Abstract
One of the major challenges in cancer chemotherapy is the development of multidrug resistance phenomenon attributed to the overexpression of ATP-binding cassette (ABC) transporter ABCB1 or ABCG2 in cancer cells. Therefore, re-sensitizing MDR cancer cells to chemotherapy by directly inhibiting the activity of ABC transporters has clinical relevance. Unfortunately, previous attempts of developing clinically applicable synthetic inhibitors have failed, mostly due to problems associated with toxicity and unforeseen drug-drug interactions. An alternative approach is by repositioning drugs with known pharmacological properties as modulators of ABCB1 and ABCG2. In this study, we discovered that the transport function of ABCB1 and ABCG2 is strongly inhibited by SIS3, a specific inhibitor of Smad3. More importantly, SIS3 enhances drug-induced apoptosis and resensitizes ABCB1- and ABCG2-overexpressing cancer cells to chemotherapeutic drugs at non-toxic concentrations. These findings are further supported by ATPase assays and by a docking analysis of SIS3 in the drug-binding pockets of ABCB1 and ABCG2. In summary, we revealed an additional action of SIS3 that re-sensitizes MDR cancer cells and a combination therapy with this drug and other chemotherapeutic agents may be beneficial for patients with MDR tumors.
Keywords: Multidrug resistance, ABCB1, ABCG2, SMAD3, SIS3
1. Introduction
One of the major challenges in cancer chemotherapy is the development of multidrug resistance (MDR) attribute to the overexpression of one of the ATP-Binding Cassette (ABC) transporters such as ABCB1 (P-glycoprotein/ MDR1) and ABCG2 (BCRP; MXR) [15, 16, 54]. These transporters can generate energy from ATP hydrolysis to actively transport anticancer drugs away from their targets within a cancer cell, resulting in MDR phenotype [15, 45, 68]. Together, ABCB1 and ABCG2 are capable of transporting a majority of conventional anticancer drugs that are functionally and structurally unrelated, including but not limited to Vinca alkaloids, anthracyclines, methotrexate, topotecan, etoposide, SN-38, as well as many protein kinase inhibitors [17, 19, 37, 63]. The overexpression of ABCB1 and/or ABCG2 has been linked to the MDR phenotype in a variety of blood and solid tumors, including acute lymphocytic leukemia (ALL), acute myelogenous leukemia (AML) [44, 52, 60], chronic lymphocytic leukemia (CLL) [33], multiple myeloma (MM) [20, 35, 39, 40, 46, 58, 59], advanced non-small cell lung cancer [71] and metastatic breast cancer [27]. Moreover, both ABCB1 and ABCG2 are highly expressed in cells forming the blood-tissue barrier sites such as gastrointestinal tract, liver, kidney and the blood-brain barrier (BBB), affecting the oral bioavailability, distribution, metabolism and elimination of most drugs in patients [8, 17, 54].
One of the most effective approaches to overcome MDR mediated by ABCB1 or ABCG2 is to directly inhibit the drug efflux function of the transporter in cancer cells [64]. Although substantial efforts have been invested in recent years to develop novel compounds that can inhibit the function of ABCB1 and ABCG2, there is still no modulator available to treat patients with MDR tumors. The problems with developing clinically applicable synthetic inhibitors are often associated with the lack of potency or selectivity, high intrinsic toxicity or adverse drug-drug interactions [50, 54]. As a result, we and others have applied the drug repositioning (or drug repurposing) approach to discover drugs with known pharmacological and toxicological properties in the treatment of MDR cancer [34, 38, 50, 64]. In recent years, many inhibitors of protein kinases and signaling molecules, especially inhibitors of the epidermal growth factor receptor (EGFR), have been shown to reverse MDR mediated by ABCB1 or ABCG2 in cancer cells [23, 26, 48, 49, 51, 65]. However, many of these inhibitors are both transport substrates and/or inhibitors of an ABC drug transporter [19]. For instance, gefitinib (Iressa) inhibits the function of ABCB1 [26], whereas ABCG2 effluxes and reduces the efficacy of gefitinib in cancer cells [13].
In the present study, we investigated the interaction of SIS3 with ABCB1 and ABCG2 in cancer cell lines. SIS3 is a specific inhibitor of Smad3, known for its potent inhibitory activity against transforming growth factor (TGF)-β1-induced phosphorylation of Smad3 [25]. Therefore, SIS3 has been used frequently as a benchmark inhibitor in studies evaluating TGF-β-regulated cellular mechanisms [9, 28, 30, 32, 41, 61, 62, 72]. Our data show that by inhibiting the drug transport function of ABCB1 and ABCG2, SIS3 enhances drug-induced apoptosis and reverses MDR in cancer cells overexpressing ABCB1 or ABCG2. In summary, our results suggest that combination therapy of SIS3 and anticancer agents may be beneficial for patients with MDR tumors, and should be further investigated.
2. Materials and methods
2.1. Chemicals
RPMI medium, Iscove’s modified Dulbecco’s medium (IMDM), Dulbecco’s Modified Eagle’s medium (DMEM), Phosphate-buffered saline (PBS), fetal calf serum (FCS), trypsin-EDTA, penicillin, and streptomycin were purchased from Gibco, Invitrogen (CA, USA). Annexin V : FITC Apoptosis Detection Kit was purchased from BD Pharmingen (San Diego, CA, USA). Tools Cell Counting (CCk-8) Kit was purchased from Biotools Co., Ltd (Taipei, Taiwan). SIS3, verapamil, Ko143 and all other chemicals were purchased from Sigma (St. Louis, MO, USA), unless stated otherwise.
2.2. Cell culture conditions
The human OVCAR-8 ovarian cancer cell line and the ABCB1-overexpressing variant NCI-ADR-RES, human KB-3–1 epidermal cancer cell line and the ABCB1-overexpressing variant KB-V-1, human MCF-7 breast cancer cell line and the ABCG2-overexpressing variants MCF7-FLV1000 and MCF7-AdVp3000, human H460 non-small cell lung cancer cell line and the ABCG2-overexpressing variant H460-MX20, as well as pcDNA3.1-HEK293, ABCB1-transfected MDR19-HEK293 and ABCG2-transfected R482-HEK293, were cultured in DMEM. The human S1 colon cancer cell line and the ABCG2-overexpressing variant S1-M1–80 were cultured in RPMI-1640. HEK293 and HEK293 transfected cells were maintained in media containing 2 mg/mL G418 [69], KB-V-1 cells were maintained in 1 mg/mL vinblastine [47], MCF7-FLV1000 cells were maintained in media containing 1 μg/mL flavopiridol [22, 42], MCF7-AdVp3000 cells were maintained in media containing 3 μg/mL doxorubicin and 5 μg/mL verapamil [22], and S1-M1–80 cells were maintained in 80 μM of mitoxantrone [69]. All cell lines were cultured in medium supplemented with 10% FCS, 2 mM L-glutamine and 100 units of penicillin/streptomycin/mL, maintained at 37 °C in 5% CO2 humidified air and placed in drug-free medium 7 days prior to assay.
2.3. Fluorescent drug accumulation assay
The intracellular accumulation of fluorescent substrates was recorded using a FACScan flow cytometer (BD Biosciences) and analyzed using CellQuest software (Becton-Dickinson) according to the method described by Gribar et al [18]. Briefly, after harvesting cells by trypsinization and centrifugation, 3 × 105 cells were resuspended in 4 mL of IMDM supplemented with 5% FCS before ABCB1 substrate calcein-AM (0.5 μM) or ABCG2 substrate pheophorbide A (1 μM) was added to the cell suspension in the presence or absence of SIS3, verapamil (an inhibitor of ABCB1), or Ko143 (an inhibitor of ABCG2), as described previously [43]. Calcein fluorescence was detected with excitation and emission wavelengths of 485 and 535 nm, whereas pheophorbide A fluorescence was detected with excitation and emission wavelengths of 395 and 670 nm.
2.4. Immunoblotting
Primary antibodies C219 (1: 3000), BXP-21 (1:15000) and α-tubulin (1:100000) were used in Western blot immunoassay to detect ABCB1, ABCG2 and positive control tubulin, respectively. The horseradish peroxidase-conjugated goat anti-mouse IgG (1:100000) was used as the secondary antibody. Signals were detected as described previously [69].
2.5. Cytotoxicity assay
Cytotoxicity assays were carried out to determine the sensitivities of cells to tested drugs according to the method described by Ishiyama et al [24]. Briefly, cells were plated in each well of 96-well plates at a density of 5000 cells per well in 100 μL of culture medium and maintained at 37 °C. After 24 h, an additional 100 μL of tested drug at various concentrations was added to each well and incubated for an additional 72 h before developing with either Cell Counting Kit-8 (CCK) or MTT reagent. CCK assay was used to determine the cytotoxicity of drugs in HEK293 and HEK293 transfected cells, whereas MTT assay was used to determine the cytotoxicity of drugs in human cancer cell lines. For the MDR reversal assays, nontoxic concentrations of SIS3 or a known inhibitor of ABCB1 or ABCG2, were added to the cytotoxicity assays. The extent of reversal was determined based on the calculated fold-reversal (FR) values, as described previously [12].
