Abstract
Due to antiretroviral therapies, HIV is now a chronic illness rather than a terminal disease. Chronic symptoms, including fatigue, should be identified and managed to prevent or minimise their potential negative consequences. We apply a Symptom Management Model to conceptualise fatigue among adolescents with HIV. In the context of minimal research, we seek to identify a research agenda for resource-constrained contexts, where HIV prevalence remains high and treatment adherence is a significant problem. By better understanding and addressing the symptom of fatigue, treatment adherence, occupational, social and emotional functioning could be improved. We highlight conceptual, methodological and measurement-related caveats.
Keywords: adolescents, fatigue, HIV, symptom management model
Introduction
It is estimated that 35 million people in the world are currently living with HIV (UNAIDS, 2015) of whom 1.8 million are children (<15 years old) who reside in low-to-middle income countries (AIDS.Gov., 2015). An estimated two-thirds of people living with HIV received antiretroviral therapy (ART) as of 2013 (UNAIDS, 2015). As a result of increasingly effective and available ARTs, HIV is now considered a chronic illness rather than a terminal disease. Therefore, identifying and managing the symptoms of the chronic illness, such as fatigue, has become a necessary health concern. Addressing fatigue may prevent or minimise potential negative health consequences for the individual, which would be of benefit to the healthcare system and society at large as well. Fatigue may also impact HIV management by affecting adherence to ART regimes due to, for example, oversleeping or impaired concentration.
Aaronson et al. (1999) defined fatigue as ‘the awareness of a decreased capacity for physical and/or mental activity due to an imbalance in the availability, utilization, and/or restoration of resources needed to perform activity’ (p. 46). Fatigue is considered to be normal in the general population, but if extreme and prolonged, can be significantly disabling. Fatigue can thus be distinguished from normal tiredness by its severity and chronicity, despite an individual taking actions that would be expected to result in relief (e.g. rest), and by its impact on functioning (Jacobsen, 2004). In a qualitative study of cancer-related fatigue, Wu and McSweeney (2007) state that fatigue is different from normal tiredness in that it is overwhelming, ongoing and unresponsive to rest. Chronic fatigue can be defined as ongoing physical and/or mental exhaustion and tiredness that persists for at least 1 month (Voss et al., 2007a).
Fatigue is a common and disabling symptom in adolescence, particularly among those living with a chronic illness. It has been found to affect approximately one-third of the general adolescent population in the United Kingdom (Rimes et al., 2007), with the lifetime prevalence estimated at 2.3 per cent among UK adolescents 8–17 year olds. Elevated rates of fatigue have been found in paediatric chronic illnesses, including in 57 per cent of those with terminal cancer (Wolfe et al., 2000), 52 per cent of those with type 1 diabetes (Levy-Marchal et al., 2001) and 60 per cent of those with multiple sclerosis (Banwell et al., 2007). Fatigue has been reported as a symptom in a case study of an adolescent with HIV (Aggarwal and Rein, 2003) but has not been more extensively studied. To our knowledge, there are no data currently available on the prevalence of fatigue among adolescents with HIV. This omission may be because the focus of research into HIV previously has been through the lens of palliative care rather than chronic illness management, with a more recent shift towards the latter as a result of improvements in antiretroviral therapies. Alternatively, it may be because adolescents with HIV tend to be clustered in low-income countries, where less attention has been paid to factors such as fatigue more broadly, with most research on fatigue having emerged from high-income countries.
The consequences of fatigue in HIV are substantial. In terms of occupational functioning, fatigue among adults with HIV has been correlated with greater functional impairment and lower survival rates (Justice et al., 1999), as well as poorer health and lower work productivity (Dibonaventura et al., 2012). In adolescents, it is likely that high levels of fatigue could impact on occupational functioning by causing an increase in school absenteeism, thus contributing to unfavourable outcomes such as academic underachievement, decreased work productivity and opportunities for employment, and ultimately, impoverishment and decreased quality of life (Harmon et al., 2008; Jong et al., 2010).
Fatigue in adults with HIV is associated with interference in family and social life (Harmon et al., 2008), possibly due to or compounded by features such as lowered motivation, difficulties with concentration, increased drowsiness and loss of patience. Fatigue among adolescents with HIV could have a similar effect on social functioning, which may be particularly detrimental at this key stage of individuation from the family and establishing one’s own close personal relationships.
Fatigue in adults has also been correlated with poorer treatment adherence, possibly due to its disruptive impact on daily routines and on concentration and memory (Gay et al., 2011). Possibly, the same may be true for adolescents with HIV who are also fatigued. Adherence depends on memory and concentration to recall that the medication needs to be taken and to see that action through to successful completion. Mental fatigue could also disrupt the process of taking medication. Alternatively, or in addition, if side effects such as fatigue, drowsiness or lack of energy are attributed to taking antiretroviral medication, adolescents might become non-adherent as a way to manage these undesirable side effects. A complicating factor in considering medication adherence in adolescents is that, at least to some extent, caregivers are likely to be involved in monitoring and prompting medication taking.
