Abstract
Objectives:
Sleep can be characterized along multiple dimensions. We investigated whether an aggregate measure of sleep health was associated with prevalent and incident clinically significant depression symptoms in a cohort of older women.
Methods:
Participants were older women (mean age 80.1 years) who completed baseline (n = 6485) and follow-up (n = 3806) visits, approximately 6 years apart, in the Study of Osteoporotic Fractures (SOF). Self-reported sleep over the past 12 months was categorized as “good” or “poor” across 5 dimensions: satisfaction with sleep duration, daytime sleepiness, mid-sleep time, sleep onset latency, and sleep duration. An aggregate measure of sleep health was calculated by summing the number of “poor” dimensions. Clinically significant depression symptoms were defined as a score ≥6 on the Geriatric Depression Scale. Relationships between sleep health and depression symptoms were evaluated with multivariate logistic regression, adjusting for health measures and medications.
Results:
Individual sleep health dimensions of sleep satisfaction, daytime sleepiness, mid-sleep time, and sleep onset latency were significantly associated with prevalent depression symptoms (odds ratios [OR] = 1.26–2.69). Sleep satisfaction, daytime sleepiness, and sleep onset latency were significantly associated with incident depression symptoms (OR = 1.32–1.79). The number of “poor” sleep health dimensions was associated in a gradient fashion with greater odds of prevalent (OR = 1.62–5.41) and incident (OR = 1.47–3.15) depression symptoms.
Conclusion:
An aggregate, multidimensional measure of sleep health was associated with both prevalent and incident clinically-significant depression symptoms in a gradient fashion. Future studies are warranted to extend these findings in different populations and with different health outcomes.
Keywords: sleep health, depression, women, epidemiology, daytime sleepiness, sleep satisfaction, mid-sleep time, sleep onset latency, sleep duration
Statement of Significance
Sleep and sleep problems can be measured across multiple dimensions of “sleep health,” including satisfaction, sleepiness, timing, and sleep continuity or efficiency. An aggregate measure of these sleep health dimensions could plausibly be related to health and human functional outcomes including depression. We examined whether an aggregate measure of sleep health was associated with prevalent and incident clinically-significant depression symptoms in a large cohort of older women. An aggregate measure of sleep health was associated with greater odds of prevalent and incident clinically-significant depression symptoms in a gradient fashion. Our findings showed that assessing multiple sleep health dimensions may provide a richer understanding of how sleep is related to depression.
INTRODUCTION
Sleep problems and depression are highly comorbid conditions. While early diagnostic classifications presumed that sleep problems such as insomnia were most often secondary to depression, more recent evidence indicates that sleep problems often precede depressive episodes.1 For instance, epidemiological studies indicate that sleep problems are associated with increased risk for depression, both cross-sectionally and longitudinally.2,3 A meta-analysis of prospective epidemiological studies showed that, among adults without depression at baseline, those with insomnia symptoms were at higher risk for development of depression than those without insomnia symptoms at baseline.2 This association has been also observed in longitudinal studies among older adults.4,5
Although previous epidemiological studies have focused mainly on the relationship between insomnia and depression, other measures of sleep–wake function have also been associated with incident depression.3 For instance, previous cross-sectional studies have reported associations between depression and subjective sleep quality,6–8 excessive daytime sleepiness,7–10 chronotype,8,11,12 sleep onset latency,13 wake after sleep onset,10 short and/or long sleep duration,7,14,15 and napping.10 Several prospective studies have reported an association between worsening depression symptoms or development of depression and baseline measures of subjective sleep quality,16,17 excessive daytime sleepiness,16,18 sleep onset latency,17 sleep–wake rhythmicity/timing,19,20 and short and long sleep duration.21
These studies show that different individual aspects of sleep are related to depression. However, individual characteristics of sleep do not occur in isolation. Sleep and sleep problems can be measured across multiple dimensions of “sleep health,” including satisfaction, sleepiness, timing, sleep continuity or efficiency, and sleep duration. These aspects or sleep are not specific to any individual sleep disorder, and indeed can be used to characterize sleep in a multidimensional fashion across all individuals.22 A composite measure of sleep health recognizes that individual dimensions of sleep always occur in conjunction with the other dimensions.
Aggregate measures of these sleep health dimensions could plausibly be related to health and functional outcomes including depression.22 Although most previous studies, including those reviewed above,6–21 have focused on the associations between individual dimensions of sleep health and depression, few have examined whether aggregate measures of sleep health are associated with depression prevalence and risk. Soehner et al.23 examined cross-sectional data from US participants meeting criteria for a major depression episode in the past year (n = 687) and reported that co-occurring insomnia and hypersomnia symptoms were associated with a more severe depression. A 7.5-year follow-up study in the United States (n = 1137) found that insomnia with short sleep duration showed higher odds of incident depression than insomnia with normal sleep duration.24 Each of these studies investigated the association between 2 dimensions of sleep health and depression, suggesting that sleep health problems may indeed increase risk in a graded fashion. Therefore, it is reasonable to investigate whether an aggregate, multidimensional measure of sleep health is associated with both cross-sectional prevalence of depression as well as the longitudinal development of depression.
In the present study, we analyzed sleep and depression symptoms data from a large cohort of community-dwelling older women. Study aims were: (1) to examine associations between individual dimensions of sleep health and the cross-sectional prevalence and development of clinically-significant depression symptoms over 6-year period; and (2) to investigate whether an aggregate measure of sleep health is associated with the cross-sectional prevalence and development of clinically significant depression symptoms in a gradient fashion, that is, whether a greater number of “poor” sleep health dimensions is associated with greater odds of clinically significant depression symptoms. We hypothesized that individual dimension of sleep health would be associated with the cross-sectional prevalence and development of depression symptoms; and that an aggregate measure of sleep health would be associated with the prevalent and incident clinically significant depression symptoms in a gradient fashion.
