Abstract
Study Objectives:
Schizophrenia patients have correlated deficits in sleep spindle density and sleep-dependent memory consolidation. In addition to spindle density, memory consolidation is thought to rely on the precise temporal coordination of spindles with slow waves (SWs). We investigated whether this coordination is intact in schizophrenia and its relation to motor procedural memory consolidation.
Methods:
Twenty-one chronic medicated schizophrenia patients and 17 demographically matched healthy controls underwent two nights of polysomnography, with training on the finger tapping motor sequence task (MST) on the second night and testing the following morning. We detected SWs (0.5–4 Hz) and spindles during non-rapid eye movement (NREM) sleep. We measured SW–spindle phase–amplitude coupling and its relation with overnight improvement in MST performance.
Results:
Patients did not differ from controls in the timing of SW–spindle coupling. In both the groups, spindles peaked during the SW upstate. For patients alone, the later in the SW upstate that spindles peaked and the more reliable this phase relationship, the greater the overnight MST improvement. Regression models that included both spindle density and SW–spindle coordination predicted overnight improvement significantly better than either parameter alone, suggesting that both contribute to memory consolidation.
Conclusion:
Schizophrenia patients show intact spindle–SW temporal coordination, and these timing relationships, together with spindle density, predict sleep-dependent memory consolidation. These relations were seen only in patients suggesting that their memory is more dependent on optimal spindle–SW timing, possibly due to reduced spindle density. Interventions to improve memory may need to increase spindle density while preserving or enhancing the coordination of NREM oscillations.
Keywords: sleep spindles, slow waves, schizophrenia, motor, procedural learning, memory consolidation.
Statement of Significance
Sleep spindles, a defining electroencephalographic feature of non-rapid eye movement sleep, act in concert with slow waves to consolidate memory. Patients with schizophrenia have a reduction in sleep spindles that correlates with impaired sleep-dependent memory consolidation. In this first investigation of slow wave–spindle coordination in schizophrenia, we demonstrate that despite markedly reduced spindles, the temporal relationship between spindles and slow waves is preserved and that it correlates with sleep-dependent memory consolidation. Together, spindle density (spindles per minute) and slow wave-spindle coordination predicted memory consolidation better than either parameter alone. These findings illuminate basic mechanisms of memory consolidation and indicate that interventions to improve memory in schizophrenia may need to both increase spindle density and preserve or enhance slow wave–spindle coordination.
INTRODUCTION
Cognitive deficits are core features of schizophrenia that often precede the onset of psychosis and are the strongest predictor of poor functional outcome.1,2 The neural bases of cognitive deficits are poorly understood and, consequently, effective treatments are lacking.3 Recent work places deficient sleep-dependent memory consolidation among the cognitive deficits of schizophrenia and implicates reduced sleep spindles, a defining electroencephalographic (EEG) feature of Stage 2 non-rapid eye movement (NREM) sleep (N2), as a potentially treatable mechanism.4 Schizophrenia patients have deficient sleep-dependent consolidation of both procedural5–10 and declarative11 memory that, in some studies, correlates with reduced sleep spindle density (spindles per minute) and number.10,11 These relations are consistent with a large basic literature showing that spindle activity correlates with measures of intelligence and sleep-dependent memory consolidation.12 Merely having enough spindles may not be sufficient—intact sleep-dependent memory consolidation is also thought to rely on the precise temporal coordination of spindles with neocortical slow waves (SWs) and hippocampal sharp wave ripples.13–17 This coordination is disrupted in a rat model of schizophrenia18 but has not been studied in patients. Since hippocampal ripples are not detectable with surface EEG, we reanalyzed the data of a previously published study10 to investigate the temporal coordination of spindles with SWs in schizophrenia and its relation to memory.
Sleep spindles, seen in the EEG as brief bursts of 12–15 Hz synchronous activity, are initiated by the thalamic reticular nucleus (TRN) and are mediated by thalamocortical networks.19 Hippocampal sharp wave ripples, which are associated with memory reactivation,20 preferentially occur in the troughs of spindles,21 which preferentially occur during SW upstates.13–16 SWs are generated within thalamocortical networks and arise from the rhythmic depolarization and hyperpolarization of cortical pyramidal neurons.22 Human and animal studies23–25 report that SWs synchronize neuronal activity in structures relevant to memory including the hippocampus and thalamus. Coordinated SW–spindle–ripple activity is theorized to redistribute recently encoded memories from temporary dependence on the hippocampus to longer term representation in the cortex.14,16,21,26–29
To investigate SW–spindle coordination, we analyzed polysomnography (PSG) data from a previously reported study of chronic medicated schizophrenia patients and demographically matched healthy controls.10,30 Using this data set, we previously reported that in the context of normal sleep architecture and quality, patients showed significant reductions in N2 sleep spindle number and density, low sigma (12–13.5 Hz) power, and cortical spindle coherence. In patients alone, spindle density correlated with overnight improvement of the finger tapping motor sequence task (MST), the most extensively validated probe of sleep-dependent memory consolidation.31–34 In the present study, we investigated whether patients show impaired SW–spindle coordination by measuring both the SW phase at the spindle peak and SW–spindle phase amplitude coupling. We then examined whether SW–spindle coordination contributes to the sleep-dependent consolidation of motor procedural memory using the MST.
METHODS AND MATERIALS
Participants
Twenty-one schizophrenia outpatients and 17 healthy control participants matched for age, sex, and parental education completed the study (Supplemental Tables S1, S2 provide patient medications and participant characteristics, respectively). All participants gave written informed consent and were paid for participation, including a monetary bonus ($.05) for each correctly typed MST sequence. The study was approved by the Institutional Review Boards of Massachusetts General Hospital (MGH), the Massachusetts Department of Mental Health, and Beth Israel Deaconess Medical Center. Data will be made available upon request.
Procedures
See10 for procedural details. Participants spent two consecutive weeknights in the MGH Clinical Research Center and were allowed to sleep up to 10 hours with PSG. They engaged in their usual daytime activities but were asked not to nap, which was confirmed by sleep diary and actigraphy. On the second night, participants trained on the MST 1 hr before their usual bedtime and were tested the following morning, 1 hr after awakening.
Finger Tapping MST
The MST involves pressing four numerically labeled keys on a standard computer keyboard with the fingers of the left hand, repeating a five-element sequence (4-1-3-2-4) “as quickly and accurately as possible” for 30 s. The numeric sequence is displayed at the top of the screen, and dots appear beneath it with each keystroke. During both training and test sessions, participants alternate tapping and resting for 30 s for a total of 12 tapping trials. The primary outcome measure is the number of correct sequences typed per trial, which reflects both the speed and the accuracy of performance. Overnight improvement is calculated as the percentage of increase in correct sequences from the last three training trials at night to the first three test trials the following morning.33
PSG
Data were digitally acquired at 100 Hz using an Embla N7000 system (Medcare Systems, Buffalo, New York) with eight EEG electrodes (F3, F4, C3, Cz, C4, Pz, O1, and O2) placed according to the 10–20 system. Electrodes were referenced to the linked mastoids. Electromyography and electrooculography were also acquired. Recordings were divided into 30-s epochs and scored according to standard criteria35 as WAKE, REM, N1, N2, and N3 by expert scorers blind to night and diagnosis. PSG data were preprocessed and analyzed using BrainVision Analyzer 2.0 (BrainProducts, Germany), MATLAB R2014a (The MathWorks, Massachusetts), and R.36 Data were filtered at 0.5–35 Hz. Artifacts identified using automated algorithms were visually confirmed and removed.