UCSF-SMM
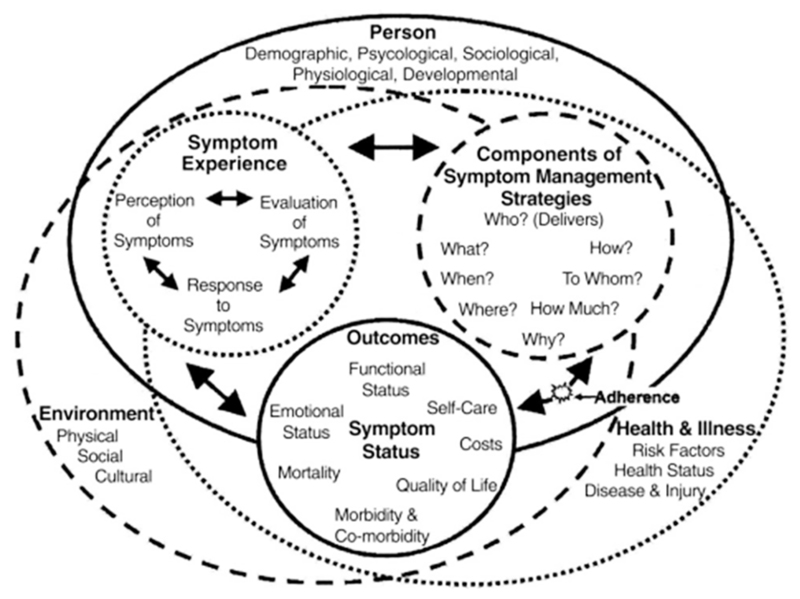
An evidence-based theoretical model that has been applied to fatigue in HIV is the University of California, San Francisco, Revised Symptom Management Model or UCSF-SMM (Dodd et al., 2001; Voss et al., 2006; see Figure 1). The UCSF-SMM is a generic multi-dimensional symptom management model that postulates interactions between the components of an individual’s experience of a symptom (their perception, evaluation and response to the symptom), the symptom management strategies they employ (what, when, where, how much) and their potential outcomes (change in severity, frequency or duration of symptom). The bidirectional link between outcomes and symptom management strategies captures the concept of adherence to the symptom management strategy (e.g., how closely an individual follows a prescribed medication regimen). These interactions are embedded within the dimensions of the person (individual variables such as demographic, psychological factors, developmental stage), the environment (social and cultural factors) and illness and health-related factors (such as health status or type of disease).
Figure 1.
The Revised University of California, San Francisco, Symptom Management Model from Dodd et al. (2001) Source: Reprinted with permission.
The SMM has been extensively applied to fatigue in adults with HIV (Corless et al., 2002, 2008; Voss, 2005; Voss et al., 2006, 2007b). It has also been proposed as a valuable model for the study of fatigue in paediatric oncology patients (Linder, 2010). However, to our knowledge, the model has not previously been applied to understanding fatigue in adolescents with HIV. It appears to be an appropriate framework, given its utility in adults with HIV who are experiencing fatigue, and its capacity to include developmental considerations, and environmental/contextual factors which may be of particular relevance to adolescents (e.g. parental factors).
We now review the existing evidence about the experience of, management for and consequences of fatigue in adolescents with HIV using the UCSF-SMM framework. We discuss the conceptual, methodological and measurement issues that arise in investigating fatigue in adolescents with HIV. We also highlight research avenues required to further develop this important yet neglected area.
Fatigue in adolescents with HIV within the SMM framework: the central components
Symptom experience
The SMM model starts with an individual’s perception of their symptoms, their evaluation of the meaning of the symptom and their response to it. Perceptions include judgements about the symptom’s severity, its cause, its treatability and the effect of symptoms on the individual’s lives and the lives of their significant others.
It has been established that adults living with HIV have considerably higher levels of fatigue than the general population (Barroso, 1999; Barroso and Voss, 2013). A review of 42 studies found the prevalence of HIV-related fatigue to range from 33 to 88 per cent, which included HIV-infected individuals who were undergoing treatment, as well as untreated individuals. The large variation in fatigue prevalence may be due to the range of instruments used to assess fatigue (Jong et al., 2010).
Extrapolating from the above research into fatigue in adults, and also fatigue in paediatric populations with chronic illnesses (Carroll et al., 2015; Gold et al., 2009; Nutini et al., 2009; Varni et al., 2004; Wolfe et al., 2000), it is likely that fatigue is a problematic symptom experienced by HIV-positive adolescents.
Symptom management strategies
The second component of the SMM consists of the strategies that individuals use to remediate or reduce their symptoms; for example, through taking medication, or seeking professional help. This component includes a consideration of the timeliness of these strategies. As fatigue has not been studied in adolescents with HIV, potential symptom management strategies are unknown. In other adolescent populations with fatigue such as those with chronic fatigue syndrome (CFS), cognitive behaviour therapy (CBT) is effective at reducing fatigue and improving functioning and results in recovery among around two-thirds of patients (Knight et al., 2013). The activity management, graded exercise, sleep management and cognitive strategies used in CBT for CFS may be adapted for fatigued adolescents living with HIV.
Outcomes
The third component of the SMM is outcomes, which includes improvements in symptom status, functioning, emotional status, mortality, morbidity/comorbidity and quality of life, as well as cost. Although not explicitly mentioned in the original model, this may encompass improvements in family life too, such as a reduction in the need for care by parents.
The inter-relations between the components of symptom experience, symptom management and outcome
Among adolescents with HIV, fatigue (symptom experience) may be a barrier to HIV symptom management via non-adherence to antiretroviral treatment possibly through poor motivation and hopelessness as part of a depression symptom cluster (Nel and Kagee, 2013), and/or through the disruptive impact of fatigue on activities of daily living and routine known to occur in adults (Harmon et al., 2008; Lee et al., 2001).
