Abstract
Background
We present an analysis of increasing rates of invasive group A streptococci (iGAS) over a 15-year period in Alberta, Canada.
Methods
From 2003 to 2017, the emm type of iGAS isolates was identified from patients with iGAS disease in Alberta. Demographic, clinical, and risk factor data were collected.
Results
A total of 3551 cases of iGAS were identified in Alberta by isolation of a GAS isolate from a sterile site. The age-standardized incidence rates of iGAS increased from 4.24/100 000 in 2003 to 10.24 in 2017. Rates (SD) were highest in those age <1 (9.69) years and 60+ (11.15) years; 57.79% of the cases were male. Commonly identified risk factors included diabetes, hepatitis C, nonsurgical wounds, addiction, alcohol abuse, drug use, and homelessness. The overall age-standardized case fatality rate was 5.11%. The most common clinical presentation was septicemia/bacteremia (41.84%), followed by cellulitis (17.25%). The top 4 emm types from 2003–2017 were emm1, 28, 59, and 12. In 2017, the top 4 emm types (emm1, 74, 101, and 59) accounted for 46.60% of cases.
Conclusions
The incidence of iGAS disease in Alberta, Canada, has increased from 2003 to 2017. This increase has been driven not by a single emm type, but rather what has been observed is a collection of common and emerging emm types associated with disease. In addition, it is also likely that societal factors are playing important roles in this increase as risk factors associated with marginalized populations (addiction, alcohol abuse, and drug use) were found to have increased during the survey period.
Keywords: Alberta, Canada, emm type, Group A streptococci, high incidence
Group A streptococci (GAS) or Streptococcus pyogenes are Gram-positive, catalase-negative bacteria. Group A streptococci cause a variety of diseases in humans, including pharyngitis, skin and soft tissue infections, rheumatic fever, rheumatic heart disease, and the severe invasive diseases necrotizing fasciitis and streptococcal toxic shock syndrome [1–7]. GAS bacteria are a global pathogen causing an estimated 1.8 million new severe disease infections annually; however, this estimate is likely an underestimation as some countries have reported increases in rates of GAS disease, notably those with low to middle incomes [1, 8, 9]. To understand the epidemiology of GAS, GAS infections can be tracked via variability in the emm gene sequence that encodes the M protein [10]. There are currently >250 different emm types and >90 sequence types based on the nucleotide variability in the 5’ end of the emm gene (ftp://ftp.cdc.gov/pub/infectious_diseases/biotech/tsemm/).
Currently no GAS vaccine exists that is in routine use; however, there has been renewed interest in development of potential GAS vaccines, many of which are M protein based [11, 12]. Due to variability in the M protein, it is important to understand the prevalent circulating M/emm types in a population and the incidence and epidemiology of invasive GAS (iGAS) in a region. This information aids in determining the need and type of potential future vaccines that are M protein based.
We previously reported rates of iGAS for the province of Alberta for the years 2000 to 2002, along with M types [13]. Here, we provide an update of iGAS rates in Alberta and emm types, clinical presentations, and risk factors from 2003 to 2017 (a 15-year period).
METHODS
Demographics and Clinical Data Collection
All cases used in the data analysis were from Alberta residents only. The population of Alberta in 2003 was 3 182 852, and in 2017, the population was 4 286 144, constituting 11.68% of the Canadian population for 2017 [14]. In Alberta, many infectious diseases including iGAS are listed as notifiable diseases, requiring reporting to the provincial Ministry of Health under the Public Health Act (https://open.alberta.ca/publications/streptococcal-disease-group-a-invasive) [15]. The notification process is to facilitate contact tracing by public health nurses, thereby allowing prophylaxis procedures to be followed if necessary. Data from both confirmed and probable cases were collected. Confirmed iGAS cases were defined as the identification of GAS from any normally sterile site including blood, CSF, brain, other sterile organs, deep tissues, joints, etc. (https://open.alberta.ca/publications/streptococcal-disease-group-a-invasive). Probable iGAS cases were defined as severe invasive disease in the absence of another etiology with isolation of GAS from a nonsterile site.
Once iGAS isolates were initially identified by a diagnostic microbiology laboratory, Public Health officials were informed, and the clinical data were collected as per routine notifiable disease requirements by trained public health nurses using a notifiable disease reporting form [15]. These data were captured in the Communicable Disease Reporting System (CDRS), held at the Ministry of Health, Alberta. The definitions used to capture clinical presentation of disease are defined in the Alberta Health Notifiable Disease Manual [16]. Clinical data from CDRS were linked with laboratory data from the Provincial Laboratory for Public Health (ProvLAB) using a Unique Lifetime identifier for each patient. Case fatality rates (CFRs) were captured if the case had died at the time the notifiable disease report form was completed. All notifiable disease reports were completed within 30 days of the initial notification of an iGAS case.
iGAS Isolates and emm Typing
iGAS isolates are required to be submitted to ProvLAB for emm typing. The methodology used to type iGAS isolates from 2003 to September 2006 was via a previously described serological typing assay, and from October 2006 to 2017 by emm typing, which was done by DNA sequencing of the M serotype–specific region of the emm gene [13, 17, 18].
Statistical Analysis
Alberta population estimates from 2003–2017 were extracted from the online Interactive Health Data Application (IHDA) database [14]. We estimated incidence rates by year of diagnosis, age group, and health zone (of patient residence at the time of diagnosis) over the study period. Incidence rates were calculated for each characteristic as number of cases per 100 000 persons per year. The Cochran-Armitage trend test was used to test for linear trend of risk factors over time. Data were analyzed using SAS 9.3 (SAS Institute Inc., Cary, NC) and graphed using OriginLab software 2018 (OriginLab Corporation, Northampton, MA).
