Abstract
Objectives:
Pharyngeal critical closing pressure (Pcrit) or collapsibility is a major determinant of obstructive sleep apnea (OSA) and may be used to predict the success/failure of non-continuous positive airway pressure (CPAP) therapies. Since its assessment involves overnight manipulation of CPAP, we sought to validate the peak inspiratory flow during natural sleep (without CPAP) as a simple surrogate measurement of collapsibility.
Methods:
Fourteen patients with OSA attended overnight polysomnography with pneumotachograph airflow. The middle third of the night (non-rapid eye movement sleep [NREM]) was dedicated to assessing Pcrit in passive and active states via abrupt and gradual CPAP pressure drops, respectively. Pcrit is the extrapolated CPAP pressure at which flow is zero. Peak and mid-inspiratory flow off CPAP was obtained from all breaths during sleep (excluding arousal) and compared with Pcrit.
Results:
Active Pcrit, measured during NREM sleep, was strongly correlated with both peak and mid-inspiratory flow during NREM sleep (r = −0.71, p < .005 and r = −0.64, p < .05, respectively), indicating that active pharyngeal collapsibility can be reliably estimated from simple airflow measurements during polysomnography. However, there was no significant relationship between passive Pcrit, measured during NREM sleep, and peak or mid-inspiratory flow obtained from NREM sleep. Flow measurements during REM sleep were not significantly associated with active or passive Pcrit.
Conclusions:
Our study demonstrates the feasibility of estimating active Pcrit using flow measurements in patients with OSA. This method may enable clinicians to estimate pharyngeal collapsibility without sophisticated equipment and potentially aid in the selection of patients for non- positive airway pressure therapies.
Keywords: collapsibility, peak inspiratory flow, active Pcrit, sleep apnea.
Statement of Significance
Recent evidence suggests that OSA is caused by the interplay of a variety of physiological variables; however, pharyngeal collapsibility remains as the key contributor in majority of patients. This is because the alternative therapies may be less likely to succeed in a severely crowded airway, even if they are correctly matched to the structure causing the upper airway collapse. Thus, clinically available methods are needed for quantifying the collapsibility during sleep. The contribution of the proposed research is a clinical methodology for estimating upper airway collapsibility (under active condition). This contribution is significant because it may ultimately aid in the selection of patients for alternative therapies, thereby potentially improving quality of life and health outcomes through improved compliance with therapy.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder characterized by repeated pharyngeal obstruction during sleep1 which causes sleep fragmentation2 and sympathetic activitation.3 Untreated OSA has also been associated with adverse neurocognitive (daytime sleepiness, reduced attention)4–6 and cardiovascular complications (hypertension, diabetes, and stroke).4,7,8 Recent evidence suggests that OSA is caused by the interplay of a variety of physiological variables, with pharyngeal collapsibility9–12 playing a prominent role in most patients.
The gold standard measurement for pharyngeal collapsibility is the pharyngeal critical pressure (Pcrit).9–21 Pcrit is determined by reducing the nasal pressure to various levels during sleep and plotting the maximum airflow for each flow-limited breath against the associated pressure. Pcrit is defined by the extrapolation of this relationship to zero flow. Pcrit can be measured in active and (relatively) passive states of pharyngeal muscle activity. Active Pcrit is determined by lowering the nasal pressure slowly to allow the dilator muscles time to activate, while passive Pcrit is determined by lowering the pressure abruptly and measuring flow before muscles have time to activate.22
Assessment of collapsibility by the Pcrit method has several problems that limit its applicability. First, complete characterization of the pressure–flow relationship (in the pressure range where flow is limited) requires the use of specialized continuous positive airway pressure (CPAP) devices that can deliver both positive and negative airway pressures. Second, measuring an individual’s Pcrit often requires trained experts to perform CPAP drops during overnight sleep studies. Consequently, Pcrit is currently only used as a research tool. Therefore, there is a need for a simple and reliable assessment of pharyngeal collapsibility during sleep.
In this study, we tested the hypothesis that upper airway collapsibility can be estimated using airflow measurements during natural sleep, without the need for CPAP manipulation. The rationale for this hypothesis is based on the fact that any change in the magnitude of the pressure or (x)-intercept of the pressure–flow plot, which is Pcrit,13–15 is reflected on the flow or (y)-intercept of the pressure–flow plot, which is the peak flow at atmospheric pressure. Thus, the y-intercept of the pressure–flow plot likely contains similar collapsibility information as the x-intercept. The advantage of using the y-intercept is that it does not require CPAP adjustments, that is, it should be equivalent to the peak flow off CPAP. To test this hypothesis, peak inspiratory flow for every breath during natural sleep (off CPAP) was determined using a specially designed automated algorithm. In addition, the mid-inspiratory flow was determined (off CPAP) to examine the effect of negative effort dependence.23 Peak and mid-inspiratory flow data were pooled and their median values were calculated for each patient and compared to separately measured active and passive Pcrits (measured during NREM sleep) using traditional methods.
METHODS
Participants
The current results were obtained by additional analysis of data obtained in the course of existing OSA phenotype investigations.24,25 Briefly, OSA patients (age: 18–60 years) with an apnea–hypopnea index (AHI) >10 events/h were invited to participate. The exclusion criteria included cardiac disease (uncontrolled hypertension or heart failure) or any other serious comorbidities and the use of medications known to influence sleep or respiration. The study was approved by the Partners Institutional Review Board. All participants provided written informed consent prior to study enrollment.
Measurements and Equipment
Participants were instrumented for a physiological polysomnographic study. Electroencephalography (EEG), chin electromyography (EMG), and electrooculography (EOG) were recorded for sleep staging. Piezoelectric bands around the chest and abdomen monitored respiratory movements. Electrocardiography (ECG), body position, and arterial oxygen saturation (SaO2) were also collected. In addition, participants wore a sealed nasal mask to facilitate airflow measurement (pneumotachograph, Hans-Rudolph, Kansas City, MO). Mask pressure was monitored with a pressure transducer (Validyne, Northridge, CA) referenced to atmosphere. To confirm flow limitation, a small flexible pressure-tipped catheter (Millar Instruments, Houston, TX) was inserted through a decongested (oxymetazoline-HCL) and anesthetized (4% lidocaine) nostril to measure the epiglottic pressure (Pepi). EEG, EMG, EOG, and respiratory movement signals were amplified using GRASS amplifiers (Grass Telefactor, West Warwick, RI), and all signals were digitized and sampled at 125 Hz (Power1401 and Spike2, Cambridge Electronic Design, Cambridge, United Kingdom).
Severity of OSA and Peak Flow Measurements
The first and last thirds of the study were used to assess peak airflow and OSA severity (assessed using the AHI) off CPAP. At least 2 hours of data off CPAP in the supine position were collected after lights out. Apneas and hypopneas were scored using standard American Academy of Sleep Medicine guidelines.26 Specifically, hypopneas were scored when there was a ≥30% reduction in airflow that lasted at least 10 seconds and was associated with ≥3% oxygen desaturation and/or an arousal (AASM recommended rule 1A). The reported peak and mid-inspiratory flow and AHI are the values while participants were in supine position and off CPAP. Flow and AHI data from non-rapid eye movement sleep (NREM) and REM sleep were analyzed separately.