METHODS
Study Participants and Data Collection
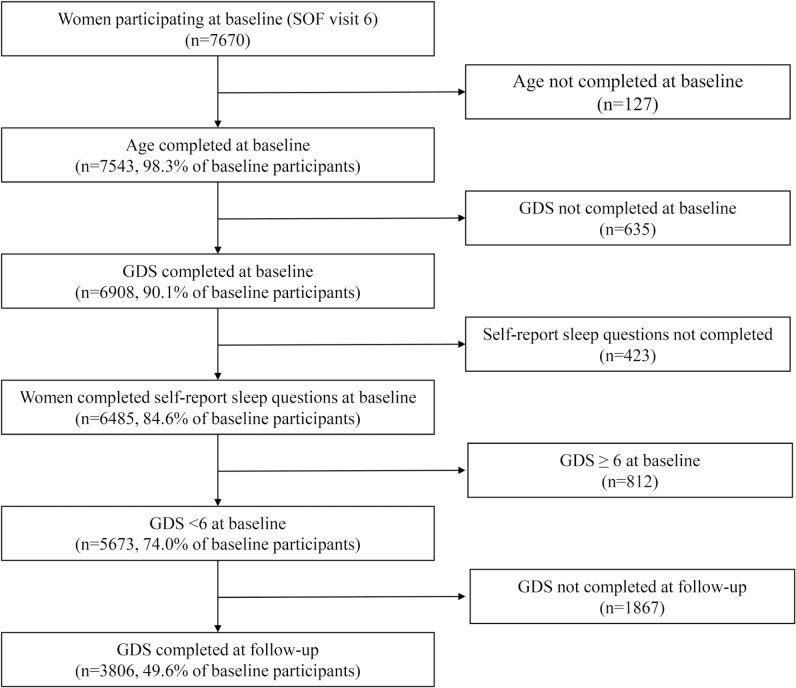
Participants were women enrolled in the Study of Osteoporotic Fractures (SOF), a multicenter, prospective cohort study of primarily Caucasian, community-dwelling women aged 65 years and older from 4 geographic areas (Portland, OR; Minneapolis, MN; Pittsburgh/Monongahela Valley, PA; Baltimore, MD). Women were recruited irrespective of bone mineral density and fracture history; those unable to walk without assistance and those with bilateral hip replacements were excluded. Women gave informed consent prior to enrollment in the study. The 9704 participants comprising the original cohort were recruited via community listings and mailed announcements between September 1986 and October 1988. Subsequently, 662 African American women were recruited between February 1997 and February 1998. A detailed description of this study was published previously.25 The current analyses focused on women participating in SOF visit 6 (1997–1998; “baseline”) and 8 (2002–2004; “follow-up”). There were 7670 participants at the baseline visit. Participants who were missing information on age (n = 127), the Geriatric Depression Scale (GDS) (n = 635), self-report sleep questions about 5 sleep health dimensions (n = 423) at the baseline visit were excluded from the analyses. We analyzed data from 6485 subjects in cross-sectional analyses. Of those, 5673 without clinically significant depression symptoms (GDS score ≥ 6) at the baseline visit were included in longitudinal analyses. We excluded those who did not complete the GDS (n = 1867) in the follow-up visit from the analyses. In total, we included data from 3806 subjects in longitudinal analyses (Figure 1).
Figure 1.
Recruitment and inclusion of participants.
Measures of Sleep Health
These SOF sleep questionnaire consisted of 12 items, including: falling asleep time, sleep onset latency, waking up time, sleep duration, napping, difficulty initiating sleep, difficulty maintaining sleep, early morning awakening, feeling unrested during the day, daytime sleepiness, satisfaction with sleep duration, and hypnotic medication use. Six self-report questions about sleep, assessed at baseline, were selected for our sleep health measure, based on 5 dimensions proposed in Buysse, 2014.22 These 5 sleep dimensions have been associated with poor health outcomes in previous studies. Each of the questions had a time frame of the past 12 months. The 6 questions were used to construct 5 sleep dimensions, which were termed satisfaction, daytime sleepiness, mid-sleep time, sleep onset latency, and sleep duration (Table 1). The first dimension refers to a specific type of sleep satisfaction, that is, the perception of the adequacy of sleep amount, but for simplicity’s sake, is termed “satisfaction” in this manuscript. Responses for each dimension were categorized as “good” or “poor” based on values previously reported in published studies,10,13,26 or on the observed distribution in our sample. In accordance with a previous study,27 mid-sleep time was calculated based on responses to falling asleep time and waking up time, using the following formula: “mid-sleep time” = “falling asleep time” + (“waking up time” − “falling asleep time”)/2. Mid-sleep time was categorized based on octiles of the mid-sleep time, and then the first (lowest score: <2:00 am) and eighth (highest score: ≥4:00 am) octiles were combined. These octiles, constituting approximately one quartile of the participants, were defined as “poor.”
Table 1.
Sleep Questions and Sleep Health Dimensions
| Sleep health dimension | Sleep survey question | Responses | Definition of “poor” sleep health |
|---|---|---|---|
| Satisfaction | Do not get enough sleep | Never (0) | Often (5–15/mo) or almost always (16–30/mo) |
| Rarely (1/mo) | |||
| Sometimes (2–4/mo) | |||
| Often (5–15/mo) | |||
| Almost always (16–30/mo) | |||
| Daytime sleepiness | Feel excessively (overly) sleepy during the day | Never (0) | Often (5–15/mo) or almost always (16–30/mo) |
| Rarely (1/mo) | |||
| Sometimes (2–4/mo) | |||
| Often (5–15/mo) | |||
| Almost always (16–30/mo) | |||
| Mid-sleep time | At what time do you usually fall asleep? | Clock time | Earlier than 2:00 am or later than/equal to 4:00 am |
| At what time do you usually wake up? | Clock time | ||
| Sleep onset latency | How many minutes does it usually take you to fall asleep at bedtime? | Number of minutes | ≥30 min10,13 |
| Sleep duration | How many hours of sleep do you usually get at night? | Number of hours | <7 h or ≥9 h26 |
An aggregate measure of sleep health was calculated by summing the number of dimensions with “poor” sleep health, and classified into 5 categories: 0, 1, 2, 3, 4, or more.
Depression Symptoms
Depression symptoms at baseline and follow-up were assessed with the 15-item GDS. The GDS was designed specifically to assess symptoms of depression in older adults, with a 1-week time frame, and does not include sleep items.28 The GDS includes binary item responses (i.e., “yes” or “no”) for the 15 items, which are summed to provide a single score (range: 0–15). Higher scores indicate increasing severity of depression symptoms. A score of 6 or higher was used to define clinically significant depression symptoms; this cutoff has a sensitivity of 90.9% and a specificity of 64.5% compared with diagnosis by DSM-IV.29
Other Measures
Sociodemographic information (race, years of education) was recorded at the original assessment. Cognitive function was assessed using the Mini Mental State Examination (MMSE),30 expressed as a continuous variable. Body mass index (BMI) was calculated using body weight and height measurements on physical examination or obtained by interview.
Additional variables obtained by self-report included age, self-reported health status, physical activity (walking for exercise), smoking status, alcohol consumption, caffeine intake, and medical history. Self-reported average daily intake of caffeinated beverages (coffee, tea, and cola with caffeine) was used to estimate the average daily caffeine intake,31 and expressed as continuous variable. Medications use during the prior 30 days was categorized according to a computerized coding dictionary.32 Hypnotic medication use in the past 12 months was assessed by self-report. Participants were asked if they had ever received a physician diagnosis of medical conditions, including hyperthyroidism, osteoporosis, Parkinson’s disease, and diabetes. For Caucasian women, data on smoking status and medical diagnoses were obtained at visit 1, because these data were not collected at baseline visit 6.
Statistical Analyses
Differences in participant characteristics according to categories of an aggregate measure of sleep health and clinically significant depression symptoms at baseline were compared using χ2 tests for categorical variables and Kruskal–Wallis or Wilcoxon rank-sum tests for continuous variables with skewed distributions (MMSE and caffeine consumption). Baseline covariates were included in multivariate models if they were significantly associated with an aggregate measure of sleep health or clinically significant depression symptoms at baseline with p < .10. These included age, race, years of education, MMSE score, BMI, self-reported health status, physical activity, alcohol use, smoking status, caffeine consumption, antidepressant use, benzodiazepine use, hypnotic medication use, and medical diagnoses (hyperthyroidism, osteoporosis, Parkinson’s disease, and diabetes).