2.6. Apoptosis assay
The percentage of apoptotic cells in the total cell population induced by the indicated regimens was determined using the conventional Annexin V-FITC and propidium iodide (PI) staining method, as described previously [23]. Briefly, cells were first treated with colchicine, topotecan, SIS3 or in combinations as indicated for 48 h before harvested, centrifuged and resuspended in FACS buffer containing 1.25 μg/mL Annexin V-FITC (PharMingen) and 0.1 mg/mL PI and incubated for 15 min at room temperature. The labeled cells (10000 per sample) were analyzed by FACScan using CellQuest software (BD Biosciences). Phosphatidylserine PS-positive and PI-negative cells were counted as early apoptotic cells with intact plasma membranes, whereas PS-positive and PI-positive cells are considered as either necrotic or late apoptotic with leaky membranes [4].
2.7. ATPase assay
The effect of SIS3 on vanadate (Vi)-sensitive ATPase activity of ABCB1 or ABCG2 was determined using membrane vesicles of High-Five cells expressing ABCB1 or ABCG2 based on the endpoint Pi assay as described previously [1].
2.8. Docking analysis of SIS3 with ABCG2 and modeled structure of ABCB1
The three dimensional structure of human ABCB1 was predicted using an automated protein homology-modeling server SWISS-MODEL. The amino acid sequence of the protein was submitted to SWISS-MODEL server and templates were searched with BLAST and HHBlits against SWISS-MODEL template library. For each identified template, its quality was predicted from features of the target-template alignment. The templates with the highest quality were then selected and built based on the target-template alignment using ProMod3 [5–7]. The energy was minimized for ABCB1 homology modeled structure based on the structure of mouse Abcb1a and ABCG2 protein structure (PDB:5NJG) [57] using Acclerys Discovery Studio 4.0. Ligand preparation and docking was performed by the CDOCKER module of the same software.
2.9. Quantification and statistical analysis
Experimental values including IC50 are presented as mean ± standard deviation (SD) calculated from at least three independent experiments. In some cases where indicated, the values are given as mean ± standard error of the mean (SEM). Curve plotting and statistical analysis were performed with KaleidaGraph (Reading, PA, USA) and GraphPad Prism (La Jolla, CA, USA) software. The improvement in fit was analyzed by two-sided Student’s t-test and labeled “statistically significant” if the probability, p, was less than 0.05.
3. Results
3.1. SIS3 inhibits ABCB1- and ABCG2-mediated drug transport
We first examined the effect of SIS3 on the drug transport function and the protein expression of ABCB1 or ABCG2. As shown in Fig. 1, the accumulation of calcein, a fluorescent product of calcein-AM, which is a substrate drug of ABCB1[21], was measured in human KB-3–1 epidermal cancer cells and the ABCB1-overexpressing variant KB-V-1cells, as well as in HEK293 cells and HEK293 cells transfected with human ABCB1 (MDR19-HEK293) treated with DMSO (solid lines), 10 μM of SIS3 (shaded, solid lines) or 10 μM of ABCB1 reference inhibitor verapamil (dotted lines). On the other hand, the accumulation of pheophorbide A (PhA), a fluorescent substrate drug of ABCG2[43], was measured in human H460 non-small cell lung cancer cells and the ABCG2-overexpressing variant H460-MX20 cells, as well as in HEK293 cells and HEK293 cells transfected with human ABCG2 (R482-HEK293) treated with DMSO (solid lines), 10 μM of SIS3 (shaded, solid lines) or 10 μM of ABCG2 reference inhibitor Ko143 (dotted lines), and analyzed as described in Materials and methods. SIS3 significantly inhibited ABCB1-medaited transport of calcein from KB-V-1 cells (Fig. 1A, right panel) and MDR19-HEK293 (Fig. 1B, right panel), as well as ABCG2-mediated transport of PhA fom H460-MX20 cells (Fig. 1C, right panel) and R482-HEK293 cells (Fig. 1D, right panel). Of note, SIS3 had no significant effect on the accumulation of fluorescent substrates in any of the drug-sensitive parental cells (Fig. 1B–1D, left panels).
Fig. 1. SIS3 inhibits drug efflux mediated by ABCB1 and ABCG2.
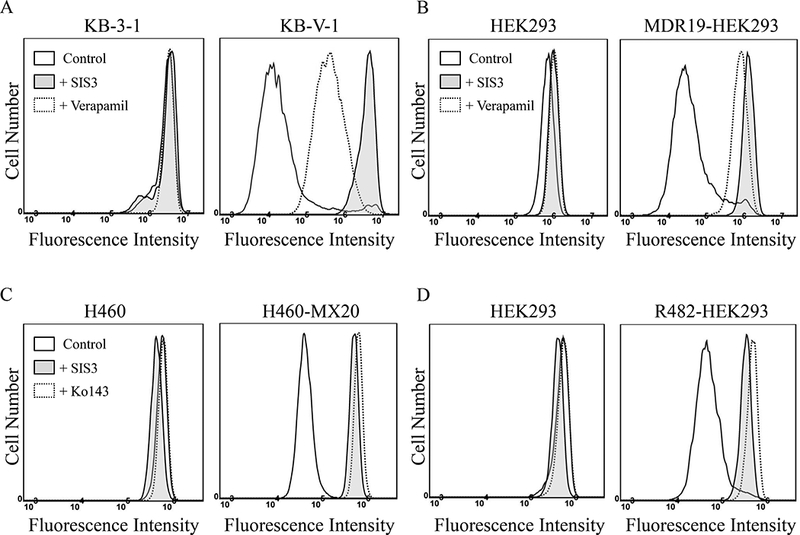
The accumulation of fluorescent calcein (0.5 μM calcein-AM) in human KB-3–1 epidermal cancer cells (A, left panel) and the ABCB1-overexpressing variant KB-V-1 cancer cells (A, right panel), as well as in human HEK293 cells (B, left panel) and MDR19-HEK293, HEK293 cells transfected with human ABCB1 (B, right panel), or 1 μM of fluorescent pheophorbide A (PhA) in human H460 lung cancer cells (C, left panel) and the ABCG2-overexpressing variant H460-MX20 cancer cells (C, right panel), as well as in human HEK293 cells (D, left panel) and R482-HEK293, HEK293 cells transfected with human ABCG2 (D, right panel), was measured and analyzed immediately by flow cytometry as described previously [70]. Experiments were carried out either in the absence (solid lines) or presence of SIS3 at10 μM (shaded, solid lines) or verapamil, a reference inhibitor for ABCB1at 20 μM (A and B, dotted lines), or Ko143, a reference inhibitor for ABCG2 at 3 μM (C and D, dotted lines). Representative histograms of at least three independent experiments are shown.
3.2. SIS3 reverses multidrug resistance mediated by ABCB1 and ABCG2
Providing that SIS3 is able to block the drug efflux function of both ABCB1 and ABCG2, we next examined the chemosensitization effect of SISs in cells overexpressing ABCB1 or ABCG2. We found that at non-toxic concentrations and without affecting the proliferation of parental cells (Fig. 2, left panels), SIS3 re-sensitized KB-V-1 cells and MDR19-HEK293 cells to paclitaxel (Fig. 2A, right panel and Table 1) and colchicine (Fig. 2B and 2C) in a concentration-dependent manner. Similarly, SIS3 reversed ABCG2-mediated drug resistance to mitoxantrone and SN-38 in S1-M1–80 cells (Fig. 2D and 2E, right panels) and R482-HEK293 cells (Table 1 and Fig. 2C). The MDR reversal effect of SIS3 was further tested in ABCB1-overexpressing NCI-ADR-RES and ABCG2-overexpressing H460-MX20 cancer cell lines. The IC50 values and the extent of reversal of multidrug-resistant cells to a particular drug by SIS3, which was calculated as the fold-reversal (FR) values [12], are summarized in Table 1. Of note, verapamil and Ko143 were used as positive controls for the reversal of drug resistance mediated by ABCB1 and ABCG2 [66]. Our results revealed that SIS3 is capable of reversing multidrug resistance mediated by ABCB1 and ABCG2 in cancer cells at concentrations below 1 μM.
Fig. 2. SIS3 reverses multidrug resistance mediated by ABCB1and ABCG2 in cancer cell lines.
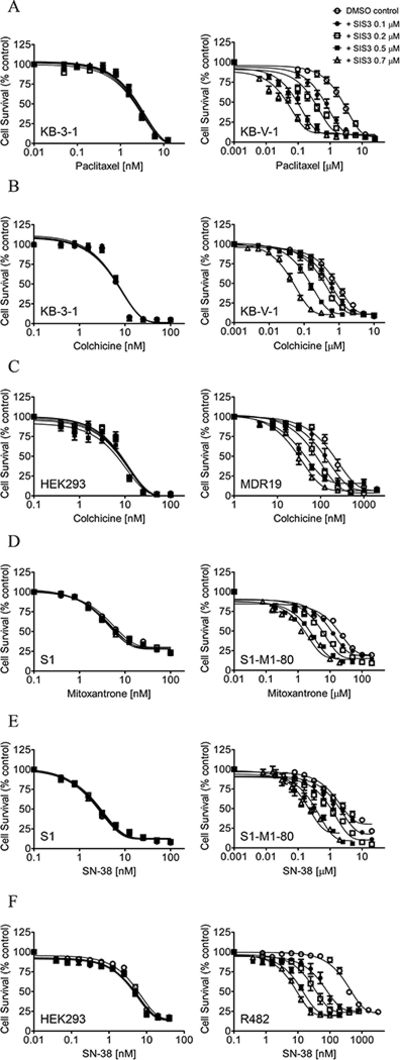
Drug-sensitive KB-3–1 cells (A and B, left panel) and MDR variant KB-V-1 cancer cells (A and B, right panel), HEK293 cells (C, left panel) and MDR19-HEK293 cells (C, right panel), drug-sensitive S1 colon cancer cells (D and E, left panel) and MDR variant S1-M1–80 cancer cells (D and E, right panel), as well as HEK293 cells (F, left panel) and R482-HEK293 cells (F, right panel), were treated with increasing concentrations of paclitaxel (A), colchicine (B and C), mitoxantrone (D), SN-38 (E and F) in the presence of DMSO (empty circles), or SIS3 at 0.1 μM (filled circles), 0.2 μM (empty squares), 0.5 μM (filled squares) or 0.7 μM (empty triangles). Points, mean values from at least three independent experiments; bars; SEM.