RESULTS
Incidence
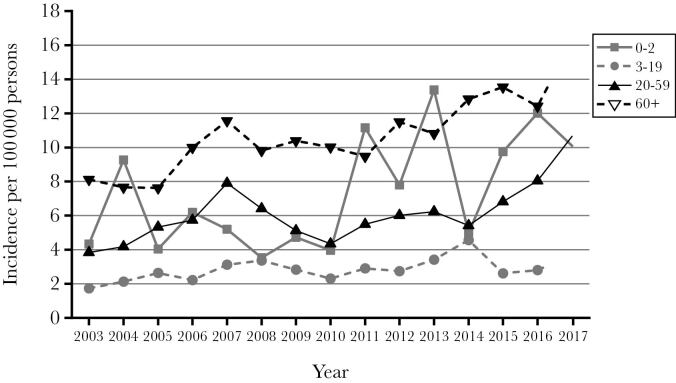
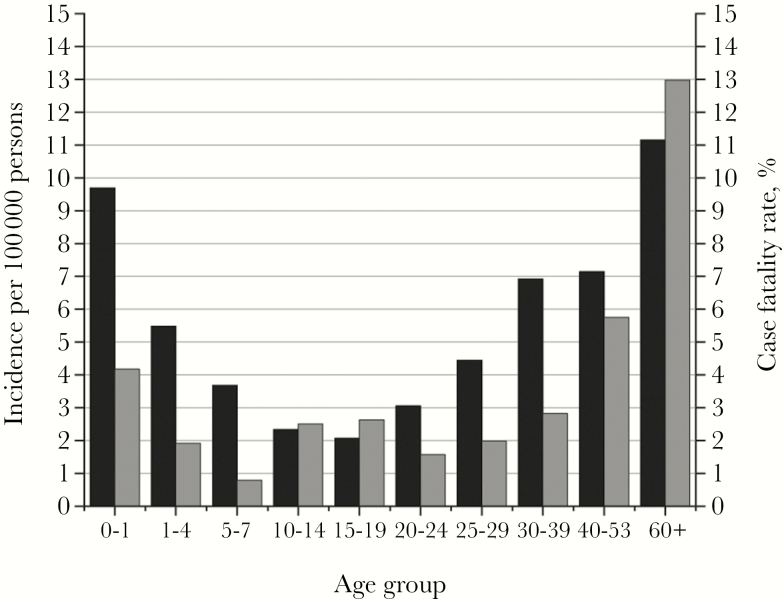
From 2003 to 2017, there were 3551 cases of both confirmed and probable iGAS reported. Probable cases were 1.2% (43 cases). Table 1 shows that the incidence of iGAS in Alberta steadily increased over the 15 years surveyed. The overall age-standardized incidence was 4.24 in 2003 and increased to 10.24 in 2017. Increasing incidence rates over the 15 years surveyed are more evident in adults (age 20 years and older) than children (age 0–19 years) (Figure 1). Overall incidence was highest at the extremes of age, with individuals <1 year of age having an incidence of 9.69 and individuals’ ≥60 years having an incidence of 11.15 (Figure 2).
Table 1.
Incidence (per 100 000), Number of Cases, Number of Deaths, and Case Fatality Rate of iGAS in Alberta, 2003–2017
| Year | Crude Incidence, % | Age-Standardizeda Incidence, % | No. Cases | No. Deaths | Crude CFR, % | Age-Standardizeda CFR, % |
|---|---|---|---|---|---|---|
| 2003 | 3.96 | 4.24 | 126 | 7 | 5.56 | 4.12 |
| 2004 | 4.38 | 4.63 | 142 | 10 | 7.04 | 6.12 |
| 2005 | 5.00 | 5.26 | 166 | 7 | 4.22 | 7.97 |
| 2006 | 5.58 | 5.93 | 191 | 10 | 5.24 | 4.20 |
| 2007 | 7.29 | 7.51 | 256 | 11 | 4.30 | 3.26 |
| 2008 | 6.15 | 6.33 | 221 | 9 | 4.07 | 3.73 |
| 2009 | 5.41 | 5.62 | 199 | 12 | 6.03 | 4.04 |
| 2010 | 4.77 | 4.98 | 178 | 7 | 3.93 | 3.39 |
| 2011 | 5.80 | 5.95 | 220 | 14 | 6.36 | 5.81 |
| 2012 | 6.29 | 6.41 | 244 | 25 | 10.25 | 8.85 |
| 2013 | 6.68 | 6.81 | 267 | 13 | 4.87 | 4.26 |
| 2014 | 6.45 | 6.61 | 265 | 19 | 7.17 | 5.92 |
| 2015 | 7.23 | 7.32 | 302 | 27 | 8.94 | 6.90 |
| 2016 | 7.91 | 7.94 | 335 | 22 | 6.57 | 4.95 |
| 2017 | 10.24 | 10.24 | 439 | 33 | 7.52 | 5.43 |
| Total or avg | 6.32 avg. | 6.52 avg. | 3551 total | 226 total | 6.36 avg. | 5.11 avg. |
Abbreviations: CFR, case fatality rate; iGAS, invasive group A streptococci.
aStandardized to 2017 Alberta population estimates.
Figure 1.
Incidence of iGAS by age in Alberta from 2003 to 2017.
Figure 2.
Incidence and case fatality rate by age for invasive group A streptococci cases from 2003 to 2017. Black bars correspond to incidence, and gray bars correspond to case fatality rate.
