A foxtail mosaic virus vector allows the rapid expression of heterologous proteins of up to 600 amino acids in length in wheat and maize.
Abstract
Rapid and cost-effective virus-derived transient expression systems for plants are invaluable in elucidating gene function and are particularly useful in plant species for which transformation-based methods are unavailable or are too time and labor demanding, such as wheat (Triticum aestivum) and maize (Zea mays). The virus-mediated overexpression (VOX) vectors based on Barley stripe mosaic virus and Wheat streak mosaic virus described previously for these species are incapable of expressing free recombinant proteins of more than 150 to 250 amino acids, are not suited for high-throughput screens, and have other limitations. In this study, we report the development of a VOX vector based on a monopartite single-stranded positive sense RNA virus, Foxtail mosaic virus (genus Potexvirus). In this vector, PV101, the gene of interest was inserted downstream of the duplicated subgenomic promoter of the viral coat protein gene, and the corresponding protein was expressed in its free form. The vector allowed the expression of a 239-amino acid-long GFP in both virus-inoculated and upper uninoculated (systemic) leaves of wheat and maize and directed the systemic expression of a larger approximately 600-amino acid protein, GUSPlus, in maize. Moreover, we demonstrated that PV101 can be used for in planta expression and functional analysis of apoplastic pathogen effector proteins such as the host-specific toxin ToxA of Parastagonospora nodorum. Therefore, this VOX vector opens possibilities for functional genomics studies in two important cereal crops.
A rapid increase in the use of next-generation genome and transcriptome sequencing technologies has facilitated the identification of long lists of candidate genes underlying traits of specific interest in plants and plant-associated organisms. These genes require functional characterization, and there has been an increasing demand for transient in planta expression systems that allow the rapid and cost-effective expression of recombinant proteins or RNA interference (RNAi)/silencing of endogenous plant genes.
Transient in planta expression systems using plant virus-mediated overexpression (VOX) vectors can provide the rapid production of heterologous recombinant proteins. Many plant viruses and primarily those with (+)-sense single-strand (ss) RNA genomes have been cloned and modified to express foreign peptides and proteins in planta. One system that is frequently used is Potato virus X (PVX [genus Potexvirus]; Chapman et al., 1992) in the model dicot species Nicotiana benthamiana. Important uses of VOX vectors include the investigation and manipulation of metabolic pathways (Majer et al., 2017), functional characterization of host disease resistance genes and pathogen effector proteins (Manning et al., 2010), and cellular protein localization studies (Zhang et al., 2013).
Plant (+)-ssRNA viruses also have been modified as vectors and used extensively for transient RNAi in virus-induced gene silencing (VIGS), which exploits an endogenous antiviral RNAi machinery to down-regulate the expression of endogenous plant genes. Until recently, only one plant virus has been used extensively for VIGS in wheat (Triticum aestivum), namely Barley stripe mosaic virus (BSMV; for review, see Lee et al., 2012). The same virus also has been adapted for VOX (Lee et al., 2012; Xu et al., 2015). Although BSMV-mediated VIGS and VOX in wheat work well, these vector systems have several limitations. First, BSMV has a tripartite RNA genome consisting of RNAs α, β, and γ, all of which need to be present in the same plant cell to initiate infection. Heterologous genes or gene fragments are usually inserted into RNAγ for expression (Yuan et al., 2011). The tripartite genome and a need to combine all three genomic RNAs for plant inoculation make the BSMV expression system only relatively low throughput. Second, as reported by different authors and confirmed in our laboratory, BSMV vectors carrying inserts larger than 450 to 500 bp are relatively unstable and also may show reduced accumulation and cell-to-cell and systemic movement in the infected plants. Although this size constraint may not be a problem for VIGS, this limits the application of VOX for the expression of relatively small (150 amino acids or less) proteins, reducing the range of possible VOX applications. Third, BSMV induces conspicuous, sometimes moderate to severe chlorotic/necrotic mosaic symptoms (depending on the host genotype), which is undesirable and can hinder the phenotypic assessment of host plants, especially when investigating cell death-related genes and pathways. Fourth, BSMV-VOX only allows the production of recombinant proteins as direct C-terminal fusions with the viral γb protein. Although some fusion-free heterologous protein can be obtained by the introduction of the self-cleaving 2A peptide immediately downstream of γb, the cleavage is rarely complete, and fusion-related cotranslational processing may interfere with the protein’s localization and/or intrinsic activity.
Two viruses in the family Potyviridae, namely Wheat streak mosaic virus (WSMV) and Triticum mosaic virus (TriMV), have been engineered to express the fluorescent reporter proteins GFP and RFP, allowing the monitoring of virus spread throughout infected tissues in wheat and maize (Zea mays) and enabling fundamental studies on virus infection biology and on mechanisms of disease resistance (Choi et al., 2000; Tatineni et al., 2015). Only TriMV allows the expression of soluble GFP and RFP (Tatineni et al., 2015), while heterologous proteins produced using WSMV often form dense aggregate-like structures (Tatineni et al., 2011), which may be undesirable. Although WSMV- and TriMV-directed protein expression is efficient and stable, these viruses have not found a wide use as vectors because of the precise engineering required to insert a gene of interest into the viral genome and the severity of symptoms induced. Potyviral genomes encode a single long polypeptide, which is then processed into 10 mature proteins by virus-encoded proteinases. The foreign genes need to be engineered into genomes of these viruses in frame with the long polypeptide-coding open reading frame (ORF) and contain at both the 5′ and 3′ flanks the sequences encoding proteinase cleavage sites required for release of the heterologous proteins from the polyprotein. Processing of these proteins from the polypeptide is not fully efficient, resulting in about 10% of the protein present as a fusion with other viral proteins (Tatineni et al., 2011). Moreover, both vectors can only be inoculated onto plants as in vitro-produced capped transcripts, a methodology that is costly and low throughput.
The monopartite potexvirus Foxtail mosaic virus (FoMV) also has been engineered as a deconstructed vector, FECT, for protein expression in plants (Liu and Kearney, 2010). This vector is unable to spread systemically and allows protein expression only in the primary inoculated leaves. Moreover, its efficiency in six different tested monocot species, including wheat and maize, is negligible (Liu and Kearney, 2010). Recently, two groups reported the development of full virus vectors based on FoMV for VIGS in several monocot species. One of these vectors was tested and shown to be efficient for VIGS in maize, sorghum (Sorghum bicolor), and green foxtail (Setaria viridis; Mei et al., 2016), and another was shown to be efficient in barley (Hordeum vulgare), foxtail millet (Setaria italica), and wheat (Liu et al., 2016). One of the key advantages of these vectors, in addition to being based on a virus with the monopartite genome, is that FoMV induces no or very mild symptoms. In this study, we report a vector based on FoMV for VOX in cereal crops such as wheat and maize that overcomes the limitations of the existing expression vectors discussed above.
RESULTS
Construction of the First-Generation FoMV Vectors for Protein Expression in Plants
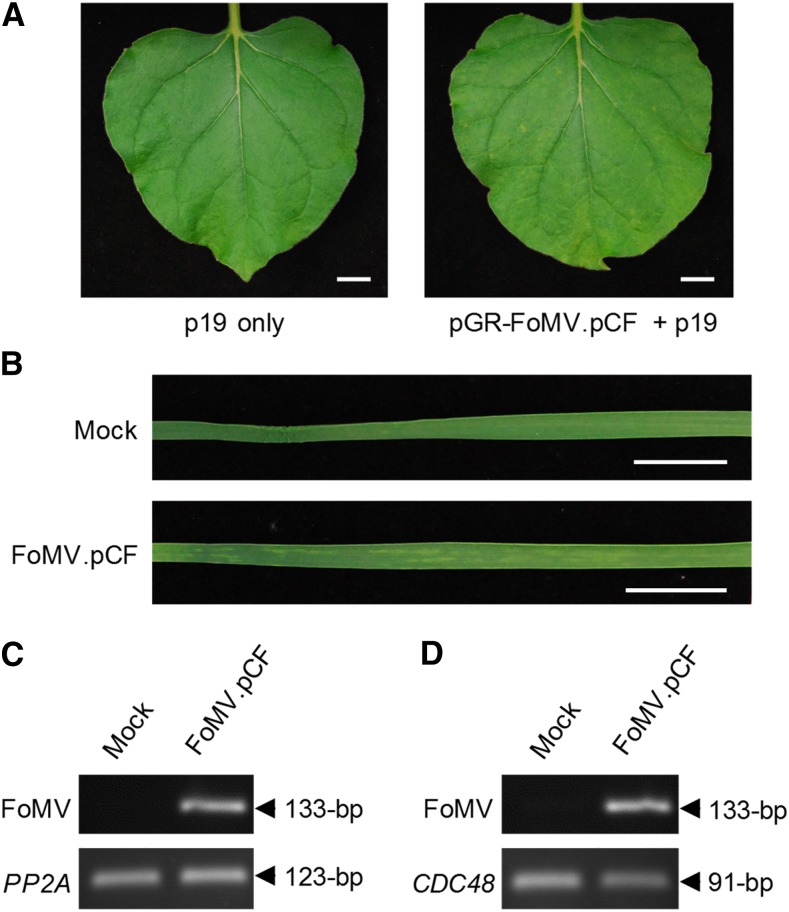
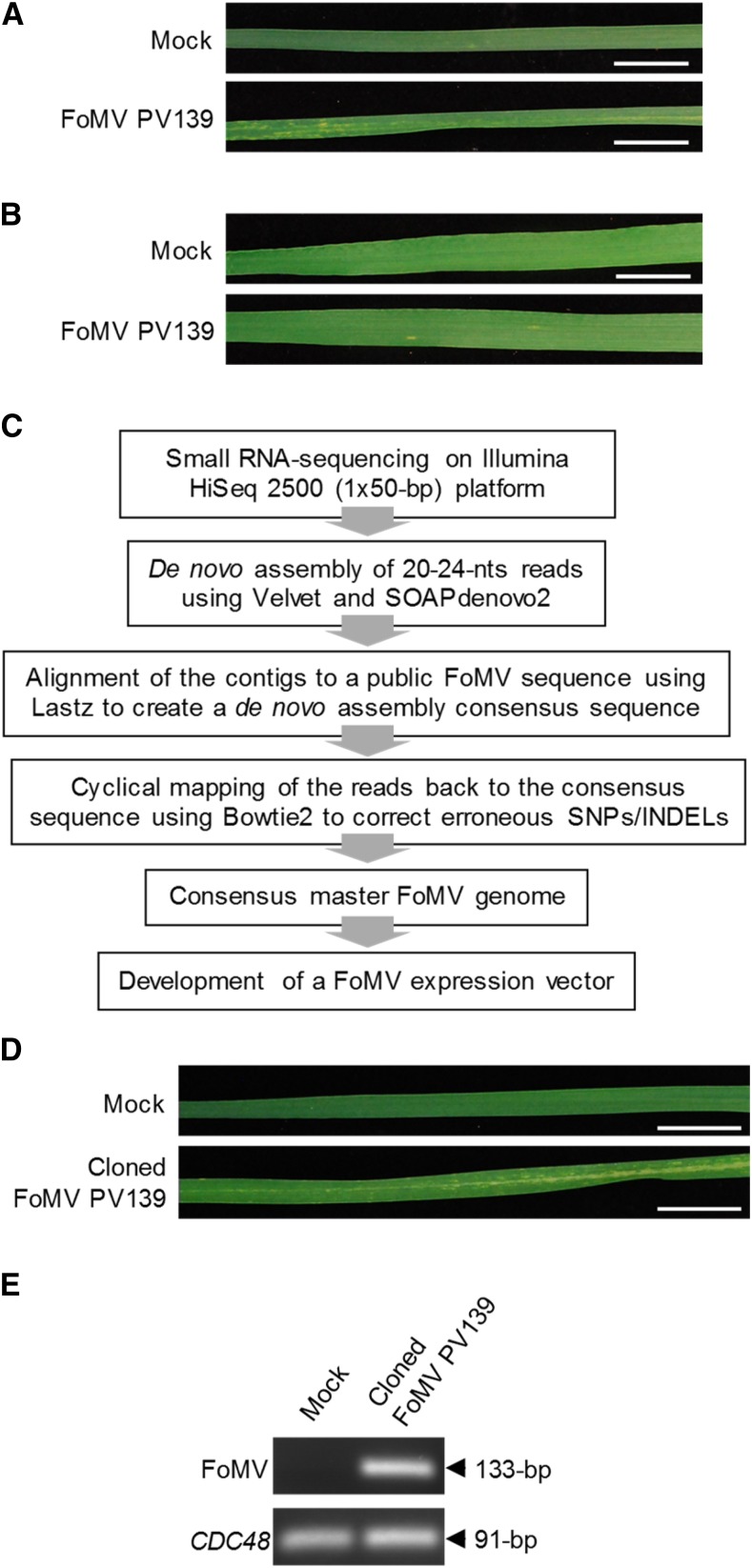
The first-generation binary FoMV VOX vectors were derived from the full-length FoMV cDNA clone pCF (Y.-H. Hsu, unpublished data). The complete PVX genome in the VIGS vector pGR106, based on the compact binary expression vector pGreen0000 (Lu et al., 2003), was replaced with the full-length FoMV genome from the cDNA clone pCF. The resulting binary plasmid, pGR-FoMV.pCF, was transformed into Agrobacterium tumefaciens and coinfiltrated into N. benthamiana leaves with a strain of A. tumefaciens carrying a standard nonviral binary vector for the expression of the Tomato bushy stunt virus (TBSV) p19 protein, a well-known suppressor of RNA silencing. Very mild chlorosis was observed in the newly emerging upper leaves of all plants from 10 d post infiltration (dpi) onward (Fig. 1). The identity of the disease-causing agent in these symptomatic plants as FoMV was confirmed using reverse transcription (RT)-PCR with primers targeting a 133-nucleotide fragment located in the FoMV ORF1 (Fig. 1). Therefore, pGR-FoMV.pCF was directly infectious when introduced into N. benthamiana plants by agroinfiltration. Agroinfiltrated leaves collected from these plants at 6 dpi served as a FoMV inoculum for the mechanical inoculation of young seedlings of wheat cv Riband. The newly developing leaves of virus-inoculated wheat plants exhibited pale green/yellowish chlorotic streaks along the leaf blade (i.e. mild mosaic) from 10 dpi onward, and the presence of FoMV in these leaves was confirmed by RT-PCR (Fig. 1).
