Abstract
Introduction:
Habitual short sleep duration is associated with adverse metabolic, cardiovascular, and inflammatory effects. Co-twin study methodologies account for familial (eg, genetics and shared environmental) confounding, allowing assessment of subtle environmental effects, such as the effect of habitual short sleep duration on gene expression. Therefore, we investigated gene expression in monozygotic twins discordant for actigraphically phenotyped habitual sleep duration.
Methods:
Eleven healthy monozygotic twin pairs (82% female; mean age 42.7 years; SD = 18.1), selected based on subjective sleep duration discordance, were objectively phenotyped for habitual sleep duration with 2 weeks of wrist actigraphy. Peripheral blood leukocyte (PBL) RNA from fasting blood samples was obtained on the final day of actigraphic measurement and hybridized to Illumina humanHT-12 microarrays. Differential gene expression was determined between paired samples and mapped to functional categories using Gene Ontology. Finally, a more comprehensive gene set enrichment analysis was performed based on the entire PBL transcriptome.
Results:
The mean 24-hour sleep duration of the total sample was 439.2 minutes (SD = 46.8 minutes; range 325.4–521.6 minutes). Mean within-pair sleep duration difference per 24 hours was 64.4 minutes (SD = 21.2; range 45.9–114.6 minutes). The twin cohort displayed distinctive pathway enrichment based on sleep duration differences. Habitual short sleep was associated with up-regulation of genes involved in transcription, ribosome, translation, and oxidative phosphorylation. Unexpectedly, genes down-regulated in short sleep twins were highly enriched in immuno-inflammatory pathways such as interleukin signaling and leukocyte activation, as well as developmental programs, coagulation cascade, and cell adhesion.
Conclusions:
Objectively assessed habitual sleep duration in monozygotic twin pairs appears to be associated with distinct patterns of differential gene expression and pathway enrichment. By accounting for familial confounding and measuring real life sleep duration, our study shows the transcriptomic effects of habitual short sleep on dysregulated immune response and provides a potential link between sleep deprivation and adverse metabolic, cardiovascular, and inflammatory outcomes.
Keywords: Sleep duration, Twins, Monozygotic, Gene expression, Leukocyte.
Statement of Significance
Habitual short sleep is common and associated with numerous untoward health outcomes including cognitive, metabolic, cardiovascular, and immunological impairment. We compared gene expression profiles of peripheral blood leukocytes between sleep duration discordant monozygotic twins to identify differentially activated transcriptional programs in these circulating immune cells and gain potential mechanistic insights linking habitual short sleep with poor outcomes. By measuring sleep duration in the natural environment we provided ecologically valid results allowing insights into the impact of “real world” chronic sleep curtailment on human health.
INTRODUCTION
Individual sleep need is both a heritable quantitative trait and a behaviorally influenced phenotype. Sleep duration heritability is substantial, estimated to be somewhere between 31% and up to 55% in classical monozygotic (MZ) twin versus dizygotic (DZ) twin studies.1–5 More recently on the timeline of human evolution societal sleep duration has abruptly eroded, with about one-third of the working population sleeping ≤6 hours per night and sleep length dropping an estimated 1.5–2 hours per night over the past century.6–8 Assuming that genetic factors determining sleep need have remained static over this same time period, environmental factors are exerting increasing influence on individual sleep duration. Modern society, with its control of light, omnipresent technology, and countless competing interests for time, along with the zeitgeist de-emphasizing sleep’s importance, has resulted in the widespread deprioritization of sleep.9 This growing disconnect between sleep need and sleep actualization has substantial adverse consequences for cognitive functioning and metabolic, cardiovascular, immunological, and psychological health.10–17
Much research has been devoted to epidemiology concerning the relationship between habitual short sleep duration and human health18–20 and to identifying genetic determinants of sleep duration and circadian functioning in humans and model organisms.21–23 Although numerous experimental studies have explored the effect of extreme, controlled sleep deprivation paradigms on mammalian physiology,24–27 ecologically valid research exploring the effect of habitual short sleep in situ has been lacking due to methodological challenges. Since individualized sleep need is variable and substantially genetically determined, finding appropriate controls is problematic given that any sleep duration may maintain physiological homeostasis for one individual but not another. Cross-over study designs attempt to address this issue, but questions regarding ecological validity remain as these studies control sleep duration rather than investigate sleep in situ.28–31 Without a biomarker or endophenotype to match subjects for sleep need, delineation of specific pathways that translate observed short sleep into disturbed physiology is difficult. The end result is scarce research exploring “real world” physiological impacts of short sleep where compensatory mechanisms may alter the sleep/physiological homeostasis relationship in unpredictable ways.
Co-twin studies, by focusing on intra-pair differences, provide a highly sensitive approach for evaluating associations of subtle laboratory or clinical findings with a specific condition. Because of the extremely tight matching between MZ twins for many potential confounders including genetic drivers of sleep need, co-twin studies of MZ siblings provide the best matched controls, thereby solving the conundrum of appropriate controls for ecologically valid sleep duration research. This method also involves fewer assumptions, requires a smaller sample size and is less susceptible to ascertainment biases than non-twin research methods.
