Abstract
Study Objectives:
Chronotype, or diurnal preference, refers to behavioral manifestations of the endogenous circadian system that governs preferred timing of sleep and wake. As variations in circadian timing and system perturbations are linked to disease development, the fundamental biology of chronotype has received attention for its role in the regulation and dysregulation of sleep and related illnesses. Family studies indicate that chronotype is a heritable trait, thus directing attention toward its genetic basis. Although discoveries from molecular studies of candidate genes have shed light onto its genetic architecture, the contribution of genetic variation to chronotype has remained unclear with few related variants identified. In the advent of large-scale genome-wide association studies (GWAS), scientists now have the ability to discover novel common genetic variants associated with complex phenotypes. Three recent large-scale GWASs of chronotype were conducted on subjects of European ancestry from the 23andMe cohort and the UK Biobank. This review discusses the findings of these landmark GWASs in the context of prior research.
Methods:
We systematically reviewed and compared methodological and analytical approaches and results across the three GWASs of chronotype.
Results:
A good deal of consistency was observed across studies with 9 genes identified in 2 of the 3 GWASs. Several genes previously unknown to influence chronotype were identified.
Conclusions:
GWAS is an important tool in identifying common variants associated with the complex chronotype phenotype, the findings of which can supplement and guide molecular science. Future directions in model systems and discovery of rare variants are discussed.
Keywords: circadian rhythms, chronotype, genome-wide association study, genetics, sleep
Statement of Significance
An individual’s tendency to be a night owl (late chronotype) or a morning lark (early chronotype) is influenced by his or her genetic makeup. Expression of chronotype (across early and late types) is normally distributed in the population, which suggests that many common genetic variants with modest effects contribute to the phenotype. In our review, we discuss 3 recent hallmark studies using genome-wide association analyses for the identification of genes associated with trait chronotype. Findings identified a number of genes with previously known roles in the circadian clock, as well as several novel gene loci that had not been previously identified as having a role in chronotype or circadian rhythms. Genome-wide analysis is an important tool for identifying novel common gene variants, which illuminates previously unknown genetic influences. Future research on the genetic basis of chronotype should consider using model systems to elucidate circadian function of novel loci.
INTRODUCTION
Circadian rhythms are cyclical changes in cellular, molecular, and biological processes that repeat approximately once every 24 h. Driven by an internal “master clock” located in the suprachiasmatic nucleus (SCN) and influenced by environmental stimuli/zeitgebers (e.g., light–dark cycles), circadian rhythms play a central role in the regulation of many aspects of physiological processes, including sleep–wake cycles. Typical endogenous circadian rhythmicity in humans tends to cycle across a period of 24.2 h with little day-to-day variation in length.1 The timing of the circadian system, however, varies considerably across individuals. Chronotype, or diurnal preference, refers to behavioral patterns or manifestations indicative of underlying circadian-governed biological processes.2 In the study of human sleep–wake regulation, chronotype represents preferred timing of sleep and wake (independent of environmental factors, such as work schedules) and corresponds to the timing of the circadian system.
The timing of circadian rhythmicity falls on a continuum, at the either end of which are individuals colloquially referred to as morning larks and night owls. Morning larks are considered to have early chronotype or advanced sleep phase (also referred to as morning type/preference). Early chronotypes prefer to awaken early in the morning and feel most active during the earlier parts of the day. Some evidence suggests that short circadian periods (e.g., <24 h) may play a role in extreme advanced sleep phase.3 Night owls, or late chronotypes with delayed sleep phase (also evening type/preference), prefer to awaken much later in the day and typically feel most active and motivated in the late evening or at night. In direct contrast to early types, some evidence suggests that individuals with extreme delayed sleep phase may have circadian periods longer than 24.2 h.4 Individuals who fall between the ends of the continuum are referred to as intermediate types. Epidemiological studies characterize chronotype as near-normally distributed with robust developmental changes in diurnal preference across the life span, i.e., earlier preference during childhood development, later in adolescence and early adulthood, then progressively earlier as age advances.2,5
GENETICS OF THE CIRCADIAN CLOCK
There has long been an interest in the genetic basis for circadian rhythmicity. Tremendous advances have been made in identifying a series of molecules that comprise a core circadian clock that exists in every cell, rather than being exclusive to the SCN. Each cell maintains its own circadian rhythmicity, and the master clock in the SCN seems to play the role of maintaining synchronization among these cellular clocks. Primarily based on work in model systems, it is now understood that the molecular circadian clock consists of a negative feedback loop involving the Period (PER1, PER2, and PER3) and Cryptochrome (CRY1 and CRY2) genes.6 Other genes involved in the molecular generation of circadian rhythms include: Casein Kinase 1δ and 1ε (CK1) and transcription factors Circadian Locomotor Output Cycles Kaput Protein (CLOCK), Brain and Muscle ARNT-like Protein (BMAL1 and BMAL2), and Neuronal Pas Domain Protein (NPAS1 and NPAS2); for greater in-depth discussion of circadian clock genes, see Lowrey and Takahashi,6 McClung,7 and Pack et al.8
GENETICS OF CIRCADIAN TIMING
In parallel with the identification of the molecular mechanisms of circadian rhythms, studies sought to determine whether chronotype, as a behavioral manifestation of circadian rhythms, is also determined by genetic factors by exploring its heritability. Several twin and family studies have estimated the heritability of chronotype to be approximately 50% based on estimates in the United States,9 United Kingdom,10 Scandinavia,11,12 and Brazil,13 whereas studies of Hutterites14 and Amazonians15 suggest lower heritability at 14% and 23%, respectively. Taken together, heritability estimates suggest that genetic factors explain a considerable proportion, up to 50%, of the population variability in circadian timing. Furthermore, the normal distribution of chronotypes indicates a polygenic basis such that multiple genes contribute modest, aggregative effects to this complex phenotype.16 Given the heritability of circadian typology and prior success in identifying the components of the molecular circadian clock, chronotype is a logical target for genetic studies.
Several studies have used a candidate-gene approach and examined the associations between circadian genes and either chronotype or circadian rhythm sleep disorders. An association was identified and supported between the 3111C allele of the CLOCK gene and eveningness,17,18 though some studies failed to replicate this finding.19–21 Associations have been found between extreme delayed sleep phase and length polymorphisms of the PER3 gene22–24 in addition to the 3111C allele of the CLOCK gene.17 Morningness has been found to be associated with polymorphisms in the clock genes PER1 and PER2 in other studies.25,26 In addition, polymorphisms in the PER3 and ARNTL2 genes are associated with chronotype in a sample of 966 British adults.27
Perhaps, the most studied human gene variant with respect to its role in diurnal preference is a PER3 polymorphism with variable number of tandem repeats (VNTR) of a 54-bp motif in exon 18 that is repeated either 4 or 5 times (see Dijk and Archer28 for review of PER3). This polymorphism is found only in primates, for which humans can be homozygotic (4/4 or 5/5) or heterozygotic (4/5) for the number of repeats.29,30 Investigations of PER3 and chronotype have offered conflicting results such that the 5-repeat allele has been found to be positively associated with morningness and earlier dim-light melatonin onset,24,31,32 but has also been shown to be elevated in individuals with extreme delayed sleep phase,33 whereas other studies have shown no association between the PER3 VNTR and diurnal preference34–37 nor in melatonin or cortisol as objective markers of circadian rhythms.38
Studies of extreme chronotype expression can yield highly informative results. Research on familial advanced sleep phase syndrome (FASPS) has identified specific genetic variants that segregate with the disorder. Linkage analysis, a statistical gene mapping approach used in affected families, has identified a rare missense mutation (i.e., replacement of a single nucleotide) in the clock component gene, PER2, which alters the circadian period and is associated with FASPS.39 However, this finding was not replicated in 2 FASPS pedigrees in Japan,40 which highlights the difficulty of replicating results of rare mutations and the limited generalizability of findings concerning extreme phenotype expressions.
