Compared with other species, rice imprinted genes are less associated with transposable elements, and the epigenetic regulation of imprinting occurs both prefertilization and postfertilization in rice.
Abstract
Genomic imprinting is an epigenetic phenomenon by which certain genes display differential expression in a parent-of-origin-dependent manner. Hundreds of imprinted genes have been identified from several plant species. Here, we identified, with a high level of confidence, 208 imprinted gene candidates from rice (Oryza sativa). Imprinted genes of rice showed limited association with the transposable elements, which contrasts with findings from Arabidopsis (Arabidopsis thaliana). Generally, imprinting in rice is conserved within a species, but intraspecific variation also was detected. The imprinted rice genes do not show signatures of selection, which suggests that domestication has had a limited evolutionary consequence on genomic imprinting. Although conservation of imprinting in plants is limited, we show that some loci are imprinted in several different species. Moreover, our results suggest that different types of epigenetic regulation can be established either before or after fertilization. Imprinted 24-nucleotide small RNAs and their neighboring genes tend to express alleles from different parents. This association was not observed between 21-nucleotide small RNAs and their neighboring genes. Together, our findings suggest that the regulation of imprinting can be diverse, and genomic imprinting has evolutionary and biological significance.
Imprinted genes are expressed in only one of the parental alleles in a parent-of-origin-dependent manner (Köhler et al., 2012; Gehring, 2013). Genes that exclusively or preferentially express the maternal or paternal alleles are termed maternally expressed genes (MEGs) or paternally expressed genes (PEGs), respectively. Genomic imprinting has been observed in many species, from mammals to flowering plants (Pires and Grossniklaus, 2014), and hundreds of putative imprinted loci, including protein-coding genes and noncoding RNAs, have been discovered in plants (Gehring et al., 2011; Hsieh et al., 2011; Luo et al., 2011; Waters et al., 2011, 2013; Wolff et al., 2011; Zhang et al., 2011, 2016; Xin et al., 2013; Pignatta et al., 2014; Xu et al., 2014; Florez-Rueda et al., 2016; Hatorangan et al., 2016; Klosinska et al., 2016). However, there is little conservation of imprinting among plant species (Waters et al., 2013; Hatorangan et al., 2016), which suggests that the evolution of genomic imprinting in plants is very rapid.
Genomic imprinting is regulated epigenetically by either DNA methylation or histone modification, and in some circumstances, both mechanisms are involved (Huh et al., 2008; Köhler and Weinhofer-Molisch, 2010). In plants, imprinting is expressed predominantly in the endosperm (Luo et al., 2011; Raissig et al., 2013; Klosinska et al., 2016), which is hypomethylated relative to the embryo and vegetative tissues (Gehring et al., 2009; Zemach et al., 2010; Rodrigues et al., 2013; Xing et al., 2015; Klosinska et al., 2016). The DNA glycosylase DEMETER (DME), which is responsible for DNA demethylation, is expressed predominantly in the central cell prior to fertilization (Choi et al., 2004). Imprinting disorders can be found in dme mutants (Hsieh et al., 2011; Wolff et al., 2011; Vu et al., 2013), indicating that proper DNA methylation status is required for the establishment of genetic imprinting in the endosperm. Several studies have revealed that imprinted genes usually neighbor transposable elements (TEs; Wolff et al., 2011; Pignatta et al., 2014; Hatorangan et al., 2016). High expression of DME in the central cell promotes the demethylation of TEs and their adjacent imprinted genes (Gehring et al., 2009). Therefore, maternal alleles of imprinted genes are activated in the central cell, but the paternal alleles are hypermethylated and remain silenced in the sperm cell, which eventually leads to the expression of only the maternal allele after fertilization. Polycomb Repressive Complex2 (PRC2)-mediated Histone H3 Lysine-27 Trimethylation (H3K27me3) is required for proper genomic imprinting (Huh et al., 2008; Köhler and Weinhofer-Molisch, 2010). Mutation of fertilization-independent endosperm (FIE), a member of the Fertilization Independent Seed (FIS)-PRC2 family, may result in the disruption of imprinting at some loci (Hsieh et al., 2011; Wolff et al., 2011).
Genetic evidence has revealed that several MEGs are likely important for seed and/or endosperm development in Arabidopsis (Arabidopsis thaliana; Chaudhury et al., 1997; Luo et al., 2000; Tiwari et al., 2008; Fitz Gerald et al., 2009; Costa et al., 2012; Liu et al., 2014). However, many mutants of paternally expressed imprinted genes show no altered phenotype (Köhler et al., 2005; Shirzadi et al., 2011; Bratzel et al., 2012; Vu et al., 2013; Wolff et al., 2015). In addition, the conservation of imprinting between closely related species is limited (Waters et al., 2013; Hatorangan et al., 2016). These observations cast doubt on the importance of genomic imprinting in plants. The kinship theory (Haig and Westoby, 1991) hypothesizes that imprinting arose as a consequence of conflict between male and female gametes. Specifically, this theory suggests that males gain fitness benefits from greater transmission of resources from the mother to the offspring, but females benefit by the suppression of growth-related demands from the offspring that are driven by paternally active genes. Numerous observations support the kinship hypothesis (Köhler et al., 2012; Pires and Grossniklaus, 2014; Rodrigues and Zilberman, 2015). However, in plants, this theory has been challenged (Rodrigues and Zilberman, 2015). Recent findings in Arabidopsis revealed that some PEGs may be involved in hybrid incompatibility (Wolff et al., 2015), implying that genomic imprinting may play a role in speciation. However, the evolutionary significance of genomic imprinting requires further investigation in different plant species.
Rice (Oryza sativa) is a model monocot and a vital agricultural crop. Consistent with results from dicots, studies in maize (Zea mays), another monocot, have revealed that differential DNA methylation and H3K27me3 modification between parental genomes are essential for the imprinting of monocots (Waters et al., 2013; Zhang et al., 2014). However, our understanding of the epigenetic regulation of genomic imprinting in rice remains limited. Through a genome-wide survey of imprinted genes from reciprocal intraspecific crosses, Luo et al. (2011) and Yuan et al. (2017) identified more than 300 imprinted candidates in rice. However, there was little overlap in the identity of these candidates between the studies (Yuan et al., 2017). The ability to identify imprinted genes is dependent largely on the availability of informative single-nucleotide polymorphisms (SNPs). Therefore, the discovery of imprinted genes from distinct intraspecific crosses is necessary to obtain greater certainty for identifying imprinted candidates. Moreover, due to its rapid evolution, variation in imprinting has been found among accessions in both Arabidopsis and maize (Waters et al., 2013; Pignatta et al., 2014). In this study, we explored imprinted genes in rice by making two independent reciprocal crosses between indica and japonica cultivars as well as crosses between cultivated and wild rice (Oryza rufipogon). Our results showed that the regulatory mechanisms of genomic imprinting in rice are diverse. These findings will help us to better understand the regulation and evolution of imprinting in plants.
RESULTS
Identification of Imprinted Genes in Cultivated Rice
To explore the imprinted genes in rice, we used three-line hybrid rice strains to construct reciprocal crosses. A cytoplasmic male sterile (CMS) line carries a sterility gene encoded in the cytoplasmic genome, such that the pollen produced is sterile. A maintainer line shares the same nuclear genome as its corresponding CMS line but has a distinct cytoplasmic genome. Due to the absence of the cytoplasmic sterility gene, the maintainer line is fertile. Using rice CMS and maintainer lines for crossing greatly increases the efficiency of hybridization and enables us to rigorously control the timing of fertilization and to avoid false hybridization events. Here, we used the japonica CMS lines (Liuqianxin-A and Yu6-A) and their maintainer lines (Liuqianxin-B and Yu6-B) and the indica CMS lines (Rongfeng-A and Wufeng-A) with their corresponding maintainer lines (Rongfeng-B and Wufeng-B) to make two distinct reciprocal cross sets, which we refer to as Liuqianxin-A/Rongfeng-B (LR), Rongfeng-A/Liuqianxin-B (RL), Yu6-A/Wufeng-B (YW), and Wufeng-A/Yu6-B (WY). The LR and RL reciprocal crosses and the YW and WY reciprocal crosses are indicated as LR-RL and YW-WY hereafter for convenience. Meanwhile, we made crosses of Wufeng-A/Wufeng-B (WW), Rongfeng-A/Rongfeng-B (RR), Liuqianxin-A/Liuqianxin-B (LL), and Yu6-A/Yu6-B (YY), which resembled the selfing of inbred lines as controls to validate imprinting.
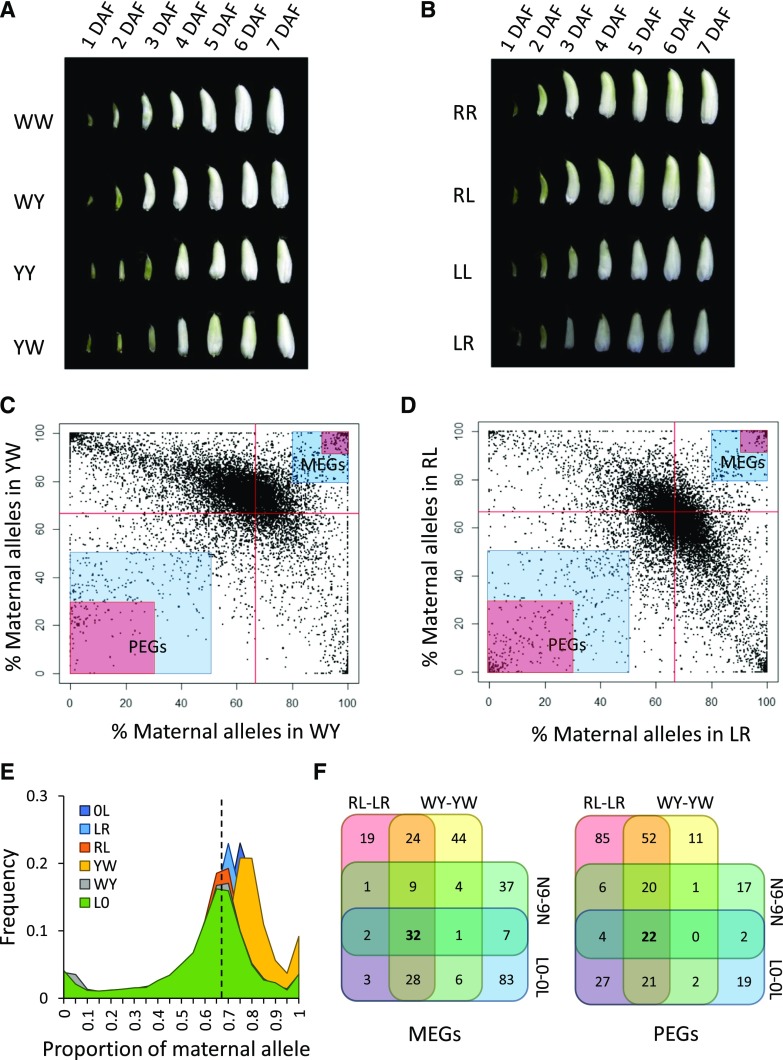
Morphological observations suggested that seeds produced by LR, RL, YW, and WY developed normally (Fig. 1, A and B). The endosperm from these reciprocal crosses was collected at 5 DAF for RNA sequencing (RNA-seq). By comparing LR-RL transcriptome data with whole genomic resequencing data from Liuqianxin-A and Rongfeng-B, we identified 62,102 SNPs from 12,299 endosperm-expressed genes in the LR-RL cross that could be used to distinguish parental alleles. Similarly, by comparing the RNA-seq data from YW-WY with genomic resequencing of Yu6-A and Wufeng-B, we identified 59,107 SNPs from 11,044 genes. In total, the parental expression of 4,946 genes in LR-RL and 6,308 genes in YW-WY deviated from the expected 2:1 maternal-to-paternal ratio (χ2 test; P < 0.05, false discovery rate [FDR] < 0.05).
