Abstract
Oestrogens are well-known proliferation and differentiation factors that play an essential role in the correct development of sex-related organs and behaviour in mammals. With the use of the ERE-Luc reporter mouse model, we show herein that throughout mouse development, oestrogen receptors (ERs) are active starting from day 12 post-conception. Most interestingly, we show that prenatal luciferase expression in each organ is proportionally different in relation to the germ layer of the origin. The luciferase content is highest in ectoderm-derived organs (such as brain and skin) and is lowest in endoderm-derived organs (such as liver, lung, thymus and intestine). Consistent with the testosterone surge occurring in male mice at the end of pregnancy, in the first two days after birth, we observed a significant increase in the luciferase content in several organs, including the liver, bone, gonad and hindbrain.
The results of the present study show a widespread transcriptional activity of ERs in developing embryos, pointing to the potential contribution of these receptors in the development of non-reproductive as well as reproductive organs. Consequently, the findings reported here might be relevant in explaining the significant differences in male and female physio-pathology reported by a growing number of studies and may underline the necessity for more systematic analyses aimed at the identification of the prenatal effects of drugs interfering with ER signalling, such as aromatase inhibitors or endocrine disrupter chemicals (EDCs).
INTRODUCTION
Oestrogens have been long known as cell proliferation and differentiation factors (Tsai and O'Malley 1994), and several lines of evidence suggest that, in the course of foetal programming, these hormones are relevant for the sexual differentiation of reproductive tissues, including the brain (Albrecht, et al. 2009; Greco, et al. 1991; Korach, et al. 1988; Nielsen, et al. 2000; Pang, et al. 1979; Phoenix, et al. 1959; Rissman, et al. 1997; Tobet, et al. 1986). Very little is known regarding the effects of these steroids in non-reproductive organs, in spite of the fact that preclinical and clinical investigations in subjects with prenatal impairment of ER signalling showed physiological alterations and increased incidence of diseases involving the cardiovascular (Conte, et al. 1994; Jones, et al. 2000; Tait, et al. 2015; Yuchi, et al. 2015), metabolic (Lapid, et al. 2014), immune (Zoller and Kersh 2006), respiratory (Thuresson-Klein, et al. 1985), and skeletal systems as well as the epidermis (Brandenberger, et al. 1997; Hanley, et al. 1996; Lemmen, et al. 1999; Takeyama, et al. 2001).
The biological effects of the steroid hormone 17β-oestradiol (E2) - the most expressed oestrogen - are predominantly mediated through two distinct ERs (ERα and ERβ) that share a common phylogenetic origin, conserved structural organization and similar mode of action (mainly as ligand-operated transcription factors) with the other members of the nuclear receptor (NR) family (Kininis and Kraus 2008; Tsai and O'Malley 1994). In addition, ERs may interfere with the signalling of other membrane receptors as well as intracellular receptors, and ERα, may associate with the plasma membrane and may activate non-nuclear signalling from this site. These rapid, non-genomic/membrane initiated steroid signals have been characterized in endothelial cells but may be present in other cellular systems (Arnal, et al. 2017). The functional activation of intracellular ERs occurs through binding with oestrogens, but unliganded ER activation may be triggered by enzymatic activities, inducing post-translational modifications (Dahlman-Wright, et al. 2006) that enable ER to release inhibitory proteins generally associated with inactive receptor proteins. ERs were the first NRs to be described (Jensen 2005; Toft and Gorski 1966), and they appear to be the closest to the ancestral steroid receptor (Thornton, et al. 2003). In addition to these intracellular proteins, a membrane receptor, G protein-coupled oestrogen receptor 1 (GPER1), may transduce oestrogen signals through non-genomic signalling (Revankar, et al. 2005).
In adult mammals, it is well known that ERs are expressed and functionally active in most reproductive and non-reproductive tissue cells (Bookout, et al. 2006; Ciocca and Roig 1995; Maggi, et al. 2004). Their expression and transcriptional activity in the course of embryo development is less studied (Brandenberger et al. 1997; Lemmen et al. 1999). In addition to selective KO mutation of ERα, ERβ, or the aromatase gene (Cyp19a1, encoding the enzyme responsible for testosterone-derived oestrogen synthesis) that highlighted the relevance of these receptors and their cognate ligands for the development of sexual organs and sexual behaviour (Kudwa, et al. 2006), the elucidation of the ER distribution and activity during implantation and embryogenesis in non-reproductive tissues is circumscribed to only a few studies (Bondesson, et al. 2015; Mogi, et al. 2015; Park, et al. 2017). However, preclinical and clinical observations in subjects carrying mutations that impair ER signalling showed deviations from the proper development of the cardiovascular system (Del Principe, et al. 2015; Tait et al. 2015), innate immune and neuro-immune communications (Zoller and Kersh 2006), pancreatic and gastric activity (Campbell-Thompson, et al. 2001; Maniu, et al. 2016), and liver functions (Barros and Gustafsson 2011; Bryzgalova, et al. 2006; Foryst-Ludwig, et al. 2008), as well as adipose (Barros and Gustafsson 2011; Lapid et al. 2014), lung (Carey, et al. 2007; Patrone, et al. 2003; Thuresson-Klein et al. 1985), kidney (Kummer, et al. 2011; Lane 2008), and epidermal tissues with the muscle-skeletal apparatus (Brandenberger et al. 1997; Hanley et al. 1996; Lemmen et al. 1999; Markiewicz, et al. 2013; Takeyama et al. 2001; Ueberschlag-Pitiot, et al. 2017; Walker and Korach 2004).