Person domain
Adolescents are developmentally distinct from adults in relation to attentional, cognitive and interpersonal functioning (Semple et al., 2006). Metacognition and executive functioning develop through adolescence (Dahl, 2004), and adolescents often have less emotional awareness and ability to label feelings than adults (Ciarrochi et al., 2008). These cognitive differences may affect the symptom experience of fatigue, which may be more focused on physical sensations and behavioural changes in activity levels and less focused on cognitive or emotional aspects of the fatigue experience (Hockenberry et al., 2003). This domain of the SMM allows for the consideration of such developmental factors and how they might influence the symptom experience, symptom management and outcomes.
The person domain also includes demographic factors. In adults with HIV, fatigue has been found to be higher in females (Breitbart et al., 1998). Being female is a predictor of fatigue among healthy individuals (Åkerstedt et al., 2004), and it has been suggested that this could be the result of increased social and family responsibility. Fatigue has also been found to be higher among older patients in some studies (Singh et al., 1997), although other studies have shown that the longer someone has experienced HIV, the lower their level of reported fatigue may be (Harmon et al., 2008), possibly due to adaptation to the fatigue and the development of coping mechanisms for it. Factors such as age and gender have yet to be examined in relation to patterns of fatigue in adolescents with HIV.
Environment domain
The environmental domain of the model captures the conditions within which the given symptom occurs, including physical, social and cultural variables. To date, little is known about how such factors might impact on fatigue in adolescents with HIV.
However, in resource-constrained environments, where the prevalence of adolescents living with HIV is highest, the physical demands of basic activities of daily living may exacerbate fatigue. For example, in South Africa, 71 per cent of pupils walk to school, and 900,000 (13%) children and young people travel for more than 30 minutes to and from school every day (Hall et al., 2014). Other requirements imposed by structural and contextual factors, such as doing household chores like carrying water, or doing menial jobs which are highly physical in nature, may also exacerbate fatigue. In adults with HIV, fatigue appears to be lower in those with a higher monthly income (Harmon et al., 2008). This may be because patients with higher income are more able to pay for people to help them out with domestic chores, thus easing the burden of physical tasks, resulting in less fatigue (Harmon et al., 2008), or that higher paying jobs may be less physically strenuous, resulting in less fatigue. Alternatively, being less fatigued may mean that a person can work for longer hours at a higher level, thus increasing earnings.
Commensurate with their developmental stage, adolescents face the task of transitioning from dependence and reliance on caregivers to independence and individuation. This influences help-seeking for symptoms, medication adherence and self-care management strategies they might employ. At the early stages of adolescence, these might be driven by caregivers to a large extent, but by the end of adolescence, they might be undertaken with considerably more autonomy.
Health and illness domain
In adults, no physiological factors have been consistently shown to predict fatigue in HIV patients. For example, there have been conflicting findings about the relationship between fatigue, CD4+ T cell counts and viral load (Barroso and Voss, 2013; Jong et al., 2010). Some HIV patients appear to develop fatigue at the early stages of the illness, and in others, it develops as the illness progresses (Holzemer, 2002). In the Fatigue in HIV Positive People study (Harmon et al., 2008), fatigue was negatively correlated with number of years living with HIV infection in a US sample of 128 HIV patients who were predominantly poor and unemployed, of whom 66 per cent were African American. Viral load was not reported in this study, although the participants had a median duration of 10 years post-HIV diagnosis and 82 per cent were on ART. This finding could suggest some degree of reduction in or adaptation to fatigue and/or the development of coping mechanisms over time and it may be that patients perceive fatigue differently over time. However, the sample was self-selecting, responding to study advertisements, with a financial incentive for participation, which poses questions about representativeness. Almost two-thirds of the participants had chronic illnesses, which are potential confounding factors. Furthermore, the cross-sectional design limits the extent to which any robust conclusions can be drawn about causality and directionality. A longitudinal follow-up of this cohort found that no physiological factors predicted greater fatigue, including hepatic function, thyroid function, HIV viral load, immunologic function, gonadal function, haematologic function and cellular injury (lactic acid) (Barroso et al., 2010).
Psychosocial factors such as depression, anxiety and social support have been more consistently related to fatigue in adults with HIV (Barroso and Voss, 2013; Jong et al., 2010). Fatigue is a common symptom of depression (APA, 2013), and in people with HIV, a strong correlation between depressed mood (assessed by self-report questionnaires such as the Beck Depression Inventory, BDI-II) and fatigue has been consistently reported in the literature (Barroso, 2001; Barroso et al., 2003, 2010; Ciesla and Roberts, 2001; Perkins et al., 1994). Of these studies, Barroso et al. (2010) showed that, over a 1-year period, changes in depression predicted changes in fatigue, both between participants and within participants. Fatigue among persons living with HIV has also been found to co-occur with anxiety (Paddison et al., 2009). In a prospective longitudinal study of 128 HIV patients, fatigue was shown to result from stressful life events, with the largest effect sizes resulting from the anxiety and depression triggered by such events (Barroso et al., 2015). Being in an anxious state, that is, a state of constant hyperarousal induces fatigue as it draws on physiological resources to prepare the body to respond to threat. Although fatigue has not been studied in adolescents, depression and anxiety are common features in adolescents with HIV (Lam et al., 2007; Naar-King et al., 2006; Pao et al., 2000).