Although results from these genetic studies are mixed, overall patterns suggest that multiple genes play important roles in influencing circadian typology. As chronotype is a complex trait, a large number of genes with modest influence contribute to phenotypic manifestation. The detection of multiple small genetic effects requires very large sample sizes, and genome-wide discovery approaches are necessary to identify novel genetic loci. Importantly, going beyond classically gene-centric approaches is essential to elucidate unexplained heritability that remains.
GENOME-WIDE DISCOVERY
The first genome-wide analysis of circadian-related phenotypes analyzed data from 749 subjects in The Sleep Heart Health Study.41 The authors employed linkage analysis that is used to map genetic loci associated with a phenotype based on observations of related individuals. Linkage analysis for habitual bedtime revealed no significant genetic associations although statistical power was likely limited by insufficient sample size for genome-wide analysis (as large samples are required to detect variants of even moderate effect, i.e., odds ratio [OR] = 1.5–2.0)42 and poor quality of genetic data. Notably, near-significant genetic associations for habitual bedtime were observed near CLOCK, PROK2, CSNK2A2, and NSPR1, all known circadian clock-related genes. However, 3 recent large-scale genome-wide association studies (GWASs) of subjects of European ancestry from the 23andMe cohort43 (23andMe, Inc., Mountain View, CA, USA), and the UK Biobank44,45 have uncovered novel gene loci associated with diurnal chronotypic preference. Here, we will compare methodologies and significant findings of each GWAS (overlap and divergence), closing with a discussion of the findings in the context of prior knowledge in this area.
Unlike candidate gene approaches, GWAS does not require any a priori knowledge of genes underlying the expression of the phenotype of interest. Indeed, GWAS is a discovery approach wherein the entire genome is searched for small genetic variations that occur more commonly in individuals with a particular phenotypic trait. Specifically, a GWAS tests associations between single nucleotide polymorphisms (SNPs; i.e., the most common type of genetic variation among people, represented by a difference in a single DNA base pair) and a phenotype of interest (often a trait or disease). GWASs are typically restricted to common genetic variants (i.e., with minor alleles occurring in more than 5% of the population), which tend to have small or modest effects on complex phenotypes. Because SNPs are often correlated with other nearby SNPs, a substantial proportion of the variability in the genome is captured by measuring several hundred thousand SNPs and imputing millions of non-genotyped SNPs.46,47
GWAS analyses involve conducting individual association models of the relationships between each SNP and the phenotype of interest (e.g., linear regression models for continuous phenotypes or logistic regression models for binary phenotypes). Due to the large number of tests resulting from all SNPs being examined individually, a multiple-comparisons correction is necessary to determine statistical significance and protect against false-positive associations. While there are different methods for performing this correction, a p-value less than 5 × 10–8 is typically required to claim genome-wide significance.47–50
The first large-scale GWAS of diurnal preference was published by Hu and colleagues43 from a European-ancestry sample of worldwide customers of 23andMe, a direct-to-consumer genotyping service. Subjects ranged from early adulthood (<30 years) to old age (>60 years; exact age range not reported). Subjects in this study responded to 2 survey items regarding natural tendency of being a night person/night owl vs. morning person/early bird. Specifically, subjects were asked twice: Are you naturally a night person or a morning person? with a unique set of response options for each administration: (1) night owl, early bird, or neither, then (2) night person, morning person, neither, it depends, or I’m not sure. Responses indicating intermediate type or ambiguity (viz. neither, it depends, and I’m not sure) were treated as missing data. Thus, those who indicated intermediate type on both items were not included in analyses, whereas those who indicated intermediate type on 1 item but also early or late type on another were classified based on their early/late responses. Subjects with diametrically opposed responses, i.e., indicating early chronotype on 1 item and late chronotype on another, were excluded from analyses entirely, resulting in 89283 unrelated individuals (38937 morning types, 50346 evening types) included in the analysis. The primary chronotype phenotype in this study was a binary outcome value (early vs. late chronotype), and analyses included as covariates age and sex. In addition, the authors adjusted for the top 5 principle components accounting for population stratification that controls for differences in allele frequencies among populations of different ancestry. The authors identified 15 loci associated with self-rated diurnal preference (Table 1, see Figure 1 for Manhattan plot that shows the genome-wide significant SNPs and nearby loci). Although Hu et al. conducted a follow-up GWAS with chronotype scored on a continuum including intermediate types, SNP association results from this analysis were only provided for 2 newly significant SNPs (designated by asterisks [*] in Table 1). Notably, because chronotype is a continuous trait, phenotyping chronotype on a continuum yields greater statistical power to detect genetic associations than operationally defining chronotype as a binary trait.
Table 1.
Gene Loci Associated With Chronotype/Diurnal Preference in 23andme And UK Biobank Genome-wide Association Analyses.