Figure 1.
Testing the infectivity of the first-generation FoMV vector pGR-FoMV.pCF in N. benthamiana and wheat. A, Upper uninoculated leaves from mock- or virus-inoculated N. benthamiana plants at 19 dpi. Bars = 20 mm. B, Upper uninoculated leaves from mock- or virus-inoculated wheat cv Riband plants at 13 dpi. Bars = 20 mm. C and D, Detection of FoMV RNA in upper uninoculated leaves from mock- or virus-inoculated N. benthamiana (C) and wheat (D) plants using RT-PCR. Housekeeping N. benthamiana Protein phosphatase 2 (PP2A; C) and wheat Cell division control 48 (CDC48; D) genes were used as loading controls.
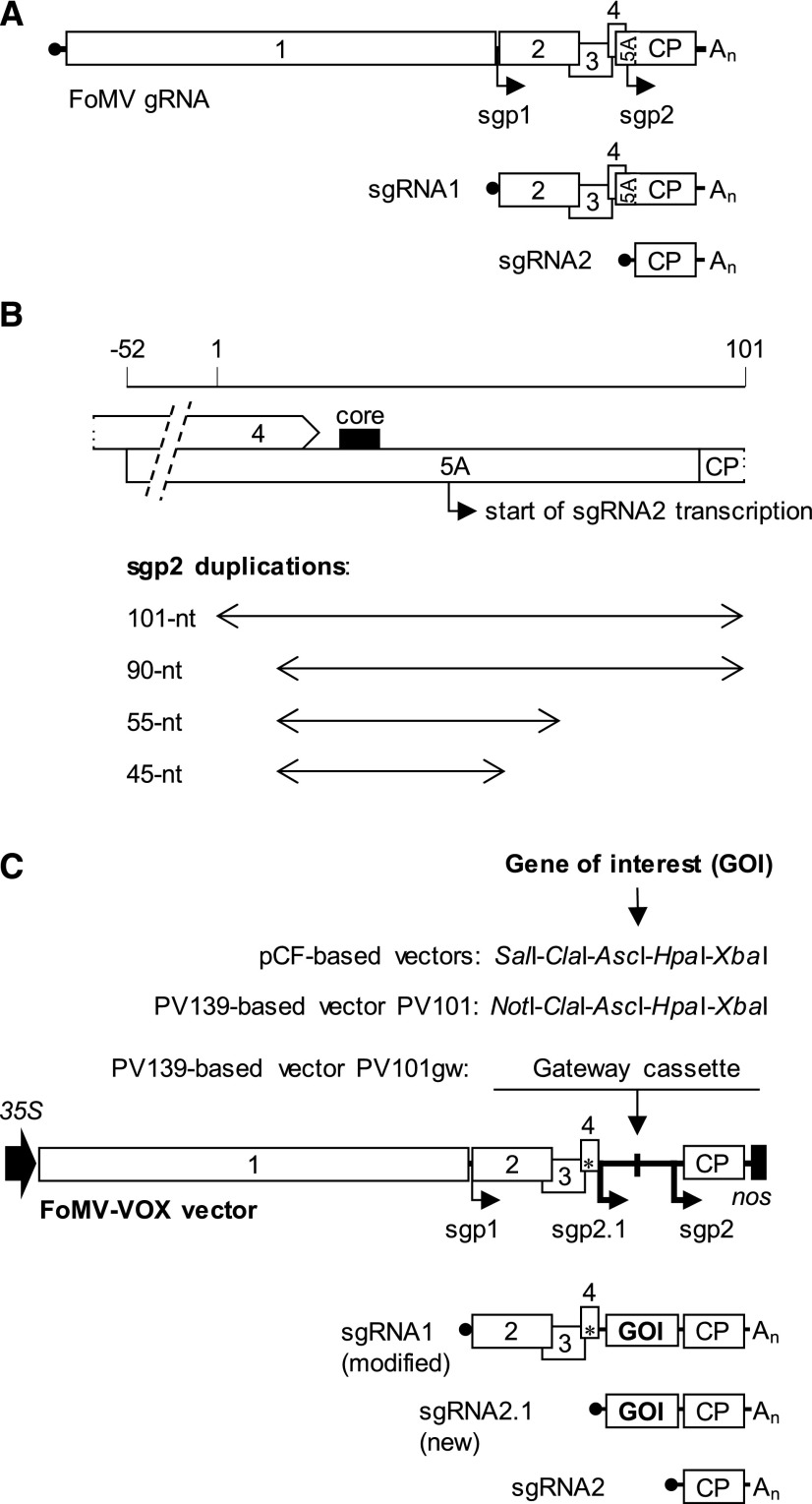
The generation of subgenomic RNAs (sgRNAs) is a mechanism used by many plant viruses with multicistronic (+)ssRNA genomes for the expression of their 3′ proximal cistrons (Sztuba-Solińska et al., 2011). The production of sgRNAs is controlled by the cis-acting promoter-like elements known as subgenomic promoters (sgps). One of the successful strategies used for the expression of heterologous proteins from genomes of viruses in the genus Potexvirus involves placing the heterologous sequence downstream of a duplicated viral coat protein (CP) sgp (Chapman et al., 1992; Lin et al., 2004; Sempere et al., 2011; Zhang et al., 2013). FoMV is known to synthesize two sgRNAs, sgRNA1 and sgRNA2, for the expression of ORF2 to ORF4 (encoding viral movement proteins) and ORF5/CP (encoding viral CP), respectively (Fig. 2). Although the precise 5′ and 3′ boundaries of FoMV CP sgp (designated as sgp2) have not been experimentally defined, an eight-nucleotide-long GUUAGGGU core element conserved in the CP sgp of other potexviruses (Dickmeis et al., 2014) is present upstream of ORF5/CP in FoMV. Using this core element as a landmark, we selected four different-sized sequences ranging from 45 to 101 nucleotides in length encompassing the core sgp2 element for duplication (Fig. 2; Supplemental Fig. S1). A multiple cloning site (MCS) containing cut sites for SalI, ClaI, AscI, HpaI, and XbaI was engineered downstream of the first sgp2 copy, sgp2.1 (Fig. 2), enabling a restriction enzyme-based insertion of a gene of interest for in planta expression. In the case of the two longer sequence duplications (i.e. 90 and 101 nucleotides long) containing a 5′ portion of ORF5/CP, the CP start codon present in sgp2 was eliminated by single-nucleotide mutagenesis that converted ATG to AGG, thus ensuring no translation of proteins other than the heterologous protein from sgRNA2.1 (Fig. 2). The four resulting expression vectors were named pCF45, pCF55, pCF90, and pCF101. In addition, ORF5A, which initiates 143 nucleotides upstream of the CP and encodes an N-terminally extended variant of CP that is dispensable for systemic infection (Robertson et al., 2000), was disrupted in each of the four FoMV expression vectors due to insertion of the MCS.
Figure 2.
Development of a FoMV expression vector. A, FoMV genome organization and expression strategy. The viral genomic RNA (gRNA) contains five major ORFs (labeled from 1 to 4 and CP), coding for the polymerase (ORF1), movement proteins (ORF2–ORF4), and CP, and a cryptic ORF5A that gives rise to an N-terminal CP extension with unknown function. ORF1 is expressed from gRNA, whereas ORF2, ORF3, and ORF4 and CP are expressed from sgRNA1 and sgRNA2, respectively, which are synthesized by the viral polymerase. The synthesis of sgRNAs is driven by subgenomic promoters sgp1 and sgp2. Black circle, mRNA cap structure; An, poly(A) tail; black arrow, sgRNA transcription start. B, A series of FoMV expression vectors were constructed by duplicating differently sized predicted sgp2 sequences, each encompassing a conserved eight-nucleotide core element (core). Duplicated sequences were placed downstream of the ORF5A start codon, disrupting the synthesis of an N-terminal CP extension. C, Schematic diagram of the constructed FoMV expression vectors. The gene of interest (GOI) in these vectors is inserted between sgp2.1 and sgp2 by restriction enzyme (isolate pCF-based vectors and PV101 based on the isolate PV139) or Gateway cloning (PV101gw) and expressed from an additional sgRNA2.1 generated from sgp2.1. The spacing between sgp2.1 and sgp2 is drawn not to scale. 35S, Cauliflower mosaic virus (CaMV) 35S promoter; nos, nopaline synthase terminator; *, start codon of ORF5A.
Heterologous Proteins Can Be Expressed from the Duplicated FoMV sgp2
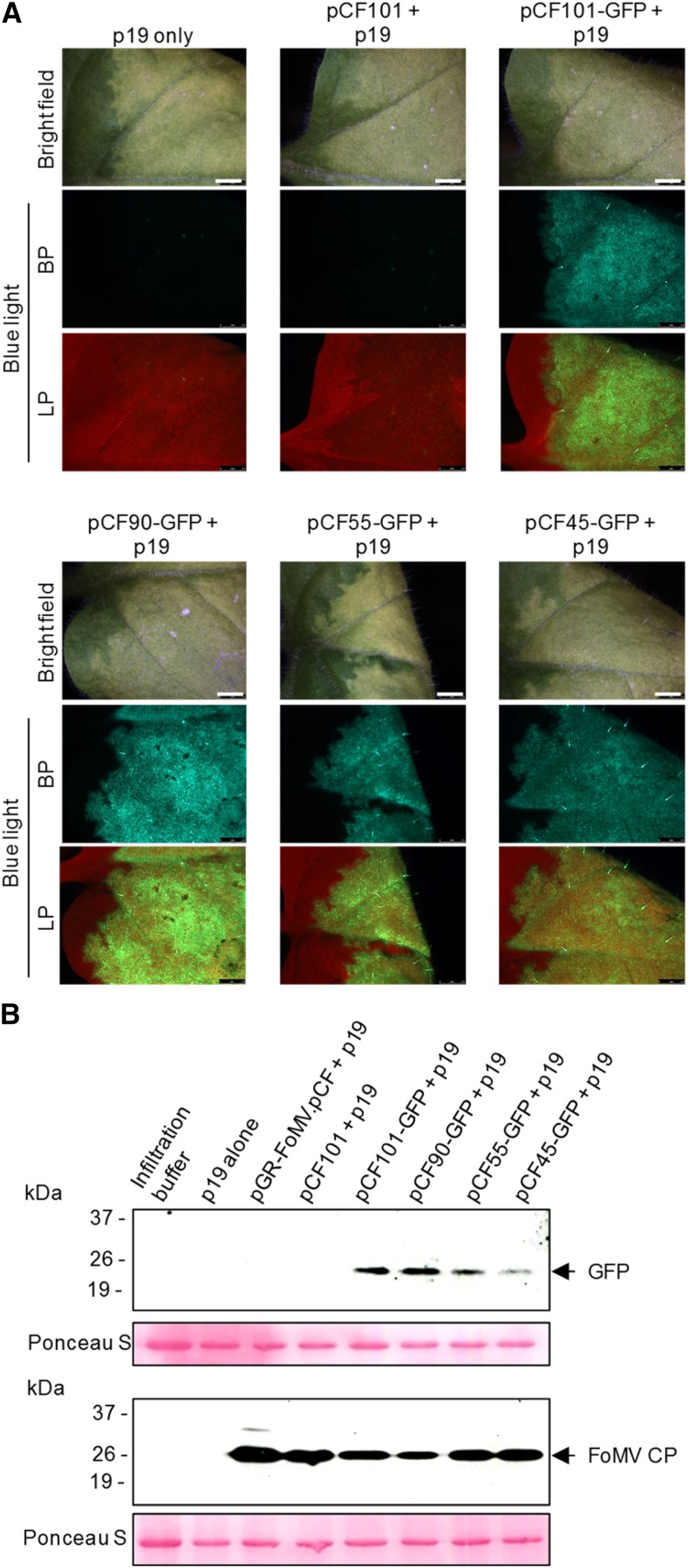
The GFP-coding gene GFP (S65T) (Heim et al., 1995) was inserted into each of the four developed FoMV VOX vectors using restriction enzyme-mediated cloning. The resulting constructs were transformed into A. tumefaciens and then coagroinfiltrated into N. benthamiana leaves with an A. tumefaciens culture carrying a plasmid for the expression of TBSV p19. At 6 dpi, GFP fluorescence was observed under a blue light in all infiltrated leaves in two independent experiments (Fig. 3), whereas no fluorescence was detected in leaves infiltrated with the empty vectors or with the TBSV p19-expressing A. tumefaciens culture only (Fig. 3). GFP also was detected using immunoblotting in leaves of N. benthamiana plants infiltrated with bacterial cultures carrying pCF45-GFP, pCF55-GFP, pCF90-GFP, and pCF101-GFP but not in plants infiltrated with A. tumefaciens carrying the empty vector pCF101, the infectious cDNA clone pGR-FoMV.pCF, or TBSV p19 (Fig. 3). Although each of the four sgp2 duplications could drive the expression of GFP, the highest expression levels were obtained consistently with the 90- and 101-nucleotide-long duplications. The 101-nucleotide-long duplication was selected for all subsequent experiments in this study.
