Abstract
Introduction:
EEG oscillations known as sleep spindles have been linked with various aspects of cognition, but the specific functions they signal remain controversial. Two types of EEG sleep spindles have been distinguished: slow spindles at 11–13.5 Hz and fast spindles at 13.5–16 Hz. Slow spindles exhibit a frontal scalp topography, whereas fast spindles exhibit a posterior scalp topography and have been preferentially linked with memory consolidation during sleep. To advance understanding beyond that provided from correlative studies of spindles, we aimed to develop a new method to systematically manipulate spindles.
Aims and Methods:
We presented repeating bursts of oscillating white noise to people during a 90-min afternoon nap. During stage 2 and slow-wave sleep, oscillations were embedded within contiguous 10-s stimulation intervals, each comprising 2 s of white noise amplitude modulated at 12 Hz (targeting slow spindles), 15 Hz (targeting fast spindles), or 50 Hz followed by 8 s of constant white noise.
Results:
During oscillating stimulation compared to constant stimulation, parietal EEG recordings showed more slow spindles in the 12-Hz condition, more fast spindles in the 15-Hz condition, and no change in the 50-Hz control condition. These effects were topographically selective, and were absent in frontopolar EEG recordings, where slow spindle density was highest. Spindles during stimulation were similar to spontaneous spindles in standard physiological features, including duration and scalp distribution.
Conclusions:
These results define a new method to selectively and noninvasively manipulate spindles through acoustic resonance, while also providing new evidence for functional distinctions between the 2 types of EEG spindles.
Keywords: sleep spindles, oscillations, memory consolidation.
Statement of Significance
Sleep spindles occur in short bursts of oscillating activity during non-rapid eye movement sleep. Spindles have been linked to numerous cognitive domains, including memory. Recent evidence suggests pharmacological manipulations can modulate spindles in humans. However, no method exists for manipulating spindles on a precise timescale and without nonspecific pharmacological side effects. Here we show that briefly oscillating sounds influence spindles in a frequency-specific manner. These results open the door to noninvasively manipulating spindles with high temporal precision in future studies in order to directly and selectively investigate sleep spindle function.
INTRODUCTION
Sleep spindles are a widely accepted physiological hallmark of sleep, particularly stage 2 and slow-wave sleep (SWS). Conventional strategies for identifying spindles in the human EEG focus on oscillatory activity between 11 and 16 Hz that is enhanced in a transient fashion, for at least 0.5 s and no longer than 2 s.1 Based largely on correlative analyses, spindles have been linked to a wide array of functions, including sleep protection, cortical development, memory and plasticity, general intelligence, and cognitive dysfunction.2
Two types of EEG spindles have been distinguished based on multiple features.3–5 First, 2 oscillation rates have been described: fast spindles have a typical rate of 13.5–16 Hz and slow spindles a typical rate of 11–13.5 Hz. Second, fast spindles display a centroparietal distribution across the scalp, whereas slow spindles display a frontal distribution, and the 2 types have been putatively localized to posterior and anterior intracranial sources, respectively.4–7 Third, the 2 types of spindles predominate at different phases of the slow oscillation (SO), with fast spindles occurring more frequently during the SO upstate than slow spindles.6,8 Fourth, pharmacological manipulations differentially affect the 2 types of spindles.9 Although these findings implicate 2 types of spindles, it has not been resolved whether fast and slow spindles reflect qualitatively different neural generating mechanisms versus the same mechanisms operative in different brain regions at different frequencies.1,10
Memory functions have received particularly strong emphasis in recent work on spindles in humans and in other mammals.2,10 For instance, 2 studies showed that administering zolpidem, a GABA agonist, increased sleep spindles and also improved declarative memory retention.11,12 However, pharmacological manipulations have limitations. They may introduce nonspecific effects (such as other changes in neuronal activity). Additionally, they alter spindle incidence over a long time-span rather than at precise times under experimental control. To gain a better understanding of the 2 spindle subtypes, researchers would benefit from methods that can modify spindles in a temporally precise manner.
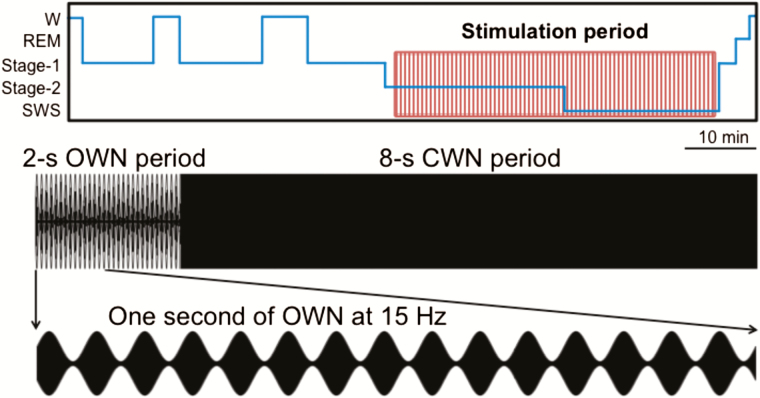
In the current study, we set out to test differences between the 2 primary spindle types using auditory stimulation that was amplitude modulated in the spindle frequency range—that is, using oscillating white noise (OWN). While participants took an afternoon nap in the laboratory, we initiated continuously repeating 10-s stimulation intervals upon online indications of stage 2 and SWS (Figure 1). These stimulation intervals included 2 s of OWN at a slow spindle frequency (12 Hz), a fast spindle frequency (15 Hz), or a non-spindle control frequency (50 Hz), followed by 8 s of constant white noise (CWN). We then measured spindles as a function of stimulation. We predicted that an increased number of fast and slow spindles would be preferentially produced during fast and slow stimulation, respectively. Furthermore, as EEG topographic evidence raises the possibility of different spindle generation mechanisms from prefrontal and centroparietal sources, we predicted that recordings from clusters of prefrontal versus centroparietal scalp electrodes would be differentially affected by the stimulation.
Figure 1.
Stimulation parameters and spindle analysis approach. Participants took a 90-min nap amidst a background of white noise. While they slept, 2-s periods of oscillating white noise (OWN) alternated with 8-s periods of constant white noise (CWN). An expanded, 1-s example of 15-Hz OWN is shown.
