Abstract
In nature, animals are exposed to a broad range of threats imposed by predators, which may strongly influence the ecology of prey species directly or indirectly by affecting their behavior via fear of predation. Here, we studied wood mice Apodemus sylvaticus behavioral and physiological responses to simulated predation risk. Risk avoidance was analyzed by live trapping with control traps and traps treated with feces of common genet Genetta genetta (direct cue of risk) under new moon nights and following by simulated full moon conditions (indirect cue). The time devoted to foraging behavior and capture time were analyzed by video recording mice activity around traps. Food intake was calculated based on the amount of bait remaining in each trap. Fecal corticosterone metabolites (FCMs) were measured by enzyme-immunoassay as indicators of physiological stress responses. Fewer wood mice were captured during full moon, yet only non-breeding adult males clearly avoided common genet odor. Mice were captured sooner at night during the simulated full moon conditions and later in predator-treated traps. Foraging activity was lower when individuals faced predator’s feces, but neither food intake nor FCM levels were affected by predation risk cues. Direct and indirect cues of predation risk selectively affected wood mice behavior, although behavioral responses seem to be modulated by different costs–benefit balances related to the individual’s perception of risk. The lack of physiological responses to predation risk cues suggests that wood mice did not perceive them as reliable stressors or the response was too small or transient to be measured by FCM.
Keywords: common genet, fecal predator cues, feeding, foraging, moonlight, predator avoidance
Predation represents one of the most important causes of death for small mammals and it strongly influences prey ecology directly through mortality (Brown et al. 1999; Hanski et al. 2001) or through indirect effects on prey demographic and behavioral responses to predators (Lima and Dill 1990; Apfelbach et al. 2005; Díaz et al. 2005; Zanette et al. 2011; Navarro‐Castilla and Barja 2014a, 2014b). Because animals are exposed to a wide range of dangers imposed by predators, they have developed a variety of predator detection mechanisms and antipredatory responses to minimize or avoid predation risk (Lima and Dill 1990; Kats and Dill 1998; Lima 1998). Thus, prey are attuned to respond in a number of behavioral and physiological ways to cues associated with predation risk that can be direct (signals associated to predators: presence, urine, feces, or sounds) or indirect (e.g., habitat complexity or environmental conditions) (Eilam et al. 1999; Orrock et al. 2004; Wróbel and Bogdziewicz 2015).
Most carnivores use secretions from glands, urine, and feces to mark their territory (Hutchings and White 2000; Barja and List 2006; Barja 2009; Martín et al. 2010; Piñeiro et al. 2012) and multiple studies have revealed that several rodent species are sensitive to the scent of potential predators, avoiding such chemical signals without needing other cues (Stoddart 1982; Dickman and Doncaster 1984; Calder and Gorman 1991; Jedrzejewski et al. 1993; Navarro‐Castilla and Barja 2014a, 2014b). Furthermore, prey species often alter their behavior in response to the auditory, visual, and chemosensory cues from predators (Lima and Dill 1990; Kats and Dill 1998; Eilam et al. 1999; Zanette et al. 2011; Clinchy et al. 2013; Tortosa et al. 2015). Thus, in that prey species are at risk of predation while performing daily activities, there are tradeoffs between antipredator behavior and other fundamental activities like foraging and feeding (Sih 1980; Brown et al. 1988; Brown 1988; Orrock et al. 2004; Gallego et al. 2017; Sánchez-González et al. 2017). In addition, antipredatory behavior can be strongly influenced by the environment. Thus, increased predation risk perception through lower habitat complexity or higher visibility has revealed that rodent species will avoid open areas and decrease activity on nights with a full moon (Kaufman and Kaufman 1982; Kotler et al. 1988; Wolfe and Summerlin 1989; Kotler et al. 1994; Brown et al. 2001; Kotler et al. 2002; Eilam 2004; Kotler et al. 2010). Few studies have attempted to determine whether prey responses to predation risk situations are influenced by individual characteristics (e.g., sex, breeding condition, and age of individuals) (Dickman and Doncaster 1984; Jedrzejewski and Jedrzejewska 1990).
Responses to predation risk should not be restricted only to behavioral responses because, under certain risky situations, prey may display physiological responses which are not translated into a modification of behavior (Eilam et al. 1999). When animals are subjected to a stressor, the hypothalamus releases corticotrophin releasing hormone inducing the anterior pituitary to secrete the adrenocorticotropic hormone (ACTH) which signals the adrenal cortex to release glucocorticoids (GC) to help the individuals to cope with the stressful situation (Sapolsky et al. 2000). Thus, GC concentrations can be used as a hormonal measure of physiological stress responses (Wingfield and Romero 2001; Möstl and Palme 2002). In fact, GC metabolites in feces have been reported in several vertebrate species as a useful non-invasive technique for assessing adrenocortical function (Möstl and Palme 2002; Monclús et al. 2006; Lepschy et al. 2007; Dantzer et al. 2010; Barja et al. 2012; Piñeiro et al. 2012; Zwijacz-Kozica et al. 2013; Navarro-Castilla et al. 2014a, 2014b). In mammals, GC plays an important role in responding to diverse factors such as social conflicts and human disturbances (Sapolsky et al. 2000; Romero 2002; Barja et al. 2007; Navarro-Castilla et al. 2014a, 2014b). Since stressful situations usually evoke an increase in GC production, predators could induce physiological responses in their prey by a physical attack but also by making them fearful of an imminent attack (Boonstra et al. 1998; Eilam et al. 1999; Hirschenhauser et al. 2000; Korte 2001; Monclús et al. 2005; Clinchy et al. 2013; Zanette et al. 2014). Similarly, increased illumination could act as a potential stressor for nocturnally active prey species. However, few studies have previously evaluated its effect on the physiological stress response (Navarro-Castilla and Barja 2014b).
In the present study, we tested whether wood mice Apodemus sylvaticus showed behavioral and physiological changes due to increased predation risk due to moonlight (i.e., natural new moon and simulated full moon conditions) and exposure to predator odor from an invasive species, the common genet Genetta genetta. Thus, we studied whether these cues of increased predation risk affected: (1) wood mouse behavior (i.e., avoidance of predator-treated traps and foraging activity), (2) food intake, and (3) physiological stress response in wood mice. Further, the influence of individual characteristics (i.e., sex, reproductive activity, and age) on these responses was also evaluated. The common genet is an important threat for small mammals, especially for wood mice (Hamdine et al. 1993; Virgós et al. 1999). Since variation in predation risk affects foraging decisions (Lima and Bednekoff 1999), we predicted that wood mice would alter their foraging behavior when confronted with common genet feces and they would also avoid entering the predator-treated traps, especially under high illumination (simulated full moon). Further, wood mice were expected to vary food intake in response to their perceived predation risk prior to entering the trap (owing both to increased illumination and the presence of predator feces), but also because of the likely detection of common genet fecal odor by individuals within treated traps. Finally, we expected that exposure to increased illumination and to common genet feces would evoke physiological stress responses in wood mice as measured by fecal GC metabolites.
Materials and Methods
Study area
Field work was carried out in the savanna-like holm oak Quercus ilex woodlands of the National Park of Cabañeros (Central Spain, 30S 385450, UTM 4353479). In this system, large oak trees grow scattered (mean tree density is 14 ha−1) on a grassland matrix with almost no shrub cover (<1%; see Pulido et al. 2001; Díaz et al. 2011).