In this study, we hypothesized that an unbiased assessment of the transcriptional landscape of peripheral blood leukocytes among MZ twins with discordant habitual sleep duration would provide novel insights into the molecular perturbations elicited by lifestyle-driven habitual sleep curtailment.
METHODS
Subjects
This study was performed at the Washington State Twin Registry, a community-based sample of twins constructed using data from the Washington State Department of Licensing. As of April 2016, the Registry consisted of over 8965 pairs. Zygosity is determined using previously validated self-report methods that are correct at least 95% of the time.32,33 For the 11 enrolled pairs in this study, MZ zygosity was confirmed by assessing twin concordance for 15 short tandem DNA repeats with the PowerPlex 16 HS System (Promega Corporation, Madison, WI).34
Every twin enrolled in the Registry completes a recruitment survey, which has included a sleep duration question since 2009. In 2006 and 2008, a health survey was mailed to more than 4000 enrolled twins that included the same sleep duration question. Sleep duration was ascertained from the question, “On average, how long do you sleep per night?” reported in hours and minutes. Out of 1284 MZ pairs, 610 were discordant by at least 60 minutes in habitual sleep duration. Because there is no standard definition of habitual sleep duration discordance for MZ twins, we adopted a 60-minute difference as the principal screening and inclusion criteria. These twin pairs were mailed an invitation letter explaining the study with a number for them to call for further screening for study eligibility. Interested twin pairs were then screened and deemed ineligible if they had a history of: diabetes, depression or depressive symptoms (PHQ-2 score > 2),35 bipolar disorder, schizophrenia, restless legs syndrome, narcolepsy, obstructive sleep apnea, insomnia, circadian rhythm sleep disorder, shift work, cigarette smoking, recreational drug use or alcohol use. Because sleep duration from the recruitment and health surveys were completed in the past, current sleep duration was screened with the same question as before, as well at the sleep timing questionnaire.36 Only twin pairs with at least 60 minutes of sleep duration discordance on either of these measures were deemed study eligible. A total of 116 twin pairs were screened for study enrollment, 70 were study eligible, 52 were enrolled, and the 11 most discordant pairs underwent gene expression analysis. All female twins had negative pregnancy tests and all twins were negative for drugs of abuse by serum drug testing.
Actigraphy
All twins were monitored concurrently for 14 days with the Actiwatch-2 actigraph worn on the non-dominant wrist starting at 5:00 pm of the first day of the research protocol (Philips Respironics, Andover, MA). The actigraph provides continuous motion data using an accelerometer to monitor the speed and degree of arm movements thus providing an indirect measure of daily sleep/wake patterns based on the assumption that movement subsides with sleep. To facilitate actigraph record scoring each twin filled out a sleep diary concurrently for the entire 14-day period. Actigraphy differentiates sleep from wake with good agreement to polysomnography.37–39 Actigram ana lysis was performed using Respironics Actiware 5 software (Philips, Respironics, Andover, MA) and previously described protocols.40 Briefly, rest intervals were set manually based on the sleep diary data provided the diary correlated within 15 minutes of expected activity level changes for bedtimes, wake times, and naptimes. If this was not the case then watch detected light levels, or, if needed, twin specified event marks, were used to determine rest intervals. The sleep period was automatically scored within the rest interval by a software-based automatic sleep scoring algorithm based on pre-set amplitude and frequency criteria for detected movements ascertained in 30-second epochs.39 Total sleep time was calculated by adding nighttime sleep plus daytime naps for the full recording period of 14 days. Total adjusted recording time was the total recording time minus the excluded interval time. Twenty-four hour sleep time was standardized by dividing the total sleep time by the total recording time and multiplying by 24 (for hours in the day) and then 60 (for minutes per hour). Epoch length was 30 seconds. The PI randomly audited 1 out of every 10 actigraph records for adherence to protocols and accuracy.
Blood Sample Acquisition and RNA Purification
On the morning of day 15 of the research protocol (directly following completion of the 14-day actigraphy assessment) all twins provided a fasting blood sample drawn directly into two PAXgene™ vacutainers (Qiagen N.V., Franklin Lakes, NJ). The RNA was separated and purified as previously described.41 Residual genomic DNA was removed with RNeasy MinElute cleanup kits (Qiagen N.V., Franklin Lakes, NJ) according to the manufacturer’s instructions. Total RNA integrity was checked using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc., Santa Clara, CA) and quantified using a Trinean DropSense96 spectrophotometer (Caliper Life Sciences, Hopkinton, MA).
Microarray Experiments
High quality RNA samples were converted to cDNA and biotin-labeled for microarray analysis using Ambion’s Illumina TotalPrep RNA Amplification kit (Life Technologies, Grand Island, NY). Labeled cRNAs were hybridized on HumanHT-12 v4 Expression BeadChips (Illumina, Inc., San Diego, CA) and image processed using an Illumina iScan system. Microarray data were assessed for quality, followed by quantile normalization using the Bioconductor package lumi.42 The dataset was initially filtered by flagging probes that fell below a low-signal threshold, which was defined by the 75th percentile of the negative control probe signals within each array. The full dataset was subsequently processed using a variance filter (ie, “shorth” function in the Bioconductor package genefilter). Approximately 10 000 transcripts passed the above filtering steps and underwent further analysis. Detailed microarray experiment description and data, meeting Minimum Information About a Microarray Experiment (MIAME) criteria, are available at Gene Expression Omnibus (GSE80612).