In view of the growing number of reports pointing to significant sex-related differences in mammalian physio-pathology and the increasing concern of the potential long-term effects of maternal exposure to EDCs and to drugs (such as aromatase inhibitors) that may interfere with oestrogen signalling during pregnancy, a better understanding of the oestrogen-dependent programmes in ontogeny is indispensable for the comprehension of the role of sex in the incidence of several pathologies and for the creation of efficacious protocols for the evaluation of EDC exposure.
To verify the extent of ER transcriptional activity during embryogenesis, we investigated the luciferase expression in the ERE-Luc reporter mouse (Ciana, et al. 2001; Ciana, et al. 2003). In this mouse, the general transcription of the firefly luciferase transgene is controlled by the activated intracellular ERs. In the last 15 years, a large number of studies carried out in different laboratories showed that, in the ERE-Luc mouse, the amount of luciferase synthesized is proportional to the ER transcriptional activity and quantifiable in vivo by bioluminescence imaging or ex vivo by means of an enzymatic assay in tissue lysates (Chambliss, et al. 2010; Humpel, et al. 2005; Klotz, et al. 2002; Mussi, et al. 2006; Patrone et al. 2003; Penza, et al. 2011; Vantaggiato, et al. 2016). These studies demonstrated: i.) a lack of interference of signals originating by the chromatin surrounding the transgene granted by the combination of the specific integration site and insulator sequences flanking the reporter (Rizzi, et al. 2017), ii. the selective response to ERs of the synthetic promoter driving luciferase transcription and iii.) that the reporter is transcriptionally viable in all tissues (as also indicated by a background reporter activity due to the TK minimal promoter).
By demonstrating that ERs are widely active during embryo development, our results will facilitate the understanding of complex functions of ERs in embryo maturation, putting novel bases for the comprehension of the involvement of sex in the incidence and progression of pathologies not strictly associated with reproductive functions. The data presented may also help with the prediction of the effects of exposure to EDCs or the administration of drugs, such as aromatase inhibitors to pregnant mothers. Therefore, the ERE-Luc reporter mice may be considered a valuable tool to unravel the effects of potential EDCs or drugs administered to pregnant mothers that can interfere with the oestrogen signalling of the foetus.
MATERIALS AND METHODS
Animals
The mice were housed with ad libitum access to standard chow (RF21, Mucedola, Italy) and water. We generated heterozygous ERE-Luc foetuses by caging homozygous ERE-Luc males with wild-type C57/Bl6j females for the night. The day after overnight mating was counted as 0.5 dpc (day post conception). Natural birth occurred on 19.5 dpc, which was counted as day 0 post-birth (P0). In the experiments with the knockouts, the mice heterozygous for aromatase (Ar+/−) from E. Simpson were crossed with ERE-Luc mice, and the progeny were as backcrossed to obtain both KO and wild-type foetuses in the same litter that were genotyped from tail-derived DNA by PCR using published primers (Fisher, et al. 1998). The sex was confirmed by PCR of the SMC locus (Agulnik, et al. 1997). All the animal experiments were carried out in accordance with the European guidelines for human animal care. The use of experimental animals was approved by the Italian Ministry of Research and University and was controlled by the panel of experts of the Department of Pharmacological and Biomolecular Sciences at the University of Milan.
Treatment with ER antagonist
ICI 182,780 powder (Sigma) was dissolved in 99% v/v ethanol to a concentration of 30 mg/mL. A total of 75 µg or 30 µg of ICI, dispersed in 100 µL of maize oil, was s.c. injected 24 hours and 6 hours before the imaging session, resulting in 1 mg/kg and 2.5 mg/kg for a 33 g female at day ~16 of pregnancy.
In vivo bioluminescence imaging
Pregnant females were anaesthetized with 50 µL s.c. injection of a water solution of 78% ketamine (Ketavet 50 mg/mL, Intervet, Peschiera Borromeo, Italy) and 15% xylazine (Rompun 20 mg/mL, Bayer, Leverkusen, Germany). Once anaesthetized, the pregnant females were shaved to allow a better measurement of the photons emitted from the foetuses. Fifteen minutes before BLI, 90 µL of a water solution of the luciferase substrate luciferin (Beetle luciferin potassium salt, Promega, Madison, WI, USA) was administered s.c. (75 mg/kg). Bioluminescence was measured by a Night Owl imaging unit (Berthold Technologies, Bad Wildbad, Germany); the mice were placed in the light-tight chamber, and their pictures were first taken with dimmed light, and then the luciferase signal was registered for 5 minutes. Merging of the images enabled visualizing body areas where photon emission occurred (luciferase signal was transformed in pseudocolours: blue-lowest, then an green, red, yellow and white as the highest signal). For quantification, photon emission was measured in the whole body areas (counts per second, cts/s). Normalization was performed using an external source of photons (Glowell, Lux Biotechnology, Edinburgh, UK) enabling measurement of the instrumental efficiency of photon counting. All the measurements were performed using WinLight32 (Berthold Technologies). After BLI, the foetuses were dissected under binocular macroscopy, and the organs shown in the figures were collected, frozen on dry-ice and stored at −80°C until assayed.