Case Demographics, Clinical Presentation, and Risk Factor Analysis
Of the 3551 cases, gender was identified for 3549; 2051 cases (57.79%) were male. The mean age was 44.86 years. The overall age-standardized CFR was 5.11% (226 deaths/3551 cases) (Table 1). The year 2010 had the lowest percentage CFR (4.12%), and 2012 had the highest (8.85%) (Table 1). The CFR in males was 5.75%, and for females it was 7.21% (chi-square = 3.08; P = .08). The highest CFR occurred in those cases age ≥60 years (12.97%; 127 deaths) (Figure 2). The incidence was higher in males (7.20) than females (5.42) overall. The most common clinical diagnosis was septicemia/bacteremia, at >40% of cases, followed by the skin-associated infections cellulitis (17.25%) and soft tissue infection (12.31%) (Table 2). Table 3 shows risk factor data presented in 3-year periods. Diabetes, hepatitis C, nonsurgical wounds, addiction, alcohol abuse, drug use, and homelessness (P < .05) were commonly found risk factors that trended upward over the 15 years surveyed. No identified risk factor significantly trended downward (Table 3). Only 2.5% (87/3511) of the cases were classed as postpartum.
Table 2.
Number of Cases of iGAS in Alberta by Clinical Diagnosis, Deaths, and CFRa
| System Affected | Clinical Presentation | No. Cases (%) | No. Deaths (CFR, %) |
|---|---|---|---|
| Blood/brain/sterile tissue | Septicemia/bacteremia | 1889 (41.84) | 118 (6.25) |
| Toxic shock syndrome | 254 (5.63) | 55 (21.65) | |
| Meningitis | 25 (0.55) | 5 (20.00) | |
| Peritonitis | 29 (0.64) | 2 (6.90) | |
| Pericarditis | 10 (0.22) | 2 (30.00) | |
| Encephalitis | 1 (0.02) | 0 (0.00) | |
| Skin/soft tissue | Cellulitis | 779 (17.25) | 22 (2.82) |
| Soft tissue infection | 556 (12.31) | 13 (2.34) | |
| Necrotizing fasciitis | 326 (7.22) | 35 (10.74) | |
| Respiratory | Pneumonia | 341 (7.55) | 31 (9.09) |
| Epiglottitis | 13 (0.29) | 2 (15.38) | |
| Bone | Joint | 255 (5.65) | 1 (0.40) |
| Osteomyelitis | 37 (0.82) | 1 (2.76) | |
| Total | 4515 (100) |
Abbreviations: CFR, case fatality rate; iGAS, invasive group A streptococci.
aMany cases had more than 1 clinical presentation.
Table 3.
Risk Factors for iGAS Disease in Alberta; 2003–2017
| 2003–2005 | 2006–2008 | 2009–2011 | 2012–2014 | 2015–2017 | ||
|---|---|---|---|---|---|---|
| Risk Factor | n (%) | n (%) | n (%) | n (%) | n (%) | P a |
| Diabetes | 13 (3.0) | 57 (8.5) | 79 (13.2) | 111 (14.3) | 193 (17.9) | <.001 |
| Hepatitis C | 16 (3.7) | 68 (10.2) | 49 (8.2) | 43 (5.5) | 106 (9.9) | .035 |
| HIV | 6 (1.4) | 20 (3.0) | 11 (1.8) | 9 (1.2) | 14 (1.3) | .109 |
| Immunocompromised | 14 (3.2) | 60 (9.0) | 50 (8.4) | 74 (9.5) | 81 (7.5) | .064 |
| Postpartum | 5 (1.2) | 20 (3.0) | 15 (2.5) | 28 (3.6) | 13 (1.2) | .513 |
| Wound (nonsurgical) | 25 (5.8) | 167 (25.0) | 159 (26.6) | 149 (19.2) | 208 (19.3) | .022 |
| Wound (surgical) | 10 (2.3) | 32 (4.8) | 30 (5.0) | 46 (5.9) | 58 (5.4) | .008 |
| Addiction abuse | 19 (4.4) | 157 (23.5) | 102 (17.1) | 136 (17.5) | 318 (29.6) | <.001 |
| Alcohol abuse | 10 (2.3) | 93 (13.9) | 75 (12.6) | 99 (12.8) | 178 (16.5) | <.001 |
| Drug use | 10 (2.3) | 97 (14.5) | 50 (8.4) | 72 (9.3) | 204 (19.0) | <.001 |
| Homelessness | 15 (3.5) | 91 (13.6) | 48 (8.0) | 59 (7.6) | 161 (15.0) | <.001 |
| Total cases, nb | 392 | 539 | 483 | 614 | 829 | - |
Years surveyed are divided into 3-year blocks.
Abbreviation: iGAS, invasive group A streptococci.
aThe Cochrane Armitage trend test was used to calculate the P value.
bMany cases had more than 1 risk factor.
emm Types
M or emm type was captured for 3102 (87.36%) of the 3551 iGAS cases identified during the survey period. Of the 3102 typed isolates, 3053 had an identifiable M or emm type (49 isolates were serologically nontypable) (Table 4). No single emm type can be attributed to the increased incidence over the 15 years, but rather the increase appears to be associated with a variety of emm types that changed in prevalence from year to year. Sixty-five emm types were identified, of which the top 30 are shown in Table 4. The top 20 emm types accounted for approximately 90% of the circulating emm types causing iGAS disease. Of these 20 emm types, 14 are part of the 30 valent M type–based vaccine [19]. Of the 65 emm types identified, 3 emm types emerged, with higher rates in 2017 than the previous 14 years. These were emm74, 76, and 81. In addition, emm101 showed higher case numbers starting in 2015 than the years before.
Table 4.