Data Analysis
We analyzed the microarray dataset using two strategies. Initially, we identified differentially expressed genes between twin-paired samples (long sleep duration vs. short sleep duration) using the Bioconductor package limma.43 False discovery rate (FDR) correction for multiple testing was applied and a combined criteria of an FDR < 0.01 and |log2 [expression ratio]| ≥ 0.322 (± 1.25-fold) was used to define statistically significant differential expression between the long versus short sleep duration groups.44 Functional enrichment analysis of the differentially expressed genes was performed with the web-based program (bioinfo.vanderbilt.edu/webgestalt) using Gene Ontology (GO) annotations.45 After correction for multiple testing, processes with adjusted enrichment p values < 0.05 were deemed significant.
Next, we undertook a more unbiased and comprehensive approach by applying Gene Set Enrichment Analysis (GSEA)46 to the entire filtered microarray dataset using 1315 curated pathways derived from multiple resources including Kyoto Encyclopedia of Genes and Genomes, Reactome, Pathway Interaction Database, and Biocarta. Random permutation analysis and an FDR < 0.05 was used to designate significant enrichment for gene sets. We performed “leading edge” analysis on the GSEA results to identify the subset of genes that significantly contributed to pathway enrichment.47 We then used hierarchical clustering based on gene set membership profile of leading edge genes to group overlapping pathways and identify larger, functionally coherent biologic modules.48,49
RESULTS
Subject Characteristics and Demographics
The twins were predominantly Caucasian, female, and educated. The mean age was 42.7 years (SD = 18.1), and the mean sleep duration for the twins as a whole was 439.2 minutes (SD = 46.8). The mean within-pair sleep duration difference was 64.4 minutes (SD = 21.2). Table 1 provides further demographic details.
Table 1.
Subject Characteristics.
| Twin pairs (N = 11, 22 total twins) | N (%) |
| MZ male–male | 2 (18%) |
| MZ female–female | 9 (82%) |
| Demographic characteristics | N (%) |
| Caucasian | 20 (91%) |
| High school graduate | 3 (14%) |
| Associates degree/some college | 4 (32%) |
| ≥ College degree | 15 (68%) |
| Study variables | Mean, SD, Range |
| Age (years) | 42.7, SD = 18.1, 20.2–70.9 |
| Sleep duration (min) | 439.2, SD = 46.8, 325.4–521.6 |
| Body mass index (kg/m2) | 24.5, SD = 4.9, 18–37 |
| Within pair sleep duration difference (min) | 64.4, SD = 21.2, 45.9–114.6 |
MZ = monozygotic; SD = standard deviation.
Discordant Sleep Duration is Associated With Differential Activation of Transcriptional Programs in PBLs
Using strict statistical criteria, we identified 575 differentially expressed transcripts in PBLs of discordant twins, of which 369 were up-regulated and 206 were down-regulated in short sleep subjects relative to their long sleep twin pairs (Figure 1A, full list available as Supplementary Table S1). The relative changes in expression were modest, with most genes being altered by less than 2-fold (Supplementary Table S1). The differentially expressed genes were enriched in distinct GO annotations, with transcripts up-regulated in short sleep mapping to ribosomal, transcription, and translational processes whereas down- regulated genes in short sleep were overrepresented across functionally diverse categories including immunity, wound healing, cell adhesion, chemotaxis, chemokine binding, and leukocyte activation (Figure 1B).
Figure 1.
Functional analysis of differentially expressed genes between sleep discordant monozygotic (MZ) twins. (A) Heatmap of 575 differentially expressed genes displaying pair-wise differences between short and long sleep MZ twin pairs after hierarchical clustering. There were 369 up-regulated genes (magenta) and 206 down-regulated genes (cyan) in short versus long sleep (complete list is available in Supplementary Table S1). Despite clear segregation between differentially up and down-regulated genes, note significant heterogeneity in expression differences among the 11 twin pairs. (B) Functional analysis of differentially expressed genes based on Gene Ontology (GO) annotations showed that distinctly different processes were enriched among up-regulated (magenta) and down-regulated (cyan) genes in short sleep. These significant functional categories have been depicted based on the relational structure of GO database.
