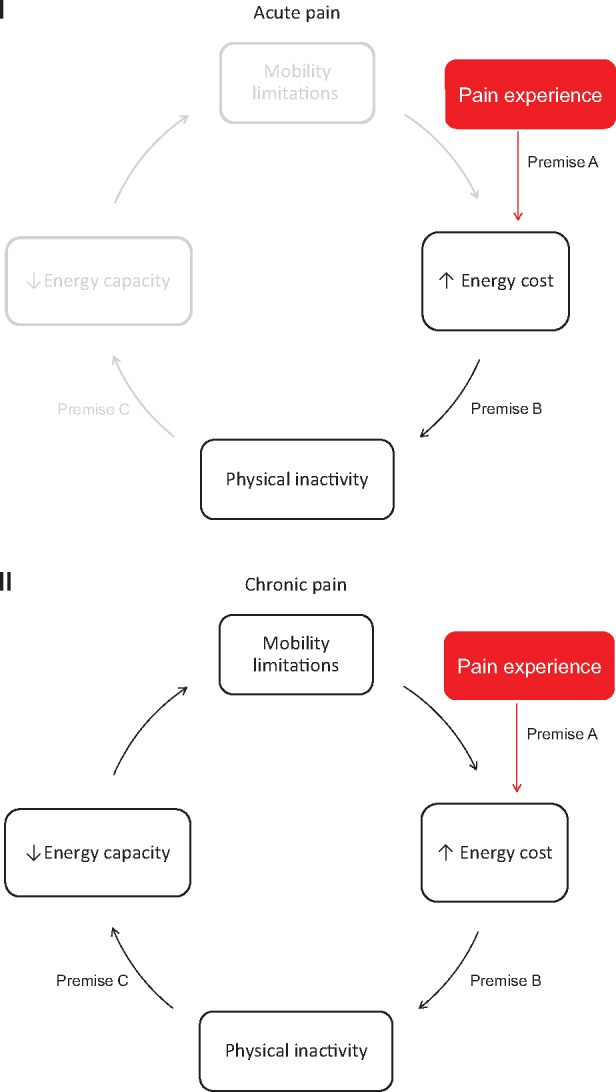
Illustration of the Pain Energy Model with premises denoted. In (I), the impact that acute pain has on this pathway is displayed. Among older adults with painful conditions: A) the pain experience increases the energy cost of mobility (i.e., energetic inefficiency), and B) increased energy cost of mobility contributes to physical inactivity. In (II), the long-term effects of pain are presented. With pain chronicity, C) persistent physical inactivity drives reductions in energy capacity. When energy capacity approaches the energetic cost of mobility, clinically relevant mobility limitations develop. These limitations, in turn, may contribute to increases in energy cost, further driving this disability cycle. Exacerbations of pain (i.e., acute-on-chronic) may also continue to drive this cycle.
Premise A: Pain Experience Increases Energy Cost of Mobility
Previous work has shown that experimentally induced pain causes resting energy expenditure to rise by nearly 62% [50], lending support to the hypothesis that acute painful sensations augment energy expenditure. Furthermore, these pain-related energetic alterations appear to carry over to fundamental activities, such as walking [51–53]. In one study, middle-aged and older adults with painful hip impairments were shown to expend more energy while walking than those without pain [53]. Similarly, Ko et al. have shown that older adults with knee pain had a higher energy cost of walking than those without knee pain [51]. In patients with intermittent vascular claudication due to peripheral arterial disease, Gardner et al. found that the energy cost of walking increased with acute pain onset and pain proliferation [52]. This evidence supports the hypothesis that the pain experience may have a universal effect on energetic efficiency, particularly within older adults with chronically painful conditions.
Pain-related changes in motor strategies are likely the mechanism by which these changes in energy efficiency occur. Gait speed shares a U-shaped relationship with walking economy [30,54]. Prior work has shown that one’s natural self-selected gait speed is the most economical, requiring the least amount of energy per unit of distance [30,54]. Walking slower than one’s self-selected pace may require less energy consumed per unit of time, but it actually requires more energy over a given distance [30,54]. If experiencing pain causes a person to limit their speed for any reason, it may drive the person below their natural walking speed, increasing their energy consumed over a fixed distance. In support of this, Ko et al. found that gait speed, along with knee range of motion, mediated the relationship between age and the energy cost of walking in older adults with knee pain [51]. Furthermore, pain may alter muscle activity during walking; for example, Ghamkhar and Kahlaee found that global trunk muscle activity increased during walking in those with chronic low back pain [55]. Increases in muscle activity likely come at a higher energetic cost. In reality, each chronically painful condition may influence walking characteristics in a unique, condition-specific way; however, we contend that these gait impairments have a common influence on energy efficiency.
