Abstract
A fundamental component of conscious experience involves a first-person perspective (1PP), characterized by the experience of being a subject and of being directed at the world. Extending earlier work on multisensory perceptual mechanisms of 1PP, we here asked whether the experienced direction of the 1PP (i.e. the spatial direction of subjective experience of the world) depends on visual-tactile-vestibular conflicts, including the direction of gravity. Sixteen healthy subjects in supine position received visuo-tactile synchronous or asynchronous stroking to induce a full-body illusion. In the critical manipulation, we presented gravitational visual object motion directed toward or away from the participant’s body and thus congruent or incongruent with respect to the direction of vestibular and somatosensory gravitational cues. The results showed that multisensory gravitational conflict induced within-subject changes of the experienced direction of the 1PP that depended on the direction of visual gravitational cues. Participants experienced more often a downward direction of their 1PP (incongruent with respect to the participant’s physical body posture) when visual object motion was directed away rather than towards the participant’s body. These downward-directed 1PP experiences positively correlated with measures of elevated self-location. Together, these results show that visual gravitational cues contribute to the experienced direction of the 1PP, defining the subjective location and perspective from where humans experience to perceive the world.
Keywords: full-body illusion, multisensory integration, first-person perspective, gravity, virtual reality, self-consciousness
Introduction
Bodily self-consciousness (BSC) is the sense of being a subject in a specific body (self-identification), of occupying a given spatial location (self-location), and it is thought to involve a first-person perspective (1PP), i.e. the experience that ‘I’ am directed at the world (Blanke and Metzinger, 2009). Converging behavioral, neuroimaging, and clinical evidence suggests that BSC depends on the integration of multisensory bodily signals in the brain [see Blanke (2012) for review]. However, while several studies investigated the neural bases of self-identification and self-location, the specific multisensory mechanisms of 1PP are poorly understood, perhaps because there is still a lack of experimental methods to induce systematic changes in 1PP under controlled conditions (Blanke, 2012; Serino et al., 2013; Pfeiffer, Serino, et al., 2014). Here, we investigate whether a fundamental aspect of the 1PP, its directedness upon the world, is affected by conflicting multisensory information about the direction of gravity.
Gravity, i.e. the constant linear attraction force by the earth’s mass, is an absolute reference of the earth-vertical direction. In order to account for the effects of gravity in perception and action, the brain estimates the direction of gravity using internal representational models (McIntyre et al., 2001; Sciutti et al., 2012). These representations depend on the integration of vestibular, somatosensory, and visual inputs, resolving sensory ambiguities (Angelaki and Cullen, 2008). Recent work also emphasizes the relevance of visual gravitational cues for the perception of self- and object-motion, as well as body orientation (Berthoz, 1991; Indovina et al., 2005; Lopez et al., 2008; De Saedeleer et al., 2013; Indovina et al., 2013). For instance in microgravity, i.e. in the absence of gravity during parabolic or space flights, astronauts may perceive a subjective vertical, or upright, direction aligned with the visual layout of the spacecraft, which may suddenly change orientation in steps of 90° or 180° angles [i.e. room-tilt illusion (Tiliket et al., 1996)]. These experiences in microgravity share aspects with altered 1PP experiences in neurological patients with out-of-body experience (OBE), i.e. who experience their 1PP located at a position outside of their physical body and as rotated by 180° with respect to their physical body position [see Lopez et al. (2008) for a detailed discussion of this issue].
Despite these observations about the experienced direction of the 1PP in microgravity and in neurological patients, these studies are expensive, rare, and often based on few participants or patients. Moreover, the experimental investigation of the associated functional and brain mechanisms is challenging for microgravity experiments. However, several recent studies under normogravity conditions investigated the brain mechanisms of the experienced direction of the 1PP using full-body illusion (FBI) paradigms in healthy subjects. The FBI consists of visuo-tactile stroking-induced changes of self-identification and self-location with respect to a fake or virtual body seen by the participant in peripersonal or extrapersonal space [see Blanke (2012); Ehrsson (2012); Pfeiffer (2015) for reviews]. When additional directional conflicts between visual, vestibular, and somatosensory gravitational cues were presented during the FBI, the experimental participants showed individual differences of their experienced direction of the 1PP (Ionta et al., 2011; Pfeiffer et al., 2013). Thus, although all participants had a supine posture (veridical gravity directed toward the participant) and viewed in a head-mounted display a prone virtual body as if seen from an elevated visuo-spatial viewpoint (visual gravity directed away from the participant), only half of the participants experienced an upward direction of the 1PP (up-group) as if they were looking upwards at a virtual body above them (i.e. the experienced direction of the 1PP was congruent with vestibular/somatosensory gravitational cues). In contrast, the remaining participants experienced a downward direction of the 1PP (down-group) as if they were looking downwards at a virtual body below them [i.e. the experienced direction of the 1PP was congruent with visual gravitational cues (Ionta et al., 2011; Pfeiffer et al., 2013)]. These individual differences of the experienced direction of the 1PP were associated with consistent changes in self-location [i.e. the experience where ‘I’ am located (Ionta et al., 2011; Pfeiffer et al., 2013)] and with individual differences in visuo-vestibular perception [i.e. subjective visual vertical perception; (Pfeiffer et al., 2013)]. Specifically, the subjective visual vertical ratings of down-group participants were more strongly biased by a visual context than ratings of up-group participants, suggesting that the experienced direction of the 1PP depends on visuo-vestibular gravitational information (Pfeiffer et al., 2013). Other FBI studies also found that the experienced direction of the 1PP depended on different types of visual cues, such as the visuo-spatial viewpoint from where the virtual body was seen (Pfeiffer, Schmutz, et al., 2014), as well as on the synchrony of visuo-tactile stroking (Guterstam et al., 2015). These previous results suggest that visual gravitational cues, in particular when related to the orientation of the seen virtual body, influence the experienced direction of the 1PP in humans. However, it is currently unclear whether these visual gravitational effects on the experienced direction of the 1PP were specific to manipulations of the virtual body, or whether such changes could be achieved also by providing contextual visual gravitational cues (Zago et al., 2011)—which would be expected based on previous studies showing visual gravitational effects on object-motion perception (McIntyre et al., 2001; Indovina et al., 2005; Zago et al., 2011).
In order to address this question, we adapted our previous FBI protocol where supine participants (veridical gravity directed toward them) viewed a prone virtual body [visual gravitational cues directed away from the participant (Pfeiffer et al., 2013)]. Here, we additionally presented gravitational virtual object motion [adapted from Senot et al. (2005), see below], which was previously employed by studies investigating contributions of visual gravity signals to object-motion perception (McIntyre et al., 2001; Indovina et al., 2005; Indovina et al., 2013). We tested whether the direction of this gravitational visual object motion would modulate the experienced direction of the 1PP during the FBI (within-subject changes of 1PP ratings). In a 2 (Stroking: synchronous, asynchronous) × 2 (Visual Gravity: toward, away) experimental design, we manipulated the synchrony of visuo-tactile stroking to either induce the FBI (synchronous stimulation) or not (asynchronous condition), while participants concurrently saw a virtual body (centrally) and a visual spherical object moving with gravitational constant acceleration either toward the participant (congruent with veridical gravity) or away from the participant (incongruent with veridical gravity)—to either side of the virtual body. We hypothesized that the direction of contextual gravitational visual object motion would influence the experienced direction of the 1PP over and above the previous effects of constant visuo-vestibular conflict (i.e. participants in supine posture and virtual body in prone posture) and would depend on visuo-tactile stimulation (Ionta et al., 2011; Pfeiffer et al., 2013; Pfeiffer, Schmutz, et al., 2014). We thus predicted changes of the experienced direction of the 1PP in the direction of the visual gravitational motion and further modulated by visuo-tactile stroking—as based on previous behavioral (Pfeiffer et al., 2013; Guterstam et al., 2015) and clinical data (Blanke et al., 2004; De Ridder et al., 2007). We also measured self-location by a mental imagery task and collected questionnaires ratings to quantify the FBI.
Materials and Methods
Participants
Sixteen undergraduate students (6 females, mean age ± SD: 22.3 ± 2.5 years) of the Ecole Polytechnique Fédérale de Lausanne participated. All were right-handed, had normal or corrected-to-normal vision, and no history of neurological or psychiatric disease. The study was conducted in accordance with the Declaration of Helsinki and the experimental protocol was approved by the local ethical committee—La Commission d’Ethique de la Recherche Clinique de la Faculté et de Medicine de l’Université de Lausanne. Participants gave their informed consent before inclusion to the study and received 30 Swiss Francs for compensation after having participated.