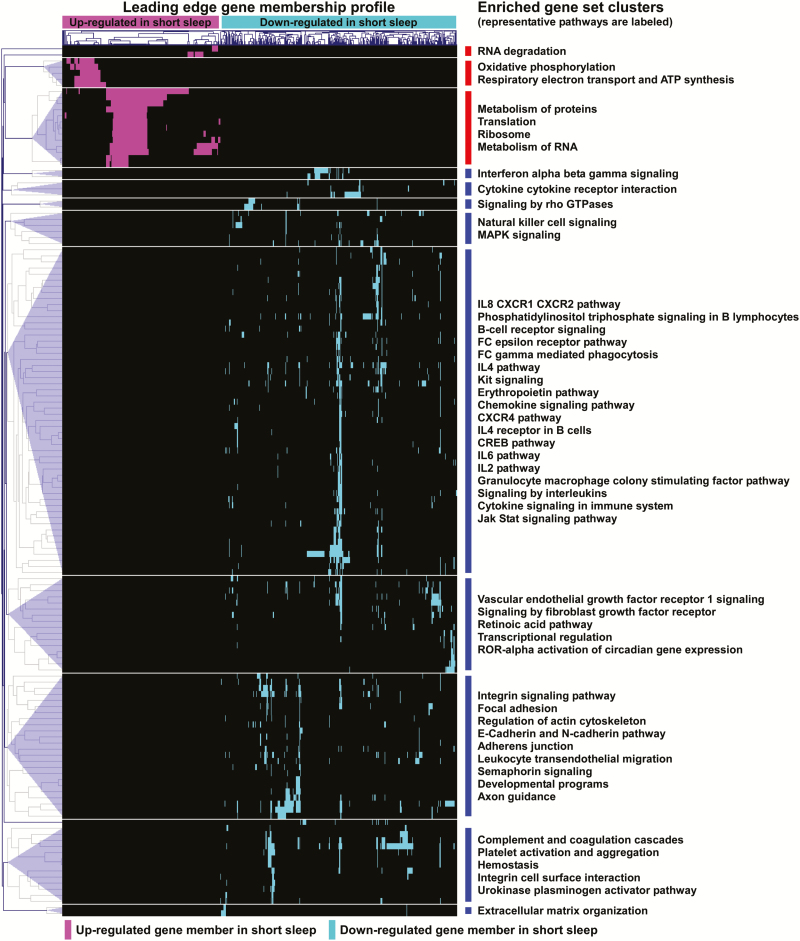
Although GO analysis of differentially expressed genes provides an overview of enriched functional categories, it does not fully exploit the genome-wide transcriptional information available, nor does it provide pathway-specific details. Therefore, we complemented our approach by performing GSEA, a more comprehensive analysis that is based on the expression pattern distribution of the entire microarray dataset. To maximize biologic relevance, we focused on ~1300 gene sets derived from curated canonical pathways. We identified 20 gene sets whose gene members were up-regulated and 124 gene sets whose members were down-regulated in subjects with short sleep duration relative to their long sleep duration twin pairs (full list available as Supplementary Table S2). We integrated these results by clustering enriched pathways together based on mutual membership of their leading edge genes. As depicted in Figure 2, this analysis defined multiple larger, “biological modules” comprised of functionally coherent processes. Enriched gene sets whose members were up-regulated in short sleep included those involved in transcription, translation, and oxidative phosphorylation. In contrast, modules with down-regulated genes in short sleep encompassed a wider functional repertoire dominated by immuno-inflammatory pathways but also included growth factor signaling, developmental programs, integrin and adhesion processes, and complement/coagulation cascade. The immunity-associated gene sets down-regulated in PBLs of short sleep duration twins included several cytokine and interleukin signaling pathways (eg, IL-2, IL-4, IL-6, and IL-8), interferon signaling, phagocytosis, granulocyte macrophage colony stimulating factor signaling, and pro-inflammatory messengers such as the Jak-Stat system.
Figure 2.
Pathway analysis of Peripheral blood leukocyte (PBL) transcriptome in monozygotic (MZ) twin pairs with discordant sleep duration. A graphical summary of significantly enriched gene sets is shown based on gene set enrichment analysis (GSEA) of 1315 curated pathways. Gene sets were deemed enriched if significant subsets of their members (“leading edge”) were either up or down-regulated in short sleep relative to long sleep twins (FDR < 0.05). Hierarchical clustering of enriched gene sets based on their membership profile revealed larger groupings of functionally similar pathways with shared genes known as “modules.” Note that several of the down-regulated modules in short sleep map to immune and inflammatory processes. Complete list is available in Supplementary Table S2.
Collectively, GSEA and GO enrichment analysis of altered gene expression profiles in discordant sleep revealed, unexpectedly, a generalized suppression of immune and inflammatory pathways in subjects with short sleep relative to their long sleep twins.
Acute Versus Chronic Reduction in Sleep Duration May Lead to Different Patterns of Activation in Immune and Inflammatory Pathways
We compared our findings to other published reports on genome-wide transcriptional consequences of sleep curtailment. An elegantly designed study by Aho et al.50 assessed the effects of forced short-term partial sleep restriction (five nights) on peripheral blood mononuclear cell (PBMC) gene expression of nine healthy volunteers. In contrast to our results, they found that short-term sleep deprivation up-regulated many immune- related GO processes, including interleukin production, leukocyte activation, immune response and B cell activation. To further explore this apparent discrepancy, we downloaded and processed their study’s raw microarray data (Affymetrix U133 Plus 2.0) from ArrayExpress repository (E-MEXP-3936). We normalized the entire dataset that also included control subjects (n = 4) and a recovery time point using Robust Multi-array Average algorithm, but focused further analysis to PBMC expression differences between baseline versus sleep restriction of the nine subjects. We observed that, similar to our experiments, alterations in gene expression were modest following reduced sleep duration. To compare the entire available transcriptional profiles between the two studies, we applied the same GSEA procedure to the Aho microarray data using ~1300 curated pathways. We used a more permissive FDR < 0.15 because very few gene sets were enriched at an FDR cutoff < 0.05. Our independent analysis confirmed their report that partial sleep restriction is associated with up-regulation of pathways mapping to immune- related processes such as interleukin activity (IL-6 and IL-3), interferon signaling, Toll pathways, B cell receptor signaling, and the inflammasome cascade (complete list available as Supplementary Table S3). However, we observed that the three pathways significantly down-regulated in forced sleep restriction were also associated with immunity, that is, natural killer cell mediated cytotoxicity, graft versus host disease, and antigen processing and presentation. Interestingly, we also found that natural killer cell signaling pathways were suppressed in our short sleep duration twins (Figure 2).