Top 30 emm Types by Year and Number of Cases
| Year | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| emm Type | 2003 | 2004 | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Total |
| 1 | 22 | 31 | 21 | 15 | 27 | 25 | 32 | 38 | 56 | 50 | 44 | 50 | 47 | 58 | 82 | 598 |
| 28 | 12 | 27 | 22 | 25 | 13 | 15 | 7 | 15 | 12 | 17 | 20 | 17 | 15 | 20 | 23 | 260 |
| 59 | 0 | 0 | 1 | 1 | 24 | 51 | 37 | 16 | 6 | 5 | 4 | 5 | 8 | 18 | 31 | 207 |
| 12 | 6 | 4 | 9 | 10 | 10 | 6 | 6 | 14 | 14 | 8 | 11 | 10 | 9 | 19 | 20 | 156 |
| 82 | 6 | 8 | 1 | 7 | 29 | 5 | 3 | 0 | 6 | 6 | 14 | 4 | 21 | 25 | 16 | 151 |
| 3 | 3 | 0 | 1 | 9 | 9 | 16 | 29 | 1 | 10 | 8 | 4 | 5 | 29 | 16 | 2 | 142 |
| 89 | 2 | 0 | 3 | 1 | 9 | 3 | 11 | 10 | 14 | 9 | 13 | 16 | 13 | 7 | 14 | 125 |
| 101 | 0 | 0 | 0 | 0 | 0 | 1 | 9 | 6 | 5 | 4 | 3 | 3 | 24 | 31 | 39 | 125 |
| 11 | 3 | 2 | 1 | 5 | 3 | 4 | 3 | 3 | 4 | 13 | 15 | 7 | 10 | 14 | 23 | 110 |
| 41 | 2 | 7 | 8 | 3 | 4 | 1 | 0 | 0 | 0 | 9 | 11 | 20 | 16 | 6 | 6 | 93 |
| 4 | 9 | 4 | 2 | 1 | 2 | 3 | 3 | 1 | 14 | 8 | 8 | 9 | 7 | 7 | 12 | 90 |
| 83 | 1 | 3 | 4 | 10 | 17 | 2 | 0 | 0 | 3 | 6 | 3 | 3 | 8 | 13 | 14 | 87 |
| 77 | 1 | 6 | 2 | 3 | 1 | 2 | 3 | 7 | 9 | 10 | 16 | 5 | 5 | 6 | 6 | 82 |
| 114 | 8 | 4 | 6 | 10 | 15 | 7 | 1 | 3 | 2 | 1 | 0 | 9 | 7 | 5 | 0 | 78 |
| 2 | 4 | 3 | 1 | 4 | 7 | 2 | 3 | 7 | 6 | 9 | 5 | 10 | 2 | 3 | 4 | 70 |
| 6 | 1 | 1 | 4 | 2 | 3 | 1 | 3 | 1 | 2 | 3 | 11 | 18 | 12 | 5 | 2 | 69 |
| 91 | 12 | 4 | 2 | 5 | 4 | 2 | 1 | 1 | 1 | 10 | 10 | 4 | 6 | 5 | 1 | 68 |
| 53 | 1 | 0 | 0 | 2 | 3 | 1 | 3 | 6 | 9 | 7 | 15 | 9 | 3 | 0 | 3 | 62 |
| 74 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 13 | 39 | 54 |
| 22 | 3 | 1 | 0 | 1 | 1 | 1 | 0 | 3 | 3 | 4 | 8 | 4 | 6 | 9 | 3 | 47 |
| 87 | 1 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 5 | 3 | 5 | 3 | 5 | 6 | 3 | 43 |
| 80 | 1 | 0 | 1 | 1 | 5 | 12 | 5 | 5 | 2 | 2 | 1 | 1 | 0 | 1 | 0 | 37 |
| 81 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 3 | 3 | 21 | 31 |
| 75 | 0 | 8 | 2 | 5 | 1 | 2 | 1 | 0 | 0 | 0 | 1 | 2 | 4 | 3 | 1 | 30 |
| 76 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 2 | 0 | 0 | 0 | 22 | 29 |
| 5 | 1 | 1 | 14 | 3 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 |
| 92 | 3 | 2 | 4 | 9 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 22 |
| 73 | 0 | 1 | 0 | 2 | 1 | 1 | 3 | 1 | 2 | 2 | 1 | 3 | 0 | 2 | 1 | 20 |
| 68 | 0 | 0 | 0 | 2 | 8 | 5 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 2 | 20 |
| 9 | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 0 | 0 | 1 | 3 | 0 | 1 | 1 | 4 | 15 |
| Others | 3 | 5 | 3 | 9 | 7 | 8 | 7 | 10 | 7 | 12 | 6 | 8 | 5 | 10 | 13 | 113 |
| NT | 7 | 11 | 20 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 49 |
| Total | 113 | 134 | 137 | 161 | 206 | 182 | 172 | 153 | 192 | 209 | 234 | 228 | 267 | 307 | 410 | 3105 |
Abbreviation: NT, nontypable.
With respect to overall numbers of cases, emm1, emm28, and emm59 accounted for more than one-third (34.33%) of all cases typed. The most common emm type was emm1, accounting for 19.28% of the total cases typed (Table 4). The percentage of emm1 typed cases compared with the overall typed case numbers by year ranged from a low of 9.43% (2006) to a high of 29.17% (2011). emm28 was the second most common emm type, with a high of 20.15% in 2004 and a low of 4.07% in 2009.