Luciferase enzymatic assay
The tissues were homogenized in 200 µL ice-cold lysis buffer (100 mM KPO4, 1 mM DTT, 4 mM EGTA, 4 mM EDTA, pH 7.8) with a micro pestle (Eppendorf); then, they underwent freezing and were thawed during centrifugation at 4900 × g, 4°C for 25 minutes. The supernatants were collected, and the protein concentrations were measured by Bradford assay following the manufacturer’s instructions (Pierce Biotech). Luciferase enzymatic activity was measured with a commercial kit (Luciferase Assay System, Promega) in a luminometer (Glomax, Promega) and was expressed as the relative light units over 10 sec/µg protein (RLU/µg proteins).
Immunohistochemistry
The mice at 17.5 dpc were fixed in 4% para-formaldehyde, embedded in paraffin, and whole-sliced. Sections of 5 µm were deparaffinised in xylene and rehydrated in decreasing ethanol concentrations. After washing, the slides were microwaved in citrate buffer, washed in PBS and incubated 15 min in 1% H2O2.
For luciferase detection
after three PBS washes, the sections were permeabilised in 0.2% Triton X-100 in PBS and blocked by incubation with 10% goat serum and 0.3% Tween 20 in PBS for 30 min at 37°C, followed by biotin blocking buffer (Vector Laboratories). The sections were incubated in a humidity chamber overnight at 4°C with rabbit anti-luciferase antibody or normal rabbit serum diluted 1:6000 in blocking buffer. The anti-luciferase antibody was provided by W. Just (Soto, et al. 1993). Next, the sections were washed in 0.3% Tween 20 PBS and were incubated at room temperature for 60 min with biotinylated goat anti-rabbit IgG (Vector Laboratories) and diluted 1:200 in 1% normal goat serum 0.3% Tween 20. Detection was performed with the Vectastain ABC kit (Vector Laboratories) according to the manufacturer’s instructions. The sections were counterstained with haematoxylin/eosin.
For ERα detection
After three PBS washes, the sections were permeabilised and blocked by incubation with 10% goat serum, 0.3% Triton-X in PBS for 120 min at RT, followed by biotin blocking buffer (Vector Laboratories). The sections were then incubated in a humidity chamber overnight at 4°C with anti-ERα antibody (polyclonal, made in rabbit, Abcam ab75635), or normal rabbit serum was diluted 1:100 in blocking buffer. Next, the sections were washed in 0.3% Tween 20 PBS and were incubated at room temperature for 60 min with biotinylated goat anti-rabbit IgG (Vector Laboratories), diluted 1:200 in 1% normal goat serum. Detection was performed with the Vectastain ABC kit (Vector Laboratories) according to the manufacturer’s instructions.
Statistical analysis
P values were calculated as described in the figure legends using GraphPad Prism version 5.01 for Windows, GraphPad Software (San Diego, California, USA).
RESULTS
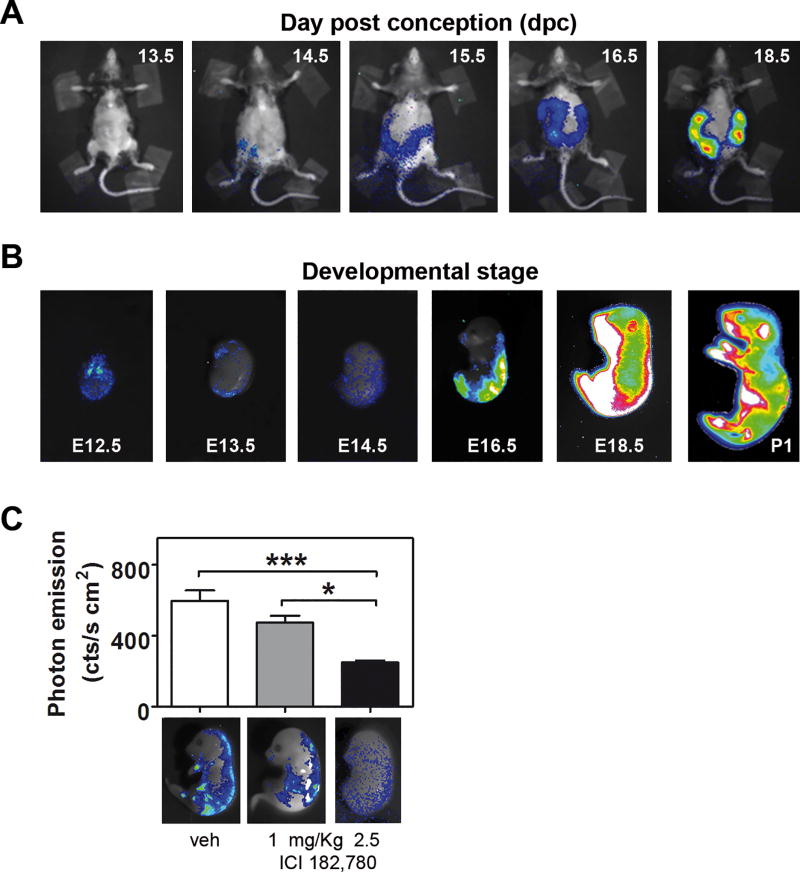
The in vivo BLI of wild-type female mice crossed with homozygote male ERE-Luc clearly showed luciferase activity in living foetuses growing in utero. Fig. 1A shows that by exposing the pregnant mothers to CCD, photon emission from the foetuses was measurable from 14.5 days post conception (dpc), increasing in the course of pregnancy, and was highest at 18.5 dpc. Considering that the mother tissues shielded some of the embryo bioluminescence, to obtain better insight into the onset of luciferase synthesis in the embryos, we investigated the BLI the pattern of ER activity in excised embryos. Therefore, the mothers were injected with luciferin and euthanized for the dissection and rapid measurement of BLI in each individual embryo (Fig. 1B). Photon emission was measurable starting at 12.5 dpc but not before. At 12.5 dpc, photon emission was quite diffuse in the whole embryo, with higher emission in the telencephalon, in the proximity of the eyes, in the heart and in the spinal ganglia. The finding of luciferase activity at 12.5 dpc was consistent with gonad differentiation from a bipotential to sexually differentiated state (as indicated by transcriptome analysis) (Munger, et al. 2013) and with the highest foetal production of oestrogens (Lemmen, et al. 2002). At 13.5 dpc, photon emission was more circumscribed to the brain and peripheral nervous system (PNS). From 16.5 dpc to 18.5 dpc, luciferase emission increased from the dorsal to the ventral skin, and one day post birth (P1), the signal was maximal in the hepatic/abdomen area and in the limbs.