The differences observed between our study and those of Aho could be due to several factors. For example, different microarray platforms were used for gene expression profiling (Illumina vs. Affymetrix). Furthermore, Aho’s study investigated PBMCs (ie, lymphocytes and monocytes), whereas we studied all circulating leukocyte populations (ie, lymphocytes, monocytes, neutrophils, and other granulocytes). Previous studies have demonstrated distinct transcriptional signatures between circulating leukocyte sub-populations in humans,51 and it is possible that sleep restriction elicits cell-type specific responses across leukocyte subsets. Nevertheless, a key difference between our study designs, namely the duration and experimental setting of sleep curtailment, was likely a critical contributor to the profound differences observed in pathway enrichment.
Taken together, the weight of available published data indicate that experimentally-induced “acute” sleep restriction is associated with activation of immune and pro-inflammatory processes, although some immune-related pathways may also be suppressed. In contrast, our study finds that “chronically” shortened sleep duration under natural conditions is associated with down-regulation of several of the same immune-mediated processes.
DISCUSSION
Despite the significant role that genes are believed to play in determining an individual’s sleep duration, progress to identify associated genes in healthy unrelated adults has been challenging. One possible explanation for the lack of replicated genetic findings in sleep duration is the small effect size of individual genes. The fact that genome-wide association studies in the scale of thousands of subjects identified very few genetic variants associated with sleep duration implies that very large sample sizes are necessary to detect individual loci.52 Genome-wide transcription profiling may serve as a useful surrogate for identifying endophenotypes of sleep duration, especially when applied to the unique genetic characteristics of MZ twins. In this study, we found distinct expression patterns in the circulating leukocytes of healthy MZ twin pairs with discordant habitual sleep duration.
We interrogated immune cells because the immune system is likely involved in producing the untoward systemic effects of short sleep. Short-term, laboratory-based sleep deprivation is associated with increased leukocyte activation with concomitant changes in pro-inflammatory cytokines such as C-reactive protein, IL-6, and tumor necrosis factor (TNF).13,53–55 Sleep loss increases transcription of IL-6 and TNF messenger RNA through activation of nuclear factor NF-κB, a transcription factor that serves a critical role in the inflammatory signaling cascade that controls cellular expression of pro-inflammatory genes.13,56,57
In contrast, we found that PBLs of twin pairs with shorter sleep duration were characterized by down-regulation of immuno-inflammatory genes and pathways. Suppression of immune responses due to sleep deprivation has been previously reported. For example, acute curtailment of sleep can influence antibody titers following vaccination,58,59 and adults with poorer response to vaccines experience higher rates of clinical illness60 supporting the concept that sleep duration influences immune response to antigenic challenge. The immunologic consequences of habitual short sleep in the natural environment have been less studied. Cohen and colleagues reported that chronic reduction in sleep duration, as assessed by self-report61 or actigraphy,62 was associated with increased susceptibility to the common cold and blunted antibody response to hepatitis B vaccination.63 A similar association between shorter sleep duration and increased incidence of common illnesses in adolescents was reported by Orzech et al.64
To our knowledge, genome-wide transcriptional consequences of chronic short versus long sleep in subjects under natural conditions have not been previously published. However, other investigators have reported on the effects of laboratory-based short-term sleep curtailment in altering gene expression. We compared our results with those of a well-designed study based on acute sleep restriction50 and found significant differences in the patterns of gene expression and pathway enrichment. Specifically, immune and inflammatory programs were generally activated in circulating leukocytes when sleep restriction is imposed for a limited duration, whereas, in our home environment-based study, chronic short sleep duration was associated with down-regulation of similar pathways. Interestingly, this comparison also revealed several overlapping themes such as suppression of processes involved in natural killer cell signaling (Supplementary Tables S2 and S3). A plausible explanation for these discordant findings is that the host response to short-term sleep restriction is quite different from its adaptive response to chronic reduction of sleep duration. While the mechanisms underlying distinct transcriptional profiles between chronic and acute sleep reduction remain to be identified, epigenetic regulation may play a potentially important role—as was recently reported in experimental sleep deprivation in humans,65 and sleep fragmentation in mice.66 Furthermore, reduced immune gene expression responses in chronic versus acute stimuli have been previously reported in several human disorders including hepatitis B infection67 and autoimmune thrombocytopenia.68 Our group recently reported that among patients with chronic obstructive pulmonary disease (COPD), those with more severe disease demonstrated a diminished cytokine response to pathogen-associated molecular patterns, possibly reflecting an adaptive host response to the increased load of bacterial colonization reported in patients with more severe COPD.69
In addition to down-regulation of immune and inflammation-related processes, we found suppression of several other biological programs in twins with shorter sleep including those mapping to development, adhesion, leukocyte migration, and hemostasis. The role of these pathways in altering leukocyte function in subjects with chronically reduced sleep duration warrants further investigation because of their association with cardiovascular and metabolic disorders. Furthermore, several epidemiologic studies have linked short sleep duration with increased risk70 and worse outcomes in certain cancers,71 and our finding that habitual curtailment of sleep is associated with dysregulated immuno-inflammatory, developmental, migration, and adhesion responses hints at possible mechanisms promoted by chronically short sleep duration.