Table 1.
Reversal effect of SIS3 on drug resistance mediated by ABCB1 and ABCG2.
| Treatment | Concentration (μM) |
Mean IC50† ± SD and (FR‡) |
|
|---|---|---|---|
| KB-3–1 [nM] | KB-V-1 [nM] | ||
| Colchicine | - | 5.09 ± 1.88 (1.0) | 808.13 ± 41.30 (1.0) |
| + SIS3 | 0.1 | 5.11 ± 1.85 (1.0) | 523.77 ± 40.35** (1.5) |
| + SIS3 | 0.2 | 5.16 ± 1.92 (1.0) | 367.13 ± 43.11*** (2.2) |
| + SIS3 | 0.5 | 5.31 ± 2.07 (1.0) | 136.60 ± 15.69*** (5.9) |
| + SIS3 | 0.7 | 5.10 ± 2.02 (1.0) | 45.51 ± 6.19*** (17.8) |
| + Verapamil | 5.0 | 2.86 ± 1.17 (1.8) | 71.44 ± 7.96*** (11.3) |
| Paclitaxel | - | 1.99 ± 0.53 (1.0) | 2273.60 ± 218.05 (1.0) |
| + SIS3 | 0.1 | 1.90 ± 0.46 (1.0) | 635.44 ± 37.69*** (3.6) |
| + SIS3 | 0.2 | 1.89 ± 0.48 (1.1) | 323.36 ± 42.78*** (7.0) |
| + SIS3 | 0.5 | 2.15 ± 0.50 (1.0) | 92.37 ± 12.79*** (24.6) |
| + SIS3 | 0.7 | 2.08 ± 0.48 (1.0) | 48.19 ± 9.47*** (47.2) |
| + Verapamil | 5.0 | 2.56 ± 0.73 (0.8) | 89.06 ± 12.26*** (25.5) |
|
OVCAR-8 [nM] |
NCI-ADR-RES [nM] |
||
| Colchicine | - | 18.62 ± 3.45 (1.0) | 1730.54 ± 317.81 (1.0) |
| + SIS3 | 0.1 | 19.29 ± 3.28 (1.0) | 1019.80 ± 216.52* (1.7) |
| + SIS3 | 0.2 | 19.74 ± 3.14 (1.0) | 681.85 ± 148.18** (2.5) |
| + SIS3 | 0.5 | 17.65 ± 3.09 (1.1) | 277.86 ± 56.22** (6.2) |
| + SIS3 | 0.7 | 16.56 ± 3.09 (1.1) | 142.47 ± 16.16** (12.1) |
| + Verapamil | 5.0 | 11.92 ± 2.56 (1.6) | 518.26 ± 96.19** (3.3) |
| Paclitaxel | - | 79.60 ± 23.93 (1.0) | 6385.00 ± 959.34 (1.0) |
| + SIS3 | 0.1 | 73.43 ± 25.60 (1.1) | 3147.20 ± 267.22** (2.0) |
| + SIS3 | 0.2 | 62.07 ± 20.33 (1.3) | 2102.74 ± 267.40** (3.0) |
| + SIS3 | 0.5 | 58.53 ± 16.68 (1.4) | 466.38 ± 46.62*** (13.7) |
| + SIS3 | 0.7 | 53.56 ± 12.95 (1.5) | 328.34 ± 51.72*** (19.4) |
| + Verapamil | 5.0 | 46.90 ± 15.15 (1.7) | 184.35 ± 22.73*** (34.6) |
|
pcDNA-HEK293 [nM] |
MDR19-HEKS293 [nM] |
||
| Colchicine | - | 8.20 ± 2.42 (1.0) | 183.66 ± 35.56 (1.0) |
| + SIS3 | 0.1 | 8.14 ± 2.26 (1.0) | 111.59 ± 23.66* (1.6) |
| + SIS3 | 0.2 | 8.09 ± 2.33 (1.0) | 66.03 ± 15.18** (2.8) |
| + SIS3 | 0.5 | 7.18 ± 1.80 (1.1) | 45.05 ± 8.65** (4.1) |
| + SIS3 | 0.7 | 7.98 ± 2.38 (1.0) | 26.76 ± 5.17** (6.9) |
| + Verapamil | 5.0 | 8.14 ± 2.44 (1.0) | 40.40 ± 9.30** (4.5) |
| Paclitaxel | - | 1.53 ± 0.26 (1.0) | 835.30 ± 128.85 (1.0) |
| + SIS3 | 0.1 | 1.24 ± 0.18 (1.2) | 479.04 ± 36.49** (1.7) |
| + SIS3 | 0.2 | 1.25 ± 0.19 (1.2) | 297.83 ± 48.57** (2.8) |
| + SIS3 | 0.5 | 1.27 ± 0.20 (1.2) | 73.94 ± 9.18*** (11.3) |
| + SIS3 | 0.7 | 1.20 ± 0.17 (1.3) | 60.43 ± 5.05*** (13.8) |
| + Verapamil | 5.0 | 0.90 ± 0.16* (1.7) | 6.14 ± 1.23*** (136.0) |
|
H460 [nM] |
H460-MX20 [nM] |
||
| SN-38 | - | 4.49 ± 1.02 (1.0) | 138.71 ± 32.27 (1.0) |
| + SIS3 | 0.1 | 4.15 ± 1.07 (1.1) | 36.67 ± 8.01** (3.8) |
| + SIS3 | 0.2 | 5.14 ± 0.90 (0.9) | 20.52 ± 5.35** (6.8) |
| + SIS3 | 0.5 | 3.76 ± 0.67 (1.2) | 11.65 ± 3.48** (11.9) |
| + SIS3 | 0.7 | 3.34 ± 0.58 (1.3) | 4.84 ± 1.28** (28.7) |
| + Ko143 | 1 | 4.10 ± 0.75 (1.1) | 4.45 ± 1.06** (31.2) |
| Mitoxantrone | - | 13.73 ± 1.19 (1.0) | 488.15 ± 81.40 (1.0) |
| + SIS3 | 0.1 | 20.54 ± 5.79 (0.7) | 342.70 ± 85.71 (1.4) |
| + SIS3 | 0.2 | 15.61 ± 4.68 (0.9) | 227.99 ± 58.92* (2.1) |
| + SIS3 | 0.5 | 16.70 ± 4.84 (0.8) | 77.22 ± 18.04** (6.3) |
| + SIS3 | 0.7 | 17.00 ± 5.41 (0.8) | 55.71 ± 21.69** (8.8) |
| + Ko143 | 1 | 15.93 ± 5.27 (0.9) | 70.14 ± 19.37** (7.0) |
|
S1 [nM] |
S1-M1–80 [μM] |
||
| SN-38 | - | 2.32 ± 0.28 (1.0) | 3.83 ± 0.65 (1.0) |
| + SIS3 | 0.1 | 2.58 ± 0.30 (0.9) | 2.33 ± 0.30* (1.6) |
| + SIS3 | 0.2 | 2.76 ± 0.32 (0.8) | 1.05 ± 0.12** (3.6) |
| + SIS3 | 0.5 | 2.36 ± 0.26 (1.0) | 0.32 ± 0.03*** (12.0) |
| + SIS3 | 0.7 | 2.31 ± 0.29 (1.0) | 0.23 ± 0.02*** (16.7) |
| + Ko143 | 1 | 2.22 ± 0.28 (1.0) | 0.05 ± 0.01*** (76.6) |
| Mitoxantrone | - | 11.48 ± 2.70 (1.0) | 24.09 ± 5.80 (1.0) |
| + SIS3 | 0.1 | 9.60 ± 2.21 (1.2) | 15.17 ± 2.82 (1.6) |
| + SIS3 | 0.2 | 9.61 ± 2.49 (1.2) | 6.95 ± 1.22** (3.5) |
| + SIS3 | 0.5 | 8.20 ± 2.01 (1.4) | 2.79 ± 0.48** (8.6) |
| + SIS3 | 0.7 | 8.03 ± 2.09 (1.4) | 1.88 ± 0.33** (12.8) |
| + Ko143 | 1 | 10.42 ± 2.56 (1.1) | 0.87 ± 0.13** (27.7) |
|
pcDNA-HEK293 [nM] |
R482-HEKS293 [nM] |
||
| SN-38 | - | 6.53 ± 0.98 (1.0) | 535.00 ± 63.14 (1.0) |
| + SIS3 | 0.1 | 5.88 ± 0.95 (1.1) | 114.69 ± 21.08*** (4.7) |
| + SIS3 | 0.2 | 5.51 ± 0.84 (1.2) | 50.09 ± 11.35*** (10.7) |
| + SIS3 | 0.5 | 5.38 ± 0.83 (1.2) | 17.61 ± 3.69*** (30.4) |
| + SIS3 | 0.7 | 5.17 ± 0.83 (1.3) | 10.96 ± 2.16*** (48.8) |
| + Ko143 | 1 | 5.50 ± 0.87 (1.2) | 7.18 ± 1.73*** (74.5) |
| Mitoxantrone | - | 0.74 ± 0.11 (1.0) | 122.15 ± 16.21 (1.0) |
| + SIS3 | 0.1 | 1.00 ± 0.13 (0.7) | 29.48 ± 2.72*** (4.1) |
| + SIS3 | 0.2 | 0.90 ± 0.13 (0.8) | 15.94 ± 2.14*** (7.7) |
| + SIS3 | 0.5 | 0.62 ± 0.09 (1.2) | 10.95 ± 1.65*** (11.2) |
| + SIS3 | 0.7 | 0.65 ± 0.07 (1.1) | 8.74 ± 1.42*** (14.0) |
| + Ko143 | 1 | 0.81 ± 0.12 (0.9) | 7.14 ± 1.10*** (17.1) |
Abbreviation: FR, fold-reversal.