We computed Pearson correlation coefficients among the 5 dimensions of poor sleep health at baseline. Correlations were computed for all participants, including those in the cross-sectional and longitudinal surveys.
Associations between the 5 individual dimensions of sleep health and clinically significant depression symptoms were analyzed using univariate and multivariate logistic regression. Cross-sectional analyses using data from baseline were conducted using a series of logistic regressions analyses for individual dimensions of sleep health, adjusting for covariates and scores on the other sleep health dimensions. Longitudinal analyses were restricted to participants without clinically significant depression symptoms (GDS score ≥ 6) at baseline. We conducted a series of logistic regression analyses to examine the association between each of the 5 dimensions of sleep health at baseline and the development of clinically significant depression symptoms at follow-up, adjusting for covariates and the other sleep health dimensions. The relationship between the aggregate measure of sleep health and clinically significant depression symptoms was analyzed using univariate and multivariate logistic regressions adjusting for the covariates listed above. All analyses were performed using SPSS 19.0 for Windows. Results were expressed as odds ratios (OR) and 95% confidence intervals (CI).
RESULTS
Characteristics of the Study Population by an Aggregate Measure of Sleep Health
Baseline characteristics of participants stratified by scores on the aggregate measure of sleep health are shown in Table 2. All covariates, except for smoking status and a diagnosis of Parkinson’s disease, were significantly associated with sleep health scores at baseline. In general, participants with a larger number of poor sleep health dimensions were older, less educated, had less alcohol use, less caffeine consumption and had more indicators of poor health status.
Table 2.
Baseline Characteristics of Participants According to an Aggregate Measure of Sleep Health
| Baseline characteristics | All participants | The number of “poor” sleep health dimensions | p | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 6485) | 0 (n = 1917) | 1 (n = 2138) | 2 (n = 1483) | 3 (n = 656) | ≥4 (n = 291) | ||||||||
| n | % | N | % | N | % | N | % | N | % | N | % | ||
| Age (y) | <.001 | ||||||||||||
| 70–74 | 328 | 5.1 | 77 | 4.0 | 106 | 5.0 | 85 | 5.7 | 49 | 7.5 | 11 | 3.8 | |
| 75–79 | 3063 | 47.2 | 1004 | 52.4 | 1008 | 47.1 | 659 | 44.4 | 267 | 40.7 | 125 | 43.0 | |
| 80–84 | 2041 | 31.5 | 584 | 30.5 | 672 | 31.4 | 477 | 32.2 | 225 | 34.3 | 83 | 28.5 | |
| 85+ | 1053 | 16.2 | 252 | 13.1 | 352 | 16.5 | 262 | 17.7 | 115 | 17.5 | 72 | 24.7 | |
| Race | <.001 | ||||||||||||
| Caucasian | 5919 | 91.3 | 1813 | 94.6 | 1966 | 92.0 | 1315 | 88.7 | 563 | 85.8 | 262 | 90.0 | |
| African American | 545 | 8.4 | 98 | 5.1 | 169 | 7.9 | 160 | 10.8 | 89 | 13.6 | 29 | 10.0 | |
| Other | 21 | 0.3 | 6 | 0.3 | 3 | 0.1 | 8 | 0.5 | 4 | 0.6 | 0 | 0.0 | |
| Years of education (y) | <.001 | ||||||||||||
| ≤8 | 509 | 7.9 | 111 | 5.8 | 154 | 7.2 | 128 | 8.7 | 75 | 11.5 | 41 | 14.3 | |
| 9–12 | 3400 | 52.7 | 990 | 51.7 | 1108 | 52.1 | 800 | 54.4 | 368 | 56.5 | 134 | 46.7 | |
| ≥13 | 2542 | 39.4 | 813 | 42.5 | 866 | 40.7 | 543 | 36.9 | 208 | 32.0 | 112 | 39.0 | |
| MMSE score (mean ± SD) | 27.8 ± 2.3 | 28.1 ± 2.0 | 27.6 ± 2.5 | 27.6 ± 2.3 | 27.4 ± 2.4 | 27.8 ± 2.4 | <.001 | ||||||
| Self-reported health status | .001 | ||||||||||||
| Excellent or good | 1022 | 15.8 | 260 | 13.6 | 339 | 15.9 | 270 | 18.2 | 117 | 17.9 | 36 | 12.4 | |
| Fair, poor, or very poor | 5462 | 84.2 | 1657 | 86.4 | 1799 | 84.1 | 1213 | 81.8 | 538 | 82.1 | 255 | 87.6 | |
| Takes walks for exercise | <.001 | ||||||||||||
| No | 3985 | 61.6 | 1049 | 54.9 | 1298 | 60.9 | 989 | 67.0 | 430 | 65.5 | 219 | 75.3 | |
| Yes | 2483 | 38.4 | 862 | 45.1 | 835 | 39.1 | 488 | 33.0 | 226 | 34.5 | 72 | 24.7 | |
| BMI, kg/m2 | .012 | ||||||||||||
| Underweight or normal weight (BMI < 25) | 2136 | 39.9 | 664 | 40.6 | 726 | 41.3 | 458 | 38.1 | 206 | 38.8 | 82 | 35.0 | |
| Overweight (BMI = 25–30) | 1978 | 36.9 | 633 | 38.7 | 634 | 36.0 | 445 | 37.1 | 184 | 34.7 | 82 | 35.0 | |
| Obese (BMI ≥ 30) | 1246 | 23.2 | 337 | 20.6 | 400 | 22.7 | 298 | 24.8 | 141 | 26.6 | 70 | 29.9 | |
| Smoking status | .349 | ||||||||||||
| Never smoked | 3971 | 61.4 | 1185 | 62.0 | 1275 | 59.7 | 939 | 63.7 | 397 | 60.8 | 175 | 60.1 | |
| Former smoker | 1936 | 29.9 | 566 | 29.6 | 662 | 31.0 | 413 | 28.0 | 207 | 31.7 | 88 | 30.2 | |
| Current smoker | 558 | 8.6 | 159 | 8.3 | 200 | 9.4 | 122 | 8.3 | 49 | 7.5 | 28 | 9.6 | |