Our study has a number of limitations. The sample size is small (N = 22), although it is comparable to previous reports describing the effects of sleep deprivation on gene expression. Furthermore, our study’s strategy of comparing MZ twin pairs provides a unique approach to reduce inter-individual variability and therefore reduce sample size requirements. Another shortcoming is our reliance on actigraphy to estimate sleep duration. However, electroencephalograph (EEG) measurements are not feasible for chronic evaluation of sleep in the natural environmental setting and previous studies have demonstrated tight correlation between actigraphic and EEG-based assessment of sleep duration.37,38 We measured PBL gene expression at a single time point and therefore did not capture temporal dynamics of transcription. We attempted to partially mitigate this limitation by ensuring blood draws of twin pairs were completed at the same time under identical conditions. Future studies exploring the temporal patterns of gene expression associated with chronic short sleep are important extensions of our project. We assessed gene transcription in a heterogeneous leukocyte cell population which may have hidden subtle cell specific differences in gene expression. Finally, our report is descriptive and does not identify a mechanism whereby the transcriptional responses of PBLs to acute versus chronic reduction in sleep duration appear to be distinctly different. Additional mechanistic studies will be required to decipher the molecular underpinnings of this finding.
In conclusion, by exploiting the unique genetic structure of MZ twins, we report on the first unbiased assessment of transcriptional signatures associated with discordant habitual sleep duration. In contrast to previous studies linking acutely imposed sleep restriction with increased inflammatory responses, we found that shorter sleep duration in the natural setting was associated with down-regulation of specific immune and inflammatory programs in circulating leukocytes. Future studies are needed to clarify the mechanisms underlying differential host response during habitual versus forced reduction in sleep duration and delineate the long-term health consequences of this phenomenon.
SUPPLEMENTARY MATERIAL
Supplementary Tables S1–S3 are available at SLEEP online.
FUNDING
This work was supported by NIH grants K23HL083350, P30NR011400, ITHS Sleep Pilot Grant, and a University of Washington General Clinical Research Center Pilot Grant
DISCLOSURE STATEMENT
None declared.
Supplementary Material
REFERENCES
- 1. Partinen M, Kaprio J, Koskenvuo M, Putkonen P, Langinvainio H. Genetic and environmental determination of human sleep. Sleep. 1983; 6(3): 179–185. [DOI] [PubMed] [Google Scholar]
- 2. de Castro JM. The influence of heredity on self-reported sleep patterns in free-living humans. Physiol Behav. 2002; 76(4–5): 479–486. [DOI] [PubMed] [Google Scholar]
- 3. Watson NF, Buchwald D, Vitiello MV, Noonan C, Goldberg J. A twin study of sleep duration and body mass index. J Clin Sleep Med. 2010; 6(1): 11–17. [PMC free article] [PubMed] [Google Scholar]
- 4. Watson NF, Harden KP, Buchwald D, et al. Sleep duration and body mass index in twins: a gene-environment interaction. Sleep. 2012; 35(5): 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heath AC, Kendler KS, Eaves LJ, Martin NG. Evidence for genetic influences on sleep disturbance and sleep pattern in twins. Sleep. 1990; 13(4): 318–335. [DOI] [PubMed] [Google Scholar]
- 6. Webb WB, Agnew HW. Are we chronically sleep deprived? Bull Psychonomic Soc. 1975; 6: 47–48. [Google Scholar]
- 7. Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007; 30(12): 1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prevention CfDCa. Short sleep duration among workers—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012; 61(16): 281–296. [PubMed] [Google Scholar]
- 9. Liu Y, Wheaton AG, Chapman DP, Cunningham TJ, Lu H, Croft JB. Prevalence of healthy sleep duration among adults–United States, 2014. MMWR Morb Mortal Wkly Rep. 2016; 65(6): 137–141. [DOI] [PubMed] [Google Scholar]
- 10. Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003; 26(2): 380–384. [DOI] [PubMed] [Google Scholar]
- 11. Ayas NT, White DP, Manson JE, et al. A prospective study of sleep duration and coronary heart disease in women. Arch Intern Med. 2003; 163(2): 205–209. [DOI] [PubMed] [Google Scholar]
- 12. Dawson D, Reid K. Fatigue, alcohol and performance impairment. Nature. 1997; 388(6639): 235. [DOI] [PubMed] [Google Scholar]
- 13. Irwin MR, Wang M, Campomayor CO, Collado-Hidalgo A, Cole S. Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation. Arch Intern Med. 2006; 166(16): 1756–162. [DOI] [PubMed] [Google Scholar]
- 14. De Jonghe S, Van Overmeire I, Poulton S, et al. Structure-activity relationship of short-chain sphingoid bases as inhibitors of sphingosine kinase. Bioorg Med Chem Lett. 1999; 9(21): 3175–3180. [DOI] [PubMed] [Google Scholar]