† IC50 values are mean ± SD calculated from dose-response curves obtained from three independent experiments using cytotoxicity assay as described in Materials and methods.
‡ FR values were calculated by dividing IC50 values of cells treated with a particular anticancer drug in the absence of SIS3 or a reference inhibitor by IC50 values of cells treated with the same anticancer drug in the presence of SIS3 or a reference inhibitor.
P < 0.05;
P < 0.01;
P < 0.001
Knowing that transient down-regulation of ABCB1 or ABCG2 can also lead to the re-sensitization of MDR cancer cells to conventional anticancer agents [11, 36], we examined effect of SIS3 on the protein expression of ABCB1 and ABCG2 in KB-V-1 and H460-MX20 cancer cells, respectively. We found that treating cancer cells with increasing concentrations of SIS3 (0 – 1 μM) for 72 h has no significant effect on ABCB1 protein expression in KB-V-1 cells (Fig. 3A) or ABCG2 protein expression in H460-MX20 cells (Fig. 3B).
Fig. 3. Effect of SIS on ABCB1 protein expression in human KB-V-1 epidermal cancer cells and ABCG2 in human H460-MX20 lung cancer cells.
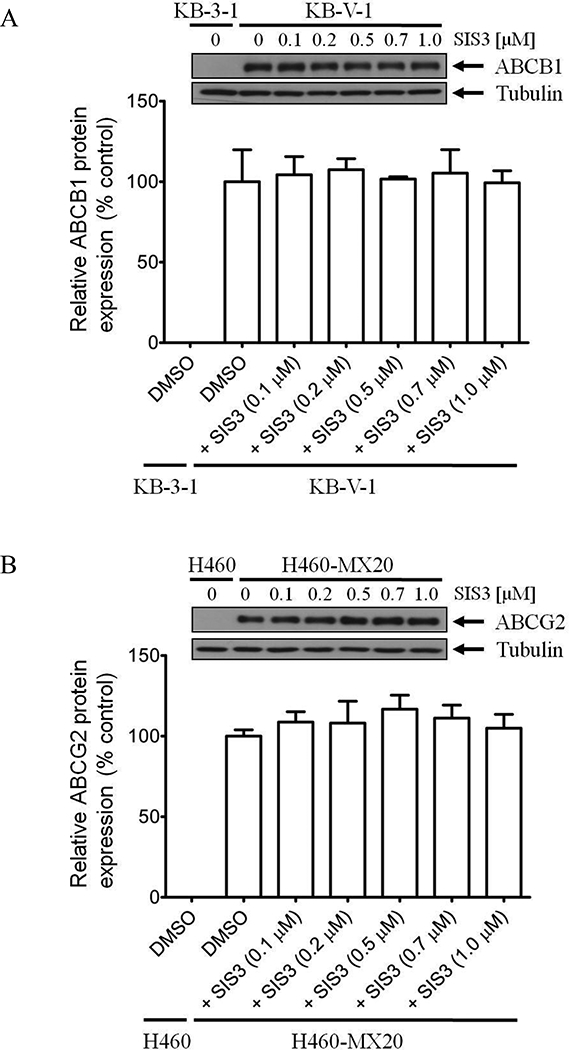
Immunoblot detection and quantification of (A) human ABCB1 in drug-sensitive human KB-3–1 cells and ABCB1-overexpressing KB-V-1 cells, or (B) human ABCG2 in drug-sensitive H460 cells and ABCG2-overexpressing H460-MX20 cells treated with DMSO (vehicle control) or increasing concentrations (0.1 – 1.0 μM) of SIS3 for 72 h according to the method described previously [65]. α-Tubulin was used as an internal loading control. Values are presented as mean ± S.D. calculated from three independent experiments.
3.3. SIS3 enhances drug-induced apoptosis in ABCB1- and ABCG2-overexpressing cancer cells
Next, we evaluated the effect of SIS3 on drug-induced apoptosis in drug-sensitive cancer cells and MDR cancer cells overexpressing ABCB1 or ABCG2. KB-3–1 and KB-V-1 cells were treated with colchicine in the presence or absence of SIS3 for 48 h, whereas S1 and S1-M1–80 cells were treated with topotecan in the presence or absence of SIS3 for 48 h, processed and analyzed as described previously [23, 65]. As shown in Fig. 4A, treatment with colchicine substantially increased the level of apoptosis from approximately 6% to 33% in KB-3–1 cells. In contrast, treatment with colchicine, a known substrate of ABCB1, only increased the level of apoptosis from approximately 8% to 10% in KB-V-1 cells. Interestingly, we found that co-treatment of SIS3 and colchicine significantly enhanced the level of apoptosis from approximately 6% to 49% in KB-V-1 cells. Similarly, treatment with topotecan increases the level of apoptosis in S1 cells, but not in S1-M1–80 cells (from approximately 6% to 7% total apoptosis), which can be significantly enhanced by SIS3, from approximately 6% to 28% total apoptosis (Fig. 4B). Of note, SIS3 alone had no significant effect on the level of apoptosis in all cell lines tested. Our results show that SIS3 enhances the chemosensitivity of MDR cancer cells overexpressing ABCB1 or ABCG2.
Fig. 4. SIS3 enhances drug-induced apoptosis in ABCB1- and ABCG2-overexpressing cancer cells.
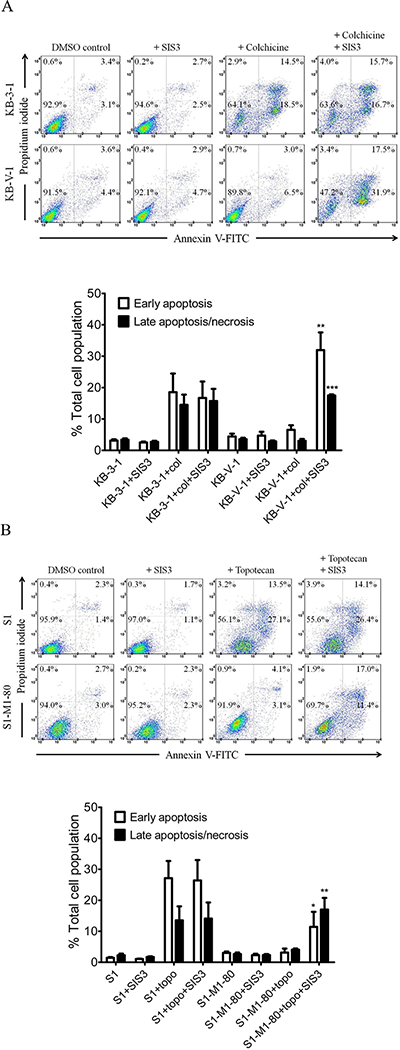
(A) Human KB-3–1 epidermal cancer cells (top dot plots panels) and MDR variant KB-V-1 cancer cells (lower dot plots panels) were treated with either DMSO (control), 1.0 μM SIS3 (+ SIS3), 0.5 μM colchicine (+ colchicine) or a combination of 0.5 μM colchicine and 1.0 μM SIS3 (+ colchicine + SIS3) for 48 h. (B) Human S1 colon cancer cells (top dot plots panel) and MDR variant S1-M1–80 cancer cells (lower dot plots panels) were treated with either DMSO (control), 1.0 μM SIS3 (+ SIS3), 5.0 μM topotecan (+ topotecan) or a combination of 5.0 μM topotecan and 1.0 μM SIS3 (+ topotecan + SIS3) for 48 h. Cells were isolated and analyzed by flow cytometry according to the method described previously [66]. Representative dot plots and the mean values of three independent experiments are shown. Quantifications of drug-induced apoptosis in cancer cell lines are presented as mean ± S.D. calculated from three independent experiments. **P < 0.01; ***P < 0.001, versus the same treatment in the absence of SIS3.