Spindle Detection
Spindles were automatically detected using a wavelet-based algorithm that has been validated against hand-counted spindles and 12–15 Hz sigma power in healthy and schizophrenia participants10 and against expert consensus spindle counts.37
SW Detection
SWs were detected using a modified version of a published algorithm14 (Supplemental Methods, Figure S1). SW number, density (number per minute), mean duration, and mean peak-to-peak amplitudes were calculated for each electrode.
SW measures were compared across electrodes, groups, and nights using linear mixed-model regression.38 Subject and night were random effects, and electrode, group, night, and their interactions were fixed effects. Night was a fixed effect to account for the systematic order of the two nights and also a random effect to account for differences between nights unrelated to order (e.g., different electrode impedances). We examined group differences in the spatial distribution of SW–spindle parameters with electrode by group interactions and post hoc generalized linear hypothesis tests as implemented by the multcomp R package.39 These tests correct for multiple comparisons while accounting for any dependencies between the electrodes.
Data from O1 and O2 were deemed unreliable and omitted from the analyses, since the SW amplitude was significantly lower than other electrodes (controls: t(1, 15) = 22.9, p < 10−6; patients: t(1, 19) = 13.25, p < 10−6), and the density of suprathreshold SWs was significantly lower than other electrodes and highly variable across the participants (Table 1).
Table 1.
SWs during N2.
| Electrodea | Night | Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F3 | F4 | Cz | C3 | C4 | Pz | Nonlearning | Learning | Controls | Patients | |
| SW density, no./min | 3.90 ± 0.90 | 3.96 ± 0.79 | 4.15 ± 1.09 | 3.10 ± 0.91 | 3.08 ± 0.91 | 3.08 ± 1.32 | 3.54 ± 1.10 | 3.48 ± 1.11 | 3.20 ± 1.01 | 3.77 ± 1.11 |
| F(5, 277) = 49.77, p < 10−6 | F(1, 37) = .82, p = .37 | F(1, 34) = 3.11, p = .09 | ||||||||
| SW p-p Amp, µV | 100 ± 22 | 100 ± 22 | 93 ± 21 | 90 ± 20 | 88 ± 20 | 84 ± 20 | 91 ± 20 | 93 ± 22 | 86 ± 17 | 97 ± 23 |
| F(5, 274) = 84.49, p < 10−6 | F(1, 35) = .72, p = .40 | F(1, 34) = 2.80, p = .10 | ||||||||
| SW Duration, s | 1.15 ± .07 | 1.15 ± .08 | 1.12 ± .07 | 1.14 ± .08 | 1.15 ± .08 | 1.16 ± .07 | 1.15 ± .07 | 1.14 ± .07 | 1.16 ± .07 | 1.12 ± .07 |
| F(5, 275) = 16.50, p < 10−6 | F(1, 35) = 8.36, p = .007 | F(1, 34) = 2.98, p = .09 | ||||||||
| Relative sigma power locked to SWs | .23 ± .09 | .23 ± .09 | .31 ± .16 | .28 ± .14 | .27 ± .13 | .37 ± .25 | .28 ± .16 | .28 ± .16 | .37 ± .19 | .21 ± .07 |
| F(5, 308) = 41.73, p < 10−6 | F(1, 307) = .002, p = .96 | F(1, 34) = 16.96, p = .0002 | ||||||||
Mean ± SD; F- and p values were derived from mixed-model regression.
SW, slow wave.
a O1 and O2 were excluded from analyses (see Methods section): SW density: O1: 0.67 ± 0.67, O2: 0.62 ± 0.66.
SW–Spindle Coordination Analyses
Overview
EEG analyses were conducted for each night (nonlearning and learning) and for N2 and N3 separately. (A) First, to visualize sigma power (12–15 Hz, spindle frequency band) in relation to SWs, we plotted spectrograms. (B) Next, we quantified the timing of spindles occurring with SWs by identifying the SW phase at the spindle peak, which is the most reliable measure of spindle timing. We also examined the consistency of this relationship for each participant. We determined whether these parameters—SW phase at spindle peak and consistency—differed by group and, to test our main hypothesis, we correlated them with sleep-dependent memory consolidation. (C) As a secondary analysis to confirm timing results, we used the standard technique, phase amplitude coupling (PAC),40–42 which uses all spindle time points to identify the SW phase at which sigma amplitude is maximal and tests the significance of this coupling.
Spectrograms of Sigma Power During SWs
Sigma power was derived for a time window of ±1.5 s centered on the negative peaks (x) of all detected SWs from multitapered spectrograms (2 tapers, 10-ms sliding window, 1 Hz bandwidth) using the Chronux toolbox in MATLAB.43 Spectrograms were time locked to SW downstates, which are typically sharper and more distinct than the longer and more attenuated upstates.14 From these spectrograms, we computed the relative sigma power within these SW time windows, This relative measure corrects for subject-specific baseline differences across the entire power spectrum.37
SW Phase at Spindle Peak
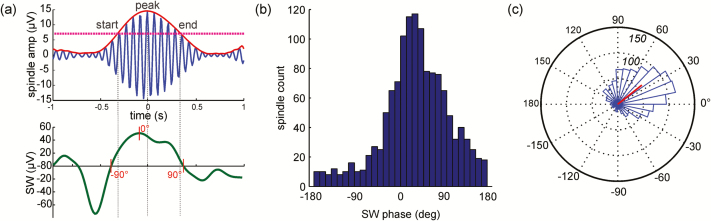
We focused on spindle peak amplitude (Figure 1a) as our most reliable index of spindle timing but also identified spindle start and end times (Supplementary Figures S2 and S3). The EEG signal was filtered in the SW (0.5–4Hz) and sigma frequency bands using a two-way least-squares finite impulse response filter as implemented by eeglab function eegfilt.44 A 2-s time window around each spindle detection point was considered. The spindle peak was defined as the point of maximum amplitude in the spindle envelope derived from the Hilbert transform45 of the EEG trace filtered in the sigma band. Spindle start and end times were identified by a threshold of 2 times the average amplitude of the entire signal envelope. The phase of the SW at the start, peak, and end of the spindle were derived from the Hilbert transform of the EEG trace filtered in the SW band. Histograms of the SW phase at these three spindle points were computed for each electrode (Figure 1, Supplementary Figure S2, S3). For each histogram, the angular mean and variance of the SW phase distribution were found using the circular statistics toolbox in MATLAB.46 For this and subsequent analyses, we considered only those spindles for which the corresponding peak-to-peak amplitude of the SW was greater than 70 μV. Thus ensured that we were analyzing prototypical SWs as defined by standard sleep scoring criteria35 and eliminated low amplitude, low frequency fluctuations, which are less likely to represent true up- and down-state activity (Supplementary Figures S2 and S3). If, after amplitude thresholding, fewer than 30 SW-associated spindles remained, the electrode was excluded from these analyses.