Fig. 1. ER is transcriptionally active in the developing mouse.
(A) In utero imaging: anaesthetized wild-type pregnant mothers carrying ERE-Luc foetuses were injected with the substrate luciferin 30 min prior to imaging, and photon emission was recorded in BLI units. Merging of the pseudo colour-transformed signal with the pregnant picture allows the identification of transcriptional active oestrogen receptors in the developing litter (blue: low, white: high). The mothers were shaved to improve photon imaging. (B) Representative BLI taken in living foetuses immediately after uterine excision and of a newborn mouse at P1. The sex of the mouse at P1 is male. (C) 24 hours and 6 hours before litter collection, pregnant females were treated with vehicle or 1 mg/kg or 2.5 mg/kg of the ER pure antagonist ICI 182,780. Foetuses were excised at 16.5 dpc and were subjected to BLI. Photon emission (cts/cm2 s) was measured in the whole (upper) foetal area and was transformed in a pseudocolour image merged on the mice pictures (lower). Bars represent the mean ± SEM. *p < 0.05 calculated with one-way ANOVA followed by Bonferroni post hoc test.
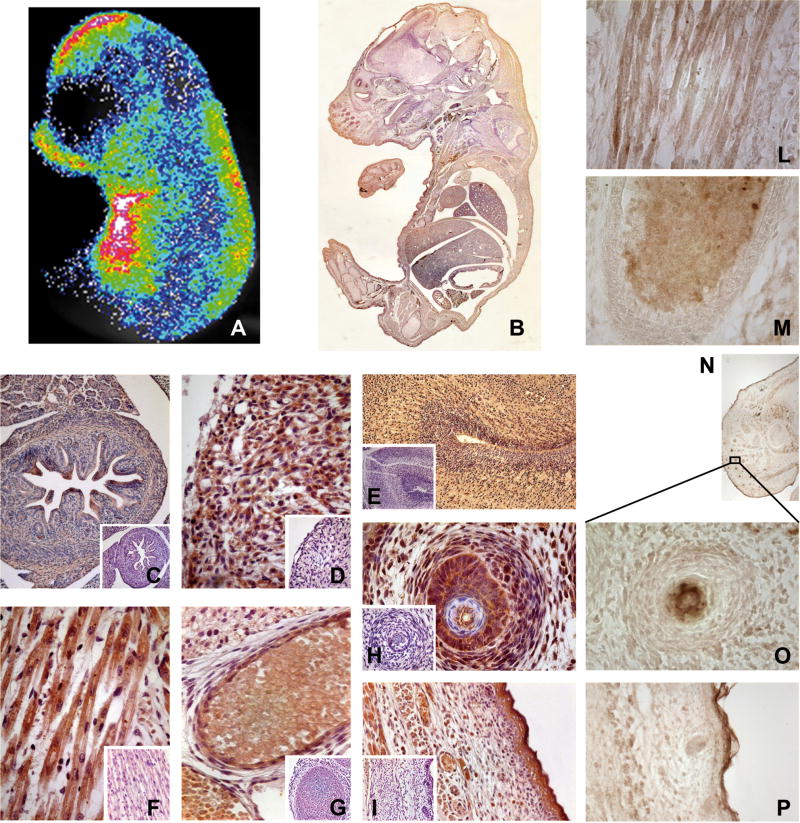
To rule out that oestrogen related receptors (ERRs), which are widely expressed in embryos (Bonnelye, et al. 1997; Luo, et al. 1997), were active on the promoter of the ERE-Luc mouse, we treated pregnant females with the ER pan-antagonist fulvestrant (ICI 182,780), which has been reported to be an agonist of ERRs (Li, et al. 2010). Treatment of the mothers with 1 mg/kg and 2.5 mg/kg ICI 182,780 was associated with a dose-dependent decrease in photon emission in foetuses (Fig. 1C), pointing to the absence of significant contributions from ERRs to luciferase synthesis. To further demonstrate the strict association between photon emission and ER-dependent luciferase expression, mice at 17.5 dpc were subjected to BLI and were subsequently whole-mounted for staining with anti-luciferase antibodies. The intensity and distribution of the peroxidase staining clearly reproduced the BLI (Fig. 2A–B) and enabled the definition of the cells expressing the reporter enzyme at the cellular level. The specificity of the luciferase staining was tested in several tissues of ERE-Luc and wild-type mice (Fig. 2 C–I) and, importantly, the immuno-decoration of luciferase reproduced the staining obtained with anti-ERα antibodies in several tissues (whole snout, muscle, bone, whiskers, skin) (Fig. 2 L–P).