Premise B: Increases in the Energy Cost of Functional Mobility Lead to a Restriction in Physical Activity
There is a well-established body of literature that suggests that physical activity levels are attenuated in older adults with chronic pain [56,57]. Typically, this is thought to occur due in no small part to psychosocial factors (e.g., fear avoidance) [58]. While there is evidence to support this point, we hypothesize that energetic inefficiency may also drive changes in physical activity levels.
Prior studies in mobility-limited patient populations serve as the best evidence for the relationship between energy cost of mobility and physical activity behavior. Maltais et al. found that, among people with cerebral palsy, worse walking economy strongly predicted lower physical activity levels [59]. In stroke survivors, prior work has shown that those with the highest levels of functional impairment have the greatest levels of energy cost of walking [60] and lowest physical activity levels [61], compared with those with little functional mobility limitation. A recent study by Danks et al. has shown that energy cost of walking is a strong predictor of daily step counts among stroke survivors [62]. Taken together, these studies suggest that energy efficiency and physical activity may be linked in other mobility-limited patient populations, such as those with painful conditions.
Premise C: Long-Term Physical Activity Restrictions Result in Further Reductions of Energy Capacity
As previously noted, there is a body of literature to suggest that the overall physical activity levels of older adults with chronic pain are reduced [56,57]. Furthermore, the relationship between low physical activity and low energy capacity is well established [63,64]. Of course, it is important to note that the influence physical activity has on energy capacity is both quantity and intensity dependent. Yet, to our knowledge, there is no conclusive evidence that examines how specific painful conditions (e.g., knee osteoarthritis or chronic low back pain) influence the different components of physical activity, such as intensity. Although energy capacity has not been studied among older adults with chronic pain specifically, younger adults with chronic low back pain have been shown to have lower energy capacity [65,66]; therefore, it is plausible that energy capacity is also reduced among geriatric chronic pain patients, given their low levels of physical activity.
The mechanisms by which physical inactivity leads to reductions in energy capacity are well documented and involve both short-term and long-term physiological changes [67,68]. Abrupt decreases in physical activity cause central cardiovascular change (i.e., decreased heart function), while persistent physical inactivity causes changes in the peripheral cardiovascular system (i.e., impaired oxygen transport between vessels and muscles) [67,68]. Further reductions in physical activity, such as those seen among older adults with painful conditions, may exacerbate these pathophysiological processes.
Discussion and Perspectives for Future Research
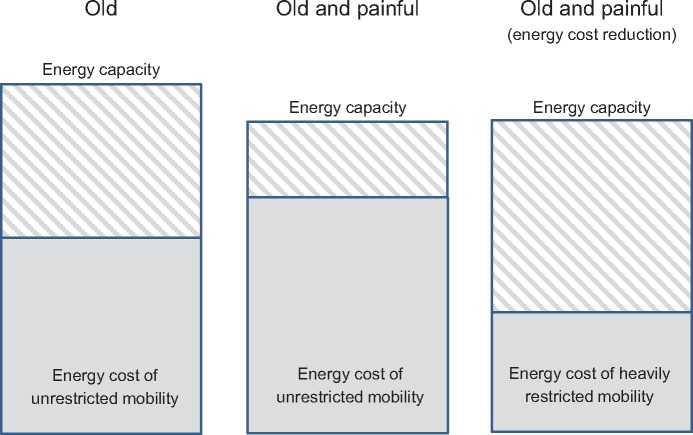
In this article, we describe how persistently painful conditions may expedite the age-related decline in functional mobility through energetic mechanisms. Gerontological experts have hypothesized that age-related mobility impairments may be the result of increased energy cost in the face of low energy capacity. In Figure 3, we summarize the effects of the Pain Energy Model in the context of the Energetic Pathway to Mobility Loss; the pain experience drives increases in the energy cost of mobility and decreases in energy capacity. As a result, functional mobility limitations develop to prevent energy cost from approaching energy capacity, thereby avoiding the risk of homeostatic collapse; however, the combined effects of both age and the pain experience result in even greater functional mobility limitations. Essentially, the pain experience accelerates the age-related decline of functional mobility.