3.4. SIS3 stimulates the ATPase activity of ABCB1 and ABCG2
In order to further understand the interaction of SIS3 with the substrate-binding sites of ABCB1 and ABCG2, we examined the effect of SIS3 on vanadate (Vi)-sensitive ATPase activity of ABCB1 and ABCG2, as well as docking analysis of SIS3 with molecular modeling of ABCB1 and ABCG2. SIS3 stimulated Vi-sensitive ABCB1 ATP hydrolysis in a concentration-dependent manner, producing approximately 250% maximum stimulation (basal, 34.6 ± 4.0 nmole Pi/min/mg protein) and the concentration required to produce 50% of the maximum stimulation (EC50) of approximately 160 nM (Fig. 5A). Similarly, SIS3 also stimulated ABCG2 ATP hydrolysis in a concentration-dependent manner, producing approximately 230% maximum stimulation (basal, 73.3 ± 3.9 nmole Pi/min/mg protein) and the EC50 of approximately 60 nM (Fig. 5B). Knowing that substrate transport mediated by ABCB1 or ABCG2 is coupled to ATP hydrolysis [2, 3], our results indicate that SIS3 is a substrate of both ABCB1 and ABCG2.
Fig. 5. SIS3 stimulates vanadate-sensitive ATPase activity of ABCB1 and ABCG2.
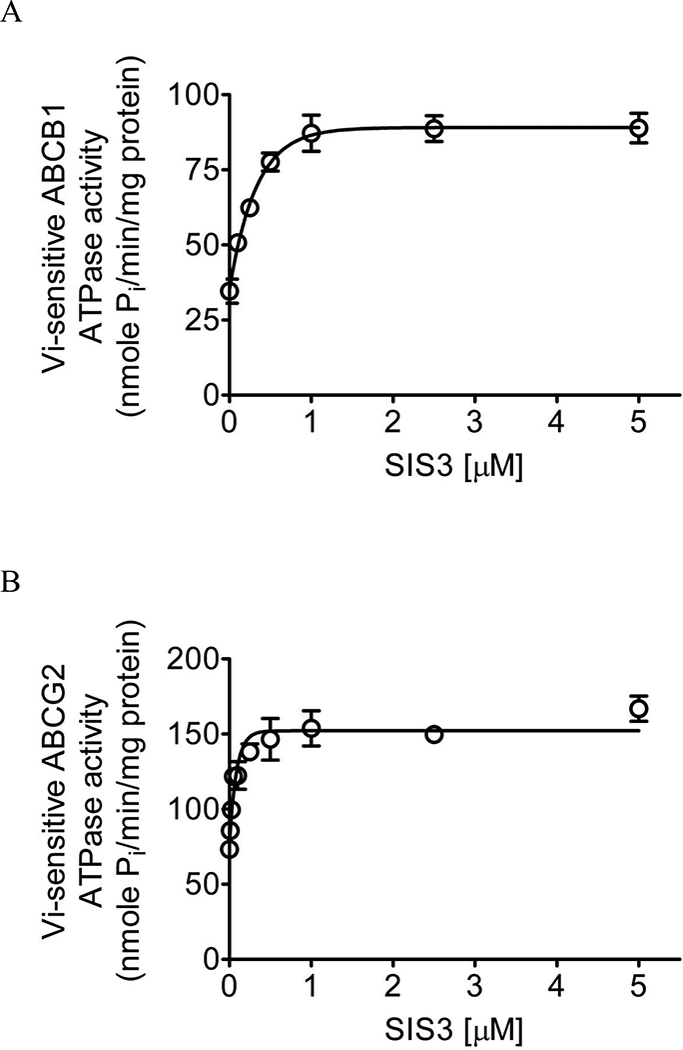
The effect of SIS3 (0 – 5 μM) on vanadate-sensitive (A) ABCB1 and (B) ABCG2 ATP hydrolysis was determined by endpoint Pi assay, as described previously [1, 67]. Values are presented as mean ± S.D. calculated from three independent experiments.
3.5. Docking analysis of SIS3 with ABCG2 and modeled structure of ABCB1
To better understand the binding of SIS3 with the transporter proteins, docking study was performed using crystal structure of ABCG2 and a homology model generated from high resolution mouse (Mus musculus) ABCB1 protein crystal structure (pdb.4Q9L) as template. Docking of SIS3 with modeled ABCB1 protein structure showed three main interactions in the probable substrate binding site [14, 55]: the aromatic rings interacted with hydrophobic residues Ala302 and Ala987 whilst the amino group on Asn842 may form hydrogen bond with the oxygen from methoxy moiety (Fig. 6A). In the docking study with ABCG2 protein, SIS3 was predicted to bind in the substrate binding cavity between two ABCG2 monomers. It is possibly stabilized by hydrophobic interactions between its aromatic rings and residues such as Phe439, Ser440 on transmembrane domain 2 (TM2) and Val546 on TM5a (Fig. 6B). Results of our ATPase activity experiments and docking analysis indicate strong interaction of SIS3 with both ABCB1 and ABCG2.
Fig. 6. Docking of SIS3 in the drug-binding pocket of ABCB1 and ABCG2.
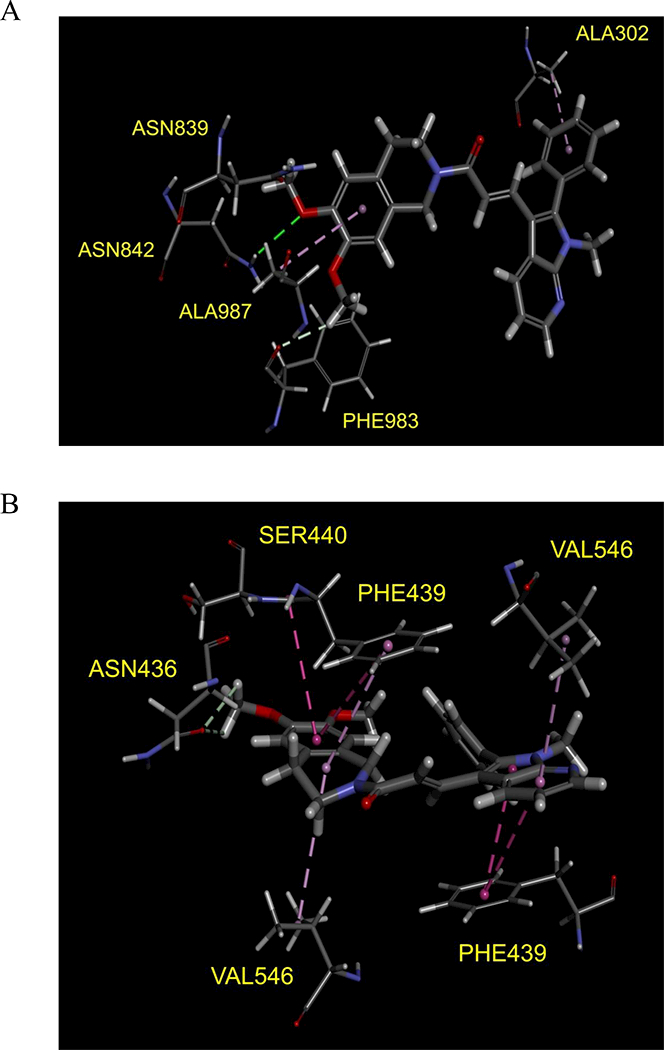
Binding modes of SIS3 with (A) homology modeled ABCB1 and (B) ABCG2 protein structure (PDB:5NJG) were predicted by Acclerys Discovery Studio 4.0 software as described in Materials and methods. SIS3 is shown as a molecular model with the atoms colored as carbon- gray, hydrogen-light gray, nitrogen-blue and oxygen-red. The same color scheme is used for interacting amino acid residues. Dotted lines indicate proposed interactions.
3.6. Overexpression of ABCB1 or ABCG2 does not reduce the chemosensitivity of cancer cells to SIS3
Knowing that SIS3 behaves as a substrate for ABCB1 and ABCG2, we examined whether cancer cells overexpressing ABCB1 or ABCG2 are resistant to SIS3. As shown in Table 2, the resistance factor (RF) value represents the extent of acquired SIS3 resistance in cancer cells caused by the overexpression of ABCB1 or ABCG2, calculated by dividing the IC50 value of SIS3 in MDR subline by the IC50 value of SIS3 in the respective parental line. The calculated RF values revealed no significant differences in the IC50 values of SIS3 in drug-sensitive cancer cells and cancer cells overexpressing ABCB1 or ABCG2. Our results show that drug-sensitive cancer cells and MDR cancer cells are equally sensitive to SIS3 treatment, suggesting that SIS3 is not actively effluxed by ABCB1 or ABCG2 in cancer cells and behaves as a high-affinity substrate for ABCB1 and ABCG2.
Table 2.
Cytotoxicity of SIS3 in human cancer cell lines overexpressing ABCB1 or ABCG2.
| Cell line | Type | Transporter expressed |
IC50 (nM)† | R.F‡ |
|---|---|---|---|---|
| KB-3–1 | epidermal | - | 10.73 ± 4.14 | 1.0 |
| KB-V-1 | epidermal | ABCB1 | 9.56 ± 3.70 | 0.9 |
| OVCAR-8 | ovarian | - | 7.58 ± 1.07 | 1.0 |
| NCI-ADR-RES | ovarian | ABCB1 | 8.79 ± 1.77 | 1.2 |
| H460 | lung | - | 9.97 ± 3.53 | 1.0 |
| H460-MX20 | lung | ABCG2 | 8.30 ± 2.12 | 0.8 |
| S1 | colon | - | 4.06 ± 0.93 | 1.0 |
| S1-M1–80 | colon | ABCG2 | 3.54 ± 0.48 | 0.9 |
| MCF7 | breast | - | 8.90 ± 2.22 | 1.0 |
| MCF7-FLV1000 | breast | ABCG2 | 11.21 ± 1.72 | 1.3 |
| MCF7-AdVp3000 | breast | ABCG2 | 10.11 ± 2.79 | 1.1 |
Abbreviation: RF, resistance factor.