Experimental setup
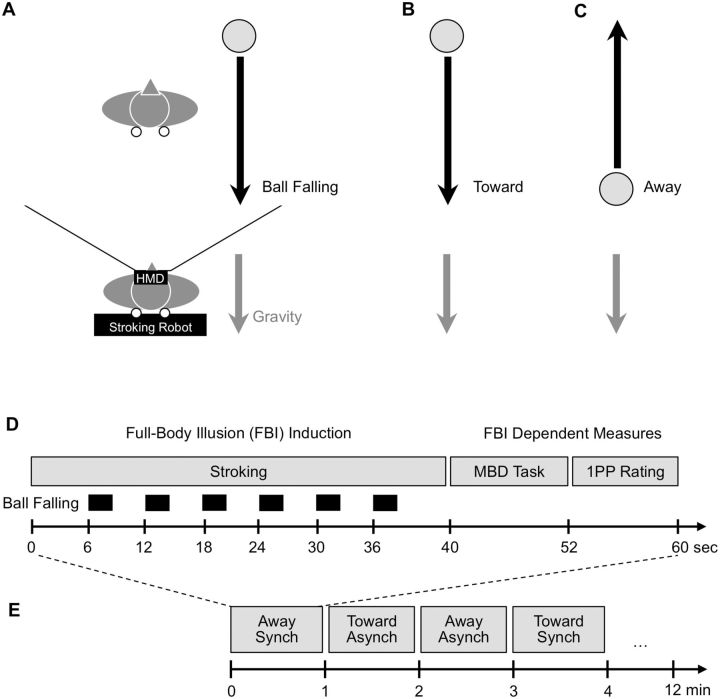
Figure 1a and b schematically shows the experimental setup, which was identical to previous experiments (Pfeiffer et al., 2013; Salomon et al., 2013; Pfeiffer, Schmutz, et al., 2014; Romano et al., 2014). In a darkened room, a custom-made robotic device (stroking robot) was horizontally placed on a table with 90 cm distance to the floor. Participants lay in supine posture on the stroking robot, which consisted of a soft-foam mattress and of two independent stroking units touching the participant at the upper back through holes in the mattress (Duenas et al., 2011; Pfeiffer et al., 2013). The participant was equipped with a head-mounted display (HMD, Virtual Research, model VR1280, http://www.virtualresearch.com, diagonal field-of-view 60°, resolution 1280 × 1024, refresh rate 60 Hz) for visual stimulus presentation and in-ear headphones for acoustic pink noise presentation masking mechanical noise of the stroking robot. A serial keypad (Targus Numeric Keypad AKP10US, http://www.targus.com) was placed under the participant’s right hand for button press responses with the right-hand index and middle fingers. A regular juggling ball (Astrix Flames-N-Games, http://flamesngames.co.uk) with 100 g weight was held by the participant in the left hand to facilitate the Mental Ball Dropping task (MBD; see below). Labview software (National Instruments, Austin Texas, http://www.ni.com/) was used to control of the stroking robot and ExpyVR software (http://lnco.epfl.ch/expyvr) was used for visual and acoustic stimuli presentation and timing.
Figure 1.
FBI experimental setup and procedure. (A) Schematic side-view of the experimental setup showing a participant lying supine on a robotic device (stroking robot), used for tactile stroking, and wearing a head-mounted display (HMD) in which the participant saw visual stroking of a virtual body (white circles; illustrating both visual and tactile stroking). Visual gravity cues consisted of a virtual ball falling (gray circle and black arrow) that was congruent or incongruent with the direction of gravity (gray arrow). An exemplar toward ball falling trial is shown. Note that during each experimental trial virtual balls repeatedly fell at random at the right side (shown here) or left side of the display. (B and C) Toward (congruent with gravitational direction) and away (incongruent with gravitational direction) visual ball falling stimuli are shown (black arrows) and gravitational direction is shown in gray. (D) Sequence of events for a single trial showing an initial FBI induction phase with continuous visuo-tactile (synchronous or asynchronous) stroking and occasional (toward or away) virtual ball falling stimuli. This was followed by the dependent measures of self-location (MBD task) and 1PP rating. (E) Randomized trial order during exemplar experimental run of 12 min duration.
Stimuli
The experimental stimuli were adapted from Pfeiffer et al. (2013). The participant lay supine (gravity directed toward the participant) and viewed on the HMD a photorealistic back-view image of a male human body (virtual body) at approximately 2 m in front of the participant. The clothing (i.e. white shirt) and the limb posture of the virtual body matched the clothing and posture of the participant during the experiment. A black colored background surrounded the virtual body such that no information about the surrounding space was visible to the participant.
Based on earlier work (Ionta et al., 2011; Pfeiffer et al., 2013), we presented static visuo-vestibular conflicts about the direction of linear gravitational acceleration. Supine participants (i.e. veridical gravity was directed toward the participant) were presented with static ‘away’ visual gravitational cues consisting of viewing in the HMD a virtual body (seen from the back) in prone posture on which linear gravitational acceleration acted along an axis through the virtual body’s back and chest showing gravitational pull on hair, clothes, and the posture of the shoulders of the virtual body directed away from our participants. In addition, we chose a distribution of light on the front and back of the virtual body that was congruent with a light following this away direction of visual gravity (Ionta et al., 2011; Pfeiffer et al., 2013). Thus, static ‘away’ visual gravitational cues were in directional conflict with ‘toward’ vestibular/somatosensory gravitational cues due to the participant’s supine posture. Of note, these static ‘away’ visual gravitational cues were consistent with dynamic ‘away’ visual gravitational cue by the ball falling manipulation (see below). However, the main manipulation of the present study was presenting different dynamic visual gravitational cues, based on related work that found effects of visual gravitational cues on self-motion perception (Indovina et al., 2013; Indovina et al., 2015) and object-motion perception (McIntyre et al., 2001; Indovina et al., 2005; Senot et al., 2005; Zago et al., 2008). For this we chose a three-dimensional white spherical object (virtual ball) that was shown on the HMD. The virtual ball initially appeared at a dedicated position in virtual space (see below) where it remained static for 1 s and subsequently accelerated for 2 s with a gravity-matching acceleration (9.81 cm/s2) along a linear trajectory in parallel to the line of sight of the participant, without colliding with the virtual body or the participant’s point of view. This procedure gives the impression that the virtual ball was falling under the influence of gravity [i.e. for similar stimulus see Senot et al. (2005)]. Critically, we manipulated the direction of virtual ball falling with respect to the participant’s physical body position (Fig. 1a–c). In one condition, the virtual ball appeared at a location far behind the virtual body and then accelerated toward the participant until it disappeared outside the field of view (‘toward’ Visual Gravity condition). In another condition, the virtual ball appeared at a location close to the participant and then accelerated away into depth until occluded by the virtual body (‘away’ Visual Gravity condition). Note that, whereas ‘toward’ ball falling was congruent with the effects of veridical gravity on physical objects viewed by the participant in supine posture, ‘away’ ball falling was incongruent with veridical gravity. The virtual ball falling stimuli thus served as a dynamic visual cue simulating different visual gravity directions. We hypothesized that these dynamic visual gravitational cues might induce stronger changes of subjective 1PP than previously observed for static visual gravitational cues only (Ionta et al., 2011; Pfeiffer et al., 2013).
In the context of these multisensory gravitational conflicts, we induced the FBI with a classic visuo-tactile stroking manipulation [see Ionta et al. (2011); Lenggenhager et al. (2009)]. We included Stroking manipulations in the present experiment both in order to induce multisensory visuo-tactile conflict, a proxy of multisensory disintegration causing OBEs and changes in the experienced 1PP in neurological patients [e.g. (Blanke et al., 2004)], and in order to assess whether strong multisensory conflicts based on combined Stroking and dynamic visual gravitational cues would induced stronger, intra-individual changes of the experienced direction of the 1PP as found previously [e.g. (Pfeiffer et al., 2013)]. Visual stroking was shown on the HMD as two red dots on the upper back of the virtual body with a diameter, position, and movement range corresponding to the tactile stroking of the participant’s back (Fig. 1a). Tactile stroking consisted of random linear strokes by two independent stroking units along the upper back, i.e. on the left and right side of the back moving in parallel to the spinal cord. On each trial and for each of the stroking units, a different pseudo-random stroking sequence was used (strokes in a 0–20 cm distance range, 2–12 cm/s velocity range, variable 0–1.5 s inter-stroke intervals). During the experiment, visual and tactile stroking was simultaneously presented either in perfect synchrony (synchronous Stroking condition) or asynchronously in terms of moment-by-moment stroking position, movement direction, and velocity. The overall amount of stroking was matched between the asynchronous sequences (asynchronous Stroking condition). As shown by many previous studies, such synchronous stroking induces increased self-identification and self-location changes toward the virtual body when compared to the asynchronous stroking control condition (Ehrsson, 2007; Lenggenhager et al., 2007).