Figure 3.
Illustration of the effects of the Pain Energy Model in the context of the Energetic Pathway to Mobility Loss. The pain experience increases the energy cost of unrestricted mobility while leading to decreases in energy capacity. As a compensatory strategy, mobility is heavily restricted to stay within the energy boundary.
It is important to note that limitations in functional mobility may arise from a number of different pathways, aside from energy expenditure [69]. For example, it has been clearly demonstrated that psychosocial factors play an important role in the pain experience. A person with pain may be fearful of exacerbating their symptoms (i.e., fear avoidance), choosing to reduce their overall physical activity, regardless of the impact that pain may have on the energy cost of mobility. While logical, we believe that the pathway from pain to mobility loss in the geriatric pain population is more complicated than driven purely by a psychosocial mechanism. While some individuals will immediately modify activities to limit pain, there are many who would choose to maintain an active lifestyle in spite of their pain but are limited by an alternate pathway. The model that we propose is not meant to replace other models of disability development in this population, but rather propose a new pathway that is unexplored. Future research should examine the unique interactions between these pathways in the progression of disability.
It is also important to note that, at present, this model is hypothetical, but very much logical given the currently available evidence; it is meant to identify and generate a conceptual pathway by which pain may influence energy expenditure. It is wholly possible that the organization of the model may, in reality, be different from the order presented in this paper. However, the current evidence supports the pathway proposed here. Future systematic investigations need to be conducted to investigate the temporal and directional nature of these relationships to establish the validity of this model. Furthermore, future studies should investigate which aspects of the pain experience (e.g., pain frequency, intensity, interference) best predict adverse changes in energy expenditure. Regardless, this model still has important clinical implications.
Energy cost and capacity may be very important outcomes in the pathway to disability. As such, it may be beneficial to use these as outcomes to gauge treatment efficacy. Although measuring these outcomes requires specific tools (i.e., metabolic gas analysis equipment), these measurements are commonly performed in specific clinical settings, such as cardiac rehabilitation centers. Collaboration between physical rehabilitation clinicians and exercise physiologists could allow for the implementation of these measurements among geriatric pain patients. Where such collaborations are not practical or possible, clinical tests exist to estimate energy capacity (i.e., VO2 Peak). For example, Simonsick et al. found that the Long Distance Corridor Walk test, a test that requires minimal training, time, and equipment, can provide a valid estimate of Peak VO2 in older adults by using a regression equation [70].
This model also has important treatment implications. Pain management interventions should be utilized to decrease the instantaneous impact that pain provocation has on the energy cost of different activities. As mentioned, the energy cost of walking can be easily measured with metabolic gas analysis equipment; clinicians can measure walking economy to gauge treatment efficacy. Next, and perhaps more importantly, geriatric pain patients may benefit from interventions aimed at improving the energetic efficiency (i.e., decrease energy cost) of mobility. This is important because deficits in mobility often persist even after pain is effectively managed. For example, patients who have undergone total knee replacement surgery report great improvements in their pain but have marginal improvements in mobility compared with preoperative status [71,72], and they continue to underperform compared with healthy older adults [73]. It is plausible that these patients continue to have higher levels of energy cost of mobility, despite resolution of their symptoms, due to compensation strategies that were learned as a result of their painful condition (e.g., impaired muscle coordination). Rehabilitation interventions exist for older adults that focus on the smoothness of walking to improve energetic efficiency [74,75], but they are not standard practice for those recovering from painful conditions. Considering the potentially important role that increased energy cost of functional mobility plays in the disability pathway among older adults with pain, clinicians may consider incorporating intervention strategies that target impairments that may be contributing to increased energy cost.
Furthermore, clinicians may focus more heavily on physical activity interventions. Although this is not a new recommendation for the treatment of patients with chronic pain, past guidelines have simply advised clinicians to encourage their patients to be more physically active [76]. Physical activity interventions are effective at improving pain-related outcomes [77–79], and clinicians may consider taking a more specific approach. Activity monitors are relatively inexpensive and easy to use. Clinicians may consider incorporating these instruments into their clinical practice to set goals and monitor patient progress. For example, prior research has shown that this is feasible to incorporate into clinical practice and effective in improving daily walking activity in patients recovering from stroke [80,81]. Clinicians may also aim to improve or preserve energy capacity in older adults with chronic pain [23,29]. Energy capacity can be improved through a variety of different exercise interventions. For example, if a patient is unable to tolerate a specific mode of exercise, such as walking for knee osteoarthritis, then a clinician may use a mode of exercise that is less provocative, such as cycling, to improve energy capacity levels. Also, a mobility aid, such as a single point cane, may be used to prevent pain exacerbation and joint degradation, which can both lead to gait impairments; clinicians, however, should still emphasize that its purpose is to allow the person to be more physically active, preserving energy capacity.