MATERIALS AND METHODS
Participants
Participants were recruited from the university community and randomly assigned to 1 of 3 conditions: 12 Hz stimulation (11 participants, 7 female), 15 Hz stimulation (11 participants, 7 female), and 50 Hz stimulation (10 participants, 6 female). The mean age was 21.3 years (range 18–25 years) and did not differ across conditions [F(2,29) < 1]. Seven other participants were excluded for not sleeping long enough for sufficient stimulation (defined as a minimum of 50 trials). There were no differences in the number of stimulations received across conditions (12 Hz: 327.1 ± 29.2, 15 Hz: 295.3 ± 26.2, 50 Hz: 272.6 ± 40.7; p > .2, all).
Stimuli
Oscillating sounds were created by modulating the amplitude of a white noise signal (42 dB) created by mixing sound frequencies from 20 to 1000 Hz with random amplitudes constant across the power spectrum (Figure 1A).13 The modulated sound alternated between 100% and 20% of its original amplitude in the form of a sine wave (Audacity software, Tremolo function). Modulations at 12 Hz, 15 Hz, or 50 Hz were made on the first 2 s of a 10-s white noise signal, such that each 2-s period of OWN was followed by 8 s of CWN.
Design
Participants napped with repeating 2-s on, 8-s off iterations of an amplitude-modulated sound that began in stage 2 sleep. The analytical approach involved comparing spindles starting during versus after the stimulation period on the following measures: density, frequency, duration, and power across electrodes.
Procedure
Participants arrived in the laboratory between 12 and 4 pm for a 2-h nap. They were asked to wake up 1 h earlier than normal the morning of the experiment to increase the chances of falling asleep in the afternoon. They were also asked to refrain from drinking alcohol the night before and from ingesting caffeine the morning of the experiment. Informed consent was obtained in advance and monetary reimbursement was provided after the experiment. Each participant was fitted with an EEG cap, and then reclined in a bed in a quiet, darkened room to try to fall asleep. The experimenter continuously monitored EEG recordings from an adjacent room to determine sleep stage (verified later using standard methods; see below). When 2 min of either stage 2 sleep or SWS was detected, trials of OWN stimulation were initiated. These trials were repeated until the experimenter determined that the participant was no longer in non-rapid eye movement (NREM) sleep. If a period of arousal from sleep was detected, OWN began again when 2 min of NREM sleep was detected. After 90 min, participants were awoken, debriefed about the nature of the experiment, and electrodes were removed. During debriefing, participants were informed about the auditory stimulation during their sleep, which they uniformly claimed to not have noticed.
EEG Acquisition and Analysis
Continuous EEG data were acquired at a sampling rate of 1000 Hz with a bandpass of 0.1–100 Hz. Tin electrodes in an elastic cap were placed at 21 standard scalp locations (FPz, Fz, Cz, Pz, Oz, FP1/2, F3/4, F7/8, C3/4, P3/4, T3/4, T5/6, and O1/2), left and right mastoids, lateral to the right eye, under the left eye, and on the chin. Data were processed offline using a combination of internal functions in EEGLAB14 and custom written scripts. Data were downsampled to 250 Hz, re-referenced to average mastoids, and filtered at 0.4–60 Hz in successive steps using a 2-way least-squares finite impulse response filter (“eegfilt” function).
Sleep stages were determined by an expert scorer according to standard criteria.15 Artifacts (large movements, blinks, arousals, and rare, large deflections in single channels) during sleep were marked separately in 5-s intervals following sleep staging.
Spindles, SOs, power spectral density measures, and continuous, Hilbert-transformed SO phase and power values were calculated using established algorithms. Each algorithm ignored 5-s intervals marked for rejection.
For spindle analyses, sleep EEG data were bandpass-filtered between 11 and 16 Hz using a 2-way least-squares finite impulse response filter and a root mean square (RMS) value was calculated using a sliding window of 148 ms for each channel separately.8 Each window thus included 37 time points (4 ms apart) and was attributed to the middle time point. A threshold was determined by multiplying the standard deviation of the entire channel’s filtered signal by 1.5. A spindle was counted whenever the RMS signal crossed this threshold continuously for 0.5 to 2 s.8 Other characteristics were calculated: spindle length (time above threshold), mean frequency (number of peaks divided by spindle length), and power (integral of RMS curve above threshold). Times for the start, negative peak (largest negative amplitude), and end of each spindle were recorded for alignment with stimulation. Spindles between 11 and 13.5 Hz were classified as slow spindles and those between 13.5 and 16 Hz were classified as fast spindles.
For analyzing SOs, sleep EEG data from stages 2 and 3 were first low-pass filtered at 3.5 Hz. A SO was counted when any series of data points included a negative peak of at least −40 μV, a peak-to-peak amplitude of at least 75 μV, and successive positive-to-negative crossings from 0.75 to 2 s, corresponding to 0.5–1.3 Hz (similar to16). For each detected SO, other characteristics were calculated: length (time between positive-to-negative and negative-to-positive zero crossings), peak-to-peak amplitude (difference between largest and smallest values), and slope (voltage change over time between the negative peak and the negative-to-positive zero crossing). Similar to spindles, the start, negative peak, and end of each SO were recorded for later alignment with stimulation. SO–spindle complexes were determined when spindles began within 0.5 s of the negative SO peak. As SOs in stages 2 and 3 either reflect the same underlying physiology or are too similar to distinguish,17 we did not set different thresholds for stage 2 and 3 spindles.16,18
Spindle density is conventionally reported as number of spindles per min. Previous evidence suggested major topographical differences in slow and fast spindle density, which we verified for spindles starting during CWN periods (Figure S1). As a crucial aim was to investigate whether effects of stimulation on spindle density varied as a function of spindle type, our analyses emphasized a cluster of 3 frontopolar electrodes (Fp1, Fpz, and Fp2) and a cluster of 3 parietal electrodes (P3, Pz, and P4).
We calculated a series of measures to approximate instantaneous fast and slow spindle density across each trial. We first counted the number of fast and slow spindles present for each 100-ms bin of each 10-s stimulation period. Total spindle counts across all stimulation intervals were then converted to instantaneous density by multiplying by 6 [60 (s / min) / 10 (s / trial)] and dividing by the total number of stimulation periods for that participant. When a spindle began in one 10-s period and ended in another, additions carried over into the next cue period and ended when the spindle ended with respect to the cue (e.g., if a spindle began 9.9 s into the 10-s period and lasted 0.7 s into the next period, then 1 spindle count was included for each 100-ms bin between 9.9 and 0.7 s). We contrasted each 100-ms bin against a baseline average computed using data from 3 to 9 s post-OWN onset, reasoning that spindles during this period were not likely to have been generated due to the prior period of OWN stimulation.