Experimental design and procedure
A 2 (Visual Gravity: toward, away) by 2 (Stroking: synchronous, asynchronous) within-subjects full-factorial experimental design was used. The four experimental conditions were presented nine times in random order. The 36 experimental trials were presented in three runs of 12 trials. Figure 1c and d show the sequence of events of an experimental trial and an experimental run. First, the FBI was induced by presenting in the HMD the virtual body and by (synchronous or asynchronous) visuo-tactile stroking during 40 s. During this period, repeated virtual ball falling stimuli, each lasting 2 s, were presented at 6, 12, 18, 24, 30, and 36 s poststimulus onset. All stimuli presented during this interval showed virtual balls falling in the same direction (away or towards) but were randomly presented at the left side (three times) or the right side (three times) of the screen in order to avoid anticipation. We chose to show only one virtual object at a time in order to avoid the induction of self-motion perception by a larger amount of coherent peripheral visual motion (Kleinschmidt et al., 2002). Immediately after that, the robotic stroking was stopped and all visual stimuli were removed from the screen upon which two dependent measures of the FBI were recorded during 14 s (see below). This was followed by a random intertrial interval of 1.5–2.5 s.
Dependent measures of the FBI
The 1PP rating served as a repeated measure of the experienced direction of participant’s 1PP [as initially introduced by (Ionta et al., 2011)]. Participants viewed at the center of the display the word ‘Orientation?’, at the bottom left side the word ‘Upwards’ and at the bottom right side the word ‘downward’. Participants were instructed that, upon viewing this display, they should answer to the question ‘Did you have the impression as if you were looking upwards at a body above you or as if you were looking downward at a body below you?’ using a two-alternative forced-choice response format, i.e. rating ‘Upwards’ by pressing the right index finger button or by rating ‘downward’ by pressing the right middle finger button. They were asked to rate their experienced direction of the 1PP they had most of the time during the previous 40-s stroking period. The judgment was given unspeeded within about 8 s after onset of the display.
The MBD task [adapted from Lenggenhager et al. (2009)] served as our measure of self-location. The MBD task began with the presentation of a white fixation cross on black background in the HMD for 1 s. This was followed by a brief acoustic beep for 500 ms, which served as a go signal for initiating mental imagery. Participants imagined releasing the juggling ball (held in the left hand) and estimated the duration of ball falling to the ground. With the right index finger, the participant pressed a button at the moment of imagined ball release from the hand, held it pressed during imagined ball falling, and released the button at the moment of imagined ball impact on the ground. Thus, the duration of button press served as response time (RT) measure, i.e. a proxy of estimated self-location above the floor. Before the experiment, the participant was seated upright on a chair and performed 20 practice trials including actual juggling ball drops from different heights and with eyes open and closed (i.e. no data were recorded for practice trials). During an experimental trial, participants performed three subsequent repetitions of the MBD task and they had 4 s to complete each repetition of the task.
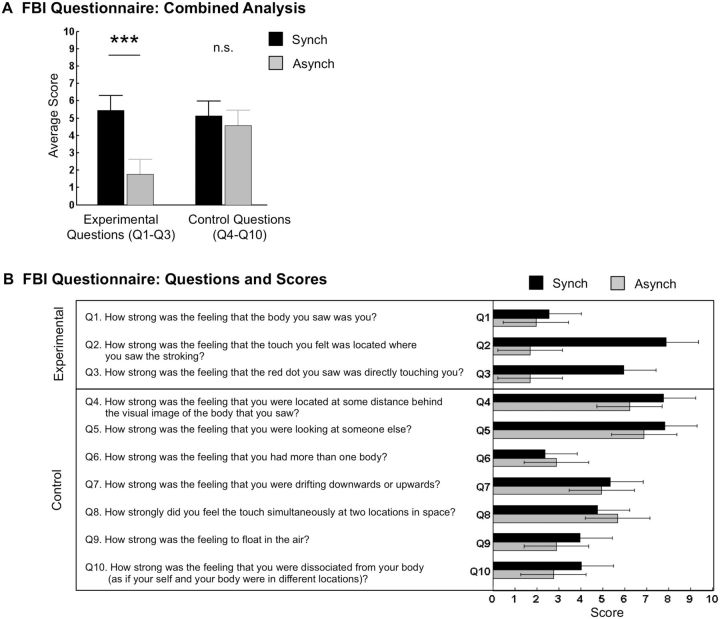
The FBI Questionnaire [adapted from Lenggenhager et al. (2009)] was administered after completion of the experiment on the robotic device. A total of 10 questions (Fig. 3b) inquired about different aspects of the illusion experience. Three questions were sensitive to the FBI experience (Experimental questions: Q1–Q3) and seven questions served as control (Control questions: Q4–Q10). The questions were presented in random order at the center of a computer screen where seated participants gave their ratings along an 11-point horizontal visual analog scale, labeled at the left side ‘weak feeling’ and at the right side ‘strong feeling.’ Ratings were given in an unspeeded fashion by button presses. Because this study primarily focused on the effects of visual gravitational cues on repeated measures of the experienced direction of the 1PP and for time-keeping reasons, we administered the FBI questionnaire twice in random order between participants: once regarding the overall synchronous Stroking trials and once regarding the asynchronous Stroking trials. The FBI questionnaire served mainly to assess that a basic FBI was induced during the experiment.
Figure 3.
Questionnaire results. (A) Combined analysis comparing critical (Q1–Q3) and control (Q4–Q10) question average scores. Results show visuo-tactile stroking dependent modulation of questionnaire scores for critical questions and no modulation for control questions. Significance levels of post-hoc comparisons are shown by stars (*P < 0.05; **P < 0.01; ***P < 0.001). (B) Questions of the FBI questionnaire and group-average scores for each questionnaire item for the synchronous and asynchronous Stroking conditions. Error bars represent 95% confidence intervals.
Analysis
The data from two participants were excluded from further statistical analysis because of a large number of MBD RT outliers (>20% of trials exceeded two times the interquartile range around the individual median, see below), suggesting low task compliance. Of the remaining sample of 14 participants, two participants completed only two out of three experimental runs due to technical problems with the HMD.
1PP ratings were quantified as proportion scores by dividing the number of ‘downward’ ratings by the total number of 1PP ratings per condition. The 1PP proportion score ranged from 0 (i.e. never rated ‘downward’) to 1 (i.e. always rated ‘downward’). Condition-wise scores from all participants were submitted to statistical analysis using a 2 (Visual Gravity: toward, away) × 2 (Stroking: synchronous, asynchronous) repeated measures ANOVA. We used Matlab (version R13, The MathWorks, Massachusetts, http://www.mathworks.ch/) and SPSS (version 17.0, IBM, http://www.ibm.com/software/analytics/spss) software for data analysis.
MBD RTs (i.e. measure of self-location) were processed by removing outlier values exceeding two times the interquartile range around the individual median RT (i.e. on average 4.5% of the RTs were removed). Condition-average RTs were then calculated and subjected to statistical analysis using a 2 (Visual Gravity: toward, away) × 2 (Stroking: synchronous, asynchronous) repeated measures ANOVA.
Based on previous results from FBI studies (Ionta et al., 2011; Pfeiffer et al., 2013) and OBEs of neurological origin (Blanke et al., 2002, 2004), we predicted that changes of the experienced direction of the 1PP would systematically relate to changes in self-location. Specifically, the downward direction of the experienced 1PP should be associated with elevated self-location (Blanke et al., 2002). In order to test this prediction, we ran correlation analysis between the repeated measures for the 1PP ratings and MBD RTs. This required z-standardization of MBD RTs (average = 0, SD = 1) immediately after outlier removal and before calculating condition-averages. Thereby the variance ranges of the resulting condition-average RTs and the 1PP proportion scores were homogenized. We then subjected the paired condition-wise RTs and 1PP scores from all participants to linear regression analysis to investigate systematic relationships between self-location and the experienced direction of the 1PP. Our correlation analysis was run on the data from all experimental conditions across all subjects.