Fig. 2. Immunostaining reveals ER activity in different tissues.
A 17.5 dpc litter was subjected both to BLI and to IHC against luciferase and ERα. (A) representative BLI of one mouse (signal was arithmetically increased with respect to Fig. 1B) and one of its whole-mount sections stained for luciferase (B). (C–I) IHC against luciferase; organ pictures were taken at 100–400× magnification: intestine (C; 100×), heart (D; 400×), hindbrain (E; 100×), muscle (F; 400×), rib (G; 400×), whisker follicle (H; 400×), skin (I; 200×). Small panels show the antibody specificity tested on a wild-type mouse. (L–P) IHC against ERα; organ pictures were taken at 100–400× magnification: muscle (L; 400×), rib (M; 400×), nose (N; reconstruction of 50×), whisker follicle (O; 400×), skin (P; 200×).
This experiment, together with previous BLI observations, was a clear indication of the widespread ER transcriptional activity. In addition to brain and reproductive organs, we found significant amounts of luciferase activity in several non-reproductive tissues. The overall analysis of luciferase showed very high staining in the epidermis and in the hindbrain. With regard to the other organs, the staining appeared to follow a gradient: highest in ectoderm-derived organs and lowest in endodermal organs.
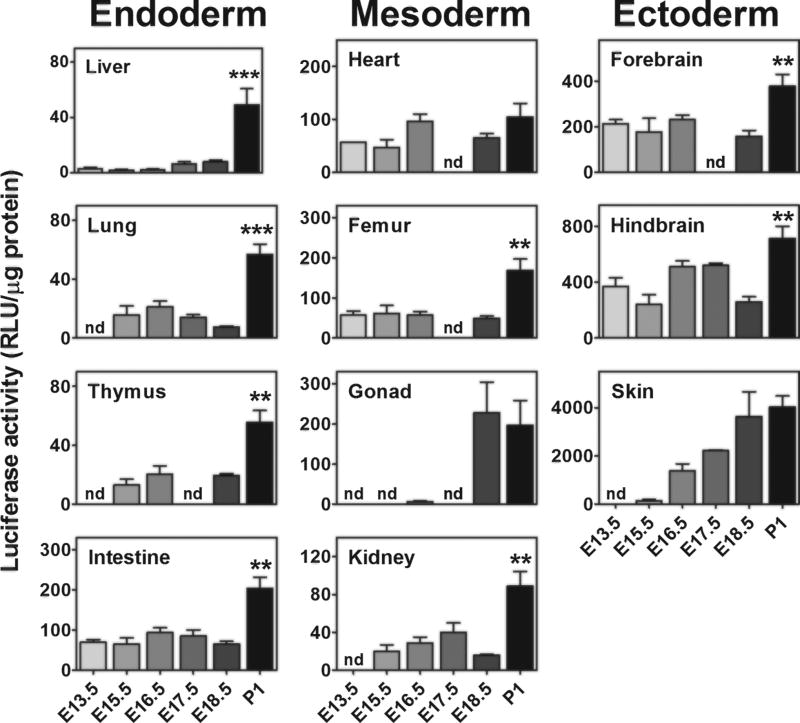
BLI, enabling the measurement of the reporter activity in living animals, is extremely useful for the spatio-temporal study of a given molecular event in single individuals; however, the two dimensional nature of the BLI outcome does not allow the exact, quantitative localization of the organ responsible for photon emission, as the deeper an organ is, the more attenuated the BLI signal, and the photon emission measured in a specific surface represents the sum of all signals coming from the different layers of tissue (Maggi and Ciana 2005). Thus, to better define the organs where ERs were most active, we carried out a series of enzymatic assays in selected tissue extracts. The measured luciferase activity changed significantly in the different organs and ranged from 10 RLU/µg protein to 4000 RLU/µg protein. In line with what was suggested by prior immuno-histochemistry analyses, we found a strict correlation between the germ layer and the amount of luciferase activity (Fig. 3). The highest expression of the reporter was found in ectoderm-derived organs (brain and skin), while in the endoderm-derived organs (liver, lung, thymus and intestine), ER activity was the lowest. Mesodermic organs (heart, femur, gonad and kidney) appeared to have an intermediate concentration of the enzyme. Fig S1 shows that in the skin, hindbrain and forebrain, luciferase is, in general, statistically more expressed than in the other tissues.
Fig. 3. Foetal ER activity shows lineage-correlation.
A pattern of ER transcriptional activity in different organs was obtained by measuring the luciferase activity on tissue lysates (enzymatic assay) obtained from the foetuses of both sexes. Each column contains organs from different germ layers. Bars represent the mean ± SEM (n = 6–10 foetuses). *p < 0.05, **p < 0.01 versus the previous stage calculated by one-way ANOVA followed by the Bonferroni post hoc test. nd = not measured.