† IC50 values are mean ± SD calculated from dose-response curves obtained from three independent experiments using cytotoxicity assay as described in Materials and methods.
‡ RF values were obtained by dividing IC50 values of ABCB1- or ABCG2- overexpressing cells by IC50 values of respective parental cells.
P < 0.05;
P < 0.01;
P < 0.001
4. Discussion
One of the significant challenges to the effective treatment of cancer is the development of multidrug resistance to chemotherapy, which is in part contributed by the overexpression of ABCB1 or ABCG2 in cancer cells [15, 37, 68]. In recent years, many attempts of developing novel inhibitors to re-sensitize MDR cancer cells to anticancer agents have failed, mostly due to problems associated with toxicity and adverse drug-drug interactions [50, 54]. This prompted us to search for drugs with known pharmacological and toxicological properties that could be re-positioned to treat MDR cancer cells that overexpress ABCB1 or ABCG2. The specific inhibitor of Smad3, SIS3, has been used on a regular basis in studies evaluating various TGF-β-regulated cellular mechanisms [9, 28, 30, 32, 41, 61, 62, 72]. More recently, SIS3 has emerged as a potential therapeutic agent in cancer treatment, capable of suppressing cancer progression and attenuating resistance to anticancer drugs in cancer cells. For instance, a recent study demonstrated that systemic treatment with SIS3 in the tumor microenvironment suppresses tumor growth, invasion and metastasis [56]. Similarly, SIS3 was able to suppress TGF-β1-induced epithelial-mesenchymal transition (EMT) in lung carcinomas [31], whereas another study demonstrated that by interrupting the TGF-β-SMAD3 pathway, SIS3 prevents the development of anti-HER2 drugs resistance and restores trastuzumab sensitivity in trastuzumab-resistant cells [10].
In the present study, we demonstrate that SIS3 inhibits the drug efflux function of ABCB1 and ABCG2. More importantly, SIS3 enhances the drug-induced apoptosis and reverses MDR-mediated by ABCB1 and ABCG2 in MDR cancer cell lines at low, nontoxic concentrations that are considerably lower than the concentrations of SIS3 used in studies evaluating TGF-β-regulated cellular mechanisms [9, 28, 30, 32, 41, 61, 62, 72]. At the same concentrations, SIS3 did not alter the protein expression of ABCB1 or ABCG2 in MDR cancer cells over a period of 72 h. In addition, the ATPase activity of ABCB1 and ABCG2 was stimulated by SIS3 in a concentration-dependent manner, which is in the same manner as other known substrate drugs for ABCB1 and ABCG2 [48, 49, 51]. The apparent EC50 value for SIS3-stimulated ABCB1 ATPase activity is approximately 160 nM, and approximately 60 nM for SIS3-stimulated ABCG2 ATPase activity, indicating a high-affinity interaction of SIS3 with ABCB1 and ABCG2. In addition, results of our docking analysis and the fact that ABCB1 and ABCG2 do not confer resistant to SIS3 in cancer cells further support the notion that SIS3 binds to the substrate-binding site of ABCB1 and ABCG2 with high enough affinity to compete with the binding of another drug substrate at the same site.
In summary, our results revealed that SIS3 is an effective modulator of ABCB1 and ABCG2, and that concomitant administration of SIS3 could potentially improve the therapeutic efficacy of other chemotherapeutic agents. Nevertheless, knowing that favorable experimental results do not necessarily translate into clinical outcomes [50], and that combination therapy may sometimes lead to unfavourable clinical responses [29, 53], SIS3 should be further evaluated in preclinical animal model studies and in clinical studies.
Fig. 7. A schematic diagram illustrating how SIS3 reverses drug resistance mediated by ABCB1 and ABCG2.
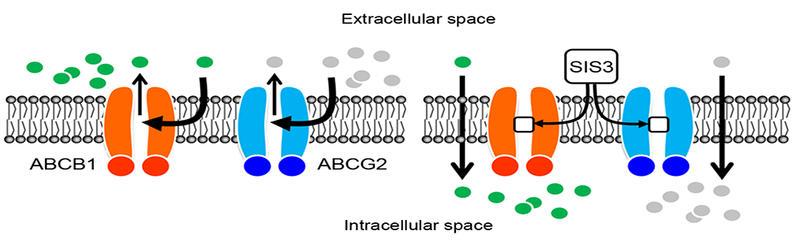
In the absence of SIS3 (left panel), ABCB1 substrate drugs (green circles) and ABCG2 substrate drugs (gray circles) are actively pumped out by ABCB1 (orange) and ABCG2 (blue), respectively, resulting in reduced intracellular drug accumulation and drug resistance. In contrast, in the presence of SIS3 (right panel), ABCB1 substrate drugs and ABCG2 substrate drugs are no longer able to access the drug binding pockets of ABCB1 and ABCG2 since SIS3 (white squares) has already occupied the sites, consequently restoring intracellular drug concentration and chemosensitivity.
Highlights.
SIS3, a specific inhibitor of Smad3, inhibits the function of ABCB1 and ABCG2.
SIS3 enhances drug-induced apoptosis in multidrug-resistant cancer cells.
SIS3 resensitizes ABCB1 and ABCG2 overexpressing cancer cells to chemotherapeutics.
ABCB1 and ABCG2 do not confer resistance to SIS3 in cancer cells.
Inclusion of SIS3 in the drug treatment may benefit patients with multidrug-resistant tumors in the future.
ACKNOWLEDGMENTS
This work was supported by funds from the Ministry of Science and Technology of Taiwan (MOST-105–2320-B-182–018 and MOST-106–2320-B-182–017 to CPW; MOST 103–2314-B-182A-099-MY1 and 103–2314-B-182A-099-MY2 to THH; MOST-102–2113-M-029–005 to YSW), Chang Gung Medical Research Program (BMRPC17, CMRPD1D0153 and CMRPD1G0112 to CPW), Taichung Veterans General Hospital (TCVGH-T1057805 to YSW) and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research to SVA and MM.
Abbreviations:
- MDR
multidrug resistance
- ABC
ATP-binding cassette
- SMAD3
Mothers against decapentaplegic homolog 3
- FCS
fetal calf serum
- CCK-8
Cell Counting Kit-8
- MTT
3-(4,5-dimethylthiazol-yl)-2,5-diphenyllapatinibrazolium bromide
- Vi
sodium orthovanadate
- IMDM
Iscove’s Modified Dulbecco’s Medium
- FR
Fold reversal
Footnotes
CONFLICT OF INTEREST
None.