Experimental design: live trapping and simulation of predation risk
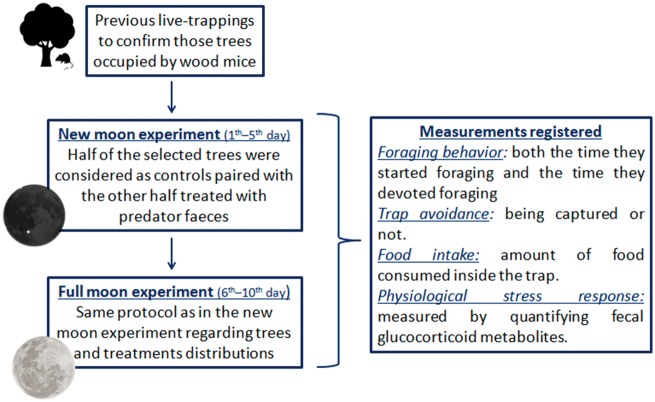
Prior to the beginning of the experimental study, to determine which trees were occupied by wood mice and to allow mice to acclimate to traps, Sherman traps were placed beneath trees (n = 170) in 2 study sites (separated by 1,500 m) over a 3-day period. Afterward, during the experimental study (Figure 1), Sherman traps (n = 2/tree) were placed in those trees (n = 40) confirmed to be occupied by wood mice. Since predator’s odors have been previously shown to evoke antipredatory responses in small mammals (Dickman and Doncaster 1984; Navarro-Castilla and Barja 2014a, 2014b), we manipulated the direct perception of predation risk through predator odor from one of the main rodent predators in the study area, the common genet G. genetta. To examine the effect of predator odor, nearby occupied trees were randomly paired and treatments (traps treated with predator odor) and control (untreated traps) were assigned to one tree of each pair at random. Mean distance between predator-treated and paired control trees was 42.79 m (range 8.20–80.36 m). Predator treatment consisted in fresh feces of common genet collected from captive animals of the Cañada Real Open Center (Madrid, Spain). To prevent volatile compounds variation in relation to seasonal or individual factors (Andreolini et al. 1987; Jemiolo et al. 1991; Hayes et al. 2006; Scordato et al. 2007; Martín et al. 2010), all collected feces were mixed to obtain a homogeneous mixture avoiding possible bias in our results. Predator treatment was made following methods by Navarro‐Castilla and Barja (2014a), 100 g of homogenized fecal sample was mixed with 100 mL of distilled water obtaining a mixture similar to real fresh feces. Predator presence was simulated by leaving an equal amount (5 g) of feces at the entrance of treated traps and it was renewed every day at dusk.
Figure 1.
Flow chart of the experimental study.
To test the effect of moonlight, the above mentioned experimental design was carried out during 5 consecutive new moon nights (20–24 March 2012); afterward, the following 5 nights we simulated full moon light conditions at the same sites by means of artificial illumination. The illumination device (composed of 3 white and 3 blue led lights grouped behind a diffusion screen simulating a diffuse light with the spectral composition of moonlight) was hung down from the tree canopy at a height of 2 m to simulate a light intensity of 1 lux at ground level (measured by means of a TES-1332A luxometer). Light intensity of 1.0 lux approximately corresponds to the maximum moonlight intensity expected during full moon nights in this region (Bünning and Moser 1969).
Sherman traps were activated at dusk, and trap checks were carried out 10–12 h later (at dawn) to minimize the time that animals were kept. Nest material (raw wool with natural lanolin) was used as bedding inside traps. All traps were baited with 4 g of toasted corn. Captured individuals were identified to species. Sex and reproductive condition was determined from external characteristics (Gurnell and Flowerdew 1994); adult males with enlarged testicles descended into the scrotal sac and females showing noticeable nipples and/or the vaginal membrane perforated were classified as reproductively active. In addition, a 100 g hand-held scale was employed to measure body weight which was used to estimate relative age following Navarro‐Castilla and Barja (2014a) (juveniles: <13 g; sub-adults: from 13 g to <20 g; adults: ≥20 g). Individuals were marked in non-conspicuous areas with harmless paints (red food coloring: Ponceau-4R E124) for individual identification and to control for recaptures. Animals were quickly handled (<1 min) and then released at the same point of capture. Manipulations of animals were done in compliance with the European Communities Council Directive 86/609/EEC for animal experiments and were carried out under the permit of the Cabañeros National Park authorities.
Mice foraging behavior and food intake
For recording wood mice foraging behavior, video-cameras (OmniVision CMOS 380 LTV, 3.6 mm lens) were mounted on a tripod 60 cm tall located 1 m away and focused on Sherman traps, covering a field of vision of 1 m2. Video-cameras were provided with ELRO dvr32 card-based recorders (settings 5 frames/s and using 16 GB recording cards replaced each day). Both the recording and the illumination devices were fully autonomous since they were powered by car batteries (70 Ah, lead-acid) attached to solar panels (ono-silicon erial P_20; 20 w). However, they were turned on each day at dusk, before opening traps and renewing predator odor. Mice foraging behavior, recorded as the time (s) since individuals appeared in the image until they went inside the trap closing it, was videotaped during trapping sessions. We also recorded at what time of the night each individual was captured allowing us to know the time spent by each individual inside traps.
To determine the amount of food eaten, bait remains were oven-dried at 50 °C (Selecta, model CONTERM 2000208) and weighed (Giros PG-500; precision 0.01 g). Body weight of individuals was positively correlated with food intake (r = 0.67, P = 0.002); therefore, food intake by an individual was divided by its body weight to control the effect of body weight on food intake.
Feces collection and fecal corticosterone metabolites quantification
Fresh feces were collected from traps where individuals were captured, if urine was detected fecal samples were excluded in order to avoid cross contamination (Touma et al. 2003). To avoid the effects of environmental conditions and microorganisms proliferation on fecal corticosterone metabolite (FCM) levels (Washburn and Millspaugh 2002; Millspaugh et al. 2003), only fresh feces (i.e., with a soft texture and not dried) were collected. Fecal samples were collected between sunrise and 2 h after; thus, by only collecting fresh feces during the early morning we avoided circadian rhythm effects on excretion patterns (Touma et al. 2003). Corticosterone peak concentrations have been observed in wood mice feces on average at 10 h after ACTH injection (range: 8–12 h; see the “results” section); therefore, fecal samples from individuals trapped >8 h were rejected to avoid any possible effect of the capture in FCM levels. Fecal samples were stored in the freezer at −20°C until analysis. To control for potential observer bias, we used blind observation by coding samples before laboratory analysis of FCM concentrations.
Extraction of FCM from fecal samples was done according to the modified method of Touma et al. (2003). Fecal samples were unfrozen and dried in the heater until constant weight. We placed 0.05 g of dry feces in assay tubes with 0.5 mL of phosphate buffer and 0.5 mL of 80% methanol, then, they were shaken for 16 h and supernatants were centrifuged at 2,500 × g for 15 min. Pellets were discarded and the fecal extracts were stored at −20 °C until analyzed. Quantification was achieved using a commercial corticosterone enzyme immunoassay (EIA) (Demeditec Diagnostics GmbH, Kiel, Germany) previously validated for measuring FCM in mice species (Abelson et al. 2016; Navarro-Castilla et al. 2017). Parallelism, accuracy, and precision tests were done to validate the EIA (Goymann et al. 1999; Young et al. 2004). Parallelism was performed with serial dilutions of fecal extracts (1:32, 1:16, 1:8, 1:4, 1:2, 1:1) resulting in a curve parallel to the standard. Accuracy (recovery) was 118.6 ± 31.7% (n = 6). Precision was tested through intra- and inter-assay coefficients of variation for 3 biological samples, being 4.7% (n = 6) and 8.2% (n = 3), respectively. In each assay, we used a standard, whose corticosterone concentration was known, included in the Demeditec kit. The assay was excluded and samples were reanalyzed if standard corticosterone concentrations deviated >10% from the expected value. The assay detection limit (sensitivity) for corticosterone metabolites was 4.1 ng/mL. Furthermore, a biological validation was carried out to confirm the suitability of the EIA for wood mouse fecal samples. Thus, following the procedure by Touma et al. (2004), we injected a high dose (60 µg/100 g of body weight) of synthetic ACTH (Synacthen Depot, Novartis, Germany) into 5 captive individuals (2 females and 3 males). Samples of each of the 5 individuals were collected within minutes after defecation and immediately stored in Eppendorfs at −20 °C until analysis. Sampling times were: 0, 2, 4, 6, 8, 10, 12, 14, 18, 22, and 26 h post-injection. FCM levels are expressed as nanograms per gram dry feces.