Upper Airway Collapsibility Measurements
The middle third of the night was dedicated to measuring Pcrit during NREM sleep. Participants slept supine and breathed on a modified CPAP device (Philips-Respironics, Murrysville, PA) capable of delivering positive and negative nasal pressures. After stable sleep was achieved, the pressure in the mask was increased to the level required to eliminate flow limitation, as determined by the airflow pattern and epiglottic pressure signals. CPAP was then reduced under 2 different conditions27: (1) The passive condition in which CPAP was reduced abruptly for 5 breaths to assess the upper airway collapsibility when the pharyngeal muscles were relatively hypotonic. In total, 214 passive drops from an optimum pressure of 8.0 (7.0–9.8) cmH2O were performed, resulting in 13 (8–16) data points per subject (Here and throughout this paper, numerical values in parentheses denote interquartile ranges,) (2) The active condition in which CPAP was reduced gradually (<1 cmH2O per min) to permit the activation of pharyngeal muscles. In total, 64 active drops were performed which resulted in 13 (9–14) data points per subject. If awakening occurred, CPAP was returned to the optimum level until stable sleep resumed.
Upper Airway Collapsibility Analysis
Passive and active Pcrit were calculated using data collected during supine NREM sleep.
-
1)
Passive upper airway collapsibility: A pressure–flow curve was constructed by plotting peak inspiratory flow versus mask pressure for each abrupt CPAP drop using linear regression. The median peak flow of breaths 3 and 4 was recorded for each drop (if flow limited; breaths 1–2 were not included due to potential lung volume effects28 and breath 5 was not included due to increasing muscle activity29). Passive collapsibility was quantified by passive Pcrit (x-intercept of the regression). We also recorded the y-intercept of the regression line ( at atmospheric pressure11,12) to see how this value compared with peak flow measurements off CPAP during spontaneous breathing (the first and last thirds of the night).
-
b)
Active upper airway collapsibility: Active Pcrit was determined as described previously.9,11,12,14,15,22 Briefly, nasal pressure was decreased gradually until apnea or awakening occurred. At the end of each pressure step, the maximal flow of the last 4 flow limited breaths were used to construct the pressure–flow plot. Similar to the passive analysis described earlier, a linear regression line was fitted to these data points. Active collapsibility was quantified by active Pcrit (x-intercept of the regression line). Again, at atmospheric pressure11,12 (the y-intercept of the regression line) was recorded for comparisons to the peak flow off CPAP.
Peak and Mid-Inspiratory Flow Analysis
Peak and mid-inspiratory flow measurements were performed during supine sleep while patients were off CPAP. The flow calculation was automated as described in the online supplement. Briefly, the following steps were performed:
-
1)
The approximate onsets of all breaths were detected automatically from peaks and troughs of the integrated flow signal (zero-mean by removing the average flow over a 9-minute window). The 9-minute interval was used to have at least 100 breaths in each window. Although the zero flow in the pneumotachograph signal is supposed to be the true zero flow, we nevertheless saw some drift and thus used the integrated flow signal to find the start and end of each breath. Furthermore, this methodology was developed with the aim of operating accurately and robustly under the condition when there is a variable but unknown DC offset (eg, low level of CPAP with small leak, flow measured in a bias flow circuit, or electrical drift in the DC baseline). Therefore, the presented method can be applied broadly to any signal estimating airflow without the need for any substantial modification.
-
2)
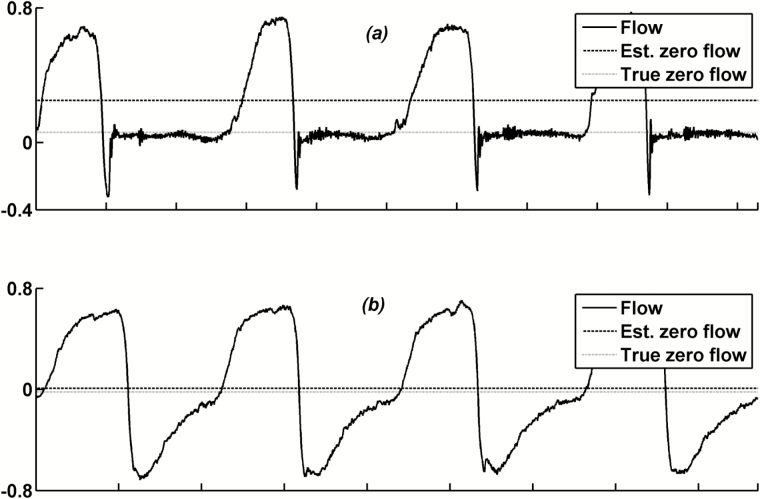
Next, breaths that contained expiratory mouth leak (EML; Figure 1, a) were detected automatically and removed from the analysis during zero flow calculation (see validation of the EML detection algorithm below). As described in the online supplement, EML was detected when there was a volume loss during expiration (due to breathing in through the nose and out through the mouth). As shown in Figure 1 a, the presence of EML leads to inaccurate estimation of true zero flow using step #1 mentioned earlier (ie, true zero flow is overestimated, resulting in an underestimated peak flow). Indeed, we have been unable at this point to develop an automated algorithm for detecting true zero flow in the presence of EML that agrees with hand detection. Because accurate detection of true zero flow is imperative to accurate estimation of peak flow, breaths with EML were excluded from the analysis during zero flow calculation. Therefore, the zero flow obtained from non-EML breaths was considered to be zero flow for EML breaths.
Figure 1.
Zero flow estimated by averaging the flow signal over a period of time does not represent the true zero flow if that period contains breaths with expiratory mouth leak (EML). (a) Breaths with expiratory flow leak. (b) Breaths without expiratory flow leak (the mean airflow is an accurate estimate of true zero flow). Est., Estimated.
A total of 10677 NREM supine sleep breaths were used to validate the EML detection algorithm, from which 1774 (16.6%) breaths were marked manually as breaths with EML. The manual scoring was based on appearance of a rapid early reduction in expiratory flow typically accompanying an abnormal increase in epiglottic pressure (Figure S2, online supplement). The algorithm correctly identified 1687 EML breaths and 8617 as non-EML breaths. This resulted in a sensitivity of 96.8% and a specificity of 95.1%. This was a crucial step in the automation process because EML occurred on at least 20% of all sleep breaths in 4 subjects and between 5% and 20% of all sleep breaths in 5 subjects. On average, the prevalence of EML was 6.6% (2.6%–20.7%) of all sleep breaths.
-
3)
Following zero-flow correction (using periods without EML), flow was reintegrated and peaks and troughs were recalculated. To remove high-frequency artifacts, the flow signal was high-pass filtered with a cutoff frequency of 20 Hz.
-
4)
Peak flow was calculated by subtracting the maximum flow (ie, first peak detected during inspiration) from the flow at the onset of the breath. For EML breath, peak flow was calculated by subtracting the maximal flow from previously calculated zero flow (from non-EML breaths). Similarly, mid-inspiratory flow was determined by subtracting the flow at the middle of inspiration from the flow at the onset of the breath.
-
5)
Apneas were filled by breaths with zero flow with a duration equal to a normal respiratory cycle (median Ttot outside apnea).
-
6)
The median of all peak and mid-inspiratory flows during supine NREM and, separately, during supine REM sleep (excluding breaths during arousals) were calculated and termed VpeakNREM, VpeakREM, VmidNREM, and VmidREM, respectively.