| SNP | EAF | Chr:Pos | Nearest gene(s) | β | Cont P | OR | Bin P | Study | Known CRS function |
|---|---|---|---|---|---|---|---|---|---|
| Loci identified in all 3 GWASs | |||||||||
| rs12736689 | 0.03 | 1:182,549,729 | RGS16, RNASEL | — | — | 0.74 | 7.00E-18 | Hu | K |
| rs1144566 | 0.97 | 1:182,569,626 | RGS16 | −0.10 | 2.62E-14 | 0.74 | 1.29E-08 | Lane | K |
| rs516134 | 0.03 | 1:182,553,693 | RGS16 | 0.08 | 9.00E-13 | 1.21 | 3.00E-12 | Jones | K |
| rs55694368 | 0.93 | 2:239,317,692 | PER2 | — | — | 0.86 | 2.60E-09 | Hu | K |
| rs35333999 | 0.043 | 2:239,161,957 | PER2 | 0.06 | 8.43E-08 | 1.21 | 9.01E-06 | Lane | K |
| rs75804782 | 0.88 | 2:239,316,043 | PER2 | 0.03 | 3.00E-07 | 1.09 | 4.00E-10 | Jones | K |
| rs3972456 | 0.29 | 7:102,436,907 | FBXL13, FAM185A | — | 0.92 | 6.00E-09 | Hu | P | |
| rs372229746 | 0.45 | 7:102,158,815 | FBXL13 | 0.03 | 5.18E-10 | 1.12 | 4.29E-07 | Lane | P |
| rs372229746 | 0.55 | 7:102,158,815 | FBXL13, ORAI2, RASA4 | 0.03 | 4.00E-09 | 1.06 | 7.00E-07 | Jones | P |
| rs10493596 | 0.24 | 1:77,713,434 | AK5 | — | 1.09 | 8.00E-12 | Hu | U | |
| rs76681500 | 0.84 | 1:77,726,241 | AK5 | –0.04 | 1.50E-12 | 0.86 | 1.77E-09 | Lane | U |
| rs11162296 | 0.16 | 1:77,700,196 | AK5, PIGK | –0.04 | 2.00E-12 | 0.93 | 1.00E-12 | Jones | U |
| Loci identified in 2 GWASs | |||||||||
| rs35833281 | 0.21 | 6:55,021,561 | HCRTR2 | — | — | 0.92 | 2.60E-09 | Hu | K |
| rs76899638 | 0.22 | 6:55,147,508 | HCRTR2 | 0.03 | 4.00E-08 | 1.05 | 2.00E-07 | Jones | K |
| rs2050122 | 0.8 | 1:19,989,205 | HTR6 | 0.03 | 4.61E-08 | 1.12 | 7.94E-07 | Lane | K |
| rs2050122 | 0.2 | 1:19,989,205 | HTR6 | 0.03 | 2.00E-08 | 1.06 | 3.00E-06 | Jones | K |
| rs4821940 | 0.55 | 22:40,659,573 | TNRC6B | 0.03 | 1.05E-08 | 1.07 | 8.56E-05 | Lane | P |
| rs4821940 | 0.45 | 22:40,659,573 | TNRC6B, SGSM3 | 0.02 | 3.00E-08 | 1.05 | 4.00E-08 | Jones | P |
| rs34714364 | 0.17 | 1:150,234,657 | APH1A, CA14 | — | — | 1.12 | 2.00E-10 | Hu | P |
| rs10157197 | 0.4 | 1:150,250,636 | APH1A | 0.03 | 1.48E-09 | 1.13 | 1.27E-11 | Lane | P |
| rs12635074 | 0.65 | 3:55,982,416 | ERC2 | –0.03 | 3.08E-08 | 0.92 | 1.20E-06 | Lane | U |
| rs11708779 | 0.32 | 3:55,934,939 | ERC2 | –0.02 | 3.00E-08 | 0.96 | 2.00E-06 | Jones | U |
| Loci identified in 1 GWAS | |||||||||
| rs1015197 | 0.6 | 1:150,250,636 | PRPF3, TARS2 | 0.03 | 1.00E-09 | 1.05 | 5.00E-07 | Jones | U |
| rs11121022 | 0.42 | 1:7,836,659 | PER3, VAMP3 | — | — | 1.07 | 2.00E-08 | Hu | K |
| rs72720396 | 0.23 | 1:91,191,582 | CALB1 | –0.03 | 1.00E-07 | 0.95 | 3.00E-08 | Jones | P |
| rs12140153 | 0.9 | 1:62,579,891 | INADL | 0.04 | 7.00E-09 | 1.07 | 4.00E-06 | Jones | P |
| rs141175086 | 0.998 | 1:780,397 | LINC01128 | –0.27 | 1.42E-04 | 0.46 | 4.38E-08 | Lane | U |
| rs1075265 | 0.52 | 2:54,354,927 | PSME4, ACYP2 | –0.03 | 2.00E-10 | 0.95 | 4.00E-08 | Jones | U |
| rs70944707 | 0.23 | 2:24,257,444 | FKBP1B | 0.03 | 3.00E-08 | 1.05 | 2.00E-05 | Jones | U |
| rs11895698 | 0.14 | 2:239,338,495 | ASB1 | 0.04 | 1.15E-08 | 1.1 | 1.30E-04 | Lane | U |
| rs1595824 | 0.49 | 2:198,874,006 | PLCL1 | — | — | 1.08 | 1.20E-10 | Hu | P |
| rs148750727 | 0.995 | 4:188,022,952 | FAT1 | 0.15 | 3.61E-06 | 2.34 | 1.58E-08 | Lane | U |
| rs9479402 | 0.01 | 6:153,135,339 | VIP | — | — | 0.69 | 3.90E-11 | Hu | K |
| rs9357620* | 0.71 | 6:13,170,634 | PHACTR1 | 0.02 | 1.70E-08 | — | Hu | U | |
| rs2948276 | 0.88 | 7:96,457,119 | DLX5, SHFM1 | — | — | 0.92 | 1.10E-08 | Hu | U |
| rs2975734* | 0.43 | 8:10,030,097 | MSRA | 0.02 | 2.10E-09 | — | Hu | K | |
| rs192534763 | 0.99 | 8:36,202,946 | UNC5D | 0.1 | 3.00E-07 | 1.25 | 2.00E-08 | Jones | U |
| rs17311976 | 0.19 | 8:131,637,337 | ADCY8 | 0.19 | 1.08E-06 | 1.13 | 3.37E-08 | Lane | P |
| rs77641763 | 0.88 | 9:140,265,782 | EXD3, GRIN1, NRARP | 0.04 | 5.00E-11 | 1.07 | 7.00E-09 | Jones | U |
| rs6582618 | 0.52 | 12:38,726,137 | ALG10B | — | — | 1.07 | 1.50E-08 | Hu | U |
| rs542675489 | 0.6 | 12:120,994,888 | RNF10 | 0.03 | 3.29E-09 | 1.09 | 1.38E-05 | Lane | U |
SNP = single nucleotide polymorphism. EAF = effect allele frequency. Chr = Chromosome. Pos = position. Cont P = significance for genetic associations in GWAS on chronotype as a continuous phenotype. β = beta coefficient, i.e., effect size for genetic associations in GWAS on chronotype as a continuous phenotype. Bin P = significance for genetic associations in GWAS on chronotype as a binary (cases vs. controls) phenotype. OR = odds ratio, i.e., effect size for genetic associations in GWAS on chronotype as a binary phenotype. Known CRS function = whether gene has a previously known association with circadian rhythms or sleep. K = Known. P = Probable. U = Unknown.
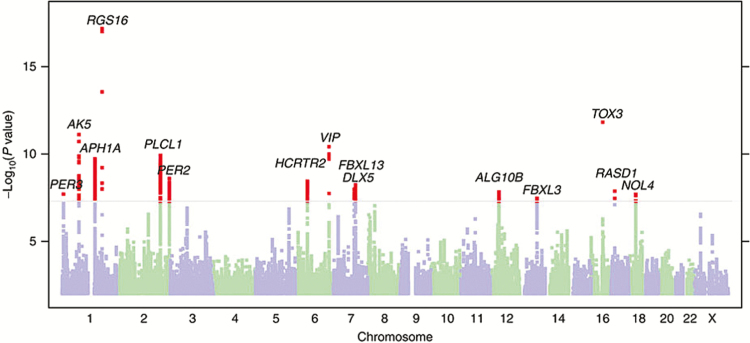
Figure 1.
Manhattan plot of genome-wide association studies of being a morning person from Hu et al. 2016.44 Presented in its originally published format, the grey line corresponds to threshold for genome-wide significance at p = 5 × 10–8, with significant results shown above this threshold in red. Gene labels are annotated as the nearby genes to the significant single nucleotide polymorphisms.
Lane et al.44 and Jones et al.45 each conducted genome-wide association analyses for chronotype on data from British, Caucasian subjects from the UK Biobank, a prospective study of >500000 people in the United Kingdom. Individuals in the UK Biobank range in age from 37 to 73 years although Lane et al.44 analyzed data only from subjects between the ages of 40 and 69 years to increase sample homogeneity. In these studies, GWASs were conducted for chronotype defined as both a binary and a continuous trait, which was self-reported in response to the single item from the Morningness–Eveningness Questionnaire (MEQ) that explains the highest fraction of variance in preferences of sleep–wake timing:51,52Do you consider yourself to be… responses to which included self-description as definitely a morning person, more a morning than evening person, more an evening person than a morning person, definitely an evening person, do not know, and prefer not to answer (subjects endorsing prefer not to answer were excluded from analyses in both studies).