Higher FCM concentrations detected in the present study are similar to those analyzed using the same methodology in another closely related rodent species, the Algerian mouse (Mus spretus), inhabiting the same study area (Navarro-Castilla et al. 2017). This may be attributable to the very low limit of detection (553 pg/mL) of the Demeditec kit, which is known to detect higher FCM concentrations than other available commercial kits (see Abelson et al. 2016).
Data analysis
Capture frequencies according to odor and moonlight treatments, as well as their interactions with individual characteristics (sex, age, and breeding condition) were analyzed by fitting log-linear models to the 5-way contingency table generated by the factors odor (control/common genet feces), moonlight (new moon/simulated full moon), sex/age (adult male, adult female, or juvenile), breeding condition (active or not), and presence/absence of capture, taking into account the structural zeros resulting from the impossibility of finding sexually active juveniles (Díaz et al. 1999; Morán-López et al. 2015). Recaptures were not taken into account in the captures frequencies tests to maintain data independence.
We used general linear models (GLMs) to analyze differences in foraging behavior due to moonlight (natural new moon/simulated full moon), treatment (control/fecal odor), sex (male/female), breeding condition (breeding/non-breeding), age (juveniles/sub-adults/adults), and recapture (new capture/recapture). We also employed GLMs to test variation in food intake (corrected by animal’s body weight); fixed factors were the same as in the foraging activity model (except age factor) and we included the time that each individual spent inside the trap as covariate. Finally, variation in FCM was analyzed by GLMs, including moon phase, treatment, sex, breeding condition, and recapture as fixed factors and body weight of individuals was included as covariate. Foraging behavior and FCM were log-transformed as needed to normalize the distributions of residuals.
The GLMs included the main effects of the factors studied and their 2-way interactions. Results were considered significant at α < 0.05. The probability of committing table-wise type-I errors was judged low (ca. 18%; 4 comprehensive test made at α = 0.05; Streiner and Norman 2011), so that we did not perform adjustments for multiple comparisons to avoid the risk of committing type-II errors (Rothman 1990; Feise 2002). Results are given as mean ± standard error (SE). We used the SPSS 15.0 statistical software (SPSS Inc., Chicago, IL, USA).
Results
Risk avoidance by wood mice
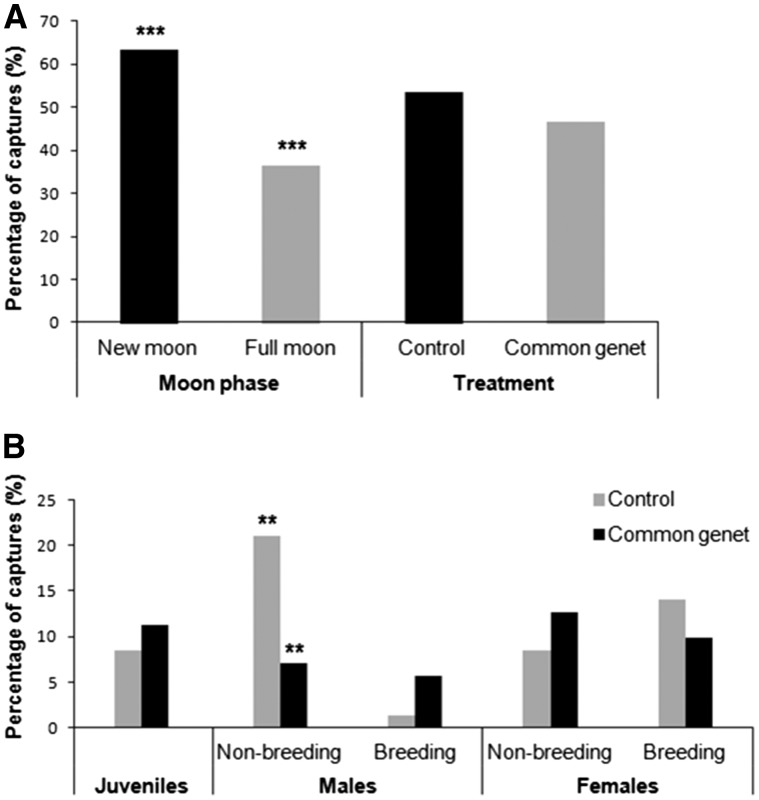
Overall, 153 wood mice (71 new captures and 82 recaptures) were captured. The study population was dominated by adults (80.3% vs. 19.7%), females (56.1% vs. 43.9% males), and reproductively active females (53% vs. 20% reproductively active males; Table 1). Regarding predation risk factors, simulated full moon conditions decreased the number of captures compared with the natural new moon phase (36.5% vs. 63.5%, respectively) while predator treatment did not significantly decrease wood mice captures (46.5% vs. 53.5% control traps) (Table 1 and Figure 2A). Nevertheless, we found a significant interaction between treatment * sex/age * breeding condition (Table 1) showing that non-breeding adult males clearly avoided common genet feces (χ2 = 7.04, df = 1, P = 0.008; Figure 2B). None of the interactions among predator risk factors were significant (Table 1).
Table 1.
Results of the fit of a log-linear model analyzing the effects of individual and predation risk factors on the capturability of wood mice
| Effect | df | G2 | P |
|---|---|---|---|
| Sex/age | 2 | 7.38 | 0.025 |
| Breeding condition | 1 | 10.68 | 0.001 |
| Sex/age * breeding condition | 1 | 6.66 | 0.010 |
| Treatment | 1 | 0.36 | 0.548 |
| Moonlight | 1 | 12.35 | 0.000 |
| Moonlight * sex/age | 2 | 3.03 | 0.220 |
| Moonlight * breeding condition | 1 | 0.15 | 0.695 |
| Treatment * moonlight | 1 | 0.04 | 0.843 |
| Treatment * sex/age | 2 | 1.94 | 0.379 |
| Treatment * breeding condition | 1 | 0.21 | 0.644 |
| Treatment * moonlight * sex/age | 2 | 0.42 | 0.809 |
| Treatment * moonlight * breeding condition | 1 | 0.14 | 0.712 |
| Treatment * sex/age * breeding condition | 1 | 6.54 | 0.011 |
| Moonlight * sex/age * breeding condition | 1 | 1.01 | 0.316 |
| Treatment * moonlight * sex/age * breeding condition | 1 | 0.97 | 1.000 |
Figure 2.
Percentage of wood mice captured in relation to direct (common genet feces) and indirect (moonlight) cues of predation risk (A). Percentage of captures according to treatment, sex/age, and breeding condition (B). Asterisks indicate significant differences between the analyzed groups (**P < 0.01; ***P < 0.001).