The FBI questionnaire scores, which were recorded at the end of the experiment related to the synchronous and asynchronous stroking, were analyzed by calculating for each participant the average scores across Experimental questions (Q1–Q3) and the average scores across Control questions (Q1–Q7) separately for the synchronous and asynchronous Stroking condition. The resulting scores were then subjected to statistical analysis using a 2 (Question: experimental, control) × 2 (Stroking: synchronous, asynchronous) repeated measures ANOVA. For all statistical analyses, an alpha threshold of 0.05 was used. Because paired t-tests for post-hoc comparisons were calculated only based on significant interactions, no correction for multiple comparisons was applied. In all figures we show 95% confidence intervals, i.e. calculated on the within-subject error term of the subject × condition interaction of the repeated-measures ANOVA used for statistical analysis, directly showing significant differences between experimental conditions (Loftus and Masson, 1994).
Predicted outcomes
Based on the idea that the experienced direction of the 1PP depends on spatial information from the body and the external world (Blanke et al., 2004; Blanke, 2012; Pfeiffer et al., 2013) and based on earlier work showing contribution of contextual visual information to the brain’s estimate of the subjective vertical, or upright, direction (Tiliket et al., 1996; McIntyre et al., 2001; Dyde et al., 2006; Indovina et al., 2013), we hypothesized that contextual dynamic visual gravitational cues would induce within-subject changes of the experienced direction of the 1PP by modulating effects based on static visual, vestibular, and somatosensory gravitational cues [i.e. individual differences of the experienced direction of the 1PP (Ionta et al., 2011; Pfeiffer et al., 2013)]. In particular, several previous studies showed strong associations between altered states of BSC in neurological patients and experimentally induced changes of BSC in healthy individuals [see Blanke et al. (2015); Pfeiffer, Serino, et al. (2014) for reviews]. For instance, during OBEs an elevated and downward-directed experienced direction of the 1PP is caused by dysfunctional multisensory integration of visual, vestibular, and somatosensory signals (Blanke et al., 2004). Similarly, in FBI studies a combination of conflicting visuo-vestibular gravitational cues and asynchronous Stroking alters the integration of vestibular, visual, and tactile signals due to spatio-temporal conflict, thereby mimicking to some extend effects of dysfunctional multisensory integration during OBEs [e.g. (Blanke and Metzinger, 2009; Ionta et al., 2011)]. We have argued before that the similarity between the experienced downward direction of the 1PP in neurological patients with during OBE and in healthy subjects as induced by the FBI (Ionta et al., 2011; Pfeiffer et al., 2013) is associated with similar brain mechanisms and processing of such spatial and temporal multisensory conflicts. Here, we hypothesized that stronger multisensory conflicts would induce more frequent illusory changes of the experienced direction of the 1PP [see Pfeiffer et al. (2013) for a related proposal]. We thus predicted to observe the highest proportion of ‘downward’ 1PP ratings for away Visual Gravity-asynchronous Stroking (i.e. dynamic and static visual cues in conflict with vestibular/somatosensory gravitational cues and visuo-tactile stroking conflict) and the lowest proportion of ‘downward’ 1PP ratings for toward Visual Gravity-synchronous Stroking (i.e. no conflict between dynamic visual and vestibular/somatosensory gravity cues and no visuo-tactile stroking conflict).
To further address whether or not the experienced direction of the 1PP and self-location depend on similar or distinct functional mechanisms (Serino et al., 2013; Pfeiffer, Serino, et al., 2014; Pfeiffer, 2015), and based on previous clinical and experimental data showing an association between an experienced downward direction of 1PP and elevated self-location levels (Blanke et al., 2002; Ionta et al., 2011), we further hypothesized a systematic relationship between the experienced direction of the 1PP and self-location. Accordingly, we hypothesized a positive correlation between more frequent ‘downward’ 1PP ratings and prolonged MBD RTs (i.e. higher self-location).
Regarding the overall experience of the FBI, we hypothesized in line with numerous previous studies that visuo-tactile stroking will induce a basic FBI experience, as reflected in higher questionnaire ratings for synchronous than asynchronous stroking for critical questionnaire items (Q1–Q3).
Results
1PP ratings
Statistical analysis of 1PP proportion scores revealed a main effect of Visual Gravity (F(1, 13) = 15.20, P = 0.002, η2 = 0.54; Fig. 2a), reflecting as predicted more frequent ‘downward’ 1PP ratings for away Visual Gravity (M = 0.60, SE = 0.07) than for toward Visual Gravity (M = 0.26, SE = 0.04), which suggests that the experienced direction of the 1PP during the FBI depends on the direction of dynamic visual gravitational motion. We note that the effect of Visual Gravity on 1PP ratings was highly consistent across subjects. Analysis of the Visual Gravity × Stroking interaction revealed a trend toward significance (F(1, 13) = 3.80, P = 0.07, η2 = 0.23). Post-hoc analyses showed for asynchronous Stroking a larger difference between toward vs. away Visual Gravity [asynchronous-toward vs. asynchronous-away: mean 1PP score difference = 0.40, SE = 0.02; post-hoc paired samples t-test: t(13) = −5.24, P = 0.0002] than for synchronous Stroking [synchronous-toward vs. synchronous-away: mean 1PP score difference = 0.28, SE = 0.03; paired samples t-test: t(13) = −2.65, P = 0.02; Fig. 2a]. No differences between synchronous and asynchronous Stroking conditions were observed for away Visual Gravity [asynchronous-away vs. synchronous-away: mean 1PP score difference = 0.07, SE = 0.05; post-hoc paired samples t-test: t(13) = −1.50, P = 0.16] and for toward Visual Gravity [asynchronous-toward vs. synchronous-toward: mean 1PP score difference = 0.05, SE = 0.04; paired samples t-test: t(13) = 1.15, P = 0.27]. Finally, no main effect of Stroking was found (F(1, 13) = 0.13, P = 0.72, η2 = 0.01).
Figure 2.
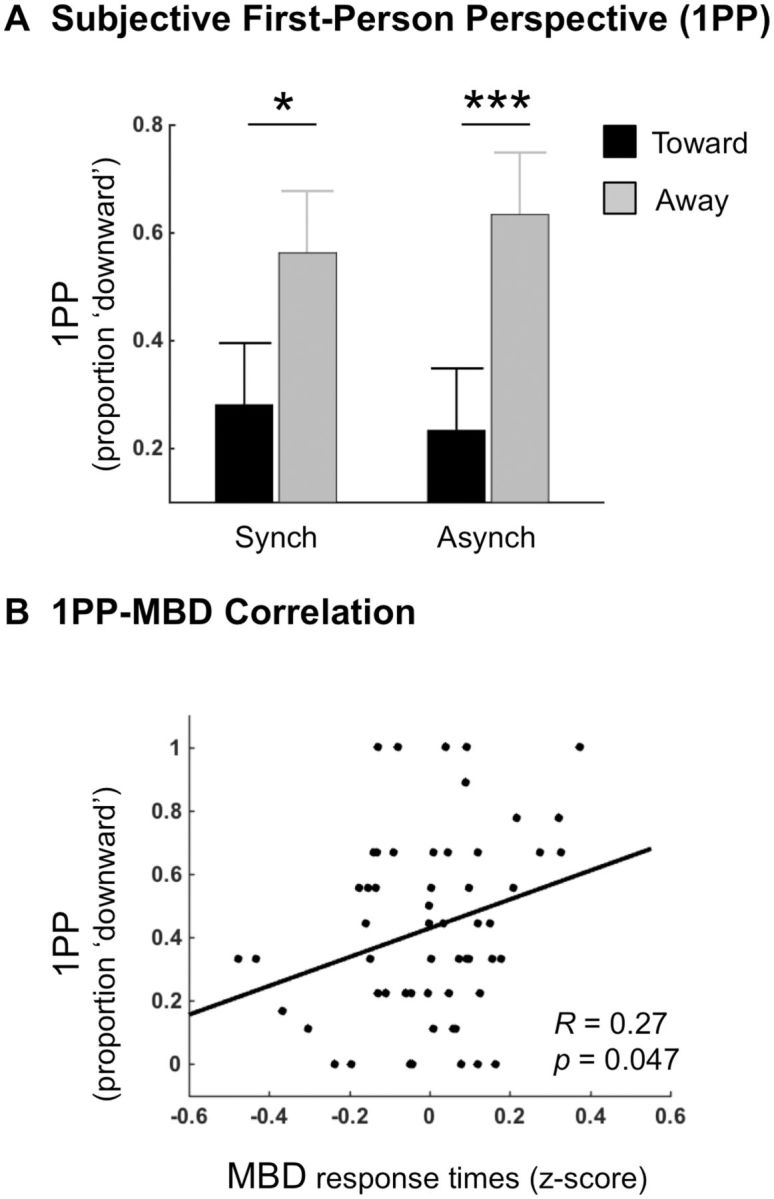
Results from the FBI. (A) Results for 1PP ratings showed higher proportions of ‘downward’ ratings for away than for toward Visual Gravity both for the synchronous (Synch) and asynchronous (Asynch) Stroking conditions. Error bars show confidence intervals (see ‘Materials and Methods’) and significance levels of post-hoc comparisons are represented by stars (*P < 0.05; **P < 0.01; ***P < 0.001). (B) Correlation analysis of 1PP ‘downward’ ratings and response times of the MBD task (self-location measure) showed a significant positive correlation, revealing that higher self-location was associated with a higher proportion of downward 1PP.