AUTHOR DECLARATION
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
We understand that the Corresponding Author is the sole contact for the Editorial process (including Editorial Manager and direct communications with the office). He/she is responsible for communicating with the other authors about progress, submissions of revisions and final approval of proofs. We confirm that we have provided a current, correct email address which is accessible by the Corresponding Author and which has been configured to accept email from:
Chung-Pu Wu : wuchung@mail.cgu.edu.tw
Megumi Murakami : megumi.murakami@nih.gov
Sung-Han Hsiao : johnson170_ya@hotmail.com
Te-Chun Liu : 716george@gmail.com
Ni Yeh: qaz00120520@gmail.com
Yan-Qing Li : hi9300190@yahoo.com.tw
Tai-Ho Hung : thh20@adm.cgmh.org.tw
Yu-Shan Wu : yushanwu@go.thu.edu.tw
Suresh. V Ambudkar : ambudkar@mail.nih.gov
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- [1].Ambudkar SV, Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells, Methods Enzymol, 292 (1998) 504–514. [DOI] [PubMed] [Google Scholar]
- [2].Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM, Biochemical, cellular, and pharmacological aspects of the multidrug transporter, Annual review of pharmacology and toxicology, 39 (1999) 361–398. [DOI] [PubMed] [Google Scholar]
- [3].Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM, P-glycoprotein: from genomics to mechanism., Oncogene, 22 (2003) 7468–7485. [DOI] [PubMed] [Google Scholar]
- [4].Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Shacter E, Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells, Nature immunology, 4 (2003) 87–91. [DOI] [PubMed] [Google Scholar]
- [5].Arnold K, Bordoli L, Kopp J, Schwede T, The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling, Bioinformatics, 22 (2006) 195–201. [DOI] [PubMed] [Google Scholar]
- [6].Benkert P, Biasini M, Schwede T, Toward the estimation of the absolute quality of individual protein structure models, Bioinformatics, 27 (2011) 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T, SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information, Nucleic acids research, 42 (2014) W252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bodo A, Bakos E, Szeri F, Varadi A, Sarkadi B, The role of multidrug transporters in drug availability, metabolism and toxicity, Toxicol Lett, 140–141 (2003)133–143. [DOI] [PubMed] [Google Scholar]
- [9].Boudreau HE, Casterline BW, Rada B, Korzeniowska A, Leto TL, Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells, Free radical biology & medicine, 53 (2012) 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chihara Y, Shimoda M, Hori A, Ohara A, Naoi Y, Ikeda JI, Kagara N, Tanei T, Shimomura A, Shimazu K, Kim SJ, Noguchi S, A small-molecule inhibitor of SMAD3 attenuates resistance to anti-HER2 drugs in HER2-positive breast cancer cells, Breast cancer research and treatment, 166 (2017) 55–68. [DOI] [PubMed] [Google Scholar]
- [11].Cuestas ML, Castillo AI, Sosnik A, Mathet VL, Downregulation of mdr1 and abcg2 genes is a mechanism of inhibition of efflux pumps mediated by polymeric amphiphiles, Bioorg Med Chem Lett, 22 (2012) 6577–6579. [DOI] [PubMed] [Google Scholar]
- [12].Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR Jr., Huang Y, Robey RW, Liang YJ, Chen LM, Shi CJ, Ambudkar SV, Chen ZS, Fu LW, Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2, Cancer Res, 68 (2008) 7905–7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Elkind NB, Szentpetery Z, Apati A, Ozvegy-Laczka C, Varady G, Ujhelly O, Szabo K, Homolya L, Varadi A, Buday L, Keri G, Nemet K, Sarkadi B, Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib), Cancer Res, 65 (2005) 1770–1777. [DOI] [PubMed] [Google Scholar]
- [14].Gade DR, Makkapati A, Yarlagadda RB, Peters GJ, Sastry BS, Rajendra Prasad VVS, Elucidation of chemosensitization effect of acridones in cancer cell lines: Combined pharmacophore modeling, 3D QSAR, and molecular dynamics studies, Computational biology and chemistry, 74 (2018) 63–75. [DOI] [PubMed] [Google Scholar]
- [15].Gillet JP, Gottesman MM, Mechanisms of multidrug resistance in cancer, Methods Mol Biol, 596 (2010) 47–76. [DOI] [PubMed] [Google Scholar]
- [16].Gottesman MM, Mechanisms of cancer drug resistance, Annual review of medicine, 53 (2002) 615–627. [DOI] [PubMed] [Google Scholar]
- [17].Gottesman MM, Fojo T, Bates SE, Multidrug resistance in cancer: role of ATP-dependent transporters, Nat Rev Cancer, 2 (2002) 48–58. [DOI] [PubMed] [Google Scholar]
- [18].Gribar JJ, Ramachandra M, Hrycyna CA, Dey S, Ambudkar SV, Functional characterization of glycosylation-deficient human P-glycoprotein using a vaccinia virus expression system, J Membr Biol, 173 (2000) 203–214. [DOI] [PubMed] [Google Scholar]
- [19].Hegedus C, Ozvegy-Laczka C, Szakacs G, Sarkadi B, Interaction of ABC multidrug transporters with anticancer protein kinase inhibitors: substrates and/or inhibitors?, Curr Cancer Drug Targets, 9 (2009) 252–272. [DOI] [PubMed] [Google Scholar]
- [20].Hofmeister CC, Yang X, Pichiorri F, Chen P, Rozewski DM, Johnson AJ, Lee S, Liu Z, Garr CL, Hade EM, Ji J, Schaaf LJ, Benson DM Jr., Kraut EH, Hicks WJ, Chan KK, Chen CS, Farag SS, Grever MR, Byrd JC, Phelps MA, Phase I trial of lenalidomide and CCI-779 in patients with relapsed multiple myeloma: evidence for lenalidomide-CCI-779 interaction via P-glycoprotein, J Clin Oncol, 29 (2011) 3427–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hollo Z, Homolya L, Davis CW, Sarkadi B, Calcein accumulation as a fluorometric functional assay of the multidrug transporter, Biochimica et biophysica acta, 1191 (1994) 384–388. [DOI] [PubMed] [Google Scholar]
- [22].Honjo Y, Hrycyna CA, Yan QW, Medina-Perez WY, Robey RW, van de Laar A, Litman T, Dean M, Bates SE, Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells, Cancer Res, 61 (2001) 6635–6639. [PubMed] [Google Scholar]
- [23].Hsiao SH, Lu YJ, Li YQ, Huang YH, Hsieh CH, Wu CP, Osimertinib (AZD9291) Attenuates the Function of Multidrug Resistance-Linked ATP-Binding Cassette Transporter ABCB1 in Vitro, Mol Pharm, (2016). [DOI] [PubMed] [Google Scholar]
- [24].Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K, A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet, Biol Pharm Bull, 19 (1996) 1518–1520. [DOI] [PubMed] [Google Scholar]
- [25].Jinnin M, Ihn H, Tamaki K, Characterization of SIS3, a novel specific inhibitor of Smad3, and its effect on transforming growth factor-beta1-induced extracellular matrix expression, Mol Pharmacol, 69 (2006) 597–607. [DOI] [PubMed] [Google Scholar]
- [26].Kitazaki T, Oka M, Nakamura Y, Tsurutani J, Doi S, Yasunaga M, Takemura M, Yabuuchi H, Soda H, Kohno S, Gefitinib, an EGFR tyrosine kinase inhibitor, directly inhibits the function of P-glycoprotein in multidrug resistant cancer cells, Lung cancer, 49 (2005) 337–343. [DOI] [PubMed] [Google Scholar]
- [27].Kovalev AA, Tsvetaeva DA, Grudinskaja TV, Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the development of primary and acquired multiple drug resistance in patients with early and metastatic breast cancer, Experimental oncology, 35 (2013) 287–290. [PubMed] [Google Scholar]
- [28].Lee YC, Hung MH, Liu LY, Chang KT, Chou TY, Wang YC, Wu YC, Lai CL, Tsai CC, Su KC, Perng DW, The roles of transforming growth factor-beta(1) and vascular endothelial growth factor in the tracheal granulation formation, Pulmonary pharmacology & therapeutics, 24 (2011) 23–31. [DOI] [PubMed] [Google Scholar]
- [29].Leggas M, Panetta JC, Zhuang Y, Schuetz JD, Johnston B, Bai F, Sorrentino B, Zhou S, Houghton PJ, Stewart CF, Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo, Cancer Res, 66 (2006) 4802–4807. [DOI] [PubMed] [Google Scholar]
- [30].Li Z, Li B, Pan J, Jin J, TNF-alpha enhances the effect of TGF-beta on Gli2 expression in the KG-1 leukemic cell line, Experimental and therapeutic medicine, 8 (2014) 676–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu Z, Zhao J, Zhang HT, JAK/STAT3 signaling is required for TGF-beta-induced epithelial-mesenchymal transition in lung cancer cells, Int J Oncol, 44 (2014) 1643–1651. [DOI] [PubMed] [Google Scholar]
- [32].Lou Z, Yang Y, Ren T, Tang S, Peng X, Lu Q, Sun Y, Guo W, Smad3 is the key to transforming growth factor-beta1-induced osteoclast differentiation in giant cell tumor of bone, Medical oncology, 30 (2013) 606. [DOI] [PubMed] [Google Scholar]
- [33].Matthews C, Catherwood MA, Larkin AM, Clynes M, Morris TC, Alexander HD, MDR-1, but not MDR-3 gene expression, is associated with unmutated IgVH genes and poor prognosis chromosomal aberrations in chronic lymphocytic leukemia, Leuk Lymphoma, 47 (2006) 2308–2313. [DOI] [PubMed] [Google Scholar]
- [34].Mistry P, Stewart AJ, Dangerfield W, Okiji S, Liddle C, Bootle D, Plumb JA, Templeton D, Charlton P, In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576, Cancer Res, 61 (2001) 749–758. [PubMed] [Google Scholar]
- [35].Nakagawa Y, Abe S, Kurata M, Hasegawa M, Yamamoto K, Inoue M, Takemura T, Suzuki K, Kitagawa M, IAP family protein expression correlates with poor outcome of multiple myeloma patients in association with chemotherapy-induced overexpression of multidrug resistance genes, American journal of hematology, 81 (2006) 824–831. [DOI] [PubMed] [Google Scholar]
