Abstract
Study Objectives:
Sleep during the biological night facilitates memory consolidation. Here we determined the impact of sleep and wake on motor skill learning (acquisition) and subsequent off-line skill improvement (memory consolidation), independent of circadian phase, and compared this to the impact of the endogenous circadian system, independent of whether sleep occurred during the biological night or day.
Methods:
Participants completed two 8-day sleep laboratory visits, adhering on one visit to a circadian aligned (“normal”) sleep schedule for the full duration of the protocol, and on the other to a circadian misaligned (12-hour inverted) schedule, with alignment during the first 3 days, a 12-hour ‘slam shift’ on Day 4, followed by circadian misalignment during the last 4 days of the protocol. Participants were repeatedly trained and tested on different versions of the finger-tapping motor sequence task across each visit.
Results:
Sleep facilitated offline memory consolidation regardless of whether it occurred during the biological day or night, while circadian phase had no significant impact. These sleep-related benefits remained after accounting for general motor speed, measured in the absence of learning. In addition, motor skill acquisition was facilitated when the training session followed shortly after sleep, without significant impact of circadian phase (biological morning vs. evening). This effect was largely driven by heightened acquisition in participants who slept during the day and were trained shortly thereafter, that is, when acquisition occurred during the biological evening. These benefits were also retained after controlling for general motor speed.
Conclusions:
Sleep benefits both the acquisition and consolidation of motor skill regardless of whether they occur during the biological day or night. After controlling for general motor speed, a critical adjustment that few studies perform, these sleep benefits remain intact. Our findings have clear implications for night shift workers who obtain their sleep during the day.
Keywords: Sleep, Circadian, Motor Skill, Memory, Acquisition, Consolidation.
Statement of Significance
There are distinct differences between the brain states associated with the diurnal and nocturnal circadian phase, which could influence how the brain processes memories. By having individuals sleep and remain wake under normal and inverted sleep schedules, the findings of this study clearly demonstrate that the neurobiology of sleep, not circadian influence, is the primary driver of enhanced motor skill learning and memory consolidation. From a public health perspective, this study highlights the mnemonic importance of sleep in individuals who may be concerned about the cognitive impact of intermittent sleep schedule inversion, as with shift workers or those who have need to sleep during the day (eg, sleep-deprived parents).
INTRODUCTION
After a new motor skill (eg, the finger-tapping motor sequence task [MST])1 is acquired, performance (measured as the number of correctly typed sequences) significantly improves across a night of sleep (ie, motor skill consolidation), whereas little to no improvement is observed across a day of wakefulness.1–5 However, the numerous studies showing this sleep-dependent benefit have employed research designs of convenience, in which participants sleep and stay awake during biologically attuned intervals (ie, the biological night and day, respectively). This design unfortunately cannot disentangle the biology associated with sleep and wake from that associated with the biological night and day, as regulated by the endogenous circadian system. One study design that has partially dissociated these biological states is the nap protocol, which has participants sleep for brief intervals during the biological day.6,7 These nap studies demonstrate that even relatively brief intervals of sleep can facilitate motor skill consolidation. However, in these studies the comparison is made between daytime nap and wake groups (ie, there were no corresponding night-time nap and wake groups), limiting the ability of such studies to fully parse the effects of circadian and sleep/wake conditions.
The mammalian circadian system is composed of the central pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus along with peripheral oscillators in virtually all tissues and organs of the body. Because the circadian system exerts biological effects across the 24-hour day, including effects on hormone concentrations,8–10 autonomic nervous system activity,10 metabolism,11 neurochemistry,12 mood13 and cognitive performance,14 its influence must be addressed in the context of memory research that makes the claim (often dogmatically) that it is the biology of sleep (not the circadian rhythm) that accounts for its effects on memory consolidation. To validate the claim that sleep (and not circadian biology) influences memory processing, sleep and wake each need to be evaluated at different circadian times (eg, evaluating the impact of sleep during the circadian day and the impact of wake during the circadian night). This would enable identification of their separate (or interacting) contributions to motor skill processing.
Scheduling sleep and wake at different circadian times also allows us to determine more clearly the impact of sleep/wake biology and circadian biology on task acquisition (ie, learning). Thus far, a preponderance of findings shows that motor skill acquisition is similar whether it occurs in the morning or evening,15,16 with exceptions.17,18 The findings, in aggregate, have been taken as a putative indication that preceding intervals of sleep/wake or biological day/night have a negligible impact on task acquisition and do not better prepare (or hinder) the brain from acquiring a task. Unfortunately, these studies cannot tell us whether the circadian system impacts task acquisition, because morning training in these cases always takes place after a full biological night of sleep and evening training occurs after a full biological day awake. Thus, the same difficulties inherent in the interpretation of motor skill consolidation effects extend to motor skill acquisition effects; participants in almost all studies slept during the biological night and stayed awake across the biological day.
To resolve this issue for both acquisition and consolidation, we assessed motor skill acquisition and consolidation in the morning and evening after participants had stayed awake during the day and slept at night as well as after participants had slept during the day and stayed awake at night.
Because of the possibility that individuals simply type faster at different circadian times and/or following long intervals of sleep or wake, we also examined “general motor (typing) speed” at each training and test session. We did this by having participants, at the end of each training and test session, perform a random sequence typing test during which no sequence learning takes place. We then introduced this non-learning version of the motor skill task as a covariate, thereby controlling for the influence of non-learning factors on the MST.