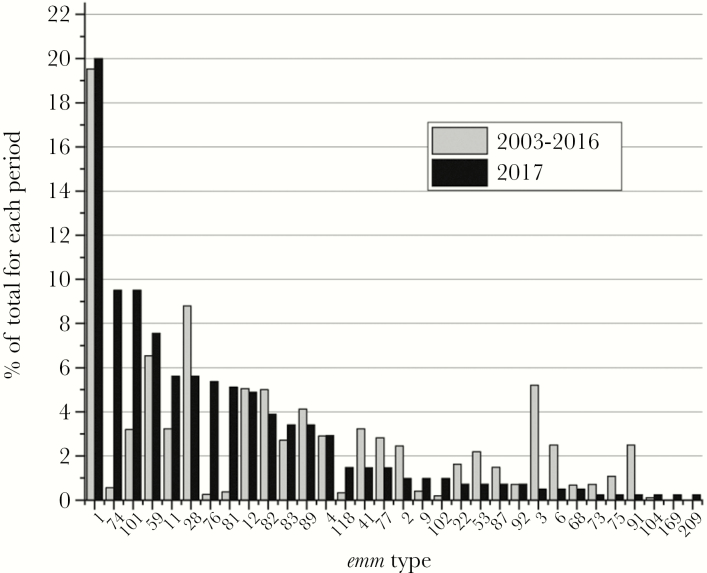
In the last year of the survey (2017), 32 different emm types were identified, with emm1 accounting for 20% (Table 4). The next most common emm types in order of prevalence for 2017 were 74 and 101, followed by 59. Together, these 4 emm types (1, 74, 101, and 59) accounted for 46.58% of the iGAS cases in 2017 (Figure 3 and Table 4). The 10 emm types (1, 74, 101, 59, 11, 28, 76, 81, 12, and 82) accounted for 316 cases or 77.07% of cases in 2017 (Figure 3).
Figure 3.
The percentage of each emm type identified in 2017 compared with the period 2003 to 2016. As 2017 had the highest incidence and is the most recent period surveyed, 2017 was compared with the past 14 years surveyed (2003–2016).
emm Type, emm Clusters, and Clinical Presentation
Table 5 shows the distribution of iGAS cases according to clade, emm type, emm cluster, and clinical presentation. The distribution of cases between clades X and Y is equal in case numbers, at 50% each. Four cluster types, A-C3, D4, E4, and E6, accounted for the majority of septicemia/bacteremia (71%), cellulitis (72%), soft tissue infections (70%), pneumonia (68.9%), and necrotizing fasciitis (77%) iGAS cases. For STSS, cluster types A-C3, A-C5, E4, and E6 were the most common, accounting for 72.4% of iGAS cases that occurred during the period surveyed.
Table 5.
Distribution of GAS Isolates by emm and emm Cluster Type in Relation to Clinical Presentation
| No. (%) of Isolates by Clinical Infection | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clade (No. of Isolates; %) | emm Type(s) (No. of Isolates) | emm Cluster | Sept/Bacterem | Cellulitis | Soft Tissue Infection | Pneum | NF | STSS | Joint | Osteo | Mening | Perito | Pericard | Epigl |
| X (1527; 50) | 4 (90) (total 90 cases) | E1 | 55 (3.1) | 16 (2.4) | 14 (3.3) | 7 (2.4) | 5 (1.8) | 7 (3.1) | 8 (3.7) | 2 (8.7) | 1 (10.0) | |||
| 27 (1), 66 (1), 68 (20), 76 (29), 90 (4), 92 (22), 104 (4), 106 (1) (total 82 cases) | E2 | 47 (2.6) | 14 (2.1) | 7 (1.7) | 6 (2.1) | 8 (2.9) | 2 (0.9) | 6 (2.7) | 2 (8.7) | |||||
| 9 (15), 25 (3), 44/61 (14), 49 (3), 58 (14), 82 (151), 87 (43), 103 (1), 113 (1), 118 (15), 183 (3), 209 (1) (total 264 cases) | E3 | 148 (8.3) | 70 (10.5) | 49 (11.7) | 19 (6.6) | 15 (5.5) | 15 (6.6) | 22 (10.0) | 3 (9.4) | 1 (10.0) | 1 (11.1) | |||
| 2 (70), 22 (47), 28 (260), 73 (20), 77 (82), 88 (1), 89 (125), 102 (9), 114 (78), 169 (1) (total 695 cases) | E4 | 405 (22.6) | 129 (19.3) | 96 (22.9) | 48 (16.6) | 56 (20.6) | 40 (17.5) | 54 (24.7) | 6 (18.8) | 3 (13.1) | 7 (30.4) | 4 (40.0) | 1 (11.1) | |
| 170 (1), 174 (4) (total 5 cases) | E5 | 1 (0.4) | ||||||||||||
| 11 (110), 48 (7), 59 (207), 75 (30), 81 (31), 94 (7), 182 (1) (total 393 cases) | E6 | 224 (12.5) | 103 (15.4) | 65 (15.5) | 33 (11.4) | 39 (14.3) | 23 (10.0) | 30 (13.7) | 2 (6.3) | 4 (17.5) | 3 (13.0) | 2 (22.2) | ||
| Y (1528; 50) | 1 (598), 227 (2) (total 600 cases) | A-C3 | 355 (19.8) | 99 (14.8) | 61 (14.6) | 86 (29.8) | 77 (28.3) | 83 (36.2) | 30 (13.7) | 5 (15.6) | 8 (34.8) | 6 (26.1) | 2 (20.0) | 4 (44.7) |
| 12 (156) (total 156 cases) | A-C4 | 97 (5.4) | 27 (4.0) | 16 (3.8) | 22 (7.6) | 13 (4.8) | 11 (4.8) | 9 (4.1) | 3 (9.4) | 1 (4.4) | ||||