Figure 1.
Slow wave (SW) phase at spindle peak. (a) Timing of one sleep spindle (top, blue trace, red envelope derived from the Hilbert transform) in relation to the corresponding SW (bottom, green trace). (b) Distribution of SW phases at spindle peaks pooled across control participants at Cz for SWs >70µV and (c) plotted on an angular histogram (numbers in italics represent spindle counts); red line represents the angular mean and variance of the phase distribution.
Measurement of the consistency of the SW phase at spindle peak (and start and end) was based on the deviation of histograms from a uniform distribution (Supplementary Figures S2 and S3). This “phase consistency” is equal to the area between the curves defined by the SW phase cumulative distribution function (CDF) and the uniform CDF, according to the Cramér-von Mises test.47 The area between the curves was estimated by numerical integration via the trapezoidal method in MATLAB (function trapz). This measure tests the degree to which the phase distribution shows a preferential peak relative to a uniform distribution and would have a value close to one when the SW phase at a specific spindle time point is consistent across spindles.
SW–spindle coordination parameters (: SW phase at spindle peak or phase consistency) were regressed on overnight MST improvement using a model that included Group and its interaction with the coordination parameter to test whether relations with MST improvement differed by group: Robust regression48 was used to limit the influence of outliers. To test whether including both spindle density and SW–spindle coordination significantly improved the prediction of overnight MST improvement over either parameter alone, we compared the residual sum of squares of the individual (spindle density or coordination parameter) versus bivariate models (spindle density, coordination, and their interactions) using analysis of variance. Data from Cz was used to be consistent with our previous reports on this dataset10,30 and because more participants (19 patients and 15 controls) had recordings and quantifiable SW–spindle coordination parameters at Cz than at other electrodes.
C. SW–Spindle Phase Amplitude Coupling
Phase Amplitude coupling (PAC)40,41 measures the degree of coupling between the phase of the SW signal and the amplitude of sigma activity, Asigma, within the associated spindle.42 It is defined as a mean complex vector which gives the preferential phase of the SW at which sigma amplitude is maximal, and the mean vector length, also known as the Modulation Index which quantifies the coupling between these two oscillations (Supplemental Methods and Figure S4 describe PAC computation).
RESULTS
We report N2 findings based on prior studies showing N2 spindle abnormalities in schizophrenia and correlations of N2 spindle activity with sleep-dependent memory consolidation.4,7,11 N3 results, which were similar, are reported in the Supplement.
Spindle Characteristics
As previously reported, compared to healthy controls, patients showed reduced spindle number (36%) and density (38%) but did not differ in the amplitude, frequency, sigma power, or duration of individual spindles based on data averaged across the two study nights.10 Spindle characteristics did not differ by night for either group.
SW Characteristics
Patients showed trends to greater SW density, greater SW peak-to-peak amplitude, and shorter SW duration relative to controls, and this did not differ by night (Table 1, Supplemetary Table S3). All SW parameters differed as a function of electrode (p < 10−6). In the combined groups, SW density and peak-to-peak amplitude were higher at Cz and frontal electrodes than at C3, C4, and Pz, consistent with the literature,49,50 and duration was shortest at Cz. There were significant group differences in the spatial variation in SW density (Group × Electrode interaction: F(5, 277) = 3.9, p = .002) and peak-to-peak amplitude (F(5, 274) = 3.6, p = .003) that were primarily driven by numerically higher SW density at Pz (z = 1.6, p = .12) and statistical trend toward higher peak-to-peak amplitude at Cz (z = 1.9, p = .09) in patients.
SW–Spindle Coordination Analyses
Except for the spectrograms, which included all detected SWs, SW–spindle coordination analyses included only SWs >70 μV peak-to-peak amplitude.
A. SW-Sigma Spectrograms
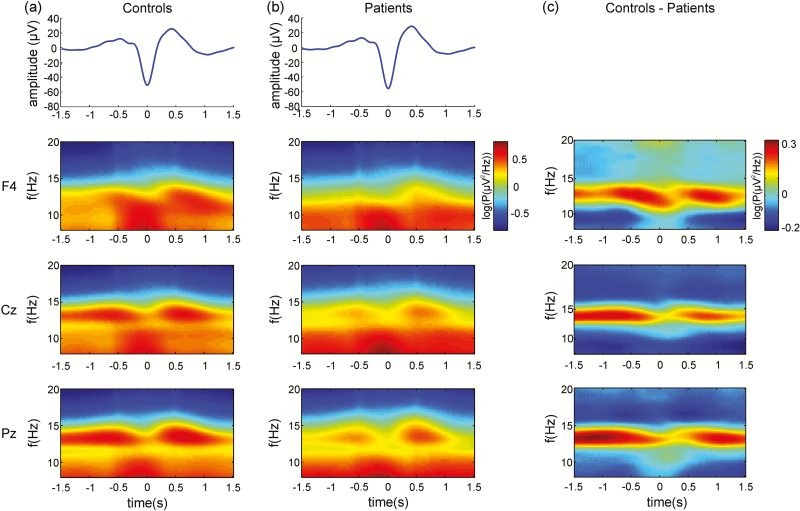
As there was no significant night effect on SW-locked sigma power, spectrograms time locked to the downstate of all detected SWs were averaged across the two nights (Table 1, Figure 2). For both groups, maximal sigma power occurred during the upstate of SWs. At all electrodes, SW-locked sigma power was significantly reduced in patients compared to controls, likely reflecting the spindle deficit.10 Results were similar for N3 (Supplementary Figure S5).
Figure 2.
Spectrograms of sigma power during slow waves (SWs) . The blue trace represents the average detected SW at F4. Spectrograms of sigma power locked to each electrode’s local SW downstate (0s) averaged across nights for (a) controls and (b) patients. For both groups, maximum sigma power occurred on the upstate of SWs. (c) Spectral difference between controls and patients. Patients showed a specific reduction in SW-locked sigma power compared to controls, reflecting their spindle deficit.