Conclusions
Energy cost of mobility and energy capacity have been shown to play an important role in the development of age-related limitations in functional mobility. Pain may play an important role in this process as painful conditions have been shown to lead to reductions in energy efficiency, energy capacity, and physical activity levels. This newly proposed model may help explain the development of mobility limitations in geriatric pain populations and warrants further investigation.
Acknowledgments
The authors are grateful to Drs. Darcy Reisman, William Farquhar, and Jenifer Pugliese for their invaluable feedback and guidance in the writing of this manuscript.
Funding sources: This work was supported by award number R01AG0412202 from the National Institute on Aging, as well as award numbers R21HD057274, K12HD055931, and T32HD007490 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Disclosure and conflicts of interest: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. None of the authors has any conflicts of interest to disclose.
References
- 1. International Association for the Study of Pain. Taxonomy. 2016. Available at: http://www.iasp-pain.org/taxonomy (accessed June 23, 2016).
- 2.Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Institutes of Health; 2011. [PubMed]
- 3. Helme RD, Gibson SJ.. The epidemiology of pain in elderly people. Clin Geriatr Med 2001;173:417–31. [DOI] [PubMed] [Google Scholar]
- 4. Weiner DK, Kim YS, Bonino P, Wang T.. Low back pain in older adults: Are we utilizing healthcare resources wisely? Pain Med 2006;72:143–50. [DOI] [PubMed] [Google Scholar]
- 5. Bressler HB, Keyes WJ, Rochon PA, Badley E.. The prevalence of low back pain in the elderly. A systematic review of the literature. Spine (Phila Pa 1976) 1999;2417:1813–9. [DOI] [PubMed] [Google Scholar]
- 6. Hicks GE, Gaines JM, Shardell M, Simonsick EM.. Associations of back and leg pain with health status and functional capacity of older adults: Findings from the retirement community back pain study. Arthritis Rheum 2008;599:1306–13. [DOI] [PubMed] [Google Scholar]
- 7. Hicks GE, Simonsick EM, Harris TB, et al. Cross-sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2005;607:882–7. [DOI] [PubMed] [Google Scholar]
- 8. Onder G, Cesari M, Russo A, et al. Association between daily pain and physical function among old-old adults living in the community: Results from the ilSIRENTE study. Pain 2006;121(1–2):53–9. [DOI] [PubMed] [Google Scholar]
- 9. Leveille SG, Ling S, Hochberg MC, et al. Widespread musculoskeletal pain and the progression of disability in older disabled women. Ann Intern Med 2001;13512:1038–46. [DOI] [PubMed] [Google Scholar]
- 10. Rudy TE, Weiner DK, Lieber SJ, Slaboda J, Boston JR.. The impact of chronic low back pain on older adults: A comparative study of patients and controls. Pain 2007;1313:293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;492:M85–94. [DOI] [PubMed] [Google Scholar]
- 12. Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;554:M221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wennie Huang WN, Perera S, VanSwearingen J, Studenski S.. Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J Am Geriatr Soc 2010;585:844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perera S, Patel KV, Rosano C, et al. Gait speed predicts incident disability: A pooled analysis. J Gerontol A Biol Sci Med Sci 2016;711:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA 2011;3051:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al-Zahrani KS, Bakheit AM.. A study of the gait characteristics of patients with chronic osteoarthritis of the knee. Disabil Rehabil 2002;245:275–80. [DOI] [PubMed] [Google Scholar]
- 17. Hicks GE, Simonsick EM, Harris TB, et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: The moderating role of back pain. J Gerontol A Biol Sci Med Sci 2005;6011:1420–4. [DOI] [PubMed] [Google Scholar]
- 18. Sowers M, Jannausch ML, Gross M, et al. Performance-based physical functioning in African-American and Caucasian women at midlife: Considering body composition, quadriceps strength, and knee osteoarthritis. Am J Epidemiol 2006;16310:950–8. [DOI] [PubMed] [Google Scholar]