| Alcohol use | <.001 | ||||||||||||
| No | 3886 | 60.0 | 1028 | 53.6 | 1261 | 59.1 | 951 | 64.2 | 441 | 67.2 | 205 | 70.4 | |
| ≤2 d/wk | 1788 | 27.6 | 601 | 31.4 | 578 | 27.1 | 373 | 25.2 | 168 | 25.6 | 68 | 23.4 | |
| ≥3 d/wk | 806 | 12.4 | 288 | 15.0 | 296 | 13.9 | 157 | 10.6 | 47 | 7.2 | 18 | 6.2 | |
| Caffeine consumption, mg/d (mean ± SD) | 155.5 ± 164.6 | 164.4 ± 162.9 | 159.9 ± 165.0 | 144.8 ± 164.0 | 145.5 ± 166.2 | 141.6 ± 167.6 | <.001 | ||||||
| Current antidepressant use | <.001 | ||||||||||||
| No | 5894 | 90.9 | 1782 | 93.0 | 1956 | 91.6 | 1327 | 89.5 | 576 | 87.8 | 253 | 86.9 | |
| Yes | 589 | 9.1 | 135 | 7.0 | 180 | 8.4 | 156 | 10.5 | 80 | 12.2 | 38 | 13.1 | |
| Current benzodiazepine use | <.001 | ||||||||||||
| No | 6053 | 93.4 | 1831 | 95.5 | 2008 | 94.0 | 1364 | 92.0 | 591 | 90.1 | 259 | 89.0 | |
| Yes | 430 | 6.6 | 86 | 4.5 | 128 | 6.0 | 119 | 8.0 | 65 | 9.9 | 32 | 11.0 | |
| Hypnotic medication use | <.001 | ||||||||||||
| Never (0), Rarely (1/mo), or Sometimes (2–4/mo) | 5781 | 89.4 | 1803 | 94.1 | 1946 | 91.3 | 1287 | 87.3 | 532 | 81.2 | 213 | 74.2 | |
| Often (5–15/mo) or Almost always (16–30/mo) | 684 | 10.6 | 113 | 5.9 | 186 | 8.7 | 188 | 12.7 | 123 | 18.8 | 74 | 25.8 | |
| Medical conditions | |||||||||||||
| Hyperthyroid disease | .002 | ||||||||||||
| No | 5852 | 92.2 | 1766 | 93.9 | 1929 | 92.1 | 1332 | 91.5 | 575 | 90.1 | 250 | 89.0 | |
| Yes | 496 | 7.8 | 114 | 6.1 | 165 | 7.9 | 123 | 8.5 | 63 | 9.9 | 31 | 11.0 | |
| Osteoporosis | <.001 | ||||||||||||
| No | 5599 | 87.3 | 1667 | 88.0 | 1872 | 88.6 | 1283 | 87.3 | 549 | 84.9 | 228 | 79.7 | |
| Yes | 811 | 12.7 | 228 | 12.0 | 241 | 11.4 | 186 | 12.7 | 98 | 15.1 | 58 | 20.3 | |
| Parkinson’s disease | .900 | ||||||||||||
| No | 6451 | 99.6 | 1908 | 99.6 | 2127 | 99.6 | 1474 | 99.5 | 652 | 99.4 | 290 | 99.7 | |
| Yes | 29 | 0.4 | 7 | 0.4 | 9 | 0.4 | 8 | 0.5 | 4 | 0.6 | 1 | 0.3 | |
| Diabetes | <.001 | ||||||||||||
| No | 6081 | 94.0 | 1842 | 96.1 | 2014 | 94.4 | 1376 | 93.2 | 583 | 89.1 | 266 | 91.4 | |
| Yes | 390 | 6.0 | 74 | 3.9 | 120 | 5.6 | 100 | 6.8 | 71 | 10.9 | 25 | 8.6 | |
N, Percent or mean ± SD shown; χ2 tests for categorical variables and Kruskal–Wallis tests for continuous variables with skewed distributions.
Characteristics of the 5 Sleep Health Dimensions at Baseline
Individual sleep health dimensions and their prevalence at baseline are shown in Table 3. The prevalence of poor sleep ranged from 10.7 for daytime sleepiness to 41.6 for sleep duration.
Table 3.
Characteristics of the 5 Sleep Health Dimensions at Baseline
| Baseline characteristics of dimensions of sleep health | Participants | |
|---|---|---|
| n | (%) | |
| Satisfaction | ||
| Never | 1787 | 27.6 |
| Rarely 1/mo | 2131 | 32.9 |
| Sometimes 2–4/mo | 1514 | 23.3 |
| Often 5–15/mo | 472 | 7.3 |
| Almost always 16–30/mo | 581 | 9.0 |
| Daytime sleepiness | ||
| Never | 1823 | 28.1 |
| Rarely 1/mo | 2231 | 34.4 |
| Sometimes 2–4/mo | 1740 | 26.8 |
| Often 5–15/mo | 491 | 7.6 |
| Almost always 16–30/mo | 200 | 3.1 |
| Mid-sleep time | ||
| <2:00 | 883 | 13.6 |
| ≥2:00 and <4:00 | 4796 | 74.0 |
| ≥4:00 | 806 | 12.4 |
| Sleep onset latency | ||
| <30 m | 4351 | 67.1 |
| ≥30 m | 2134 | 32.9 |
| Sleep duration | ||
| <7 h | 1848 | 28.5 |
| ≥7 h and <9 h | 3785 | 58.4 |
| ≥9 h | 852 | 13.1 |
Correlation Among the 5 Dimensions of Sleep Health
Pearson correlation coefficients among the 5 dimensions of sleep health are shown in Table 4. All Pearson correlation coefficients were <0.3, suggesting relatively weak relationships among the 5 dimensions of sleep health.
Table 4.
Pearson Correlation Coefficient Among 5 Dimensions of Sleep Health at Baseline
| Satisfaction | Daytime sleepiness | Extreme mid-sleep time | Long sleep onset latency | Extreme sleep duration | |
|---|---|---|---|---|---|
| Satisfaction | — | 0.276 | 0.080 | 0.174 | 0.165 |
| Daytime sleepiness | 0.243 | — | 0.072 | 0.085 | 0.106 |
| Extreme mid-sleep time | 0.071 | 0.039 | — | 0.074 | 0.140 |
| Long sleep onset latency | 0.168 | 0.066 | 0.048 | — | 0.139 |
| Extreme sleep duration | 0.198 | 0.105 | 0.128 | 0.164 | — |
Numbers in the upper right portion of the figure are participants including in the cross-sectional survey. Numbers in the left lower portion of the figure are participants including in the longitudinal survey.
Association Between Individual Sleep Health Dimensions and Presence/Development of Clinically Significant Depression Symptoms
Associations between the 5 individual dimensions of sleep health and clinically significant depression symptoms in the cross-sectional and longitudinal studies are shown in Table 5.
Table 5.