Finally, we assessed whether spindle boosts could be artifacts of the OWN due merely to direct auditory synchronization (as in the auditory steady-state response or ASSR). We conducted fast Fourier transform analyses on 2-s segments of OWN and all 2-s segments of CWN that did not contain spindles (e.g., 2–4 s, 4–6 s, 6–8 s, and 8–10 s post-OWN onset). These analyses involved computing power using the fast Fourier transform within 1-Hz windows around the matching stimulation frequency for each condition (i.e., 11.5–12.5 Hz for 12 Hz stimulation; 14.5–15.5 Hz for 15 Hz stimulation). For the topographical similarity analyses, we ran correlations between z-scored spindle boosts and ASSR boosts, in both cases using comparisons between OWN versus CWN across electrodes.
RESULTS
Auditory Oscillations Increased Spindle Incidence Relative to Control Sounds in a Frequency-Specific Manner
To determine the impact of auditory oscillations on spindle incidence, we contrasted spindle incidence during OWN and CWN periods. To analyze spindle timing, we grouped spindles by their start time (i.e., the moment the bandpass-filtered RMS signal crossed a specific threshold; see Materials and Methods).
We predicted that OWN stimulation would increase spindles of matching frequencies. To test this prediction, we categorized results according to whether OWN stimulation modulated spindles at the frequency that matched or did not match the OWN frequency. Slow spindles in the 12 Hz condition and fast spindles in the 15 Hz condition were considered matching, whereas fast spindles in the 12 Hz condition and slow spindles in the 15 Hz conditions were considered non-matching. Furthermore, to ask whether spindle modification by OWN differed topographically, we focused analyses on a frontopolar and parietal cluster of electrodes during the CWN (non-stimulation) period (Figure S1). We analyzed data from each cluster separately.
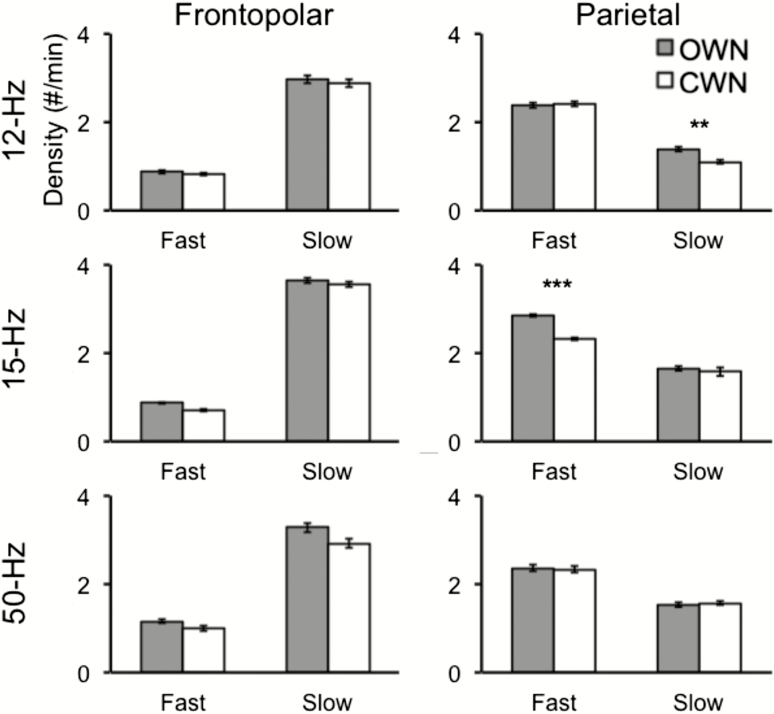
As shown in Figure 2, OWN increased spindles for matching frequencies only in the parietal cluster. We submitted spindle density measures to a 2 (match status: matching vs. non-matching) × 2 (sound type: OWN vs. CWN) ANOVA for each electrode cluster. In the frontopolar cluster, we found no significant main effects or interactions [F(1, 21) < 2.4, p > .1, all]. In the parietal cluster, we found a significant main effect for sound type [OWN: 2.06 ± 0.95; CWN: 1.9 ± 0.88, F(1,21) = 11.1, p = .003], indicating that there were more spindles during OWN than CWN. The main effect for match status was not significant [F(1, 21) < 1], whereas the interaction between match status and sound type was significant [F(1, 21) = 31.9, p < .001]. Thus, the OWN-based spindle increase differed according to whether the OWN frequency matched the spindle frequency. Indeed, spindles matching the OWN frequency were significantly more prevalent during OWN than during CWN [2.12 ± 1.14 vs. 1.71 ± 0.97, respectively; t(21) = 6.37, p < .001], whereas there was no difference for non-matching frequencies [2.01 ± 0.76 vs. 1.99 ± 0.79, respectively; t(21) = 0.24, p = .81]. Confirming this effect on spindles for each stimulation frequency separately, slow spindles significantly increased during 12 Hz OWN compared to CWN [1.38 ± 0.16 vs. 1.10 ± 0.13, respectively; t(10) = 3.16, p = .01], and fast spindles significantly increased during 15 Hz OWN compared to CWN [2.84 ± 0.34 vs. 2.32 ± 0.30, respectively; t(10) = 6.83, p < .001]. OWN spindle increases were comparable in the 2 conditions, computed as percentage increase, with a 25.4% increase with 12 Hz stimulation and a 22.4% increase with 15 Hz stimulation.
Figure 2.
Oscillating white noise (OWN) increased spindles relative to constant white noise (CWN) in a frequency-specific manner. For the parietal electrode cluster (right column), 12 and 15 Hz stimulation enhanced slow and fast spindles, respectively. For both electrode clusters, 50 Hz stimulation showed no significant changes in spindle density. Error bars denote SEM. **p < .01, ***p < .001.
Finally, as 50 Hz OWN did not match either spindle frequency, we tested its impact on spindles separately for each cluster and spindle frequency. The 50 Hz OWN stimulation did not increase spindles relative to CWN in either cluster or spindle frequency [t(9) < 0.41, p > .6].