- 19. White DK, Niu J, Zhang Y.. Is symptomatic knee osteoarthritis a risk factor for a trajectory of fast decline in gait speed? Results from a longitudinal cohort study. Arthritis Care Res (Hoboken) 2013;652:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coyle P, Sions J, Velasco T, Hicks G.. Older adults with chronic low back pain: A clinical population vulnerable to frailty? J Frailty Aging 2015;44:188–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vlaeyen JW, Linton SJ.. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain 2000;853:317–32. [DOI] [PubMed] [Google Scholar]
- 22. Verbunt JA, Seelen HA, Vlaeyen JW, van der Heijden GJ, Knottnerus JA.. Fear of injury and physical deconditioning in patients with chronic low back pain. Arch Phys Med Rehabil 2003;848:1227–32. [DOI] [PubMed] [Google Scholar]
- 23. Cress ME, Meyer M.. Maximal voluntary and functional performance levels needed for independence in adults aged 65 to 97 years. Phys Ther 2003;831:37–48. [PubMed] [Google Scholar]
- 24. Morey MC, Pieper CF, Cornoni-Huntley J.. Is there a threshold between peak oxygen uptake and self-reported physical functioning in older adults? Med Sci Sports Exerc 1998;308:1223–9. [DOI] [PubMed] [Google Scholar]
- 25. Myers J, Prakash M, Froelicher V, et al. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;34611:793–801. [DOI] [PubMed] [Google Scholar]
- 26. Blair SN, Kohl HW, Paffenbarger RS, et al. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 1989;26217:2395–401. [DOI] [PubMed] [Google Scholar]
- 27. Schrack JA, Simonsick EM, Ferrucci L.. The energetic pathway to mobility loss: An emerging new framework for longitudinal studies on aging. J Am Geriatr Soc 2010;58(suppl 2):S329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schrack JA, Simonsick EM, Chaves PH, Ferrucci L.. The role of energetic cost in the age-related slowing of gait speed. J Am Geriatr Soc 2012;6010:1811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schrack JA, Simonsick EM, Ferrucci L.. The relationship of the energetic cost of slow walking and peak energy expenditure to gait speed in mid-to-late life. Am J Phys Med Rehabil 2013;921:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ralston HJ. Energy-speed relation and optimal speed during level walking. Int Z Angew Physiol 1958;174:277–83. [DOI] [PubMed] [Google Scholar]
- 31. Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 2005;1125:674–82. [DOI] [PubMed] [Google Scholar]
- 32. Shephard RJ. Maximal oxygen intake and independence in old age. Br J Sports Med 2009;435:342–6. [DOI] [PubMed] [Google Scholar]
- 33. Fleg JL, O'Connor F, Gerstenblith G, et al. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol (1985) 1995;783:890–900. [DOI] [PubMed] [Google Scholar]
- 34. Hawkins S, Wiswell R.. Rate and mechanism of maximal oxygen consumption decline with aging: Implications for exercise training. Sports Med 2003;3312:877–88. [DOI] [PubMed] [Google Scholar]
- 35. Martin PE, Rothstein DE, Larish DD.. Effects of age and physical activity status on the speed-aerobic demand relationship of walking. J Appl Physiol (1985) 1992;731:200–6. [DOI] [PubMed] [Google Scholar]
- 36. Knaggs JD, Larkin KA, Manini TM.. Metabolic cost of daily activities and effect of mobility impairment in older adults. J Am Geriatr Soc 2011;5911:2118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schols AM, Fredrix EW, Soeters PB, Westerterp KR, Wouters EF.. Resting energy expenditure in patients with chronic obstructive pulmonary disease. Am J Clin Nutr 1991;546:983–7. [DOI] [PubMed] [Google Scholar]
- 38. Riley M, Elborn JS, McKane WR, et al. Resting energy expenditure in chronic cardiac failure. Clin Sci (Lond) 1991;806:633–9. [DOI] [PubMed] [Google Scholar]
- 39. Wert DM, Brach J, Perera S, VanSwearingen JM.. Gait biomechanics, spatial and temporal characteristics, and the energy cost of walking in older adults with impaired mobility. Phys Ther 2010;907:977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kang HG, Dingwell JB.. Separating the effects of age and walking speed on gait variability. Gait Posture 2008;274:572–7. [DOI] [PubMed] [Google Scholar]
- 41. O'Connor SM, Xu HZ, Kuo AD.. Energetic cost of walking with increased step variability. Gait Posture 2012;361:102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carter CS, Sonntag WE, Onder G, Pahor M.. Physical performance and longevity in aged rats. J Gerontol A Biol Sci Med Sci 2002;575:B193–7. [DOI] [PubMed] [Google Scholar]