Overall, participants completed two 8-day in-laboratory visits. During one visit, participants adhered to a “normal” 11 pm–7 am sleep schedule. During the other visit their sleep schedule was inverted by 12hours, such that participants had a scheduled sleep episode during the biological day (11 am–7 pm) and stayed awake during the biological night.
In addition to providing basic insights into the sleep and circadian regulation of motor skill acquisition and consolidation, another benefit of this design is that it tests learning and memory consolidation under conditions that correspond to what night shift workers experience when their sleep schedules are inverted as a condition of their employment.
METHODS
Aspects of this study, designed to test separate hypotheses, have previously been published.19–21
Study Participants
Sixteen healthy participants (7 female/9 male, mean age = 28.4 ± 8.9 (SD), age range: 20–49; BMI = 24.5 ± 2.8) participated in this study. The sample was characterized by heterogeneous occupational and educational backgrounds, with all participants having at least completed high school. Participants completed a variable number of training and test sessions across the four conditions (NightSleep: mean age = 27.8 ± 9.8, 5 female/4 male; DayWake: mean age = 27.6 ± 9.2, 5 female/5 male; NightWake: mean age = 28.5 ± 9.2, 5 female/9 male; DaySleep: mean age = 29.0 ± 9.4, 5 female/8 male). Participants provided written informed consent. This study was approved by the Institutional Review Board of Brigham and Women’s Hospital, Boston.
Procedure
For 17 ± 3 days prior to each laboratory visit, participants adhered to a normal sleep schedule with an 8-hour sleep opportunity each night and with instructions to sleep from 11 pm to 7 am the night before both laboratory visits to facilitate acclimation to the laboratory sleep schedule.
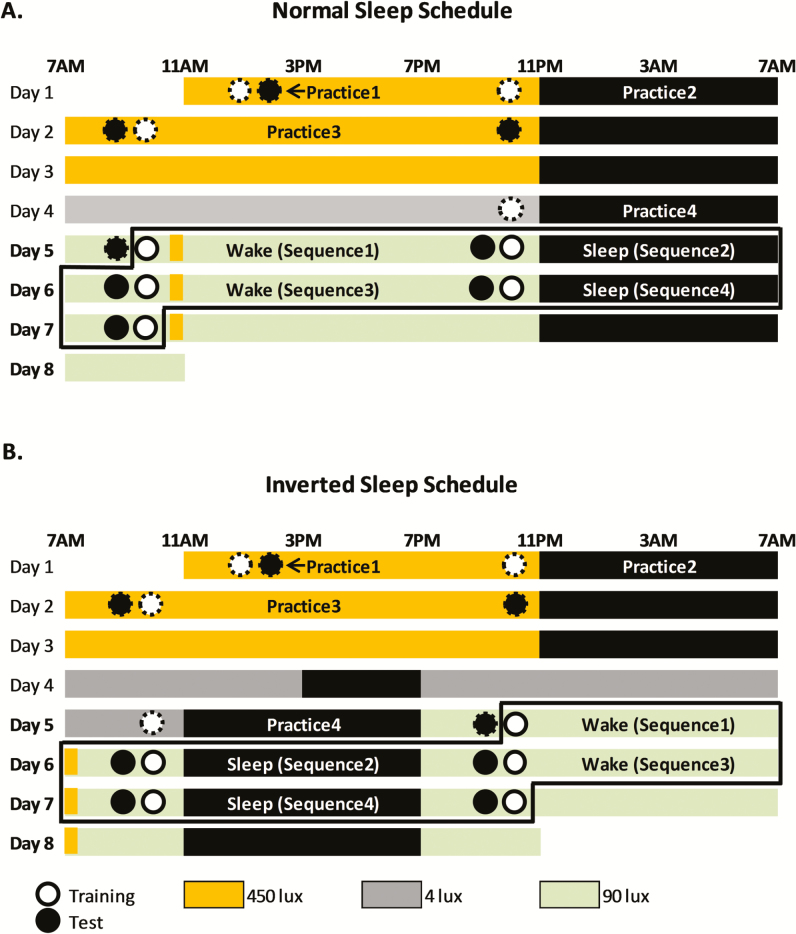
Participants remained in a designated room at the Center for Clinical Investigations at Brigham and Women’s Hospital for the duration of each of the two 8-day visits. During the circadian aligned (“normal sleep”) protocol, sleep was scheduled from 11 pm to 7 am on each of the 8 days, with MST testing (for analysis) occurring within the period from Day 5-Morning to Day 7-Morning (Figure 1). During the circadian misaligned (“inverted sleep”) protocol, sleep was scheduled from 11 pm to 7 am on days 1–3. On Day 4, participants remained awake until 3 pm, at which time they were given a 4-hour sleep opportunity. From the end of this sleep opportunity onwards, the participants remained on the 12-hour inverted sleep/wake schedule, with MST testing (for analysis) occurring within the period from Day 5-Night to Day 7-Night. The two visits were separated by 2–8 weeks (4 ± 2 [SD]), and the order of the circadian aligned and circadian misaligned protocols was randomized.
Figure 1.