From their GWAS analyses, Lane et al.44 excluded shift workers, subjects taking sleep medications, and those who responded do not know to the chronotype item, resulting in a sample size of n = 100420. The authors conducted 2 parallel GWASs: a continuous chronotype outcome (1–4, definitely a morning person to definitely an evening person) and a binary comparison of the 2 extreme phenotypes (26948 definite morning types vs. 8724 definite evening types). Genetic association analysis was performed using a SNPTEST while controlling for age, sex, and 10 principal components of ancestry and genotyping array (2 different, though highly similar, Affymetrix arrays were used for different subjects in the UK Biobank). The authors identified 12 significant loci and 1 suggestive secondary signal associated with chronotype (Table 1).
Jones and colleagues45 also conducted parallel GWASs for continuous and binary operationalizations of chronotype. However, their continuous chronotype was coded differently such that the response of do not know served as a proxy for intermediate type, rather than excluded as missing data. Thus, their continuous variable included 5 groups (i.e., definite morning, moderate morning, intermediate, moderate evening, definite evening types) rather than 4 as in the Lane et al study. Chronotypes were then normalized for analysis through adjustment of the raw phenotype for age, gender, and study center, followed by inverse normalization of the resulting residuals. Unlike Lane et al who compared just extreme phenotypes in their binary GWAS, Jones et al collapsed across definite and moderate groups within morning and evening types, resulting in a sample size of 114765 in the GWAS of binary chronotype (compared to n = 35672 in the GWAS of extreme phenotypes in Lane et al.). In addition, exclusionary criteria differed from the prior 2 investigations such that Jones et al. excluded patients with diabetes who reported insulin use within the first year of diagnosis, patients with diabetes diagnosed younger than the age of 35 years or with no known age of diagnosis, and patients with diabetes diagnosed within a year of the study. These exclusions were due to the authors' focus on the relationship between chronotype and diabetes. The authors used linear mixed models to conduct GWAS analyses to account for population structure and subject relatedness, while controlling for age, sex, study center, and genotyping array. Jones et al.45 identified 16 significant loci associated with chronotype in the UK Biobank (Table 1).
All 3 GWASs independently supported associations with chronotype for genes PER2, RGS16, FBXL13, and AK5 based on nearby significant SNPs. Of these identified genes, PER2 and RGS16 are both known for their roles in circadian regulation. The degradation of PER2, the speed of which influences circadian period length, is governed by Casein kinase 1’s phosphorylation of DEC2 (BHLHE41).53 A regulator of G-protein signaling 16, RGS16, has been shown to regulate Gαi/o-mediated cyclic adenosine monophosphate synthesis in the SCN. RGS16 deficiency/ablation leads to delayed dorsomedial PER1 expression resulting in circadian period lengthening in mice, highlighting the association between chronotype and RGS16.54 The F-Box protein, FBXL13, has a suspected circadian influence,55–57 whereas AK5 had not previously been linked to chronotype or circadian processes.58 Regarding FBXL13, the SCFFBXL3 complex regulates expression of CRY and PER through ubiquitination and degradation of CRY proteins via the SCFFBXl3 ubiquitin ligase complex, and mutations in FBXL3 are associated with lengthened circadian periods in mice.55–57 Adenylate kinase 5, i.e., AK5, has been shown to regulate the adenine nucleotides within cells58 but has not been tied to chronotype or the circadian pacemaker prior to these studies.
Five additional genes were found to be associated with chronotype in 2 of the 3 studies (Table 1). Hypocretin (orexin) Receptor Type 2 (HCRTR2) is a gene that regulates the expression of the G protein–coupled hypocretin receptor type 2. Deficient hypocretin systems are thought to be associated with a reduction of wakefulness and circadian alertness signals in narcolepsy.59 5-Hydroxytryptamine (serotonin) Receptor 6 (HTR6) has not been previously linked to chronotype but is known for its connection to sleep regulation in rats such that 5-HTR6 agonists lead to reductions in sleep.60,61 Trinucleotide Repeat Containing 6B (TNRC6B) is a protein-coding gene that belongs to the GW182 family of proteins, which are necessary for micro-RNA gene silencing in animal cells. These proteins have been linked to circadian behavior in Drosophila62 and bound to circadian transcription factors in mouse liver,63 although these GWASs offer the first evidence linking this gene to human circadian typology. Both Hu et al. and Lane et al. identified the association of APH1A and chronotype. APH1A encodes a subunit of the γ-secretase complex that cleaves the amyloid-β precursor protein.64 Hu et al. highlighted that amyloid-β levels are higher in wakefulness than sleep and are affected by manipulations of the hypocretin (orexin) system,65,66 in addition to clearance by the glymphatic system of the brain during sleep.67 The last gene to be implicated by 2 independent GWASs was ERC2 (ELKS/RAB6-Interacting/CAST Family Member 2), a protein-coding gene belonging to the Rab3-interacting molecule-binding protein family involved in the regulation of neurotransmitter release through involvement in the organization of the cytomatrix at the nerve terminals active zone. This gene has no previously known association with sleep or circadian rhythmicity.
Highlighting the genetic complexity of chronotype as well as the limitations of genome-wide association analysis, 24 SNPs across these 3 GWASs were genome-wide significant in just 1 of the 3 studies (see Table 1 for full list). Unlike the replicated genes discussed above, relationships of these unreplicated genes with chronotype were largely unclear, e.g., ALG10B, LINC01128, and UNC5D. Even so, 5 genes with well-documented circadian and/or sleep regulatory roles—PER3, Vasoactive Intestinal Peptide (VIP),68FBXL3, Methionine Sulfoxide Reductase A (MSRA), and RAS Dexamethasone-Induced 1 (RASD1)—were significant in 1 of the 3 GWASs. Given the lack of replication across studies, these associations, particularly for those with no known circadian roles, should be interpreted with great caution.
Importantly, of the 9 genes identified in multiple studies (4 found in all 3 studies and 5 found in 2 studies), only 3 SNPs were shared: rs372229746 in FBXL13, rs2050122 in HTR6, and rs4821940 in TNRC6B. Effects of these shared SNPs on chronotype were in the same direction and exhibited similar magnitudes. Only the studies by Lane and Jones shared SNPs in common, given the usage of the same genotype data from the UK Biobank. Both Lane et al. and Jones et al. attempted to replicate the findings from the 23andMe SNPs reported in the Hu study. In Lane et al.’s GWAS of extreme chronotype, 8 of the 15 significant SNPs identified in Hu et al. were found to have genome-wide significance, and all 15 signals had the same direction of effect in both studies. In a follow-up meta-analysis of both samples, Lane et al. identified 3 additional SNPs near PER3, VIP, and TOX3. In the Jones study, the authors conducted a meta-analysis on their UK Biobank sample and the 23andMe cohort from the Hu study. Findings supported 13 of Jones et al.’s initially identified 16 loci to be associated with chronotype although 2 signals were in the opposite direction.