Association Between Individual Sleep Health Dimensions and Presence/Development of Clinically Significant Depression Symptoms in the Cross-Sectional and the Longitudinal Studies
| Baseline items | Cross-sectional study | Longitudinal study (6 y later) | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |||
| Dimensions of sleep health | ||||||||
| Satisfaction (poor vs. fair) | ||||||||
| Univariate model | 2.31 | 1.95 | 2.74 | <.001 | 1.87 | 1.46 | 2.41 | <.001 |
| Multivariate model 1 | 1.99 | 1.59 | 2.49 | <.001 | 1.70 | 1.26 | 2.28 | <.001 |
| Multivariate model 2 | 1.34 | 1.05 | 1.72 | .017 | 1.39 | 1.01 | 1.90 | .042 |
| Daytime sleepiness (poor vs. fair) | ||||||||
| Univariate model | 4.36 | 3.64 | 5.22 | <.001 | 2.48 | 1.83 | 3.35 | <.001 |
| Multivariate model 1 | 3.19 | 2.50 | 4.06 | <.001 | 2.08 | 1.45 | 2.99 | <.001 |
| Multivariate model 2 | 2.69 | 2.08 | 3.48 | <.001 | 1.79 | 1.23 | 2.61 | <.001 |
| Mid-sleep time (<2:00 and ≥4:00 vs. ≥2:00 and <4:00) | ||||||||
| Univariate model | 1.79 | 1.54 | 2.09 | <.001 | 1.43 | 1.15 | 1.79 | <.001 |
| Multivariate model 1 | 1.42 | 1.15 | 1.75 | <.001 | 1.30 | 1.00 | 1.69 | .047 |
| Multivariate model 2 | 1.26 | 1.02 | 1.56 | .034 | 1.24 | 0.95 | 1.61 | .119 |
| Sleep onset latency (≥30 m vs. <30 m) | ||||||||
| Univariate model | 2.02 | 1.74 | 2.35 | <.001 | 1.72 | 1.39 | 2.12 | <.001 |
| Multivariate model 1 | 2.01 | 1.65 | 2.44 | <.001 | 1.44 | 1.13 | 1.85 | <.001 |
| Multivariate model 2 | 1.78 | 1.45 | 2.18 | <.001 | 1.32 | 1.03 | 1.71 | .031 |
| Sleep duration (<7 h and ≥9 h vs. ≥7 h and <9 h) | ||||||||
| Univariate model | 1.95 | 1.68 | 2.26 | <.001 | 1.27 | 1.03 | 1.56 | .024 |
| Multivariate model 1 | 1.49 | 1.23 | 1.81 | <.001 | 1.27 | 1.00 | 1.61 | .051 |
| Multivariate model 2 | 1.19 | 0.97 | 1.47 | .089 | 1.07 | 0.83 | 1.38 | .589 |
Model 1: Adjusted for age, race, years of education, MMSE, self-reported health status, takes walks for exercise, BMI, alcohol use, smoke status, caffeine consumption, antidepressant use, benzodiazepine use, hypnotic medication use, hyperthyroidism, osteoporosis, Parkinson’s disease, and diabetes. Model 2: Adjusted for age, race, years of education, MMSE, self-reported health status, takes walks for exercise, BMI, alcohol use, smoke status, caffeine consumption, antidepressant use, benzodiazepine use, hypnotic medication use, hyperthyroidism, osteoporosis, Parkinson’s disease, diabetes, and other sleep health dimensions.
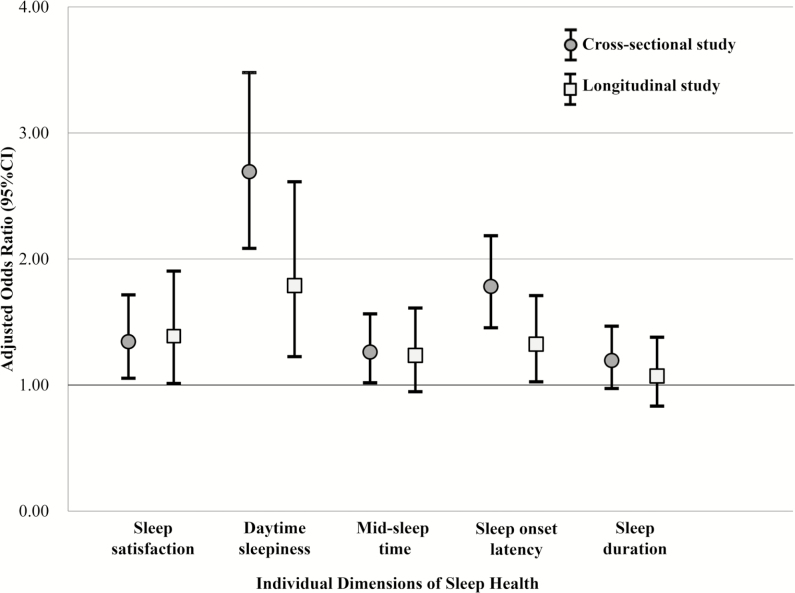
The prevalence of clinically significant depression symptoms at baseline was 12.5%. Multivariate logistic regression analyses revealed that 4 of the 5 dimensions of poor sleep health, including satisfaction, daytime sleepiness, mid-sleep time, and sleep onset latency were significantly associated with clinically significant depression symptoms at baseline, with ORs ranging from 1.26 for mid-sleep time to 2.69 for daytime sleepiness (Figure 2).
Figure 2.
Association between individual sleep health dimensions and presence/development of clinically significant depression symptoms in the cross-sectional (SOF, V6) and longitudinal (SOF, V6–V8) studies.
Among individuals without clinically significant depression symptoms at baseline, the prevalence of such symptoms at follow-up was 10.9%. Multivariate logistic regression analyses revealed that 3 of 5 dimensions of poor sleep health, including satisfaction, daytime sleepiness, and sleep onset latency were significantly associated with incident clinically significant depression symptoms over 6 years, with ORs ranging from 1.32 for sleep onset latency to 1.79 for daytime sleepiness (Figure 2).
Associations Between an Aggregate Measure of Sleep Health and Presence/Development of Clinically Significant Depression Symptoms
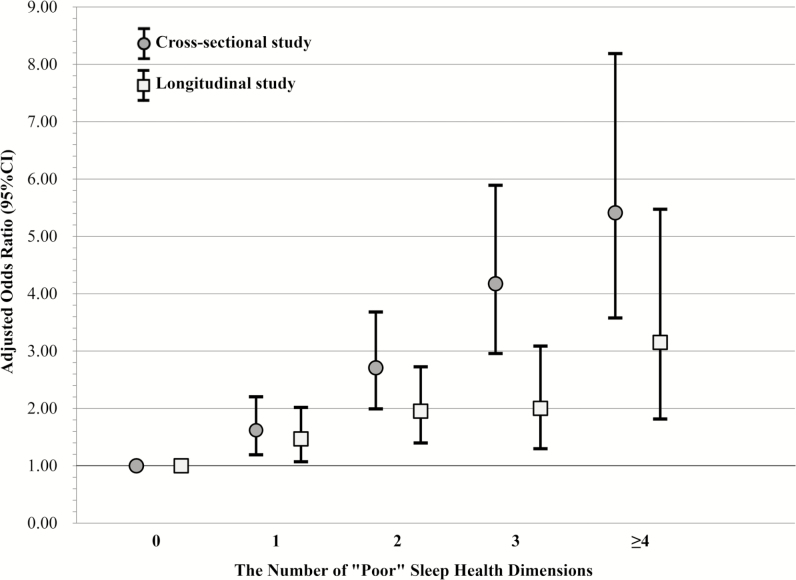
Associations between the aggregate measure of sleep health and clinically significant depression symptoms in cross-sectional and longitudinal studies are shown in Table 6. Multivariate logistic regression analyses revealed that the aggregate measure of sleep health showed a gradient effect: higher levels of poor sleep health were associated with greater odds of prevalent clinically significant depression symptoms (ORs 1.62–5.41; p-trend < .001) and the longitudinal development of clinically significant depression symptoms (ORs 1.47–3.15; p-trend < .001). To illustrate these relationships, Figure 3 presents the associations between the aggregate measure of sleep health and clinically significant depression symptoms in cross-sectional and longitudinal analyses.