Study Protocol. A. Normal sleep condition: Sleep was scheduled to occur 11 pm–7 am each night of the 8-day visit. B. Inverted sleep condition: On Days 1–3, was scheduled from 11 pm–7am. On the morning of Day 4, participants transitioned to a 12-hour inverted sleep schedule. On Days 5–8, the sleep opportunity was scheduled during the day and wakefulness at night. Each test session was always followed by a new training session, except for Practice 1, where test occurred immediately after training. Practice sequences are indicated by circles with dashed outline. MST sessions on Days 5–8 (circles with solid outline) were analyzed. After each training and test session, participants completed a random sequence typing test to assess motor performance in the absence of learning. The black box in 1A and 1B encompasses the training and test sessions that were analyzed for this study.
Light intensity during the scheduled wake episodes on the first 3 Days was 450 lux to facilitate circadian entrainment to the imposed sleep/wake cycle. Light levels were dim on Day 4 and Day 4–5 (in the Normal and Inverted Sleep protocol, respectively) to enable dim light melatonin assessments. On Day 5–8, during the scheduled wake episodes, light levels were 90 lux to simulate regular indoor light intensity during work shifts, with the exception of a brief 30-minute 450 lux light exposure to simulate the morning commute, in both the Normal and Inverted Sleep protocol. Lights were completely off during each sleep episode (Figure 1).
On Days 1–4, participants trained and tested on four “practice” MST sequences to ensure familiarity with the MST (see below). These were not included in any analyses. Data from the subsequent four MST sequences, on Days 5–8, were analyzed. See Supplementary Figure S1 for trial-by-trial performance across the four conditions for analyzed sequences (Days 5–8). In the normal sleep protocol, participants continued a nocturnal sleep schedule on Days 5–8, and were tested over biological nights of sleep (NightSleep) and biological days of wake (DayWake). In the inverted sleep protocol, after a 12-hour inversion of the participants’ sleep schedule, participants were tested over biological days of sleep (DaySleep) and biological nights of wake (NightWake).
Motor Sequence Task (MST)
The MST1 was used to assess motor skill acquisition (performance at the end of training) and consolidation (measured as delayed performance improvement across 12 hours without intervening practice; ie, reflecting “off-line processing”), further detailed below. During each training session participants typed a 5-digit sequence using the four fingers (excluding the thumb) of the non-dominant hand (eg, 4-1-3-2-4) during a series of twelve 30-s trials, with a 30-s rest period after each trial (thus each training session was completed in 12 minutes). Participants were instructed to repeatedly type the sequence as “quickly and accurately as possible.” At the testing session, always 12 hours later, participants again performed 12 trials of the same sequence, to test motor skill consolidation. Ten minutes after completing each test session, participants were trained on a new MST sequence. To assess general typing speed (independent of learning), a 1-minute random number typing test, using the same 4 fingers, was performed 5 minutes after each 12-trial training session and each 12-trial test session, in which participants typed a series of 5-digit random number sequences. Familiarization with the MST occurred on Days 1–4, during which participants practiced on four MST sequences that were simpler versions of the sequences used for analysis on Days 5–8. This familiarization period was implemented because participants entered the study with a range of typing abilities, and the first 4 days were spent acclimating to the laboratory conditions prior to onset of the normal or inverted sleep schedule on Days 5–8. On Days 5–8 of both laboratory visits, participants underwent the training-test regimen with four different sequences, and each morning and evening session was carefully timed so that each started within a few minutes of 10 am/10 pm. Pre-sleep training started approximately 1 hour prior to lights out, and post-sleep training started approximately 3 hours after lights on. As in past studies using the MST, each unique 5-digit sequence conformed to the following rules: the first and last numbers of the sequence were the same; no other number appeared twice in a row within the sequence (eg, 4-1-1-3-4 would not be used); each of the four numbers (1, 2, 3 and 4) was used in each sequence.
As in previous research, training performance (acquisition) was defined as the average number of correct sequences typed per 30 seconds across the last 3 training trials (trials 10–12). Test performance was defined as the average number of correct sequences per 30 seconds across the first 3 test trials (thus prior to substantial additional learning), and consolidation was defined as the numeric improvement from training to test performance. Percent improvement was defined as (100 * (test−training)/training). General typing speed at each training/test session was defined as the number of correct sequences typed per 30 seconds (to match the duration of each training and test trial) during the 1-minute random sequence test that followed each training and test session. The random sequences were pseudo-randomly generated 5-digit sequences arranged side-by-side (one space between each sequence) across the computer monitor (eg, 21434 21414 14314…), with each sequence composed of the digits 1, 2, 3, and 4, and with no digits appearing twice in a row within a sequence. Post-training and post-test random sequence performance was averaged to compute general typing speed (motor performance independent of sequence learning) for each session.
Sixteen participants completed n = 82 training-test session pairs that were used for analysis (NightSleep, n = 16; DayWake, n = 17; NightWake, n = 27; DaySleep, n = 22). Table 1 lists the completed sequences (a complete training and test session for a given sequence) for each participant across Days 5–8 for Visit 1 and Visit 2. To be included in the data analysis the following criteria had to be met:
Table 1.