B. SW Phase at Spindle Peak
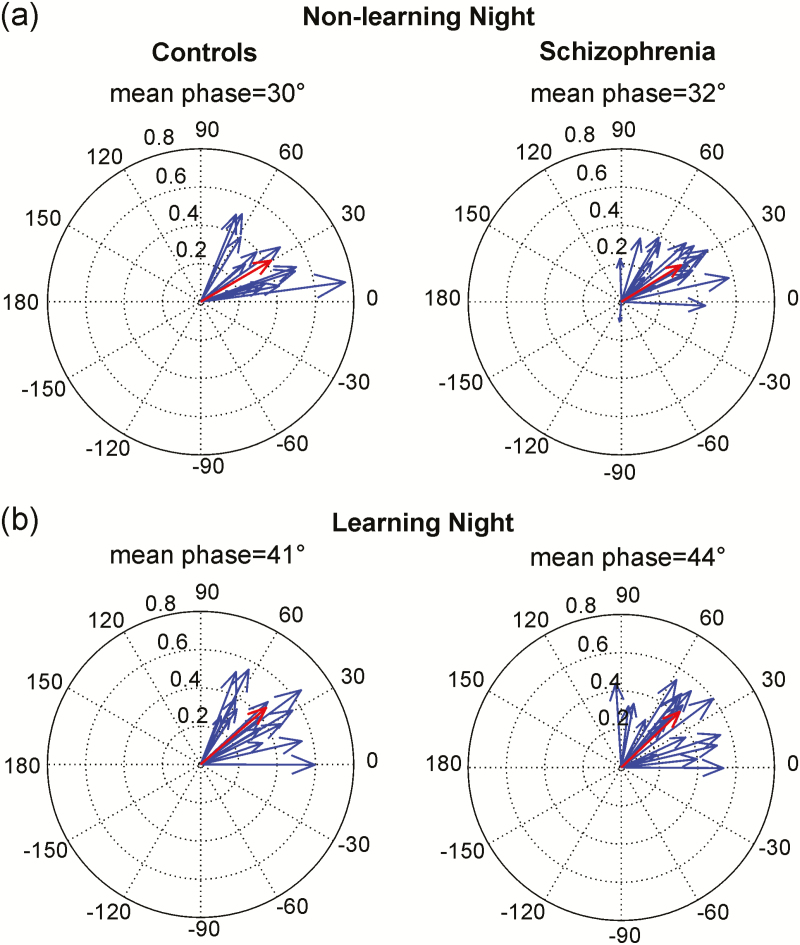
On average, spindles peaked during the upstate of SWs following the positive peak (0°) irrespective of night and group. Spindles peaked later in the SW upstate on the learning versus nonlearning night (F(1,28) = 5.94, p = .02), but the groups did not differ in the mean SW phase of spindle peaks at Cz on either night (nonlearning night: Figure 3a; Controls: 30°, Patients: 32°; learning night: Figure 3b; Controls: 41°; Patients: 44°). SW–spindle phase consistency did not differ between the groups and was similar at every electrode (Supplementary Table S4). This was also true for spindle starts and ends (Supplementary Figure S2 and S3). Similar findings were found for analysis of these relationships during N3 (Supplementary Figure S6 and Table S5).
Figure 3.
Mean slow wave (SW) phase at spindle peak at Cz. (a) Nonlearning and (b) learning nights. Blue arrows represent the mean SW phase for each participant; the length of each arrow (vector) represents the variance for that participant. Red arrows represent group means and their magnitude represents the between-subject variance.
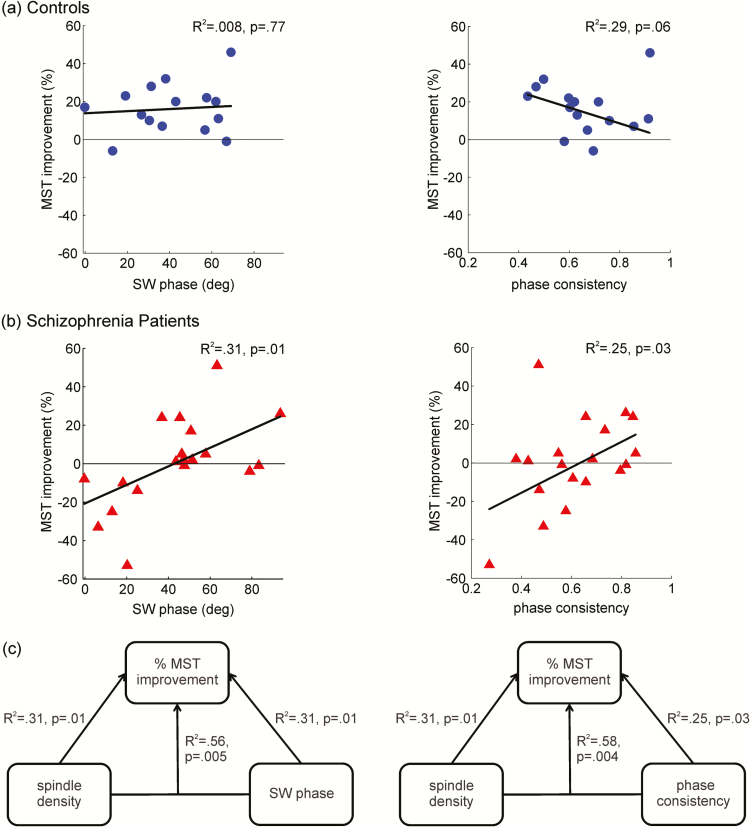
We next examined these parameters in relation to MST overnight improvement. We previously reported that MST improvement correlated with spindle density in schizophrenia but not in controls.10 Extending these results, SW–spindle coordination on the learning night also correlated with overnight improvement, for both SW phase at spindle peak (R2 = 0.31, p = .01) and SW–spindle phase consistency (R2 = 0.25, p = .03; Figure 4b) only in patients. The later within the SW upstate the spindle peaked and the more consistent this timing, the greater the overnight improvement. Controls, in contrast, showed no correlation of SW phase at spindle peak with MST improvement (R2 = 0.008, p = .77) and only a trend-level correlation of phase consistency with MST improvement (R2 = 0.29, p = .06; Figure 4a) that was not in the predicted direction. Regression analyses revealed that the slope of the relations between MST improvement and coordination differed significantly between groups for phase consistency (p = .03), with patients having a stronger positive relationship but did not differ significantly for SW phase at spindle peak (p = .15).
Figure 4.
Relations of slow wave (SW)–spindle coordination with sleep-dependent memory consolidation. Data from Cz. (a) Controls: SW phase at spindle peak and phase consistency did not show significant correlations with overnight motor sequence task (MST) improvement. (b) Patients: Both SW phase at spindle peak and phase consistency predicted overnight MST memory improvement (ie, memory consolidation). (c) Models that included both spindle density and SW–spindle coordination (either SW phase at spindle peak or phase consistency) predicted overnight MST improvement better than either parameter alone.
Both spindle density and SW phase at spindle peak predicted overnight MST improvement in schizophrenia, and together they predicted improvement significantly better than either parameter alone (Figure 4c; spindle density vs. bivariate model: F(2,15) = 4.1, p = .04; SW phase at spindle peak vs. bivariate model: F(2,15) = 4.2, p = .04). The same was true for spindle density and phase consistency (Figure 4c; spindle density vs. bivariate model: F(2,15) = 4.7, p = .03; phase consistency vs. bivariate model: F(2,15) = 7.8, p = .005). When both SW phase at spindle peak and phase consistency were entered into a bivariate model, they each explained a significant proportion of the variance in overnight improvement (R2 = 0.65; SW phase at spindle peak p = .001, phase consistency p = .002), suggesting that each contributes to memory consolidation.