Mice foraging behavior and food intake
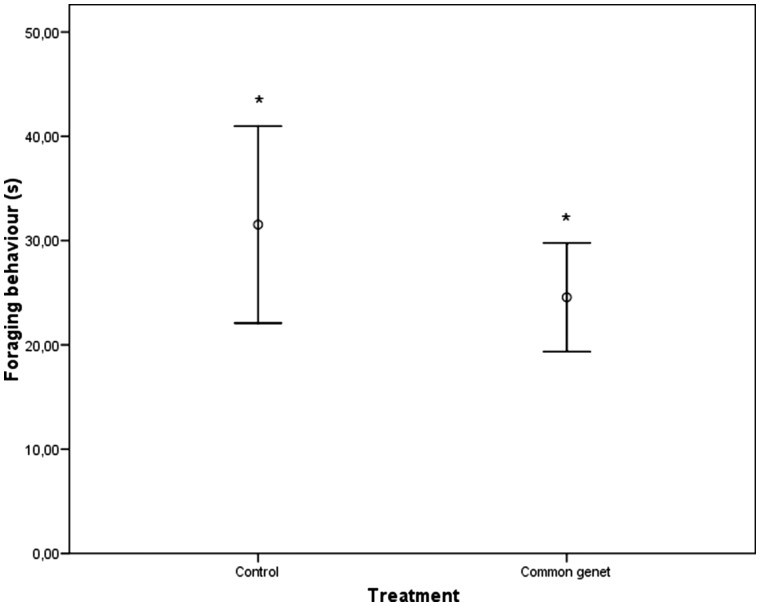
Wood mice were captured sooner at night during simulated full moon conditions (5 h 1′ ± 34′ after trap activation) than during new moon nights (6 h 45′± 34′) (F1,76 = 5.77, P = 0.019). Further, individuals were captured sooner in control traps (at 5 h 5′± 34′) compared with traps treated with common genet feces in which individuals were captured later during the night (6 h 40′± 34′) (F1,76 = 4.66, P = 0.034). Treatment was the only significant factor explaining the variation found in foraging behavior before entering traps (Table 2); individuals spent less time foraging when they were subjected to predator fecal cues (24.56 ± 2.60 s) than when they faced control traps (31.54 ± 4.67 s; Table 2 and Figure 3). The amount of food consumed was not related to the amount of time that animals spent inside traps, or by their previous capture history. Further, neither common genet feces nor illumination influenced food intake. Only breeding condition emerged as a significant factor (Table 3), with breeding individuals having a significantly lower food intake (0.091 ± 0.008 g/g) than non-breeding individuals (0.174 ± 0.013 g/g). Interactions among factors were not statistically significant.
Table 2.
Results of GLMs testing for the effects of predation risk and individual factors on wood mice foraging behavior
| Factor | df | F | P |
|---|---|---|---|
| Moonlight | 1 | 0.301 | 0.587 |
| Treatment | 1 | 6.945 | 0.013 |
| Sex | 1 | 1.176 | 0.286 |
| Breeding condition | 1 | 2.554 | 0.120 |
| Relative age | 1 | 1.680 | 0.202 |
| Recapture | 1 | 1.366 | 0.271 |
| Error | 59 |
Figure 3.
Effect of treatment (control vs. common genet) on wood mice foraging behavior (s, mean ± SE). Significant differences are indicated by asterisks (*P < 0.05).
Table 3.
Food intake by wood mice in relation to predation risk and individual factors
| Factor | df | F | P |
|---|---|---|---|
| Moonlight | 1 | 3.579 | 0.065 |
| Treatment | 1 | 1.432 | 0.238 |
| Sex | 1 | 0.019 | 0.890 |
| Breeding condition | 1 | 8.486 | 0.006 |
| Recapture | 1 | 6.563 | 0.231 |
| Time inside trap | 4 | 2.608 | 0.114 |
| Error | 55 |
Physiological stress response
In the biological validation experiment, measured FCM baseline levels (prior to injection) for each tested individual ranged from 13,120 to 40,420 ng/g feces. In the 5 individuals, the corticosterone EIA detected an average increase in FCM concentrations ranging from 116% to 247% within 8–12 h of the injection event. Subsequent to that, a downward trend toward baseline FCM values was detected within 12–18 h, validating the corticosterone EIA for the analysis of wood mouse fecal samples.
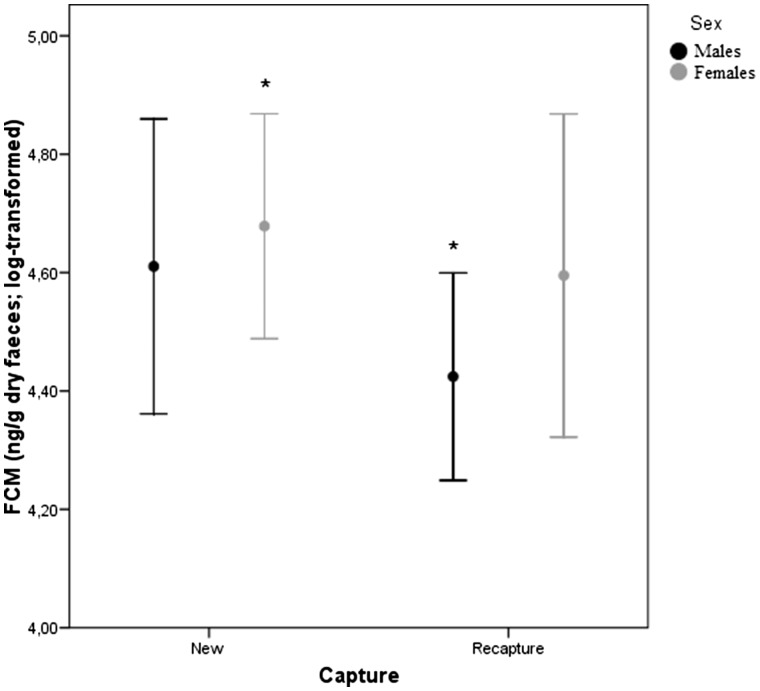
FCM levels were analyzed in 107 fresh fecal samples. Neither moonlight nor predator odor emerged as significant factors influencing FCM levels. Factors explaining the variation found in FCM concentrations are presented in Table 4. Body weight of individuals was positively correlated with FCM levels (Table 4). Overall, FCM levels were lower in males (130,695 ± 53,407 ng/g dry feces) than in females (138,762 ± 41,306 ng/g dry feces) and individuals showed lower FCM levels when they were recaptured (84,928 ± 35,891 ng/g dry feces) than when they were captured for the first time (165,002 ± 47,982 ng/g dry feces). However, the only significant interaction between sex * recapture revealed that significant differences in FCM were between females captured for the first time and recaptured males (Table 4 and Figure 4).
Table 4.
Results of a general lineal model testing the effects of individual and predation risk factors on fecal glucocorticoid metabolites in wood mice
| Factor | df | F | P |
|---|---|---|---|
| Moonlight | 1 | 2.690 | 0.105 |
| Treatment | 1 | 0.025 | 0.875 |
| Sex | 1 | 0.177 | 0.675 |
| Breeding condition | 1 | 0.170 | 0.681 |
| Recapture | 1 | 1.235 | 0.270 |
| Body weight | 1 | 5.660 | 0.020 |
| Sex * recapture | 1 | 4.178 | 0.044 |
| Error | 90 |
Figure 4.
Log-transformed concentrations (mean ± SE) of fecal glucocorticoid metabolites (FCM, ng/g dry feces) in males and females in relation to new captures or recaptures.