Sequences Completed by Each Participant.
| Sequence number | Sequence number | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participant | Visit 1 | 1 | 2 | 3 | 4 | Visit 2 | 1 | 2 | 3 | 4 |
| 1 | Inverted | W | S | W | S | |||||
| 2 | Inverted | W | S | W | S | |||||
| 3 | Normal | W | S | W | S | |||||
| 4 | Normal | W | S | W | S | Inverted | W | S | W | S |
| 5 | Inverted | W | S | W | S | |||||
| 6 | Normal | W | Inverted | W | S | W | S | |||
| 7 | Normal | W | S | W | S | |||||
| 8 | Inverted | W | S | W | S | Normal | W | S | S | |
| 9 | Inverted | W | S | W | S | |||||
| 10 | Normal | W | S | W | Inverted | W | S | W | ||
| 11 | Normal | W | S | W | Inverted | W | S | W | S | |
| 12 | Inverted | W | W | S | Normal | W | S | W | S | |
| 13 | Inverted | W | S | W | S | Normal | W | S | W | S |
| 14 | Inverted | W | S | W | S | |||||
| 15 | Inverted | W | S | W | ||||||
| 16 | Inverted | W | Normal | W | S | |||||
S = training and test were performed across a participant’s sleep period; W = training and test were performed across a participant’s wake period. Participants had to complete the training and test session for each sequence and each sequence had to meet the criteria for analysis.
-
1.
For a 30-second trial to be included in data analysis there could be no gaps between key presses greater than 5000 ms, a lapse likely due to the distraction of the participant from the task. Only 1.5% of all trials were excluded on this basis.
-
2.
For the training and test data for any one finger- tapping sequence to be included in data analysis, the participant had to have completed the last 3 training and first 3 test trials for that sequence, as well as the random sequence tests that followed the training and test sessions. Furthermore, no more than 3 of the 12 trials could have been excluded from analysis in either the training or test session.
-
3.
To ensure comparable experience with the MST task, participants had to complete sessions in the correct order starting from Sequence 1 (eg, Sequence 1, 2, and 3 or Sequence 1 and 2). On the other hand, if a Sequence was completed, but it did not meet one or more of the above three criteria to be included in data analysis, the next Sequences could still be used for analysis (eg, see in Table 1, Sequence 2 for Participant 12 was completed but excluded from analysis.
-
4.
Participants had to perform the test session 12 hours after the training session. If a training or test session could not be completed as scheduled, that sequence’s data were excluded from analysis. Because of the way the MST software was configured, when a participant missed a session, all subsequent sessions in that visit were invalid, because incorrect sequences were presented at all subsequent sessions. This occurred with Participant 6 and 16, for one of the two visits (Table 1).
Table 1 presents the sequences participants completed (training and testing) and for which they met criteria for analysis. The primary reasons for sequences being omitted were that a participant did not return for a visit (no completed sequences), the participant missed a training/test session, which invalidated the timings for the rest of the visit, or performance did not meet one or more of the above criteria.
Sleep Recordings
Sleep recordings were conducted after the training session of Sequence 4 during both visits and were scored from the C3 and C4 electrodes. Sleep stages were scored using AASM scoring rules. Analyzed sleep parameters are listed in Table 2.
Table 2.
Sleep Parameters in the Night Sleep and Day Sleep Conditions.
| Night Sleep | Day Sleep | Night vs. Day | |
|---|---|---|---|
| (n = 6) | (n = 10) | p | |
| TST (min) | 444.3 ± 5.4 | 386.4 ± 13.8 | .0006 |
| SE% | 92.5 ± 1.1 | 80.4 ± 2.9 | .0006 |
| SOL (min) | 46.8 ± 30.1 | 9.5 ± 2.7 | .13 |
| WASO (min) | 36.2 ± 5.4 | 93.9 ± 13.8 | .0005 |
| N1 (min) | 18.7 ± 5.1 | 29.3 ± 4.7 | .004 |
| N2 (min) | 214.2 ± 19.7 | 168.6 ± 14.5 | .03 |
| N3 (min) | 109.2 ± 16.7 | 107.7 ± 8.0 | .65 |
| REM (min) | 102.3 ± 12.0 | 80.9 ± 5.8 | .002 |
| N1% | 4.2 ± 1.1 | 7.6 ± 1.2 | .06 |
| N2% | 48.2 ± 4.5 | 43.1 ± 2.8 | .14 |
| N3% | 24.5 ± 3.6 | 28.1 ± 2.2 | .22 |
| REM% | 23.1 ± 2.7 | 21.1 ± 1.6 | .03 |
SE = Sleep Efficiency; SOL = Sleep Onset Latency; TST = Total Sleep Time; WASO = Wake After Sleep Onset. p-values are based on linear mixed models analysis with condition (Night Sleep vs. Day Sleep) entered as a fixed factor. Data are displayed as means ± SEMs.
Statistical Analysis
Linear mixed model analyses were conducted unless otherwise specified. For motor skill consolidation, biological time (day/night) and state (sleep/wake) of the 12 hours between training and testing were used as fixed factors. For motor skill acquisition, biological time (morning/evening) and state (after sleep/after wake) were used as fixed factors. Analyses were based on the MST training and test metrics used in numerous past studies (unadjusted performance), as well as improved metrics that take into account general motor speed (adjusted performance), in which general typing speed was added as a covariate in the mixed model analyses. Sleep parameters were entered as covariates in the mixed models analysis to assess the relationship between sleep and MST acquisition and consolidation. Differences in sleep parameters for the DaySleep and NightSleep groups were analyzed as fixed factors in the mixed models analysis.