C. SW–Spindle PAC
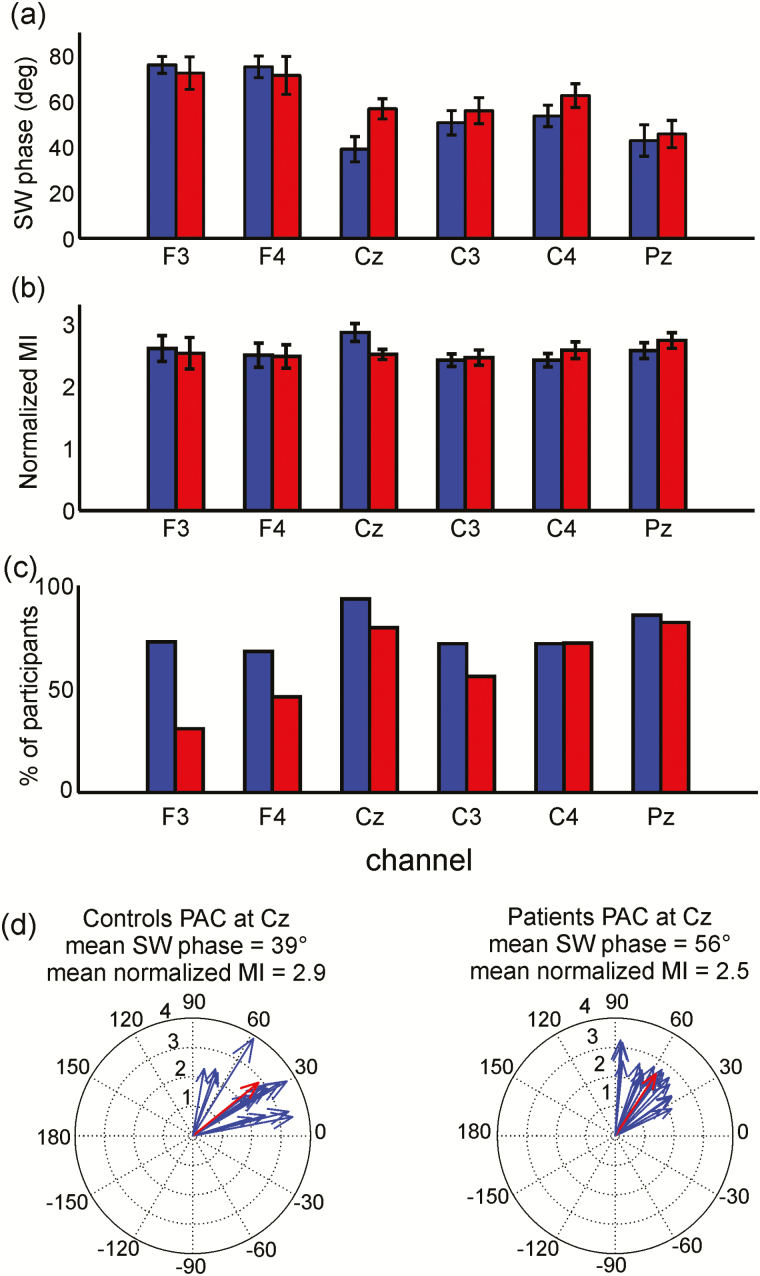
PAC results were consistent with our primary analyses of SW phase at spindle peak. At each electrode, only participants with significant PAC results (as determined by MI permutations) were included in the mixed-model regression (Figure 5c). Since there was no main effect of night on either the SW phase (F(1, 27.5) = .36, p = .55) or the MI (F(1, 35) = .47, p = .49), PAC results were averaged across nights (Figure 5). There was a main effect of electrode (F(5, 181) = 26, p < 10−6), indicating that maximum sigma amplitude occurred earlier within the SW at centroparietal than frontal electrodes. While SW phase at maximum sigma amplitude did not differ by group (mixed-model regression F(1, 34.4) = .54, p = .47) and was similar to the SW phase at spindle peak (Figure 3), there was a significant Group × Electrode interaction (F(5, 180) = 4.6, p = .001) reflecting that patients had a later SW phase than controls at Cz (z = 2.7, p = .03) but did not differ at other electrodes. Finally, the MI (ie, degree of SW–spindle coupling) did not differ by electrode, group, or night. N3 results were similar (Supplement, Figure S7).
Figure 5.
Phase amplitude coupling (PAC) of slow waves (SWs) with spindles. Significant PAC results averaged across nights; controls (blue), patients (red). Error bars represent standard error of the mean (SEM) across participants. (a) The SW phase of maximal sigma amplitude during spindle time windows did not significantly differ between the groups. (b) Modulation index (MI) normalized by the null distribution did not vary spatially or differ between groups. (c) Percentage of participants with significant PAC as determined by MI permutations. (d) PAC for controls and patients at Cz. Blue arrows represent mean significant PAC for each participant, that is, the mean SW phase (vector angle) and mean normalized MI (vector length). Red arrows represent group means.
DISCUSSION
In this first investigation of the coordination of sleep spindles with SWs in schizophrenia, we demonstrate that in the context of markedly reduced spindle density and number, the temporal relationship between spindles and SWs is largely preserved. As in prior studies of healthy individuals,23,25 in both healthy and schizophrenia participants, spindles started on the rising phase of the SW upstate, peaked after its maximum, and ended during the downstate. In the schizophrenia group alone, the later in the SW upstate that spindles peaked and the more consistent this timing, the greater the sleep-dependent memory consolidation. Each coordination parameter (SW phase at spindle peak and phase consistency) predicted memory consolidation independent of spindle density. Together, spindle density and SW–spindle coordination predicted memory consolidation better than either parameter alone. These findings suggest that both the density of spindles and their coordination with SWs contribute to sleep-dependent memory consolidation in schizophrenia.
The finding of similar SW–spindle coordination between patients and controls was consistent across three methods, two study nights, and N2 and N3. First, using spectrograms, sigma power was highest during SW upstates in both the groups. (The reduction in SW-locked sigma power in schizophrenia likely reflects their significantly lower spindle number and density.) Second, spindles peaked at approximately the same phase of the upstate of SWs (~43°) and with similar consistency in both the groups. Third, PAC, which uses all spindle time points, but only participants with significant coupling, showed that the SW phase at which sigma amplitude was maximal was similar to the SW phase at spindle peak. While there was no overall group difference in PAC, patients showed a later SW phase at Cz but did not differ at other electrodes. These findings suggest that SW–spindle coordination is intact in chronic medicated patients with schizophrenia.