DISCUSSION
The results of these 3 GWAS reports highlight the potential of discovery-based approaches for the identification of novel candidate genes involved in chronotype, circadian rhythms, and circadian rhythm sleep disorders, while also pointing to the need for large sample sizes to find common genetic variants with small effects for complex phenotypes. In particular, GWAS have demonstrated promise in revealing unexpected genetic effects that have gone undetected in more traditional candidate gene studies.69,70 Despite some differences in phenotyping and analytic approach, there was a good deal of consistency across studies with 9 genes identified in at least 2 of the 3 main studies reviewed. However, given that 2 studies both used data from the UK Biobank, one might expect the results of these analyses to be more similar. There are several important lessons that can be drawn from these studies.
Importance of Rigorous and Consistent Phenotyping
Multiple methods exist to capture the timing of the circadian system, including assessments of core body temperature, dim light melatonin onset, self-reported diurnal preference, and actigraphy- or diary-based midpoint of sleep.71 For large-sample research endeavors, non-invasive, cost-effective, and expeditious assessments are needed to capture the phenotypes of the chronotype. At its most rudimentary level, chronotype can be assessed with a single question such as those used by 23andMe and the UK Biobank.1 While these questions have face validity, sole reliance on a single, subjective item to define the chronotype phenotype may lead to difficulties regarding individual differences in beliefs about what constitutes morning or evening types and fails to capture much of the variability in chronotype within the population.2 Measurement error from non-rigorous phenotyping of complex traits decreases the signal-to-noise ratio and attenuates that measure’s ability to demonstrate association with genetic variants, thus reducing power to detect significant genome-wide associations. Furthermore, although circadian rhythms play important roles in its expression, chronotype is influenced by several other genetic and environmental factors, which can add further noise to the phenotype and reduce statistical power. Thus, non-rigorous phenotyping can yield unreliable significant findings that may be less likely to be replicated in subsequent studies.72
In the 3 large GWASs reviewed above, chronotype was determined by 1 or 2 self-report questions. Specifically, Lane et al.44 and Jones et al.45 used a single item of the MEQ, whereas Hu et al.44 measured chronotype using 2 identically worded items (Are you naturally a night person or a morning person?) with 2 similar sets of response options. Although the differences between these items are slight, they likely contributed to disparate findings between results from the 23andMe and UK Biobank samples, highlighting the impact that small variations in phenotype definition can have on genome-wide associations. Moreover, even when the measure for phenotyping was consistent across analyses (both within studies conducting parallel GWASs and across the 2 separate studies analyzing UK Biobank data), differences in response coding (e.g., binary vs. continuous, treatment of ambiguous answers) affect measurement error and yield disparate findings. These are the same issues that plague other epidemiological research and produce varying rates of disease prevalence and incidence. Future endeavors should prioritize more rigorous and consistent phenotyping strategies including the use of standardized self-report instruments, clinical interviews, or objective measures of sleep–wake periodicity (e.g. actigraphy), as GWASs will have greater statistical power and produce more reliable results when phenotypic information is grounded on a solid base of defining criteria. There is also a critical need to validate commonly used self-reported items by comparing them to biological markers of circadian rhythms to determine which question(s) most closely relate(s) to endogenous processes.
Analytic Approaches Yield Differing GWAS Results
Differences in the analysis plan, including decisions defining the sample and selections regarding covariates, statistical tests, and methods to address confounders, may also produce disparate findings across GWASs. The first critical analytic decisions regard inclusion and exclusion criteria. Notably, all 3 GWASs analyzed data from subjects with European Ancestry to increase sample homogeneity and accounted for residual population structure in their models. Where Hu et al.43 and Lane et al.44 excluded individuals based on genetic relatedness, Jones et al.45 statistically adjusted for relatedness in their analyses. In addition, Lane et al. excluded shift workers and subjects taking sleep medications, as shift work and hypnotics may potentially influence self-reported chronotype. The Jones study, however, excluded individuals based on diabetic and autoimmune considerations (see above).
Where Hu et al. and Lane et al. used regression for their genome-wide analyses, Jones et al employed linear mixed modeling that allowed adjusting for between-subjects genetic relatedness. Furthermore, the Lane study broke from convention by using a less robust “expected” genotype count (also known as the posterior mean73 or allele dosage74) in SNPTEST, rather than more traditionally employed frequentist test statistics (e.g., chi-square statistics for case–control studies, the score test for quantitative trait analyses). Lane et al.’s choice of model reflects efforts to account for uncertainty in imputed SNP quality; in particular, difficult-to-impute SNPs, i.e., those with low information scores following imputation. While both models asymptotically approximate each other, frequentist test statistics (using likelihood estimation) tend to result in larger effect sizes for difficult-to-impute SNPs.73 Thus, frequentist test statistics may overestimate genetic effects, which may have influenced why the Lane study chose a different, more conservative, statistical model. Although all studies adjusted for age, sex, and population structure, differences of methods of statistical adjustment and in covariate selection existed across studies. It is unclear whether different estimation techniques (e.g., regression vs. linear mixed modeling) and covariate selection contributed to the disparate results across studies. However, the use of multiple approaches can be beneficial by allowing for sensitivity analysis such that genetic associations that are significant across different strategies are more likely to be true findings.
The Complementary Nature of GWAS and Molecular Studies
Findings from the GWAS analyses confirmed the roles of known circadian genes for influencing chronotype, a behavioral phenotype. Even so, perhaps the most exciting findings from these GWASs center on the significant SNPs near genes with little to no known history of circadian influence, such as AK5, APH1A, and ERC2 among many others. Indeed, although many classical clock genes were significantly associated with chronotype in these GWASs, a critical strength of these exploratory techniques is the ability to identify novel genetic etiology of these complex phenotypes. Owing to discovery of the novel loci and newly implicated genes, efforts should be directed toward replicating these findings and elucidating their roles in circadian regulation and chronotype. Comparison of phenotype-associated variants to expression quantitative trait loci variants can help prioritize a gene and mechanism for functional follow-up.75,76 As genome-wide discovery attempts to establish the genetic architecture and functional genomic model of circadian rhythms and chronotype, studies using model systems can reveal the molecular mechanisms modulating circadian processes. Molecular studies can be used to support associations identified through genome-wide analysis by examining these identified regions in finer detail and finding a potential mechanism by which they act on chronotype. Indeed, genetic model systems for studying sleep and sleep–wake disorders are needed to reveal the molecular basis of chronotype and circadian typology. Genes with no previously known circadian role should now be investigated in model systems to determine whether these genes do have a hitherto unknown role in circadian regulation.77 Importantly, failure to replicate GWAS findings in molecular investigations may reveal erroneous associations from GWAS results.