RESULTS
Motor Skill Consolidation Across Sleep/Wake and Across Biological Day/Biological Night
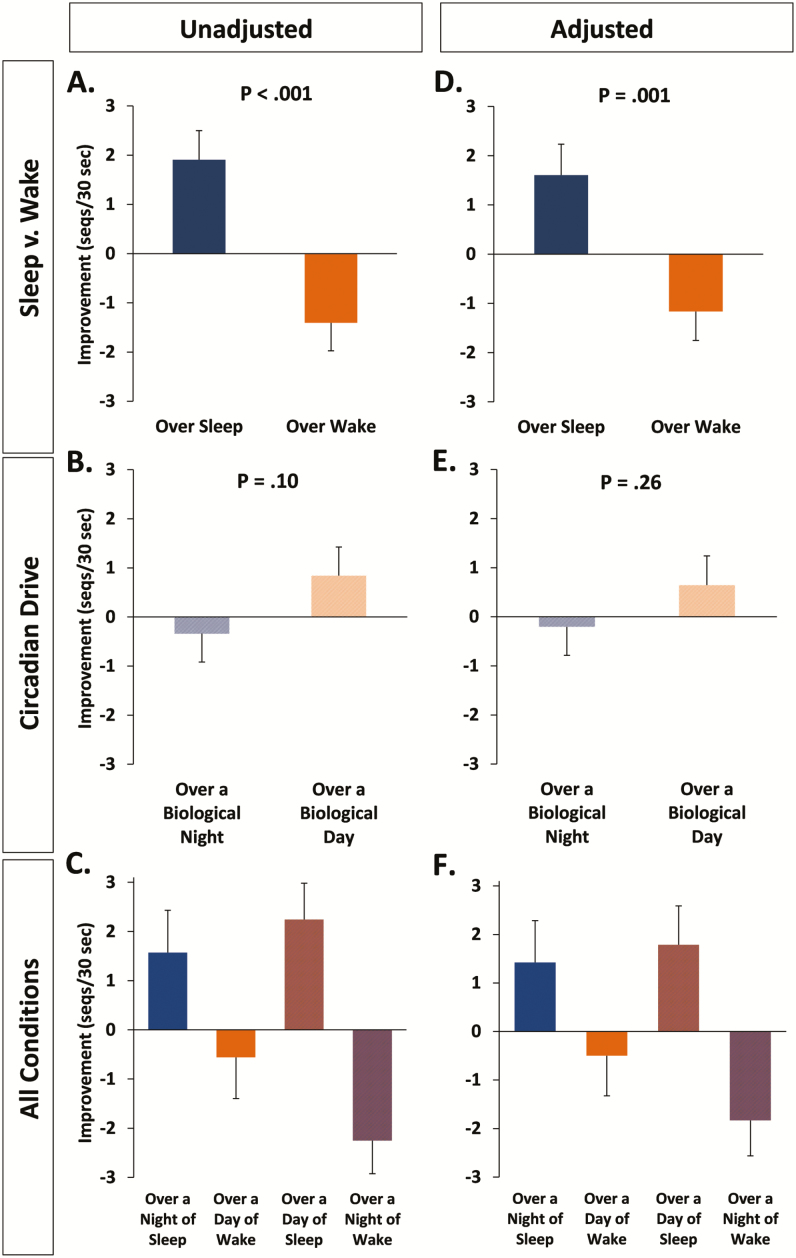
Independent of circadian phase, we found that sleep led to much greater off-line motor skill improvement (ie, consolidation) than did wake, with periods of wake leading to performance decrements (sleep conditions: +1.9 ± 0.6 sequences (+10.1 ± 2.9%), wake conditions: −1.4 ± 0.6 (−4.2 ± 2.8%), F1,64.5 = 21.85, p < .001; Figure 2A). All means/SEMs are the marginal means derived from the linear mixed models analyses. Motor skill improvement was not significantly different across the biological day versus the biological night (biological night: −0.3 ± 0.6 sequences (+0.2 ± 2.9%); biological day: +0.8 ± 0.6 sequences (+5.8 ± 2.9%); F1,64.3 = 2.79, p = .10; Figure 2B). Note: the opposite signs for numeric and percent change reflect the closeness of both values to zero and the nature of averaged percentages. There was no interaction between sleep/wake condition and biological day/night, that is, no effect of inverting the sleep schedule on consolidation (F1,75.3 = 0.45, p = .51; Figure 2C).
Figure 2.
MST Consolidation. Change in MST performance (consolidation) across sleep/wake and biological day/night. Panels A, B, C (left side of Figure) represent MST analyses without adding general motor speed as a covariate, a method of analysis in line with previous MST studies. Panels D, E, and F (right side of Figure) represent MST consolidation results after adding general motor speed as a covariate. Bars show the estimated means ± SEMs from the mixed model analyses.
As demonstrated in previous studies examining MST performance across nocturnal sleep and daytime wake, we found that motor performance improved significantly from training to testing if participants obtained sleep during the biological night (t15 = 2.42, p = .029). We observed a non-significant decline in performance if participants stayed awake during the biological day (t16 = 0.72, p = .48).