Discussion
Risk avoidance by wood mice
A decrease in activity during full moon conditions has been described as a generalized antipredatory behavior in prey species (Kotler et al. 2010; Penteriani et al. 2013). This common behavioral response could explain why fewer wood mice were captured during the simulated full moon conditions, matching this result with the moonlight avoidance found in wood mice under natural full moon nights (Navarro-Castilla and Barja 2014b). The number of newly captured individuals during the full moon treatment could also have been influenced by the design of our study, because it was carried out after the new moon treatment and had a smaller pool of potential new animals to catch. However, the reduction in the number of both newly captured and recaptured individuals supported the overall negative effect of increased illumination on wood mice foraging. Therefore, moonlight can indicate a higher risk of predation since an individual’s vulnerability to a predator depends partly upon visibility. On the other hand, predator odor might provide direct information on predation risk even when the predator is absent at the time of detection. In this study, the number of captures decreased in traps treated with common genet feces, coincident with several studies where avoiding areas marked by predators was common in small mammals (Dickman and Doncaster 1984; Calder and Gorman 1991; Russell and Banks 2007). However, this antipredatory response varied depending on individual characteristics, being significantly different from random expectations for non-breeding adult males only. Coincident with these results, Dickman and Doncaster (1984) showed that male wood mice exhibited a higher avoidance of predator feces than females did. However, in our case, breeding males did not show such avoidance. Their social, sexual, and territorial-related behaviors during the breeding season (Montgomery and Gurnell 1985) and their attraction to new objects (Brown 1969) could be possible explanations for the results. Similar differences in response to predator cues due to sex and breeding condition were also found in bank voles Clethrionomys glareolus by Jedrzejewski and Jedrzejewska (1990). Therefore, sex and breeding condition differences in the responses to predation risk suggest that gonadal hormones may be involved in the mediation of the antipredatory responses (Perrot-Sinal et al. 1999). On the other hand, young mammals typically devote less time to predator detection (Arenz and Leger 2000) which could explain why juveniles were equally captured although predation risk cues increased. Therefore, while indirect risk cues (moonlight) seem to be perceived by most individuals as a more reliable indicator of enhanced predation risk (Orrock et al. 2004), responses to direct cues (predator feces) are not generalized, but vary among individuals according to the individual’s characteristics and in all likelihood, their previous experience (Lima and Bednekoff 1999).
Mice foraging behavior and food intake
Predation risk perception may influence animal daily decision making to choose when, where, and how long to forage. According to Lima and Bednekoff (1999), under high risk situations prey reduce time spent in daily activities to optimize the energy spent on antipredatory behavior. Several studies have reported that under high levels of risk, individuals decreased mobility and concentrated foraging activity in safer habitats (Lima and Dill 1990; Díaz 1992; Kotler et al. 2002). Our results also showed predation risk influencing foraging behavior. Thus, when traps were treated with common genet feces, wood mice apparently delayed foraging close to these traps and were captured later at night. Despite that delay, once individuals were detected in the vicinity of a trap, they devoted less time to foraging, entering feces-treated traps more rapidly than control traps. In relation to moonlight, wood mice were trapped sooner during simulated full moon conditions, but no effect of moonlight was found on foraging behavior. These results perfectly match those of Díaz et al. (2005), who found that wood mice reduced foraging behavior in response to the presence or activity of common genets but there was no effect of moonlight on foraging activity. Apart from effects of the different nature and meaning of both predation risk cues (direct cues indicate predator presence nearby, whereas indirect cues just general levels of danger), results may be also explained by temporal variation in their intensity. Predator odor intensity surely decreased through the night, so that wood mice delayed foraging and foraged more rapidly when facing predator feces, consistent with this decreasing intensity of the direct cue. However, moonlight intensity was constant over the night, so that perception of risk, and hence responses, did not decrease. According to Lima and Bednekoff (1999), animals under longer periods of high risk (e.g., full moon nights) are forced to decrease antipredatory behavior and forage to meet their energy demands. This hypothesis could explain why although moonlight is supposed to increase perceived predation risk and wood mice were expected to decreased food intake we did not find significant differences. Alternatively, as the full moon experiment was carried out after the new moon sampling, this might be an effect of treatment order (i.e., first new moon directly following by the full moon experiments) and previous experience. Thus, as a result of being repeatedly captured during both moon phases, wood mice may have become accustomed and would have valued the benefits of obtaining food over the risk perceived via illumination. Regarding individual factors, only breeding condition led to significant differences in food intake. Overall, prey have to trade off food and safety under each situation, but they also have to prioritize between different daily activities. In this regard, we conservatively speculate that breeding individuals could be more careful under risky situations reducing feeding and allocating more time to survive and breed.
Physiological stress response
Generally, short-term GC secretion last only a few hours and promotes successful adaptive responses to a stressful stimulus (Wingfield and Romero 2001), whereas chronic stress occurs when individuals experienced either multiple, frequent exposure to stressors, and/or long-term continuous exposure to stressors which generates elevated and prolonged high GC levels exceeding the individual level of beneficial adaptation and leading to pathological consequences (Möstl and Palme 2002; Sapolsky 2002; Romero 2004). Physiological responses due to simulated predation risk by owl calls were previously found for voles and mice (Eilam et al. 1999). However, studies where predation risk was simulated with predator odor did not evoke any physiological response in different rodent species (bank voles and weasels: Ylönen et al. 2006; meadow voles and weasels: Fletcher and Boonstra 2006). In the present study, neither moonlight nor exposure to predator feces had any influence on FCM in wood mice, a result similar to the lack of effect of natural moonlight conditions and red fox fecal odor on the physiological stress response of wood mice found by Navarro-Castilla and Barja (2014b). Thus, perceived predation risk does not appear to be sufficient to elicit increased FCM levels in the wood mouse. Both the delay in approaching feces-treated traps and reduced foraging behavior when in proximity to such traps could reduce the individual’s perceived predation risk so as to preclude a physiological stress response, or diminish that response in magnitude or duration to the point that it escaped detection by our FCM assay. Alternatively, inter-individual variation in FCM levels could result in insufficient statistical power to detect significant differences among predation risk treatments. Regarding the effect of individual factors, we found that body weight of individuals, which is closely related to the age of individuals (Gurnell and Flowerdew 1994), was positively correlated with FCM levels. Adults may have exhibited higher FCM levels as a consequence of their breeding condition, that is, changes due to pregnancy and lactancy (Bauman 2000; Strier et al. 2003; Reeder and Kramer 2005), as well as to social interactions among adult males (Rogovin et al. 2003). Alternatively, individuals might simply display age-related physiological responses to cope with stressors (Hauger et al. 1994). The interaction between sex * recapture also showed a significant influence on FCM levels, which could also indicate a greater stress response by females to the novel testing environment. Higher GC levels in females have been previously found in this and other rodent species (Touma et al. 2004; Navarro-Castilla et al. 2014a, 2014b), which could be attributed primarily to differences in the metabolism and/or excretion of GCs between both sexes (Touma et al. 2003).