Figure 3.
Expression of GFP from the first-generation FoMV VOX vectors in N. benthamiana. A series of four expression vectors generated through the duplication of 45-, 55-, 90-, and 101-nucleotide sequences spanning the predicted sgp2 sequences were generated based on the full-length infectious FoMV cDNA clone pCF. A, N. benthamiana leaves coinfiltrated with one of the A. tumefaciens strains carrying either the empty vector pCF101 or pCF101-GFP, pCF90-GFP, pCF55-GFP, or pCF45-GFP and an A. tumefaciens strain carrying a construct for the expression of the gene-silencing suppressor p19. Representative infiltrated leaves were photographed at 6 dpi with a fluorescence stereomicroscope mounted with band-pass (BP) and long-pass (LP) filters using identical acquisition settings. Bars = 2.5 mm. B, Immunodetection of FoMV CP and GFP in pooled agroinfiltrated leaves from three individuals sampled at 3 dpi using the corresponding antibodies. Equal loading was verified by staining the membranes with Ponceau S.
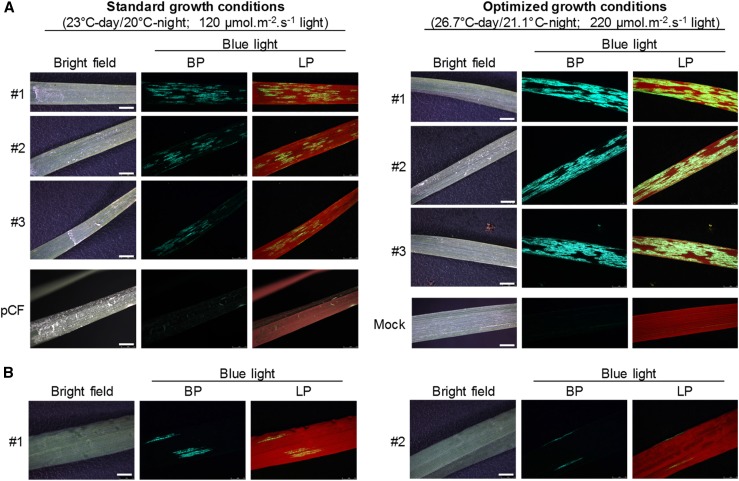
The pCF101-GFP-agroinfiltrated N. benthamiana leaves harvested at 7 dpi served as a virus inoculum for rub inoculation of leaf 1 (L1) and L2 of young, two-leaf-stage wheat cv Riband seedlings grown under standard conditions (day/night temperatures of 23°C/20°C, 16 h of light at ∼120 μmol m−2 s−1, and ∼65% relative humidity). GFP fluorescence was observed in the virus-inoculated leaves at 8 dpi (Fig. 4), but no fluorescence was observed in any of the upper uninoculated leaves, and the majority of inoculated plants did not develop any systemic symptoms even after more than 21 dpi. Temperature is known to be one of the key environmental factors influencing virus infection. For example, it has been reported previously that growing plants at temperatures below 24°C results in a delay in the onset of FoMV-induced symptoms (Paulsen and Niblett, 1977). To assess whether growth conditions could influence heterologous protein expression from the FoMV vector, we raised and maintained wheat plants post inoculation with pCF101-GFP either under standard conditions (as above) or under a slightly elevated temperature regime (day/night temperatures of 26.7°C/21.1°C, 16 h of light at ∼220 μmol m−2 s−1, and ∼65% relative humidity). GFP fluorescence observed in the leaves inoculated with pCF101-GFP in two independent experiments was more intense and appeared to cover wider areas of leaf blades under the higher temperature conditions (Fig. 4). Furthermore, under these warmer conditions, sparse fluorescence foci were observed also in the second systemically infected leaf L4 (Fig. 4) in five out of eight plants inoculated with pCF101-GFP, whereas no systemic fluorescence was detected in the 10 plants inoculated under standard growth conditions. We concluded that elevated temperature can indeed enhance the FoMV-mediated expression of heterologous proteins and that our first-generation vectors, although efficient in expressing GFP in the inoculated leaves of wheat, performed unsatisfactorily in terms of heterologous protein expression in systemically infected tissues.
Figure 4.
Influence of growth conditions on FoMV-mediated protein expression. A, Seedlings of wheat cv Riband grown under standard or optimized growth conditions were inoculated with pCF101-GFP. Mock-inoculated plants or plants inoculated with the wild-type FoMV pCF served as negative controls. Inoculated leaves (L2) from three representative individual plants were photographed at 8 dpi using a fluorescence stereomicroscope mounted with band-pass (BP) and long-pass (LP) filters. B, Scant green fluorescent foci observed in the upper uninoculated leaves (L4) of some pCF101-GFP-inoculated plants grown under optimized conditions at 18 dpi. All fluorescence images in A and B were taken using identical acquisition settings. Bars = 2.5 mm.
Construction of the Second-Generation FoMV Vector for Improved Protein Expression in Plants
The first-generation FoMV VOX vectors were based on the pCF cDNA clone derived from the FoMV isolate PV139 that was maintained on barley (Y.-H. Hsu, personal communication). Viral cDNA clones are traditionally produced by RT of isolated viral genomic RNA followed by insertion of the full-length cDNA genome into a plasmid under the control of a prokaryotic or eukaryotic promoter. This methodology does not ensure that the cloned virus genome is as infectious as the starting virus material for several reasons. First, the replication of RNA viruses is known to be error prone; therefore, during infection of the host, clouds of closely related viral genome sequence variants, known as quasi-species, are generated (Domingo et al., 2012). Therefore, the cloned viral genome may represent one of the suboptimal genome variants. Second, RT itself is an error-prone process and, hence, unwanted inauspicious mutations can occasionally be introduced during the cDNA synthesis step as well. More recently, small RNA sequencing (sRNA-seq)-based approaches have proven to be very valuable for reconstituting full-length viral RNA genomes or identifying new plant viruses (Kreuze, 2014; Seguin et al., 2014). These approaches rely on the fact that virus-infected plants accumulate high levels of virus-derived small interfering RNAs produced through the action of the natural antiviral RNA-silencing machinery (Csorba and Burgyán, 2016). Therefore, we chose to use sRNA-seq followed by de novo sequence reads assembly as an unbiased approach to identify the consensus fittest FoMV isolate PV139 genome variant, hereafter referred to as the master genome, produced in wheat.
The starting material for these experiments was lyophilized leaves of sorghum plants infected with the FoMV isolate PV139 that had been maintained on sorghum to prevent contamination with WSMV (Seifers et al., 1999). Inoculation of this virus onto wheat cv Riband or Chinese Spring typically induced mild chlorotic mosaic symptoms on the upper uninoculated leaves from 7 to 10 dpi onward (Fig. 5), whereas only sporadic mild chlorotic streaks were observed on the newly emerging leaves of cv Bobwhite from 14 dpi onward (Fig. 5). Therefore, cv Chinese Spring and Riband were considered good hosts for maintaining this isolate of FoMV. Total RNA extracted from pooled leaf tissue from the infected cv Chinese Spring and Riband plants after the seventh consecutive serial passage was used for sRNA-seq (Fig. 5). The consensus FoMV master genomes obtained following the assembly of sequence reads corresponding to the 20- to 24-nucleotide fraction of small RNAs were identical between the two biological replicates. The full-length FoMV genome sequence obtained differed from that of FoMV pCF (also originally derived from PV139) at 85 nucleotide positions located both in cistrons and in noncoding sequences (Supplemental Table S1). The full-length FoMV cDNA containing a 40-nucleotide-long 3′ terminal poly(A) tail was produced by gene synthesis and cloned into the backbone of the binary vector pGR106 (replacing the PVX cDNA). The resulting pGR-FoMV.PV139 plasmid was transformed into A. tumefaciens and coagroinfiltrated into N. benthamiana leaves with the p19-carrying A. tumefaciens strain, as described above. Upper noninfiltrated leaves of these plants developed very mild chlorosis similar to that observed previously for pGR-FoMV.pCF (Fig. 1). Agroinfiltrated N. benthamiana leaves collected at 6 dpi served as a source of inoculum for rub inoculation of young two-leaf-stage seedlings of wheat cv Riband. At 10 dpi, all inoculated wheat plants developed systemic mosaic (Fig. 5) similar to that induced by the original virus inoculum (i.e. that prepared from the infected wheat leaf tissue sampled after seven rounds of virus passaging through wheat). The presence of FoMV was confirmed by RT-PCR (Fig. 5).
Figure 5.
Generation of a full-length infectious cDNA clone of the FoMV isolate PV139. A and B, Symptoms observed on the upper uninoculated leaves of wheat cv Riband (A) and Bobwhite (B) plants infected with the original FoMV PV139 at 21 dpi. No symptoms were observed in mock-inoculated plants. Bars = 20 mm. C, Pipeline used to obtain a consensus master genome sequence of FoMV isolate PV139 starting from a small RNA fraction purified from the FoMV-infected leaf material. INDEL, Insertion/deletion polymorphism; SNP, single-nucleotide polymorphism. D, Symptoms observed on the upper uninoculated leaves of wheat cv Riband plants infected with the full-length infectious FoMV PV139 cDNA clone at 24 dpi. No symptoms were observed in mock-inoculated plants. Bars = 20 mm. E, Detection of FoMV RNA in the upper uninoculated leaves of wheat cv Riband plants infected with the FoMV PV139 cDNA clone by RT-PCR. Wheat CDC48 was used as a loading control.
The pGR-FoMV.PV139 was used to develop a second-generation FoMV expression vector using the same methodology as described above for pCF101. A 101-nucleotide-long fragment spanning the core FoMV sgp2 sequence was duplicated, and an MCS containing recognition sites for NotI, ClaI, AscI, HpaI, and XbaI was inserted downstream of the first sgp2 copy. The resulting vector was named PV101. An empty PV101 vector was modified further by replacing the MCS with the Gateway cassette to produce a second prototype vector, PV101gw, which allows the insertion of heterologous sequences using recombination-based cloning.