Figure 1.
Identification of imprinted genes in rice. A, Seed morphology of Wufeng-A × Wufeng-B (WW), Wufeng-A × Yu6-B (WY), Yu6-A × Yu6-B (YY), and Yu6-A × Wufeng-B (YW) at 1 to 7 d after fertilization (DAF). B, Seed morphology of Rongfeng-A × Rongfeng-B (RR), Rongfeng-A × Liuqianxin-B (RL), Liuqianxin-A × Liuqianxin-B (LL), and Liuqianxin-A × Rongfeng-B (LR) from 1 to 7 DAF. C and D, Allele-specific expression analysis in WY-YW (C) and RL-LR (D). The highlighted areas indicate moderately (2-fold higher than expected; blue) and strongly (5-fold higher than expected; red) imprinted genes. Red lines denote the 0.67 expected value. The proportion of maternal alleles is calculated by fragments per kilobase million (FPKM)maternal/(FPKMmaternal + FPKMpaternal). E, Distribution of the proportion of maternal alleles at each locus with informative SNPs in WY, YW, RL, LR, Longtepu × 02428 (L0), and 0L. The dashed line indicates the 0.67 expected value. F, Venn diagrams of imprinted genes identified from MY-YM, RL-LR, L0-0L, and Nipponbare and 9311 (N9-9N) reciprocal crosses.
Next, we surveyed parent-of-origin effects of these genes by examining expression levels that were greater than 2-fold (greater than 4:1 for moderate MEGs and less than 2:2 for moderate PEGs) or 5-fold (greater than 10:1 for strong MEGs and less than 1:5 for strong PEGs) relative to the expected expression levels in both directions of the reciprocal crosses (Fig. 1, C and D). Therefore, for MEGs, the proportion of maternal alleles should be greater than 0.8 [4/(1+4)] or 0.91 [10/(1+10)], while for PEGs, it should be less than 0.5 [2/(2+2)] or 0.29 [2/(2+5)]. According to this criterion, there were 177 and 118 MEGs identified from LR-RL using the 0.8 and 0.91 thresholds, respectively. There were 347 and 148 MEGs in the YW-WY hybrids (Supplemental Table S1). Moreover, there were 504 moderate PEGs and 237 strong PEGs identified from LR-LR and 362 moderate PEGs and 129 strong PEGs identified from YW-WY (Supplemental Table S1).
In general, we identified more MEGs but fewer PEGs in the YW-WY cross. By analyzing the ratio of (maternal transcripts):(maternal transcripts + paternal transcripts) at each locus, we found that the peak of the YW distribution (0.75–0.8) was shifted from the expected value of 0.67 (Fig. 1E), suggesting that, on average, the mother Yu6-A contributed more mRNA transcripts than the expected 2:1 ratio. A similar trend also was observed in the LR cross, albeit much less substantial than that in YW (Fig. 1E). In contrast, most genes did not deviate from the expected ratio of 2:1 in WY and RL (Fig. 1E). Given that the Yu6-A CMS line is completely sterile and cannot self-fertilize, this deviation cannot be caused by false hybridization events. Rice endosperm is easily separated from maternal tissues at 5 DAF, and the two independent YW RNA-seq replicates were highly correlated (R2 = 0.9873), indicating that the contamination of maternal tissues was unlikely. We used the genomic sequence of Nipponbare, a japonica rice variety, as the reference for read mapping. Therefore, it is possible that mapping bias could cause this deviation. However, given that RR and WW are closely related (http://ricedata.cn) but LR did not show a deviation as extreme as YW, it is unlikely that mapping bias caused the deviation.
In light of the observations that indica/indica crosses (WW and RR) exhibited faster early endosperm development than the japonica/japonica crosses (LL and YY) and the hybrid seeds were more similar to their maternal parent (Fig. 1, A and B), we propose that the maternal contribution at 5 DAF is caused by slower endosperm development and a slower maternal-to-zygotic transition when japonica rice is used as the mother. To confirm this hypothesis, we reanalyzed the RNA-seq data of endosperm at 7 DAF from the Longtepu (indica) and 02428 (japonica) reciprocal crosses (L0-0L; Yuan et al., 2017). Consistent with our hypothesis, when the 02428 line was used as the mother, the peak of the distribution of (maternal transcripts):(maternal transcripts + paternal transcripts) shifted to 0.75 (Fig. 1E). These findings suggest that, when japonica is used as the mother, it contributes more to early endosperm development of the japonica/indica hybrid. To minimize the influence of this bias, we focused mainly on the 208 strongly imprinted genes (greater than 5-fold bias) shared between LR-RL and YW-WY in the following studies (Fig. 1F).
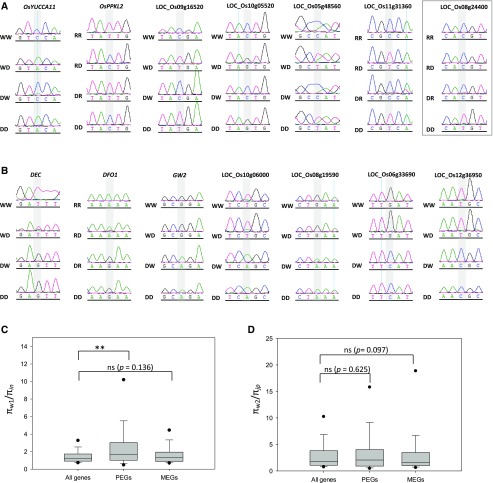
Using reverse transcription (RT)-PCR and sequencing, we confirmed that all 14 of the tested candidates were imprinted genes (Supplemental Fig. S1A). We also performed single-colony sequencing and pyrosequencing (Supplemental Fig. S1, B–D) to confirm the imprinting status of two quantitative trait loci, Grain Weight2 (GW2) and protein phosphatase with Kelch-like repeat domain2 (OsPPKL2), for rice seed development (Song et al., 2007; Zhang et al., 2012b), suggesting that genome imprinting may function directly for seed development in rice.
For MEGs, Gene Ontology (GO) analysis showed enrichment for the term DNA binding (P = 0.00031, FDR = 0.017), whereas for PEGs, the terms transferase activity (P = 6.3e-05, FDR = 0.0014), kinase activity (P = 6.3e-05, FDR = 0.0014), and protein binding (P = 0.001, FDR = 0.015) were overrepresented. These results suggested that PEGs and MEGs might have different functions. Interestingly, we noticed that several of the imprinted genes were involved in epigenetic regulation (Supplemental Table S2). A previous study reported that OsFIE1 was the only imprinted PRC2 gene in rice (Luo et al., 2009). Here, we found that rice Embryonic Flower2a (OsEMF2a), a homolog of the Su(z)12 family, also was imprinted, which was validated by different approaches (Supplemental Fig. S2).
Comparison with Previously Identified Imprinted Genes in Rice
Luo et al. (2011) conducted a genome-wide survey of imprinted genes in rice using the japonica variety Nipponbare and the indica variety 9311 as the parents (N9-9N). Approximately 74% (53/72) of the PEGs and 53% (49/93) of the MEGs identified overlapped with those identified in this study (Fig. 1F). Similarly, about 80% (76/97) of the PEGs and 44% (72/162) of the MEGs discovered from L0-0L (Yuan et al., 2017) were included in our list of candidates (Fig. 1F). However, there were fewer overlaps between N9-9N and L0-0L (Yuan et al., 2017). In total, 300 MEGs and 289 PEGs have been identified from rice (Fig. 1F; Supplemental Table S1), with PEGs being more conserved than MEGs. About 54.3% of the PEGs (157/289) could be found in at least two sets of the four reciprocal crosses (RL-LR, WY-YW, N9-9N, and L0-0L), while only 39% of the MEGs (117/300) coincided among the different combinations.
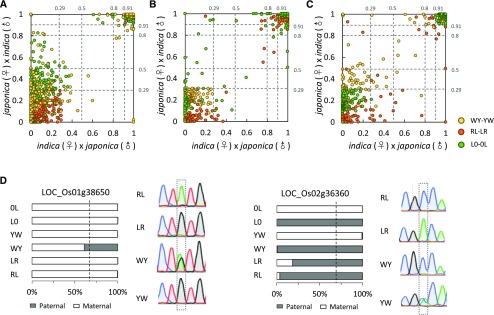
Most of the imprinted genes identified in one cross also tended to be imprinted in other reciprocal crosses (Fig. 2, A–C; Supplemental Fig. S3). The ones found in only one set of reciprocals usually lacked informative SNPs or sufficient reads to identify if they were imprinted in other crosses (Supplemental Fig. S3). For example, among the 156 imprinted genes identified in the other two studies but not in this study (Fig. 1F), 62 (40%) lacked polymorphisms or expression in WY-YW and LR-RL.
Figure 2.
Intraspecific imprinting variation in rice. A to C, Proportion of maternal alleles [FPKMmaternal/(FPKMmaternal + FPKMpaternal)] in the reciprocal crosses. A, Proportion of maternal alleles in the imprinted genes identified from RL-LR in WY-YW and L0-0L. B, Proportion of maternal alleles in the imprinted genes identified from WY-YW in RL-LR and L0-0L. C, Proportion of maternal alleles of imprinted genes identified from L0-0L in RL-LR and WY-YW. Most of the imprinted genes identified from one reciprocal cross set are imprinted in other sets. D, Examples of allele-specific imprinting in rice. The bar charts show the proportion of parental alleles in different crosses; the sequencing diagrams show the validation of intraspecific imprinting variation using RT-PCR sequencing. Dashed lines indicate the 0.67 expected value, and the informative SNPs are boxed.
Allele-specific imprinting has been found in maize and Arabidopsis (Waters et al., 2013; Pignatta et al., 2014). To test for allele-specific imprinting in rice, we used a cutoff criterion. If a strong imprinted gene identified in one reciprocal cross set failed to show moderate imprinting (less than 2-fold deviation from the 2:1 expected ratio in other reciprocal crosses), we considered it as an allele-specific imprinted gene. Seventy allele-specific imprinted genes were found in rice (Supplemental Table S3). Given that the mother plant likely contributes more to the 5-DAF endosperm of the japonica (♀)/indica (♂) hybrid than expected (Fig. 1E), the number of allele-specific imprinted genes could be overestimated in this study. However, PEGs should not be affected by the maternal bias. We did find some PEGs showing allele-specific imprinting (Supplemental Table S3), which confirmed the existence of allele-specific imprinting in rice. For example, LOC_Os02g36360 was identified as a PEG in RL-LR and L0-0L but not in WY-YW (Fig. 2D; Supplemental Table S3).