- 15. Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006; 29(8): 1009–1014. [DOI] [PubMed] [Google Scholar]
- 16. Watson NF. Stroke and sleep specialists: an opportunity to intervene? J Clin Sleep Med. 2010; 6(2): 138–139. [PMC free article] [PubMed] [Google Scholar]
- 17. Troxel WM, Kupfer DJ, Reynolds CF, III, et al. Insomnia and objectively measured sleep disturbances predict treatment outcome in depressed patients treated with psychotherapy or psychotherapy-pharmacotherapy combinations. J Clin Psychiatry. 2012; 73(4): 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J. 2011; 32(12): 1484–1492. [DOI] [PubMed] [Google Scholar]
- 19. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010; 33: 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab. 2010; 24(5): 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andretic R, Franken P, Tafti M. Genetics of sleep. Annu Rev Genet. 2008; 42: 361–388. [DOI] [PubMed] [Google Scholar]
- 22. Kelly JM, Bianchi MT. Mammalian sleep genetics. Neurogenetics. 2012; 13(4): 287–326. [DOI] [PubMed] [Google Scholar]
- 23. Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011; 146(2): 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aldabal L, Bahammam AS. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir Med J. 2011; 5: 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009; 51(4): 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999; 354(9188): 1435–1439. [DOI] [PubMed] [Google Scholar]
- 27. Spiegel K, Tasali E, Leproult R, Van Cauter E. Effects of poor and short sleep on glucose metabolism and obesity risk. Nat Rev Endocrinol. 2009; 5(5): 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Broussard JL, Ehrmann DA, Van Cauter E, Tasali E, Brady MJ. Impaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover study. Ann Med. 2012; 157(8): 549–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. St-Onge MP, O’Keeffe M, Roberts AL, RoyChoudhury A, Laferrère B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012; 35(11): 1503–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang H, Durocher JJ, Larson RA, Dellavalla JP, Carter JR. Total sleep deprivation alters cardiovascular reactivity to acute stressors in humans. J Appl Physiol (1985). 2012; 113(6): 903–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pellegrino R, Sunaga DY, Guindalini C, et al. Whole blood genome-wide gene expression profile in males after prolonged wakefulness and sleep recovery. Physiol Genomics. 2012; 44(21): 1003–1012. [DOI] [PubMed] [Google Scholar]
- 32. Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma). 1979; 28(3): 225–2236. [DOI] [PubMed] [Google Scholar]
- 33. Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam era Twin Registry: an approach using questionnaires. Clin Genet. 1989; 35(6): 423–432. [DOI] [PubMed] [Google Scholar]
- 34. Edwards A, Civitello A, Hammond HA, Caskey CT. DNA typing and genetic mapping with trimeric and tetrameric tandem repeats. Am J Hum Genet. 1991; 49(4): 746–756. [PMC free article] [PubMed] [Google Scholar]
- 35. Kroenke K, Spitzer RL, Williams JB. The Patient health questionnaire-2: validity of a two-item depression screener. Med Care. 2003; 41(11): 1284–1292. [DOI] [PubMed] [Google Scholar]
- 36. Monk TH, Buysse DJ, Kennedy KS, Pods JM, DeGrazia JM, Miewald JM. Measuring sleep habits without using a diary: the sleep timing questionnaire. Sleep. 2003; 26(2): 208–212. [DOI] [PubMed] [Google Scholar]
- 37. Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992; 15(5): 461–469. [DOI] [PubMed] [Google Scholar]
- 38. Sadeh A, Sharkey KM, Carskadon MA. Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994; 17(3): 201–207. [DOI] [PubMed] [Google Scholar]
- 39. Webster JB, Kripke DF, Messin S, Mullaney DJ, Wyborney G. An activity-based sleep monitor system for ambulatory use. Sleep. 1982; 5(4): 389–399. [DOI] [PubMed] [Google Scholar]
- 40. Taibi DM, Landis CA, Vitiello MV. Concordance of polysomnographic and actigraphic measurement of sleep and wake in older women with insomnia. J Clin Sleep Med. 2013; 9(3): 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chai V, Vassilakos A, Lee Y, Wright JA, Young AH. Optimization of the PAXgene blood RNA extraction system for gene expression analysis of clinical samples. J Clin Lab Anal. 2005; 19(5): 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Du P, Kibbe WA, Lin SM. Lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008; 24(13): 1547–1548. [DOI] [PubMed] [Google Scholar]
- 43. Smyth GK. Limma: linear models for microarray data. In: Gentleman VCR, Dudoit S, Irizarry R, Huber W, eds. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York, NY: Springer, 2005: 397–420. [Google Scholar]
- 44. Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003; 19(3): 368–375. [DOI] [PubMed] [Google Scholar]
- 45. Wang J, Duncan D, Shi Z, Zhang B. WEB-based GEne SeT AnaLysis Toolkit (WebGestalt): update 2013. Nucleic Acids Res. 2013; 41:W77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005; 102(43): 15545–15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Subramanian A, Kuehn H, Gould J, Tamayo P, Mesirov JP. GSEA-P: a desktop application for gene set enrichment analysis. Bioinformatics. 2007; 23(23): 3251–3253. [DOI] [PubMed] [Google Scholar]