LIMITATIONS AND FUTURE DIRECTIONS
Common vs. Rare Variants
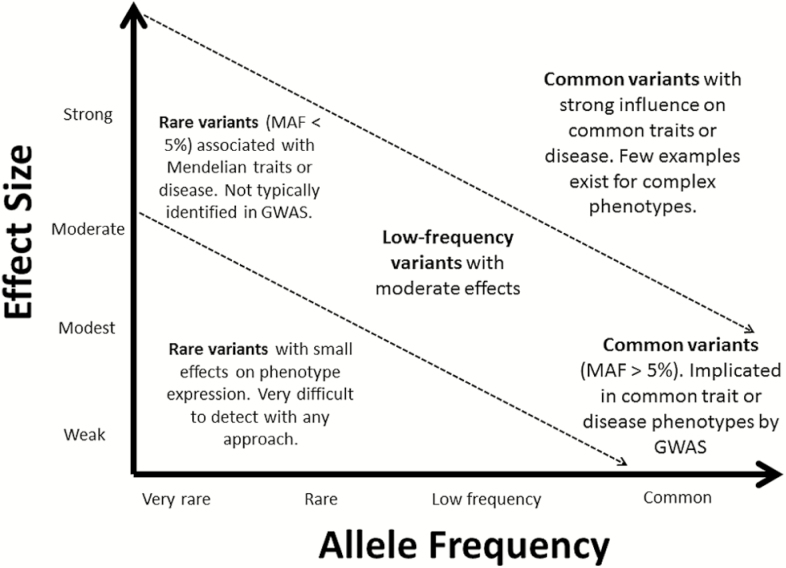
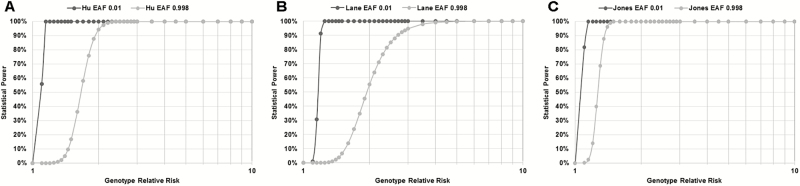
While genome-wide discovery has greatly advanced our knowledge of the genetic basis of sleep and circadian regulation, genetic variants obtained through genotyping chips used in GWAS are restricted to common SNPs with minor allele frequencies (MAF) greater than 5%. Importantly, common variants identified in GWAS typically result in modest influences on the manifestation of complex phenotypes (Figure 2). Despite very large samples in the 3 GWASs we reviewed, differences in findings across studies are likely influenced by effect allele frequency (EAF) and the effect size that each allele contributes. The graphs in Figure 3 show that the more common an effect allele is in the population (i.e., higher EAF), the greater the phenotypic effect must be to detect a genetic association. This demonstrates that achieving sufficient statistical power to detect effects of common alleles requires a greater phenotypic effect at that allele. The complicating matter, however, is that the frequency of effect alleles and their magnitudes of effect are typically inversely related, that is, the more common an effect allele is, the more modest its effect is likely to be (Figure 2).78,79 Over the range of effect sizes (OR = 1.04–2.34) noted in GWASs of Hu, Lane, and Jones, it is not surprising that there was divergence between significant alleles, given the sample sizes and group proportions in each of the studies. The next-step research on common alleles contributing to chronotype may utilize polygenic risk scores, which aggregate the multiple small effects of common variants to effectively explain much of the heritability of complex/polygenic traits,80 such as prior work on schizophrenia81 and height.82 Derivation of polygenic risk scores would allow for the examination of whether genetic load for late chronotype is associated with phenotypic variations in mood, sleep, or other circadian-linked health outcomes.83
Figure 2.
Relationship between allele frequency and effect on phenotype expression. Common variants with minor allele frequencies (MAF)>5% typically boast weak or modest effects on expression of complex phenotypes. The effects of common variants are often detected using genome-wide association studies (GWASs). In contrast, rarer variants typically wield stronger effects and contribute to more extreme expressions of complex phenotypes. Rare variants, however, are not often detected using genome-wide analysis.
Figure 3.
Comparison of statistical power in the 3 genome-wide association studies (GWASs). Comparisons of the 3 different study designs (number of cases:number of controls) and their power to detect alleles with varying genetic relative risk over the range of effect allele frequencies (EAF). The differences in the power over the range of genotype relative risks clearly indicate the impact of sample size (Jones et al. > Hu et al. > Lane et al.), which figures most prominently at higher allele frequencies. Power estimates were performed using the CaTS power calculator (http://csg.sph.umich.edu//abecasis/cats/)89 for all studies, using a significance threshold of 5 × 10−8, prevalence of morningness phenotype of 0.652 (estimated from total UK Biobank cohort), and EAF minimum (0.01) and minimum (0.998) values used to reflect the range across studies.
In contrast, rare gene variants (i.e., SNPs with minor allele frequencies less than 5%) often exert larger effects on phenotype expression but are not typically identified using genome-wide discovery techniques due to their low frequency (see Pack et al.8 for review). Given that chronotype is relatively normally distributed in the population, a large number of common variants with small effects contribute to its expression. Even so, rare variants, quite possibly in the same genes as known common variants, likely result in greater magnitude of influence on chronotype when present. The influence of rare variants is particularly germane to extreme expressions of chronotype and circadian pathology, such as in FASPS.3,41 Given that rare variants may disproportionately influence a trait at the same loci as the common variants identified, fine mapping of these regions may help elucidate the roles that genes in the region play in chronotype expression, beyond simple linkage disequilibrium (i.e., associations between nearby genetic loci) with plausible sleep-related genes.84
Demonstrating the significant impact of rare variants, He et al.85 studied 2 genetically related subjects with markedly advanced circadian phase and short sleep compared to other members in their family and the general population. Despite habitual sleep periods of approximately 22:00 to 04:00, these individuals denied sleep-related daytime impairment. After sequencing all clock and clock-related genes, He et al. identified a rare point mutation in DEC2 as complicit in their unique and extreme sleep phenotypes. The authors then replicated the effect of this mutation in transgenic mice and Drosophila. Pellegrino and colleagues extended this line of research by demonstrating that genes with rare variants with large effects may harbor other such mutations. Specifically, Pellegrino et al.85 sequenced DEC2 in 2 human samples—one from a twin study87 and another from a study on sleep deprivation88—and found 2 new mutations in DEC2 associated with short sleep. Taken together, these findings highlight the importance of advancements in genetic sequencing and targeted efforts to identify and characterize rare variants associated with sleep and circadian phenotypes.
Further elucidation of both the common and rare variants constituting the genetic basis of chronotype will provide critical insights into the molecular underpinnings of the circadian system. This knowledge will enhance the understanding of the etiology of circadian rhythm and other illnesses with known circadian abnormalities, including major depression and cardiometabolic disorders. These discoveries may lead to potential novel pharmacologic and non-pharmacologic treatment options that target the circadian system. For instance, the creation of personalized genetic risk profiles for circadian abnormalities may be used to guide the design and assignment of shift work schedules to reduce risk for shift work disorder in the future.
FUNDING
This work was supported by NIH grant T32 HL110952-04.
DISCLOSURE STATEMENT
Dr Gehrman has had research grant support from Merck, Inc. and has served as a consultant for Johnson & Johnson and General Sleep Corporation. The other authors reported no financial conflicts of interest.