PEGs in Rice Show Weak Associations with TEs and Tend To Be Clustered in the Genome
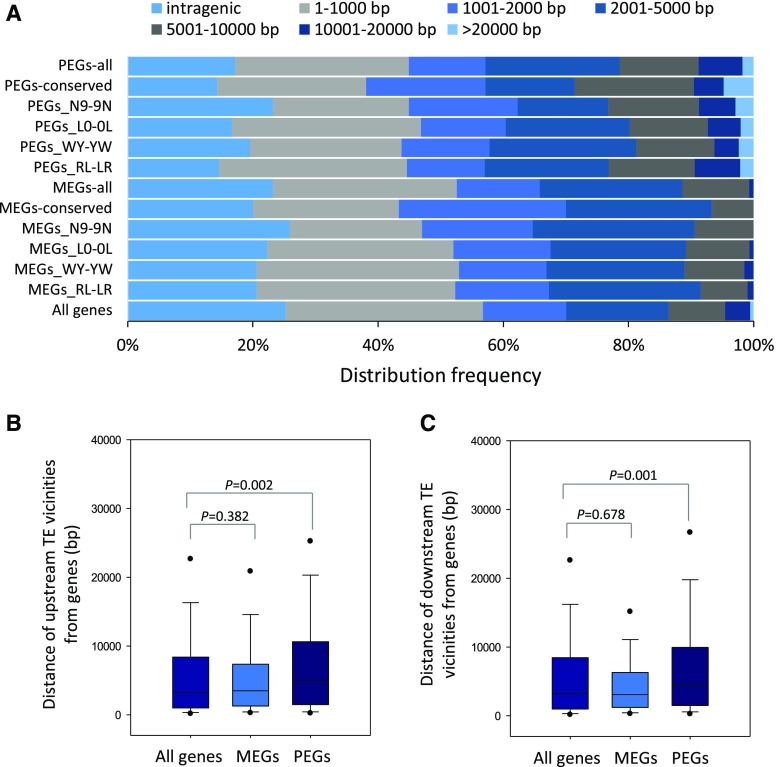
TEs are enriched in the proximity of imprinted genes in Arabidopsis and Capsella rubella (Wolff et al., 2011; Pignatta et al., 2014; Hatorangan et al., 2016). Yuan et al. (2017) found that miniature inverted-repeat TEs were abundant near imprinted genes of rice. However, Luo et al. (2011) did not find TE enrichment in rice. We comprehensively searched for TEs in the rice genome and found 149,007 TEs, which consisted of approximately 36% of the rice genome. We did not find enrichment of TEs within 2-kb flanking regions of imprinted genes in rice. When using the united imprinted genes of RL-LR, WY-YW, N9-9N, and L0-0L for analysis, about 66% of MEGs and 57% of PEGs have a TE within the vicinity of the 2-kb flanking region. By contrast, more than 70% of the genes in the rice genome have a TE located within 2 kb (Fig. 3A). The chance of finding a PEG close to a TE was lower than that for MEGs (Fig. 3A). Consistent results were obtained when different MEGs and PEGs identified from distinct reciprocal crosses were included (Fig. 3A). To minimize the influence of variation in TEs across different varieties, we used the 32 MEGs and 22 PEGs (Supplemental Table S1) that were most highly conserved among RL-LR, WY-YW, N9-9N, and L0-0L for the analysis. The conserved PEGs continued to show a weaker association with TEs (Fig. 3A). Moreover, the distance of MEGs to their closest upstream and downstream TE did not differ from a whole-genome control but was smaller than that for PEGs (Fig. 3, B and C). These data imply that imprinted genes, especially PEGs, showed limited association with TEs in rice.
Figure 3.
TEs in rice do not enrich with PEGs or MEGs. A, Frequency distribution showing the distance of rice imprinted genes to their nearest TE. There is no TE enrichment in the 2-kb region flanking MEGs or PEGs. PEGs-all and MEGs-all indicate the combined PEGs and MEGs found in RL-LR, WY-YW, N9-9N, and L0-0L. PEGs-conserved and MEGs-conserved indicate the common PEGs and MEGs shared among RL-LR, WY-YW, N9-9N, and L0-0L. B and C, The combined PEGs of rice are found farther upstream (B) and downstream (C) of TEs compared with the combined set of MEGs; the overall distances of combined MEGs to their upstream (B) and downstream (C) TE vicinities do not differ from all genes encoded in the rice genome. A Kruskal-Wallis one-way analysis was used for statistical analysis. Error bars indicate sd.
Mammalian imprinted genes usually are clustered in the genome (Edwards and Ferguson-Smith, 2007). However, this distribution pattern was not found in plants, although some microclusters were observed across the genomes of different plant species (Gehring et al., 2011; Luo et al., 2011; Wolff et al., 2011; Zhang et al., 2011). We used the set of imprinted genes found in MY-YM and RL-LR for clustering analysis. Across a sliding window, we found that the 113 imprinted genes were organized into 51 clusters, using the 13,821 genes with informative SNPs and expression in 5-DAF endosperm as the control. The clusters are distributed unevenly across the genome (Supplemental Fig. S4). Over 34% (86/215) of the rice PEGs were located within clusters, while fewer than 16% (27/215) of the MEGs were in clusters. In general, imprinted genes in the same cluster displayed a consistent maternal or paternal expression pattern (Supplemental Table S4).
Expression Patterns of Imprinted Genes
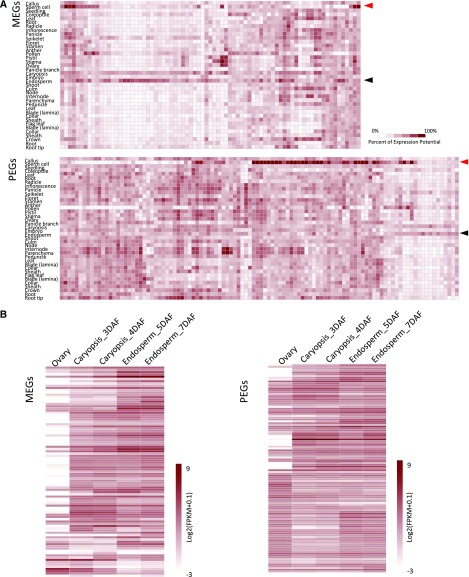
We used Genevestigator to study the gene expression of the imprinted candidates in rice. More than half of the common MEGs of RL-LR and WY-YW were expressed preferentially in the endosperm (Fig. 4A; Supplemental Fig. S5). The expression preference was more apparent with the set of MEGs shared by RL-LR, WY-YW, and N9-9N (Supplemental Fig. S6A). Overall, the PEGs did not display preferential expression in the endosperm (Fig. 4A; Supplemental Fig. S6B). Dynamic expression analysis of the imprinted genes during early seed development indicated that many MEGs and some PEGs were not expressed in the first 2 to 3 DAF (Fig. 4B; Supplemental Fig. S6, C and D). Their expression became activated at 3 to 4 DAF in the endosperm but usually not in the embryo (Supplemental Fig. S6C).
Figure 4.
Expression profiles of imprinted genes in rice. A, Expression analysis of the common MEGs (top) and PEGs (bottom) of RL-LR and WY-YW by Genevestigator. Red and black arrowheads indicate sperm cell and endosperm, respectively. B, Dynamic expression patterns of the MEGs (left) and PEGs (right) in developing Nipponbare seed.
As demonstrated in Arabidopsis, the MEGs are activated in the central cell before fertilization (Huh et al., 2008). However, we noted that only approximately half of the MEGs were expressed in the ovary, whereas the rest were activated in the endosperm after fertilization (Fig. 4A; Supplemental Fig. S7, A and B). Similarly, approximately only half of the PEGs showed the highest expression in sperm cells (Fig. 4A; Supplemental Fig. S7A). By surveying publicly available transcriptome data, we found that the MEG expression level was generally low in rice sperm cells (Supplemental Fig. S7C). However, some MEGs were expressed highly in sperm cells (Supplemental Fig. S7, A, D, and E).
Epigenetic Regulation of Gene Imprinting in Rice
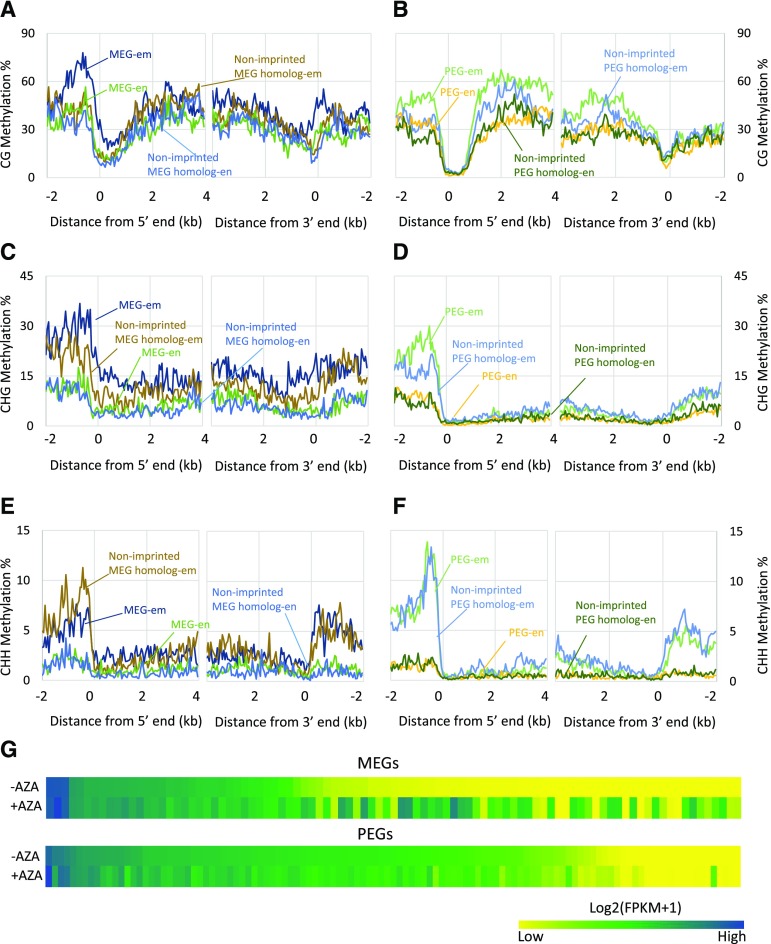
By reanalyzing the publicly available rice DNA methylome from Nipponbare (Zemach et al., 2010), we found that DNA methylation profiles of imprinted genes (RL-LR and YW-WY commons) were quite similar in the embryo, roots, and shoot, but there was clear hypomethylation in endosperm (Supplemental Fig. S8). In terms of the promoter region, imprinted genes generally exhibited higher CG and CHG methylation levels than their nonimprinted paralogs in the embryo (Fig. 5, A–D). Moreover, MEGs had higher CG and CHG methylation levels than PEGs (Fig. 5, A–D). In the endosperm, CG methylation of the promoter regions of imprinted genes was reduced substantially (Fig. 5, A and B; Supplemental Fig. S9), while nonimprinted homologous genes were found at background levels in the embryo (Fig. 5, A and B). Promoter CHG methylation of imprinted genes was comparable to that of corresponding nonimprinted homologs in the endosperm (Fig. 5D). The CHH methylation level in the endosperm was low, and there were no differences between imprinted genes and their homologs (Fig. 5, E and F). In the embryo, gene bodies showed higher CG methylation of PEGs compared with MEGs (Fig. 5A). However, the higher CG methylation of PEGs in gene bodies was not detected in the endosperm (Fig. 5B). These results are in agreement with the findings that PEGs are enriched for differentially methylated regions in gene bodies, while MEGs tend to show differentially methylated regions in promoter regions (Rodrigues et al., 2013). In addition, MEGs showed more CHG methylation in the gene body than nonimprinted MEG homologs and PEGs in the embryo (Fig. 5, C and D). The difference between MEGs and their nonimprinted homologs was not observed in the endosperm, although MEGs were still highly methylated relative to PEGs (Fig. 5D).