The FoMV Vector PV101 Provides Improved Expression of Heterologous Proteins in Wheat
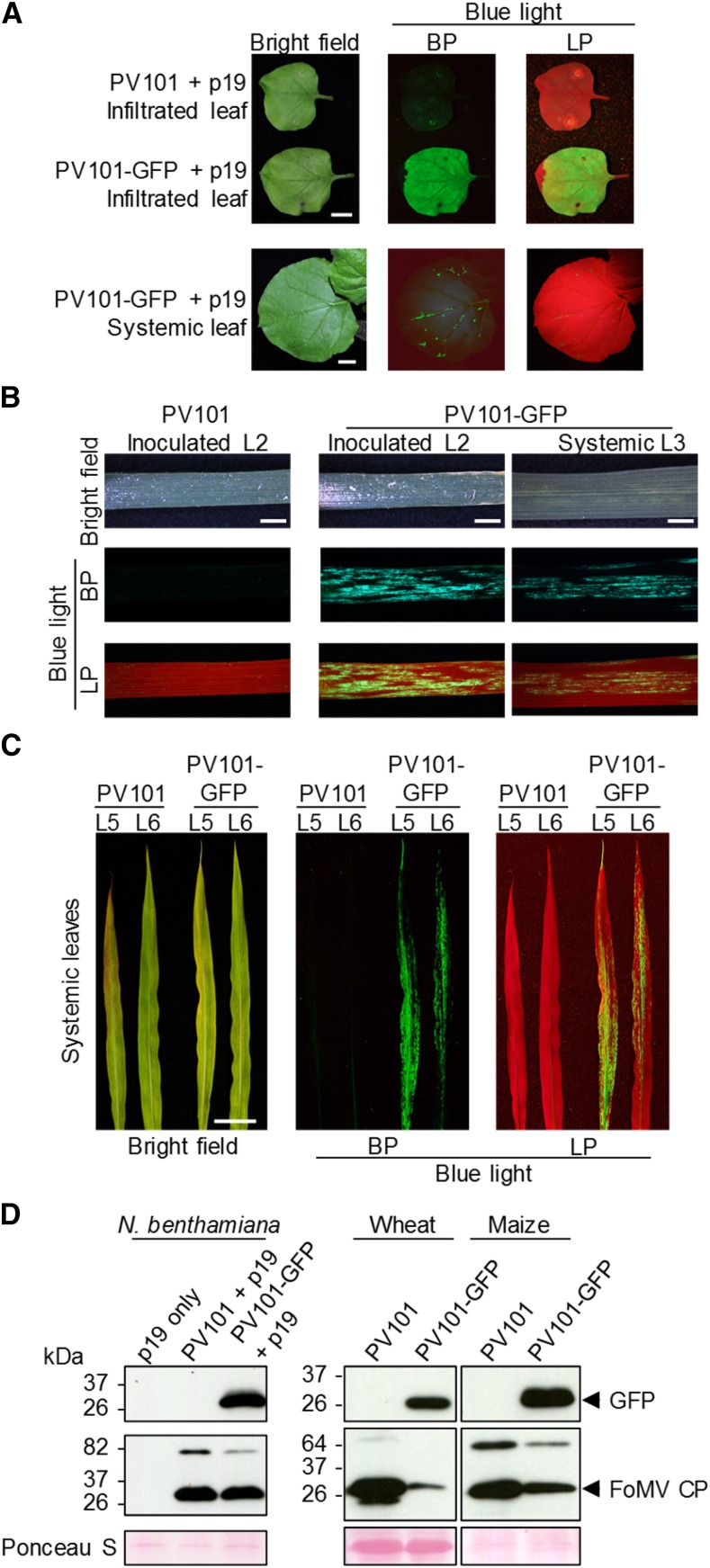
The GFP (S65T) gene was inserted into the PV101 vector using restriction enzyme-mediated cloning, and the resulting plasmid PV101-GFP was transformed into A. tumefaciens and agroinfiltrated into N. benthamiana leaves. At 7 dpi, GFP fluorescence was observed in the infiltrated leaves under blue light, but no fluorescence was detected in leaves infiltrated with an empty vector (Fig. 6). At 14 dpi, GFP fluorescence was observed around veins in the upper uninoculated leaves (Fig. 6), demonstrating the ability of PV101-GFP to replicate and to move systemically in this plant species. GFP also was detected using immunoblotting in the upper uninoculated leaves of PV101-GFP-infiltrated N. benthamiana plants but not in plants infiltrated with the empty vector PV101 (Fig. 6).
Figure 6.
Expression of GFP using the second-generation FoMV vector PV101 in plants. A, Directly inoculated (via coinfiltration with A. tumefaciens strains carrying PV101-GFP and a construct for the expression of the gene-silencing suppressor p19) and upper uninoculated leaves of N. benthamiana plants at 7 and 14 dpi, respectively. Bars = 20 mm. B, Directly inoculated and upper uninoculated (systemic) leaves of wheat cv Riband at 7 and 14 dpi, respectively. Bars = 2.5 mm. C, Systemically infected leaves of maize line B73 plants at 20 dpi. Bar = 50 mm. Photographs were taken using a camera (A and C) or a fluorescence stereomicroscope (B) mounted with band-pass (BP) and long-pass (LP) filters. D, Immunodetection of GFP and FoMV-CP in pooled systemically infected leaves from three individuals of different plant species sampled at 14 dpi using the corresponding antibodies. The presence of fluorescence in PV101-GFP-infected plants was checked before sampling. Equal loading was verified by staining the membranes with Ponceau S.
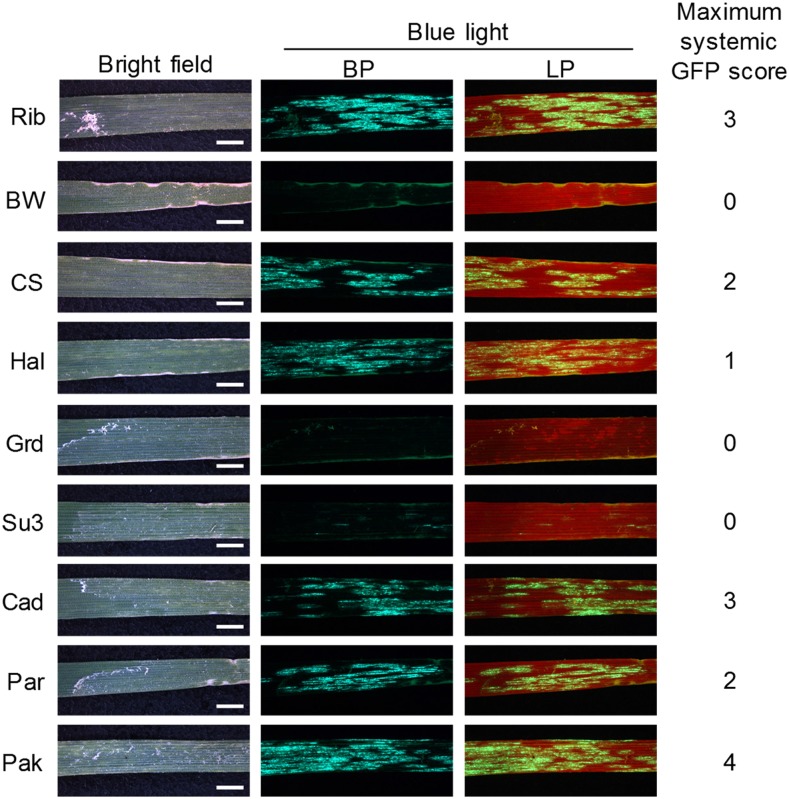
Wheat cv Riband seedlings, grown under day/night temperatures of 26.7°C/21.1°C and inoculated using sap from leaves of N. benthamiana plants agroinfiltrated with PV101-GFP, showed GFP fluorescence in both inoculated and systemically infected leaves (Fig. 6). In the inoculated leaves, GFP fluorescence was observed from 4 dpi onward and lasted for at least another 10 d (Supplemental Fig. S2). GFP fluorescence was readily detectable in the first two systemically infected leaves, L3 and L4, at 11 and 17 dpi, respectively. Although fluorescence in these PV101-GFP infected leaves was rather patchy (Fig. 6), PV101 vector clearly outperformed pCF101 (note a very limited systemic fluorescence observed in leaves of pCF101-GFP infected plants; Fig. 4). GFP in systemically infected wheat leaves also was detected by immunoblotting (Fig. 6). Considerably less FoMV CP accumulated in the systemically infected leaves of wheat plants inoculated with PV101-GFP compared with those inoculated with an empty vector (Fig. 6). This might reflect a better spread of the empty PV101 virus and/or a negative effect of the inserted GFP gene on CP expression. To assess accurately the GFP expression levels in the systemically infected wheat leaves and across different individuals, we developed a GFP fluorescence scoring system (Supplemental Fig. S3) utilizing a scale from 0 to 4 (with 0 indicating no GFP fluorescence and 4 indicating abundant GFP fluorescence foci). The extent of GFP expression was found to be heterogenous in different plants and even in different systemically infected leaves of the same plant. Roughly 85% of L3, L4, and L5 that emerged post inoculation scored only 0 and 1, with the remaining ∼15% showing scores of 2 or 3 (Supplemental Fig. S3). Although L6 and newer leaves showed virus-induced mosaic symptoms, no GFP fluorescence was detected in any of these leaves, indicating that FoMV-GFP was relatively unstable during wheat infection. We then tested the performance of PV101-GFP on 36 other wheat cultivars, the majority of which had been bred in Europe. Moderate levels of GFP fluorescence were observed in the inoculated leaves on all the European cultivars assessed (Fig. 7; Supplemental Table S2). Seven cultivars (i.e. ∼22% of all tested), including Bobwhite, showed no GFP fluorescence and no or very limited systemic mosaic symptoms in any of the assessed inoculated or upper uninoculated leaves, indicating that these cultivars were either partially or fully resistant to PV101-GFP. The FoMV-locally susceptible cultivars displayed variable maximum levels of GFP fluorescence in the systemically infected leaves, from low (score = 1) and patchy (score = 2) to sometimes almost uniform throughout the entire leaf blades (score = 4), as in cv Pakito (Fig. 7). In summary, our data indicate that FoMV-directed heterologous protein expression is likely to be achieved in many European wheat cultivars, whereas at least some of the non-European wheat genotypes may be poor hosts for PV101 under the growth conditions tested.
Figure 7.
Influence of the wheat genotype on FoMV-mediated protein expression. PV101-GFP-inoculated leaves (L2) of different wheat cultivars were photographed at 8 dpi using a fluorescence stereomicroscope mounted with band-pass (BP) and long-pass (LP) filters. All fluorescence images were taken using identical acquisition settings. The maximum scores for GFP fluorescence coverage in systemically infected leaves of wheat cv Riband (Rib), Bobwhite (BW), Chinese Spring (CS), Halberd (Hal), Grandin (Grd), Sumai 3 (Su3), Cadenza (Cad), Paragon (Par), and Pakito (Pak) are indicated. Data are from at least 16 plants from three independent experiments. Bars = 2.5 mm.
An expression construct produced by recombining the coding sequence of GFP (S65T) into the Gateway-enabled vector PV101gw also was fully infectious, and GFP expression was detected in the infected plant tissues (Supplemental Fig. S4).
A FoMV vector for VIGS has been described (Liu et al., 2016). This vector, pFoMV-sg, was developed through the duplication of a 170-nucleotide-long sequence that encompassed not only sgp2 but also an entire ORF5A located upstream of ORF5/CP. To investigate whether this longer sgp2 duplication also may be suitable for driving the expression of heterologous proteins, we constructed an additional vector based on pGR-FoMV.PV139 that contained a 169-nucleotide-long duplication of sgp2 equivalent to that in the VIGS vector pFoMV-sg (Supplemental Fig. S5). The resulting vector PV169 expressed GFP in both N. benthamiana and wheat; however, the observed GFP fluorescence in the infected leaves was less intense than that in the PV101-GFP infected leaves, and the PV169-GFP-infiltrated N. benthamiana leaves accumulated less GFP but noticeably more viral CP than the PV101-GFP infiltrated leaves, as determined by immunoblotting (Supplemental Fig. S5).
The FoMV Vector PV101 Efficiently Expresses Heterologous Proteins in Maize
Next, we assessed whether PV101 could be used for VOX in another major cereal crop, maize. Young (three- to four-leaf stage) seedlings of maize line B73 with a sequenced genome (Schnable et al., 2009) were inoculated by rubbing L1, L2, and L3 with the PV101-GFP-containing sap from infected N. benthamiana plants as described above. Isolated GFP foci were observed in the systemically infected leaves from 7 dpi, and GFP fluorescence spread was observed from 14 dpi onward (Fig. 6). GFP was readily detected in systemic leaves using immunoblotting (Fig. 6). In some maize B73 plants, GFP fluorescence was detected in all systemically infected leaves up to and including L7, and relatively high GFP fluorescence scores (scores = 3 or 4) were regularly observed (Supplemental Fig. S3). Therefore, maize line B73 appears to be an acceptable host for FoMV PV139.
The FoMV Vector PV101 Can Be Used for the Expression of Proteins as Large as 600 Amino Acids
PV101 was tested for the in planta expression of large proteins. In this experiment, a synthetic gusA gene based on the sequence from Staphylococcus spp., encoding the 600-amino acid-long protein GUSPlus (Broothaerts et al., 2005), was cloned into PV101. GUSPlus expression was observed in the PV101-GUSPlus-inoculated leaves of both wheat and maize seedlings (Fig. 8). GUSPlus expression in the systemically infected wheat leaves appeared to be more limited than the expression of GFP (Fig. 8). This suggests a negative association between insert length and vector stability. Slightly higher levels of GUSPlus expression were observed in the upper uninoculated leaves of wheat cv Pakito (Fig. 8), which agrees well with the fact that this same cultivar showed the best GFP fluorescence scores in the systemically infected leaves among all wheat cultivars tested (Fig. 7).
Figure 8.