The above analysis demonstrates stimulation has a topographically specific effect on spindles. To test across-cluster differences directly, we computed a spindle-boost effect for each electrode cluster and participant as the increase in spindles matching the OWN oscillation frequency during OWN versus CWN [(matching-frequency spindle density during OWN − matching-frequency spindle density during CWN) − (non-matching-frequency spindle density during OWN − non-matching-frequency spindle density during CWN)]. A paired t-test showed that the spindle-boost effect was significantly greater for the parietal cluster (0.39 ± 0.07) than the frontopolar cluster [0.05 ± 0.10; t(21) = 2.89, p = .008].
SOs Did Not Drive the Spindle Enhancement Effect
Auditory stimuli during sleep often elicit SOs. SOs occur during SWS as well as stage 2 sleep, during which they are typically referred to as K-complexes.19 We investigated how OWN affected SO incidence during NREM sleep by contrasting the density of SOs (SOs/min) that began in OWN versus CWN periods (i.e., when voltage crossed from positive to negative). We focused on the parietal cluster, as it showed modulation by OWN. The parietal cluster exhibited higher SO density during OWN than CWN in both the 12 Hz condition [5.05 ± 1.61 vs. 4.84 ± 1.62 SOs/min, t(10) = 2.86, p = .01] and the 15 Hz condition [4.56 ± 1.07 vs. 4.21 ± 1.04 SOs/min, t(10) = 2.52, p = .03], but not the 50 Hz condition [4.20 ± 1.61 vs. 4.19 ± 1.61 SOs/min, t(9) < 0.1, p = 0.96].
As spindles frequently occur during the SO upstate,8 we next investigated whether OWN-related spindles were preferentially associated with induced SOs. We separately measured the density of parietal spindles occurring with accompanying SOs and without them. In the 12 Hz condition, there was no OWN-related increase for SO–slow spindle complexes [0.16 ± 0.07 vs. 0.14 ± 0.05; t(10) < 0.6, p > .6], but there was for slow spindles without SOs [1.22 ± 0.23 vs. 0.95 ± 0.11; t(10) = 2.86, p = .01]. In the 15 Hz group, there was a significant OWN-related increase for SO–fast spindle complexes [0.35 ± 0.07 vs. 0.25 ± 0.07; t(10) = 2.4, p = .03] and for fast spindles without SOs [2.49 ± 0.32 vs. 2.07 ± 0.27; t(10) = 5.0, p < .001]. These results demonstrate that induced SOs were not necessary for spindle enhancement effects. Though SOs occurred far more commonly in the frontopolar cluster [across both conditions—frontopolar SO density: 9.58 ± 1.51; parietal SO density: 4.84 ± 0.96, t(10) = 6.06; p < .001], consistent with the known frontal predominance of slow waves,19 frontopolar SOs and SO–spindle complexes were not more common during OWN than CWN in either condition [t(10) < 2.05, p > .05, all].
Spindles Were Reliably Influenced After One Second of OWN
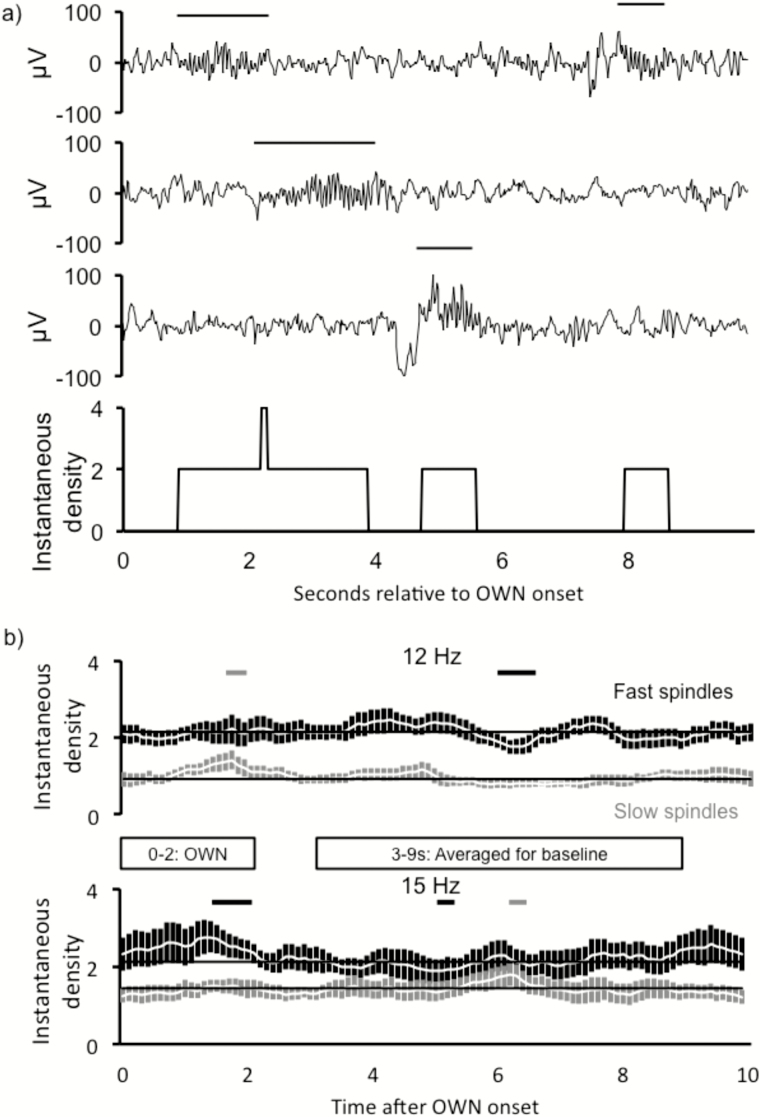
To investigate the precise timing of spindle incidence with respect to OWN onset, we found the instantaneous spindle density for each 100-ms time bin of each trial (with trial defined as the 10-s on–off period locked to the OWN onset). Essentially, this measure probes whether a spindle is present within each time bin on each trial and normalizes these totals by the number of trials for each participant (see Materials and Methods and Figure 3A for more details).
Figure 3.