Figure 5.
DNA methylation of imprinted genes in rice. A to F, Average CG (A and B), CHG (C and D), and CHH (E and F) methylation profiles of the MEGs and their most similar nonimprinted homologs (A, C, and E) and the PEGs and their most similar nonimprinted homologs (B, D, and F). em, Embryo; en, endosperm. G, Heat maps of the expression changes of MEGs (top) and PEGs (bottom) in response to AZA, a DNA methylation inhibitor. −AZA, AZA-free seedlings; +AZA, AZA-treated seedlings.
Intraspecific variations of methylation can lead to allele-specific imprinting in plants (Pignatta et al., 2014). To eliminate the impact of the Nipponbare genotype on methylation patterns, we used the highly conserved imprinted genes (22 PEGs and 32 MEGs shared across all four crosses) to investigate the effects of methylation on genomic imprinting. We found that the methylation profiles of the conserved PEGs and their nonimprinted homologs were similar in the embryo and endosperm, suggesting that methylation has quite limited effects on the imprinting of conserved PEGs (Supplemental Fig. S10).
Many MEGs are not expressed in tissues besides the hypomethylated endosperm, while PEGs are expressed broadly, and imprinting shows a limited association with DNA methylation. Therefore, the repression of DNA methylation may induce the ectopic expression of some MEGs. To test this hypothesis, we applied 5-aza-2′-deoxycytidine (AZA), a DNA methylation inhibitor, to the seedlings of Kitaake (japonica) for 10 d. Genome-wide transcriptome analysis showed that the expression of many imprinted genes shared between the RL-LR and WY-YW crosses was disturbed as expected, with the MEGs showing a different response to AZA in comparison with the PEGs (Fig. 5G; Supplemental Fig. S11A). The vast majority of PEGs exhibited few expression changes, while approximately 60% of MEGs showed at least a 2-fold up-regulation (Supplemental Fig. S11A). We also noticed that many of the activated MEGs were not expressed in seedlings under normal conditions (Supplemental Fig. S11, B and D), implying that decreased methylation caused the ectopic expression of some MEGs.
Generally, imprinted genes with high expression in seedlings were not sensitive to AZA (Supplemental Fig. 11C). We speculated that the imprinting regulation of these genes also may rely on PRC2-mideated histone modification or some other mechanisms, such as chromatin structure. Malone et al. (2011) profiled H3K27me3 histone modification in young 6- to 7-DAF rice endosperm. Using these data, we found that approximately 30% (34/115) of the PEGs common to the RL-LR and WY-YW crosses coincided with H3K27me3 peaks within the gene body or in the 2-kb flanking regions, whereas only 17% (16/93) of MEGs coincided with H3K27me3 peaks (Supplemental Table S5).
Association of Genomic Imprinting with Imprinted 24-Nucleotide Small RNAs
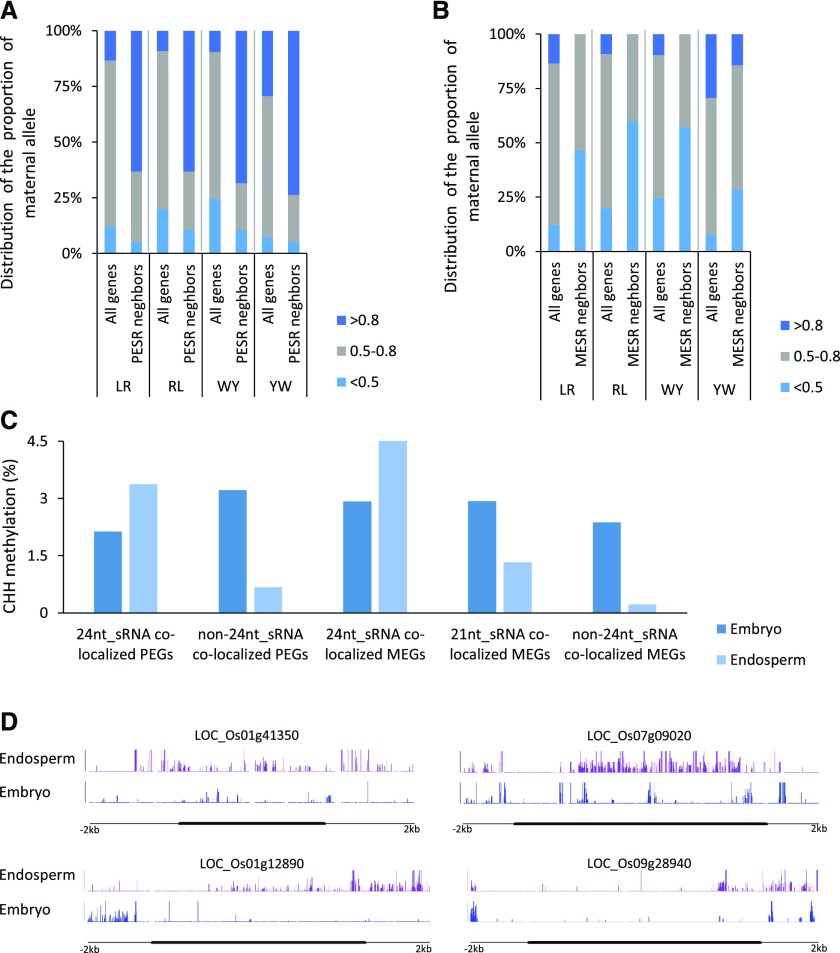
Previous studies suggested that small RNAs (sRNAs) may be involved in the regulation of imprinting (Rodrigues et al., 2013; Pignatta et al., 2014; Yuan et al., 2017). Several dozen imprinted sRNAs have been found in rice (Rodrigues et al., 2013; Yuan et al., 2017). Here, we found that more than 36% (28/77) of the imprinted sRNA loci colocalize with the imprinted genes in rice (Supplemental Table S6). Interestingly, the bias in the direction of imprinted 24-nucleotide sRNA loci was opposite to its neighboring imprinted genes (Supplemental Table S6), suggesting that paternally expressed sRNAs associate with MEGs and vice versa. To confirm this tendency, we investigated the parental expression of the flanking genes of the imprinted 24-nucleotide sRNAs. Forty-two of the 93 genes had informative SNPs and showed expression in the endosperm. Our results showed that the paternal sRNA neighbors usually exhibited maternal expression bias (more than 80% of transcripts were maternal), while maternal sRNA neighbor genes displayed paternal expression bias (fewer than 50% of transcripts were maternal) in comparison with the whole genome (Fig. 6, A and B). These results suggest that an imprinted 24-nucleotide sRNA is able to oppositely target its genic vicinity for imprinting.
Figure 6.
The genic neighbors of imprinted 24-nucleotide sRNAs show opposite parental expression bias and high CHH methylation in endosperm. A, Distribution frequency of the proportion of maternal alleles (FPKMmaternal/FPKMmaternal + FPKMpaternal) of the genes that group with a paternally expressed 24-nucleotide sRNA (PESR) and all genes encoded by the genome in different crosses. B, Distribution frequency of the proportion of maternal alleles of genes that group with a maternally expressed 24-nucleotide sRNA (MESR) and all genes encoded by the genome in different crosses. C, CHH methylation of the imprinted genes that colocalize with 24-nucleotide sRNAs and the imprinted genes that colocalized with 21-nucleotide sRNAs in embryo and endosperm. D, Examples of CHH methylation increased imprinted genes that grouped with 24-nucleotide sRNAs.
We next compared the CHH methylation levels of the imprinted genes that either overlapped or did not overlap imprinted 24-nucleotide sRNAs, because 24-nucleotide sRNAs are able to promote CHH methylation through the RNA-directed DNA methylation pathway. The MEGs and PEGs around 24-nucleotide sRNAs both showed high CHH methylation levels in the endosperm (Fig. 6, C and D; Supplemental Fig. S12A), which is opposite to the observation that imprinted genes showed a lower overall methylation level in the endosperm (Supplemental Fig. S8). We also analyzed the CG and CHG methylation of the imprinted genes neighboring imprinted 24-nucleotide sRNAs. In comparison with the embryo, these MEGs generally showed lower CG and CHG methylation in the endosperm. However, the 24-nucleotide sRNA-associated PEGs exhibited a similar CHG methylation level in the endosperm and embryo (Supplemental Fig. S12, B and C).
Notably, aside from the 24-nucleotide sRNAs, all five of the imprinted 21-nucleotide sRNAs showed the same maternal expression bias as the MEGs with which they overlapped (Supplemental Table S6; Yuan et al., 2017), which likely formed imprinted clusters. Moreover, the CHH methylation pattern of the 21-nucleotide sRNA-associated imprinted genes resembled that of the control (Fig. 6C).
Domestication Shows Limited Influence on Genomic Imprinting
The conservation of imprinting between cultivated rice and wild rice remains unknown. We made interspecific crosses between cultivated rice and Dongxiang wild rice (designated as D hereafter). Using an RT-PCR sequencing approach, 13 of the 14 confirmed imprinted genes of cultivated rice (Supplemental Fig. S1) also displayed parent-of-origin expression patterns in these crosses (Fig. 7, A and B). These results suggest that the regulation of genomic imprinting is conserved between cultivated rice and wild rice, and domestication likely has few consequences for the evolution of genomic imprinting in rice.
Figure 7.