Statistical Analyses
Data are expressed as the median (interquartile range) unless otherwise specified. Pearson’s correlation assessed associations between peak flow and pharyngeal collapsibility. A linear multiple regression (with backward elimination) analysis was performed to examine the association between active Pcrit and peak flow while including clinical variables such as AHI, percent hypopneas, and nadir SaO2 as covariates. Changes in peak and mid-inspiratory flow and Pcrit from NREM to REM were assessed using Wilcoxon signed-rank test. Value of p < .05 was considered as statistically significant.
RESULTS
Patient Characteristics
A total of 14 participants (age: 55 (51–61) years, 5 females) with a body mass index of 30.4 (29.2–33.1) kg/m2 were studied (Table 1).
Table 1.
Participants’ characteristics (N = 14).
| Age, years | 55(51–61) |
|---|---|
| M: F | 9: 5 |
| Body mass index, kg/m2 | 30.4 (29.2–33.1) |
| AHI-Total, events/h | 29.8 (15.6–44.1) |
| AHI-NREM Supine, events/h | 38.4 (20.5–51.8) |
| % Hypopnea | 75.2 (37.8–90.2) |
| Average SaO2 (%) | 97.5 (96.9–98.3) |
| Nadir SaO2 (%) | 89.5 (88.3–91.8) |
| ArI- NREM Supine, events/hr | 49.9 (37.3–66.2) |
| TST, minutes | 256.0 (199.5–279.8) |
| NREM1 Sleep (%TST) | 30.2 (24.0–35.5) |
| NREM2 Sleep (%TST) | 51.5 (49.4–55.1) |
| NREM3 Sleep (%TST) | 4.5 (0.1–13.5) |
| REM Sleep (%TST) | 12.8 (7.6–17.9) |
| SE (%) | 60.4 (47.4–66.6) |
AHI, apnea-hypopnea index; NREM, non-REM sleep; SaO2, oxyhemoglobin saturation; TST, total sleep time; SE, sleep efficiency; Pcrit, pharyngeal closing pressure (x-intercept of pressure-flow curve); Arl, arousal index.
Data are presented as median (interquartile range) unless otherwise specified.
Upper Airway Collapsibility
Table 2 shows the passive and active Pcrits determined during the middle third of the night. Active Pcrit was ~3 cmH2O lower than passive Pcrit (−3.5 (−5.0 to −0.9) cmH2O versus −0.7 (−2.8 to 0.1) cmH2O, p < .05). at atmospheric pressure (y-intercept) was significantly lower in passive than in active conditions (0.09 (0.0–0.19) L/s versus 0.18 (0.10–0.30) L/s, p < .01; Table 2).
Table 2.
Pharyngeal Collapsibility Quantified in Passive and Active States (N = 14).
| Measure | State | Median (IQR) | Wilcoxon test of difference in median (z-value, p value) |
|---|---|---|---|
| Pcrit (cmH 2 O) | Passive | −0.7 (−2.8 to 0.1) | (2.3, .012a) |
| Active | −3.5 (−5.0 to −0.9) | ||
| (L/s) | Passive | 0.09 (0.0 to 0.19) | (2.5, .006 a) |
| Active | 0.18 (0.10 to 0.30) |
IQR, interquartile range; Pcrit, pharyngeal closing pressure (x-intercept of pressure-flow curve); , at atmospheric pressure (y-intercept of the pressure-flow regression line).
a statistically significant.
Example Traces
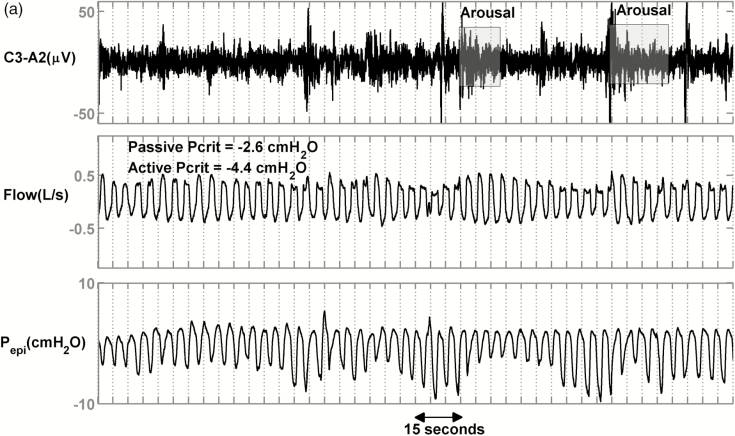
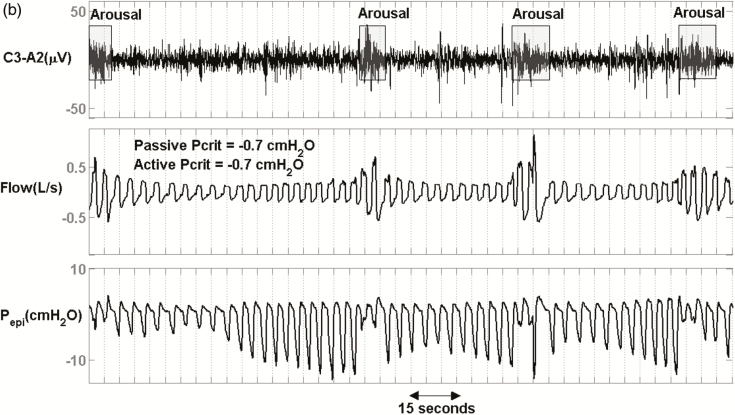
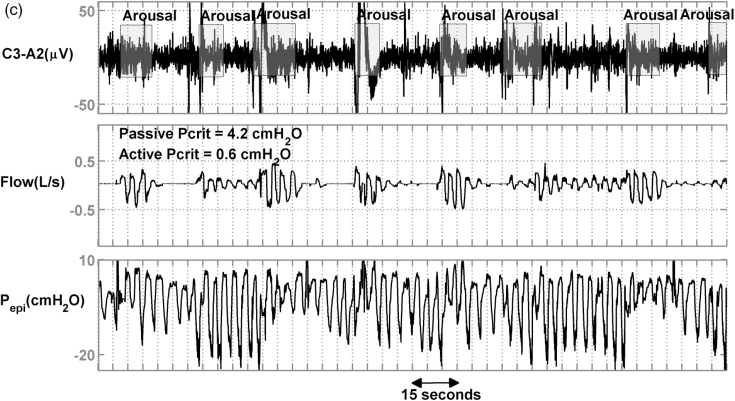
Figure 2 shows the raw data for 3 representative patients with severe OSA. Figure 2a displays a patient with less collapsible airway (passive Pcrit = −2.6 cmH2O, active Pcrit = −4.4 cmH2O) and relatively high median peak flow (0.33 L/s). Figure 2b shows a patient with moderate collapsibility values (passive Pcrit = −0.7 cmH2O, active Pcrit = −0.7 cmH2O) and a lower median peak flow (0.24 L/s, 27% less than first patient). Figure 2c shows a patient with high collapsibility values (passive Pcrit = +4.2 cmH2O, active Pcrit = +0.6 cmH2O) and a lower median peak flow (0.23 L/s, 30% less than the first patient). Despite different collapsibilities, reflected in peak flow, all 3 patients had similar severities of OSA (NREM Supine AHI = 44, 38 and 32 events/hr) and similar proportion of events that were hypopneas rather than apneas (80%, 97%, and 79%).