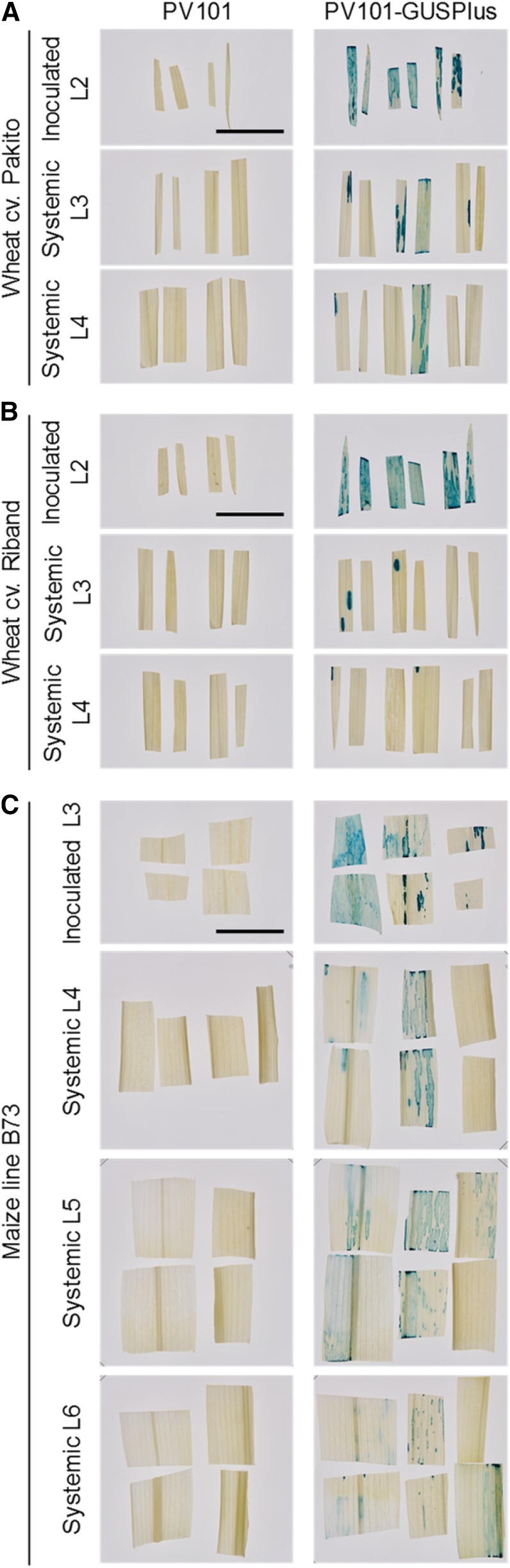
Expression of the 600-amino acid-long protein GUSPlus using the second-generation FoMV vector PV101. PV101-GUSPlus was inoculated onto wheat cv Pakito (A) and Riband (B) and onto maize line B73 (C), and GUSPlus activity in leaf samples from inoculated plants was detected by histochemical staining with 5-bromo-4-chloro-3-indolyl-β-d-GlcA. An empty vector, PV101, was used as a control. Samples of inoculated leaves were taken at 9 dpi. Samples of first systemic wheat (L3) and maize (L4) leaves were taken at 15 dpi, and samples of second systemic wheat leaves (L4) and second and third maize leaves (L5 and L6) were taken at 22 dpi. Each leaf piece comes from a different individual plant. PV101- and PV101GUSPlus-infected material was sampled from four representative individuals from two independent experiments and from six representative individuals from three independent experiments, respectively. Bars = 20 mm.
In contrast to the suboptimal performance of PV101-GUSPlus in the systemically infected wheat leaves, adequate levels of GUSPlus expression were observed in upper uninoculated maize leaves (Fig. 8). The percentage of symptomatic maize plants showing systemic GUSPlus expression varied from 33% to 83% between three independent experiments (Supplemental Table S3). The best GUSPlus expression levels were observed consistently in L5, with GUS staining less intense and somewhat patchier in L4 and L6 (Fig. 8).
In conclusion, our study demonstrates that the FoMV vector PV101 can be used to express heterologous proteins of up to 600 amino acids in the inoculated leaves of wheat and maize and in up to three consecutive systemically infected leaves in maize.
The FoMV Vector PV101 Can Be Used as a Tool for the Expression of Pathogen Effector Proteins
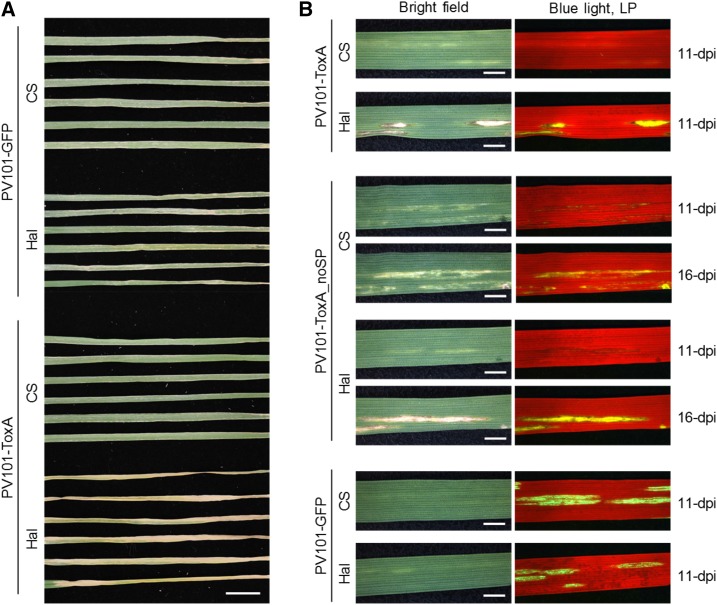
The functional analysis of genes encoding small secreted effector proteins predicted in the genomes of wheat-infecting fungal pathogens currently relies on labor-intensive methods when assessing their function in wheat. Typical experiments involve the expression of candidate effectors in heterologous in vitro systems (such as the yeast Pichia pastoris or Escherichia coli) followed by syringe infiltration of purified effectors into wheat leaves and analysis of the resulting induced phenotypes/responses. To test the suitability of the vector for this purpose, we cloned the full-length coding sequence of the well-studied necrotrophic effector ToxA (Friesen et al., 2006; Liu et al., 2006) from Parastagonospora nodorum, the causal agent of glume blotch disease in wheat, into PV101. ToxA is known to induce necrosis on wheat cultivars carrying the corresponding sensitivity gene Tan spot necrosis 1 (Tsn1; Friesen et al., 2006; Faris et al., 2010). As expected, only ToxA-sensitive wheat cv Halberd but not ToxA-insensitive wheat cv Chinese Spring developed necrosis in both inoculated and systemically infected leaves when inoculated with the PV101-SnToxA construct (Fig. 9). A mature version of ToxA (without its native signal peptide) also was cloned into PV101. The resulting PV101-ToxA_noSP induced necrosis in systemic leaves of wheat cv Halberd only, but the necrosis was delayed by at least 5 d in comparison with the necrosis induced by the full-length ToxA in two independent experiments. This indicates that the secretion of ToxA into the apoplastic space is not absolutely required, which agrees well with the previous work by Manning and Ciuffetti (2005) demonstrating that ToxA is imported within the cell in Tsn1 wheat lines. PV101-GFP was used as a control in these experiments and induced only mild chlorotic mosaics on both cultivars (Fig. 9). Therefore, we conclude that the FoMV vector PV101 has great potential for VOX applications such as medium- to high-throughput screens for necrosis or cell death-inducing candidate fungal effectors.
Figure 9.
FoMV-mediated expression of the necrotrophic fungal effector ToxA from P. nodorum. A, PV101-GFP- or PV101-ToxA-inoculated leaves (L2) of wheat cv Chinese Spring (CS; ToxA insensitive) and Halberd (Hal; ToxA sensitive) seedlings at 6 dpi from one of the two replicated experiments. Bar = 20 mm. B, Upper uninoculated leaves (L3) from wheat cv Chinese Spring and Halberd plants inoculated with PV101 carrying either full-length SnToxA effector protein with its native secretion signal peptide or its mature version without signal peptide (ToxA_noSP) at 11 dpi (and 16 dpi where indicated). Photographs were taken from the same leaf areas under white light or blue light using a fluorescence stereomicroscope mounted with a long-pass filter (LP). Bars = 2.5 mm.
DISCUSSION
Here, we report the development of a vector, based on the FoMV, for the expression of heterologous proteins, including large fusion-free proteins of up to 600 amino acids in size and pathogen-secreted effector proteins, for applications in two major crops, wheat and maize. This vector, PV101, induced only mild symptoms in both wheat and maize (as well as in a laboratory dicotyledonous host, N. benthamiana), which agrees well with findings from studies of FoMV by others (Paulsen and Niblett, 1977; Robertson et al., 2000; Liu et al., 2016; Mei et al., 2016). This lack of strong symptoms is a very useful feature when determining plant phenotypes induced by heterologous proteins, especially those predicted to induce or regulate plant defense, cell death, and/or senescence pathways. However, appropriate controls must be included when designing a VOX experiment, since the presence of mild symptoms indicates that some changes in plant physiology do occur during FoMV infection.
The PV101 VOX vector is superior to the existing monocot expression vectors in several other respects. Specifically, this study shows that PV101 can carry the 1,800-nucleotide-long GUSPlus gene coding sequence, representing an increase of about 30% in the FoMV genome length, whereas BSMV-derived vectors do not tolerate inserts larger than ∼450 to 500 nucleotides (Lee et al., 2012) and potyvirus-derived vectors can stably express only moderately sized proteins such as RFP (237 amino acids), GFP (238 amino acids), and neomycin phosphotransferase II (264 amino acids) but not proteins that are as large as GUS (603 amino acids; Choi et al., 2000; Tatineni et al., 2011, 2015). Furthermore, PV101 allows the expression of heterologous proteins in their native forms, including those with the N-terminal signal sequences for secretion outside the plant cell, whereas heterologous proteins expressed from potyviruses WSMV and TriMV or from BSMV must be processed from polyproteins by the native or heterologous proteases, a process that is only 90% efficient at best. In addition, expression vectors based on FoMV, a potexvirus with the monopartite genome, that utilize a strategy of heterologous protein expression from subgenomic RNA have greater potential for applications requiring higher throughput. As a proof of concept, we engineered a second prototype FoMV vector, PV101gw, enabling high-throughput cloning of heterologous sequences using the Gateway recombination technology. Although not yet fully tested for its ability to express a range of different proteins in wheat, this vector was effective in expressing GFP in the infected plant tissues (Supplemental Fig. S4).
Two different FoMV vectors for VIGS in monocots, including wheat and maize, have been described recently (Liu et al., 2016; Mei et al., 2016). The pFoMV-V vector (Mei et al., 2016) was designed for cloning the heterologous sequences using the engineered XbaI and XhoI restriction enzymes immediately downstream of the stop codon of the viral CP ORF. This vector cannot be used for protein expression because the gene of interest, cloned downstream of the CP ORF, would be in the second position on the coat protein subgenomic RNA and, therefore, would remain silent. Another published FoMV VIGS vector, pFoMV-sg (Liu et al., 2016), was developed using a similar strategy to that used in this study and involved the duplication of a predicted sgp2. However, by contrast to PV101 bearing a relatively short 101-nucleotide sgp2 duplication, pFoMV-sg contained a 170-nucleotide-long duplication, which spanned the sgp2 core sequence as well as a dispensable ORF5A (Liu et al., 2016). Here, we showed that this longer duplication of predicted sgp2, although able to direct the expression of heterologous proteins, is less effective than the 101-nucleotide sgp2 sequence duplication. The vector, PV169, constructed to replicate the sgp2 duplication in pFoMV-sg, produced less GFP and more viral CP in the inoculated plants than PV101 (Supplemental Fig. S5). Therefore, it may be concluded that longer sgp2 promoter duplications, as in pFoMV-sg and PV169, somehow confer reduced insert stability. Although insert stability in pFoMV-sg was not investigated specifically in the study by Liu et al. (2016), those authors reported that a 200-bp coding region fragment of the barley Phytoene desaturase (HvPDS) gene cloned into pFoMV-sg induced only a very limited silencing of the endogenous PDS in barley, whereas 110- to 120-bp-long inserts containing direct inverted repeat fragments of various monocot plant genes induced efficient silencing of targeted endogenes. A more efficient gene silencing with VIGS vectors carrying 110- to 120-bp inserts may be explained at least in part by their potentially higher stability over those bearing inserts longer than 200 bp.
Our current FoMV VOX procedure involves propagation of the PV101-derived constructs in N. benthamiana prior to their inoculation onto monocot plants. This limits the experimental throughput. Furthermore, avoiding this first step of virus inoculum buildup in N. benthamiana may be necessary when testing constructs for the expression of generic cell death-inducing proteins (Lacomme and Santa Cruz, 1999; Tang et al., 2015; Kettles et al., 2018), predicted proteinaceous pathogen-associated molecular patterns (Franco-Orozco et al., 2017), or certain candidate pathogen effector proteins that may be recognized by the corresponding immune receptors in this plant species (Dagvadorj et al., 2017; Kettles et al., 2017). Therefore, we assessed the possibility of delivering the FoMV VOX vector directly into wheat leaves using infiltration with the recA-deficient A. tumefaciens strain COR308 harboring the disarmed pTi derivative plasmid pMP90 and the helper plasmid pCH32, which provide extra copies of the virA and virG genes (Hamilton, 1997), and a corresponding protocol that was claimed to be efficient in delivering BSMV-derived gene-silencing constructs directly to wheat leaves (Panwar et al., 2013). However, all our attempts to inoculate wheat cv Riband and Pakito directly with PV101-GFP or pGR-FoMV-PV139 using this approach were unsuccessful (data not shown). Other means of the direct delivery of virus vectors, in which the transcription of the viral genomes is under the control of the CaMV 35S promoter, to monocot plants have been reported. For example, microprojectile particle bombardment was used to deliver BSMV VIGS and VOX vectors (Meng et al., 2009; Xu et al., 2015) and the recently developed pFoMV-V VIGS vector (Mei et al., 2016) directly to barley and maize leaves, respectively. A procedure for inducing Tobacco rattle virus-mediated VIGS in wheat and maize was described recently (Zhang et al., 2017). It involves the use of vacuum infiltration and cocultivation with A. tumefaciens in Luria-Bertani medium supplemented with acetosyringone, Cys, and Tween 20. Further studies are needed to investigate whether any of the methods described above may be used for direct inoculation of the PV101-derived expression constructs directly into wheat, maize, and other monocot crops.