Detection of gene imprinting status in cultivated rice × wild rice reciprocal crosses. A and B, Imprinting status of the PEGs (A) and MEGs (B) identified from RL-LR and WY-YW in cultivated rice × wild rice reciprocal crosses. Polymorphic sites are shaded in gray. W, R, and D indicate Wufeng, Rongfeng, and Dongxiang wild rice, respectively. The gene that does not display a parent-of-origin expression pattern is boxed. C and D, Differences in π between W1-type wild rice and indica (C) and W2-type wild rice and japonica (D). A Mann-Whitney rank sum test was used for statistical analyses. **, Significant difference at P < 0.01. The bars indicate 15th/75th percentiles, and the dots indicate 5th/95th percentiles. All genes encoded by the rice genome are used as the control.
We further investigated the demographic history of imprinted loci. Recent evidence suggests that W1-type wild rice is closely related to indica, whereas the W2 type is closer to japonica rice (Xu et al., 2011; Huang et al., 2012). Here, we used πw1/πin and πw2/πja to reveal differences in nucleotide diversity (π) between wild rice and indica or japonica. In indica, the πw1/πin of PEGs was significantly higher than that of the whole genome (P < 0.001; Fig. 7C). However, for japonica, the imprinted genes showed no evidence of being selected (Fig. 7D). We did not find differences between πw1/πin and πw2/πja for MEGs relative to the rest of the indica and japonica genomes (Fig. 7, C and D). Taken together, we inferred that most of the imprinted genes were not selected during domestication.
Genomic Imprinting Is Conserved at Some Loci in Plants
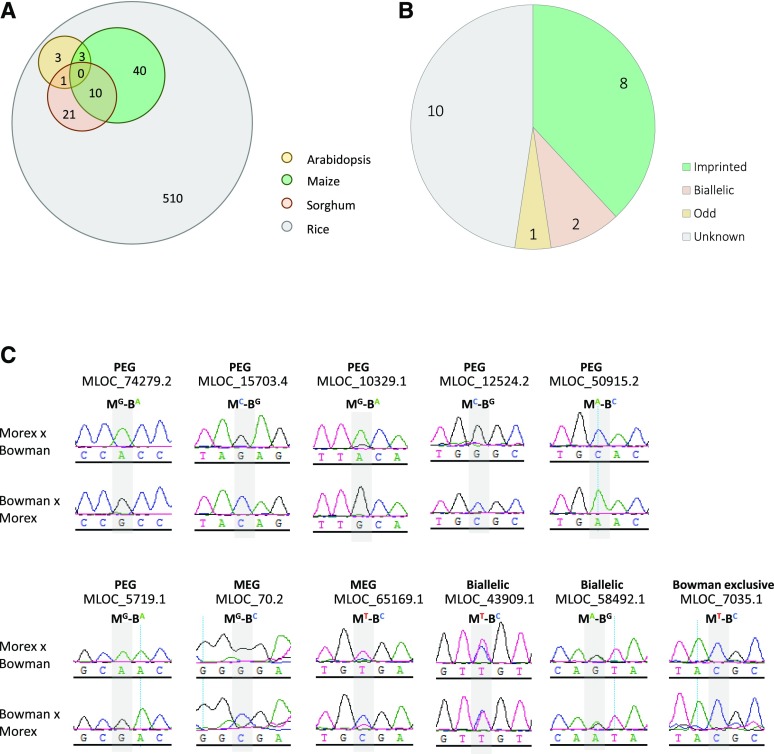
We identified orthologs to imprinted genes in rice by searching the genomes of other plant species. Among the top hits, we found that seven, 53, and 32 homologs in Arabidopsis, maize, and sorghum (Sorghum bicolor), respectively, also were imprinted (Fig. 8A), which indicated that about 13% (78/588) of the imprinted genes in rice also may be imprinted in other plant species. When using the top three hits, the number increased to 24% (143/589; Supplemental Table S7). In comparison with Arabidopsis and sorghum, maize showed more imprinting conservation with rice. Sixty-three imprinted genes of rice had maize homologs (Supplemental Table S7). However, we found some conserved imprinted loci among plant species (Supplemental Table S7). To test for conservation, we constructed reciprocal crosses of barley (Hordeum vulgare) using the varieties Morex and Bowman as the parents. First, we searched for the most similar barley orthologs of some conserved imprinted genes (Supplemental Table S7) using the rice protein sequences as queries. By searching for information deposited in the Gramene database (ensembl.gramene.org/Hordeum_vulgare/Info/Index), we designed primers to amplify cDNA fragments with potentially informative SNPs. Among the 21 genes we tested, 10 lacked informative SNPs or expression in the endosperm (Fig. 8B). Of the remaining 11 genes, two were identified as MEGs and six as PEGs (Fig. 8C). In addition, one showed odd monoallelic expression in a parent-of-origin-independent manner and two were biallelic (Fig. 8C). In total, approximately 73% (8/11) of the genes showed parent-of-origin expression patterns, which confirmed that the imprinting was conserved in some loci across plants, particularly in monocots.
Figure 8.
Some loci exhibit conserved imprinting in plants. A, Venn diagram analysis of imprinting conservation in plants. Only a few of the most similar rice homologs (top one hit) of the imprinted genes found in maize, sorghum, and Arabidopsis show imprinting in rice. B, Many of the conserved imprinting loci are imprinted in barley. Conserved imprinted genes refer to those identified from at least two species of rice, maize, sorghum, and Arabidopsis. The top three BLAST hits (e < 10e-10) are used to evaluate imprinting conservation. C, Validation of the imprinting status of 11 conserved imprinted loci in barley. M and B indicate Morex and Bowman, respectively, and the superscripts indicate the polymorphic nucleotides of each allele. Polymorphic sites are highlighted in gray.
DISCUSSION
By generating reciprocal crosses of indica and japonica rice varieties, we identified 208 imprinted genes (Supplemental Table S1). In this study, we compared the rice imprinted genes with those identified from other plant species. We found that 13% to 24% of the rice imprinted genes had imprinted homologs in at least two of the species (Fig. 8A; Supplemental Table S7). This finding is consistent with previous estimates that about 20% of imprinted genes are conserved between species (Waters et al., 2013; Hatorangan et al., 2016). We randomly chose imprinted genes identified from at least two species to detect the imprinting status of their most similar homologs in barley. More than 70% of the genes with informative SNPs showed parent-of-origin expression patterns (Fig. 8B), which revealed conservation of imprinting at some loci in monocots. Klosinska et al. (2016) found that parentally biased expression patterns were conserved between Arabidopsis lyrata and Arabidopsis. Here, we found that 13 out of the 14 imprinted candidates identified from cultivated rice also showed parent-of-origin expression in the cultivated rice and wild rice interspecific reciprocal crosses (Fig. 7, A and B), indicating that the regulation of genomic imprinting in the two rice species is conserved. The process of rice domestication likely imposed limited selection on the evolution of imprinting, although PEGs tended to be selected during indica rice domestication (Fig. 7C).
Allele-specific imprinting has been found in plants (Waters et al., 2013; Pignatta et al., 2014). Here, we discovered 70 imprinted genes showing parent-of-origin-dependent expression in certain genotypes but not in others (Supplemental Table S3). As an example, OsEMF2a, a PRC2 family member, was reported as a nonimprinted gene in Nipponbare/IR64 crosses (Luo et al., 2009). However, we showed that it is a maternally expressed imprinted gene, either in RL-LR or WY-YW. This provided a case that imprinting may change for some important genes in different crosses. Notably, the OsEMF2a corresponding Su(z)12 member, Fertilization Independent Seed2 (FIS2), in Arabidopsis also is imprinted (Jullien et al., 2006).
Auxin is important for triggering central cell division and for maintaining endosperm development (Bernardi et al., 2012; Figueiredo et al., 2015). OsYUCCA11 of rice, ZmYUCCA1 of maize, and AtYUCCA10 of Arabidopsis are orthologs, and all were identified as PEGs (Supplemental Table S7). Tryptophan aminotransferase related1 (TAR1), another auxin biosynthetic gene, was identified as a PEG in several plant species (Supplemental Table S7). OsTAR1 and OsYUCCA11 contribute to auxin accumulation during rice grain development (Abu-Zaitoon et al., 2012). Likewise, ZmYUCCA1 is important for the endosperm development of maize (Bernardi et al., 2012). These findings indicate that genomic imprinting may have evolutionary significance and function directly in auxin-mediated seed development. Xin et al. (2013) also found that many auxin-related genes showed expression bias in maize. In addition, we noted that several genes identified as PEGs in different plant species were associated with epigenetic regulation, including chromatin-remodeling factors, histone H3K9 methyltransferase genes, and variant in methylation genes (Supplemental Table S7). For example, Arabidopsis SU(VAR)3-9 Homolog7 encodes a SET domain protein and acts as a histone methyltransferase for the epigenetic control of gene expression, which is essential for establishing postzygotic hybridization barriers (Wolff et al., 2015). Imprinting is conserved between orthologs of rice and maize, which led us to hypothesize that these genes may contribute to reproductive isolation in both monocots and dicots.
A preferential expression pattern of MEGs in the endosperm was observed in rice (Fig. 4A; Supplemental Fig. S6A) as well as in Arabidopsis, maize, and sorghum (Wolff et al., 2011; Waters et al., 2013; Klosinska et al., 2016; Zhang et al., 2016); however, this preference was not observed for rice PEGs (Fig. 4A; Supplemental Fig. S6B). We inferred that MEGs may be more important for proper endosperm development than PEGs. In agreement with this hypothesis, mutations in PEGs usually were not accompanied by endosperm defects (Wolff et al., 2015), while many MEG mutants exhibited endosperm or seed abnormalities (Chaudhury et al., 1997; Luo et al., 2000; Tiwari et al., 2008; Fitz Gerald et al., 2009; Costa et al., 2012; Liu et al., 2014). Rice PEGs showed higher GC methylation of gene bodies in tissues other than endosperm (Supplemental Fig. S8A), which is consistent with the finding that the CG methylation of gene bodies correlates with transcriptional activity (Zilberman et al., 2007).
Studies from Brassicaceae species revealed that many imprinted genes often were located physically in the vicinity of TEs (Wolff et al., 2011; Pignatta et al., 2014; Hatorangan et al., 2016). TEs were found to be activated in central cells as well as in the vegetative cells of pollen (Slotkin et al., 2009; Schoft et al., 2011; Calarco et al., 2012; Ibarra et al., 2012). Genomic imprinting was believed to be associated with the dynamic regulation of TEs in the reproductive cells and endosperm (Gehring et al., 2009). The asymmetrical activation of TEs in the central and sperm cell leads to monoallelic expression after fertilization. However, our study suggested that TEs are not more abundant near imprinted rice genes (Fig. 3). Interestingly, rice PEGs showed a lower association with TEs than MEGs (Fig. 3). This is contrary to the findings in Arabidopsis (Pignatta et al., 2014). Unlike the gene silencing observed in Arabidopsis, many TEs are not silenced in the sperm cells of rice (Anderson et al., 2013). We assumed that the regulation of imprinting in rice may not be the same as that found in Arabidopsis. Notably, in comparison with the PEGs, the promoters of MEGs showed higher methylation in endosperm, which contrasts with findings from Arabidopsis and maize (Pignatta et al., 2014; Zhang et al., 2014). However, this result is consistent with our observation that the rice MEGs have a higher probability of neighboring with TEs in the promoter regions than in the rice PEGs (Fig. 3). In agreement with previous findings in Arabidopsis and maize (Zhang et al., 2014; Moreno-Romero et al., 2016), our methylomic analysis and AZA treatment revealed that the activation of the MEGs showed more association with DNA hypomethylation (Fig. 5), while H3K27me3 modification is more important for the imprinting of PEGs (Supplemental Fig. S10). Further investigation of the differential DNA methylation and histone modification between parental alleles is necessary.