Figure 2.
Raw data of 3 representative patients with similar apnea–hypopnea index (AHIs) and percent hypopneas but different peak flows and Pcrits. (a) An obstructive sleep apnea (OSA) patient with an AHI of 44 events/h, an active Pcrit of −4.4 cmH2O, and hypopneas that exhibited a mild reduction in peak flow (VpeakNREM = 0.33 L/s). (b) An OSA patient with an AHI of 38 events/h, an active Pcrit of −0.7 cmH2O, and hypopneas that exhibited a moderate reduction in peak flow (VpeakNREM = 0.24 L/s). (c) An OSA patient with an AHI of 32 events/h, an active Pcrit of 0.6 cmH2O, and hypopneas that exhibited a severe reduction in peak flow (VpeakNREM = 0.23 L/s, 30% less than patient (a)).
Peak and Mid-Inspiratory Flow Versus Collapsibility in NREM
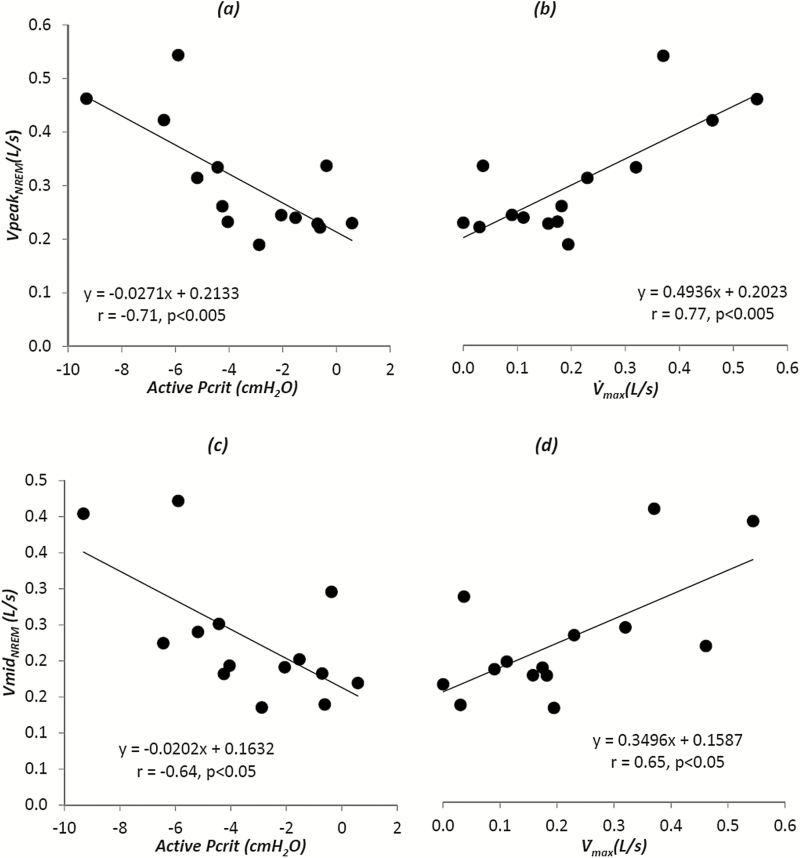
A significant relationship was found among VpeakNREM, the median peak flow obtained when patients were off CPAP, and active Pcrit (r = −0.71, p < .005; Figure 3, left top panel) and between VpeakNREM and at atmospheric pressure (y-intercept of the pressure–flow curve; r = 0.77, p < .005; Figure 3, right top panel), suggesting that peak flow during NREM sleep is a valid estimate of the active collapsibility during NREM sleep. A weaker but still significant correlation was found between VmidNREM, the median mid-inspiratory flow obtained when patients were off CPAP, and active Pcrit (r = −0.64, p < .05; Figure 3, left bottom panel) and between VmidNREM and at atmospheric pressure (y-intercept of the pressure–flow curve; r = 0.65, p < .05; Figure 3, right bottom panel), indicating that the relationship between active Pcrit and flow measurements holds true, despite the presence of negative effort dependence. However, there was no relationship between VpeakNREM or VmidNREM and passive Pcrit during NREM sleep (r = −0.007, p = 0.98 and r = −0.1, p = .73, respectively; Table 3). This suggests that NREM flow measurements are not a measure of collapsibility under hypotonic conditions.
Figure 3.
There was a strong correlation between peak flow during supine non-rapid eye movement sleep (NREM) sleep and active pharyngeal collapsibility as determined by either the active Pcrit (a) or the active (b) (y-intercept of the pressure-flow graph) during NREM sleep. There was a weaker but significant correlation between mid-inspiratory flow during NREM sleep and active pharyngeal collapsibility as determined by either the active Pcrit (c) or the active (d) (y-intercept of the pressure-flow graph) during NREM sleep.
Table 3.
Correlation Coefficients Between Peak and Mid-Inspiratory Flow Measurements and Passive Collapsibility Values, AHI, and %Hypopnea.
| Measure | Vpeak NREM | Vmid NREM | Vpeak REM a | Vmid REM a | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Passive Pcrit | −0.007 | .98 | −0.1 | .73 | −0.46 | .18 | −0.42 | .22 |
| Active Pcrit | −0.71 | <.005 | −0.64 | <.05 | −0.22 | .54 | −0.09 | .80 |
| AHI | 0.21 | .47 | −0.19 | .52 | −0.32 | .37 | 0.38 | .27 |
| %Hypopnea | 0.05 | .87 | 0.24 | .40 | −0.05 | .91 | 0.36 | .31 |
a N = 10 (4 excluded patients had <50 breaths in REM sleep); VpeakNREM is the median peak flow of all breaths during NREM sleep (excluding during arousal); VmidNREM is the median mid-inspiratory flow of all breaths during NREM sleep (excluding during arousal); VpeakREM includes breaths during REM sleep; VmidREM is the median mid-inspiratory flow of all breaths during REM sleep (excluding during arousal); Pcrit is the pharyngeal closing pressure (x-intercept of pressure-flow curve); AHI is the apnea–hypopnea index; %Hypopnea is the number of hypopneas / total number of respiratory events (hypopneas + apneas). AHI and %Hypopnea were calculated separately in NREM and REM and were correlated with the corresponding Vpeak reported here.
Peak Flow During Selected Periods
In addition to the analysis mentioned earlier in which we took all breaths during NREM sleep (excluding breaths associated with arousals), we also tested whether the breaths selected from certain periods of NREM sleep would improve the correlation between active Pcrit and peak flow. We selected breaths from the following periods thought to contain increased pharyngeal muscle activity: (1) stable breathing periods30 and (2) the last 15 seconds of each respiratory event.31 The correlation coefficient in both cases was −0.69 (p < .01), which is similar to what we found using all NREM sleep breaths. These data suggest that more accurate estimates of active collapsibility were not achieved by choosing breaths under these selected conditions.