Screening a collection of 37 wheat cultivars with PV101-GFP revealed that hexaploid wheat is not universally susceptible to FoMV. Some cultivars, largely those of non-European origin, showed partial or complete virus resistance (Fig. 7; Supplemental Table S2). Thus, we recommend testing the chosen wheat cultivars for their susceptibility to FoMV before planning a PV101-VOX study. The same applies to VOX studies involving maize, because some maize inbred lines (e.g. Mo17) have been reported to be resistant to FoMV (Ji et al., 2010; Mei et al., 2016). Maize inbred lines shown previously to be susceptible to pFoMV-V (Mei et al., 2016) also are likely to be susceptible to PV101, because both these vectors originate from the same FoMV isolate, namely PV139.
Our main initial objective was to develop a virus-based expression vector for wheat. Therefore, the FoMV isolate PV139 selected for this study was first passaged several times through wheat, and the VOX vector PV101 was derived from the most highly abundant and, hence, most likely the fittest FoMV quasi-species accumulating in this experimental host. Nevertheless, somewhat surprisingly, maize was found to be a better systemic host for PV101 than wheat. That is, a nearly uniform GFP fluorescence across entire leaf blades was observed more frequently in maize plants systemically infected with PV101-GFP than in wheat (Supplemental Fig. S3). Also, the expression of a larger protein, GUSPlus, was more stable and noticeably more efficient in maize (Fig. 8). These data suggest that FoMV may be naturally better adapted to infect maize than wheat. In this regard, it is interesting that FoMV PV139 was isolated originally from green foxtail and foxtail millet growing as weeds in a maize field (Paulsen and Niblett, 1977). Also, FoMV has been isolated previously from sorghum (Seifers et al., 1999). It is quite remarkable that the only natural hosts reported for FoMV as well as the seemingly best experimental host, maize, all are species with the C4 photosynthetic pathway. Leaves of C4 plants have Kranz-type anatomy, in which the vascular bundle is surrounded by the organelle-rich vascular bundle sheath cells and this tissue layer further surrounded by the radially arranged mesophyll cells. This anatomy facilitates the transport of photosynthetic assimilates between the different cell types. The leaf anatomy of C3 plants is very different, with the mesophyll cells being well developed relative to the organelle-poor vascular bundle sheath cells. Another key difference between leaves of C4 and C3 plants is that C4 leaves have much denser networks of small longitudinal and transverse veins (Brown and Hattersley, 1989). It seems possible that the leaf anatomy of C4 plants is more favorable to FoMV spread and, ultimately, to the vector performance than the anatomy of C3 plants (e.g. wheat).
CONCLUSION
Here, we developed a FoMV vector, PV101, for transient protein expression in wheat and maize and demonstrated the successful expression of a wide range of native proteins from 178 amino acids long (ToxA) to at least approximately 600 amino acids long (GUSPlus). Although not tested specifically here, it is likely that PV101 can be used for VOX in plants such as sorghum, green foxtail, and foxtail millet, which are natural hosts for FoMV, as well as in several other monocots, including important crops such as barley, oat (Avena sativa), and rye (Secale cereale) that can be infected systemically with FoMV under laboratory conditions (Paulsen and Niblett, 1977). Furthermore, FoMV-mediated VOX using PV101 and PV101gw can be used in medium-throughput screens, and the vector can be modified further to allow rapid restriction endonuclease-independent cloning and, thereby, increase experimental throughput. This opens a wide range of applications where an easy and rapid method of heterologous protein expression in monocots is needed. For example, PV101 may be used in screens for cell death activity of secreted or cytosolic candidate pathogen effectors in wheat, maize, or other monocot crops or model species, or in screens for proteins with putative insecticide or antifungal activities. We are positive that many other interesting and useful applications for PV101 will soon be found by the scientific research community.
MATERIALS AND METHODS
Plants and Growth Conditions
Nicotiana benthamiana plants were grown in a controlled-environment room with day/night temperatures of 23°C/20°C at 60% relative humidity and a 16-h photoperiod with approximately 130 μmol m−2 s−1 light. Maize (Zea mays) and bread wheat (Triticum aestivum) were grown in a controlled-environment room with day/night temperatures of 26.7°C/21.1°C at around 65% relative humidity and a 16-h photoperiod with approximately 220 μmol m−2 s−1 light.
Serial Passaging of the FoMV Isolate PV139 through Wheat
At least four young, 10 to 11 d old, wheat plants of three different wheat cultivars (cv Chinese Spring, Riband, and Bobwhite) were rub inoculated with the sap prepared by grinding 70 mg of freeze-dried FoMV PV139-infected sorghum (Sorghum bicolor ‘Asgrow’; XP6105) leaves in 1.5 mL of deionized water supplemented with 15 mg of coarse Celite 545 AW (Sigma-Aldrich). At 5 min or more post inoculation, the plants were lightly misted with tap water to remove residual abrasive, covered with clear plastic bags or propagator lids, and incubated overnight at no or very low light, following which plants were returned to standard growth conditions. Systemically infected leaves showing moderate mosaic symptoms were collected at 14 to 21 dpi and used as a source of inoculum for rub inoculation of batches of wheat plants. This passaging sequence was repeated seven times.
sRNA-Seq
Systemic leaves of three cv Riband and three cv Chinese Spring FoMV-infected wheat plants were collected at 26 d after the seventh passaging and pooled. Total RNA was extracted using TRIzol (Invitrogen) following the manufacturer’s instructions, with the exception that the chloroform extraction was repeated once. Total RNA was treated with DNase I (Promega) at 1 unit per μg of RNA for 35 min at 37°C and then purified by extraction with phenol-chloroform and then with chloroform, followed by standard precipitation with ethanol. RNA pellets were resuspended in nuclease-free water (Promega), and RNA quality was assessed using a 2100 Bioanalyzer (Agilent Technologies). Total RNA samples from two independent pools of leaves were used for sRNA-seq on an Illumina HiSeq 2500 (1 × 50 bp) platform at Fasteris.
Assembly of sRNA-Seq Reads to Obtain a Consensus Master Genome of FoMV Isolate PV139
The adapter-trimmed small RNA reads in the range between 20 and 24 nucleotides were assembled de novo into contigs using Velvet (version 1.2.09; Zerbino, 2010) with the best k-mer value of 19 and using SOAPdenovo2 (version 2.04; Luo et al., 2012) with a multi-k-mer setting of 19 to 24. The resulting assemblies were aligned using Lastz (version 1.02.00; Harris, 2007) with a publicly available FoMV genomic RNA sequence (GenBank accession no. EF630359.1) to orientate the de novo assembly contigs and create a de novo assembly consensus sequence. The small RNA reads were then mapped back to the assemblies using Bowtie2 (version 2.2.0; Langmead and Salzberg, 2012) using default settings with an end-to-end mapping algorithm in a cyclical method of improvement to check for concordance and to correct any erroneous small insertions/deletions and single-nucleotide polymorphisms from the de novo assemblies.
Construction of an Infectious Full-Length cDNA Clone of the FoMV Isolate pCF RNA Genome
All PCRs described below were conducted using the high-fidelity DNA polymerases Phusion (New England Biolabs) or Platinum SuperFi (Invitrogen) unless stated otherwise, and all the constructs were verified by Sanger sequencing.
The genome of FoMV isolate pCF was cloned from a previously available cDNA clone called pCF into a binary vector as follows. The CaMV 35S promoter sequence was amplified by PCR from the vector pGR106 (Lu et al., 2003) with the oligonucleotides 5′35Sp and 5′FoMV-3′35Sp (Supplemental Table S4). The 5′ part of FoMV isolate pCF was amplified by PCR from the plasmid pCF with the oligonucleotides 3′35Sp-5′FoMV and SpeI-FoMV1040R (Supplemental Table S4). The two resulting amplicons were fused by PCR with the oligonucleotides 5′35Sp and SpeI-FoMV1040R using a 38-nucleotide-long complementary region that was artificially introduced at the 3′ extremity of 35S promoter and at the 5′ extremity of FoMV amplicons. The resulting 35S-5′-FoMV fragment was then cloned between EcoRV and SpeI recognition sites into an EcoRV- and SpeI-digested pGR106 vector backbone to produce the plasmid pGR-5′-pCF. Finally, the 3′ part of FoMV pCF was obtained by BlpI and XbaI digestion of the pCF plasmid, and this fragment was then inserted into the BlpI- and SpeI-digested pGR-5′-pCF. The resulting construct was named pGR-FoMV.pCF. In this binary plasmid, the FoMV genome is under the control of the CaMV 35S promoter and is flanked at the 3′ end by the Agrobacterium tumefaciens nos terminator sequence.
Construction of First-Generation FoMV VOX Vectors Based on the Isolate pCF
First, a general cloning plasmid, pMA-RQ (Thermo Fisher Scientific), was modified by introducing SpeI and XhoI restriction sites into the multiple cloning site, creating the plasmid pBxs. This was done using oligonucleotide-directed mutagenesis on the whole plasmid (Silva et al., 2017) and primers pBxs-fw and pBxs-rev. The FoMV cDNA clone pCF was then digested with SpeI plus XhoI, and a fragment corresponding to the 1,627-nucleotide-long 3′ most portion of the FoMV genome, including a 65-nucleotide-long poly(A) tail, was cloned into SpeI- plus XhoI-digested pBxs, generating the plasmid pB-F. Then, an MCS containing sites for digestion with the restriction enzymes SalI, ClaI, AscI, HpaI, and XbaI was inserted into pB-F immediately upstream of the predicted FoMV CP sgp2 by oligonucleotide-directed mutagenesis using two consecutive PCR cycles and primers pB-Fmcs-10-fw1 and pB-Fmcs-10-rev1 for the first reaction and pB-Fmcs-10-fw2 and pB-Fmcs-10-rev2 for the second reaction. The resulting plasmid, pBFmcs-10, was digested with SphI and AscI and ligated with a gene-synthesized DNA fragment (Invitrogen), digested with the same two enzymes, comprising a 101-bp sequence of the predicted FoMV sgp2 (nucleotides 5,280–5,380 in FoMV pCF) and a downstream MCS containing restriction sites for SalI, ClaI, AscI, HpaI, and XbaI, generating the plasmid pB-Fsgp2-101/pCF. Three other predicted FoMV sgp2 sequences of 90, 55, and 45 nucleotides (90-bp sequence, nucleotides 5,291–5,380 in FoMV pCF; 55-bp sequence, nucleotides 5,291–5,345; 45-bp sequence, nucleotides 5,291–5,324) were produced by gene synthesis upstream of the above-mentioned MCS, digested with SphI and AscI, and ligated into a SphI- plus AscI-digested pBF-mcs plasmid produced by oligonucleotide-directed mutagenesis on the plasmid pBF using two consecutive PCR cycles and primers pB-Fmcs-fw1 and pB-Fmcs-rev1 for the first reaction and pB-Fmcs-fw2 and pB-Fmcs-rev2 for the second reaction. The corresponding plasmids were named pB-Fsgp2-90/pCF, pB-Fsgp2-55/pCF, and pB-Fsgp2-45/pCF, respectively.
The coding sequence of the S65T variant of GFP (Heim et al., 1995) was amplified from the plasmid pActIsGFP using the oligonucleotides GFP5′-ClaI-fw and GFP3′-XbaI-rev. The corresponding amplicon was digested with ClaI plus XbaI and cloned into ClaI- plus XbaI-digested pB-Fsgp2-101/pCF, pB-Fsgp2-90/pCF, pB-Fsgp2-55/pCF, and pB-Fsgp2-45/pCF. The generated plasmids were named pB-Fsgp2-101/pCF-GFP, pB-Fsgp2-90/pCF-GFP, pB-Fsgp2-55/pCF-GFP, and pB-Fsgp2-45/pCF-GFP. The empty and the GFP-containing pB-Fsgp2-/pCF plasmids were digested by SpeI and XhoI and inserted into SpeI- plus XhoI-digested pGR.FoMV.pCF. The obtained empty VOX vectors were named pCF101, pCF90, pCF55, and pCF45, whereas their GFP-containing counterparts were named pCF101-GFP, pCF90-GFP, pCF55-GFP, and pCF45-GFP, respectively.
FoMV pCF-derived expression constructs were inoculated onto plants and analyzed as specified in the sections below and are summarized in Supplemental Table S5.
Construction of an Infectious Full-Length cDNA Clone of the FoMV Isolate PV139 RNA Genome
A commercially synthesized (Invitrogen) full-length cDNA copy of the FoMV isolate PV139 was cloned into the pGR106 vector backbone between EcoRV and AflII restriction nuclease sites, generating the binary plasmid pGR-FoMV.PV139. A full-length FoMV cDNA in this plasmid was flanked at the 5′ end by the CaMV 35S promoter and at the 3′ end by the nos terminator sequence.