Frequency-specific spindle increases occurred most strongly between 1 and 2 s of oscillating white noise (OWN) onset. (A) Method for calculating instantaneous spindle density relative to OWN onset. Three sample traces are shown with spindles occurring at different times relative to OWN onset at time 0. For each 100-ms interval during a given 10-s period, the number of spindles counted in the interval (spindles either starting during the interval or continuing into the interval) was converted into an instantaneous spindle density measure. (B) Significant frequency-specific increases manifested during 1–2 s post-OWN onset. Fast and slow spindle counts are presented in light and dark gray, respectively. Color-coded horizontal significance bars signify times during which spindle counts deviated from baseline (average count during 3–9 s post-cue).
As shown in Figure 3B, OWN frequency–specific spindle increases typically occurred 1–2 s into the trial (pair-wise p values remained below 0.05 for the following post OWN-onset intervals: 12 Hz slow spindles: 1.2–1.7 s; 15 Hz fast spindles: 1.2–1.8 s). This analysis revealed no significant increases just before the OWN sound or between 0 and 1 s post-OWN-onset, though the 15Hz group had at least 200 ms of marginal values during both intervals.
Additionally, we found effects around 5.5–6.5 s for spindles of non–OWN-like frequencies. Slow spindles in the 15 Hz condition significantly increased (between 5.92 and 6.34 s) and fast spindles in the 12Hz condition significantly decreased (between 5.83–6.51 s). Unlike the effects at 1–2 s, these later effects were not predicted in advance.
Comparable Spindles With Versus Without Stimulation
We next asked whether spindles during OWN and CWN differed in any basic characteristics. We created independent contrasts for spindle frequency, duration, and power at the parietal cluster. As expected, we saw a significant decrease in frequency in the 12 Hz condition [t(10) = –3.7, p = .004], a marginal increase in frequency in the 15 Hz condition [t(10) = 1.7, p = .13], and a significant interaction between the 2 groups [F(1, 20) = 13.0, p = .002]. No significant differences were found for either duration or power (Table S1). There was a marginal trend for a decrease in duration in the 15 Hz condition for spindles during OWN than CWN [t(10) = –1.9, p = .09; there was no relationship in the 12 Hz condition (t(10) = –0.2, p > .8)]. We also analyzed topographic power measures across all electrodes, with average power for spindles beginning during each period at each electrode submitted to a 21 (electrodes) × 2 (sound type: OWN vs. CWN) ANOVA. We used fast spindle power in the 15 Hz condition and slow spindle power in the 12 Hz condition. We found no significant main effects or interaction (all F < 3.5, p > .1; Figure S2). The topography of spindle power during OWN periods, when spindles were enhanced, was thus indistinguishable from that during CWN periods.
OWN did not Produce Large Frequency-Specific EEG Responses During Periods Without Spindles
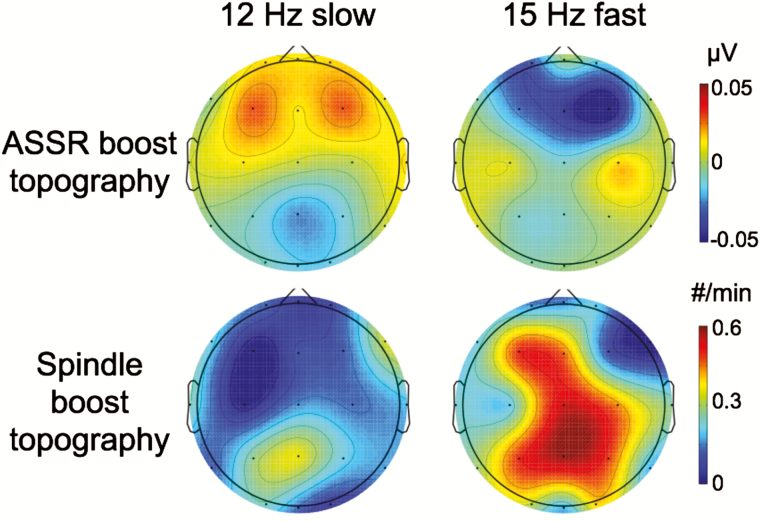
An alternative conceptualization of the above results is that the apparent spindle increase merely reflected a summation of subthreshold spindle responses with auditory entrainment at the precise stimulation frequency (i.e., steady-state responses). If this scenario were operative, one would expect to observe an increase in EEG power at the OWN frequency during OWN intervals. We thus focused an analysis on OWN and CWN intervals that did not contain spindles. We did not find significantly greater frequency-specific responses for OWN than CWN in either condition at any electrode [t(10) < 2, p > .07, all, uncorrected]. Furthermore, the topography of the ASSR bore no similarity to that of the spindle boost effect (r < 0.3, p > .3, both; Figure 4).
Figure 4.
Auditory steady-state response (ASSR) analyses revealed no stimulus-evoked power differences for corresponding frequencies. (Top) Plotted are ASSR differences for 2-s segments of OWN − CWN without spindles. No electrodes showed significant differences between OWN and CWN. (Bottom) Spindle boost topographies are presented as a comparison to ASSR results. No cross-electrode topographical correlations were found between the ASSR and spindle boost effect for either condition.
Sleep Architecture and Spindle Densities did not Differ as a Function of Stimulation Frequency
We asked whether there were differences in sleep architecture among conditions in the present experiment. No differences were found in any sleep stage [Table S2; F(2, 31) < 1.1, p > .37, all stages]. Likewise, fast and slow spindle density did not differ among conditions [Table S2; F(2, 31) < 1.1, p > .35, all types and clusters].
DISCUSSION
We showed that human sleep spindles can be altered in a frequency-specific and temporally precise manner using an acoustic resonance method that is non-pharmacological and non-invasive. In response to 2-s periods of auditory oscillatory stimulation at 12 and 15 Hz, corresponding slow and fast spindle boosts relative to control periods were observed in EEG recordings from parietal scalp locations, but not frontal scalp locations. Whereas oscillating sounds also increased SOs, spindle boosts did not rely on these increases.
The finding that parietal slow spindles were preferentially increased may seem surprising, because slow spindles are most prevalent over frontal scalp regions.4 However, data from intracranial electrodes in epilepsy patients suggest that a simple dichotomous mapping of slow=frontal and fast=parietal is an oversimplification—spindles within the frontal lobe were preferentially slow, whereas spindles within the parietal lobe showed a broad distribution of faster and slower frequencies.6 Accordingly, parietal scalp recordings are likely sensitive to both slow and fast spindles, such that OWN stimulation could enhance both slow parietal spindles and fast parietal spindles.