Peak Flow During REM
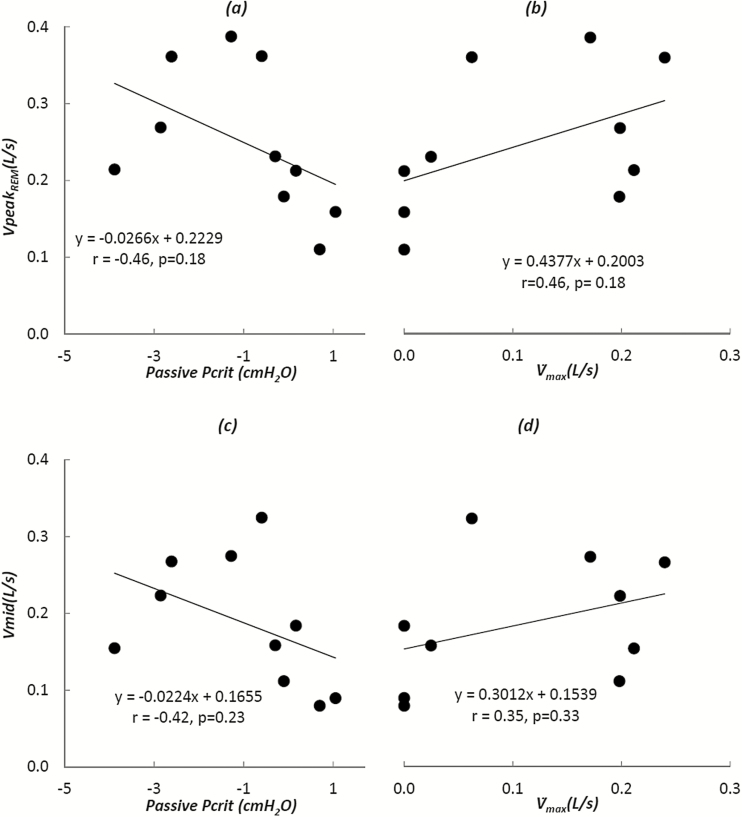
On the other hand, previous studies21,32 suggest that the muscle activity is low in REM sleep and may better represent the hypotonic conditions under which passive Pcrit is determined. For this reason, the correlation coefficient between peak and mid-inspiratory flow during REM sleep (VpeakREMand VmidREM, respectively) and passive Pcrit, obtained during NREM sleep, was calculated (Figure 4). Ten patients who had at least 50 breaths in REM sleep (excluding arousals) were included. As shown in Figure 4a, there was a moderate but nonsignificant relationship between passive Pcrit and VpeakREM or VmidREM (r = −0.46, p = .18 and r = −0.42, i = .23; Figure 4). Therefore, we did not find that passive collapsibility during NREM sleep could be estimated by selecting peak flow during REM sleep.
Figure 4.
Peak flow during supine rapid eye movement sleep (REM) sleep and its relationships with passive pharyngeal collapsibility as determined by either the passive Pcrit (a) or the passive (b) (y-intercept of the pressure-flow graph) during non-rapid eye movement sleep (NREM) sleep. Similarly, there was a nonsignificant relationship between mid-inspiratory flow during supine REM sleep and passive pharyngeal collapsibility values as determined by either the passive Pcrit (c) or the passive (d) (y-intercept of the pressure-flow graph) during NREM sleep.
Peak Flow Detects the Increased Collapsibility From NREM to REM
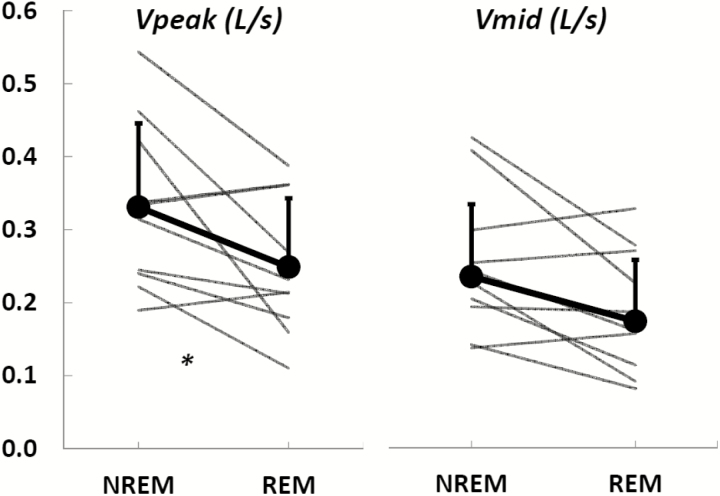
In addition to the correlation analysis between Pcrits, obtained from NREM sleep, and peak flow during NREM and REM sleep, we also wanted to confirm that our peak flow measurements detected the anticipated decrease in peak flow from NREM to REM sleep, likely, as a result of general worsening of sleep apnea in REM.21,30,32–37 Indeed, a significant reduction in peak flow from NREM sleep (VpeakNREM) to REM sleep (VpeakREM) was observed (0.32 (0.24–0.42)L/s versus 0.22 (0.18–0.36)L/s, N = 10, p < .02; Figure 5, left panel). However, the reduction in mid-inspiratory flow from NREM sleep (VmidNREM) to REM sleep (VmidREM) was not significant (0.23 (0.19–0.28)L/s versus 0.17 (0.12–0.26)L/s, N = 10, p = .2; Figure 5, right panel). Table 4 reports on the clinical measurements (AHI and percent hypopnea) and flow measurements (number of breaths analyzed, median peak and mid-inspiratory values and interquartile ranges) for each participant during NREM and REM sleep.
Figure 5.
Peak inspiratory flow (left panel) during non-rapid movement sleep (NREM) sleep is significantly higher than that during rapid movement sleep (REM) sleep. In contrast, the reduction in mid-inspiratory flow (right panel) from NREM sleep to REM sleep is not significant.
Table 4.
Peak and Mid-Inspiratory Flow Characteristics During Non-REM and REM Sleep.