Many MEGs are activated before fertilization in Arabidopsis. For example, the PRC2 components MEA and FIS2 are highly expressed in central cells and gradually repressed after fertilization (Luo et al., 2000; Baroux et al., 2006). The hypomethylated environment of the central cell (Gehring et al., 2006; Jullien et al., 2006; Park et al., 2016) promotes maternal allele expression in the endosperm, whereas the paternal allele remains hypermethylated and silenced in the sperm cell and endosperm. According to this model, MEG expression should persist immediately after fertilization. However, we found many rice MEGs that were activated specifically at 3 to 4 DAF in the endosperm (Fig. 4B). As an example, the PRC2 component gene OsFIE1 is an exclusively endosperm-expressed gene (Zhang et al., 2012a), but OsFIE1 was not detectable until 3 DAF (Supplemental Fig. S6D). Although we do not have evidence showing OsFIE1 to be absent in central cells, our findings indicated that the regulation of imprinting in OsFIE1 is distinct from that of MEA and FIS2 in Arabidopsis. Extensive activation of imprinted genes after fertilization also was observed in maize (Xin et al., 2013). The Arabidopsis MEG Formin5 was activated specifically in the endosperm (Fitz Gerald et al., 2009). These findings suggest that, for some MEGs, differential imprinting between two parental alleles is expressed in the endosperm but not in germ cells. Xing et al. (2015) found a dramatic decrease in global DNA methylation in the endosperm of rice from 2 to 3 DAF. The methylation level of endosperm is even lower than that of the central cell (Park et al., 2016). Imprinting of these genes could be caused by asymmetric DNA demethylation in the endosperm, or the regulator of these genes is expressed only after fertilization.
We also noticed that several MEGs were expressed in sperm cells (Supplemental Fig. S7) but their paternal alleles were silenced after fertilization. In addition to the establishment of imprinting in the central cell, this finding suggests that plants can apply selective imprinting markers to the paternal genome to repress gene expression after fertilization. The underlying mechanism needs further investigation. We found that more than 50% of PEGs were expressed in sperm cells (Fig. 4A). Anderson et al. (2013) showed low expression of Methyltransferase1 and high expression of ROS1a, a DNA glycosylase reversing DNA methylation, in rice sperm cells. These results suggested that, similar to what happens in central cells, the hypomethylated environment in the sperm cell facilitates the paternal monoallelic expression of PEGs in the endosperm. The RNA-directed DNA methylation pathway regulates paternal genomic imprinting in Arabidopsis (Vu et al., 2013). However, by surveying the transcriptome, Anderson et al. (2013) revealed no evidence for RNA interference activity in sperm cells of rice. Nonetheless, our result indicates that the 24-nucleotide sRNAs may be involved in the regulation of genomic imprinting in rice (Supplemental Table S6). The genic vicinities of imprinted sRNAs generally showed opposite parental expression patterns relative to the imprinted sRNAs (Fig. 7, A and B).
Several imprinted genes found in rice have been studied functionally. Among them, GW2 and OsPPKL2 are characterized as two quantitative trait loci that determine seed size in rice, and they also function in grain filling and starch accumulation (Song et al., 2007; Zhang et al., 2012b). Gibberellin-Regulated Calcineurin B-like2 of rice is involved in the development of the aleurone layer (Hwang et al., 2005). Notably, Os8N3, which shows copper reallocation activity, was identified initially as a disease-resistance gene and then found to be essential for pollen and seed development (Yang et al., 2006; Yuan et al., 2010). The Carbon Starved Anther gene triggers the expression of sugar partitioning and metabolic genes during pollen and seed development (Zhang et al., 2010). These genes appear to be involved in the reallocation of resources and nutrients from maternal tissues to offspring, which provides support for the kinship theory and suggests that at least some imprinted genes are indispensable for proper seed development. Interestingly, the activation of many imprinted genes in the endosperm at 3 to 4 DAF (Fig. 4B; Supplemental Fig. S6) coincides with the cellularization event in rice (Ishikawa et al., 2011; Chen et al., 2016). Genetic evidence showed that rice MADS box gene87, a transiently activated MEG, can promote endosperm proliferation and repress its cellularization (Ishikawa et al., 2011; Chen et al., 2016). In contrast, the MEG OsFIE1 promotes cellularization (Folsom et al., 2014). In conclusion, many imprinted genes in rice appear to have biological significance for rice seed development (Yuan et al., 2017).
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Cultivated rice (Oryza sativa) Wufeng-A and -B, Rongfeng-A and -B, Liuqianxin-A and -B, Yu6-A and -B, and wild rice (Oryza rufipogon) were grown in Nanchang, Jiangxi Province, China. Wild rice typically is an outcrossing plant. Therefore, to avoid variation within the genome, Dongxiang wild rice was self-fertilized by bagging for six generations and then used for crossing. The RL-LR and WY-YW reciprocal crosses were performed in the summer of 2014. Plants were grown in a paddy field under regular conditions. The ovary donors were emasculated before flowering and moved to a growth chamber on a 14-h/10-h light/dark cycle at 30°C. At 5 DAF, the endosperm was carefully collected with a pipette to avoid maternal tissue contamination according to the method used by Luo et al. (2011). Two (WY-YW) or three (LR-RL) replicates were used for the mining of imprinted genes. Each replicate was pooled with 20 to 30 endosperm. The japonica varieties Nipponbare and Kitaake were grown in a growth chamber (14-h/10-h light/dark cycle, 30°C) and labeled when flowering. The caryopsis at 0 to 3 DAF or endosperm at 5 to 7 DAF were collected for RNA isolation. Barley (Hordeum vulgare) varieties Morex and Bowman were planted in an experimental plot in Chengdu, Sichuan Province, China, with regular nutrient and water management. Reciprocal crosses were performed in the field. The ovary donors were emasculated before flowering. At 10 DAF, the endosperm of the hybrid seeds was collected.
For the AZA treatment assay, Kitaake seeds were sterilized and grown on one-half-strength Murashige and Skoog medium with 70 mg L−1 AZA for 10 d in a growth chamber at 28°C and a 14-h/10-h light/dark cycle. The controls were grown under the same conditions, except that the medium was AZA free. Seedlings were collected for RNA-seq. Three biological replicates were used, each one comprising approximately 10 individuals.
DNA and RNA Isolation and Quantitative PCR Assay
DNA was extracted from 100 mg of leaves from Liuqianxin-A, Yu6-A, RR, and WW plants using the EasyPure Plant Genomic DNA Kit (Trans) following the manufacturer’s suggested procedures. RNA was extracted from endosperm or seedlings using the Plant RNA Kit (Omega) and treated with an RNA-free DNase set (Omega) to remove DNA contamination according to the manufacturer’s protocol.
cDNA was synthesized using PrimeScript RT Master Mix (Takara). Two microliters of the 1:5 diluted cDNA was used for quantitative PCR in a 20-μL reaction using AceQ qPCR SYBR Green Master Mix (Vazyme). The reactions were performed using the CFX Connect Real-Time System (Bio-Rad). The proteasome gene (LOC_Os03g63430) was used as an endogenous control. For each sample, we performed three independent biological replicates with three technical replicates each. Each biological replicate was pooled with 20 to 30 endosperm. The relative expression level was calculated by the ΔΔCt approach. All primers used in this study can be found in Supplemental Table S8.
DNA Resequencing, RNA-Seq, and Imprinted Gene Identification
Sheared DNA was used for library preparation following the protocols for the NEBNext Ultra DNA Library Prep Kit for Illumina. The libraries were quantified using a Qubit 2.0 Fluorometer and Agilent Bioanalyzer 2100 (Agilent Technologies) and then sequenced on the Illumina HiSeq-2500 platform. The integrity of the purified RNA was inspected using the Agilent Bioanalyzer 2100 (Agilent Technologies) and subjected to RNA-seq library preparation with the TruSeq RNA Kit version 2 (Illumina). Libraries were sequenced on the Illumina HiSeq platform.
Raw reads were processed using the Trimmomatic version 0.32 package to remove adaptors and discard low-quality reads. Filtered reads were aligned to the rice reference genome (MSU version 7.0; rice.plantbiology.msu.edu) using Bowtie2. Two mismatches were allowed for alignments. Reads that mapped to multiple sites were discarded. GATK was used to process aligned reads for SNP calling. The obtained SNPs were filtered by genome coverage, and SNPs with depths < 11 were removed. RNA alignment was carried out by Bowtie2. Then, samtools (version 0.1.16) was used to process the aligned reads and call SNPs with default parameters. RNA SNPs were aligned to DNA SNPs with in-house Perl script, with depths < 11 removed. Reads that aligned to multiple loci were discarded. Imprinted genes were identified by calculating the ratio of maternal to paternal reads at each SNP site that deviated from the expected 2:1 ratio using χ2 tests.
Validation of Imprinted Genes
Primers were designed to amplify 300- to 500-bp gene fragments harboring informative SNPs from the cDNA of reciprocal hybrids and of the parents. The PCR products were purified for Sanger sequencing or cloned into pEAZY-Blunt Zero (Trans) for a single-colony sequencing assay; at least 12 colonies were sequenced. For the validation of GW2 and OsPPKL2 using pyrosequencing, genes were amplified with 2× EPIK Amplification Mix (Bioline) using cDNA as the template. Fifteen- to 20-μL PCR products were sequenced using the PyroMark Q96 (Qiagen) platform. The primers used for the assays are listed in Supplemental Table S8.
Epigenetic Analysis of Imprinted Genes
Rice bisulfite-sequencing data from root, shoot, embryo, and endosperm were obtained from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database (accession no. GSE22591). The reads were aligned to the rice reference genome using Bismark Extractor software, allowing two mismatches per read. Reads that mapped to multiple positions were discarded. The average methylation level was calculated at each C as CG, CHG, and CHH of the PEGs, MEGs, MEG homologs, and PEG homologs and visualized in each 50-bp bin.
Data on H3K27me3 in rice endosperm were obtained from the NCBI GEO database (accession no. GSE27048). Reads were aligned to the rice genome using Bowtie2. Two mismatches per read were allowed, and reads that mapped to more than one site were removed. The program MACS was used to process aligned reads and call peaks as described by Malone et al. (2011). Peaks with fold change greater than 5 in comparison with the mock treatment and q < 0.05 were kept for further analysis.