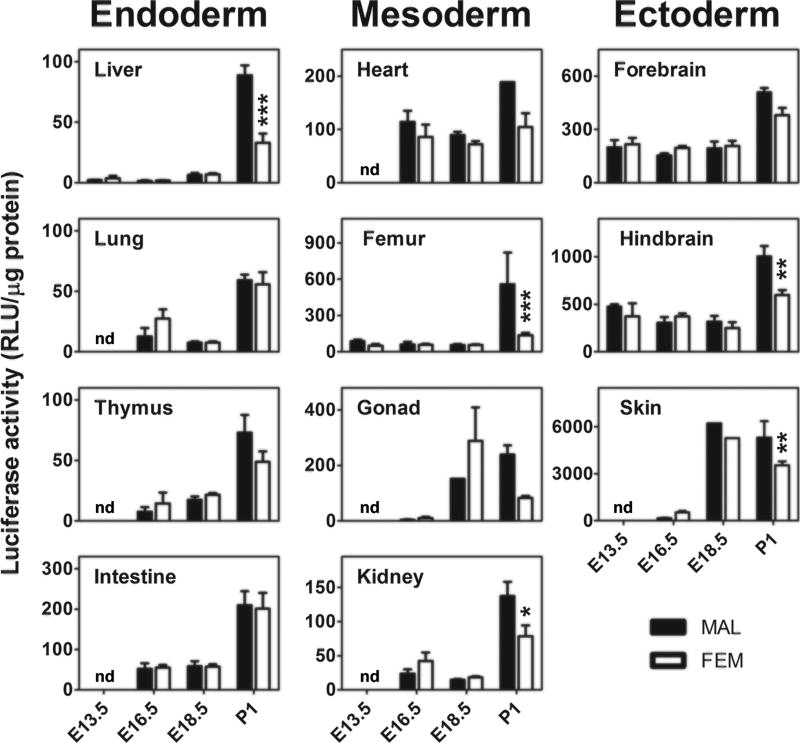
Next, we stratified the data in relation to sex (Fig. 4). No major differences were found prior to birth. The only exception was represented by the gonads at 18.5 dpc, where females had more luciferase than males, an effect possibly associated with the very high expression of ERα protein in the ovary (Nielsen et al. 2000) at this embryonal stage. At P1, there was a generalized increase in luciferase activity in most organs. In the liver, bone, gonads and hindbrain, the increase observed in luciferase expression was significantly higher in males.
Fig. 4. Gender specificity in ER activity during development.
The same data shown in Fig. 3 (luciferase enzymatic assay) were stratified for sex. Bars represent the mean ± SEM (n = 4–6). *p < 0.05, **p < 0.01 compared with opposite gender calculated with unpaired student’s t test. nd = not measured.
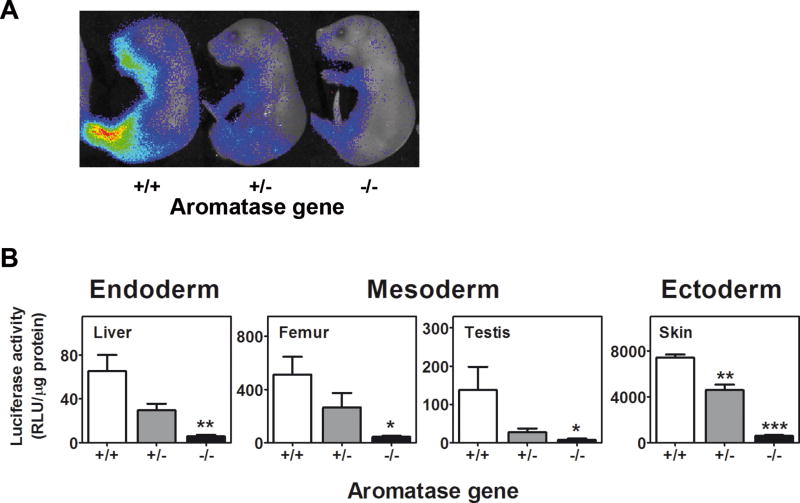
This result was consistent with the fact that male, not female, gonads synthesize steroids just prior to and after birth and that the testosterone synthesized is aromatized to E2 in several organs (Jones et al. 2000). When we crossed the ERE-Luc with the aromatase KO mice, we failed to see such an effect in male livers and femurs as well as the testis and skin of pups at P1 (Fig. 5). In the ArKOxERE-Luc mice, the luciferase activity was comparable with the levels recorded through previous foetal stages (Fig. 3; RLU/µg protein: liver 5.67 ± 1.33; femur 42.67 ± 9.28; testis 6.67 ± 3.67; skin 572 ± 75.50), which provided further support for the hypothesis that the increased activity of the reporter observed in males only at P1 was induced by the oestrogens derived from the surge of testosterone production by male gonads (Clarkson and Herbison 2016). In addition, the observation of a basal level of ER activity in the different organs of ArKOxERE-Luc mice suggested that the unliganded ERs could be activated by factors other than circulating oestrogens.
Fig. 5. Lack of aromatase activity regulates ER activity in males.
ERE-Luc mice were bred with Ar+/− mice, and the progeny were backcrossed to obtain the same litter Ar−/− and Ar+/+ × ERE-Luc mice. (A) Newborn P1 mice were subjected to BLI. (B) Luciferase activity was measured in tissue lysates (enzymatic assay) in which gender difference was previously observed (Fig. 4). Bars represent the mean ± SEM (n = 4–6 foetuses). *p < 0.05, **p < 0.01 versus Ar+/+ × ERE-Luc calculated by one-way ANOVA followed by Bonferroni post hoc test.