Construction of the FoMV VOX Vectors PV101 and PV101gw
The FoMV isolate PV139-derived expression vector PV101, enabling the integration of genes of interest using a standard restriction enzyme cloning, was constructed as follows. A fragment of pB-Fsgp2/pCF-101 was amplified by PCR (PCR1) with primers PV101-F2 and PV101-R2. A second PCR (PCR2) was done using the vector pGR-FoMV.PV139 as the template and primers PV101-F3 and PVsorg-8R. A fusion PCR (PCR3) was then carried out using a 1:1 mixture of amplicons produced in PCR1 and PCR2 as the template and primers PV101-F2 and PVsorg-8R. Finally, a fourth PCR (PCR4) was performed using the vector pGR-FoMV.PV139 as the template and primers PVsorg-6F and PV101-R1. Amplicons produced in PCR3 and PCR4 were then assembled into SpeI- plus AvrII-digested plasmid pGR-FoMV.PV139 using the NEBuilder HiFi DNA assembly system (New England Biolabs) following the manufacturer’s protocol to obtain the vector PV101.
A Gateway-compatible FoMV VOX vector was created as follows. A first PCR (PCR1) was conducted using pGR-FoMV.PV139 as the template and the oligonucleotides PVsorg-6F and PV101gw-R1. A Gateway cassette was amplified by PCR (PCR2) from the vector pGWB605 (Nakamura et al., 2010) using the oligonucleotides PV101gw-F2′ and PV101gw-R2′. A third amplicon (PCR3) was produced from pGR-FoMV.PV139 with the oligonucleotides PV101gw-F3 and PVsorg-8R. Amplicons from PCR1, PCR2, and PCR3 were assembled into SpeI- plus AvrII-digested pGR-FoMV.PV139 using the NEBuilder HiFi DNA assembly system to obtain the vector PV101gw.
Cloning of Genes Encoding Reporter Proteins and a Fungal Necrotrophic Effector Protein into the FoMV Isolate PV139-Derived Expression Vectors
The coding sequence of the S65T variant of GFP was amplified from the plasmid pActIsGFP using the oligonucleotides GFP5′-ClaI-fw and GFP3′-XbaI-rev. The corresponding amplicon was digested with ClaI plus XbaI and cloned into ClaI- plus XbaI-digested PV101 to create PV101-GFP. The GFP coding sequence also was amplified from pActIsGFP using the oligonucleotides attB1-GFP-F and attB2-GFP-R, and the obtained amplicon was recombined into the Gateway-enabled FoMV vector PV101gw using BP Clonase II enzyme mix (Invitrogen) following the manufacturer’s protocol, to produce PV101gw-GFP.
The coding sequence of the Parastagonospora nodorum ToxA gene was amplified from the plasmid pDONR207-ToxA+SP-STOP using the oligonucleotides ClaI-ToxA-F and XbaI-ToxA-R. The amplicon was then digested using ClaI plus XbaI and cloned into ClaI- plus XbaI-digested PV101 to produce PV101-ToxA.
The coding sequence of GUSPlus (Broothaerts et al., 2005) in the plasmid pRRes104.293 served as a template for two PCRs producing partially overlapping amplicons using primers ClaI-woGUS-F1 and woGUS-R1 (PCR1) and woGUS-F2 and XbaI-woGUS-R2 (PCR2). The amplicons from PCR1 and PCR2 were then fused together using an additional cycle of PCR and the oligonucleotides ClaI-woGUS-F1 and XbaI-woGUS-R2. The resulting amplicon, containing a GUSPlus coding sequence with the internal ClaI recognition site removed, was digested using ClaI and XbaI and cloned into ClaI- plus XbaI-digested PV101 to obtain PV101-GUSPlus.
FoMV PV139-derived expression constructs were inoculated onto plants and analyzed as specified in the sections below and are summarized in Supplemental Table S5.
Inoculation of N. benthamiana, Wheat, and Maize Seedlings with the FoMV Expression Constructs
FoMV PV101, PV101gw, and other expression constructs derived from these binary vectors and the plasmid pBIN61-p19 for the expression of the well-known RNA-silencing suppressor protein p19 from TBSV were introduced into the A. tumefaciens strain GV3101 pCH32 pSa-Rep (Hellens et al., 2000) by electroporation. Bacterial cultures were obtained by inoculating single colonies in liquid Luria-Bertani medium supplemented with gentamycin (25 μg mL−1) and kanamycin (50 μg mL−1) followed by incubation at 28°C for 20 h under constant shaking (250 rpm). A. tumefaciens cells were pelleted at 2,013g for 20 min at 17°C and then resuspended in an infiltration medium containing 10 mm MES, pH 5.6, 10 mm MgCl2, and 100 μm acetosyringone. Bacterial suspensions were adjusted to an OD600 of 1.2 to 1.5 and incubated at room temperature for at least 3 h. To initiate infection in N. benthamiana plants, each FoMV vector-containing A. tumefaciens suspension was mixed with an equal volume of pBIN61-P19-containing A. tumefaciens suspension and then pressure infiltrated into the abaxial side of fully expanded leaves of three to four young, six- to eight-leaf-stage, seedlings using a needleless syringe.
To initiate infection in wheat and maize plants, leaves of young seedlings were rub inoculated using a FoMV-containing sap prepared from N. benthamiana leaves agroinfiltrated as described above and harvested at 5 to 7 dpi. The sap, produced by finely grinding N. benthamiana leaves in 0.67% (w/v) deionized water using a mortar and pestle, was supplemented with 1% (w/v) Celite 545 AW (Sigma-Aldrich) and used for rub inoculation of the first two leaves of two-leaf-stage wheat seedlings or the first three leaves of three- to four-leaf-stage maize seedlings. At 5 min or more post inoculation, leaves were sprayed with tap water to remove the residual sap and Celite, and plants were bagged and kept under high humidity and low light for ∼24 h before they were returned to the standard growth conditions. Typically, each different FoMV was inoculated to at least six wheat plants and/or at least four maize plants per experiment. Each experiment was repeated at least twice.
RT-PCR
Total RNA was extracted from FoMV- or mock-inoculated or -agroinfiltrated plants using TRIzol (Invitrogen) and then treated with RQ1 RNase-free DNase (Promega) following the manufacturer’s instructions. First-strand cDNAs were produced using SuperScript III Reverse Transcriptase (Invitrogen) and random hexamer primers following the manufacturer’s protocol and used as templates for PCR with the GoTaq G2 DNA Polymerase (Promega). For the detection of FoMV, the oligonucleotides qFoMV3464F and qFoMV3597R, spanning nucleotides 3,464 to 3,597 in ORF1, were used. The housekeeping internal control N. benthamiana PP2A gene and the wheat CDC48 gene (Paolacci et al., 2009) were detected by PCR using the oligonucleotides NbPP2AF/NbPP2A (Liu et al., 2012) and TaCDC48F/TaCDC48R (Lee et al., 2014), respectively.
Immunodetection of GFP and FoMV CP
Aliquots of 50 to 100 mg of leaf samples were ground in 1.5-mL tubes with 6% (v/w) suspension buffer (100 mm Tris-HCl, pH 8, and 1 mm dl-DTT) using micropestles and centrifuged at 16,100g for 1 min to pellet any cell debris. One hundred microliters of leaf extract was supplemented with 33 μL of 4× Laemmli extraction buffer (8% [w/v] SDS, 20% [v/v] 2-mercaptoethanol, 40% [w/v] glycerol, 0.008% [w/v] Bromophenol Blue, and 0.25 m Tris-HCl, pH 6.8) and incubated at 95°C for 5 min to allow the denaturation of proteins. The samples were loaded onto a 16% (v/v) SDS-polyacrylamide gel. Proteins were separated by electrophoresis in 25 mm Tris, 192 mm Gly, and 0.1% (v/v) SDS and then electrotransferred to a nitrocellulose membrane (Protran Premium 0.45 NC; GE Healthcare Life Sciences) for 90 min at 90 V in 25 mm Tris, 192 mm Gly, and 20% (v/v) methanol. After transfer, the membranes were stained by incubating in a Ponceau S (Sigma-Aldrich) solution (5% [v/v] acetic acid and 0.1% [w/v] Ponceau S) for 5 min to verify that equal total protein was loaded for each sample. The membranes were then destained by rinsing in PBS-T buffer (50 mm Tris, 150 mm NaCl, and 0.1% [v/v] Tween 20) and then blocked in PBS-T supplemented with 5% (w/v) dry milk for 45 min at room temperature and under constant shaking (70 rpm). Blocked membranes were incubated overnight at 4°C under constant shaking with primary antibodies diluted in PBS-T supplemented with 5% (w/v) dry milk. The rabbit anti-GFP tag monoclonal antibody (G10362; Invitrogen) used in Figure 2 was diluted at 1:200. The anti-GFP polyclonal antibody (A-11122; Invitrogen) used in Figure 6 and Supplemental Figure S5 was diluted at 1:2,000, and the rabbit anti-FoMV-CP polyclonal antibody (ChinaPeptides) was diluted at 1:5,000. Nonbound antibodies were eliminated by rinsing the membranes three times in PBS-T for 15 min at room temperature under constant shaking. Incubation with secondary antibody (goat anti-rabbit peroxidase antibody; A0545; Sigma-Aldrich) diluted at 1:10,000 in PBS-T plus 5% (w/v) dry milk was performed for 3 h at room temperature under constant shaking. Nonbound antibodies were removed by rinsing the membranes three times in PBS-T for 10 min at room temperature under constant shaking. Membrane-bound immune complexes were revealed with the ECL Prime kit (GE Healthcare Life Sciences). Chemiluminescence signals were visualized using Hyperfilm ECL (GE Healthcare Life Sciences).
GUS Staining
Leaf segments were placed onto six-well plates (Thermo Fisher Scientific) and immersed in staining solution (57 mm sodium phosphate dibasic, 42 mm sodium phosphate monobasic, 10 mm EDTA, 0.01% [v/v] Triton X-100, 1 mm potassium ferricyanide, 1 mm potassium ferrocyanide, and 0.5 mg mL−1 5-bromo-4-chloro-3-indolyl-β-d-GlcA [Thermo Fisher Scientific]). The samples were then vacuum infiltrated several times for 10 min to help the staining solution penetrate leaves. The plates were sealed with cling film and incubated in the dark at 37°C for up to 24 h. The staining solution was removed, and the samples were destained in 70% ethanol at room temperature and under constant agitation. Ethanol was changed every 24 to 48 h until completion of the destaining process. Samples were illuminated using a white light box (Edvotek) for photography with a Nikon D90 camera.
Stereomicroscopy
Plant samples were observed with a Leica M205 FA stereomicroscope (Leica Microsystems). Fluorescence was visualized using either a GFP2 filter set (excitation filter, 460–500 nm; long-pass filter, 510 nm) or a GFP3 filter set (excitation filter, 450–490 nm; band-pass filter, 500–550 nm). Photographs were taken using Leica LAS AF software (Leica Microsystems).
Photography
Full leaf photographs were taken using a Nikon D90 camera (N. benthamiana and wheat) or an Olympus OM-D E-M1 Mark II camera (maize). For the fluorescence photography, plants were illuminated with blue light (440–460 nm excitation) using a Dual Fluorescent Protein flashlight (NightseaA). Long-pass (510 nm) or band-pass (500–555 nm) filters (Midwest Optical Systems) were mounted onto the camera objectives to block blue or blue plus red light, respectively, reflected from the excitation source. Maize full leaf fluorescence photographs were taken using the Live Composite Time mode of the Olympus OM-D E-M1 Mark II camera.
Accession Numbers
Raw next-generation sRNA-seq data have been deposited to the European Nucleotide Archive under accession number PRJEB21979. The complete sequences of FoMV pCF and PV139 were submitted to GenBank under accession numbers MF573298 and MF573299, respectively.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Sequences of the four differently sized sgp2 duplications used for the development of a series of FoMV vectors.
Supplemental Figure S2. Progression of PV101-mediated GFP expression over time in directly inoculated wheat leaves.
Supplemental Figure S3. Assessment of PV101-mediated GFP expression in systemically infected wheat and maize leaves.
Supplemental Figure S4. Gateway-enabled FoMV vector PV101gw-mediated expression of GFP in N. benthamiana.
Supplemental Figure S5. Comparison of protein expression efficiency from FoMV vectors PV101 and PV169.
Supplemental Table S1. Single-nucleotide polymorphisms identified between genomes of FoMV pCF and FoMV PV139.
Supplemental Table S2. Assessment of PV101-mediated GFP expression in wheat cultivars of diverse geographical origins.
Supplemental Table S3. FoMV-directed expression of GUSPlus in maize line B73.
Supplemental Table S4. Oligonucleotides used in this study.
Supplemental Table S5. Summary of the experiments involving FoMV expression vectors carried out in this study.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank Yau-Heiu Hsu (Graduate Institute of Biotechnology, National Chung University) and Jeff Ackerman and Dallas Seifers (Kansas State University Agricultural Research Center) for kindly providing the infectious FoMV cDNA clone pCF and the FoMV isolate PV139, respectively. We also thank Robert Stebbins (U.S. Department of Agriculture, Agricultural Research Service, North Central Regional Plant Introduction Station) for providing seed of the maize inbred line B73; Pauline Bansept-Basler (Syngenta), Beat Keller (University of Zurich), and Jeff Ellis, Rohit Mago, and Peter Dodds (Commonwealth Scientific and Industrial Research Organization) for providing seed of various wheat cultivars; Guus Bakkeren (Agriculture and Agri-Food Canada) for providing the A. tumefaciens strain COR308 UIA143 pMP90 pCH32; David Baulcombe (University of Cambridge) for the PVX expression vector pGR106; Patrice Dunoyer (Institut de Biologie Moléculaire des Plantes du Centre National de la Recherche Scientifique, Université de Strasbourg) for the plasmid pBIN61-p19; Caroline Sparks (Rothamsted Research) for the plasmid pActIsGFP; Alison Huttly (Rothamsted Research) for the plasmid pRRes104.293; and Graeme Kettles (Rothamsted Research) for the plasmid pDONR207-ToxA+SP-STOP. We are grateful to Monika Spiller and Scott Young for comments on the article and to Michael Hammond-Kosack for assistance with photography.