Self-location (MBD response times)
Analysis of MBD RTs showed no significant main effects nor an interaction [Visual Gravity main effect: F(1, 13) = 2.19, P = 0.16, η2 = 0.14; Stroking main effect: F(1, 13) = 3.19, P = 0.10, η2 = 0.20; Visual Gravity × Stroking interaction: F(1, 13) = 0.52, P = 0.46, η2 = 0.04], suggesting that overall self-location, as measured by the MBD task, was not modulated by the present experimental conditions.
Correlation analysis
Correlation analysis between 1PP proportion scores and MBD RTs across experimental conditions showed a significant positive correlation (R = 0.27, P = 0.047), reflecting that more frequent ‘downward’ 1PP ratings were associated with prolonged MBD RTs, i.e. higher self-location (Fig. 2b).
FBI questionnaire ratings
Combined FBI questionnaire scores (i.e. condition-wise averages of experimental questions Q1–Q3 vs. control questions Q4–Q10; see ‘Materials and Methods’) were analyzed using a 2 (Question: experimental, control) × 2 (Stroking: synchronous, asynchronous) repeated-measures ANOVA, which showed a main effect of Question (F(1, 13) = 6.34, P = 0.03, η2 = 0.33), a main effect of Stroking [F(1, 13) = 46.82, p < 0.001, η2 = 0.78] and a critical Question × Stroking interaction [F(1, 13) = 32.59, P < 0.001, η2 = 0.71; Fig. 3a]. In order to uncover the meaning of the interaction, post-hoc comparisons using paired-samples t-tests were conducted between the synchronous and asynchronous Stroking conditions separately for the experimental and control question scores. Post-hoc comparisons revealed a significant difference for experimental questions [t(13) = 8.32, P < 0.001], reflected an overall higher score for synchronous Stroking (M = 6.45, SE = 0.50) than for asynchronous Stroking (M = 2.64, SE = 0.40). In contrast, no difference between synchronous and asynchronous Stroking conditions was found for control questions [t(13) = 1.55, P = 0.15]. Figure 3b shows question-wise average ratings to the FBI questionnaire.
Discussion
We investigated whether combined visuo-vestibular and visuo-tactile spatial conflicts impact on a key component of human BSC: the experienced direction of the 1PP. Further, we asked how experimental changes of the experienced direction of the 1PP relate to self-location and whether predicted changes of self-identification could be induced under these conditions. Healthy participants were presented with multisensory conflicts between contextual dynamic visual and static vestibular-somatosensory gravitational cues to test whether such stimulations would induce predictable changes of the experienced direction of the 1PP at the single-subject level. Our results showed, first, that we were able to induce changes of the experienced direction of the 1PP by manipulating visual gravity cues and that these effects exceeded the degree of previously reported between-subjects changes of the experienced direction of the 1PP induced by constant visual-vestibular gravitational conflicts (Pfeiffer et al., 2013; Pfeiffer, Schmutz, et al., 2014). Second, our data suggest that Stroking might further modulate Visual Gravity effects on the experienced direction of the 1PP because the Visual Gravity × Stroking interaction was close to significant and might have reached significance level for a larger subject sample. Thus, illusory downward direction of the 1PP was more frequently reported for asynchronous than for synchronous visuo-tactile stroking. Next, our results showed an association between frequency of ‘downward’ 1PP ratings and magnitude of self-location in line with previous data (Ionta et al., 2011; Pfeiffer et al., 2013). However, because Visual Gravity or Stroking did not affect self-location, it remains unclear how these manipulations contributed to the association between 1PP and self-location. Finally, we found that despite these multisensory conflicts, we induced changes of self-identification that depended on visuo-tactile stroking, comparable to previous FBI studies [see Blanke (2012); Ehrsson (2012) for reviews]. In the following, we discuss these results in separate sections for the experienced direction of the 1PP, self-location, and self-identification.
1PP: Dependence on visual gravitational motion
Participants more frequently experienced a downward direction of the 1PP when the direction of visual gravitational motion cues (i.e. directed away from the participant) was congruent with static visual gravitational cues (i.e. directed away) and both visual cues were incongruent with vestibular and somatosensory gravitational cues (i.e. always directed toward the supine participant; see Fig. 1a–c). The present findings show that the experienced direction of the 1PP depends on the direction of visual gravitational cues and that coherence of dynamic and static visual gravitational cues (i.e. away) induced stronger, within-subject changes of the experienced direction of the 1PP than previously observed for static visual gravitational cues alone (Ionta et al., 2011; Pfeiffer et al., 2013). In particular, this previous work tested the effect of visual gravitational cues that were constantly present during the experiment (i.e. the prone virtual body suggesting visual gravity directed away from the participant) or the vestibular/somatosensory cues signaled by the participant’s supine posture (suggesting gravity directed toward the participant). The present work, instead, shows that additional dynamic visual gravitational cues systematically and more strongly influence 1PP ratings. These previous FBI studies about visual contributions to the experienced direction of the 1PP induced an experienced downward direction of the 1PP [i.e. congruent with the visual stimulus) in 30–50% of the tested participants (Ionta et al., 2011; Pfeiffer et al., 2013)]. An experienced downward direction of the 1PP was furthermore systematically related to visual biases of visuo-vestibular integration during subjective visual vertical ratings (Pfeiffer et al., 2013), supporting the proposal that the experienced direction of the 1PP depends on integrated visuo-vestibular information (Lopez et al., 2008; Pfeiffer, Serino, et al., 2014). In addition to such visual gravitational cues, visuo-spatial viewpoint manipulations, and visual cues about the virtual body have also been shown to affect the experienced direction of the 1PP (Pfeiffer, Schmutz, et al., 2014). Here, we extended these previous observations by showing that contextual dynamic visual gravitational cues induced more pronounced and within-subject changes of the experienced direction of the 1PP (i.e. 40% ‘downward’ 1PP rating difference between away vs. toward Visual Gravity) than previously observed [i.e. 15% ‘downward’ 1PP rating difference between down-group vs. up-group participants; see Experiment 2 in Pfeiffer et al. (2013)]. This effect is particularly relevant considering that static multisensory gravitational cues were constantly present throughout the experiment, whereas visual gravitational object motion was presented occasionally during single trials. Thus, the present study describes a new experimental protocol that induces these changes of the experienced direction of the 1PP on a trial-by-trial basis, whereas earlier descriptions were based on single overall ratings (Ionta et al., 2011; Pfeiffer et al., 2013).