Because the age range of the sample (20–49y) was larger than most past studies on sleep and memory, and because age can impact learning and memory, we added age as a covariate in the linear mixed models analysis. Analyzing the data with age as a covariate had no appreciable impact on any of the MST acquisition and consolidation analyses.
Correcting for General Motor Speed
Performance on the 1-minute random-sequence typing test was used as a measure of general motor speed independent of learning. After adding general typing speed as a covariate in the statistical model, we continued to see a robust benefit of sleep on MST improvement compared to wake (F1,65.1 = 11.95, p = .001; Figure 2D). Uncorrected, there was a hint that circadian drive may impact MST improvement (see above and Figure 2B). However, when general typing speed was added to the model as a covariate, both the magnitude and significance of the difference in improvement across the biological day versus night were diminished (F1,64.5 = 1.31, p = .26; Figure 2E). As was the case for uncorrected performance improvement, the interaction between sleep/wake and biological day/night was not significant after correcting for general typing speed (F1,74.5 = 0.42, p = .52; Figure 2F).
Motor Skill Acquisition
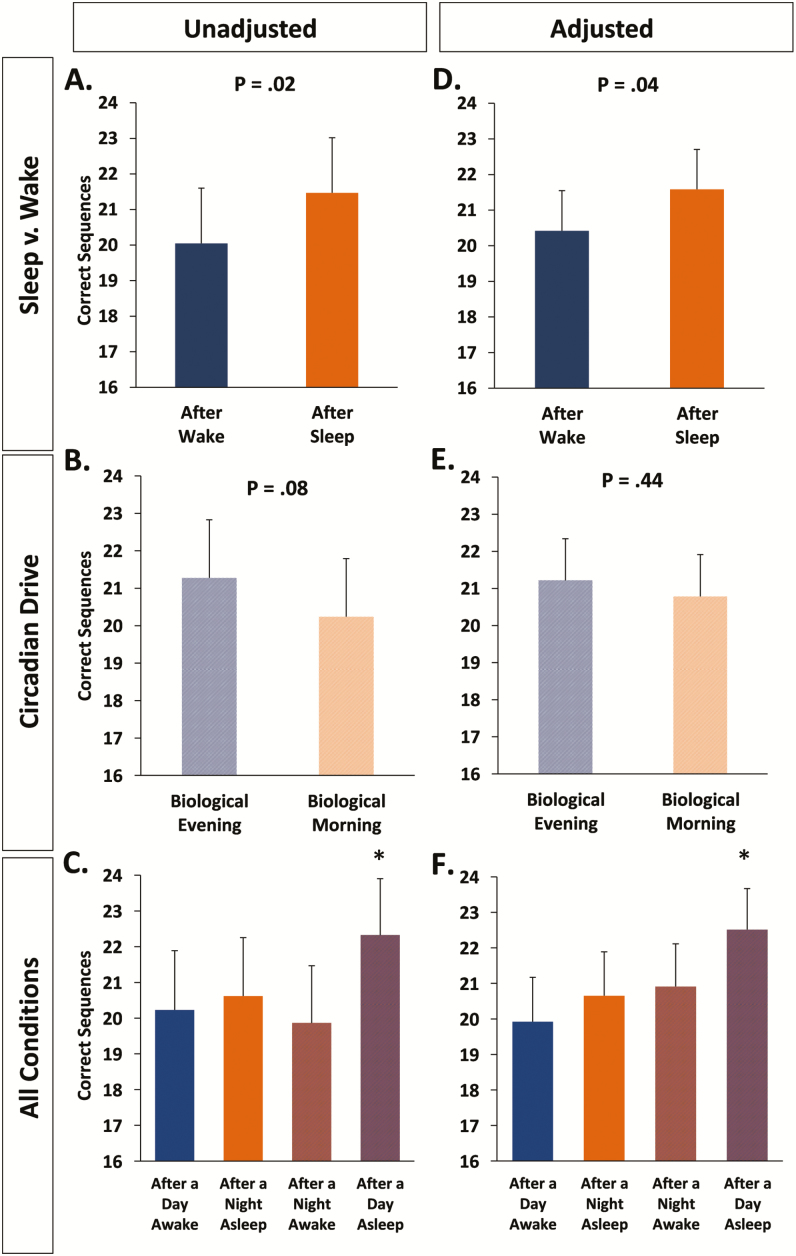
Motor skill acquisition during training benefitted from occurring following sleep as opposed to following wake (post-sleep: 21.5 ± 1.5, post-wake: 20.0 ± 1.6, F1,63.9 = 6.01, p = .02, Figure 3A). There was also a statistical trend toward greater acquisition in the biological evening than morning (biological morning: 20.2 ± 1.6, biological evening: 21.3 ± 1.6; F1,63.9 = 3.17, p = .08; Figure 3B), but no interaction between sleep/wake and biological day/night (F1,63.9 = 0.86, p = .36; Figure 3C).
Figure 3.