Overall, wood mice behavioral changes found under the predation risk situations studied likely reduced the probability of an encounter with a predator, but they imply important trade-offs between the benefits of safety from predation and the costs associated with missing opportunities for foraging or reproduction (Abrams 1986; Lima and Dill 1990; Brown et al. 1999; Brown and Kotler 2004). Besides being an invasive species, common genets defecate in latrines, so their feces may be less indicative of their presence or movement patterns, and therefore, a generalized antipredatory response would not lead to survival benefits that would outweigh the cost of lost foraging opportunities. Thus, wood mice are expected to exhibit different antipredatory responses only when they have an accurate assessment of the current predation risk, and making decisions, choosing those behavioral options which maximize their fitness, for example, delaying and reducing foraging activity when facing common genet fecal cues. Nevertheless, behavioral responses seem to depend on context, past experience, and individual variation (Gorman and Trowbridge 1989). The apparent absence of a GC stress responses to factors that are presumably related to predation risk (i.e., moonlight and predator odor) suggests that these factors are not perceived as reliable stressors by wood mice. So, making decisions by altering behavioral responses seem to be better, faster, and a more useful option to maximize fitness.
Author Contributions
Conceptualization: A.N.C., I.B., and M.D.; Methodology: A.N.C.; Data analysis: A.N.C., I.B., and M.D.; Writing—original draft preparation: A.N.C.; Writing—review and editing: A.N.C., I.B., and M.D.; and Funding acquisition: A.N.C., I.B., and M.D.
The authors are very grateful to Miguel Fernández, Verónica Alonso, David López, and Javier Jiménez for their help during field work. They thank the authorities and staff of the Cabañeros National Park who allowed them to develop their studies there. They also thank Dr Vincenzo Penteriani for their generous help in reviewing a previous version of this manuscript, and Dr James Hare and 2 anonymous reviewers for their useful comments and suggestions to improve the present work.
Funding
This work was partially supported by the project RISKDISP (CGL2009-08430/BOS) and Comunidad de Madrid together with the European Social Fund and Universidad Autónoma de Madrid (CCG10-UAM/AMB-5325). This study is a contribution to the projects MONTES [CSD2008-00040], RISKDISP [CGL2009-08430], and VULGLO [CGL2010-22180-C03-03], funded by the Spanish Ministerio de Ciencia e Innovación; 096/2002 and 003/2007, funded by the Organismo Autónomo Parques Nacionales; and ANASINQUE [PGC2010-RNM-5782], funded by the Junta de Andalucía. Á.N.-C. was supported by a FPU scholarship [AP2008-03430] from the Ministerio de Educación y Ciencia of Spain.
References
- Abelson KSP, Kalliokoski O, Teilmann AC, Hau J, 2016. Applicability of commercially available ELISA kits for the quantification of faecal immunoreactive corticosterone metabolites in mice. In Vivo 30:739–744. [DOI] [PubMed] [Google Scholar]
- Abrams PA, 1986. Is predator–prey coevolution an arms race? Trends Ecol Evol 1:108–110. [DOI] [PubMed] [Google Scholar]
- Andreolini F, Jemiolo B, Novotny M, 1987. Dynamics of excretion of urinary chemosignals in the house mouse Mus musculus during the natural estrous cycle. Experientia 43:998–1002. [DOI] [PubMed] [Google Scholar]
- Apfelbach R, Blanchard CD, Blanchard RJ, Hayes RA, McGregor IS, 2005. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci Biobehav Rev 29:1123–1144. [DOI] [PubMed] [Google Scholar]
- Arenz CL, Leger DW, 2000. Antipredator vigilance of juvenile and adult thirteen-lined ground squirrels and the role of nutritional need. Anim Behav 59:535–541. [DOI] [PubMed] [Google Scholar]
- Barja I, 2009. Decision making in plant selection during the faecal-marking behaviour of wild wolves. Anim Behav 77:489–493. [Google Scholar]
- Barja I, Escribano G, Lara C, Virgós E, Benito J. et al. , 2012. Non-invasive monitoring of adrenocortical activity in European badgers Meles meles and effects of sample collection and storage on faecal cortisol metabolite concentrations. Anim Biol 62:419–432. [Google Scholar]
- Barja I, List R, 2006. Faecal marking behaviour in ringtails Bassariscus astutus during the non-breeding period: spatial characteristics of latrines and single faeces. Chemoecology 16:219–222. [Google Scholar]
- Barja I, Silván G, Rosellini S, Piñeiro A, González-Gil A. et al. , 2007. Stress physiological responses to tourist pressure in a wild population of European pine marten. J Steroid Biochem 104:136–142. [DOI] [PubMed] [Google Scholar]
- Bauman DE, 2000. Regulation of nutrient partitioning during lactation: homeostasis and homeorhesis revisited In: Cronjé PB, editor. Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction. New York: CAB Publishing, 311–327. [Google Scholar]
- Boonstra R, Hik D, Singleton GR, Tinnikov A, 1998. The impact of predator-induced stress on the snowshoe hare cycle. Ecol Monogr 79:371–394. [Google Scholar]
- Brown JS, 1988. Patch use as an indicator of habitat preference, predation risk, and competition. Oecologia 22:37–47. [Google Scholar]
- Brown JS, Kotler BP, 2004. Hazardous duty pay and the foraging cost of predation. Ecol Lett 7:999–1014. [Google Scholar]
- Brown JS, Kotler BP, Bouskila A, Bouskila A, 2001. The ecology of fear and the foraging game between owls and gerbils. Ann Zool Fenn 38:71–87. [Google Scholar]
- Brown JS, Kotler BP, Smith RJ, Wirtz WO, 1988. The effects of owl predation on the foraging behavior of heteromyid rodents. Oecologia 76:408–415. [DOI] [PubMed] [Google Scholar]
- Brown JS, Laundré JW, Gurung M, 1999. The ecology of fear: optimal foraging, game theory, and trophic interactions. J Mammal 80:385–399. [Google Scholar]
- Brown LE, 1969. Field experiments on the movements of Apodemus sylvaticus L. using trapping and tracking techniques. Oecologia 2:198–222. [DOI] [PubMed] [Google Scholar]
- Bünning E, Moser I, 1969. Interference of moonlight with the photoperiodic measurement of time by plants, and their adaptive reaction. Proc Natl Acad Sci USA 62:1018–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder CJ, Gorman ML, 1991. The effects of red fox Vulpes vulpes faecal odours on the feeding behaviour of Orkney voles Microtus arvalis. J Zool 224:599–606. [Google Scholar]
- Clinchy M, Sheriff MJ, Zanette LY, 2013. Predator-induced stress and the ecology of fear. Funct Ecol 27:56–65. [Google Scholar]
- Dantzer B, McAdam AG, Palme R, Fletcher QE, Boutin S. et al. , 2010. Fecal cortisol metabolite levels in free-ranging North American red squirrels: assay validation and the effects of reproductive condition. Gen Comp Endocrinol 167:279–286. [DOI] [PubMed] [Google Scholar]
- Díaz M, 1992. Rodent seed predation in cereal crop areas of central Spain: effects of physiognomy, food availability, and predation risk. Ecography 15:77–85. [Google Scholar]
- Díaz M, Alonso CL, Arroyo L, Bonal R, Muñoz A. et al. , 2011. Desarrollo de un protocolo de seguimiento a largo plazo de los organismos clave para el funcionamiento de los bosques mediterráneos In: Ramírez L, Asensio B, editors. Proyectos de Investigación en Parques Nacionales: 2007–2010. Madrid: Organismo Autónomo Parques Nacionales, 47–75. [Google Scholar]