| Subject # | NREM | REM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AHI | %Hypopnea | #Breaths | Vpeak | Vmid | AHI | %Hypopnea | #Breaths | Vpeak | Vmid | |
| 1 | 39.1 | 12 | 1376 | 0.22 (0–0.32) | 0.14 (0–0.26) | 64.9 | 25 | 716 | 0.11 (0–0.20) | 0.08 (0–0.14) |
| 2 | 54.7 | 29 | 903 | 0.26 (0.15–0.36) | 0.18 (0.06–0.28) | N/A | N/A | 0 | N/A | N/A |
| 3 | 24.3 | 69 | 1404 | 0.31 (0.27–0.38) | 0.24 (0.19–0.29) | 0 | N/A | 91 | 0.23 (0.20–0.26) | 0.16 (0.12–0.19) |
| 4 | 54.4 | 95 | 930 | 0.23 (0.17–0.29) | 0.19 (0.12–0.25) | N/A | N/A | 0 | N/A | N/A |
| 5 | 10.5 | 100 | 3016 | 0.46 (0.41–0.54) | 0.40 (0.36–0.50) | 13.0 | 100 | 277 | 0.27 (0.21–0.34) | 0.22 (0.17–0.30) |
| 6 | 32.0 | 79 | 1766 | 0.23 (0.19–0.27) | 0.17 (0.13–0.21) | N/A | N/A | 0 | N/A | N/A |
| 7 | 19.2 | 73 | 3295 | 0.19 (0.17–0.24) | 0.14 (0.10–0.17) | 32.5 | 23 | 867 | 0.21 (0.15–0.27) | 0.15 (0.10–0.20) |
| 8 | 37.7 | 96 | 991 | 0.24 (0.19–0.31) | 0.18 (0.15–0.24) | N/A | N/A | 0 | N/A | N/A |
| 9 | 43.9 | 80 | 303 | 0.33 (0.27–0.39) | 0.25 (0.17–0.31) | 24.0 | 0 | 262 | 0.36 (0.27–0.45) | 0.27 (0.13–0.37) |
| 10 | 42.0 | 30 | 657 | 0.42 (0.20–0.59) | 0.22 (0.05–0.39) | 0 | N/A | 71 | 0.16 (0.13–0.17) | 0.09 (0.08–0.09) |
| 11 | 9.3 | 55 | 1265 | 0.24 (0.19–0.32) | 0.20 (0.15–0.27) | 69.9 | 27 | 281 | 0.18 (0.05–0.26) | 0.11 (0.03–0.20) |
| 12 | 64.0 | 94 | 1373 | 0.24 (0.19–0.30) | 0.19 (0.15–0.25) | 60.0 | 100 | 50 | 0.21 (0.16–0.25) | 0.18 (0.14–0.22) |
| 13 | 12.8 | 77 | 2075 | 0.54 (0.42–0.69) | 0.42 (0.29–0.56) | 22.0 | 0 | 164 | 0.39 (0.28–0.63) | 0.28 (0.16–0.46) |
| 14 | 96.0 | 32 | 417 | 0.34 (0.10–0.51) | 0.30 (0.08–0.44) | 37.5 | 100 | 52 | 0.36 (0.29–0.50) | 0.33 (0.25–0.46) |
NREM, non-rapid movement sleep; REM, rapid movement sleep; AHI, apnea–hypopnea index; %Hypopnea, the number of hypopneas / total number of respiratory events (hypopneas + apneas); #Breaths, number of breaths analyzed; Vpeak, median peak inspiratory flow during sleep excluding arousals; Vmid, mid-inspiratory flow during sleep excluding arousals.
Comparison With Clinical Measurements
It may be argued that the AHI or the percent of the respiratory events that were hypopneas (percent hypopnea) adequately reflect the underlying collapsibility. For this reason, we calculated the univariate correlation coefficient between NREM Pcrit (passive and active) and nadir SaO2, NREM AHI, and NREM percent hypopnea. The only significant correlation was observed between active Pcrit and nadir SaO2 (r = −0.58, p < .05). No other significant relationship was observed (0.15 < p < .9), indicating that neither the AHI nor the percent hypopnea is a surrogate of passive or active collapsibility. In addition, to examine the correlation between active Pcrit and peak flow in the presence of clinical covariates, a multiple linear regression analysis including active Pcrit (as the dependent variable), peak flow, nadir SaO2, AHI, and percent hypopnea was performed. Peak flow was the only significant variable (p < .05) after adjusting for clinical covariates. Likewise, the AHI and the percent hypopnea did not show any significant relationships with flow metrics proposed in this study (p > .05; Table 3), suggesting that our metrics do not estimate the AHI or the percent hypopnea. The significance of this is that VpeakNREM, VpeakREM, VmidNREM, and VmidREM are continuous measurements of collapsibility and are not meant to predict the AHI (which is a frequency measure) and/or percent hypopnea (which is a relative frequency measure).
DISCUSSION
The major conclusion of the current study is that active pharyngeal collapsibility during NREM sleep, as quantified by the active Pcrit, can be estimated from peak airflow measurements during NREM sleep.
Upper Airway Collapsibility and Peak Flow
Upper airway collapsibility, as quantified by Pcrit,9–12,14,15,22,27,38 varies from being quite negative in normal subjects without any evidence of reduced airflow12 to being positive in patients with obstructive sleep apnea.11 Gleadhill et al. showed that the active Pcrit is lowest in snorers, patients with predominantly obstructive hypopneas, and highest in patients with predominantly obstructive apneas9 (hypopneas versus apneas were scored using thermistors which is a less accurate than the pneumotach used in our study). Our data demonstrate that in those with OSA, the median peak flow during sleep reflects the degree of pharyngeal compromise, independent of the AHI, revealed by multiple regression analysis.
Poor Correlation Between Collapsibility and Routine Clinical Variables
While more severe collapsibility is expressed as a lower peak flow, this lower flow does not translate into a greater frequency of respiratory events. Previous studies demonstrated that Pcrit explains only 6% of the variation in AHI within OSA patients,10 and ~21% of variation in respiratory disturbance index within control (NREM respiratory disturbance index <10) and OSA patients.39 Also, Younes found that in patients with zero at atmospheric pressure (positive passive Pcrit), AHI could range from 0 to 160 events/h.40 Similarly, in this study, the AHI did not have any significant correlation with the passive or active Pcrit (p >.05). This is likely because the AHI is a frequency measure and dependent on respiratory control factors such as arousability and chemosensitivity.40
Likewise, the percent of respiratory events that are hypopneas (number of hypopneas/total number of events) was not significantly correlated with passive or active collapsibility (p > .05). The absence of a correlation is likely because majority of events are hypopneas, and hypopneas may reflect a wide range of peak flows (30%–90% reduction from baseline). The finding that neither AHI nor percent hypopnea reflects the typical peak flow observed during sleep is illustrated in Figure 2. The patient shown in Figure 2a had mild reductions in flow along with periods of stable breathing, whereas the patient shown in Figure 2b had moderate reductions in flow, and the patient shown in Figure 2c had mostly severe reductions in flow mixed with apneas. However, all 3 had approximately the same AHI and percent hypopneas, despite clearly different peak flow profiles and Pcrit values. Thus, peak flow gives a more precise and continuous measurement of differences in collapsibility than the dichotomous classification of hypopneas versus apneas.
We emphasize that the primary objective of the present study was not to correlate collapsibility with routine clinical variables including the AHI. This poor correlation is not a specific conclusion of this study. However, the absence of a strong correlation indicates that peak flow provides unique information about collapsibility (active Pcrit) that is not otherwise available from routine clinical variables.
Sleep State Effects
The lack of correlation between NREM peak flow measurements and NREM passive collapsibility (Table 3) suggests that the upper airway muscles must be somewhat active during NREM sleep. On the other hand, REM sleep is known to be associated with lower muscle activity21,32 and therefore might better represent the hypotonic conditions under which passive Pcrit is determined. However, we observed a nonsignificant linear relationship between NREM passive Pcrit and VpeakREM (Figure 4). Furthermore, a significant reduction in peak flow from NREM to REM sleep was also observed (Figure 5). This is in agreement with general increase in OSA severity in REM, as suggested by decreased muscle tone,21,30,32 reduced chemosensitivity,33,34 and impaired genioglossus responsiveness to negative pressure.35–37 However, whether impaired anatomy is also a contributing factor remains unknown, as some studies reported similar passive Pcrit in REM and NREM sleep,15,41 whereas some reported an increase in passive Pcrit (higher collapsibility) in REM sleep.29,42
Although peak flow generally decreases from NREM to REM sleep, 3 patients had slightly increased peak flow during REM sleep (Table 4: subjects 7, 9, and 14). Subjects 9 and 14 had more stable sleep (with respect to AHI) in REM and subject 7 had a higher AHI in REM sleep. However, all these patients had substantial stable breathing during REM sleep with high peak flow which affected their average median peak flow. This was not detectable with clinical measurements such as AHI or percent hypopnea.