Table 6.
Association Between an Aggregate Measure of Sleep Health and Presence/Development of Clinically Significant Depression Symptoms in the Cross-Sectional and the Longitudinal Analyses
| Baseline items | Participants | Univariate model | Multivariate modela | ||||||
|---|---|---|---|---|---|---|---|---|---|
| An aggregate measures of sleep health | (%) | OR | 95% CI | p-trend | OR | 95% CI | p-trend | ||
| Cross-sectional study | |||||||||
| 0 | 29.6 | 1.00 | (reference) | <.001 | 1.00 | (reference) | <.001 | ||
| 1 | 33.0 | 2.05 | 1.60 | 2.63 | 1.62 | 1.19 | 2.20 | ||
| 2 | 22.9 | 3.46 | 2.70 | 4.42 | 2.71 | 1.99 | 3.68 | ||
| 3 | 10.1 | 6.07 | 4.64 | 7.94 | 4.17 | 2.96 | 5.89 | ||
| ≥4 | 4.5 | 9.61 | 7.01 | 13.18 | 5.41 | 3.58 | 8.19 | ||
| Longitudinal study (6 y later) | |||||||||
| 0 | 34.1 | 1.00 | (reference) | <.001 | 1.00 | (reference) | <.001 | ||
| 1 | 33.2 | 1.51 | 1.15 | 2.00 | 1.47 | 1.07 | 2.02 | ||
| 2 | 21.1 | 2.10 | 1.57 | 2.81 | 1.95 | 1.40 | 2.73 | ||
| 3 | 8.5 | 2.14 | 1.47 | 3.12 | 2.00 | 1.30 | 3.09 | ||
| ≥4 | 3.1 | 4.62 | 2.91 | 7.32 | 3.15 | 1.81 | 5.47 | ||
Adjusted for age, race, years of education, MMSE, self-reported health status, takes walks for exercise, BMI, alcohol use, smoke status, caffeine consumption, antidepressant use, benzodiazepine use, hypnotic medication use, hyperthyroidism, osteoporosis, Parkinson’s disease, and diabetes.
Figure 3.
Associations between an aggregate measure of sleep health and presence/development of clinically significant depression symptoms in the cross-sectional (SOF, V6) and longitudinal (SOF, V6–V8) studies.
Additional Analyses
Associations of mid-sleep time (early, intermediate, and late) and sleep duration (short, normal, and long) with the presence/development of clinically significant depression symptoms in cross-sectional and longitudinal analyses are shown in Supplementary Table S1. In the univariate cross-sectional analyses, both mid-sleep time and sleep duration showed a U-shaped association with presence of clinically significant depression symptoms. Multivariate logistic regression analyses showed that early mid-sleep time (<2:00), short sleep duration (<7 h), and long sleep duration (≥9 h) were significantly associated with prevalent clinically significant depression symptoms at baseline. Multivariate logistic regression analyses showed that early mid-sleep time (<2:00), and short sleep duration (<7 h) were significantly associated with incident clinically significant depression symptoms over 6 years.
DISCUSSION
In a large sample of community-dwelling older women in the United States, we found that both individual sleep dimensions and an aggregate measure of sleep health were associated with prevalent depression and the longitudinal development of clinically significant depression symptoms. For the aggregate measure, the increased odds of depression symptoms occurred in a graded fashion: the greater the number of “poor” sleep health dimensions, the greater the cross-sectional and longitudinal risk for clinically significant depression symptoms. To our knowledge, this is the first study to investigate associations between an aggregate measure of sleep health dimensions and the presence and development of clinically significant depression symptoms. Although many previous studies have documented health risks associated with individual dimensions of sleep health such as short and long sleep duration,26 our findings suggest that other characteristics of sleep may also confer risk, and that these sleep characteristics have additive effects. Examining multivariate sleep health profiles may advance our understanding of the relationships between sleep, health, and disease.
In the present study, poor satisfaction (specifically, the feeling of not getting enough sleep) was significantly associated with prevalence of clinically significant depression symptoms at baseline, as well as being significantly associated with greater odds of development of clinically significant depression symptoms. Several previous cross-sectional epidemiological studies in Western and Asian countries reported the association between sleep satisfaction and depression.6–8 Significant associations between sleep satisfaction and the onset of depression have also been reported in several prospective studies across different countires.16,17 The results of present study are consistent with these previous studies. However, measures of sleep satisfaction differ among these studies. Our item measured the individual’s judgment of whether they got “enough sleep,” which likely incorporates some judgment about sleep duration as well as satisfaction. Assessments used in other studies more specifically measured sleep quality or sleep satisfaction.17,33,34 Nevertheless, we observed a low Pearson’s correlation between the sleep satisfaction item and the actual sleep duration item, suggesting that participants distinguished these 2 dimensions of sleep.
Of the individual sleep health dimensions, daytime sleepiness had the strongest positive association with clinically significant depression symptoms in both the cross-sectional and longitudinal analyses. Numerous previous cross-sectional studies have reported significant associations between increased daytime sleepiness and depression, independent of insomnia symptoms and sleep duration.7–9 Extending beyond cross-sectional data, prospective studies in young18 and older adults16 have reported that excessive daytime sleepiness is a significant predictor of subsequent depression. Thus, the results of the present study are again in agreement with findings from previous studies.
Previous cross-sectional epidemiological studies have revealed that chronotype is associated with depression, with increased risk in self-reported “evening types,” that is, individuals who prefer sleeping at later times.8,11,12 However, the association between early sleep timing and depression is controversial, with some epidemiological studies reporting lower prevalence of depression in morning types compared to intermediate types,8,12 and at least one study suggesting that the prevalence of depression was higher in morning type compared to intermediate types.11 Others have evaluated mid-sleep time as a behavioral index of chronotype, finding that both late and early timing was associated with greater symptoms of depression compared to intermediate sleep timing.35,36 In the present study, mid-sleep time showed a U-shaped association with concurrent clinically significant depression symptoms in the univariate analyses. Therefore, categorization of both very early and very late mid-sleep times into a single “poor” category seemed appropriate. Cross-sectional analyses indicated a significant association between extreme mid-sleep times and the prevalence of clinically significant depression symptoms. In longitudinal analyses, extreme mid-sleep times were again associated with increased odds ratio for the development of clinically significant depression symptoms, although the association was no longer significant after adjustment for the other sleep health, suggesting that longitudinal associations between extreme mid-sleep time and development of clinically significant depression symptoms are mediated by the other sleep health dimensions. Further studies are required to examine the complex interactions among mid-sleep time, the other sleep health dimensions, and depression. Furthermore, distinctions between chronotype as a habitual measure of sleep timing preference, and actual mid-sleep times, may be important.