Footnotes
This work was supported by the Rothamsted-Syngenta Alliance (RoSy 2020 Project CP8.1) and Biotechnology and Biological Sciences Research Council through the Institute Strategic Program Grants “20:20 Wheat” (BB/J/00426X/1) and “Designing Future Wheat” (BB/P016855/1).
[CC-BY]: Article free via Creative Commons CC-BY 4.0 license.
References
- Broothaerts W, Mitchell HJ, Weir B, Kaines S, Smith LMA, Yang W, Mayer JE, Roa-Rodríguez C, Jefferson RA (2005) Gene transfer to plants by diverse species of bacteria. Nature 433: 629–633 [DOI] [PubMed] [Google Scholar]
- Brown RH, Hattersley PW (1989) Leaf anatomy of C3-C4 species as related to evolution of C4 photosynthesis. Plant Physiol 91: 1543–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman S, Kavanagh T, Baulcombe D (1992) Potato virus X as a vector for gene expression in plants. Plant J 2: 549–557 [DOI] [PubMed] [Google Scholar]
- Choi IR, Stenger DC, Morris TJ, French R (2000) A plant virus vector for systemic expression of foreign genes in cereals. Plant J 23: 547–555 [DOI] [PubMed] [Google Scholar]
- Csorba T, Burgyán J (2016) Antiviral silencing and suppression of gene silencing in plants. In Wang A, Zhou X, eds, Current Research Topics in Plant Virology. Springer International Publishing, Cham, Switzerland, pp 1–33 [Google Scholar]
- Dagvadorj B, Ozketen AC, Andac A, Duggan C, Bozkurt TO, Akkaya MS (2017) A Puccinia striiformis f. sp. tritici secreted protein activates plant immunity at the cell surface. Sci Rep 7: 1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickmeis C, Fischer R, Commandeur U (2014) Potato virus X-based expression vectors are stabilized for long-term production of proteins and larger inserts. Biotechnol J 9: 1369–1379 [DOI] [PubMed] [Google Scholar]
- Domingo E, Sheldon J, Perales C (2012) Viral quasispecies evolution. Microbiol Mol Biol Rev 76: 159–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faris JD, Zhang Z, Lu H, Lu S, Reddy L, Cloutier S, Fellers JP, Meinhardt SW, Rasmussen JB, Xu SS, et al. (2010) A unique wheat disease resistance-like gene governs effector-triggered susceptibility to necrotrophic pathogens. Proc Natl Acad Sci USA 107: 13544–13549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco-Orozco B, Berepiki A, Ruiz O, Gamble L, Griffe LL, Wang S, Birch PRJ, Kanyuka K, Avrova A (2017) A new proteinaceous pathogen-associated molecular pattern (PAMP) identified in ascomycete fungi induces cell death in Solanaceae. New Phytol 214: 1657–1672 [DOI] [PubMed] [Google Scholar]
- Friesen TL, Stukenbrock EH, Liu Z, Meinhardt S, Ling H, Faris JD, Rasmussen JB, Solomon PS, McDonald BA, Oliver RP (2006) Emergence of a new disease as a result of interspecific virulence gene transfer. Nat Genet 38: 953–956 [DOI] [PubMed] [Google Scholar]
- Hamilton CM. (1997) A binary-BAC system for plant transformation with high-molecular-weight DNA. Gene 200: 107–116 [DOI] [PubMed] [Google Scholar]
- Harris RS. (2007) Improved Pairwise Alignment of Genomic DNA. PhD thesis. Pennsylvania State University, State College [Google Scholar]
- Heim R, Cubitt AB, Tsien RY (1995) Improved green fluorescence. Nature 373: 663–664 [DOI] [PubMed] [Google Scholar]
- Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM (2000) pGreen: a versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol 42: 819–832 [DOI] [PubMed] [Google Scholar]
- Ji Q, Yang B, Lee M, Chen Y, Lubberstedt T (2010) Mapping of quantitative trait loci/locus conferring resistance to Foxtail mosaic virus in maize using the intermated B73 × Mo17 population. Plant Breed 129: 721–723 [Google Scholar]
- Kettles GJ, Bayon C, Canning G, Rudd JJ, Kanyuka K (2017) Apoplastic recognition of multiple candidate effectors from the wheat pathogen Zymoseptoria tritici in the nonhost plant Nicotiana benthamiana. New Phytol 213: 338–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettles GJ, Bayon C, Sparks CA, Canning G, Kanyuka K, Rudd JJ (2018) Characterization of an antimicrobial and phytotoxic ribonuclease secreted by the fungal wheat pathogen Zymoseptoria tritici. New Phytol 217: 320–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuze J. (2014) siRNA deep sequencing and assembly: piecing together viral infections. In Gullino ML, Bonants PJM, eds, Detection and Diagnostics of Plant Pathogens. Springer, Dordrecht, The Netherlands, pp 21–38 [Google Scholar]
- Lacomme C, Santa Cruz S (1999) Bax-induced cell death in tobacco is similar to the hypersensitive response. Proc Natl Acad Sci USA 96: 7956–7961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Hammond-Kosack KE, Kanyuka K (2012) Barley stripe mosaic virus-mediated tools for investigating gene function in cereal plants and their pathogens: virus-induced gene silencing, host-mediated gene silencing, and virus-mediated overexpression of heterologous protein. Plant Physiol 160: 582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Rudd JJ, Hammond-Kosack KE, Kanyuka K (2014) Mycosphaerella graminicola LysM effector-mediated stealth pathogenesis subverts recognition through both CERK1 and CEBiP homologues in wheat. Mol Plant Microbe Interact 27: 236–243 [DOI] [PubMed] [Google Scholar]
- Lin MK, Chang BY, Liao JT, Lin NS, Hsu YH (2004) Arg-16 and Arg-21 in the N-terminal region of the triple-gene-block protein 1 of Bamboo mosaic virus are essential for virus movement. J Gen Virol 85: 251–259 [DOI] [PubMed] [Google Scholar]
- Liu Z, Kearney CM (2010) An efficient Foxtail mosaic virus vector system with reduced environmental risk. BMC Biotechnol 10: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Shi L, Han C, Yu J, Li D, Zhang Y (2012) Validation of reference genes for gene expression studies in virus-infected Nicotiana benthamiana using quantitative real-time PCR. PLoS ONE 7: e46451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Xie K, Jia Q, Zhao J, Chen T, Li H, Wei X, Diao X, Hong Y, Liu Y (2016) Foxtail mosaic virus-induced gene silencing in monocot plants. Plant Physiol 171: 1801–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Friesen TL, Ling H, Meinhardt SW, Oliver RP, Rasmussen JB, Faris JD (2006) The Tsn1-ToxA interaction in the wheat-Stagonospora nodorum pathosystem parallels that of the wheat-tan spot system. Genome 49: 1265–1273 [DOI] [PubMed] [Google Scholar]
- Lu R, Malcuit I, Moffett P, Ruiz MT, Peart J, Wu AJ, Rathjen JP, Bendahmane A, Day L, Baulcombe DC (2003) High throughput virus-induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO J 22: 5690–5699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo R, Liu B, Xie Y, Li Z, Huang W, Yuan J, He G, Chen Y, Pan Q, Liu Y, et al. (2012) SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer E, Llorente B, Rodríguez-Concepción M, Daròs JA (2017) Rewiring carotenoid biosynthesis in plants using a viral vector. Sci Rep 7: 41645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning VA, Ciuffetti LM (2005) Localization of Ptr ToxA produced by Pyrenophora tritici-repentis reveals protein import into wheat mesophyll cells. Plant Cell 17: 3203–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning VA, Chu AL, Scofield SR, Ciuffetti LM (2010) Intracellular expression of a host-selective toxin, ToxA, in diverse plants phenocopies silencing of a ToxA-interacting protein, ToxABP1. New Phytol 187: 1034–1047 [DOI] [PubMed] [Google Scholar]
- Mei Y, Zhang C, Kernodle BM, Hill JH, Whitham SA (2016) A Foxtail mosaic virus vector for virus-induced gene silencing in maize. Plant Physiol 171: 760–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Moscou MJ, Wise RP (2009) Blufensin1 negatively impacts basal defense in response to barley powdery mildew. Plant Physiol 149: 271–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Mano S, Tanaka Y, Ohnishi M, Nakamori C, Araki M, Niwa T, Nishimura M, Kaminaka H, Nakagawa T, et al. (2010) Gateway binary vectors with the bialaphos resistance gene, bar, as a selection marker for plant transformation. Biosci Biotechnol Biochem 74: 1315–1319 [DOI] [PubMed] [Google Scholar]
- Panwar V, McCallum B, Bakkeren G (2013) Endogenous silencing of Puccinia triticina pathogenicity genes through in planta-expressed sequences leads to the suppression of rust diseases on wheat. Plant J 73: 521–532 [DOI] [PubMed] [Google Scholar]
- Paolacci AR, Tanzarella OA, Porceddu E, Ciaffi M (2009) Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol Biol 10: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen AQ, Niblett CL (1977) Purification and properties of Foxtail mosaic virus. Phytopathology 67: 1346–1351 [Google Scholar]
- Robertson NL, French R, Morris TJ (2000) The open reading frame 5A of Foxtail mosaic virus is expressed in vivo and is dispensable for systemic infection. Arch Virol 145: 1685–1698 [DOI] [PubMed] [Google Scholar]
- Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, Pasternak S, Liang C, Zhang J, Fulton L, Graves TA, et al. (2009) The B73 maize genome: complexity, diversity, and dynamics. Science 326: 1112–1115 [DOI] [PubMed] [Google Scholar]
- Seguin J, Rajeswaran R, Malpica-López N, Martin RR, Kasschau K, Dolja VV, Otten P, Farinelli L, Pooggin MM (2014) De novo reconstruction of consensus master genomes of plant RNA and DNA viruses from siRNAs. PLoS ONE 9: e88513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifers DL, Harvey TL, Haber S, She YM, Chernushevich I, Ens W, Standing KG (1999) Natural infection of sorghum by Foxtail mosaic virus in Kansas. Plant Dis 83: 905–912 [DOI] [PubMed] [Google Scholar]
- Sempere RN, Gómez P, Truniger V, Aranda MA (2011) Development of expression vectors based on Pepino mosaic virus. Plant Methods 7: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva D, Santos G, Barroca M, Collins T (2017) Inverse PCR for point mutation introduction. Methods Mol Biol 1620: 87–100 [DOI] [PubMed] [Google Scholar]
- Sztuba-Solińska J, Stollar V, Bujarski JJ (2011) Subgenomic messenger RNAs: mastering regulation of (+)-strand RNA virus life cycle. Virology 412: 245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C, Wei J, Han Q, Liu R, Duan X, Fu Y, Huang X, Wang X, Kang Z (2015) PsANT, the adenine nucleotide translocase of Puccinia striiformis, promotes cell death and fungal growth. Sci Rep 5: 11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatineni S, McMechan AJ, Hein GL, French R (2011) Efficient and stable expression of GFP through Wheat streak mosaic virus-based vectors in cereal hosts using a range of cleavage sites: formation of dense fluorescent aggregates for sensitive virus tracking. Virology 410: 268–281 [DOI] [PubMed] [Google Scholar]
- Tatineni S, McMechan AJ, Bartels M, Hein GL, Graybosch RA (2015) In vitro transcripts of wild-type and fluorescent protein-tagged Triticum mosaic virus (family Potyviridae) are biologically active in wheat. Phytopathology 105: 1496–1505 [DOI] [PubMed] [Google Scholar]
- Xu W, Meng Y, Surana P, Fuerst G, Nettleton D, Wise RP (2015) The knottin-like Blufensin family regulates genes involved in nuclear import and the secretory pathway in barley-powdery mildew interactions. Front Plant Sci 6: 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C, Li C, Yan L, Jackson AO, Liu Z, Han C, Yu J, Li D (2011) A high throughput Barley stripe mosaic virus vector for virus induced gene silencing in monocots and dicots. PLoS ONE 6: e26468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino DR. (2010) Using the Velvet de novo assembler for short-read sequencing technologies. Curr Protoc Bioinformatics 11: 11.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang L, Hunter D, Voogd C, Joyce N, Davies K (2013) A Narcissus mosaic viral vector system for protein expression and flavonoid production. Plant Methods 9: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yu D, Zhang Y, Liu K, Xu K, Zhang F, Wang J, Tan G, Nie X, Ji Q, et al. (2017) Vacuum and co-cultivation agroinfiltration of (germinated) seeds results in Tobacco rattle virus (TRV) mediated whole-plant virus-induced gene silencing (VIGS) in wheat and maize. Front Plant Sci 8: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]