DISCUSSION
The present study demonstrates that, in the course of embryogenesis, ER transcriptional activity is not restricted to the reproductive organs. Indeed, ERs appear to be particularly active in tissues originating from the ectoderm, such as the brain and skin, and have a lower yet still significant action in tissues, such as the gonads, kidney, bone, and hearth, deriving from the mesoderm. Quite interesting is the fact that ERs are minimally active in endodermic tissues, such as the lung, liver, thymus and intestine.
The mechanisms associated with this such a differential pattern of ER activity in the organs studied in relation to the germ-layer of origin remain to be defined. An initial explanation could be associated with a differential, tissue-specific expression of the two isoforms of ER. The lack of a detailed localization study describing the relative content of ERα and ERβ in embryogenesis does not allow us to define whether the differences of ERs activity observed are due to a germ-layer-specific expression of each ER or to changes in their relative concentration with consequent potential homo- or hetero-dimerization. A second potential explanation resides in ligand availability, as it is conceivable that, depending of the stage of development, the oestrogens produced may have differential ability to reach the organs deriving from each of the three germ layers. The main source of steroid hormones during pregnancy is the maternal-placental-foetal unit (MPF unit) (Becker 2001). Measurements of oestrogen concentration in maternal blood showed a steady increase throughout pregnancy, with a peak at 17.5 dpc (Barkley, et al. 1977); in the foetus, the peak of oestrogen synthesis is at 12.5 dpc (Lemmen et al. 2002). However, to be transported by the plasma, these lipophilic molecules associate with the alpha-fetoprotein (AFP), and it has been hypothesized that the high concentration of AFP produced may sequester most of the steroids produced by the MPF and may protect the foetus from the effects of maternal steroid hormones (Bakker, et al. 2006; De Mees, et al. 2006; Mendel 1989; Savu, et al. 1981). Our data clearly show germ layer-dependent ER transcriptional activity, which that could be due to either the existence of a tissue-specific uptake of circulating oestrogens or to ER unliganded activation. Indeed, endocytic pathways were described as necessary to carry steroids inside the cells (Hammes, et al. 2005), but we still do not know whether such transport proteins are present in the embryo and are localized differentially in the various tissues. Alternatively, ERs may be differentially activated by means of a localized pattern of expression of genes encoding factors known to be able to activate these nuclear receptors in a unliganded way (such as EGF, IGF or others) (Bondy, et al. 1990; Partanen 1990).
Regardless of the mechanisms involved, the number of tissues where ER is transcriptionally active in the course of development is suggestive of a significant biological function of the hormone-receptor complex in both reproductive and non-reproductive organs. Unfortunately, the developmental effects of the deletion of genes encoding ERα and ERβ or both isoforms have been only slightly investigated. This lack of study is possibly due the fact that none of the initial studies described major phenotypes, and both males and females survived to adulthood (Couse, et al. 1999; Krege, et al. 1998; Lubahn, et al. 1993), even with fertility problems, which is particularly relevant in the ERα null mice (Dupont, et al. 2000; Krege et al. 1998; Walker and Korach 2004). However, more recent studies carried out in adults demonstrated that the deletion of ERα or ERβ is associated with malfunctions of several organs considered to be non-essential for reproduction, such as the lung, liver, adipose tissue, heart, kidney, thymus, bone, and skin (Carey et al. 2007; Barros and Gustafsson 2011; Foryst-Ludwig et al. 2008; Lapid et al. 2014; Del Principe et al. 2015; Tai et al. 2015; Kummer et al. 2011; Zoller and Kersh 2006; Brandenberger et al. 1997; Lemmen et al. 1999; Markiewicz et al. 2013; Takeyama et al. 2001; Walker and Korach 2004). In the perinatal mouse brain, the spatio-temporal expression of ERα and ERβ was reported to contribute to organize sex differences that are not associated with reproduction, such as the stress response and cognition (Mogi et al. 2015).
It is conceivable that the sexual dimorphism observed in the prevalence, course, and severity of many common diseases, including cardiovascular diseases, autoimmune diseases, and asthma, may originate from a sex-specific genetic architecture that is created in the course of development (also through ER activity) and that results in a male and female differential endocrine susceptibility and gene regulation, particularly in sex steroid responsive genes. The relevance of oestrogen programming mechanisms within the mammalian foetus and perinatal time period is underlined by studies on liver metabolism that are strictly regulated by sex and where the expression and activation of hepatic ER in the course of embryo maturation and in adult life plays a prominent role (Della Torre et al., 2018) on energy metabolism (Maniu et al. 2016; Yuchi et al. 2015), gastric functions (Campbell-Thompson et al. 2001), adrenal and renal activity (Inamdar, et al. 2015; Walker, et al. 2009), cardiovascular activity (Del Principe et al. 2015), and immune functions (Zoller and Kersh 2006).
In conclusion, the results from this study and those mentioned above point to a substantial relevance of ER signalling for the correct development of mammalian embryos, and they highlight the necessity to increase our knowledge with more systematic studies aimed at understanding the necessity of these hormones, particularly for the correct development and functioning of non-reproductive organs. Such studies should not be confined to prenatal development and pups, but should be extended to adults, possibly challenged with appropriate stimuli, enabling us to test their ability to respond to alimentary and environmental stimuli, with a particular emphasis on endocrine disrupters known to preferentially act through ERs.