Our results showed that longer sleep onset latency (≥30 m) was significantly associated with both the prevalence and the development of clinically significant depression symptoms. Findings from epidemiological studies that examined associations between sleep onset latency and depression have shown variable results. For example, a cross-sectional study in older adults showed an association between long sleep onset latency and depression,13 whereas no significant associations were reported between depression and longer sleep onset latency,10 or sleep latency assessed with the Pittsburgh Sleep Quality Index.37 In prospective studies among older adults, longer sleep onset latency indicated a significant association with worsening of depression symptoms in one study,17 whereas it exhibited no significant association with depression in another study.34 Although our findings demonstrated cross-sectional and longitudinal associations between sleep onset latency and clinically significant depression symptoms in older women, other indicators of sleep continuity or efficiency should be examined in subsequent studies.
Multiple studies have reported associations between both short and/or long sleep duration and depression, and some cross-sectional studies have revealed a U-shaped association.7,14,15,38 In the present study, both short and long sleep duration were significantly associated with prevalence of clinically significant depression symptoms. Therefore, the categorization of both short and long sleep duration into single “poor” sleep duration group in our study seems appropriate. Results of prospective studies have been inconsistent, with some studies reporting significant associations between short sleep duration and depression,39 and other studies failing to find such an effect.5,17,34,39–41 A recent meta-analysis of prospective studies reported a significant increase in the risk of onset of depression in both short- and long-sleepers.21 However, as Zhai et al.21 pointed out, confounding factors were not well addressed in that report. In the present study, extreme sleep duration was associated with an increased odds ratio for the prevalence and development of clinically significant depression symptoms in unadjusted models. However, these associations were no longer significant after adjustment for multiple health problems, medications, and the other individual indices of sleep health. Thus, complex interactions among sleep dimensions and other health factors may explain our findings.
This is the first study to demonstrate a significant association between the presence/development of clinically significant depression symptoms and an aggregate multivariate measure of sleep health. Several previous studies have reported that a more limited number of sleep health, most notably insomnia (poor satisfaction/quality) with short objective sleep duration, are associated with adverse health outcomes, including hypertension,42,43 diabetes,42 neurocognitive impairment,42 and mortality.42 Taken together, our findings and previous studies suggest that multivariate measures of sleep may prove to be more useful than individual measures for assessing health risks.
There are several possible interpretations of our findings. First, the number of “poor” sleep health dimensions may be a marker for overall severity of sleep disturbances. Previous studies have reported that the severity of sleep problems is associated with the onset of depression,24,41 perceived mental health status,44 and quality of life.45 Second, it is possible that each sleep health dimension increases depression risk via different mechanisms. To date, no definitive psychophysiological mechanisms identified to explain the pathways through which disturbed sleep increases depression symptoms or risk. Hypothesized pathways include neurotransmitter imbalance,3 phase-advance theories,3 the S-deficiency hypothesis,3 overactivity of the hypothalamic-pituitary-adrenal axis,3 and sleep-related alterations in neural circuits regulating affect.46 The interaction of several such mechanisms, indicated by different types of sleep disturbance, may increase the presence/development of depression. Finally, disturbed sleep and depression may reflect a common background factors such as chronic stress or unmeasured medical disease.
Despite the richness of these cross-sectional and longitudinal data, several limitations deserve consideration. First, the sample comprised exclusively older women. Despite statistical adjustment for confounding variables, other unmeasured factors may have influenced these results. Nor do our data address associations among sleep health and depression in men or racial/ethnic minority groups. Second, the sleep evaluation consisted only of retrospective self-report questionnaire data. Future studies may examine multivariate sleep measures based on behavioral (actigraphy) or physiological (polysomnography) methods. Third, the present study used the GDS to define clinically significant depression symptoms, but a previous medical history of depression and existence of other mental disorders were not investigated. Fourth, the sleep questions used a time frame of the past 12 months, which does not reflect normal variations in sleep, or shorter-term changes in sleep that might be associated more strongly with depression. Finally, the results from cross-sectional study could not determine whether depression preceded or resulted from poor sleep health.
Our findings suggest that assessing multiple sleep health dimensions may provide a richer understanding of how sleep is related to other health problems, compared to the traditional approach of examining one dimension at a time. This approach recognizes that different sleep characteristics occur in conjunction with each other, and may have additive or interactive effects on health. The results also raise the possibility that interventions focusing on multiple sleep health dimensions could potentially reduce risk for other health problems. Further studies examining multivariate measures of sleep health in other samples and with other health outcomes are warranted.
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
FUNDING
The Study of Osteoporotic Fractures is supported by National Institutes of Health funding. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 AG005407, R01 AR35582, R01 AR35583, R01 AR35584, R01 AG005394, R01 AG027574, R01 AG027576, and R01 AG026720. Stephen F. Smagula is supported by T32 MH019986. Makoto Uchiyama is supported by a Research Grant from the Japan Society for Promoting Science and Technology Agency (26507012, 2014–2017).
DISCLOSURE STATEMENT
SA-I is a consultant for Merck, Purdue, Eisai, Jansen, and Pfizer. YK has received grant support from Eisai Japan. MU is on the speakes’ bureau for Astellas Pharma, Eisai, Meiji Seika Pharma, MSD, Pfizer Japan, and Takeda Pharmaceutical. MU is a consultant for Taisho Pharmaceutical, and Kao Corporation. DJB is a consultant for Cerêve, Inc., Emmi Solutions, and Philips Respironics. DJB is supported by the NIH grants. DJB has Intellectual Property Rights for Pittsburgh Sleep Quality Index (PSQI). The other authors have indicated no financial conflicts of interest.
Supplementary Material
ACKNOWLEDGMENT
Analysis was performed at Nihon University School of Medicine.