It may also be argued that the reduction in peak flow, as a surrogate of active Pcrit, from NREM to REM sleep should be proportionally related to the increase in active Pcrit from NREM to REM (higher collapsibility) or similarly the gap between the active and the passive Pcrits diminishes in REM sleep. As a result, the sleep stage-dependent variations in active Pcrit may be captured by determining the peak flow in both REM and NREM sleep. However, this cannot be confirmed using the data presented in this study and requires further experiments involving Pcrit measurement in REM sleep.
Automation
In addition to the observed correlation between NREM peak flow and active Pcrit, this study also demonstrates that peak flow can be quantified automatically. However, one of the major obstacles to automated peak flow detection was the presence of EML (Figure 1). Therefore, identification and removal of EML ensured correctly identifying the start and end of each breath.
Clinical Implications
The significance of the findings in this study is that automated assessment of peak flow during sleep could be potentially used in a clinical setting to estimate the active collapsibility, which may ultimately aid in the selection of patients for alternative therapies. It is known that OSA is commonly and effectively treated with CPAP, which pneumatically keeps the airway open during sleep.4 Nonetheless, many patients cannot tolerate CPAP or cannot use it on a regular basis for an effective outcome.43 Alternative therapies, such as oral appliances44 and surgery,45 are often unpredictable in their efficacy and have a limited effect size on the airway anatomy. One important contributing factor to the variable response to these alternative therapies may be that the airway is too collapsible to be held open by oral appliances or removal of tissue. It is known that Pcrit changes along a continuum from healthy subjects (negative Pcrit) to severe sleep apnea patients (positive Pcrit). As a result it has been suggested that patients whose sleep apnea is resolved with weight loss demonstrate reduced collapsibility.18 In addition, responders to uvulopalatopharyngoplasty demonstrated a significant reduction of 6.5 cmH2O in their active Pcrit.19 This study suggests that simple airflow measurements can be used to estimate collapsibility and thereby possibly guide therapeutic choices. However, more research is needed to demonstrate that this is possible.
Limitations
One of the limitations of the current study is that airflow was recorded using a pneumotachograph. For a routine polysomnography study, flow is estimated by recording nasal pressure. Therefore, there is a need to adjust the algorithm to correctly identify peak flow using nasal pressure signals. However, we believe this is highly feasible. Also, visual detection of arousals could lead to inclusion and/or exclusion of some of the marginal breaths. This could bias the peak flow measurements by including breaths during arousal (normally breaths with large peak flow) and/or excluding breaths before arousal (depending on prearousal condition these breaths may have very low peak flows). We attempted to deal with this limitation by calculating median peak flow (insensitive to outliers) per subject instead of mean peak flow. Alternatively, objective measurements of beginning and end of arousals could reduce this bias. Another limitation is that peak flow may be variable throughout the night as a result of variation in muscle activity and sleep depth (stages within NREM). This limitation also applies to the gold standard measurement of collapsibility (Pcrit). However, by measuring peak flow over many breaths in different sleep stages, a typical collapsibility for the night is obtained which may actually be more representative of the physiology than a Pcrit measurement at one given point in time. The third limitation is that this method was tested in a relatively small population of 14 OSA subjects. However, a robust association between peak flow and active Pcrit was observed which speaks to the strength of the relationship (Figure 3). Finally, whether the algorithm presented can discriminate among patients whose active Pcrits are positive (eg, +2 cmH2O versus +5 cmH2O) may not be clinically important, as such patients would likely require CPAP to open their obstructed airway (ie, they would likely fail non-CPAP therapies such as oral appliances and oxygen therapy). However, this requires further investigation.
CONCLUSION
The current study demonstrates the feasibility of estimating pharyngeal collapsibility using peak flow in patients with OSA. This method may enable clinicians to automatically estimate pharyngeal collapsibility in a noninvasive manner and may ultimately help clinicians select patients for appropriate therapy.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at SLEEP online.
FUNDING
This work was performed at the Brigham and Women’s Hospital and Harvard Medical School and was supported by funding from Fan Hongbing, President of OMPA Corporation, Kaifeng, China, Philips Respironics research grant, the National Institutes of Health grants R01 (HL102321 and P01 NIH HL095491), as well as the Harvard Catalyst Clinical Research Center (UL1 RR 025758-01). SAS was supported by the National Health and Medical Research Council of Australia (1053201, 1035115) and Menzies Foundation and is currently supported by the American Heart Association (15SDG25890059) and an American Thoracic Society Foundation Unrestricted Grant. BAE is supported by the National Health and Medical Research Council (NHMRC) of Australia’s CJ Martin Overseas Biomedical Fellowship (1035115). LT-M is supported by the American Heart Association (15POST25480003). This research project received generous funding from Fan Hongbing, President of OMPA Corporation, Kaifeng, China. This work was also supported by Philips Respironics research grant, the National Institutes of Health grants R01 HL102321 and P01 NIH HL095491 as well as the Harvard Catalyst Clinical Research Center (UL1 RR 025758-01). SAS was supported by an NHMRC Early Career Fellowship and R.G. Menzies award (1053201) and the American Heart Association (11POST7360012, 15SDG25890059). MDOM was supported by Capes Foundation, Ministry of Education of Brazil.
DISCLOSURE STATEMENT
AA, SAS, MDOM, PRG, BAE, and JB declare no conflicts of interest. LT-M serves as a consultant for Novion Pharmaceuticals Inc. DPW receives salary from Apnicure Inc and serves as a consultant for Philips Respironics and Night Balance. AW receives research support from Philips Respironics.