- Díaz M, Santos T, Tellería JL, 1999. Effects of forest fragmentation on the winter body condition and population parameters of an habitat generalist, the wood mouse Apodemus sylvaticus: a test of hypotheses. Acta Oecol 20:39–49. [Google Scholar]
- Díaz M, Torre I, Peris A, Tena L, 2005. Foraging behavior of wood mice as related to presence and activity of genets. J Mammal 86:1178–1185. [Google Scholar]
- Dickman CR, Doncaster CP, 1984. Responses of small mammals to red fox Vulpes vulpes odour. J Zool 204:521–531. [Google Scholar]
- Eilam D, 2004. Locomotor activity in common spiny mice Acomys cahirinuse: the effect of light and environmental complexity. BMC Ecol 4:16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam D, Dayan T, Ben-Eliyahu S, Schulman I, Shefer G. et al. , 1999. Differential behavioural and hormonal responses of voles and spiny mice to owl calls. Anim Behav 58:1085–1093. [DOI] [PubMed] [Google Scholar]
- Feise RJ, 2002. Do multiple outcome measures require P-value adjustment? BMC Med Res Methodol 2:8.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher QE, Boonstra R, 2006. Do captive male meadow voles experience acute stress in response to weasel odour? Can J Zool 84:583–588. [Google Scholar]
- Gallego D, Morán-López T, Torre I, Navarro-Castilla Á, Barja I. et al. , 2017. Context dependence of acorn handling by the Algerian mouse Mus spretus. Acta Oecol 8:1–7. [Google Scholar]
- Gorman ML, Trowbridge BJ, 1989. The role of odor in the social lives of carnivores In: Gittleman JL, editor. Carnivore Behavior, Ecology, and Evolution. New York: Cornell University Press, 57–88. [Google Scholar]
- Goymann W, Möstl E, Van’t Hof T, East ML, Hofer H, 1999. Noninvasive fecal monitoring of glucocorticoids in spotted hyenas, Crocuta crocuta. Gen Comp Endocrinol 114:340–348. [DOI] [PubMed] [Google Scholar]
- Gurnell J, Flowerdew JR, 1994. Live Trapping Small Mammals: A Practical Guide. 3rd edn London: The Mammal Society. [Google Scholar]
- Hamdine W, Thévenot M, Sellami M, De Smet K, 1993. Régime alimentaire de la genette (Genetta genetta Linné, 1758) dans le Parc national du Djurdjura, Algérie. Mammalia 57:9–18. [Google Scholar]
- Hanski I, Henttonen H, Korpimäki E, Oksanen L, Turchin P, 2001. Small-rodent dynamics and predation. Ecology 82:1505–1520. [Google Scholar]
- Hauger R, Thrivikraman K, Plotsky P, 1994. Age-related alterations of hypothalamic–pituitary–adrenal axis function in male Fischer rats. Endocrinology 134:1528–1536. [DOI] [PubMed] [Google Scholar]
- Hayes RA, Morelli TL, Wright PC, 2006. Volatile components of lemur scent secretions vary throughout the year. Am J Primatol 68:1202–1207. [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K, Möstl E, Wallner B, Dittami J, Kotrschal K, 2000. Endocrine and behavioural responses of male greylag geese Anser anser to pairbond challenges during the reproductive season. Ethology 106:63–77. [Google Scholar]
- Hutchings MR, White PCL, 2000. Mustelid scent‐marking in managed ecosystems: implications for population management. Mamm Rev 30:157–169. [Google Scholar]
- Jedrzejewski W, Jedrzejewska B, 1990. Effect of a predator’s visit on the spatial distribution of bank voles: experiments with weasels. Can J Zool 68:761–824. [Google Scholar]
- Jedrzejewski W, Rychlik L, Jedrzejewska B, 1993. Responses of bank voles to odours of seven species of predators: experimental data and their relevance to natural predator–vole relationships. Oikos 68:251–257. [Google Scholar]
- Jemiolo B, Xie TM, Andreolini F, Baker AEM, Novotny M, 1991. The t complex of the mouse: chemical characterization by urinary volatile profiles. J Chem Ecol 17:353–367. [DOI] [PubMed] [Google Scholar]
- Kats LB, Dill LM, 1998. The scent of death: chemosensory assessment of predation risk by prey animals. Ecoscience 5:361–394. [Google Scholar]
- Kaufman DW, Kaufman GA, 1982. Effect of moonlight on activity and microhabitat use by Ord’s kangaroo rat Dipodomys ordii. J Mammal 63:309–312. [Google Scholar]
- Korte SM, 2001. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci Biobehav Rev 25:117–142. [DOI] [PubMed] [Google Scholar]
- Kotler BP, Ayal Y, Subach A, 1994. Effects of predatory risk and resource renewal on the timing of foraging activity in a gerbil community. Oecologia 100:391–396. [DOI] [PubMed] [Google Scholar]
- Kotler BP, Brown J, Mukherjee S, Berger-Tal O, Bouskila A, 2010. Moonlight avoidance in gerbils reveals a sophisticated interplay among time allocation, vigilance and state-dependent foraging. Proc R Soc B 277:1469.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler BP, Brown JS, Dall SRX, Gresser S, Ganey D. et al. , 2002. Foraging games between gerbils and their predators: temporal dynamics of resource depletion and apprehension in gerbils. Evol Ecol Res 4:495–518. [Google Scholar]
- Kotler BP, Brown JS, Smith RJ, Wirtz WO, 1988. The effects of morphology and body size on rates of owl predation on desert rodents. Oikos 53:145–152. [Google Scholar]
- Lepschy M, Touma C, Hruby R, Palme R, 2007. Non-invasive measurement of adrenocortical activity in male and female rats. Lab Anim 41:372–387. [DOI] [PubMed] [Google Scholar]
- Lima SL, 1998. Stress and decision making under the risk of predation: recent developments from behavioral, reproductive, and ecological perspectives. Adv Stud Behav 27:215–290. [Google Scholar]
- Lima SL, Bednekoff PA, 1999. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659. [DOI] [PubMed] [Google Scholar]
- Lima SL, Dill LM, 1990. Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. [Google Scholar]
- Martín J, Barja I, López P, 2010. Chemical scent constituents in feces of wild Iberian wolves Canis lupus signatus. Biochem Syst Ecol 38:1096–1102. [Google Scholar]
- Millspaugh JJ, Washburn BE, Milanick MA, Slotow R, van Dyk G, 2003. Effects of heat and chemical treatments on fecal glucocorticoid measurements: implications for sample transport. Wildl Soc B 31:399–406. [Google Scholar]
- Monclús R, Rödel HG, Palme R, Von Holst D, de Miguel J, 2006. Non-invasive measurement of the physiological stress response of wild rabbits to the odour of a predator. Chemoecology 16:25–29. [Google Scholar]
- Monclús R, Rödel HG, Von Holst D, de Miguel J, 2005. Behavioural and physiological responses of naïve European rabbits to predator odour. Anim Behav 70:753–761. [Google Scholar]
- Montgomery WI, Gurnell J, 1985. The behaviour of Apodemus. Symp Zool Soc Lond 55:89–115. [Google Scholar]
- Morán-López T, Fernández M, Alonso CL, Flores-Rentería D, Valladares F. et al. , 2015. Effects of forest fragmentation on the oak-rodent mutualism. Oikos 124:1482–1491. [Google Scholar]
- Möstl E, Palme R, 2002. Hormones as indicators of stress. Domest Anim Endocrinol 23:67–74. [DOI] [PubMed] [Google Scholar]
- Navarro-Castilla Á, Barja I, 2014a. Antipredatory response and food intake in wood mice Apodemus sylvaticus under simulated predation risk by resident and novel carnivorous predators. Ethology 120:90–98. [Google Scholar]