- 43. Ferrucci L, Schrack JA, Knuth ND, Simonsick EM.. Aging and the energetic cost of life. J Am Geriatr Soc 2012;609:1768–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Priede IG. Natural selection for energetic efficiency and the relationship between activity level and mortality. Nature 1977;2675612:610–1. [DOI] [PubMed] [Google Scholar]
- 45. Wax TM, Goodrick CL.. Nearness to death and wheelrunning behavior in mice. Exp Gerontol 1978;13(3–4):233–6. [DOI] [PubMed] [Google Scholar]
- 46. Schrack JA, Zipunnikov V, Simonsick EM, Studenski S, Ferrucci L.. Rising energetic cost of walking predicts gait speed decline with aging. J Gerontol A Biol Sci Med Sci 2016;71(7):947–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lumley MA, Cohen JL, Borszcz GS, et al. Pain and emotion: A biopsychosocial review of recent research. J Clin Psychol 2011;679:942–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. World Health Organization. Health topics: Physical activity. 2016. Available at: http://www.who.int/topics/physical_activity/en/. (accessed June 23, 2016).
- 49. Kohl HW, Craig CL, Lambert EV, et al. The pandemic of physical inactivity: Global action for public health. Lancet 2012;3809838:294–305. [DOI] [PubMed] [Google Scholar]
- 50. Holland-Fischer P, Greisen J, Grøfte T, et al. Increased energy expenditure and glucose oxidation during acute nontraumatic skin pain in humans. Eur J Anaesthesiol 2009;264:311–7. [DOI] [PubMed] [Google Scholar]
- 51. Ko SU, Simonsick EM, Ferrucci L.. Gait energetic efficiency in older adults with and without knee pain: Results from the Baltimore Longitudinal Study of Aging. Age (Dordr) 2015;371:9754.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gardner AW, Ritti-Dias RM, Stoner JA, et al. Walking economy before and after the onset of claudication pain in patients with peripheral arterial disease. J Vasc Surg 2010;513:628–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gussoni M, Margonato V, Ventura R, Veicsteinas A.. Energy cost of walking with hip joint impairment. Phys Ther 1990;705:295–301. [DOI] [PubMed] [Google Scholar]
- 54. Malatesta D, Simar D, Dauvilliers Y, et al. Energy cost of walking and gait instability in healthy 65- and 80-yr-olds. J Appl Physiol (1985) 2003;956:2248–56. [DOI] [PubMed] [Google Scholar]
- 55. Ghamkhar L, Kahlaee AH.. Trunk muscles activation pattern during walking in subjects with and without chronic low back pain: A systematic review. PM R 2015;75:519–26. [DOI] [PubMed] [Google Scholar]
- 56. Griffin DW, Harmon DC, Kennedy NM.. Do patients with chronic low back pain have an altered level and/or pattern of physical activity compared to healthy individuals? A systematic review of the literature. Physiotherapy 2012;981:13–23. [DOI] [PubMed] [Google Scholar]
- 57. Stubbs B, Binnekade TT, Soundy A, et al. Are older adults with chronic musculoskeletal pain less active than older adults without pain? A systematic review and meta-analysis. Pain Med 2013;149:1316–31. [DOI] [PubMed] [Google Scholar]
- 58. Larsson C, Ekvall Hansson E, Sundquist K, Jakobsson U.. Impact of pain characteristics and fear-avoidance beliefs on physical activity levels among older adults with chronic pain: A population-based, longitudinal study. BMC Geriatr 2016;16:50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maltais DB, Pierrynowski MR, Galea VA, Bar-Or O.. Physical activity level is associated with the O2 cost of walking in cerebral palsy. Med Sci Sports Exerc 2005;373:347–53. [DOI] [PubMed] [Google Scholar]
- 60. Reisman DS, Rudolph KS, Farquhar WB.. Influence of speed on walking economy poststroke. Neurorehabil Neural Repair 2009;236:529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Roos MA, Rudolph KS, Reisman DS.. The structure of walking activity in people after stroke compared with older adults without disability: A cross-sectional study. Phys Ther 2012;929:1141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Danks KA, Pohlig RT, Roos MA, Wright TR, Reisman D.. The relationship between walking capacity, biopsychosocial factors, self-efficacy and walking activity in individuals post stroke. J Neurol Phys Ther 2016;404:232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Berthouze SE, Minaire PM, Castells J, et al. Relationship between mean habitual daily energy expenditure and maximal oxygen uptake. Med Sci Sports Exerc 1995;278:1170–9. [PubMed] [Google Scholar]