REFERENCES
- 1. Czeisler CA, Gooley J. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol 200772: 579–597. [DOI] [PubMed] [Google Scholar]
- 2. Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007; 11(6): 429–438. [DOI] [PubMed] [Google Scholar]
- 3. Jones CR, Campbell SS, Zone SE, et al. Familial advanced sleep-phase syndrome: A short-period circadian rhythm variant in humans. Nat Med. 1999; 5(9): 1062–1065. [DOI] [PubMed] [Google Scholar]
- 4. Sack R, Auckley D, Auger R, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. Sleep. 2007; 30(11): 1484–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walch OJ, Cochran A, Forger DB. A global quantification of “normal” sleep schedules using smartphone data. Sci Adv. 2016; 2(5): e1501705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lowrey PL, Takahashi JS. Genetics of circadian rhythms in Mammalian model organisms. Adv Genet. 2011; 74: 175–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007; 114(2): 222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pack AI, Keenan BT, Byrne EM, Gehrman PR. Genetics and genomic basis of sleep disorders in humans. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 6th ed St. Louis, MO: Elsevier; 2016. [Google Scholar]
- 9. Hur Y-M, Bouchard TJ, Jr, Lykken DT. Genetic and environmental influence on morningness–eveningness. Personality Indiv. Differ. 1998; 25(5): 917–925. [Google Scholar]
- 10. Barclay NL, Eley TC, Buysse DJ, Archer SN, Gregory AM. Diurnal preference and sleep quality: same genes? A study of young adult twins. Chronobiol Int. 2010; 27(2): 278–296. [DOI] [PubMed] [Google Scholar]
- 11. Vink JM, Groot AS, Kerkhof GA, Boomsma DI. Genetic analysis of morningness and eveningness. Chronobiol Int. 2001; 18(5): 809–822. [DOI] [PubMed] [Google Scholar]
- 12. Koskenvuo M, Hublin C, Partinen M, Heikkilä K, Kaprio J. Heritability of diurnal type: a nationwide study of 8753 adult twin pairs. J Sleep Res. 2007; 16(2): 156–162. [DOI] [PubMed] [Google Scholar]
- 13. von Schantz M, Taporoski TP, Horimoto AR, et al. Distribution and heritability of diurnal preference (chronotype) in a rural Brazilian family-based cohort, the Baependi study. Scientific Rep. 2015; 5 : 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Klei L, Reitz P, Miller M, et al. Heritability of morningness-eveningness and self-report sleep measures in a family-based sample of 521 hutterites. Chronobiol Int. 2005; 22(6): 1041–1054. [DOI] [PubMed] [Google Scholar]
- 15. Aguiar GF, da Silva HP, Marques N. Patterns of daily allocation of sleep periods: a case study in an Amazonian riverine community. Chronobiologia. 1991; 18(1): 9–19. [PubMed] [Google Scholar]
- 16. Landolt H-P, Dijk D-J. Genetic and genomic basis of sleep in healthy humans.6th ed NYC: Elsevier; 2016. Kryger MH, Roth T, eds. Principles and Practice of Sleep Medicine. [Google Scholar]
- 17. Katzenberg D, Young T, Finn L, et al. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998; 21(6): 569–576. [DOI] [PubMed] [Google Scholar]
- 18. Mishima K, Tozawa T, Satoh K, Saitoh H, Mishima Y. The 3111T/C polymorphism of hClock is associated with evening preference and delayed sleep timing in a Japanese population sample. Am J Med Genet B Neuropsychiatr Genet. 2005; 133B(1): 101–104. [DOI] [PubMed] [Google Scholar]
- 19. Robilliard DL, Archer SN, Arendt J, et al. The 3111 Clock gene polymorphism is not associated with sleep and circadian rhythmicity in phenotypically characterized human subjects. J Sleep Res. 2002; 11(4): 305–312. [DOI] [PubMed] [Google Scholar]
- 20. Iwase T, Kajimura N, Uchiyama M, et al. Mutation screening of the human Clock gene in circadian rhythm sleep disorders. Psychiatry Res. 2002; 109(2): 121–128. [DOI] [PubMed] [Google Scholar]
- 21. Pedrazzoli M, Louzada FM, Pereira DS, et al. Clock polymorphisms and circadian rhythms phenotypes in a sample of the Brazilian population. Chronobiol Int. 2007; 24(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 22. Ebisawa T, Uchiyama M, Kajimura N, et al. Association of structural polymorphisms in the human period3 gene with delayed sleep phase syndrome. EMBO Rep. 2001; 2(4): 342–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pereira DS, Tufik S, Louzada FM, et al. Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: does latitude have an influence upon it? Sleep. 2005; 28(1): 29–32. [PubMed] [Google Scholar]
- 24. Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003; 26(4): 413–415. [DOI] [PubMed] [Google Scholar]
- 25. Carpen JD, Archer SN, Skene DJ, Smits M, von Schantz M. A single-nucleotide polymorphism in the 5’-untranslated region of the hPER2 gene is associated with diurnal preference. J Sleep Res. 2005; 14(3): 293–297. [DOI] [PubMed] [Google Scholar]
- 26. Carpen JD, von Schantz M, Smits M, Skene DJ, Archer SN. A silent polymorphism in the PER1 gene associates with extreme diurnal preference in humans. J Hum Genet. 2006; 51(12): 1122–1125. [DOI] [PubMed] [Google Scholar]
- 27. Parsons MJ, Lester KJ, Barclay NL, et al. Polymorphisms in the circadian expressed genes PER3 and ARNTL2 are associated with diurnal preference and GNβ3 with sleep measures. J Sleep Res. 2014; 23(5): 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med Rev. 2010; 14(3): 151–160. [DOI] [PubMed] [Google Scholar]
- 29. Jenkins A, Archer SN, von Schantz M. Expansion during primate radiation of a variable number tandem repeat in the coding region of the circadian clock gene period3. J Biol Rhythms. 2005; 20(5): 470–472. [DOI] [PubMed] [Google Scholar]
- 30. Sabino FC, Ribeiro AO, Tufik S, et al. Evolutionary history of the PER3 variable number of tandem repeats (VNTR): idiosyncratic aspect of primate molecular circadian clock. PLoS One. 2014; 9(9): e107198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Drake C.L., Belcher R., Howard R., Roth T., Levin A.M., Gumenyuk V. (2015). Length polymorphism in the Period 3 gene is associated with sleepiness and maladaptive circadian phase in night-shift workers. Journal of sleep research, 24(3), 254–261. [DOI] [PubMed] [Google Scholar]
- 32. Kunorozva L, Stephenson KJ, Rae DE, Roden LC. Chronotype and PERIOD3 variable number tandem repeat polymorphism in individual sports athletes. Chronobiol Int. 2012; 29(8): 1004–1010. [DOI] [PubMed] [Google Scholar]
- 33. Pereira DS, Tufik S, Louzada FM, et al. Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: does latitude have an influence upon it? Sleep. 2005; 28(1): 29–32. [PubMed] [Google Scholar]
- 34. Perea CS, Niño CL, López-León S, et al. Study of a Functional Polymorphism in the PER3 Gene and Diurnal Preference in a Colombian Sample. Open Neurol J. 2014; 8: 7–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Osland TM, Bjorvatn BR, Steen VM, Pallesen Sl. Association study of a variable-number tandem repeat polymorphism in the clock gene PERIOD3 and chronotype in Norwegian university students. Chronobiol Int. 2011; 28(9): 764–770. [DOI] [PubMed] [Google Scholar]
- 36. An H, Zhu Z, Zhou C, et al. Chronotype and a PERIOD3 variable number tandem repeat polymorphism in Han Chinese pilots. Int J Clin Exp Med. 2014; 7(10): 3770–3776. [PMC free article] [PubMed] [Google Scholar]
- 37. Barclay NL, Eley TC, Mill J, et al. Sleep quality and diurnal preference in a sample of young adults: associations with 5HTTLPR, PER3, and CLOCK 3111. Am J Med Genet B Neuropsychiatr Genet. 2011; 156B(6): 681–690. [DOI] [PubMed] [Google Scholar]
- 38. Viola AU, Archer SN, James LM, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007; 17(7): 613–618. [DOI] [PubMed] [Google Scholar]
- 39. Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001; 291(5506): 1040–1043. [DOI] [PubMed] [Google Scholar]
- 40. Xu Y, Padiath QS, Shapiro RE, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005; 434(7033): 640–644. [DOI] [PubMed] [Google Scholar]