Methodological/conceptual issues
Quantifying fatigue as part of the symptom experience is complicated due to the lack of a consensus framework, a variety of definitions and terminology (Aaronson et al., 1999) and the subjective and multi-dimensional nature of the symptoms (Crichton et al., 2015). Dilemmas continue to exist around when ‘normal’ fatigue becomes ‘abnormal’ or pathological, whether duration or severity of fatigue, or both, should be considered, and how to make an inherently subjective experience quantifiable in a meaningful way.
Fatigue can be a core symptom of HIV or a side effect of ART (health and illness-related factors), but also a symptom of co-morbid mental health problems such as depression and anxiety (person-related factors). Therefore, distinguishing fatigue and depression is an important methodological issue, given the overlap in symptoms. Both fatigue and depression are multi-dimensional constructs, encompassing cognitive, behavioural, emotional and physiological aspects. Around 1 in 10 HIV-positive adults have depression (Ciesla and Roberts, 2001) and at least 1 in 3 report problems with fatigue (Jong et al., 2010). Therefore, although for some people with HIV, this might be due to their co-morbid mood difficulties, others have fatigue but do not have co-morbid mood difficulties, so depression cannot fully account for all fatigue in people with HIV. The consensus from the literature appears to be that it is possible, albeit complex, to distinguish fatigue from depression (Jacobsen, 2004; Levine and Greenwald, 2009). It is possible that people with HIV may be fatigued, or depressed, both, or neither (see Table 1).
Table 1.
Separating out individuals with HIV who are fatigued from those who are depressed (based on APA DSM-5 major depressive disorder criteria and White, 2006).
| HIV non-depressed | HIV depressed | |
|---|---|---|
| HIV non-fatigued | May be asymptomatic if HIV is well-controlled with ART (viral load undetectable, high CD4+ cell counts). No significant impact on social, occupational or educational functioning. |
Low mood and/or high levels or irritability. Markedly diminished interest or pleasure in almost all activities. High self-reproach, guilt, low self-esteem and suicidal ideation with themes of abandonment and loss. Depressive symptomatology has a significant impact on social, occupational or educational functioning, or cause significant distress. |
| HIV fatigued |
Significant levels of fatigue. Maintain a strong interest in life and often overestimate how much they can do. Fatigue has a significant impact on social, occupational or educational functioning. |
Markedly diminished interest or pleasure in almost all activities most of the day, most days. Features include high self-reproach, guilt, low self-esteem and suicidal ideation with themes of abandonment and loss. High levels of fatigue. Depressive symptomatology and fatigue have a significant impact on social, occupational or educational functioning, or cause significant distress. |
Measurement issues
A person-related domain measurement issue for all of the studies that have examined depression and fatigue in adults is that questionnaires used to assess depression commonly include somatic symptoms that overlap with symptoms of fatigue (Jong et al., 2010) and/or symptoms of HIV. Psychometric measures of depression, frequently used in health settings, such as the Hospital Anxiety and Depression Scale (HADS) have been shown to be useful as a measure of general distress (Norton et al., 2013), but are not specific enough for diagnosing depression and are also unlikely to differentiate between fatigue (which may result in individuals scoring on depression) and depression itself.
Furthermore, in paediatric chronic illness populations, there is a paucity of research into fatigue. Eddy and Cruz (2007) argue that the existing research is limited by the consideration of fatigue as a dichotomous variable (yes-no) or the use of a visual analogue scale to measure this complex and multi-dimensional construct. Fatigue fluctuates naturally over the course of development and is higher in late adolescence for example (Ter Wolbeek et al., 2006). Thus, measurement of fatigue needs to take into consideration the person-related factors of developmental stage and age-related norms. Additionally, a review by Crichton et al. (2015) highlighted the lack of robust, well-validated measures of fatigue for paediatric populations. The Paediatric Quality of Life Inventory (PedsQL) Multidimensional Fatigue Scale (MFS) is the only instrument which has robust measurement properties (Crichton et al., 2015), although it is considered difficult to score and the copyright is held by a primary researcher. Other promising measures include the Fatigue Scales (Child, Adolescent and Parent versions) and the Patient-Reported Outcomes Measurement Instrument System (PROMIS). The former is brief to administer and easy to score, but only considers the intensity dimension of fatigue, while the latter requires computer literacy and access, and is lengthy, taking at least 30 minutes in adolescents. None of these measures have been used in a sample of adolescents with HIV. The fluctuating and unpredictable nature of fatigue further complicates measurement (Hewlett et al., 2011b).
Defining a research agenda for fatigue among HIV-infected adolescents
Many of the components of the UCSF-SMM are unknown in adolescents with HIV, as the extent of the fatigue, its correlates and potential management among adolescents with HIV are yet to be investigated. However, it seems likely that fatigue presents a considerable problem in adolescents with HIV that may have serious ramifications, possibly impacting the medical management of HIV itself as well as on other outcomes such as school attendance and attainment, employment and quality of life.
Emergent research questions include the following.
Symptom experience
To establish whether fatigue is experienced in the same way in adolescents with HIV as in other populations where more extensive fatigue research has been conducted, qualitative research to explore the nature and subjective experience of fatigue in this population is indicated. This will help to establish whether the construct of fatigue applies in a conceptually similar way, and therefore, whether existing measures of fatigue may be appropriate, or whether more culturally sensitive measures may need to be developed. To establish the intensity, severity and duration of fatigue, quantitative research using existing or newly developed measures is indicated. Careful consideration of how best to recruit representative samples (e.g. through schools) is required.