- 64. Talbot LA, Metter EJ, Fleg JL.. Leisure-time physical activities and their relationship to cardiorespiratory fitness in healthy men and women 18-95 years old. Med Sci Sports Exerc 2000;322:417–25. [DOI] [PubMed] [Google Scholar]
- 65. Smeets RJ, Wittink H, Hidding A, Knottnerus JA.. Do patients with chronic low back pain have a lower level of aerobic fitness than healthy controls? Are pain, disability, fear of injury, working status, or level of leisure time activity associated with the difference in aerobic fitness level? Spine (Phila Pa 1976) 2006;311:90–7; discussion 98. [DOI] [PubMed] [Google Scholar]
- 66. Smeets RJ, van Geel KD, Verbunt JA.. Is the fear avoidance model associated with the reduced level of aerobic fitness in patients with chronic low back pain? Arch Phys Med Rehabil 2009;901:109–17. [DOI] [PubMed] [Google Scholar]
- 67. Convertino VA. Cardiovascular consequences of bed rest: Effect on maximal oxygen uptake. Med Sci Sports Exerc 1997;292:191–6. [DOI] [PubMed] [Google Scholar]
- 68. Neufer PD. The effect of detraining and reduced training on the physiological adaptations to aerobic exercise training. Sports Med 1989;85:302–20. [DOI] [PubMed] [Google Scholar]
- 69. Ferrucci L, Cooper R, Shardell M, et al. Age-related change in mobility: Perspectives from life course epidemiology and geroscience. J Gerontol A Biol Sci Med Sci 2016;719:1184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Simonsick EM, Fan E, Fleg JL.. Estimating cardiorespiratory fitness in well-functioning older adults: Treadmill validation of the long distance corridor walk. J Am Geriatr Soc 2006;541:127–32. [DOI] [PubMed] [Google Scholar]
- 71. Mizner RL, Petterson SC, Clements KE, et al. Measuring functional improvement after total knee arthroplasty requires both performance-based and patient-report assessments: A longitudinal analysis of outcomes. J Arthroplasty 2011;265:728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stevens-Lapsley JE, Schenkman ML, Dayton MR.. Comparison of self-reported knee injury and osteoarthritis outcome score to performance measures in patients after total knee arthroplasty. PM R 2011;36:541–9; quiz 549. [DOI] [PubMed] [Google Scholar]
- 73. Bade MJ, Kohrt WM, Stevens-Lapsley JE.. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther 2010;409:559–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Brach JS, Vanswearingen JM.. Interventions to improve walking in older adults. Curr Transl Geriatr Exp Gerontol Rep 2013;24:230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Brach JS, Lowry K, Perera S, et al. Improving motor control in walking: a randomized clinical trial in older adults with subclinical walking difficulty. Arch Phys Med Rehabil 2015;963:388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Delitto A, George SZ, Van Dillen LR, et al. Low back pain. J Orthop Sports Phys Ther 2012;424:A1–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Krein SL, Kadri R, Hughes M, et al. Pedometer-based internet-mediated intervention for adults with chronic low back pain: Randomized controlled trial. J Med Internet Res 2013;158:e181.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. McDonough SM, Tully MA, Boyd A, et al. Pedometer-driven walking for chronic low back pain: A feasibility randomized controlled trial. Clin J Pain 2013;2911:972–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Roddy E, Zhang W, Doherty M.. Aerobic walking or strengthening exercise for osteoarthritis of the knee? A systematic review. Ann Rheum Dis 2005;644:544–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Danks KA, Roos MA, McCoy D, Reisman DS.. A step activity monitoring program improves real world walking activity post stroke. Disabil Rehabil 2014;3626:2233–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Danks KA, Pohlig R, Reisman DS.. Combining fast-walking training and a step activity monitoring program to improve daily walking activity after stroke: A preliminary study. Arch Phys Med Rehabil 2016;97(suppl 9):S185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]