The dissociation between intact SW–spindle coordination and deficient spindle number and density suggests that they rely on different neural mechanisms. SWs emerge spontaneously in the neocortex, even after cortical deafferentation51 but can also be elicited by optogenetic manipulation of TRN and thalamocortical neurons,52,53 suggesting that thalamic input shapes SW expression.22 In contrast, spindle frequency rhythms are seen in the isolated TRN54,55 but not in the isolated cortex or other thalamic nuclei.55,56 Cortical feedback to the TRN and thalamocortical neurons, however, synchronizes spindles across cortical regions57,58 and can initiate and terminate spindles.59 Thus, in the intact brain, both spindles and SWs are the products of thalamocortical circuits. While SWs contribute to spindle synchronization, initiation, and duration, spindles do not appear to trigger SWs or affect their duration.60 This relative independence of SWs from mediation by spindles may account for why the spindle deficit in schizophrenia is not paralleled by a SW or SW–spindle coordination deficit.
Our findings regarding SW abnormalities in schizophrenia are inconclusive. Patients showed trends to greater SW density and peak-to-peak amplitude and shorter SW duration. There was no deficit in N2 delta power. Previous investigations of SW activity in schizophrenia have been inconsistent, reporting no differences during NREM sleep in medicated patients,61,62 reduced N3 SW count and delta power in antipsychotic-naive and unmedicated patients,63,64 and reduced delta power in N3 but not N2 in medicated patients.65 These inconsistencies may reflect differences in definitions of delta power, sleep stages considered, and medication status of the participants.
Although the groups were similar in SW–spindle coordination, only in patients did coordination predict sleep-dependent memory consolidation. Patients whose spindles peaked later in the upstate of the SW and for whom this temporal relation was more consistent showed better memory consolidation. Adding either coordination parameter to spindle density in a model predicting sleep-dependent memory consolidation resulted in a significantly stronger prediction than either parameter alone, suggesting that both SW–spindle coordination and spindle density are important for memory consolidation in schizophrenia. We can only speculate why we did not see similar relations of either spindle density10 or SW–spindle coordination to memory in healthy controls. This may reflect that controls showed a more restricted range of overnight MST improvement (Figure 4: controls SD = 13%; patients SD = 23%). Another possibility is that patients are more sensitive to variation in SW–spindle coordination due to their spindle deficit. The overall reduction in spindles may lead to fewer spindles that are optimally coordinated with SWs to consolidate memory. For controls, spindle density and the number of coordinated SW–spindle events may be sufficient and not rate limiting for memory consolidation.
These findings raise the question of whether enhancing spindles while preserving or improving SW–spindle coordination might improve sleep-dependent memory consolidation in schizophrenia. In healthy individuals, increasing spindles with zolpidem,66,67 increasing sigma activity with transcranial stimulation,68,69 and enhancing the synchronization of sigma activity with slow oscillations using auditory closed-loop stimulation70 all improve memory, while transcranial stimulation that decreases sigma activity impairs memory.71 Only a few studies have attempted to improve cognition in schizophrenia by manipulating sleep oscillations. In a small sample of patients, 0.75 Hz transcranial stimulation during N2 did not significantly alter sleep but improved word list recall.72 In a small preliminary study, eszopiclone, which acts on γ-aminobutyric acid (GABA)-ergic neurons in the TRN,73 significantly increased spindles in schizophrenia but not sleep-dependent memory.30 In another study that did not include PSG, longer term administration of eszopiclone improved working memory in schizophrenia but not symptoms.74 The effects of eszopiclone on SW–spindle coordination in schizophrenia are now being investigated. This body of work provides an impetus to develop and test novel therapies for spindle deficits and synchronization to improve cognition.
A limitation to the interpretation of our findings is that our patients were treated with medications that affect sleep.75 As previously reported, antipsychotic medication dose, measured by chlorpromazine equivalents, did not significantly correlate with any spindle parameter,10 SW–spindle phase (R = 0.17, p = .20) or phase consistency (R = 0.19, p = .52). In addition, we recently reported spindle deficits in antipsychotic-naive early course patients with schizophrenia (but not to those with other psychotic disorders) and nonpsychotic first-degree relatives. These deficits correlated with IQ and executive function.76 These data suggest that spindle deficits in schizophrenia are not a side effect of antipsychotic medications or chronicity and may instead be endophenotypes that are linked to risk genes and contribute to cognitive dysfunction.4
It is possible that larger samples would have revealed clinically meaningful group differences in SW–spindle coordination. Based on the small effect sizes for SW–spindle phase and phase consistency, and the consistent lack of significant group differences across electrodes, however, this seems unlikely. Other study limitations include that our sparse EEG array does not allow a fuller exploration of the spatial characteristics of spindles associated with memory. We were also not able to examine the coordination of SWs and spindles with hippocampal ripples, since ripples are not seen on scalp recordings. Animal models may be necessary to evaluate the contribution of coordination between all three NREM oscillations (SWs, spindles, and ripples) to memory and how this is affected by potential interventions. Recent studies suggest distinctions between fast and slow frequency spindles, with regard to cortical generators,77 mnemonic function,58 and relation to SW phase.25,78 As the present study was restricted to the standard definition of spindle frequency (12–15 Hz;35,79), future work is needed to address whether spindles in the fast and slow halves of this range, as well as in lower frequency bands, are preserved in schizophrenia and contribute to sleep-dependent memory consolidation.
In summary, despite marked spindle deficits, the temporal coordination of SWs and spindles is preserved in chronic medicated schizophrenia. Moreover, SW–spindle coordination correlated with overnight improvement on a procedural memory task, suggesting a role in sleep-dependent memory consolidation. This implies that interventions aimed at ameliorating deficient sleep-dependent memory consolidation in schizophrenia will need to both increase spindle density and preserve (or improve) the coordination of NREM oscillations. A greater understanding of the role of the coordination of NREM oscillations in memory may open new avenues for treating cognitive deficits in schizophrenia.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at SLEEP online.
FUNDING
This work was supported by the National Institutes of Health R01 MH092638 to DSM and RS; R01 MH048832 to RS; 1UL1TR001102-01 to Harvard Catalyst; Grant #30675 from Sunovion Pharmaceuticals, Inc. (formerly Sepracor, Inc.) to DSM; an NARSAD Young Investigator Award from the Brain and Behavior Research Foundation to CD; Medical Research Council grant G1002064 for support of MWJ and UB; and Rubicon grant from The Netherlands Organization for Scientific Research to RC. MWJ received research support from Eli Lilly & Co.
DISCLOSURE STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Laura Lewis, PhD, and Shaun Purcell, PhD, for their comments on the manuscript.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources, the National Center for Advancing Translational Science, or the National Institutes of Health. All other authors report no biomedical financial interests or potential conflicts of interest.
This study is not a clinical trial.
Institution at which work was performed:
Department of Psychiatry, Massachusetts General Hospital and Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA.