| 3 (142) (total 142 cases) | A-C5 | 84 (4.7) | 26 (3.9) | 17 (4.1) | 21 (7.3) | 14 (5.1) | 20 (8.7) | 10 (4.6) | 1 (3.1) | 1 (4.4) | 2 (8.7) | 1 (10.0) | 1 (11.1) | |
| 100 (1), 115 (2) (total 3 cases) | D2 | 1 (0.1) | 1 (0.2) | 1 (0.5) | 3 (9.4) | |||||||||
| 123 (2), 217 (1) (total 3 cases) | D3 | 1 (0.1) | 1 (0.2) | 1 (0.2) | 1 (0.4) | |||||||||
| 33 (1), 41 (93), 43 (1), 53 (62), 64 (1), 80 (37), 83 (87), 91 (68), 101 (125), 223 (1) (total 476 cases) | D4 | 289 (16.1) | 150 (22.5) | 71 (17.0) | 32 (11.1) | 38 (14.0) | 15 (6.6) | 40 (18.3) | 6 (18.8) | 2 (8.7) | 1 (4.3) | |||
| 5 (22) (total 22 cases) | M5 | 7 (0.4) | 2 (0.3) | 2 (0.5) | 2 (0.9) | 1 (0.4) | 1 (0.5) | 1 (4.3) | ||||||
| 6 (69) (total 69 cases) | M6 | 41 (2.9) | 11 (1.6) | 4 (1.0) | 10 (3.5) | 2 (0.9) | 6 (2.6) | 5 (2.3) | 1 (3.1) | 3 (13.0) | ||||
| 23 (1) (total 1 case) | M23 | 1 (0.1) | ||||||||||||
| 74 (54) (total 54 cases) | M74 | 36 (2.0) | 19 (2.8) | 16 (3.8) | 4 (1.4) | 2 (0.9) | 6 (2.6) | 2 (0.9) | 2 (6.3) | 1 (10.0) | ||||
| 122 (1) (total 1 case) | M122 | 1 (0.1) | ||||||||||||
| 218 (1) (total 1 case) | M218 | 1 (0.1) | ||||||||||||
| Outlier (1; 0.03) | 111 (1) (total 1 case) | M111 | 1 (0.5) | |||||||||||
| Total | 1793 | 668 | 419 | 289 | 272 | 229 | 219 | 32 | 23 | 23 | 10 | 9 | ||
Abbreviations: Epigl, epliglottis; GAS, group A streptococci; Mening, meningitis; NF, necrotizing fasciitis; Osteo, osteomyelitis; Pericard, pericarditis; Perito, peritonitis; Pneum, pneumonia; Sept/bactem, septicemia/bacteremia; STSS, streptococcal toxic shock syndrome.
Seasonality
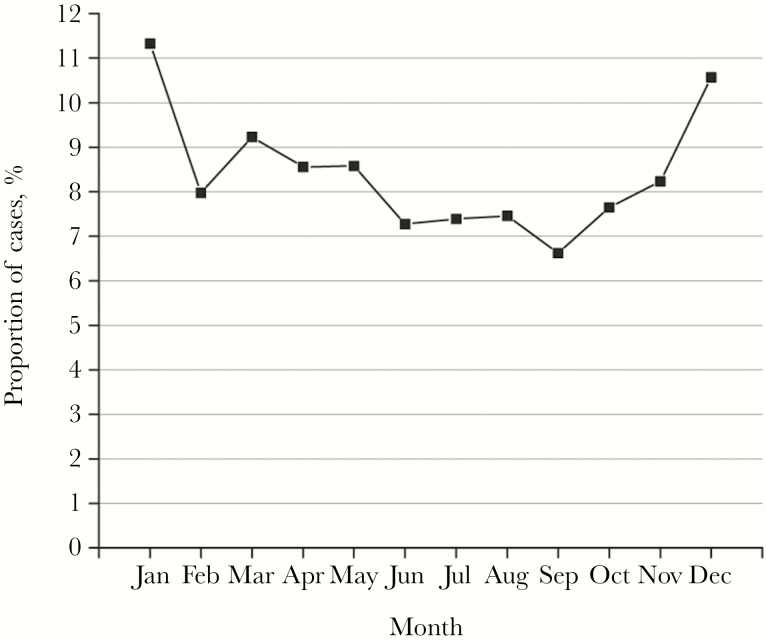
Figure 4 displays the number of cases identified by month for each year surveyed. Presenting the 15 years surveyed as 1 calendar year shows that a peak in iGAS cases generally occurred in the winter months of January and December, with a low in the month of September (Figure 4).
Figure 4.
Invasive group A streptococci (iGAS) cases by month. The average proportion of cases by month expressed as a percentage of iGAS cases by month that occurred over the 15 years surveyed.
DISCUSSION
We have provided a report on 15 years of iGAS disease in Alberta, Canada, during which incidence rose from 4.24 (2003) to 10.24 (2017). Recent surveillance from the Active Bacterial Core surveillance program (a program representing approximately 10% of the US population) also documented a rise in rates [20]. This program showed an incidence of 3.4 in 2012 rising to 5.8 in 2016, suggesting that increases in iGAS are occurring throughout North America [20].
In addition to the United States, the Public Health Agency of Canada has been reporting incidence of iGAS for Canada since 2000. In 2000, Canadian incidence was 2.81 climbing to 5.28 (2015, last reported year; http://disease.canada.ca/notifiable/charts?c=yl). Recently, the British Columbia Centre for Disease Control (BCCDC) released a report describing iGAS incidence in British Columbia at 8.4 in 2017 [21]. Based on these reports and our data, it is evident that iGAS rates in parts of North America have risen in the last decade.