It remains unresolved whether slow and fast spindles share a common generating mechanism or depend on unique mechanisms with multiple qualitative differences.1,10 However, the present results favor the latter possibility, given that spindles at parietal locations were sensitive to stimulation at both frequencies, whereas spindles at frontal locations were sensitive to neither type of stimulation. Other sources of evidence also support this conceptualization of spindles. In a combined EEG/fMRI study, slow and fast spindles appeared to differentially engage different areas of the brain, with only fast spindles recruiting the hippocampus.5 The 2 types also differ in their relationship to SOs, as fast spindles are preferentially linked with the SO upstate, when hippocampal ripples occur.6,8 These differences align well with findings that fast spindles more typically correlate with declarative memory measures than slow spindles18,20,21 (though see22). Additionally, the same pharmacological manipulation can differentially modulate the 2 types of spindles, with concurrent increases in slow and decreases in fast spindle activity.9
Our finding that spindles from the parietal EEG cluster were preferentially sensitive to stimulation adds further support to a qualitative difference between frontal and parietal spindle types. At the same time, the results conflict with the generalization that slow spindles are uniquely frontal and fast spindles uniquely parietal.
Many lines of evidence implicate sleep spindles in memory and plasticity. On the cellular level, spindles elicit calcium neuronal influx, which is necessary for long-term potentiation (LTP),2 and spindle-like trains of neuronal activity increase LTP.23 On a systems level, spindles overlap with ripple events in the hippocampus24–26 as well as blood oxygen level–dependent activity in the medial temporal lobe5,27 and neocortical regions,28 possibly indicative of a unique opportunity for hippocampal–neocortical communication to aid memory consolidation.29 Accordingly, spindles increase after learning within subjects and correlate positively with memory measures.30–34 Additionally, drugs that increase spindles improve memory.11,12 Methods aimed at directly altering spindles—such as auditory oscillations, transcranial electrical stimulation, or optogenetics in non-human animals—may open the door to real-time, causal evidence for the spindle–memory link and help to elucidate the relevant neural mechanisms.
The current results importantly add to other aspects of the literature on sleep spindles. In particular, our results relate to 3 sources of evidence suggesting that spindles protect organisms from awakening.
First, neuronal responses to stimuli are dampened during spindles in EEG35–37 and fMRI.38,39 These suppressions may reflect a type of sensory gating that inhibits the sensory relay centers in the thalamus, as the thalamic reticular nucleus (RT) neurons interact with thalamocortical neurons needed to relay visual, auditory, and somatosensory signals to the cortex.40 In the present study, OWN had its greatest effect on spindles after 1 s, not immediately. This finding suggests that OWN primarily influenced the spindle generation process rather than the ongoing architecture of sleep spindles, perhaps because responses to stimuli are dampened during spindles.
Second, various results show that spindles lessen the likelihood of arousal. Stronger acoustic stimuli are needed to arouse subjects during spindles.35 Individuals with higher spindle thresholds have elevated arousal thresholds.41 Hypersomnia patients have elevated spindle counts.42 Insomnia drugs that boost spindles increase arousal thresholds in humans.43 Genetically manipulated mice with greater spindle rates show elevated arousal thresholds.44 Finally, frequent optogenetic stimulation of the RT at spindle frequencies boosts spindles45 and in turn extends sleep.46 Though much remains to be learned about the mechanisms that OWN stimulation procedures put into motion, we speculate that the auditory oscillations may enhance spindles via repetitive RT burst firing as in the aforementioned optogenetic study.
Third, spindles increase in response to visual, auditory, and somatosensory stimuli.37,47 These findings suggest that sleep spindles may act to suppress continued responses to stimuli to prevent arousals. The present procedures diverge from those generally used to show spindle increases to sensory stimulation, as OWN constitutes a decrease in the overall auditory stimulation amplitude. The frequency-specific response to OWN demonstrates that spindle generation mechanisms respond to external stimuli in a nuanced manner.
Although the present results offer new opportunities to non-invasively and non-pharmacologically manipulate sleep spindles, a few caveats should be mentioned. First, although oscillating sounds increased spindles relative to control periods, it remains to be seen whether repeated stimulation could increase overall spindle density relative to that in a sleep period with no stimulation at all. The results provide a convincing proof-of-concept demonstration but do not include all possible control comparisons, and thus we infer that OWN stimulation influenced spindle prevalence in a relative manner, during discrete periods of time, rather than producing a global enhancement of spindles. Although CWN stimulation closely resembles a condition of no stimulation at all (given that some external noise is practically unavoidable), it remains possible that a period of spindle refractoriness might generally follow stimulation so as to negate global effects.2,48,49 Second, although the basic features of spindles did not differ for those that onset during OWN versus those that onset during CWN, spontaneous spindles during oscillatory stimulation may have masked weak differences between induced and spontaneous spindles. Third, our data cannot speak to other possible functional differences such as thalamic bursting patterns or the alignment between spindles and hippocampal sharp-wave ripple complexes theorized to underlie declarative memory consolidation.26,50 Evidence from other recording methodologies may be highly informative, in that intracranial spindles are often locally rather than globally distributed6,51 and magnetoencephalographic spindles have multiple asynchronous generators52 and complex connectivity patterns.53 Fourth, although the changes in spindle prevalence observed between 5.5 and 6.5 s after OWN onset were not predicted, it will be interesting to see whether they replicate in future experiments. Finally, our study leaves many intriguing questions unanswered: Do stimulation effects extend to nocturnal sleep? What results would be obtained if multiple stimulation frequencies were used in sequence? What other types of oscillating stimulation can be used? Do effects persist over multiple sessions? Does this sort of stimulation produce any negative consequences? And how might spindle stimulation be combined with the presentation of learning-related stimuli that reactivate memories during sleep?54–57
The present demonstration of enhanced spindles provides new insights into spindle subtyping as well as into how external stimulation can influence spindles. Future use of spindle-manipulation methods could produce additional causal evidence on the role of spindles in cognitive function. This line of experimentation could also help to elucidate mechanisms of spindle generation, potentially contributing to the understanding and treatment of populations with abnormally infrequent spindles, such as people with schizophrenia,58,59 people with dyslexia,60 or the elderly.1,61
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
FUNDING
This work was supported by NIH grant T32-AG020418, F31-MH100958, and CV Starr Fellowship (JWA), and a Faculty Research Grant from the University Research Grants Committee at Northwestern University (KAP).