- Navarro-Castilla Á, Barja I, 2014b. Does predation risk, through moon phase and predator cues, modulate food intake, antipredatory and physiological responses in wood mice Apodemus sylvaticus? Behav Ecol Sociobiol 68:1505–1512. [Google Scholar]
- Navarro-Castilla Á, Barja I, Olea PP, Piñeiro A, Mateo-Tomás P. et al. , 2014a. Are degraded habitats from agricultural crops associated with elevated faecal glucocorticoids in a wild population of common vole Microtus arvalis? Mammal Biol 79:36–43. [Google Scholar]
- Navarro-Castilla Á, Díaz M, Barja I, 2017. Does ungulate disturbance mediate behavioural and physiological stress responses in Algerian mice Mus spretus? A wild exclosure experiment. Hystrix J. doi: 10.4404/hystrix-28.2-12332. [Google Scholar]
- Navarro-Castilla Á, Mata C, Ruiz-Capillas P, Palme R, Malo JE. et al. , 2014b. Are motorways potential stressors of roadside wood mice Apodemus sylvaticus populations? PLoS One 9:e91942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrock JL, Danielson BJ, Brinkerhoff RJ, 2004. Rodent foraging is affected by indirect, but not by direct, cues of predation risk. Behav Ecol 15:433–437. [Google Scholar]
- Penteriani V, Kuparinen A, del Mar Delgado M, Palomares F, López-Bao JV. et al. , 2013. Responses of a top and a meso predator and their prey to moon phases. Oecologia 173:753–766. [DOI] [PubMed] [Google Scholar]
- Perrot-Sinal T, Kavaliers M, Ossenkopp KP, 1999. Changes in locomotor activity following predator odor exposure are dependent on sex and reproductive status in the meadow vole In: Johnston RE, Müller-Schwarze D, Sorensen PW, editors. Advances in Chemical Signals in Vertebrates. New York: Kluber Academic/Plenum Publishers, 497–504. [Google Scholar]
- Piñeiro A, Barja I, Silván G, Illera JC, 2012. Effects of tourist pressure and reproduction on physiological stress response in wildcats: management implications for species conservation. Wildl Res 39:532–539. [Google Scholar]
- Pulido FJ, Dı´az M, Hidalgo SJ, 2001. Size structure and regeneration of Spanish holm oak Quercus ilex forests and dehesas: effects of agroforestry use on their long-term sustainability. Forest Ecol Manage 146:1–13. [Google Scholar]
- Reeder DM, Kramer KM, 2005. Stress in free-ranging mammals: integrating physiology, ecology, and natural history. J Mammal 86:225–235. [Google Scholar]
- Rogovin K, Randall JA, Kolosova I, Moshkin M, 2003. Social correlates of stress in adult males of the great gerbil Rhombomys opimus in years of high and low population densities. Horm Behav 43:132–139. [DOI] [PubMed] [Google Scholar]
- Romero LM, 2002. Seasonal changes in plasma glucocorticoid concentrations in free-living vertebrates. Gen Comp Endocrinol 128:1–24. [DOI] [PubMed] [Google Scholar]
- Romero LM, 2004. Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol 19:249–255. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, 1990. No adjustments are needed for multiple comparisons. Epidemiology 1:43–46. [PubMed] [Google Scholar]
- Russell BG, Banks PB, 2007. Do Australian small mammals respond to native and introduced predator odours? Aust J Ecol 32:277–286. [Google Scholar]
- Sánchez-González B, Barja I, Navarro-Castilla Á, 2017. Wood mice modify food intake under different degrees of predation risk: influence of acquired experience and degradation of predator’s faecal volatile compounds. Chemoecology 27:115–122. [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU, 2000. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21:55–89. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, 2002. Endocrinology of the stress-response. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, Editors. Behavioral Endocrinology. Cambridge: MIT Press, 409–450.
- Scordato ES, Dubay G, Drea CM, 2007. Chemical composition of scent marks in the ringtailed lemur Lemur catta: glandular differences, seasonal variation, and individual signatures. Chem Senses 32:493–504. [DOI] [PubMed] [Google Scholar]
- Sih A, 1980. Optimal behavior: can foragers balance two conflicting demands? Science 210:1041–1043. [DOI] [PubMed] [Google Scholar]
- Stoddart DM, 1982. Demonstration of olfactory discrimination by the short-tailed vole Microtus agrestis L. Anim Behav 30:293–294. [Google Scholar]
- Streiner DL, Norman GR, 2011. Correction for multiple testing: is there a resolution? Chest 140:16–18. [DOI] [PubMed] [Google Scholar]
- Strier KB, Lynch JW, Ziegler TE, 2003. Hormonal changes during the mating and conception seasons of wild northern muriquis Brachyteles arachnoides hypoxanthus. Am J Primatol 61:85–99. [DOI] [PubMed] [Google Scholar]
- Tortosa FS, Barrio IC, Carthey AJR, Banks PB, 2015. No longer naïve? Generalized responses of rabbits to marsupial predators in Australia. Behav Ecol Sociobiol 69:1649–1655. [Google Scholar]
- Touma C, Palme R, Sachser N, 2004. Analyzing corticosterone metabolites in fecal samples of mice: a noninvasive technique to monitor stress hormones. Horm Behav 45:10–22. [DOI] [PubMed] [Google Scholar]
- Touma C, Sachser N, Möstl E, Palme R, 2003. Effects of sex and time of day on metabolism and excretion of corticosterone in urine and feces of mice. Gen Comp Endocrinol 130:267–278. [DOI] [PubMed] [Google Scholar]
- Virgós E, Llorente M, Cortés Y, 1999. Geographical variation in genet (Genetta genetta L.) diet: a literature review. Mamm Rev 29:117–126. [Google Scholar]
- Washburn BE, Millspaugh JJ, 2002. Effects of simulated environmental conditions on glucocorticoid metabolite measurements in white-tailed deer feces. Gen Comp Endocrinol 127:217–222. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Romero LM, 2001. Adrenocortical responses to stress and their modulation in free‐living vertebrates In: McEwen BS, Goodman HM, editors. Handbook of Physiology—Coping with the Environment: Neural and Endocrine Mechanisms. New York: Oxford University Press, 211–234. [Google Scholar]
- Wolfe JL, Summerlin CT, 1989. The influence of lunar light on nocturnal activity of the old-field mouse. Anim Behav 37:410–414. [Google Scholar]
- Wróbel A, Bogdziewicz M, 2015. It is raining mice and voles: which weather conditions influence the activity of Apodemus flavicollis and Myodes glareolus?. Eur J Wildl Res 61:475–478. [Google Scholar]
- Ylönen H, Eccard JA, Jokinen I, Sundell J, 2006. Is the antipredatory response in behaviour reflected in stress measured in faecal corticosteroids in a small rodent? Behav Ecol Sociobiol 60:350–358. [Google Scholar]
- Young KM, Walker SL, Lanthier C, Waddell WT, Monfort SL. et al. , 2004. Noninvasive monitoring of adrenocortical activity in carnivores by fecal glucocorticoid analyses. Gen Comp Endocrinol 137:148–165. [DOI] [PubMed] [Google Scholar]
- Zanette LY, White AF, Allen MC, Clinchy M, 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334:1398–1401. [DOI] [PubMed] [Google Scholar]
- Zanette LY, Clinchy M, Suraci JP, 2014. Diagnosing predation risk effects on demography: can measuring physiology provide the means? Oecologia 176:637–651. [DOI] [PubMed] [Google Scholar]
- Zwijacz-Kozica T, Selva N, Barja I, Silván G, Martínez-Fernández L. et al. , 2013. Concentration of fecal cortisol metabolites in chamois in relation to tourist pressure in Tatra National Park (South Poland). Acta Theriol 58:215–222. [Google Scholar]