Bisulfite Sequencing
Equal amounts of genomic DNA isolated from 6-DAF endosperm and embryo were treated with sodium bisulfite, according to the protocol provided by the manufacturer (EZ DNA Methylation Kit; Zymo Research). Two microliters of the treated DNA was used as a template for PCR. The primers used are listed in Supplemental Table S8. The PCR products were then cloned into pEAZY-Blunt Zero (Trans), and at least 10 colonies were selected for Sanger sequencing.
Characterization of Imprinted Genes
Information on imprinted sRNAs in rice endosperm was obtained from previous studies (Rodrigues et al., 2013; Yuan et al., 2017). Gene expression data were obtained from Genevestigator, RiceXPro (http://ricexpro.dna.affrc.go.jp), and NCBI GEO (accession nos. GSE50777 and GSE40674). Expression profiles of the imprinted genes in different tissues were generated by Genevestigator, using the complete expression data set from wild-type rice collected by Genevestigator. We used the transcriptome data of early-developing seeds from rice deposited in RiceXPro (http://ricexpro.dna.affrc.go.jp) to analyze the expression patterns of the shared imprinted genes among RL-LR, WY-YW, and N9-9N. A Venn diagram was created using the Web-based tool VENNY 2.1 (http://bioinfogp.cnb.csic.es/tools/venny/index.html). GO analysis was performed with the online Singular Enrichment Analysis tool provided by AgriGO (http://bioinfo.cau.edu.cn/agriGO/). Gene clustering analysis was conducted with the software REEF (Coppe et al., 2006) with window size 100 kb, step size 50 kb, and default statistical settings. Genes expressed in the endosperm at 5 DAF that showed polymorphisms between the RL-LR and WY-YW parents were used as the reference for the GO analysis and the clustering analysis. For the annotation of TEs, we downloaded all of the TE families in rice (http://genome.arizona.edu/cgi-bin/rite/index.cgi) as the source for RepeatModeler modeling to identify repeat element boundaries and family relationships. We then combined the database with the Dfam database and used RepeatMasker to screen DNA sequences for TEs in Nipponbare.
Differential Expression Analysis
The raw reads were filtered by Seqtk before mapping to the genome using Tophat (version 2.0.9). Gene fragments were counted using HTSeq followed by TMM (trimmed mean of M values) normalization. Significantly differentially expressed genes were identified based on an FDR value above the threshold (q < 0.05) and fold change greater than 2 using edgeR software.
Evolutionary Analysis of Imprinted Genes
We used the published resequencing data from 50 japonica and 25 wild rice (Xu et al., 2011) to calculate the polymorphisms across all W1-type wild rice and indica, and W2-type wild rice and japonica, to test for the selection of the imprinted genes in rice. The sequence diversity ratio in each 5-kb sliding window (2.5-kb step size) was calculated using License (version 3.0). A Mann-Whitney rank sum test was used to determine statistical significance in SigmaPlot 12.5.
Accession Numbers
The RNA-seq data have been deposited into the GEO database (GSE113769; http://www.ncbi.nlm.nih.gov/geo).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Validation of the imprinting status of selected candidates.
Supplemental Figure S2. OsEMF2a is a MEG in rice.
Supplemental Figure S3. Heat map of the proportion of maternal alleles of all the imprinted genes in different cross combinations.
Supplemental Figure S4. Distribution of imprinted clusters in the rice genome.
Supplemental Figure S5. Confirmation of the expression patterns of randomly selected MEGs and PEGs by quantitative PCR.
Supplemental Figure S6. Expression profiles of the high-convincing imprinted candidates (RL-LR, WY-YW, and N9-9N commons) and their most similar homologs in rice.
Supplemental Figure S7. Gene expression of the imprinted candidates in reproductive cells or tissues.
Supplemental Figure S8. DNA methylation profiles of the imprinted genes (RL-LR and WY-YW commons) in root, shoot, embryo, and endosperm in the context of CG, CHG, and CHH in rice.
Supplemental Figure S9. DNA methylation in promoter regions of the randomly selected imprinted genes.
Supplemental Figure S10. DNA methylation profiles of the imprinted genes (RL-LR, WY-YW, N9-9N, and L0-0L commons) and their most similar homologs in embryo and endosperm in the context of CG, CHG, and CHH of rice.
Supplemental Figure S11. Differential responses of PEGs and MEGs to the methylation inhibitor AZA in rice seedlings.
Supplemental Figure S12. DNA methylation of the imprinted genes colocalized with imprinted small RNAs.
Supplemental Table S1. Imprinted genes identified in rice.
Supplemental Table S2. Epigenetic and auxin-related imprinted genes of rice.
Supplemental Table S3. Allele-specific imprinting of rice.
Supplemental Table S4. Imprinted clusters of rice.
Supplemental Table S5. Enrichment of H3K27me3 in the 2-kb flanking regions of imprinted genes in rice endosperm
Supplemental Table S6. Imprinted genes adjacent to imprinted small RNAs in rice.
Supplemental Table S7. Conservation of some imprinted loci in different plant species.
Supplemental Table S8. Primers used in this study.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Footnotes
This research was supported by grants from the National Key R&D Program of China (2016YFD0100902 to C.C.), Natural Science Foundation of Jiangsu Province (BK20150446 to C.C.), National Natural Science Foundation of China (31571623 to C.C. and 31671964 to R.C.), and the Priority Academic Development of Jiangsu Higher Education Institutions.
Articles can be viewed without a subscription.
References
- Abu-Zaitoon YM, Bennett K, Normanly J, Nonhebel HM (2012) A large increase in IAA during development of rice grains correlates with the expression of tryptophan aminotransferase OsTAR1 and a grain-specific YUCCA. Physiol Plant 146: 487–499 [DOI] [PubMed] [Google Scholar]
- Anderson SN, Johnson CS, Jones DS, Conrad LJ, Gou X, Russell SD, Sundaresan V (2013) Transcriptomes of isolated Oryza sativa gametes characterized by deep sequencing: evidence for distinct sex-dependent chromatin and epigenetic states before fertilization. Plant J 76: 729–741 [DOI] [PubMed] [Google Scholar]
- Baroux C, Gagliardini V, Page DR, Grossniklaus U (2006) Dynamic regulatory interactions of Polycomb group genes: MEDEA autoregulation is required for imprinted gene expression in Arabidopsis. Genes Dev 20: 1081–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi J, Lanubile A, Li QB, Kumar D, Kladnik A, Cook SD, Ross JJ, Marocco A, Chourey PS (2012) Impaired auxin biosynthesis in the defective endosperm18 mutant is due to mutational loss of expression in the ZmYuc1 gene encoding endosperm-specific YUCCA1 protein in maize. Plant Physiol 160: 1318–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratzel F, Yang C, Angelova A, López-Torrejón G, Koch M, del Pozo JC, Calonje M (2012) Regulation of the new Arabidopsis imprinted gene AtBMI1C requires the interplay of different epigenetic mechanisms. Mol Plant 5: 260–269 [DOI] [PubMed] [Google Scholar]
- Calarco JP, Borges F, Donoghue MTA, Van Ex F, Jullien PE, Lopes T, Gardner R, Berger F, Feijó JA, Becker JD, et al. (2012) Reprogramming of DNA methylation in pollen guides epigenetic inheritance via small RNA. Cell 151: 194–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhury AM, Ming L, Miller C, Craig S, Dennis ES, Peacock WJ (1997) Fertilization-independent seed development in Arabidopsis thaliana. Proc Natl Acad Sci USA 94: 4223–4228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Begcy K, Liu K, Folsom JJ, Wang Z, Zhang C, Walia H (2016) Heat stress yields a unique MADS box transcription factor in determining seed size and thermal sensitivity. 171: 606–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y, Harada JJ, Goldberg RB, Fischer RL (2004) An invariant aspartic acid in the DNA glycosylase domain of DEMETER is necessary for transcriptional activation of the imprinted MEDEA gene. Proc Natl Acad Sci USA 101: 7481–7486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe A, Danieli GA, Bortoluzzi S (2006) REEF: searching REgionally Enriched Features in genomes. BMC Bioinformatics 7: 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LM, Yuan J, Rouster J, Paul W, Dickinson H, Gutierrez-Marcos JF (2012) Maternal control of nutrient allocation in plant seeds by genomic imprinting. Curr Biol 22: 160–165 [DOI] [PubMed] [Google Scholar]
- Edwards CA, Ferguson-Smith AC (2007) Mechanisms regulating imprinted genes in clusters. Curr Opin Cell Biol 19: 281–289 [DOI] [PubMed] [Google Scholar]
- Figueiredo DD, Batista RA, Roszak PJ, Köhler C (2015) Auxin production couples endosperm development to fertilization. Nat Plants 1: 15184. [DOI] [PubMed] [Google Scholar]
- Fitz Gerald JN, Hui PS, Berger F (2009) Polycomb group-dependent imprinting of the actin regulator AtFH5 regulates morphogenesis in Arabidopsis thaliana. Development 136: 3399–3404 [DOI] [PubMed] [Google Scholar]
- Florez-Rueda AM, Paris M, Schmidt A, Widmer A, Grossniklaus U, Städler T (2016) Genomic imprinting in the endosperm is systematically perturbed in abortive hybrid tomato seeds. Mol Biol Evol 33: 2935–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsom JJ, Begcy K, Hao X, Wang D, Walia H (2014) Rice Fertilization-Independent Endosperm1 regulates seed size under heat stress by controlling early endosperm development. Plant Physiol 165: 238–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M. (2013) Genomic imprinting: insights from plants. Annu Rev Genet 47: 187–208 [DOI] [PubMed] [Google Scholar]
- Gehring M, Huh JH, Hsieh TF, Penterman J, Choi Y, Harada JJ, Goldberg RB, Fischer RL (2006) DEMETER DNA glycosylase establishes MEDEA polycomb gene self-imprinting by allele-specific demethylation. Cell 124: 495–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M, Bubb KL, Henikoff S (2009) Extensive demethylation of repetitive elements during seed development underlies gene imprinting. Science 324: 1447–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring M, Missirian V, Henikoff S (2011) Genomic analysis of parent-of-origin allelic expression in Arabidopsis thaliana seeds. PLoS ONE 6: e23687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haig D, Westoby M (1991) Genomic imprinting in endosperm: its effect on seed development in crosses between species, and between different ploidies of the same species, and its implications for the evolution of apomixis. Philos Trans R Soc B Biol Sci 333: 1–13 [Google Scholar]
- Hatorangan MR, Laenen B, Steige KA, Slotte T, Köhler C (2016) Rapid evolution of genomic imprinting in two species of the Brassicaceae. Plant Cell 28: 1815–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh TF, Shin J, Uzawa R, Silva P, Cohen S, Bauer MJ, Hashimoto M, Kirkbride RC, Harada JJ, Zilberman D, et al. (2011) Regulation of imprinted gene expression in Arabidopsis endosperm. Proc Natl Acad Sci USA 108: 1755–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Kurata N, Wei X, Wang ZX, Wang A, Zhao Q, Zhao Y, Liu K, Lu H, Li W, et al. (2012) A map of rice genome variation reveals the origin of cultivated rice. Nature 490: 497–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JH, Bauer MJ, Hsieh TF, Fischer RL (2008) Cellular programming of plant gene imprinting. Cell 132: 735–744 [DOI] [PubMed] [Google Scholar]