INSTITUTION AT WHICH THE WORK WAS PERFORMED
Northwestern University.
OFF-LABEL OR INVESTIGATIONAL USE
None.
CLINICAL TRIAL
None.
DISCLOSURE STATEMENT
None declared.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Julia Mossbridge for help with generating the auditory stimuli, Jinkyung (Julie) Bae for help with data collection, and Laura Batterink for insightful comments on the manuscript. This work comprised a chapter of JWA’s dissertation, originally published in January, 2015. JWA and KAP designed the research. JWA performed the research and analyzed the data. JWA and KAP wrote the paper.
REFERENCES
- 1. De Gennaro L, Ferrara M. Sleep spindles: an overview. Sleep Med Rev. 2003; 7(5): 423–440. [DOI] [PubMed] [Google Scholar]
- 2. Astori S, Wimmer RD, Lüthi A. Manipulating sleep spindles–expanding views on sleep, memory, and disease. Trends Neurosci. 2013; 36(12): 738–748. [DOI] [PubMed] [Google Scholar]
- 3. Gibbs F, Gibbs E. An Atlas of Electroencephalography. Vol 1 Cambridge: Addison-Wesley Press; 1950. [Google Scholar]
- 4. Anderer P, Klösch G, Gruber G, et al. Low-resolution brain electromagnetic tomography revealed simultaneously active frontal and parietal sleep spindle sources in the human cortex. Neuroscience. 2001; 103(3): 581–592. [DOI] [PubMed] [Google Scholar]
- 5. Schabus M, Dang-Vu TT, Albouy G, et al. Hemodynamic cerebral correlates of sleep spindles during human non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2007; 104(32): 13164–13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andrillon T, Nir Y, Staba RJ, et al. Sleep spindles in humans: insights from intracranial EEG and unit recordings. J Neurosci. 2011; 31(49): 17821–17834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peter-Derex L, Comte JC, Mauguière F, Salin PA. Density and frequency caudo-rostral gradients of sleep spindles recorded in the human cortex. Sleep. 2012; 35(1): 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mölle M, Bergmann TO, Marshall L, Born J. Fast and slow spindles during the sleep slow oscillation: disparate coalescence and engagement in memory processing. Sleep. 2011; 34(10): 1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ayoub A, Aumann D, Hörschelmann A, et al. Differential effects on fast and slow spindle activity, and the sleep slow oscillation in humans with carbamazepine and flunarizine to antagonize voltage-dependent Na+ and Ca2+ channel activity. Sleep. 2013; 36(6): 905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rasch B, Born J. About sleep’s role in memory. Physiol Rev. 2013; 93(2): 681–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaestner EJ, Wixted JT, Mednick SC. Pharmacologically increasing sleep spindles enhances recognition for negative and high-arousal memories. J Cogn Neurosci. 2013; 25(10): 1597–1610. [DOI] [PubMed] [Google Scholar]
- 12. Mednick SC, McDevitt EA, Walsh JK, et al. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013; 33(10): 4494–4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sherman A, Grabowecky M, Suzuki S. Auditory rhythms are systemically associated with spatial-frequency and density information in visual scenes. Psychon Bull Rev. 2013; 20(4): 740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004; 134(1): 9–21. [DOI] [PubMed] [Google Scholar]
- 15. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Scoring Stages of Human Subjects. Bethesda, MD: U.S. Department of Health, Education and Welfare, Public Health Services; 1968. [Google Scholar]
- 16. Mölle M, Born J. Slow oscillations orchestrating fast oscillations and memory consolidation. Prog Brain Res. 2011; 193: 93–110. [DOI] [PubMed] [Google Scholar]
- 17. Cash SS, Halgren E, Dehghani N, et al. The human K-complex represents an isolated cortical down-state. Science. 2009; 324(5930): 1084–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ngo HV, Martinetz T, Born J, Mölle M. Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron. 2013; 78(3): 545–553. [DOI] [PubMed] [Google Scholar]
- 19. Halász P. K-complex, a reactive EEG graphoelement of NREM sleep: an old chap in a new garment. Sleep Med Rev. 2005; 9(5): 391–412. [DOI] [PubMed] [Google Scholar]
- 20. Ngo HV, Miedema A, Faude I, Martinetz T, Mölle M, Born J. Driving sleep slow oscillations by auditory closed-loop stimulation-a self-limiting process. J Neurosci. 2015; 35(17): 6630–6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saletin JM, Goldstein AN, Walker MP. The role of sleep in directed forgetting and remembering of human memories. Cereb Cortex. 2011; 21(11): 2534–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lustenberger C, Wehrle F, Tüshaus L, Achermann P, Huber R. The multidimensional aspects of sleep spindles and their relationship to word-pair memory consolidation. Sleep. 2015; 38(7): 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rosanova M, Ulrich D. Pattern-specific associative long-term potentiation induced by a sleep spindle-related spike train. J Neurosci. 2005; 25(41): 9398–9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mölle M, Yeshenko O, Marshall L, Sara SJ, Born J. Hippocampal sharp wave-ripples linked to slow oscillations in rat slow-wave sleep. J Neurophysiol. 2006; 96(1): 62–70. [DOI] [PubMed] [Google Scholar]
- 25. Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998; 21(5): 1123–1128. [DOI] [PubMed] [Google Scholar]
- 26. Clemens Z, Mölle M, Eross L, et al. Fine-tuned coupling between human parahippocampal ripples and sleep spindles. Eur J Neurosci. 2011; 33(3): 511–520. [DOI] [PubMed] [Google Scholar]
- 27. Andrade KC, Spoormaker VI, Dresler M, et al. Sleep spindles and hippocampal functional connectivity in human NREM sleep. J Neurosci. 2011; 31(28): 10331–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bergmann TO, Mölle M, Diedrichs J, Born J, Siebner HR. Sleep spindle-related reactivation of category-specific cortical regions after learning face-scene associations. Neuroimage. 2012; 59(3): 2733–2742. [DOI] [PubMed] [Google Scholar]
- 29. Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010; 11(2): 114–126. [DOI] [PubMed] [Google Scholar]