Symptom management strategies
The cognitive behavioural model of fatigue, which has been applied to illnesses such as CFS (Moss-Morris et al., 2011), rheumatoid arthritis (Hewlett et al., 2011a) and multiple sclerosis (Carroll et al., 2015), aims to explain the onset and maintenance of fatigue and could conceivably apply to HIV. Biological disease characteristics such as infection may trigger the onset of fatigue, which is then maintained by cognitive, behavioural and emotional factors. Cognitive factors include illness perceptions and beliefs about the controllability and predictability of the chronic illness (HIV). Behavioural responses to fatigue include over-exertion or excessive rest. Emotional factors include depression and anxiety, which are common features in adolescents with HIV (Lam et al., 2007; Naar-King et al., 2006; Pao et al., 2000). The cognitive and behavioural responses to fatigue in this population have not been investigated, and therefore, further research is needed to explore these factors and the appropriateness of this model in adolescents with HIV.
Assuming that the cognitive behavioural model is applicable to adolescents with HIV who are experiencing fatigue, cognitive behavioural interventions used successfully in adolescents with CFS (Lloyd et al., 2012; Nijhof et al., 2011) could be trialled in this population. These interventions target patterns of activity, aiming to achieve consistent amounts of activity and to gradually increase these, thus combating the vicious cycles of over-exertion and excessive rest. Where necessary, interventions also seek to identify and address unhelpful illness perceptions and cognitions. Improving sleep hygiene is also a focus of intervention, and where necessary, mood and anxiety are addressed. Similar CBT approaches have proved effective in reducing fatigue and improving functioning in adults with cancer (Gielissen et al., 2007), and in adults with multiple sclerosis, for example (Van Kessel et al., 2008). These kinds of interventions could be adapted and trialled in this population, potentially leading to potential improvements in adherence to ART, social and educational/occupational functioning. It would be important to design the interventions such that they could be delivered by low-cost healthcare workers in clinic locations.
Outcomes
Further research is needed to explore the impact of fatigue on functioning, and on well-being, and the impact of fatigue on quality of life, as well as the impact on significant others such as parents and siblings. Important variables to measure will therefore include fatigue using validated self-report scales, distress, functional impairment/disability, sleep and biological markers of HIV infection such as viral load and CD4+ T cell count.
Health and illness factors
To understand the symptom experience of fatigue, and how it fits more widely into the individual’s life, it will be important to establish what the health and illness-related correlates of fatigue are, including the relationship between fatigue and pain, and fatigue and sleep. Measures that enable these to be separated out in a meaningful way will be needed, and this could be usefully complimented by qualitative research exploring these factors through the lived experience of HIV.
Person
Factors such as age and gender, as well as developmental factors will need to be investigated in relation to patterns of fatigue in adolescents with HIV. The age criterion for adolescents also requires some attention. We propose using the World Health Organization’s definition of adolescent which is between the ages of 10 and 19 (WHO, 2016).
Environment
Cultural expressions of fatigue have also been under-investigated. To date, most research on fatigue among people living with HIV has been conducted among people in North America and Europe. Yet, HIV prevalence in many countries in Africa and Asia is high, in addition to high prevalence in specific groups such as sex workers, intravenous drug users, and men who have sex with men, all of whom are represented among adolescents.
Measurement issues
None of the existing fatigue measures have been well validated outside of Western populations. Thus, the extent to which fatigue assessment instruments, such as the Chalder Fatigue Scale (Chalder et al., 1993), which has been extensively used in research with both adults and children, might be globally applicable is unclear, and this needs to be established to bring clarity around the environment-related domain.
This programme of research would indicate whether fatigue, as a common symptom in HIV, could or should be a treatment target in adolescents. The SMM could be a useful theoretical framework for linking the research findings to symptom management and outcomes. Remediating fatigue by individuals learning self-management strategies, particularly at the early life stage of adolescence, could potentially improve outcomes by increasing quality of life, decreasing school absenteeism and underachievement, and improving the future prospects of adolescents with HIV who are likely to spend many years managing HIV as a chronic illness. It could also improve their adherence to ART, decreasing HIV-related mortality and morbidity, and the associated healthcare costs. Achieving symptom management requires research into all aspects of the SMM model.