REFERENCES
- 1. Murphy MJ, Peterson MJ. Sleep disturbances in depression. Sleep Med Clin. 2015; 10(1): 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011; 135(1–3): 10–19. [DOI] [PubMed] [Google Scholar]
- 3. Rumble ME, White KH, Benca RM. Sleep disturbances in mood disorders. Psychiatr Clin North Am. 2015; 38(4): 743–759. [DOI] [PubMed] [Google Scholar]
- 4. Brabbins CJ, Dewey ME, Copeland JRM, et al. Insomnia in the elderly: prevalence, gender differences and relationships with morbidity and mortality. Int J Geriatr Psychiatry. 1993; 8: 473–480. [Google Scholar]
- 5. Yokoyama E, Kaneita Y, Saito Y, et al. Association between depression and insomnia subtypes: a longitudinal study on the elderly in Japan. Sleep. 2010; 33(12): 1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chapman DP, Presley-Cantrell LR, Liu Y, Perry GS, Wheaton AG, Croft JB. Frequent insufficient sleep and anxiety and depressive disorders among U.S. community dwellers in 20 states, 2010. Psychiatr Serv. 2013; 64(4): 385–387. [DOI] [PubMed] [Google Scholar]
- 7. Kaneita Y, Ohida T, Uchiyama M, et al. The relationship between depression and sleep disturbances: a Japanese nationwide general population survey. J Clin Psychiatry. 2006; 67(2): 196–203. [DOI] [PubMed] [Google Scholar]
- 8. Kitamura S, Hida A, Watanabe M, et al. Evening preference is related to the incidence of depressive states independent of sleep-wake conditions. Chronobiol Int. 2010; 27(9–10): 1797–1812. [DOI] [PubMed] [Google Scholar]
- 9. Bixler EO, Vgontzas AN, Lin HM, Calhoun SL, Vela-Bueno A, Kales A. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005; 90(8): 4510–4515. [DOI] [PubMed] [Google Scholar]
- 10. Maglione JE, Ancoli-Israel S, Peters KW, et al. Depressive symptoms and subjective and objective sleep in community-dwelling older women. J Am Geriatr Soc. 2012; 60(4): 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hidalgo MP, Caumo W, Posser M, Coccaro SB, Camozzato AL, Chaves ML. Relationship between depressive mood and chronotype in healthy subjects. Psychiatry Clin Neurosci. 2009; 63(3): 283–290. [DOI] [PubMed] [Google Scholar]
- 12. Merikanto I, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, Partonen T. Circadian preference links to depression in general adult population. J Affect Disord. 2015; 188: 143–148. [DOI] [PubMed] [Google Scholar]
- 13. Sukegawa T, Itoga M, Seno H, et al. Sleep disturbances and depression in the elderly in Japan. Psychiatry Clin Neurosci. 2003; 57(3): 265–270. [DOI] [PubMed] [Google Scholar]
- 14. Furihata R, Uchiyama M, Suzuki M, et al. Association of short sleep duration and short time in bed with depression: a Japanese general population survey. Sleep Biol Rhythms. 2015; 13(2): 136–145. [Google Scholar]
- 15. Krueger PM, Friedman EM. Sleep duration in the United States: a cross-sectional population-based study. Am J Epidemiol. 2009; 169(9): 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaussent I, Bouyer J, Ancelin ML, et al. Insomnia and daytime sleepiness are risk factors for depressive symptoms in the elderly. Sleep. 2011; 34(8): 1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maglione JE, Ancoli-Israel S, Peters KW, et al. ; Study of Osteoporotic Fractures Research Group. Subjective and objective sleep disturbance and longitudinal risk of depression in a cohort of older women. Sleep. 2014; 37(7): 1179–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996; 39(6): 411–418. [DOI] [PubMed] [Google Scholar]
- 19. Smagula SF, Ancoli-Israel S, Blackwell T, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group. Circadian rest-activity rhythms predict future increases in depressive symptoms among community-dwelling older men. Am J Geriatr Psychiatry. 2015; 23(5): 495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smagula SF, Boudreau RM, Stone K, et al. ; Osteoporotic Fractures in Men (MrOS) Research Group. Latent activity rhythm disturbance sub-groups and longitudinal change in depression symptoms among older men. Chronobiol Int. 2015; 32(10): 1427–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhai L, Zhang H, Zhang D. Sleep duration and depression among adults: a meta-analysis of prospective studies. Depress Anxiety. 2015; 32(9): 664–670. [DOI] [PubMed] [Google Scholar]
- 22. Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014; 37(1): 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soehner AM, Kaplan KA, Harvey AG. Prevalence and clinical correlates of co-occurring insomnia and hypersomnia symptoms in depression. J Affect Disord. 2014; 167: 93–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernandez-Mendoza J, Shea S, Vgontzas AN, Calhoun SL, Liao D, Bixler EO. Insomnia and incident depression: role of objective sleep duration and natural history. J Sleep Res. 2015; 24(4): 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cummings SR, Black DM, Nevitt MC, et al. Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet. 1993; 341(8837): 72–75. [DOI] [PubMed] [Google Scholar]
- 26. Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015; 38(8): 1161–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003; 18(1): 80–90. [DOI] [PubMed] [Google Scholar]
- 28. Yesavage JA, Sheikh JI. 9/Geriatric Depression Scale (GDS). Clin Gerontol. 1986; 5: 165–173. [Google Scholar]
- 29. Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999; 14(10): 858–865. [DOI] [PubMed] [Google Scholar]
- 30. Teng EL, Chui HC. The mModified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987; 48(8): 314–318. [PubMed] [Google Scholar]
- 31. Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996; 34(1): 119–129. [DOI] [PubMed] [Google Scholar]
- 32. Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994; 10(4): 405–411. [DOI] [PubMed] [Google Scholar]
- 33. Lacruz ME, Schmidt-Pokrzywniak A, Dragano N, et al. Depressive symptoms, life satisfaction and prevalence of sleep disturbances in the general population of Germany: results from the Heinz Nixdorf Recall study. BMJ Open. 2016; 6(1): e007919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paudel M, Taylor BC, Ancoli-Israel S, et al. Sleep disturbances and risk of depression in older men. Sleep. 2013; 36(7): 1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Antypa N, Vogelzangs N, Meesters Y, Schoevers R, Penninx BW. Chronotype associations with depression and anxiety disorders in a large cohort study. Depress Anxiety. 2016; 33(1): 75–83. [DOI] [PubMed] [Google Scholar]
- 36. Levandovski R, Dantas G, Fernandes LC, et al. Depression scores associate with chronotype and social jetlag in a rural population. Chronobiol Int. 2011; 28(9): 771–778. [DOI] [PubMed] [Google Scholar]
- 37. Potvin O, Lorrain D, Belleville G, Grenier S, Préville M. Subjective sleep characteristics associated with anxiety and depression in older adults: a population-based study. Int J Geriatr Psychiatry. 2014; 29(12): 1262–1270. [DOI] [PubMed] [Google Scholar]
- 38. van den Berg JF, Luijendijk HJ, Tulen JH, Hofman A, Neven AK, Tiemeier H. Sleep in depression and anxiety disorders: a population-based study of elderly persons. J Clin Psychiatry. 2009; 70(8): 1105–1113. [DOI] [PubMed] [Google Scholar]
- 39. Gehrman P, Seelig AD, Jacobson IG, et al. Predeployment sleep duration and insomnia symptoms as risk factors for new-onset mental health disorders following military deployment. Sleep. 2013; 36(7): 1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997; 146(2): 105–114. [DOI] [PubMed] [Google Scholar]
- 41. Szklo-Coxe M, Young T, Peppard PE, Finn LA, Benca RM. Prospective associations of insomnia markers and symptoms with depression. Am J Epidemiol. 2010; 171(6): 709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vgontzas AN, Fernandez-Mendoza J, Liao D, Bixler EO. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013; 17(4): 241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bathgate CJ, Edinger JD, Wyatt JK, Krystal AD. Objective but not subjective short sleep duration associated with increased risk for hypertension in individuals with insomnia. Sleep. 2016; 39(5): 1037–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Furihata R, Uchiyama M, Takahashi S, et al. The association between sleep problems and perceived health status: a Japanese nationwide general population survey. Sleep Med. 2012; 13(7): 831–837. [DOI] [PubMed] [Google Scholar]
- 45. Schubert CR, Cruickshanks KJ, Dalton DS, Klein BE, Klein R, Nondahl DM. Prevalence of sleep problems and quality of life in an older population. Sleep. 2002; 25(8): 889–893. [PubMed] [Google Scholar]
- 46. Gujar N, Yoo SS, Hu P, Walker MP. Sleep deprivation amplifies reactivity of brain reward networks, biasing the appraisal of positive emotional experiences. J Neurosci. 2011; 31(12): 4466–4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.