- [36].Natarajan K, Bhullar J, Shukla S, Burcu M, Chen ZS, Ambudkar SV, Baer MR, The Pim kinase inhibitor SGI-1776 decreases cell surface expression of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and drug transport by Pim-1-dependent and -independent mechanisms, Biochem Pharmacol, 85 (2013)514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Noguchi K, Katayama K, Sugimoto Y, Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics, Pharmacogenomics and personalized medicine, 7 (2014) 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pajeva IK, Sterz K, Christlieb M, Steggemann K, Marighetti F, Wiese M, Interactions of the multidrug resistance modulators tariquidar and elacridar and their analogues with P-glycoprotein, ChemMedChem, 8 (2013) 1701–1713. [DOI] [PubMed] [Google Scholar]
- [39].Pilarski LM, Belch AR, Intrinsic expression of the multidrug transporter, P-glycoprotein 170, in multiple myeloma: implications for treatment, Leuk Lymphoma, 17 (1995) 367–374. [DOI] [PubMed] [Google Scholar]
- [40].Pilarski LM, Szczepek AJ, Belch AR, Deficient drug transporter function of bone marrow-localized and leukemic plasma cells in multiple myeloma, Blood, 90 (1997) 3751–3759. [PubMed] [Google Scholar]
- [41].Qureshi HY, Ricci G, Zafarullah M, Smad signaling pathway is a pivotal component of tissue inhibitor of metalloproteinases-3 regulation by transforming growth factor beta in human chondrocytes, Biochimica et biophysica acta, 1783 (2008) 1605–1612. [DOI] [PubMed] [Google Scholar]
- [42].Robey RW, Medina-Perez WY, Nishiyama K, Lahusen T, Miyake K, Litman T, Senderowicz AM, Ross DD, Bates SE, Overexpression of the ATP-binding Cassette Half-Transporter, ABCG2 (MXR/BCRP/ABCP1), in Flavopiridol-resistant Human Breast Cancer Cells, Clin Cancer Res, 7 (2001) 145–152. [PubMed] [Google Scholar]
- [43].Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, P Mistry SE Bates, Pheophorbide a is a specific probe for ABCG2 function and inhibition, Cancer Res, 64 (2004) 1242–1246. [DOI] [PubMed] [Google Scholar]
- [44].Ross DD, Karp JE, Chen TT, Doyle LA, Expression of breast cancer resistance protein in blast cells from patients with acute leukemia, Blood, 96 (2000) 365–368. [PubMed] [Google Scholar]
- [45].Sarkadi B, Homolya L, Szakacs G, Varadi A, Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system, Physiological reviews, 86 (2006) 1179–1236. [DOI] [PubMed] [Google Scholar]
- [46].Schwarzenbach H, Expression of MDR1/P-glycoprotein, the multidrug resistance protein MRP, and the lung-resistance protein LRP in multiple myeloma, Medical oncology, 19 (2002) 87–104. [DOI] [PubMed] [Google Scholar]
- [47].Shen DW, Fojo A, Chin JE, Roninson IB, Richert N, Pastan I, Gottesman MM, Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification, Science, 232 (1986) 643–645. [DOI] [PubMed] [Google Scholar]
- [48].Shi Z, Peng XX, Kim IW, Shukla S, Si QS, Robey RW, Bates SE, Shen T, Ashby CR Jr., Fu LW, Ambudkar SV, Chen ZS, Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance, Cancer Res, 67 (2007) 11012–11020. [DOI] [PubMed] [Google Scholar]
- [49].Shi Z, Tiwari AK, Shukla S, Robey RW, Kim IW, Parmar S, Bates SE, Si QS, Goldblatt CS, Abraham I, Fu LW, Ambudkar SV, Chen ZS, Inhibiting the function of ABCB1 and ABCG2 by the EGFR tyrosine kinase inhibitor AG1478, Biochem Pharmacol, 77 (2009) 781–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Shukla S, Wu CP, Ambudkar SV, Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges, Expert Opin Drug Metab Toxicol, 4 (2008) 205–223. [DOI] [PubMed] [Google Scholar]
- [51].Sodani K, Tiwari AK, Singh S, Patel A, Xiao ZJ, Chen JJ, Sun YL, Talele TT, Chen ZS, GW583340 and GW2974, human EGFR and HER-2 inhibitors, reverse ABCG2- and ABCB1-mediated drug resistance, Biochem Pharmacol, 83 (2012) 1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Steinbach D, Sell W, Voigt A, Hermann J, Zintl F, Sauerbrey A, BCRP gene expression is associated with a poor response to remission induction therapy in childhood acute myeloid leukemia, Leukemia, 16 (2002) 1443–1447. [DOI] [PubMed] [Google Scholar]
- [53].Stewart CF, Leggas M, Schuetz JD, Panetta JC, Cheshire PJ, Peterson J, Daw N, Jenkins JJ 3rd, Gilbertson R, Germain GS, Harwood FC, Houghton PJ, Gefitinib enhances the antitumor activity and oral bioavailability of irinotecan in mice, Cancer Res, 64 (2004) 7491–7499. [DOI] [PubMed] [Google Scholar]
- [54].Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM, Targeting multidrug resistance in cancer, Nature reviews, 5 (2006) 219–234. [DOI] [PubMed] [Google Scholar]
- [55].Tajima Y, Nakagawa H, Tamura A, Kadioglu O, Satake K, Mitani Y, Murase H, Regasini LO, Bolzani Vda S, Ishikawa T, Fricker G, Efferth T, Nitensidine A, a guanidine alkaloid from Pterogyne nitens, is a novel substrate for human ABC transporter ABCB1, Phytomedicine : international journal of phytotherapy and phytopharmacology, 21 (2014) 323–332. [DOI] [PubMed] [Google Scholar]
- [56].Tang PM, Zhou S, Meng XM, Wang QM, Li CJ, Lian GY, Huang XR, Tang YJ, Guan XY, Yan BP, To KF, Lan HY, Smad3 promotes cancer progression by inhibiting E4BP4-mediated NK cell development, Nature communications, 8 (2017) 14677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP, Structure of the human multidrug transporter ABCG2, Nature, 546 (2017) 504–509. [DOI] [PubMed] [Google Scholar]
- [58].Tsubaki M, Satou T, Itoh T, Imano M, Komai M, Nishinobo M, Yamashita M, Yanae M, Yamazoe Y, Nishida S, Overexpression of MDR1 and survivin, and decreased Bim expression mediate multidrug-resistance in multiple myeloma cells, Leuk Res, 36 (2012) 1315–1322. [DOI] [PubMed] [Google Scholar]
- [59].Turner JG, Gump JL, Zhang C, Cook JM, Marchion D, Hazlehurst L, Munster P, Schell MJ, Dalton WS, Sullivan DM, ABCG2 expression, function, and promoter methylation in human multiple myeloma, Blood, 108 (2006) 3881–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Uggla B, Stahl E, Wagsater D, Paul C, Karlsson MG, Sirsjo A, Tidefelt U, BCRP mRNA expression v. clinical outcome in 40 adult AML patients, Leuk Res, 29 (2005) 141–146. [DOI] [PubMed] [Google Scholar]
- [61].Voloshenyuk TG, Landesman ES, Khoutorova E, Hart AD, Gardner JD, Induction of cardiac fibroblast lysyl oxidase by TGF-beta1 requires PI3K/Akt, Smad3, and MAPK signaling, Cytokine, 55 (2011) 90–97. [DOI] [PubMed] [Google Scholar]
- [62].Watanabe T, Yasue A, Tanaka E, Inhibition of transforming growth factor beta1/Smad3 signaling decreases hypoxia-inducible factor-1alpha protein stability by inducing prolyl hydroxylase 2 expression in human periodontal ligament cells, Journal of periodontology, 84 (2013) 1346–1352. [DOI] [PubMed] [Google Scholar]
- [63].Wu C-P, Hsieh C-H, Wu Y-S, The Emergence of Drug Transporter-Mediated Multidrug Resistance to Cancer Chemotherapy, Molecular Pharmaceutics, 8 (2011) 1996–2011. [DOI] [PubMed] [Google Scholar]
- [64].Wu CP, Calcagno AM, Ambudkar SV, Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies, Current molecular pharmacology, 1 (2008) 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Wu CP, Hsiao SH, Murakami M, Lu MJ, Li YQ, Hsieh CH, Ambudkar SV, Wu YS, Tyrphostin RG14620 selectively reverses ABCG2-mediated multidrug resistance in cancer cell lines, Cancer Lett, 409 (2017) 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Wu CP, Hsiao SH, Su CY, Luo SY, Li YQ, Huang YH, Hsieh CH, Huang CW, Human ATP-Binding Cassette transporters ABCB1 and ABCG2 confer resistance to CUDC-101, a multi-acting inhibitor of histone deacetylase, epidermal growth factor receptor and human epidermal growth factor receptor 2, Biochem Pharmacol, 92 (2014) 567–576. [DOI] [PubMed] [Google Scholar]
- [67].Wu CP, Hsieh CH, Hsiao SH, Luo SY, Su CY, Li YQ, Huang YH, Huang CW, Hsu SC, Human ATP-Binding Cassette Transporter ABCB1 Confers Resistance to Volasertib (BI 6727), a Selective Inhibitor of Polo-like Kinase 1, Mol Pharm, 12 (2015) 3885–3895. [DOI] [PubMed] [Google Scholar]
- [68].Wu CP, Hsieh CH, Wu YS, The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy, Mol Pharm, 8 (2011) 1996–2011. [DOI] [PubMed] [Google Scholar]
- [69].Wu CP, Shukla S, Calcagno AM, Hall MD, Gottesman MM, Ambudkar SV, Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance linked ABCG2 transporter, Mol Cancer Ther, 6 (2007) 3287–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wu CP, Sim HM, Huang YH, Liu YC, Hsiao SH, Cheng HW, Li YQ, Ambudkar SV, Hsu SC, Overexpression of ATP-binding cassette transporter ABCG2 as a potential mechanism of acquired resistance to vemurafenib in BRAF(V600E) mutant cancer cells, Biochem Pharmacol, 85 (2013) 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Yoh K, Ishii G, Yokose T, Minegishi Y, Tsuta K, Goto K, Y Nishiwaki T Kodama, M. Suga, A. Ochiai, Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer, Clin Cancer Res, 10 (2004) 1691–1697. [DOI] [PubMed] [Google Scholar]
- [72].Zhao B, Guan H, Liu JQ, Zheng Z, Zhou Q, Zhang J, Su LL, Hu DH, Hypoxia drives the transition of human dermal fibroblasts to a myofibroblast-like phenotype via the TGF-beta1/Smad3 pathway, International journal of molecular medicine, 39 (2017) 153–127909731 [DOI] [PMC free article] [PubMed] [Google Scholar]