- 48. Saeed AI, Sharov V, White J, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003; 34(2): 374–378. [DOI] [PubMed] [Google Scholar]
- 49. Gharib SA, Loth DW, Soler Artigas M, et al. Integrative pathway genomics of lung function and airflow obstruction. Hum Mol Genet. 2015; 24(23): 6836–6848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Aho V, Ollila HM, Rantanen V, et al. Partial sleep restriction activates immune response-related gene expression pathways: experimental and epidemiological studies in humans. PLoS One. 2013; 8(10):e77184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Palmer C, Diehn M, Alizadeh AA, Brown PO. Cell-type specific gene expression profiles of leukocytes in human peripheral blood. BMC Genomics. 2006; 7: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gottlieb DJ, Hek K, Chen TH, et al. Novel loci associated with usual sleep duration: the CHARGE Consortium Genome-Wide Association Study. Mol Psychiatry. 2015; 20(10): 1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frey DJ, Fleshner M, Wright KP., Jr The effects of 40 hours of total sleep deprivation on inflammatory markers in healthy young adults. Brain Behav Immun. 2007; 21(8): 1050–1057. [DOI] [PubMed] [Google Scholar]
- 54. Patel SR, Zhu X, Storfer-Isser A, et al. Sleep duration and biomarkers of inflammation. Sleep. 2009; 32(2): 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vgontzas AN, Zoumakis E, Bixler EO, et al. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004; 89(5): 2119–2126. [DOI] [PubMed] [Google Scholar]
- 56. Irwin MR, Carrillo C, Olmstead R. Sleep loss activates cellular markers of inflammation: sex differences. Brain Behav Immun. 2010; 24(1): 54–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Irwin MR, Wang M, Ribeiro D, et al. Sleep loss activates cellular inflammatory signaling. Biol Psychiatry. 2008; 64(6): 538–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lange T, Perras B, Fehm HL, Born J. Sleep enhances the human antibody response to hepatitis A vaccination. Psychosom Med. 2003; 65(5): 831–835. [DOI] [PubMed] [Google Scholar]
- 59. Spiegel K, Sheridan JF, Van Cauter E. Effect of sleep deprivation on response to immunization. JAMA. 2002; 288(12): 1471–1472. [DOI] [PubMed] [Google Scholar]
- 60. McGlone FB, Arden NH. Impact of influenza in geriatrics and action plan for prevention and treatment. Am J Med. 1987; 82: 55–57.3103437 [Google Scholar]
- 61. Cohen S, Doyle WJ, Alper CM, Janicki-Deverts D, Turner RB. Sleep habits and susceptibility to the common cold. Archives of Internal Medicine. 2009; 169: 62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Prather AA, Janicki-Deverts D, Hall MH, Cohen S. Behaviorally assessed sleep and susceptibility to the common cold. Sleep. 2015; 38(9): 1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Prather AA, Hall M, Fury JM, et al. Sleep and antibody response to hepatitis B vaccination. Sleep. 2012; 35(8): 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Orzech KM, Acebo C, Seifer R, Barker D, Carskadon MA. Sleep patterns are associated with common illness in adolescents. J Sleep Res. 2014; 23(2): 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nilsson EK, Boström AE, Mwinyi J, Schiöth HB. Epigenomics of total acute sleep deprivation in relation to genome-wide DNA methylation profiles and RNA expression. OMICS. 2016; 20(9): 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cortese R, Khalyfa A, Bao R, Andrade J, Gozal D. Epigenomic profiling in visceral white adipose tissue of offspring of mice exposed to late gestational sleep fragmentation. Int J Obes (Lond). 2015; 39(9): 1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. TrehanPati N, Geffers R, Sukriti et al. Gene expression signatures of peripheral CD4+ T cells clearly discriminate between patients with acute and chronic hepatitis B infection. Hepatology. 2009; 49(3): 781–790. [DOI] [PubMed] [Google Scholar]
- 68. Jernås M, Hou Y, Strömberg Célind F, et al. Differences in gene expression and cytokine levels between newly diagnosed and chronic pediatric ITP. Blood. 2013; 122(10): 1789–1792. [DOI] [PubMed] [Google Scholar]
- 69. Fan VS, Gharib SA, Martin TR, Wurfel MM. COPD disease severity and innate immune response to pathogen-associated molecular patterns. Int J Chron Obstruct Pulmon Dis. 2016; 11: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xiao Q, Signorello LB, Brinton LA, Cohen SS, Blot WJ, Matthews CE. Sleep duration and breast cancer risk among black and white women. Sleep Med. 2016; 20: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Phipps AI, Bhatti P, Neuhouser ML, et al. Pre-diagnostic sleep duration and sleep quality in relation to subsequent cancer survival. J Clin Sleep Med. 2016; 12(4): 495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.