REFERENCES
- 1. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000; 26(1): 119–136. [DOI] [PubMed] [Google Scholar]
- 2. Kahn RS, Keefe RSE. Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry. 2013; 70(10): 1107–1112. [DOI] [PubMed] [Google Scholar]
- 3. Sergi MJ, Green MF, Widmark C, et al. Social cognition [corrected] and neurocognition: effects of risperidone, olanzapine, and haloperidol. Am J Psychiatry. 2007; 164(10): 1585–1592. [DOI] [PubMed] [Google Scholar]
- 4. Manoach DS, Pan JQ, Purcell SM, Stickgold R. Reduced sleep spindles in schizophrenia: a treatable endophenotype that links risk genes to impaired cognition? Biol Psychiatry. 2016; 80(8): 599–608. doi: 10.1016/j.biopsych.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manoach DS, Cain MS, Vangel MG, Khurana A, Goff DC, Stickgold R. A failure of sleep-dependent procedural learning in chronic, medicated schizophrenia. Biol Psychiatry. 2004; 56(12): 951–956. [DOI] [PubMed] [Google Scholar]
- 6. Manoach DS, Thakkar KN, Stroynowski E, et al. Reduced overnight consolidation of procedural learning in chronic medicated schizophrenia is related to specific sleep stages. J Psychiatr Res. 2010; 44(2): 112–120. doi: 10.1016/j.jpsychires.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seeck-Hirschner M, Baier PC, Sever S, Buschbacher A, Aldenhoff JB, Göder R. Effects of daytime naps on procedural and declarative memory in patients with schizophrenia. J Psychiatr Res. 2010; 44(1): 42–47. doi: 10.1016/j.jpsychires.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 8. Genzel L, Ali E, Dresler M, Steiger A, Tesfaye M. Sleep-dependent memory consolidation of a new task is inhibited in psychiatric patients. J Psychiatr Res. 2011; 45(4): 555–560. [DOI] [PubMed] [Google Scholar]
- 9. Genzel L, Dresler M, Cornu M, et al. Medial prefrontal-hippocampal connectivity and motor memory consolidation in depression and schizophrenia. Biol Psychiatry. 2015; 77(2): 177–186. [DOI] [PubMed] [Google Scholar]
- 10. Wamsley EJ, Tucker MA, Shinn AK, et al. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012; 71(2): 154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Göder R, Graf A, Ballhausen F, et al. Impairment of sleep-related memory consolidation in schizophrenia: relevance of sleep spindles? Sleep Med. 2015; 16(5): 564–569. [DOI] [PubMed] [Google Scholar]
- 12. Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011; 35(5): 1154–1165. [DOI] [PubMed] [Google Scholar]
- 13. Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995; 15(1 Pt 2): 604–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mölle M, Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog Brain Res. 2011; 193: 93–110. [DOI] [PubMed] [Google Scholar]
- 15. Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res. 2012; 76(2): 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Staresina BP, Bergmann TO, Bonnefond M, et al. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat Neurosci. 2015; 18(11): 1679–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maingret N, Girardeau G, Todorova R, Goutierre M, Zugaro M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat Neurosci. 2016; 19(7): 959–964. [DOI] [PubMed] [Google Scholar]
- 18. Phillips KG, Bartsch U, McCarthy AP, et al. Decoupling of sleep-dependent cortical and hippocampal interactions in a neurodevelopmental model of schizophrenia. Neuron. 2012; 76(3): 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993; 262(5134): 679–685. [DOI] [PubMed] [Google Scholar]
- 20. Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994; 265(5172): 676–679. [DOI] [PubMed] [Google Scholar]
- 21. Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998; 21(5): 1123–1128. [DOI] [PubMed] [Google Scholar]
- 22. Crunelli V, David F, Lőrincz ML, Hughes SW. The thalamocortical network as a single slow wave-generating unit. Curr Opin Neurobiol. 2015; 31: 72–80. doi: 10.1016/j.conb.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 23. Mölle M, Marshall L, Gais S, Born J. Grouping of spindle activity during slow oscillations in human non-rapid eye movement sleep. J Neurosci. 2002; 22(24): 10941–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mölle M, Eschenko O, Gais S, Sara SJ, Born J. The influence of learning on sleep slow oscillations and associated spindles and ripples in humans and rats. Eur J Neurosci. 2009; 29(5): 1071–1081. [DOI] [PubMed] [Google Scholar]
- 25. Mölle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011; 34(10): 1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mölle M, Marshall L, Gais S, Born J. Learning increases human electroencephalographic coherence during subsequent slow sleep oscillations. Proc Natl Acad Sci U S A. 2004; 101(38): 13963–13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clemens Z, Mölle M, Eross L, Barsi P, Halász P, Born J. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007; 130(Pt 11): 2868–2878. [DOI] [PubMed] [Google Scholar]
- 28. Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci (Regul Ed). 2007; 11(10): 442–450. [DOI] [PubMed] [Google Scholar]
- 29. Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013; 93(2): 681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wamsley EJ, Shinn AK, Tucker MA, et al. The effects of eszopiclone on sleep spindles and memory consolidation in schizophrenia: a randomized placebo-controlled trial. Sleep. 2013; 36(9): 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karni A, Meyer G, Rey-Hipolito C, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA. 1998; 95(3): 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007; 2(4): e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002; 35(1): 205–211. [DOI] [PubMed] [Google Scholar]
- 34. Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003; 425(6958): 616–620. [DOI] [PubMed] [Google Scholar]
- 35. Iber C, Ancoli-Isreal S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specification.1st ed Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 36. R Core Team. R: a language and environment for statistical computing 2014. GBIF.ORG. http://www.R-project.org/. Accessed November 5, 2015
- 37. Warby SC, Wendt SL, Welinder P, et al. Sleep-spindle detection: crowdsourcing and evaluating performance of experts, non-experts and automated methods. Nat Methods. 2014; 11(4): 385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models using lme4 arXiv:14065823 [stat] June 2014. http://arxiv.org/abs/1406.5823.