Further, our results suggest that this multisensory gravitational effect on 1PP ratings also depended on multisensory body-related signals. We found a trend toward an interaction between visual gravity and visuo-tactile stroking, reflected in a larger effect of Visual Gravity on 1PP ratings during the asynchronous than the synchronous Stroking condition. We note that because of the small sample size (14 participants) tested here, this statistical trend of the Visual Gravity × Stroking interaction should be considered preliminary evidence requiring further confirmation by future studies. It is also worth noting that these results were collected from supine participants, i.e. vestibular and somatosensory gravitational cues were always directed toward the participant. Thus, it remains to be determined how different body tilts or positions (e.g. prone, standing) would affect visual gravitational effects on the experienced direction of the 1PP. Nonetheless, this effect pattern is in line with previous experimental (Ionta et al., 2011; Pfeiffer et al., 2013) and clinical studies (Blanke et al., 2004) showing that during asynchronous stroking or body-related multisensory disintegration, an illusory downward direction of the experienced direction of the 1PP is more frequently induced. Thus, during OBEs an elevated and downward-directed experienced direction of the 1PP is caused by dysfunctional multisensory integration of visual, vestibular, and somatosensory signals (Blanke et al., 2004). Similarly, in FBI studies a combination of conflicting visuo-vestibular gravitational cues and asynchronous Stroking alters the integration of vestibular, visual, and tactile signals due to spatio-temporal conflict, thereby mimicking to some extend effects of dysfunctional multisensory integration during OBEs [e.g. (Blanke and Metzinger, 2009; Ionta et al., 2011)]. We have argued before that the similarity between the experienced downward direction of the 1PP in neurological patients with OBE and in healthy subjects as induced by the FBI (Ionta et al., 2011; Pfeiffer et al., 2013) is associated with similar brain mechanisms and processing of such spatial and temporal multisensory conflicts. Importantly, this pattern of down-looking direction of the 1PP during asynchronous stimulation is consistent across studies, different subject samples, and different stimulation robots [for discussion see Pfeiffer et al. (2013)]. Indeed, previous work has found higher levels of self-location in the asynchronous vs. synchronous Stroking conditions (Ionta et al., 2011; Pfeiffer et al., 2013). The present data extend these previous observations by showing that asynchronous visuo-tactile stimulation in the away conditions leads to the highest proportion of ‘downward’ 1PP ratings, further confirmed by the present correlation analysis between 1PP ratings and self-location measures (see below). We note that here 1PP ratings were always assessed related to presentation of multisensory gravitational cues and Stroking in order to measure the effects of multisensory conflict on the experienced direction of the 1PP. Future studies should also measure 1PP ratings during baseline conditions (e.g. without visuo-tactile stimulation) in order to quantify the experienced direction of the 1PP in the presence of vestibular-somatosensory gravitational cues alone.
Past research has provided solid evidence that gravity signals contribute to visual perception. For instance, visual object motions comparable to those used in our study were shown to affect behavioral performance in ball-interception tasks and also modulated brain activity in the posterior insula cortex [i.e. a core region of the human vestibular cortical network (Lopez et al., 2012; zu Eulenburg et al., 2012)]. These findings are in accord with the idea that internal models of gravity are automatically and routinely engaged in visual motion perception (McIntyre et al., 2001; Indovina et al., 2005; Senot et al., 2005). Similar results were found for visual self-motion perception (De Saedeleer et al., 2013; Indovina et al., 2013). Our results extend these observations on visuo-vestibular processing to BSC, showing that the experienced direction of 1PP depends on visual information about gravity, resembling altered own-body and verticality perception during room-tilt illusions in microgravity (Tiliket et al., 1996; Lopez et al., 2008).
What functional mechanisms might explain these directional effects of visual cues on the experienced direction of the 1PP, as observed in the present and previous FBI studies (Pfeiffer et al., 2013; Pfeiffer, Schmutz, et al., 2014; Guterstam et al., 2015)? Perception in general and the experienced direction of the 1PP depend on the integration of information from different senses. It is currently accepted that the cues from multiple sensory modalities are not homogeneously taken into account during multisensory perception, but they are weighted depending on the role and reliability of individual senses for each specific context (Helbig and Ernst, 2007; Stein and Stanford, 2008; Burr and Gori, 2012; Chen et al., 2013a, 2013b). We propose that sensory weighting underlies the present effects, in line with experimental evidence for Bayes-optimal visual-vestibular cue integration (Fetsch et al., 2007; Prsa et al., 2012; Rosenberg and Angelaki, 2014; Sunkara et al., 2015) Specifically, we propose that in motionless supine posture, the constant vestibular and somatosensory cues signaling the location and orientation of the participant’s body might be overridden by dynamic visual gravitational cues determining the experienced direction of the 1PP. Differences in weighing of visual over vestibular cues and their effects on the experienced direction of the 1PP are also in line with the observation that dysfunctional visuo-vestibular multisensory processing leads to changes in the experienced direction of the 1PP during OBEs (Blanke et al., 2004), which most often occur in supine posture (Green, 1968; Kovacs et al., 2008).
This proposal may also account for our observation of a trend toward a significant Visual Gravity × Stroking interaction. In line with previous data, we here found the highest proportion of ‘downward’ 1PP ratings in the asynchronous stroking condition [Fig. 2a (Pfeiffer et al., 2013)]. In line with recent models of the rubber hand illusion (Samad et al., 2015), we propose that combination of a visuo-vestibular gravitational conflict and a visuo-tactile Stroking conflict during the FBI modulates reliance, or weighting, of sensory inputs for multisensory processing. Thus, directional conflict of visual and vestibular gravitational cues induces ambiguity about the direction of gravity and results in illusory changes of the experienced direction of the 1PP. In addition, during synchronous Stroking the brain might rely on integrated visual-somatosensory cues, whereas during asynchronous Stroking (because of stronger visuo-tactile conflict) the brain relies more on visual cues further enhancing the effects of visual-gravitational cues on the experienced direction of the 1PP. The present results thus demonstrate the crucial role of visual gravitational cues in determining the experienced direction of the 1PP in the context of the FBI. Nonetheless, it should be noted that different functional and brain mechanisms are involved in the rubber hand illusion and the FBI [see Blanke et al. (2015); Lenggenhager and Lopez (2015) for recent reviews]. We further argue that this is likely related to the higher levels of self-location reported in previous work in the asynchronous vs. synchronous conditions (Ionta et al., 2011; Pfeiffer et al., 2013), more compatible with a downward than upward direction of the experienced direction of the 1PP. The present correlation analysis between 1PP ratings and self-location measures also supports this point (for discussion see next section). Such an interpretation is in line with clinical data showing that OBEs, which are marked by disembodied, elevated, and downward-directed experienced direction of the 1PP, were induced by electrical interference or damage of brain regions functionally involved in multisensory integration (Blanke et al., 2004). In consequence, multisensory disintegration likely underlies the OBE phenomenology resembling experimental changes of both self-location and of the experienced direction of the 1PP as found here. Such an explanation is further in line with the hypothesis that the 1PP is functionally distinct from self-identification (i.e. which depends on visuo-tactile integration), in line with the present results [for further discussion of this issue see Blanke (2012); Pfeiffer (2015); Pfeiffer, Serino, et al. (2014); Serino et al. (2013)].
Self-location: association with 1PP
The positive correlation between self-location and the experienced direction of the 1PP indicates that higher levels of self-location are associated with more frequent downward-directed 1PP experiences. We note again that this pattern is strikingly similar to the phenomenology of OBEs, during which subjects typically experience a downward-directed 1PP anchored to an elevated aerial self-location (Blanke et al., 2004). This has also been observed during experimentally induced states of altered BSC in healthy subjects during the FBI (Ionta et al., 2011; Pfeiffer et al., 2013). In particular, in our previous studies, the experienced downward direction of the 1PP was associated with an elevated aerial self-location and a stroking-dependent drift in self-location in the downward direction (associated with vestibular sensations); on the contrary, an experienced ‘upward’ direction of 1PP was associated with a lower self-location and a stroking-dependent drift in self-location in upward direction (Ionta et al., 2011; Pfeiffer et al., 2013). These changes of self-location further depended on visuo-tactile stroking synchrony, which induced self-location changes toward the seen virtual body location, as compared to asynchronous stroking (Lenggenhager et al., 2007, 2009). Although, we did not find effects of visuo-tactile stroking on self-location in the present study (see discussion below), our measures of self-location and the experienced downward direction of the 1PP were positively correlated. Moreover, numerous previous studies showed that self-location depends on the integration of multisensory bodily-related signals and can be experimentally manipulated by tactile signals presented in synchrony with visual signals at a distant spatial location. Indeed, self-location changes due to visuo-tactile manipulations during FBI are directed toward the spatial location of the visual stimulus (Lenggenhager et al., 2007; Aspell et al., 2009; Lenggenhager et al., 2009; Noel et al., 2015), suggesting that visual cues affect self-location. Here, we showed that dynamic visual gravitational cues affected the experienced direction of the 1PP, extending previous FBI studies (Ionta et al., 2011; Pfeiffer et al., 2013; Pfeiffer, Schmutz, et al., 2014). Thus, visual cues seem to play an important role for both self-location and the experienced direction of the 1PP, compatible with associations previously observed between self-location and experienced direction of the 1PP (Ionta et al., 2011; Pfeiffer et al., 2013). These findings stimulated discussion on whether both spatial aspects of BSC, 1PP and self-location, might be functionally dissociable, or whether they rely on common mechanisms (Serino et al., 2013). Our study design did not directly address the question of whether self-location and 1PP can be separated; however, the fact that dynamic visual gravitational manipulations strongly affected the experienced direction of the 1PP and that ‘downward’ 1PP ratings correlated with elevated self-location suggest that self-location and 1PP share functional mechanisms.