Acknowledgements
We would like to thank the Departments of Psychology at the Universities of Bath and Stellenbosch for supporting our collaborative relationship.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: M.E.L. is funded by the National Institute for Health Research (Doctoral Research Fellowship, DRF-2016-09-021). This report is independent research. The views expressed in this publication are those of the authors(s) and not necessarily those of the NHS, The National Institute for Health Research or the Department of Health.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Reprints and permissions: sagepub.co.uk/journalsPermissions.nav
References
- Aaronson LS, Teel CS, Cassmeyer V, et al. Defining and measuring fatigue. Image: The Journal of Nursing Scholarship. 1999;31(1):45–50. doi: 10.1111/j.1547-5069.1999.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Aggarwal M, Rein J. Acute human immunodeficiency virus syndrome in an adolescent. Pediatrics. 2003;112(4):E323–E324. doi: 10.1542/peds.112.4.e323. [DOI] [PubMed] [Google Scholar]
- AIDS.Gov. The global HIV/AIDS epidemic. 2015 Available at: https://www.aids.gov/hiv-aids-basics/hiv-aids-101/global-statistics/
- Åkerstedt T, Knutsson A, Westerholm P, et al. Mental fatigue, work and sleep. Journal of Psychosomatic Research. 2004;57(5):427–433. doi: 10.1016/j.jpsychores.2003.12.001. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Banwell B, Krupp L, Kennedy J, et al. Clinical features and viral serologies in children with multiple sclerosis: A multinational observational study. The Lancet Neurology. 2007;6(9):773–781. doi: 10.1016/S1474-4422(07)70196-5. [DOI] [PubMed] [Google Scholar]
- Barroso J. A review of fatigue in people with HIV infection. Journal of the Association of Nurses in AIDS Care. 1999;10(5):42–49. doi: 10.1016/S1055-3290(06)60342-7. [DOI] [PubMed] [Google Scholar]
- Barroso J. ‘Just worn out’: A qualitative study of HIV-related fatigue. In: Funk SG, Leeman J, Tornquist, editors. Key Aspects of Preventing and Managing Chronic Illness. New York: Springer; 2001. pp. 183–194. [Google Scholar]
- Barroso J, Voss JG. Fatigue in HIV and AIDS: An analysis of evidence. Journal of the Association of Nurses in AIDS Care. 2013;24(1):S5–S14. doi: 10.1016/j.jana.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Barroso J, Carlson JR, Meynell J. Physiological and psychological markers associated with HIV-related fatigue. Clinical Nursing Research. 2003;12(1):49–68. doi: 10.1177/1054773803238740. [DOI] [PubMed] [Google Scholar]
- Barroso J, Hammill BG, Leserman J, et al. Physiological and psychosocial factors that predict HIV-related fatigue. AIDS and Behavior. 2010;14(6):1415–1427. doi: 10.1007/s10461-010-9691-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso J, Leserman J, Harmon JL, et al. Fatigue in HIV-infected people: A three-year observational study. Journal of Pain and Symptom Management. 2015;50(1):69–79. doi: 10.1016/j.jpainsymman.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitbart W, Mcdonald MV, Rosenfeld B, et al. Fatigue in ambulatory AIDS patients. Journal of Pain and Symptom Management. 1998;15(3):159–167. doi: 10.1016/s0885-3924(97)00260-1. [DOI] [PubMed] [Google Scholar]
- Carroll S, Chalder T, Hemingway C, et al. Understanding fatigue in paediatric multiple sclerosis: A systematic review of clinical and psychosocial factors. Developmental Medicine & Child Neurology. 2015;58:229–239. doi: 10.1111/dmcn.12964. [DOI] [PubMed] [Google Scholar]
- Chalder T, Berelowitz G, Pawlikowska T, et al. Development of a fatigue scale. Journal of Psychosomatic Research. 1993;37(2):147–153. doi: 10.1016/0022-3999(93)90081-p. [DOI] [PubMed] [Google Scholar]
- Ciarrochi J, Heaven PCL, Supavadeeprasit S. The link between emotion identification skills and socio-emotional functioning in early adolescence: A 1-year longitudinal study. Journal of Adolescence. 2008;31(5):565–582. doi: 10.1016/j.adolescence.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. American Journal of Psychiatry. 2001;158:725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Corless IB, Bunch EH, Kemppainen JK, et al. Self-care for fatigue in patients with HIV. Oncology Nursing Forum. 2002;29:E60–69. doi: 10.1188/02.ONF.E60-E69. [DOI] [PubMed] [Google Scholar]
- Corless IB, Voss JG, Nicholas PK, et al. Fatigue in HIV/AIDS patients with comorbidities. Applied Nursing Research. 2008;21(3):116–122. doi: 10.1016/j.apnr.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Crichton A, Knight S, Oakley E, et al. Fatigue in child chronic health conditions: A systematic review of assessment instruments. Pediatrics. 2015;135(4):E1015–E1031. doi: 10.1542/peds.2014-2440. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: A period of vulnerabilities and opportunities (keynote address) Annals of the New York Academy of Sciences. 2004;1021(1):1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Dibonaventura MD, Gupta S, Cho M, et al. The association of HIV/AIDS treatment side effects with health status, work productivity, and resource use. AIDS Care. 2012;24(6):744–755. doi: 10.1080/09540121.2011.630363. [DOI] [PubMed] [Google Scholar]
- Dodd M, Janson S, Facione N, et al. Advancing the science of symptom management. Journal of Advanced Nursing. 2001;33(5):668–676. doi: 10.1046/j.1365-2648.2001.01697.x. [DOI] [PubMed] [Google Scholar]