Drivers in Alberta responsible for increased incidence are not readily clear and are likely multifactorial. Contributors likely include a collection of various risk factors for iGAS disease. The risk factors in Table 3 that trended upward over the 15 years surveyed for iGAS were diabetes, hepatitis C, nonsurgical wounds, drug abuse, alcohol abuse, addiction, and homelessness. These risk factors have been described as key players for patients with iGAS infections in other surveys [22–24]. For example, in the large emm59 outbreak that occurred in western Canada from 2006 to 2009, alcohol abuse, illicit drug use, and homelessness were considered significant risk factors [22].
It is interesting that the overall CFR in our survey of 6.36% is lower than reported in other surveys; however, this may be due to not following iGAS cases for a full 30 days after initial diagnosis. Lamagni et al. reported a CFR of 20% for 3422 cases in the United Kingdom [25]. Recently, Teatero et al. reported a CRF of 19% for iGAS infections with emm89 alone and 20% with emm1 in metropolitan Toronto from 2000 to 2014 using a definition of death within 30 days of positive culture [26]. Although the Toronto CFRs are emm type specific and not overall CFRs, CFRs of approximately 20% are similar to those reported in the United Kingdom [25]. A recent report from the BCCDC documented a CFR of 4% for 2017, similar to Alberta [21]. Alberta and British Columbia are neighboring provinces, suggesting that the similarly low CFR rates in Alberta are not inaccurate.
With respect to emm types, the data show that emm types rose and fell in prevalence over the 15 years surveyed; therefore, no single emm type can account for the overall rise in incidence. It is important to note that the rise in the case numbers associated with various emm types was especially evident in 2017. emm types 1, 74, 101, and 59 accounted for close to 50% of the cases typed in 2017. Previous investigators have shown the emergence of a virulent M1 clone that has disseminated worldwide starting in the 1990s [27]. Although a worldwide virulent M1 strain likely contributes to increases for this emm type, it is unlikely the only reason for the increase in emm1 seen, especially for 2017. It is likely that the increase is multifactorial, involving both the GAS strain and the affected population having significant risk factors for the disease.
The second most common emm types in 2017 were emm74 and emm101. Data from the Centers for Disease Control and Prevention’s Active Bacterial Core surveillance program (2010–2016) show that emm74 was documented once from a case in Maryland in 2010 and that emm101 has not been documented [20]. Although emm74 increases were not evident in the United States in the years surveyed, increases in emm74 have been reported in Ontario and other parts of Canada recently [28].
emm101 was first identified in Alberta in 2009, with the highest number of cases in 2017. In Canada, Athey et al. documented emm101 as 1 of the 6 most prevalent emm types from 2011 to 2013, identified from cases in Thunder Bay, Ontario [23]. The recent BCCDC report for 2017 listed emm101 as the third most common emm type in British Columbia, at 11% [21].
Increased rates of emm59 cases in Canada were first described in 2006, and since this outbreak, increased rates of emm59 cases have been reported in the United States (MT, WY, AZ, NM) [22, 29, 30]. In the description of the emm59 outbreak in Canada, emm59 cases were associated with alcohol abuse, illicit drug use, and homelessness [22]. Based on the risk factors described in this report, which are similar to the risk factors for the emm59 outbreak in 2006–2009, it would not be surprising to see emm59 case numbers rise again.
Potential GAS vaccines have focused on M protein as a vaccine component. For 1 vaccine, investigators used the variable portion of the M protein and incorporated 30 M types in 1 formulation [19]. Multivalent vaccines have the potential problem that M types can change over time, resulting in lack of coverage for specific circulating emm types. This could potentially be addressed through development of emm cluster–based vaccines as there is a limited number of cluster types, in comparison with the number of emm types [31]. In our survey, a breakdown of emm types by cluster type suggests that a vaccine for Alberta should encompass members of 5 cluster types, A-C3, D4, E3, E4, and E6, out of the 19 cluster types identified. These 5 cluster types constitute close to 80% of the emm types that were identified in our survey.
Although this survey provides important information regarding iGAS disease in Alberta, there are limitations. The described survey is retrospective and not prospective. A retrospective study does not allow investigators to go back to cases and interview patients for information that may not have been collected at the onset. Also, a passive surveillance system was used to collect the data; therefore, the possibility exists that cases may not have been reported. Another limitation is that not all cases had a GAS isolate for typing (87% were typed). However, having close to 90% of isolates emm-typed over a 15-year period still provides useful information regarding circulating emm types.
In summary, rates of iGAS disease in Alberta, Canada, have risen since 2003, reaching >10/100 000 in 2017. No single emm type alone can be attributed to the higher rates but rather a collection of common and new emerging emm types are evident. Another driver contributing is likely the growing number of marginalized and homeless people.