- 39. Bretz F, Hothorn T, Westfall P. Multiple Comparisons Using R.Boca Raton, FL: Chapman and Hall; 2010. [Google Scholar]
- 40. Canolty RT, Edwards E, Dalal SS, et al. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006; 313(5793): 1626–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Khan S, Gramfort A, Shetty NR, et al. Local and long-range functional connectivity is reduced in concert in autism spectrum disorders. Proc Natl Acad Sci U S A. 2013; 110(8): 3107–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cox R, van Driel J, de Boer M, Talamini LM. Slow oscillations during sleep coordinate interregional communication in cortical networks. J Neurosci. 2014; 34(50): 16890–16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mitra P, Bokil H. Observed Brain Dynamics.New York, NY: Oxford University Press; 2007. [Google Scholar]
- 44. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004; 134(1): 9–21. [DOI] [PubMed] [Google Scholar]
- 45. Rosenblum MG, Pikovsky AS, Kurths J. Phase synchronization of chaotic oscillators. Phys Rev Lett. 1996; 76(11): 1804–1807. [DOI] [PubMed] [Google Scholar]
- 46. Philipp B. CircStat: A MATLAB Toolbox for Circular Statistics. J Stat Software. 2009; 31(10): 1–21. https://www.jstatsoft.org/article/view/v031i10. [Google Scholar]
- 47. Anderson TW. On the Distribution of the Two-Sample Cramer-von Mises Criterion. Ann Math Statist. 1962; 33(3): 1148–1159. [Google Scholar]
- 48. Andersen R. Modern Methods for Robust Regression.Los Angeles, Calif: SAGE Publications, Inc; 2007. [Google Scholar]
- 49. Finelli LA, Borbély AA, Achermann P. Functional topography of the human nonREM sleep electroencephalogram. Eur J Neurosci. 2001; 13(12): 2282–2290. [DOI] [PubMed] [Google Scholar]
- 50. Riedner BA, Vyazovskiy VV, Huber R, et al. Sleep Homeostasis and Cortical Synchronization: III. A High-Density EEG Study of Sleep Slow Waves in Humans. Sleep. 2007; 30(12): 1643–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Timofeev I, Grenier F, Bazhenov M, Sejnowski TJ, Steriade M. Origin of slow cortical oscillations in deafferented cortical slabs. Cereb Cortex. 2000; 10(12): 1185–1199. [DOI] [PubMed] [Google Scholar]
- 52. Poulet JFA, Fernandez LMJ, Crochet S, Petersen CCH. Thalamic control of cortical states. Nat Neurosci. 2012; 15(3): 370–372. [DOI] [PubMed] [Google Scholar]
- 53. Lewis LD, Voigts J, Flores FJ, et al. Thalamic reticular nucleus induces fast and local modulation of arousal state. eLife Sciences. 2015; 4: e08760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jacobsen RB, Ulrich D, Huguenard JR. GABA B and NMDA receptors contribute to spindle-like oscillations in rat thalamus in vitro. J Neurophysiol. 2001; 86(3): 1365–1375. [DOI] [PubMed] [Google Scholar]
- 55. Fuentealba P, Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog Neurobiol. 2005; 75(2): 125–141. [DOI] [PubMed] [Google Scholar]
- 56. Steriade M, Domich L, Oakson G, Deschênes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987; 57(1): 260–273. [DOI] [PubMed] [Google Scholar]
- 57. Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996; 274(5288): 771–774. [DOI] [PubMed] [Google Scholar]
- 58. De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003; 7(5): 423–440. [DOI] [PubMed] [Google Scholar]
- 59. Bonjean M, Baker T, Lemieux M, Timofeev I, Sejnowski T, Bazhenov M. Corticothalamic feedback controls sleep spindle duration in vivo. J Neurosci. 2011; 31(25): 9124–9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Valencia M, Artieda J, Bolam JP, Mena-Segovia J. Dynamic interaction of spindles and gamma activity during cortical slow oscillations and its modulation by subcortical afferents. PLoS One. 2013; 8(7): e67540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ferrarelli F, Huber R, Peterson MJ, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007; 164(3): 483–492. [DOI] [PubMed] [Google Scholar]
- 62. Ferrarelli F, Peterson MJ, Sarasso S, et al. Thalamic dysfunction in schizophrenia suggested by whole-night deficits in slow and fast spindles. Am J Psychiatry. 2010; 167(11): 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hiatt JF, Floyd TC, Katz PH, Feinberg I. Further evidence of abnormal non-rapid-eye-movement sleep in schizophrenia. Arch Gen Psychiatry. 1985; 42(8): 797–802. [DOI] [PubMed] [Google Scholar]
- 64. Keshavan MS, Reynolds CF, Miewald MJ, et al. Delta sleep deficits in schizophrenia: evidence from automated analyses of sleep data. Arch Gen Psychiatry. 1998; 55(5): 443–448. [DOI] [PubMed] [Google Scholar]
- 65. Göder R, Aldenhoff JB, Boigs M, Braun S, Koch J, Fritzer G. Delta power in sleep in relation to neuropsychological performance in healthy subjects and schizophrenia patients. J Neuropsychiatry Clin Neurosci. 2006; 18(4): 529–535. doi:10.1176/appi.neuropsych.18.4.529. [DOI] [PubMed] [Google Scholar]
- 66. Kaestner EJ, Wixted JT, Mednick SC. Pharmacologically Increasing Sleep Spindles Enhances Recognition for Negative and High-arousal Memories. J Cogn Neurosci. 2013; 25(10): 1597–1610. [DOI] [PubMed] [Google Scholar]
- 67. Mednick SC, McDevitt EA, Walsh JK, et al. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013; 33(10): 4494–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006; 444(7119): 610–613. [DOI] [PubMed] [Google Scholar]
- 69. Del Felice A, Magalini A, Masiero S. Slow-oscillatory Transcranial Direct Current Stimulation Modulates Memory in Temporal Lobe Epilepsy by Altering Sleep Spindle Generators: A Possible Rehabilitation Tool. Brain Stimul. 2015; 8(3): 567–573. [DOI] [PubMed] [Google Scholar]
- 70. Ngo HV, Martinetz T, Born J, Mölle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013; 78(3): 545–553. [DOI] [PubMed] [Google Scholar]
- 71. Marshall L, Kirov R, Brade J, Mölle M, Born J. Transcranial electrical currents to probe EEG brain rhythms and memory consolidation during sleep in humans. PLoS One. 2011; 6(2): e16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Göder R, Baier PC, Beith B, et al. Effects of transcranial direct current stimulation during sleep on memory performance in patients with schizophrenia. Schizophrenia Research. 2013; 144(1): 153–154. [DOI] [PubMed] [Google Scholar]
- 73. Jia F, Goldstein PA, Harrison NL. The modulation of synaptic GABA(A) receptors in the thalamus by eszopiclone and zolpidem. J Pharmacol Exp Ther. 2009; 328(3): 1000–1006. [DOI] [PubMed] [Google Scholar]
- 74. Tek C, Palmese LB, Krystal AD, et al. The impact of eszopiclone on sleep and cognition in patients with schizophrenia and insomnia: a double-blind, randomized, placebo-controlled trial. Schizophr Res. 2014; 160(1–3): 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Krystal AD, Goforth HW, Roth T. Effects of antipsychotic medications on sleep in schizophrenia. Int Clin Psychopharmacol. 2008; 23(3): 150–160. [DOI] [PubMed] [Google Scholar]
- 76. Manoach DS, Demanuele C, Wamsley EJ, et al. Sleep spindle deficits in antipsychotic-naïve early course schizophrenia and in non-psychotic first-degree relatives. Front Hum Neurosci. 2014; 8: 762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Schabus M, Dang-Vu TT, Albouy G, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. PNAS. 2007; 104(32): 13164–13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Klinzing JG, Mölle M, Weber F, et al. Spindle activity phase-locked to sleep slow oscillations. NeuroImage. 2016; 134: 607–616. [DOI] [PubMed] [Google Scholar]
- 79. Kales A, Rechtschaffen A; University of California LA, Brain Information Service; NINDB Neurological Information Network (U.S.). . A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Bethesda, MD: U.S. National Institute of Neurological Diseases and Blindness, Neurological Information Network; 1968. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.