- Hwang YS, Bethke PC, Cheong YH, Chang HS, Zhu T, Jones RL (2005) A gibberellin-regulated calcineurin B in rice localizes to the tonoplast and is implicated in vacuole function. Plant Physiol 138: 1347–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarra CA, Feng X, Schoft VK, Hsieh TF, Uzawa R, Rodrigues JA, Zemach A, Chumak N, Machlicova A, Nishimura T, et al. (2012) Active DNA demethylation in plant companion cells reinforces transposon methylation in gametes. Science 337: 1360–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa R, Ohnishi T, Kinoshita Y, Eiguchi M, Kurata N, Kinoshita T (2011) Rice interspecies hybrids show precocious or delayed developmental transitions in the endosperm without change to the rate of syncytial nuclear division. Plant J 65: 798–806 [DOI] [PubMed] [Google Scholar]
- Jullien PE, Kinoshita T, Ohad N, Berger F (2006) Maintenance of DNA methylation during the Arabidopsis life cycle is essential for parental imprinting. Plant Cell 18: 1360–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klosinska M, Picard CL, Gehring M (2016) Conserved imprinting associated with unique epigenetic signatures in the Arabidopsis genus. Nat Plants 2: 16145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C, Weinhofer-Molisch I (2010) Mechanisms and evolution of genomic imprinting in plants. Heredity (Edinb) 105: 57–63 [DOI] [PubMed] [Google Scholar]
- Köhler C, Page DR, Gagliardini V, Grossniklaus U (2005) The Arabidopsis thaliana MEDEA Polycomb group protein controls expression of PHERES1 by parental imprinting. Nat Genet 37: 28–30 [DOI] [PubMed] [Google Scholar]
- Köhler C, Wolff P, Spillane C (2012) Epigenetic mechanisms underlying genomic imprinting in plants. Annu Rev Plant Biol 63: 331–352 [DOI] [PubMed] [Google Scholar]
- Liu P, Qi M, Wang Y, Chang M, Liu C, Sun M, Yang W, Ren H (2014) Arabidopsis RAN1 mediates seed development through its parental ratio by affecting the onset of endosperm cellularization. Mol Plant 7: 1316–1328 [DOI] [PubMed] [Google Scholar]
- Luo M, Bilodeau P, Dennis ES, Peacock WJ, Chaudhury A (2000) Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proc Natl Acad Sci USA 97: 10637–10642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Platten D, Chaudhury A, Peacock WJ, Dennis ES (2009) Expression, imprinting, and evolution of rice homologs of the polycomb group genes. Mol Plant 2: 711–723 [DOI] [PubMed] [Google Scholar]
- Luo M, Taylor JM, Spriggs A, Zhang H, Wu X, Russell S, Singh M, Koltunow A (2011) A genome-wide survey of imprinted genes in rice seeds reveals imprinting primarily occurs in the endosperm. PLoS Genet 7: e1002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone BM, Tan F, Bridges SM, Peng Z (2011) Comparison of four ChIP-seq analytical algorithms using rice endosperm H3K27 trimethylation profiling data. PLoS ONE 6: e25260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Romero J, Jiang H, Santos-González J, Köhler C (2016) Parental epigenetic asymmetry of PRC2-mediated histone modifications in the Arabidopsis endosperm. EMBO J 35: 1298–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Kim MY, Vickers M, Park JS, Hyun Y, Okamoto T, Zilberman D, Fischer RL, Feng X, Choi Y, et al. (2016) DNA demethylation is initiated in the central cells of Arabidopsis and rice. Proc Natl Acad Sci USA 113: 15138–15143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatta D, Erdmann RM, Scheer E, Picard CL, Bell GW, Gehring M (2014) Natural epigenetic polymorphisms lead to intraspecific variation in Arabidopsis gene imprinting. eLife 3: e03198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires ND, Grossniklaus U (2014) Different yet similar: evolution of imprinting in flowering plants and mammals. F1000Prime Rep 6: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raissig MT, Bemer M, Baroux C, Grossniklaus U (2013) Genomic imprinting in the Arabidopsis embryo is partly regulated by PRC2. PLoS Genet 9: e1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues JA, Zilberman D (2015) Evolution and function of genomic imprinting in plants. Genes Dev 29: 2517–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues JA, Ruan R, Nishimura T, Sharma MK, Sharma R, Ronald PC, Fischer RL, Zilberman D (2013) Imprinted expression of genes and small RNA is associated with localized hypomethylation of the maternal genome in rice endosperm. Proc Natl Acad Sci USA 110: 7934–7939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoft VK, Chumak N, Choi Y, Hannon M, Garcia-Aguilar M, Machlicova A, Slusarz L, Mosiolek M, Park JS, Park GT, et al. (2011) Function of the DEMETER DNA glycosylase in the Arabidopsis thaliana male gametophyte. Proc Natl Acad Sci USA 108: 8042–8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirzadi R, Andersen ED, Bjerkan KN, Gloeckle BM, Heese M, Ungru A, Winge P, Koncz C, Aalen RB, Schnittger A, et al. (2011) Genome-wide transcript profiling of endosperm without paternal contribution identifies parent-of-origin-dependent regulation of AGAMOUS-LIKE36. PLoS Genet 7: e1001303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin RK, Vaughn M, Borges F, Tanurdzić M, Becker JD, Feijó JA, Martienssen RA (2009) Epigenetic reprogramming and small RNA silencing of transposable elements in pollen. Cell 136: 461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XJ, Huang W, Shi M, Zhu MZ, Lin HX (2007) A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase. Nat Genet 39: 623–630 [DOI] [PubMed] [Google Scholar]
- Tiwari S, Schulz R, Ikeda Y, Dytham L, Bravo J, Mathers L, Spielman M, Guzmán P, Oakey RJ, Kinoshita T, et al. (2008) MATERNALLY EXPRESSED PAB C-TERMINAL, a novel imprinted gene in Arabidopsis, encodes the conserved C-terminal domain of polyadenylate binding proteins. Plant Cell 20: 2387–2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu TM, Nakamura M, Calarco JP, Susaki D, Lim PQ, Kinoshita T, Higashiyama T, Martienssen RA, Berger F (2013) RNA-directed DNA methylation regulates parental genomic imprinting at several loci in Arabidopsis. Development 140: 2953–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Makarevitch I, Eichten SR, Swanson-Wagner RA, Yeh CT, Xu W, Schnable PS, Vaughn MW, Gehring M, Springer NM (2011) Parent-of-origin effects on gene expression and DNA methylation in the maize endosperm. Plant Cell 23: 4221–4233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Bilinski P, Eichten SR, Vaughn MW, Ross-Ibarra J, Gehring M, Springer NM (2013) Comprehensive analysis of imprinted genes in maize reveals allelic variation for imprinting and limited conservation with other species. Proc Natl Acad Sci USA 110: 19639–19644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff P, Weinhofer I, Seguin J, Roszak P, Beisel C, Donoghue MTA, Spillane C, Nordborg M, Rehmsmeier M, Köhler C (2011) High-resolution analysis of parent-of-origin allelic expression in the Arabidopsis endosperm. PLoS Genet 7: e1002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff P, Jiang H, Wang G, Santos-González J, Köhler C (2015) Paternally expressed imprinted genes establish postzygotic hybridization barriers in Arabidopsis thaliana. eLife 4: 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Yang R, Li G, Chen H, Laurie J, Ma C, Wang D, Yao Y, Larkins BA, Sun Q, et al. (2013) Dynamic expression of imprinted genes associates with maternally controlled nutrient allocation during maize endosperm development. Plant Cell 25: 3212–3227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing MQ, Zhang YJ, Zhou SR, Hu WY, Wu XT, Ye YJ, Wu XX, Xiao YP, Li X, Xue HW (2015) Global analysis reveals the crucial roles of DNA methylation during rice seed development. Plant Physiol 168: 1417–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Dai M, Li F, Liu A (2014) Genomic imprinting, methylation and parent-of-origin effects in reciprocal hybrid endosperm of castor bean. Nucleic Acids Res 42: 6987–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Liu X, Ge S, Jensen JD, Hu F, Li X, Dong Y, Gutenkunst RN, Fang L, Huang L, et al. (2011) Resequencing 50 accessions of cultivated and wild rice yields markers for identifying agronomically important genes. Nat Biotechnol 30: 105–111 [DOI] [PubMed] [Google Scholar]
- Yang B, Sugio A, White FF (2006) Os8N3 is a host disease-susceptibility gene for bacterial blight of rice. Proc Natl Acad Sci USA 103: 10503–10508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Chen S, Jiao W, Wang L, Wang L, Ye W, Lu J, Hong D, You S, Cheng Z, et al. (2017) Both maternally and paternally imprinted genes regulate seed development in rice. New Phytol 216: 373–387 [DOI] [PubMed] [Google Scholar]
- Yuan M, Chu Z, Li X, Xu C, Wang S (2010) The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell 22: 3164–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemach A, Kim MY, Silva P, Rodrigues JA, Dotson B, Brooks MD, Zilberman D (2010) Local DNA hypomethylation activates genes in rice endosperm. Proc Natl Acad Sci USA 107: 18729–18734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Liang W, Yang X, Luo X, Jiang N, Ma H, Zhang D (2010) Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. Plant Cell 22: 672–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Cheng Z, Qin R, Qiu Y, Wang JL, Cui X, Gu L, Zhang X, Guo X, Wang D, et al. (2012a) Identification and characterization of an epi-allele of FIE1 reveals a regulatory linkage between two epigenetic marks in rice. Plant Cell 24: 4407–4421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhao H, Xie S, Chen J, Xu Y, Wang K, Zhao H, Guan H, Hu X, Jiao Y, et al. (2011) Extensive, clustered parental imprinting of protein-coding and noncoding RNAs in developing maize endosperm. Proc Natl Acad Sci USA 108: 20042–20047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Xie S, Dong X, Zhao X, Zeng B, Chen J, Li H, Yang W, Zhao H, Wang G, et al. (2014) Genome-wide high resolution parental-specific DNA and histone methylation maps uncover patterns of imprinting regulation in maize. Genome Res 24: 167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Li N, He W, Zhang H, Yang W, Liu B (2016) Genome-wide screen of genes imprinted in sorghum endosperm, and the roles of allelic differential cytosine methylation. Plant J 85: 424–436 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang J, Huang J, Lan H, Wang C, Yin C, Wu Y, Tang H, Qian Q, Li J, et al. (2012b) Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc Natl Acad Sci USA 109: 21534–21539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberman D, Gehring M, Tran RK, Ballinger T, Henikoff S (2007) Genome-wide analysis of Arabidopsis thaliana DNA methylation uncovers an interdependence between methylation and transcription. Nat Genet 39: 61–69 10.1038/ng1929 [DOI] [PubMed] [Google Scholar]