MST Acquisition. MST acquisition was significantly better when sleep preceded the MST training session (Panels A, D). Overall, biological time did not significantly influence task acquisition (Panels B, E).The post-sleep/wake difference in acquisition (A, D) was due to the superior performance after participants slept during the day. The asterisk in Panels C and F describes the difference between the Day Sleep and other three conditions (all p-values < .05, except the After Night Sleep–After Day Sleep comparison, p = .057 [Unadjusted]). Bars show the estimated means ± SEMs from the mixed model analyses.
Correcting for General Motor Speed
When correcting for the influence of general motor speed on MST acquisition, we found that the difference in motor skill acquisition following sleep versus following wake remained significant (F1,62.3 = 4.43, p = .04; Figure 3D), while the circadian effect showed no hint of a significant effect (F1,64.0 = 0.60, p = .44; Figure 3E). Interestingly, the interaction became significant after adjusting for general motor speed (F1,69.1 = 4.14, p = .046; Figure 3F). When training occurred in the biological morning, there was no substantial influence of prior sleep versus wake, but when training occurred in the biological evening, motor skill acquisition was substantially better after prior sleep than after prior wake. The appearance of a significant interaction after adjustment for general motor speed resulted from an upward adjustment of motor skill acquisition after a night awake due to a lower general motor speed in the biological morning after a night awake (Figure 3F). Of note, independent of whether or not adjusting for general motor speed, MST acquisition was greatest during the biological evening following daytime sleep, with performance in that condition being greater than any of the other three conditions (p-values < .05, except the After Night Sleep–After Day Sleep comparison, p = .057(Unadjusted), Figure 3F). Consistent with past findings,2 sleep did not benefit MST acquisition compared to wake under aligned circadian conditions (ie, when sleep occurred during the biological night and wake occurred during the biological day; p = .66 unadjusted; p = .40 adjusted).
Sleep Parameters and MST Performance
When entered as covariates in the mixed models analysis, none of the recorded sleep parameters correlated with MST acquisition or consolidation. There were, however, differences in sleep between participants that slept during the day and those that slept at night (see Table 2).
DISCUSSION
Teasing apart the circadian and sleep contributions to motor skill acquisition and consolidation has been an elusive goal in the science of sleep and memory consolidation. What past studies have been able to demonstrate is that when sleep occurs at night and wake occurs during the day, there are no effects of time of day on task acquisition (Figure 3C and F), while there are consistent benefits of sleep for memory consolidation: participants become faster on the task after nighttime sleep as opposed to after daytime wake (Figure 2C and F).1,2 Because the neurobiology associated with the nocturnal circadian phase is markedly different from that during the diurnal phase, it raises the question of how the circadian and sleep contributions to these processes can be separated, especially in light of reports suggesting that circadian factors can influence motor learning and memory consolidation.18,22 The current study addresses these issues by examining motor skill acquisition and consolidation following daytime and nighttime wake and sleep under controlled laboratory conditions. We demonstrate that sleep optimizes task acquisition when it precedes initial learning. However, it should be noted that this effect was largely driven by improved task acquisition following Day-Sleep (an experimental condition that has not previously been studied), suggesting that the interacting effects of prior sleep and the circadian phase of assessment may account for this acquisition boost that could not be revealed in studies that only examine acquisition performance following Night-Sleep and Day-Wake. Additionally, the time of day of learning did not affect task acquisition, which confirms the results of many past studies (however, see Rickard and Cai, 200817). The second novel finding of this study regards the process of offline motor memory consolidation that follows task acquisition. We demonstrate that only sleep, and not wake, regardless of when it occurs (either during the biological day or night) facilitates MST performance. The time of day of the offline consolidation period per se, whether occurring across the biological day or biological night, did not significantly impact how performance changes from training to test.
We further validated the performance outcomes of this study by controlling for performance on a non-learning random motor sequence task, which allowed us to correct for participants’ general motor speed. When adding general motor speed as a covariate in the linear mixed models analysis, sleep retained its beneficial effect on task acquisition when it preceded each learning session, and sleep retained its beneficial effect on consolidation when sleep occurred during the subsequent offline consolidation period.
Regarding the analysis of sleep recordings, and their impact on MST performance, we were not surprised to find that sleep parameters differed between participants that slept during the day as opposed to night, with participants sleeping less during the day than at night. We were surprised, however, that stage 2 sleep (total minutes and percentage of total sleep time) did not predict MST consolidation in either the DaySleep, NightSleep, or combined groups, as in previous studies.1,3 Our study may have been underpowered to detect a significant association between sleep stages and sleep consolidation. Alternatively, this association may be specific for night-time sleep on which the previous data were based. Future studies are required to clarify the interaction between sleep stages and circadian phase on memory consolidation.
Based on previously published 24-hour circulating melatonin and cortisol data collected on Day 5/6 and Day 7/8 of both the circadian alignment and misalignment protocols of the current study, our circadian misalignment protocol resulted in misalignment between the central circadian pacemaker and the 24-hour environmental and behavioral cycles.19 Despite the behavioral and environmental cycle shifting by 12 hours in the misalignment protocol, the average observed profile of melatonin and cortisol only delayed by ~1 hour on Day 5–6 and ~3 hour on Day 7–8 compared to the alignment protocol, consistent with the circadian system shifting relatively slowly.