- Eddy L, Cruz M. The relationship between fatigue and quality of life in children with chronic health problems: A systematic review. Journal for Specialists in Pediatric Nursing. 2007;12(2):105–114. doi: 10.1111/j.1744-6155.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- Gay C, Portillo CJ, Kelly R, et al. Self-reported medication adherence and symptom experience in adults with HIV. Journal of the Association of Nurses in AIDS Care. 2011;22(4):257–268. doi: 10.1016/j.jana.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielissen MF, Verhagen CA, Bleijenberg G. Cognitive behaviour therapy for fatigued cancer survivors: Long-term follow-up. British Journal of Cancer. 2007;97(5):612–618. doi: 10.1038/sj.bjc.6603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Mahrer NE, Yee J, et al. Pain, fatigue and health-related quality of life in children and adolescents with chronic pain. Clinical Journal of Pain. 2009;25(5):407–412. doi: 10.1097/AJP.0b013e318192bfb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall K, Meintjes H, Sambu W, et al. Demography of South Africa’s children. In: Mathews S, Jamieson L, Lake L, et al., editors. South African Child Gauge. Cape Town, South Africa: University of Cape Town; 2014. pp. 90–93. [Google Scholar]
- Harmon JL, Barroso J, Pence BW, et al. Demographic and illness-related variables associated with HIV-related fatigue. Journal of the Association of Nurses in AIDS Care. 2008;19(2):90–97. doi: 10.1016/j.jana.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett S, Chalder T, Choy E, et al. Fatigue in rheumatoid arthritis: Time for a conceptual model. Rheumatology. 2011a;50(6):1004–1006. doi: 10.1093/rheumatology/keq282. [DOI] [PubMed] [Google Scholar]
- Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (Braf Mdq), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (Braf Nrs) for severity, effect, and coping, Chalder Fatigue Questionnaire (Cfq), Checklist Individual Strength (Cis20r and Cis8r), Fatigue Severity Scale (Fss), Functional Assessment Chronic Illness Therapy (Fatigue) (Facit-F), Multi-Dimensional Assessment of Fatigue (Maf), Multi-Dimensional Fatigue Inventory (Mfi), Pediatric Quality of Life (Pedsql) Multi-Dimensional Fatigue Scale, Profile of Fatigue (Prof), Short Form 36 Vitality Subscale (Sf-36 Vt), and Visual Analog Scales (Vas) Arthritis Care and Research. 2011b;63(Suppl. 11):S263–S286. doi: 10.1002/acr.20579. [DOI] [PubMed] [Google Scholar]
- Hockenberry MJ, Hinds PS, Barrera P, et al. Three instruments to assess fatigue in children with cancer: The child, parent and staff perspectives. Journal of Pain and Symptom Management. 2003;25(4):319–328. doi: 10.1016/s0885-3924(02)00680-2. [DOI] [PubMed] [Google Scholar]
- Holzemer WL. HIV and AIDS: The symptom experience: What cell counts and viral loads won’t tell you. The American Journal of Nursing. 2002;102(4):48–52. doi: 10.1097/00000446-200204000-00023. [DOI] [PubMed] [Google Scholar]
- Jacobsen PB. Assessment of fatigue in cancer patients. Journal of the National Cancer Institute: Monographs. 2004;32:93–97. doi: 10.1093/jncimonographs/lgh010. [DOI] [PubMed] [Google Scholar]
- Jong E, Oudhoff LA, Epskamp C, et al. Predictors and treatment strategies of HIV-related fatigue in the combined antiretroviral therapy era. AIDS. 2010;24(10):1387–1405. doi: 10.1097/QAD.0b013e328339d004. [DOI] [PubMed] [Google Scholar]
- Justice AC, Rabeneck L, Hays RD, et al. Sensitivity, specificity, reliability, and clinical validity of provider-reported symptoms: A comparison with self-reported symptoms. Journal of Acquired Immune Deficiency Syndromes. 1999;21(2):126–133. [PubMed] [Google Scholar]
- Knight SJ, Scheinberg A, Harvey AR. Interventions in pediatric chronic fatigue syndrome/myalgic encephalomyelitis: A systematic review. Journal of Adolescent Health. 2013;53(2):154–165. doi: 10.1016/j.jadohealth.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Lam PK, Naar-King S, Wright K. Social support and disclosure as predictors of mental health in HIV-positive youth. AIDS Patient Care and STDs. 2007;21(1):20–29. doi: 10.1089/apc.2006.005. [DOI] [PubMed] [Google Scholar]
- Lee KA, Portillo CJ, Miramontes H. The influence of sleep and activity patterns on fatigue in women with HIV/AIDS. Journal of the Association of Nurses in AIDS Care. 2001;12:19–27. doi: 10.1177/105532901773742257. [DOI] [PubMed] [Google Scholar]
- Levine J, Greenwald BD. Fatigue in Parkinson disease, stroke, and traumatic brain injury. Physical Medicine and Rehabilitation Clinics. 2009;20(2):347–361. doi: 10.1016/j.pmr.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Levy-Marchal C, Patterson C, Green A, et al. Geographical variation of presentation at diagnosis of Type I diabetes in children: The EURODIAB study. Diabetologia. 2001;44(3):B75–B80. doi: 10.1007/pl00002958. [DOI] [PubMed] [Google Scholar]
- Linder L. Analysis of the UCSF Symptom Management Theory: Implications for pediatric oncology nursing. Journal of Pediatric Oncology Nursing. 2010;27(6):316–324. doi: 10.1177/1043454210368532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd S, Chalder T, Sallis HM, et al. Telephone-based guided self-help for adolescents with chronic fatigue syndrome: A nonrandomised cohort study. Behaviour Research and Therapy. 2012;50(5):304–312. doi: 10.1016/j.brat.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Moss-Morris R, Spence M, Hou R. The pathway from glandular fever to chronic fatigue syndrome: Can the cognitive behavioural model provide the map? Psychological Medicine. 2011;41(05):1099–1107. doi: 10.1017/S003329171000139X. [DOI] [PubMed] [Google Scholar]