Why did the present experimental manipulation not affect experienced self-location depending on the chosen experimental conditions? We argue that this might depend on the specific visual contextual stimulation used in the present study. As the specific measure of self-location used here (MBD task) requires a representation of distance to the ground floor, both the static and the dynamic visual stimuli that we used suggested a virtual space reaching far into depth, which probably made it difficult for our participants to form a clear mental representation of ground floor, necessary to perform the task. In previous studies, which observed a modulation of MBD RTs (Lenggenhager et al., 2009; Ionta et al., 2011), a surface supporting the virtual body was shown and their absence in the present study may have affected the present self-location data based on the MBD task.
Self-identification: dependence on visuo-tactile stroking
Subjective ratings for critical questionnaire items (Q1–Q3) showed higher scores for synchronous than for asynchronous visuo-tactile Stroking, indicating that the FBI was induced (weaker though than in previous publications; see below). In contrast, Stroking did not modulate control question ratings (Q4–Q10). These results are in line with numerous previous studies using different variants of the FBI or related illusions (Blanke, 2012; Lenggenhager and Lopez, 2015). Our study extends these results by showing that the presentation of dynamic visual gravitational motion did not abolish the induction of the FBI. Indeed, one might have anticipated that virtual object motion captures attention to a location in the visual field other than that of the virtual body, thus distracting participants from the critical visuo-tactile stimulation for the FBI, or, alternatively, that the visual object approaching the body might represent a potential threat [see e.g. (Ehrsson, 2007; Ehrsson et al., 2007)]. However, from the present data it seems that these mechanisms did not interfere with the induction of the FBI, probably because the virtual objects were never (visually) colliding with the observer’s viewpoint or the virtual body and the virtual ball only appeared during relatively short periods.
Next, we found that self-identification (Q1) ratings in the present study were overall lower as compared to previous FBI studies using the same experimental setup (Pfeiffer et al., 2013; Salomon et al., 2013; Pfeiffer, Schmutz, et al., 2014; Romano et al., 2014). This might be related to the stronger multisensory gravitational conflicts (static and/or dynamic gravity cues), in line with a previous study showing a reduction of self-identification by stronger visuo-vestibular gravity conflicts [(Pfeiffer et al., 2013), Experiment 1]. We note that the present study did not address how multisensory gravitational conflict affects self-identification, but focused on the question how gravitational effects impact the experience direction of the 1PP and self-location [approach similar to Ionta et al. (2011); Pfeiffer et al. (2013)]. Based on our data, it remains thus unclear how self-identification relates to the experience direction of the 1PP, which will be an important issue to address by future studies (Blanke, 2012; Serino et al., 2013; Pfeiffer, Serino, et al., 2014; Pfeiffer, 2015). In addition, although on average self-identification (Q1) ratings were higher for synchronous than asynchronous Stroking, these differences were not statistically significant and smaller than stroking effects observed in related FBI studies [e.g. (Petkova and Ehrsson, 2008; Maselli and Slater, 2013; Peck et al., 2013; Guterstam et al., 2015)]. This might be related to the fact that our visual stimuli differed from those used in previous FBI studies, which used real-time videos of the participant’s physical body (Ehrsson, 2007; Lenggenhager et al., 2007), a first-person visual viewpoint of the mannequin/virtual body [e.g. (Petkova and Ehrsson, 2008)] and showed the room in which participants were located. These visual cues highly resembled the physical conditions of the experimental room and the participant and might have induced overall high ratings for the self-identification (independent of stroking manipulations). In contrast, in our study we showed to all participants the same unknown virtual body, located at a distance and provided no information about the experimental room, likely associated with overall low ratings of self-identification.
Finally, we propose that associations between asynchronous Stroking and downward-directed 1PP, as well as between 1PP and self-location as observed here and in previous work, might be related to an overall weaker self-identification during asynchronous stroking, which might further enhance effects of visuo-vestibular gravitational conflict on the experienced direction of the 1PP. Indeed, asynchronous stroking is typically associated with lower self-identification, and less self-location drift toward the virtual body, compared to synchronous Stroking [e.g. (Lenggenhager et al., 2009; Ionta et al., 2011; Pfeiffer et al., 2013)]. Thus, during asynchronous Stroking the virtual body is experienced as another person located at a remote location in front, and might merely serve as a visuo-spatial reference in the external world inducing, due to static downward-directed visual gravitational cues, a downward-directed experienced direction of the 1PP and elevated self-location experience [see Pfeiffer, Schmutz, et al. (2014) for a related proposal]. Based on our data it remains however unclear how self-identification relates to experienced direction of the 1PP, which will be an important issue to address by future studies (Blanke, 2012; Serino et al., 2013; Pfeiffer, Serino, et al., 2014; Pfeiffer, 2015).
Conclusion
Exposing healthy participants to visuo-vestibular gravitational conflicts while inducing the FBI resulted in predictable within-subjects changes of the experienced direction of the 1PP, characterized by an inversion of 180° (downward-directed 1PP experience) with respect to participant’s supine body orientation. The present findings corroborate anecdotal data about neurologically-induced changes in the experienced direction of the 1PP and self-location in patients with OBEs and present a novel method that allows testing one of the most enigmatic aspects of BSC: the directedness of human experience as subject.
Acknowledgements
This work was supported by the Swiss National Science Foundation SINERGIA Grant CRSII1-125135, the European Science Foundation FP7 project VERE, and the Bertarelli Foundation to Olaf Blanke and by the Volkswagen Stiftung (UnBoundBody project, ref. 85 639) and the University of Bologna (RFO) to Andrea Serino. The authors declare that the research reported in this article has been conducted in the absence of financial and commercial relationships that may be construed as a potential conflict of interest. Data are available upon request.