Acknowledgments
The authors would like to thank the clinical diagnostic microbiology laboratories in Alberta for identifying iGAS isolates and submitting them to the ProvLAB for emm typing.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005; 5:685–94. [DOI] [PubMed] [Google Scholar]
- 2. Carapetis JR. Rheumatic heart disease in developing countries. N Engl J Med 2007; 357:439–41. [DOI] [PubMed] [Google Scholar]
- 3. Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev 2000; 13:470–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nelson GE, Pondo T, Toews K, et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005–2012. Clin Infect Dis 2016; 63:478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seckeler MD, Hoke TR. The worldwide epidemiology of acute rheumatic fever and rheumatic heart disease. Clin Epidemiol 2011; 3:67–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Steer AC, Lamagni T, Curtis N, Carapetis JR. Streptococcal disease epidemiology, pathogenesis and management. Drugs 2012; 72:1213–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Watkins DA, Johnson CO, Colquhoun SM, et al. Global, regional and national burden of rheumatic heart disease, 1990–2015. N Engl J Med 2017; 377:713–22. [DOI] [PubMed] [Google Scholar]
- 8. Seale AC, Davies MR, Anampiu K, et al. Invasive group A streptococcus infection among Children, Rural Kenya. Emerg Infect Dis 2016; 22:224–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Steer AC, Jenney AJ, Oppedisano F, et al. High burden of invasive beta-haemolytic streptococcal infections in Fiji. Epidemiol Infect 2008; 136:621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smeesters PR, McMillan DJ, Sriprakash KS. The streptococcal M protein: a highly versatile molecule. Trends Microbiol 2010; 18:275–82. [DOI] [PubMed] [Google Scholar]
- 11. Dale JB, Batzloff MR, Cleary P, et al. Current approaches to group A streptococcal vaccine development. In: Ferretti JJ, Stevens DL, Fischetti VA, eds. Streptococcus pyogenes: Basic Biology to Clinical Manifestations. Oklahoma City: University of Oklahoma Health Sciences Center; 2016: 27–53. [Google Scholar]
- 12. Good MF, Pandey M, Batzloff MR, Tyrrell GJ. Strategic development of the conserved region of the M protein and other candidates as vaccines to prevent infection with group A streptococci. Expert Rev Vaccines 2015; 14:1459–70. [DOI] [PubMed] [Google Scholar]
- 13. Tyrrell GJ, Lovgren M, Kress B, Grimsrud K. Invasive group A streptococcal disease in Alberta, Canada (2000 to 2002). J Clin Microbiol 2005; 43:1678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Government of Alberta. Interactive Health Data Application. 2018. Available at: http://www.ahw.gov.ab.ca/IHDA_Retrieval/. Accessed 27 January 2018. [Google Scholar]
- 15. Alberta Health. Public health notifiable disease management criteria. Available at: https://open.alberta.ca/publications/ndr-manual-9th-edition. Accessed 2 February 2018. [Google Scholar]
- 16. Alberta Health. Notifiable Disease Report (NDR) Manual, Ninth Edition. Available at: https://open.alberta.ca/dataset/43769dee-7d57-4d9a-b863-7be03e7f6f00/resource/e84919a3-3df5-4cd8-9568-f1f44bd411a3/download/nd-report-manual-2018.pdf. Accessed 2 February 2018. [Google Scholar]
- 17. Centers for Disease Control and Prevention. Protocol for emm typing. Available at: https://www.cdc.gov/streplab/protocol-emm-type.html. Accessed 15 January 2018. [Google Scholar]
- 18. Centers for Disease Control and Prevention. Streptococcus pyogenes emm Sequence Database. 2018. Available at: https://www2a.cdc.gov/ncidod/biotech/strepblast.asp. Accessed 15 January 2018. [Google Scholar]
- 19. Dale JB, Penfound TA, Chiang EY, Walton WJ. New 30-valent M protein-based vaccine evokes cross-opsonic antibodies against non-vaccine serotypes of group A streptococci. Vaccine 2001; 29:8175–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Centers for Disease Control and Prevention. Active Bacterial Core surveillance (ABCs) surveillance reports. Available at: https://www.cdc.gov/abcs/reports-findings/surv-reports.html. Accessed 21 June 2018. [Google Scholar]
- 21. http://www.bccdc.ca/resource-gallery/Documents/Statistics%20and%20Research/Statistics%20and%20Reports/Immunization/Coverage/BC%20iGas%202017%20Epi%20Summary.pdf. Accessed 19 March 2018.
- 22. Tyrrell GJ, Lovgren M, St Jean T, et al. Epidemic of group A streptococcus M/emm59 causing invasive disease in Canada. Clin Infect Dis 2010; 51:1290–7. [DOI] [PubMed] [Google Scholar]
- 23. Athey TB, Teatero S, Sieswerda LE, et al. High incidence of invasive group A streptococcus disease caused by strains of uncommon emm types in Thunder Bay, Ontario, Canada. J Clin Microbiol 2016; 54:83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bocking N, Matsumoto C, Loewen K, et al. High incidence of invasive group A streptococcal infections in remote indigenous communities in Northwestern Ontario, Canada. Open Forum Infect Dis 2016; 4:ofw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lamagni TL, Neal S, Keshishian C, et al. Predictors of death after severe Streptococcus pyogenes infection. Emerg Infect Dis 2009; 15:1304–7. [DOI] [PubMed] [Google Scholar]
- 26. Teatero S, Coleman BL, Beres SB, et al. Rapid emergence of a new clone impacts the population at risk and increases the incidence of type emm89 group A streptococcus invasive disease. Open Forum Infect Dis 2017; 4:ofx042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nasser W, Beres SB, Olsen RJ, et al. Evolutionary pathway to increased virulence and epidemic group A streptococcus disease derived from 3615 genome sequences. Proc Natl Acad Sci U S A 2014; 111:E1768–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Teatero S, McGeer A, Tyrrell GJ, et al. Canada-wide epidemic of emm74 group A streptococcus invasive disease. Open Forum Infect Dis 2018; 5:ofy085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown CC, Olsen RJ, Fittipaldi N, et al. Spread of virulent group A streptococcus type emm59 from Montana to Wyoming, USA. Emerg Infect Dis 2014; 20:679–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Engelthaler DM, Valentine M, Bowers J, et al. Hypervirulent emm59 clone in invasive group A streptococcus outbreak, Southwestern United States. Emerg Infect Dis 2016; 22:734–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sanderson-Smith M, De Oliveira DM, Guglielmini J, et al. ; M Protein Study Group A systematic and functional classification of Streptococcus pyogenes that serves as a new tool for molecular typing and vaccine development. J Infect Dis 2014; 210:1325–38. [DOI] [PMC free article] [PubMed] [Google Scholar]