The findings of this study, collected under well-controlled laboratory conditions, provide a clear picture of the effects of sleep and circadian drive on motor skill acquisition and consolidation. Similar to previous studies,15 which have typically relied on more homogenous samples (young college students), we observed a 15–20% greater consolidation benefit during sleep as opposed to wake, which suggests that this sleep-related benefit is robust and well-conserved across different research designs. Unfortunately, because some participants occasionally failed to begin typing at the start of a trial, and due to infrequent technical issues associated with running the MST, we could not obtain full data from all participants (see Table 1). Nonetheless, we ensured the validity of data processing by only analyzing data that conformed to strict requirements as outlined in the methods. We feel that the size of the data set under these conditions was more than adequate to test our hypotheses. Future studies are required to test whether these sleep-specific benefits generalize to other learning and memory domains. Additionally, while the study design allowed us to tease apart the effects of sleep/wake episodes and circadian biology, we only collected data from participants under completely inverted sleep conditions, during which sleep was misaligned approximately 180° from normal. Therefore, we are unable to generalize these results to other circadian time points for initial learning, and circadian intervals for offline consolidation. However, the inversion of participants’ sleep schedules under this experimental paradigm allowed us to determine the impact on learning and memory while participants underwent a sleep schedule that is comparable to that experienced by shift workers who work the “graveyard” shift and sleep during the day. In this regard, the findings of this study are clear: even under conditions simulating those observed in night shift workers, sleep continues to benefit motor skill acquisition and consolidation.
FUNDING
This study was supported by NHLBI Grant R01 HL094806 (to FAJLS.) and NIMH Grant R01 MH048832 (to RS). The project described was supported by Clinical Translational Science Award UL1RR025758 to Harvard University and Brigham and Women’s Hospital from the National Center for Research Resources.
DISCLOSURE STATEMENT
FAJLS has received lecture fees from Bayer HealthCare. None of these relationships are related to the present article. None of the other authors declare any disclosures.
SUPPLEMENTARY MATERIAL
Supplementary data are available at SLEEP online.
Supplementary Material
ACKNOWLEDGMENTS
We thank the research volunteers and Brigham and Women’s Hospital’s Center for Clinical Investigation nursing and technical staff. This study did not involve off-label or investigational drug use. This study is not a clinical trial. This study was performed at the Center for Clinical Investigation, Brigham and Women’s Hospital, Boston, MA 02115.
REFERENCES
- 1. Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002; 35(1): 205–211. [DOI] [PubMed] [Google Scholar]
- 2. Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003; 10(4): 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tucker MA, Fishbein W. The impact of sleep duration and subject intelligence on declarative and motor memory performance: how much is enough? J Sleep Res. 2009; 18(3): 304–312. [DOI] [PubMed] [Google Scholar]
- 4. Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proc Natl Acad Sci U S A. 2002; 99(18): 11987–11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fischer S, Nitschke MF, Melchert UH, Erdmann C, Born J. Motor memory consolidation in sleep shapes more effective neuronal representations. J Neurosci. 2005; 25(49): 11248–11255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishida M, Walker MP. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007; 2(4): e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci. 2007; 10(9): 1206–1213. [DOI] [PubMed] [Google Scholar]
- 8. Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999; 54: 97–130; discussion 130. [PubMed] [Google Scholar]
- 9. Morris CJ, Aeschbach D, Scheer FA. Circadian system, sleep and endocrinology. Mol Cell Endocrinol. 2012; 349(1): 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scheer FA, Hu K, Evoniuk H, et al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A. 2010; 107(47): 20541–20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qian J, Scheer FA. Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol Metab. 2016; 27(5): 282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012; 35: 445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wirz-Justice A. Biological rhythm disturbances in mood disorders. Int Clin Psychopharmacol. 2006; 21(suppl 1): S11–S15. [DOI] [PubMed] [Google Scholar]
- 14. Goel N, Basner M, Rao H, Dinges DF. Circadian rhythms, sleep deprivation, and human performance. Prog Mol Biol Transl Sci. 2013; 119: 155–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walker MP. A refined model of sleep and the time course of memory formation. Behav Brain Sci. 2005; 28(1): 51–64; discussion 64–104. [DOI] [PubMed] [Google Scholar]
- 16. Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011; 35(5): 1154–1165. [DOI] [PubMed] [Google Scholar]
- 17. Rickard TC, Cai DJ, Rieth CA, Jones J, Ard MC. Sleep does not enhance motor sequence learning. J Exp Psychol Learn Mem Cogn. 2008; 34(4): 834–842. [DOI] [PubMed] [Google Scholar]
- 18. Keisler A, Ashe J, Willingham DT. Time of day accounts for overnight improvement in sequence learning. Learn Mem. 2007; 14(10): 669–672. [DOI] [PubMed] [Google Scholar]
- 19. Morris CJ, Yang JN, Garcia JI, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci U S A. 2015; 112(17): E2225–E2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Morris CJ, Purvis TE, Hu K, Scheer FA. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 2016; 113(10): E1402–E1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Morris CJ, Garcia JI, Myers S, Yang JN, Trienekens N, Scheer FA. The human circadian system has a dominating role in causing the morning/evening difference in diet-induced thermogenesis. Obesity (Silver Spring). 2015; 23(10): 2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pan SC, Rickard TC. Sleep and motor learning: Is there room for consolidation? Psychol Bull. 2015; 141(4): 812–834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.