References
- Angelaki DE, Cullen KE. Vestibular system: the many facets of a multimodal sense. Ann Rev Neurosci 2008; 31: 125–50. [DOI] [PubMed] [Google Scholar]
- Aspell JE, Lenggenhager B, Blanke O. Keeping in touch with one's self: multisensory mechanisms of self-consciousness. Plos One 2009;4: e6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoz A. Reference frames for the perception and control of movement. In: Paillard J. (ed.), Brain and Space. New York: Oxford University Press, 1991, 81–111. [Google Scholar]
- Blanke O. Multisensory brain mechanisms of bodily self-consciousness. Nat Rev Neurosci 2012;13: 556–71. [DOI] [PubMed] [Google Scholar]
- Blanke O, Landis T, Spinelli L, et al. Out-of-body experience and autoscopy of neurological origin. Brain 2004;127: 243–58. [DOI] [PubMed] [Google Scholar]
- Blanke O, Metzinger T. Full-body illusions and minimal phenomenal selfhood. Trends Cogn Sci 2009;13: 7–13. [DOI] [PubMed] [Google Scholar]
- Blanke O, Ortigue S, Landis T, et al. Stimulating illusory own-body perceptions. Nature 2002;419: 269–70. [DOI] [PubMed] [Google Scholar]
- Blanke O, Slater M, Serino A. Behavioral, neural, and computational principles of bodily self-consciousness. Neuron 2015;88: 145–66. [DOI] [PubMed] [Google Scholar]
- Burr D, Gori M. Multisensory integration develops late in humans. In: Murray MM, Wallace MT. (eds), The Neural Bases of Multisensory Processes. CRC Press: Boca Raton, FL, 2012. [PubMed] [Google Scholar]
- Chen X, Deangelis GC, Angelaki DE. Diverse spatial reference frames of vestibular signals in parietal cortex. Neuron. 2013a. doi: 10.1016/j.neuron.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Deangelis GC, Angelaki DE. Eye-centered representation of optic flow tuning in the ventral intraparietal area. J Neurosci 2013b; 33: 18574–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D, Van Laere K, Dupont P, et al. Visualizing out-of-body experience in the brain. N Engl J Med 2007;357: 1829–33. [DOI] [PubMed] [Google Scholar]
- De Saedeleer C, Vidal M, Lipshits M, et al. Weightlessness alters up/down asymmetries in the perception of self-motion. Exp Brain Res 2013;226: 95–106. [DOI] [PubMed] [Google Scholar]
- Duenas J, Chapuis D, Pfeiffer C, et al. Neuroscience robotics to investigate multisensory integration and bodily awareness. In: Conference Proceedings: IEEE Engineering in Medicine and Biology Society, Boston, MA, 2011, 8348–52. [DOI] [PubMed] [Google Scholar]
- Dyde RT, Jenkin MR, Harris LR. The subjective visual vertical and the perceptual upright. Exp Brain Res 2006; 173: 612–22. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH. The experimental induction of out-of-body experiences. Science 2007; 317: 1048. [DOI] [PubMed] [Google Scholar]
- Ehrsson HH. The concept of body ownership and it's relationship to multisensory integration. In Stein B. (ed.), The New Handbook of Multisensory Processes. Cambridge, MA: MIT Press, 2012. [Google Scholar]
- Ehrsson HH, Wiech K, Weiskopf N, et al. Threatening a rubber hand that you feel is yours elicits a cortical anxiety response. Proc Natl Acad Sci 2007; 104: 9828–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetsch CR, Wang S, Gu Y, et al. Spatial reference frames of visual, vestibular, and multimodal heading signals in the dorsal subdivision of the medial superior temporal area. J Neurosci 2007; 27: 700–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CE. Out-of-body Experiences. London: Hamish Hamilton, 1968. [Google Scholar]
- Guterstam A, Bjornsdotter M, Gentile G, et al. Posterior cingulate cortex integrates the senses of self-location and body ownership. Curr Biol 2015; 25: 1416–25. [DOI] [PubMed] [Google Scholar]
- Helbig HB, Ernst MO. Optimal integration of shape information from vision and touch. Exp Brain Res 2007; 179: 595–606. [DOI] [PubMed] [Google Scholar]
- Indovina I, Maffei V, Bosco G, et al. Representation of visual gravitational motion in the human vestibular cortex. Science 2005; 308: 416–19. [DOI] [PubMed] [Google Scholar]
- Indovina I, Maffei V, Pauwels K, et al. Simulated self-motion in a visual gravity field: sensitivity to vertical and horizontal heading in the human brain. Neuroimage 2013; 71: 114–24. [DOI] [PubMed] [Google Scholar]
- Indovina I, Mazzarella E, Maffei V, et al. Sound-evoked vestibular stimulation affects the anticipation of gravity effects during visual self-motion. Exp Brain Res 2015. doi: 10.1007/s00221-015-4306-9. [DOI] [PubMed] [Google Scholar]
- Ionta S, Heydrich L, Lenggenhager B, et al. Multisensory mechanisms in temporo-parietal cortex support self-location and first-person perspective. Neuron 2011; 70: 363–74. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Thilo KV, Buchel C, et al. Neural correlates of visual-motion perception as object- or self-motion. NeuroImage 2002; 16: 873–82. [DOI] [PubMed] [Google Scholar]
- Kovacs G, Raabe M, Greenlee MW. Neural correlates of visually induced self-motion illusion in depth. Cerebral Cortex 2008; 18: 1779–87. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B, Lopez C. Vestibular contributions to the sense of body, self, and others. In: Metzinger T, Windt JM. (eds), Open MIND. Frankfurt: MIND-Group, 2015. [Google Scholar]
- Lenggenhager B, Mouthon M, Blanke O. Spatial aspects of bodily self-consciousness. Conscious Cogn 2009;18: 110–17. [DOI] [PubMed] [Google Scholar]
- Lenggenhager B, Tadi T, Metzinger T, et al. Video ergo sum: manipulating bodily self-consciousness. Science 2007;317: 1096–99. [DOI] [PubMed] [Google Scholar]
- Loftus GR, Masson M. Using confidence intervals in within-subjects designs. Psychon Bull Rev 1994;1: 476–90. [DOI] [PubMed] [Google Scholar]
- Lopez C, Blanke O, Mast FW. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience 2012;212: 159–79. [DOI] [PubMed] [Google Scholar]
- Lopez C, Halje P, Blanke O. Body ownership and embodiment: vestibular and multisensory mechanisms. Clin Neurophysiol 2008;38: 149–61. [DOI] [PubMed] [Google Scholar]
- Maselli A, Slater M. The building blocks of the full body ownership illusion. Front Hum Neurosci 2013;7: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre J, Zago M, Berthoz A, et al. Does the brain model Newton’s laws? Nat Neurosci 2001;4: 693–94. [DOI] [PubMed] [Google Scholar]
- Noel JP, Pfeiffer C, Blanke O, et al. Peripersonal space as the space of the bodily self. Cognition 2015;144: 49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck TC, Seinfeld S, Aglioti SM, et al. Putting yourself in the skin of a black avatar reduces implicit racial bias. Conscious Cogn 2013;22: 779–87. [DOI] [PubMed] [Google Scholar]
- Petkova VI, Ehrsson HH. If I were you: perceptual illusion of body swapping. Plos One 2008;3: e3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer C. Multisensory spatial mechanisms of the bodily self and social cognition-A commentary on Vittorio Gallese and Valentina Cuccio. In: Metzinger T, Windt JM. (eds), Open MIND. Frankfurt: MIND-Group, 2015. [Google Scholar]
- Pfeiffer C, Lopez C, Schmutz V, et al. Multisensory origin of the subjective first-person perspective: visual, tactile, and vestibular mechanisms. Plos One 2013; 8: e61751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer C, Schmutz V, Blanke O. Visuospatial viewpoint manipulation during full-body illusion modulates subjective first-person perspective. Exp Brain Res. 2014. doi: 10.1007/s00221-014-4080-0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C, Serino A, Blanke O. The vestibular system: a spatial reference for bodily self-conciousness. Front Integrat Neurosci. 2014. doi: 10.3389/fnint.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prsa M, Gale S, Blanke O. Self-motion leads to mandatory cue fusion across sensory modalities. J Neurophysiol 2012; 108: 2282–91. [DOI] [PubMed] [Google Scholar]
- Romano D, Pfeiffer C, Maravita A, et al. Illusory self-identification with an avatar reduces arousal responses to painful stimuli. Behav Brain Res 2014; 261: 275–81. [DOI] [PubMed] [Google Scholar]
- Rosenberg A, Angelaki DE. Gravity influences the visual representation of object tilt in parietal cortex. J Neurosci 2014; 34: 14170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R, Lim M, Pfeiffer C, et al. Full body illusion is associated with widespread skin temperature reduction. Front Behav Neurosci 2013; 7: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad M, Chung AJ, Shams L. Perception of body ownership is driven by Bayesian sensory inference. Plos One 2015; 10: e0117178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciutti A, Demougeot L, Berret B, et al. Visual gravity influences arm movement planning. J Neurophysiol 2012; 107: 3433–45. [DOI] [PubMed] [Google Scholar]
- Senot P, Zago M, Lacquaniti F, et al. Anticipating the effects of gravity when intercepting moving objects: differentiating up and down based on nonvisual cues. J Neurophysiol 2005; 94: 4471–80. [DOI] [PubMed] [Google Scholar]
- Serino A, Alsmith A, Costantini M, et al. Bodily ownership and self-location: components of bodily self-consciousness. Conscious Cogn 2013; 22: 1239–52. [DOI] [PubMed] [Google Scholar]
- Stein BE, Stanford TR. Multisensory integration: current issues from the perspective of the single neuron. Nat Rev Neurosci 2008; 9: 255–66. [DOI] [PubMed] [Google Scholar]
- Sunkara A, DeAngelis GC, Angelaki DE. Role of visual and non-visual cues in constructing a rotation-invariant representation of heading in parietal cortex. Elife 2015; 4: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiliket C, Ventre-Dominey J, Vighetto A, et al. Room tilt illusion. A central otolith dysfunction. Arch Neurol 1996;53: 1259–64. [DOI] [PubMed] [Google Scholar]
- Zago M, La Scaleia B, Miller WL, et al. Coherence of structural visual cues and pictorial gravity paves the way for interceptive actions. J Vis 2011;11: 13. [DOI] [PubMed] [Google Scholar]
- Zago M, McIntyre J, Senot P, et al. Internal models and prediction of visual gravitational motion. Vis Res 2008;48: 1532–38. [DOI] [PubMed] [Google Scholar]
- zu Eulenburg P, Caspers S, Roski C, et al. Meta-analytical definition and functional connectivity of the human vestibular cortex. Neuroimage 2012; 60: 162–69. [DOI] [PubMed] [Google Scholar]