- 41. Gottlieb DJ, O’Connor GT, Wilk JB. Genome-wide association of sleep and circadian phenotypes. BMC Med Genet. 2007; 8(Suppl 1): S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Spencer CC, Su Z, Donnelly P, Marchini J. Designing genome-wide association studies: sample size, power, imputation, and the choice of genotyping chip. PLoS Genet. 2009; 5(5): e1000477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hu Y, Shmygelska A, Tran D, Eriksson N, Tung JY, Hinds DA. GWAS of 89,283 individuals identifies genetic variants associated with self-reporting of being a morning person. Nat Commun. 2016; 7: 10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lane JM, Vlasac I, Anderson SG, et al. Genome-wide association analysis identifies novel loci for chronotype in 100,420 individuals from the UK Biobank. Nat commun. 2016; 7 : 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones SE, Tyrrell J, Wood AR, et al. Genome-wide association analyses in 128,266 individuals identifies new morningness and sleep duration loci. PLoS Genet. 2016; 12(8): e1006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002; 296(5576): 2225–2229. [DOI] [PubMed] [Google Scholar]
- 47. Altshuler D, Daly MJ, Lander ES. Genetic mapping in human disease. Science. 2008; 322(5903): 881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McCarthy MI, Abecasis GR, Cardon LR, et al. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet. 2008; 9(5): 356–369. [DOI] [PubMed] [Google Scholar]
- 49. Pe’er I, Yelensky R, Altshuler D, Daly MJ. Estimation of the multiple testing burden for genomewide association studies of nearly all common variants. Genet Epidemiol. 2008; 32(4): 381–385. [DOI] [PubMed] [Google Scholar]
- 50. Chanock SJ, Manolio T, Boehnke M, et al. Replicating genotype-phenotype associations. Nature. 2007; 447(7145):655–660. [DOI] [PubMed] [Google Scholar]
- 51. Taillard J, Philip P, Chastang JF, Bioulac B. Validation of Horne and Ostberg morningness-eveningness questionnaire in a middle-aged population of French workers. J Biol Rhythms. 2004; 19(1): 76–86. [DOI] [PubMed] [Google Scholar]
- 52. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976; 4(2): 97–110. [PubMed] [Google Scholar]
- 53. Xu Y, Toh KL, Jones CR, Shin JY, Fu YH, Ptácek LJ. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007; 128(1): 59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Doi M, Ishida A, Miyake A, et al. Circadian regulation of intracellular G-protein signalling mediates intercellular synchrony and rhythmicity in the suprachiasmatic nucleus. Nat Commun. 2011; 2: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Godinho SI, Maywood ES, Shaw L, et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007; 316(5826): 897–900. [DOI] [PubMed] [Google Scholar]
- 56. Siepka SM, Yoo SH, Park J, et al. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007; 129(5): 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Busino L, Bassermann F, Maiolica A, et al. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007; 316(5826): 900–904. [DOI] [PubMed] [Google Scholar]
- 58. Van Rompay AR, Johansson M, Karlsson A. Identification of a novel human adenylate kinase. Eur J Biochem. 1999; 261(2): 509–517. [DOI] [PubMed] [Google Scholar]
- 59. Taheri S, Zeitzer JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu Rev Neurosci. 2002; 25: 283–313. [DOI] [PubMed] [Google Scholar]
- 60. Ly S, Pishdari B, Lok LL, Hajos M, Kocsis B. Activation of 5-HT6 receptors modulates sleep-wake activity and hippocampal theta oscillation. ACS Chem Neurosci. 2013; 4(1): 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Monti JM, Jantos H, Schechter LE. The effects of systemic and local microinjection into the central nervous system of the selective serotonin 5-HT6 receptor agonist WAY-208466 on sleep and wakefulness in the rat. Behav Brain Res. 2013; 249: 65–74. [DOI] [PubMed] [Google Scholar]
- 62. Zhang Y, Emery P. GW182 controls Drosophila circadian behavior and PDF-receptor signaling. Neuron. 2013; 78(1): 152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Koike N, Yoo SH, Huang HC, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012; 338(6105): 349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Francis R, McGrath G, Zhang J, et al. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell. 2002; 3(1): 85–97. [DOI] [PubMed] [Google Scholar]
- 65. Kang JE, Lim MM, Bateman RJ, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009; 326(5955): 1005–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Roh JH, Huang Y, Bero AW, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of β-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012; 4(150): 150ra122–150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Xie L, Kang H, Xu Q, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013; 342(6156): 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nature Neurosci. 2005; 8(4): 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005; 308(5720): 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rioux JD, Xavier RJ, Taylor KD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007; 39(5): 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Anna W. How to measure circadian rhythms in humans. Medicographia. 2007; 29(1): 84–90. [Google Scholar]
- 72. Ioannidis JP, Thomas G, Daly MJ. Validating, augmenting and refining genome-wide association signals. Nat Rev Genet. 2009; 10(5): 318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guan Y, Stephens M. Practical issues in imputation-based association mapping. PLoS Genet. 2008; 4(12): e1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Li Y, Willer C, Sanna S, Abecasis G. Genotype imputation. Annu Rev Genomics Hum Genet. 2009; 10: 387–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Emilsson V, Thorleifsson G, Zhang B, et al. Genetics of gene expression and its effect on disease. Nature. 2008; 452(7186): 423–428. [DOI] [PubMed] [Google Scholar]
- 76. Nica AC, Montgomery SB, Dimas AS, et al. Candidate causal regulatory effects by integration of expression QTLs with complex trait genetic associations. PLoS Genet. 2010; 6(4): e1000895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011; 146(2): 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hong EP, Park JW. Sample size and statistical power calculation in genetic association studies. Genomics Inform. 2012; 10(2): 117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Park J-H, Gail MH, Weinberg CR, et al. Distribution of allele frequencies and effect sizes and their interrelationships for common genetic susceptibility variants. Proceedings of the National Academy of Sciences. 2011; 108(44): 18026–18031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013; 9(3): e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009; 460(7256): 748–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang J, Benyamin B, McEvoy BP, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010; 42(7): 565–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fabbian F, Zucchi B, Giorgi AD, et al. Chronotype, gender and general health. Chronobiol Intl. 2016; 1–20. [DOI] [PubMed] [Google Scholar]
- 84. McCarthy MI. Exploring the unknown: assumptions about allelic architecture and strategies for susceptibility variant discovery. Genome Med. 2009; 1(7): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. He Y, Jones CR, Fujiki N, et al. The transcriptional repressor DEC2 regulates sleep length in mammals. Science. 2009; 325(5942): 866–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pellegrino R, Kavakli IH, Goel N, et al. A novel BHLHE41 variant is associated with short sleep and resistance to sleep deprivation in humans. Sleep. 2014; 37(8): 1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kuna ST, Maislin G, Pack FM, et al. Heritability of performance deficit accumulation during acute sleep deprivation in twins. Sleep. 2012; 35(9): 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Goel N, Banks S, Mignot E, Dinges DF. DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue. Neurology. 2010; 75(17): 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Skol AD, Scott LJ, Abecasis GR, Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nat Genet. 2006; 38(2): 209–213. [DOI] [PubMed] [Google Scholar]