- Naar-King S, Templin T, Wright K, et al. Psychosocial factors and medication adherence in HIV-positive youth. AIDS Patient Care & STDs. 2006;20(1):44–47. doi: 10.1089/apc.2006.20.44. [DOI] [PubMed] [Google Scholar]
- Nel A, Kagee A. The relationship between depression, anxiety and medication adherence among patients receiving antiretroviral treatment in South Africa. AIDS Care. 2013;25(8):948–955. doi: 10.1080/09540121.2012.748867. [DOI] [PubMed] [Google Scholar]
- Nijhof SL, Bleijenberg G, Uiterwaal CS, et al. Fatigue in teenagers on the internet – The FITNET Trial. A randomized clinical trial of web-based cognitive behavioural therapy for adolescents with chronic fatigue syndrome: Study protocol. [Isrctn59878666] BMC Neurology. 2011;11:23. doi: 10.1186/1471-2377-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton S, Cosco T, Doyle F, et al. The Hospital Anxiety and Depression Scale: A meta confirmatory factor analysis. Journal of Psychosomatic Research. 2013;74(1):74–81. doi: 10.1016/j.jpsychores.2012.10.010. [DOI] [PubMed] [Google Scholar]
- Nutini M, Karczewski M, Capoor J. Fatigue in children with neurologic impairments. Physical Medicine and Rehabilitation Clinics of North America. 2009;20(2):339–346. doi: 10.1016/j.pmr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Paddison J, Fricchione G, Gandhi RT, et al. Fatigue in psychiatric HIV patients: A pilot study of psychological correlates. Psychosomatics. 2009;50(5):455–460. doi: 10.1176/appi.psy.50.5.455. [DOI] [PubMed] [Google Scholar]
- Pao M, Lyon M, D’Angelo LJ, et al. Psychiatric diagnoses in adolescents seropositive for the human immunodeficiency virus. Archives of Pediatrics & Adolescent Medicine. 2000;154(3):240–244. doi: 10.1001/archpedi.154.3.240. [DOI] [PubMed] [Google Scholar]
- Perkins DO, Stern RA, Golden RN. Mood disorders in HIV infection: Prevalence and risk factors in a nonepicenter of the AIDS epidemic. American Journal of Psychiatry. 1994;151:233–236. doi: 10.1176/ajp.151.2.233. [DOI] [PubMed] [Google Scholar]
- Rimes K, Goodman R, Hotopf M, et al. Incidence, prognosis, and risk factors for fatigue and chronic fatigue syndrome in adolescents: A prospective community study. Pediatrics. 2007;119(3):E603–E609. doi: 10.1542/peds.2006-2231. [DOI] [PubMed] [Google Scholar]
- Semple RJ, Lee J, Miller LF. Mindfulness-based cognitive therapy for children. In: Baer R, editor. Mindfulness-Based Treatment Approaches: Clinician’s Guide to Evidence Base and Applications. Burlington, MA: Academic Press; 2006. pp. 143–166. [Google Scholar]
- Singh N, Squier C, Sivek C, et al. Psychological stress and depression in older patients with intravenous drug use and human immunodeficiency virus infection: Implications for intervention. International Journal of STD & AIDS. 1997;8(4):251–255. doi: 10.1258/0956462971920000. [DOI] [PubMed] [Google Scholar]
- Ter Wolbeek M, Van Doornen LJ, Kavelaars A, et al. Severe fatigue in adolescents: A common phenomenon? Pediatrics. 2006;117(6):E1078–E1086. doi: 10.1542/peds.2005-2575. [DOI] [PubMed] [Google Scholar]
- UNAIDS. Joint United Nations Programme on HIV/AIDS AIDS by the numbers. Geneva: 2015. [accessed 9 May 2017]. Available at: http://aidsinfo.unaids.org/ [Google Scholar]
- Van Kessel K, Moss-Morris R, Willoughby E, et al. A randomized controlled trial of cognitive behavior therapy for multiple sclerosis fatigue. Psychosomatic Medicine. 2008;70(2):205–213. doi: 10.1097/PSY.0b013e3181643065. [DOI] [PubMed] [Google Scholar]
- Varni JW, Burwinkle TM, Szer IS. The PedsQL Multidimensional Fatigue Scale in pediatric rheumatology: Reliability and validity. The Journal of Rheumatology. 2004;31(12):2494–2500. [PubMed] [Google Scholar]
- Voss JG. Predictors and correlates of fatigue in HIV/AIDS. Journal of Pain and Symptom Management. 2005;29(2):173–184. doi: 10.1016/j.jpainsymman.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Voss JG, Dodd M, Portillo C, et al. Theories of fatigue: Application in HIV/AIDS. Journal of the Association of Nurses in AIDS Care. 2006;17(1):37–50. doi: 10.1016/j.jana.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Voss JG, Portillo CJ, Holzemer WL, et al. Symptom cluster of fatigue and depression in HIV/AIDS. Journal of Prevention & Intervention in the Community. 2007a;33(1–2):19–34. doi: 10.1300/J005v33n01_03. [DOI] [PubMed] [Google Scholar]
- Voss JG, Sukati NA, Seboni NM, et al. Symptom burden of fatigue in men and women living with HIV/AIDS in Southern Africa. Journal of the Association of Nurses in AIDS Care. 2007b;18(4):22–31. doi: 10.1016/j.jana.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of life in children with cancer. New England Journal of Medicine. 2000;342(5):326–333. doi: 10.1056/NEJM200002033420506. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Adolescents: Health risks and solutions. [accessed 1 December 2016];2016 ( http://www.who.int/mediacentre/factsheets/fs345/en/
- Wu HS, McSweeney M. Cancer-related fatigue: ‘It’s so much more than just being tired’. European Journal of Oncology Nursing. 2007;11(2):117–125. doi: 10.1016/j.ejon.2006.04.037. [DOI] [PubMed] [Google Scholar]