Supplementary Material
REFERENCES
- 1. Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002; 165(9): 1217–1239. [DOI] [PubMed] [Google Scholar]
- 2. Colt HG, Hass H, Rich GB. Hypoxemia vs sleep fragmentation as cause of excessive daytime sleepiness in obstructive sleep apnea. Chest. 1991; 100(6): 1542–1548. [DOI] [PubMed] [Google Scholar]
- 3. Davies CW, Crosby JH, Mullins RL, Barbour C, Davies RJ, Stradling JR. Case-control study of 24 hour ambulatory blood pressure in patients with obstructive sleep apnoea and normal matched control subjects. Thorax. 2000; 55(9): 736–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. White DP, Younes MK. Obstructive Sleep Apnea. In: Comprehensive Physiology. Wiley-Blackwell; 2012:2541–2594. [DOI] [PubMed] [Google Scholar]
- 5. Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS). Sleep. 2000; 23(Suppl 4): S102–S108. [PubMed] [Google Scholar]
- 6. Reynolds AC, Banks S. Total sleep deprivation, chronic sleep restriction and sleep disruption. Prog Brain Res. 2010; 185: 91–103. [DOI] [PubMed] [Google Scholar]
- 7. Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000; 283(14): 1829–1836. [DOI] [PubMed] [Google Scholar]
- 8. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342(19): 1378–1384. [DOI] [PubMed] [Google Scholar]
- 9. Gleadhill IC, Schwartz AR, Schubert N, Wise RA, Permutt S, Smith PL. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis. 1991; 143(6): 1300–1303. [DOI] [PubMed] [Google Scholar]
- 10. Sforza E, Petiau C, Weiss T, Thibault A, Krieger J. Pharyngeal Critical Pressure in Patients with Obstructive Sleep Apnea Syndrome. Am J Respir Crit Care Med. 1999; 159(1): 149–157. [DOI] [PubMed] [Google Scholar]
- 11. Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure-flow relationships in obstructive sleep apnea. J Appl Physiol (1985). 1988; 64(2): 789–795. [DOI] [PubMed] [Google Scholar]
- 12. Schwartz AR, Smith PL, Wise RA, Gold AR, Permutt S. Induction of upper airway occlusion in sleeping individuals with subatmospheric nasal pressure. J Appl Physiol (1985). 1988; 64(2): 535–542. [DOI] [PubMed] [Google Scholar]
- 13. Boudewyns A, Punjabi N, Van dH, et al. Abbreviated Method for Assessing Upper Airway Function in Obstructive Sleep Apnea. Chest. 2000; 118(4): 1031–1041. [DOI] [PubMed] [Google Scholar]
- 14. Patil SP, Punjabi NM, Schneider H, O’Donnell CP, Smith PL, Schwartz AR. A simplified method for measuring critical pressures during sleep in the clinical setting. Am J Respir Crit Care Med. 2004; 170(1): 86–93. [DOI] [PubMed] [Google Scholar]
- 15. Schwartz AR, O’Donnell CP, Baron J, et al. The Hypotonic Upper Airway in Obstructive Sleep Apnea: Role of structures and neuromuscular activity. Am J Respir Crit Care Med. 1998; 157(4 Pt 1): 1051–1057. [DOI] [PubMed] [Google Scholar]
- 16. Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013; 188(8): 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gold AR, Marcus CL, Dipalo F, Gold MS. Upper airway collapsibility during sleep in upper airway resistance syndrome. Chest. 2002; 121(15): 1531–1540. [DOI] [PubMed] [Google Scholar]
- 18. Schwartz AR, Gold AR, Schubert N, et al. Effect of Weight Loss on Upper Airway Collapsibility in Obstructive Sleep Apnea. Am Rev Respir Dis. 1991; 144(3 Pt 1): 494–498. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz AR, Schubert N, Rothman W, et al. Effect of uvulopalatopharyngoplasty on upper airway collapsibility in obstructive sleep apnea. Am Rev Respir Dis. 1992; 145(3): 527–532. [DOI] [PubMed] [Google Scholar]
- 20. Schwartz AR, Smith PL, Wise RA, Bankman I, Permutt S. Effect of positive nasal pressure on upper airway pressure-flow relationships. J Appl Physiol (1985). 1989; 66(4): 1626–1634. [DOI] [PubMed] [Google Scholar]
- 21. Chin C-, Kirkness JP, Patil SP, et al. Compensatory responses to upper airway obstruction in obese apneic men and women. J Appl Physiol. 2011; 112(3): 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patil SP, Schneider H, Marx JJ, Gladmon E, Schwartz AR, Smith PL. Neuromechanical control of upper airway patency during sleep. J Appl Physiol (1985). 2007; 102(2): 547–556. [DOI] [PubMed] [Google Scholar]
- 23. Genta PR, Owens RL, Edwards BA, et al. Influence of pharyngeal muscle activity on inspiratory negative effort dependence in the human upper airway. Respir Physiol Neurobiol. 2014; 201: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sands SA, Edwards BA, Terrill PI, et al. Phenotyping Sleep Apnea Using Polysomnography: Upper Airway Collapsibility and Responsiveness. American Thoracic Society. 2016; Accepted for presentation. [Google Scholar]
- 25. Taranto-Montemurro L Sands SA Edwards BA, . et al. Desipramine improves upper airway collapsibility and reduces OSA severity in patients with minimal muscle compensation Eur Respir J. 2016; 48(5): 1340–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012; 8(5): 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wellman A, Edwards BA, Sands SA, et al. A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985). 2013; 114(7): 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Owens RL, Edwards BA, Sands SA, et al. Upper airway collapsibility and patterns of flow limitation at constant end-expiratory lung volume. J Appl Physiol (1985). 2012; 113(5): 691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carberry JC, Jordan AS, White DP, Wellman A, Eckert DJ. Upper Airway Collapsibility (Pcrit) and Pharyngeal Dilator Muscle Activity are Sleep Stage Dependent. Sleep. 2016; 39(3): 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jordan AS, White DP, Lo Y, et al. Airway Dilator Muscle Activity and Lung Volume During Stable Breathing in Obstructive Sleep Apnea. Sleep. 2008; 32(3): 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xiao SC, He BT, Steier J, Moxham J, Polkey MI, Luo YM. Neural Respiratory Drive and Arousal in Patients with Obstructive Sleep Apnea Hypopnea. Sleep. 2015; 38(6): 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eastwood PR, Szollosi I, Platt PR, Hillman DR. Comparison of upper airway collapse during general anaesthesia and sleep. Lancet. 2002; 359(9313): 1207–1209. [DOI] [PubMed] [Google Scholar]
- 33. Douglas NJ, White DP, Weil JV, Pickett CK, Hudgel DW, Zwillich CW. Hypoxic Ventilatory Response Decreases during Sleep in Men. Am Rev Respir Dis. 1982; 125(3): 286–289. [DOI] [PubMed] [Google Scholar]
- 34. White DP, Douglas NJ, Pickett CK, Weil JV, Zwillich CW. Hypoxic ventilatory response during sleep in normal premenopausal women. Am Rev Respir Dis. 1982; 126(3): 530–533. [DOI] [PubMed] [Google Scholar]
- 35. Eckert DJ, McEvoy RD, George KE, Thomson KJ, Catcheside PG. Genioglossus reflex inhibition to upper-airway negative-pressure stimuli during wakefulness and sleep in healthy males. J Physiol. 2007; 581(Pt 3): 1193–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shea SA, Edwards JK, White DP. Effect of wake-sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol. 1999; 520(Pt 3): 897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Issa FG, Edwards P, Szeto E, Lauff D, Sullivan C. Genioglossus and breathing responses to airway occlusion: effect of sleep and route of occlusion. J Appl Physiol (1985). 1988; 64(2): 543–549.m [DOI] [PubMed] [Google Scholar]
- 38. Sands SA, Eckert DJ, Jordan AS, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014; 190(8): 930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kirkness JP, Schwartz AR, Schneider H, et al. Contribution of male sex, age, and obesity to mechanical instability of the upper airway during sleep. J Appl Physiol (1985). 2008; 104(6): 1618–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003; 168(6): 645–658. [DOI] [PubMed] [Google Scholar]
- 41. Penzel T, Möller M, Becker HF, Knaack L, Peter JH. Effect of sleep position and sleep stage on the collapsibility of the upper airways in patients with sleep apnea. Sleep. 2001; 24(1): 90–95. [DOI] [PubMed] [Google Scholar]
- 42. Issa FG, Sullivan CE. Upper airway closing pressures in obstructive sleep apnea. J Appl Physiol. 1984; 57(2): 520–527. [DOI] [PubMed] [Google Scholar]
- 43. Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993; 147(4): 887–895. [DOI] [PubMed] [Google Scholar]
- 44. Chan AS, Cistulli PA. Oral appliance treatment of obstructive sleep apnea: an update. Curr Opin Pulm Med. 2009; 15(6): 591–596. [DOI] [PubMed] [Google Scholar]
- 45. Kezirian EJ, Goldberg AN. Hypopharyngeal surgery in obstructive sleep apnea: an evidence-based medicine review. Arch Otolaryngol Head Neck Surg. 2006; 132(2): 206–213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.