- 30. Eschenko O, Mölle M, Born J, Sara SJ. Elevated sleep spindle density after learning or after retrieval in rats. J Neurosci. 2006; 26(50): 12914–12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gais S, Mölle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. J Neurosci. 2002; 22(15): 6830–6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heib DP, Hoedlmoser K, Anderer P, Gruber G, Zeitlhofer J, Schabus M. Oscillatory theta activity during memory formation and its impact on overnight consolidation: a missing link? J Cogn Neurosci. 2015; 27(8w): 1648–1658. [DOI] [PubMed] [Google Scholar]
- 33. Johnson LA, Blakely T, Hermes D, Hakimian S, Ramsey NF, Ojemann JG. Sleep spindles are locally modulated by training on a brain-computer interface. Proc Natl Acad Sci USA. 2012; 109(45): 18583–18588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Studte S, Bridger E, Mecklinger A. Nap sleep preserves associative but not item memory performance. Neurobiol Learn Mem. 2015; 120: 84–93. [DOI] [PubMed] [Google Scholar]
- 35. Yamadori A. Role of the spindles in the onset of sleep. Kobe J Med Sci. 1971; 17(3): 97–111. [PubMed] [Google Scholar]
- 36. Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993; 262(5134): 679–685. [DOI] [PubMed] [Google Scholar]
- 37. Cote KA, Epps TM, Campbell KB. The role of the spindle in human information processing of high-intensity stimuli during sleep. J Sleep Res. 2000; 9(1): 19–26. [DOI] [PubMed] [Google Scholar]
- 38. Dang-Vu TT, Bonjean M, Schabus M, et al. Interplay between spontaneous and induced brain activity during human non-rapid eye movement sleep. Proc Natl Acad Sci USA. 2011; 108(37): 15438–15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schabus M, Dang-Vu TT, Heib DP, et al. The fate of incoming stimuli during NREM sleep is determined by spindles and the phase of the slow oscillation. Front Neurol. 2012; 3: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Destexhe A, Sejnowski TJ. Interactions between membrane conductances underlying thalamocortical slow-wave oscillations. Physiol Rev. 2003; 83(4): 1401–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dang-Vu TT, McKinney SM, Buxton OM, Solet JM, Ellenbogen JM. Spontaneous brain rhythms predict sleep stability in the face of noise. Curr Biol. 2010; 20(15): R626–R627. [DOI] [PubMed] [Google Scholar]
- 42. Bové A, Culebras A, Moore JT, Westlake RE. Relationship between sleep spindles and hypersomnia. Sleep. 1994; 17(5): 449–455. [DOI] [PubMed] [Google Scholar]
- 43. Johnson LC, Hanson K, Bickford RG. Effect of flurazepam on sleep spindles and K-complexes. Electroencephalogr Clin Neurophysiol. 1976; 40(1): 67–77. [DOI] [PubMed] [Google Scholar]
- 44. Wimmer RD, Astori S, Bond CT, et al. Sustaining sleep spindles through enhanced SK2-channel activity consolidates sleep and elevates arousal threshold. J Neurosci. 2012; 32(40): 13917–13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Halassa MM, Siegle JH, Ritt JT, Ting JT, Feng G, Moore CI. Selective optical drive of thalamic reticular nucleus generates thalamic bursts and cortical spindles. Nat Neurosci. 2011; 14(9): 1118–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kim A, Latchoumane C, Lee S, et al. Optogenetically induced sleep spindle rhythms alter sleep architectures in mice. Proc Natl Acad Sci USA. 2012; 109(50): 20673–20678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sato Y, Fukuoka Y, Minamitani H, Honda K. Sensory stimulation triggers spindles during sleep stage 2. Sleep. 2007; 30(4): 511–518. [DOI] [PubMed] [Google Scholar]
- 48. Bal T, McCormick DA. What stops synchronized thalamocortical oscillations? Neuron. 1996; 17(2): 297–308. [DOI] [PubMed] [Google Scholar]
- 49. von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993; 261(5119): 361–364. [DOI] [PubMed] [Google Scholar]
- 50. Born J, Wilhelm I. System consolidation of memory during sleep. Psychol Res. 2012; 76(2): 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nir Y, Staba RJ, Andrillon T, et al. Regional slow waves and spindles in human sleep. Neuron. 2011; 70(1): 153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dehghani N, Cash SS, Rossetti AO, Chen CC, Halgren E. Magnetoencephalography demonstrates multiple asynchronous generators during human sleep spindles. J Neurophysiol. 2010; 104(1): 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zerouali Y, Lina JM, Sekerovic Z, et al. A time-frequency analysis of the dynamics of cortical networks of sleep spindles from MEG-EEG recordings. Front Neurosci. 2014; 8: 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Antony JW, Gobel EW, O’Hare JK, Reber PJ, Paller KA. Cued memory reactivation during sleep influences skill learning. Nat Neurosci. 2012; 15(8): 1114–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rudoy JD, Voss JL, Westerberg CE, Paller KA. Strengthening individual memories by reactivating them during sleep. Science. 2009; 326(5956): 1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rasch B, Büchel C, Gais S, Born J. Odor cues during slow-wave sleep prompt declarative memory consolidation. Science. 2007; 315(5817): 1426–1429. [DOI] [PubMed] [Google Scholar]
- 57. Bendor D, Wilson MA. Biasing the content of hippocampal replay during sleep. Nat Neurosci. 2012; 15(10): 1439–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ferrarelli F, Huber R, Peterson MJ, et al. Reduced sleep spindle activity in schizophrenia patients. Am J Psychiatry. 2007; 164(3): 483–492. [DOI] [PubMed] [Google Scholar]
- 59. Phillips KG, Bartsch U, McCarthy AP, et al. Decoupling of sleep-dependent cortical and hippocampal interactions in a neurodevelopmental model of schizophrenia. Neuron. 2012; 76(3): 526–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bruni O, Ferri R, Novelli L, et al. Sleep spindle activity is correlated with reading abilities in developmental dyslexia. Sleep. 2009; 32(10): 1333–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Crowley K, Trinder J, Kim Y, Carrington M, Colrain IM. The effects of normal aging on sleep spindle and K-complex production. Clin Neurophysiol. 2002; 113(10): 1615–1622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.