Supplementary Material
Fig. S1. Developmental changes in ER activation suggest the germ-layer origin of the tissues. Luciferase activity was measured in tissue lysates (enzymatic assay) at E16.5, E18.5 and P1. Bars represent the mean ± SEM (n = 6–20). The square brackets are meant to encompass all the tissues significantly different with regard to the skin (#) and hindbrain (*). The statistics of the comparisons with the forebrain are shown with circles (°).
°p < 0.05, °°p < 0.01 and °°°p < 0.001 versus forebrain, ***p < 0.001 versus hindbrain, ###p < 0.001 versus skin calculated by one-way ANOVA followed by Bonferroni post hoc test.
Acknowledgments
FUNDING
This work was supported by grants from the European Community (EWA: STREP EWA LSHM-CT-2005-518245; Ways: ERC-Advanced Grant 322977) and NIH (RO1-AF027713).
We thank E. Simpson (Monash University, Victoria, Australia) for providing the heterozygous for aromatase mice. We acknowledge E. Faggiani and M. Rebecchi for their skilful technical assistance during genotyping.
Footnotes
DECLARATION of INTEREST
The authors do not have any conflicts of interest.
AUTHOR CONTRIBUTION
S.D.T. carried out all the final analyses and control experiments. G.R. carried out most of the experimental work, together with L.O. C.M. performed the immunostaining experiments. P.C. contributed in conceiving the study and shared his expertise on the use of ERE-Luc mice. A.M. conceived the project, wrote the manuscript and supervised the entire project.
References
- Agulnik AI, Bishop CE, Lerner JL, Agulnik SI, Solovyev VV. Analysis of mutation rates in the SMCY/SMCX genes shows that mammalian evolution is male driven. Mammalian genome : official journal of the International Mammalian Genome Society. 1997;8:134–138. doi: 10.1007/s003359900372. [DOI] [PubMed] [Google Scholar]
- Albrecht ED, Lane MV, Marshall GR, Merchenthaler I, Simorangkir DR, Pohl CR, Plant TM, Pepe GJ. Estrogen promotes germ cell and seminiferous tubule development in the baboon fetal testis. Biology of reproduction. 2009;81:406–414. doi: 10.1095/biolreprod.108.073494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnal JF, Lenfant F, Metivier R, Flouriot G, Henrion D, Adlanmerini M, Fontaine C, Gourdy P, Chambon P, Katzenellenbogen B, et al. Membrane and Nuclear Estrogen Receptor Alpha Actions: From Tissue Specificity to Medical Implications. Physiological reviews. 2017;97:1045–1087. doi: 10.1152/physrev.00024.2016. [DOI] [PubMed] [Google Scholar]
- Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nature neuroscience. 2006;9:220–226. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- Barkley MS, Michael SD, Geschwind II, Bradford GE. Plasma testosterone during pregnancy in the mouse. Endocrinology. 1977;100:1472–1475. doi: 10.1210/endo-100-5-1472. [DOI] [PubMed] [Google Scholar]
- Barros RP, Gustafsson JA. Estrogen receptors and the metabolic network. Cell metabolism. 2011;14:289–299. doi: 10.1016/j.cmet.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Becker KL, editor. Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Bondesson M, Hao R, Lin CY, Williams C, Gustafsson JA. Estrogen receptor signaling during vertebrate development. Biochimica et biophysica acta. 2015;1849:142–151. doi: 10.1016/j.bbagrm.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondy CA, Werner H, Roberts CT, Jr, LeRoith D. Cellular pattern of insulin-like growth factor-I (IGF-I) and type I IGF receptor gene expression in early organogenesis: comparison with IGF-II gene expression. Molecular endocrinology. 1990;4:1386–1398. doi: 10.1210/mend-4-9-1386. [DOI] [PubMed] [Google Scholar]
- Bonnelye E, Vanacker JM, Spruyt N, Alric S, Fournier B, Desbiens X, Laudet V. Expression of the estrogen-related receptor 1 (ERR-1) orphan receptor during mouse development. Mechanisms of development. 1997;65:71–85. doi: 10.1016/s0925-4773(97)00059-2. [DOI] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human foetus. The Journal of clinical endocrinology and metabolism. 1997;82:3509–3512. doi: 10.1210/jcem.82.10.4400. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, et al. Evidence that oestrogen receptor-alpha plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49:588–597. doi: 10.1007/s00125-005-0105-3. [DOI] [PubMed] [Google Scholar]
- Campbell-Thompson M, Reyher KK, Wilkinson LB. Immunolocalization of estrogen receptor alpha and beta in gastric epithelium and enteric neurons. The Journal of endocrinology. 2001;171:65–73. doi: 10.1677/joe.0.1710065. [DOI] [PubMed] [Google Scholar]
- Carey MA, Card JW, Bradbury JA, Moorman MP, Haykal-Coates N, Gavett SH, Graves JP, Walker VR, Flake GP, Voltz JW. Spontaneous airway hyperresponsiveness in estrogen receptor-alpha-deficient mice. American journal of respiratory and critical care medicine. 2007;175:126–135. doi: 10.1164/rccm.200509-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, et al. Non-nuclear estrogen receptor alpha signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. The Journal of clinical investigation. 2010;120:2319–2330. doi: 10.1172/JCI38291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciana P, Di Luccio G, Belcredito S, Pollio G, Vegeto E, Tatangelo L, Tiveron C, Maggi A. Engineering of a mouse for the in vivo profiling of estrogen receptor activity. Molecular endocrinology. 2001;15:1104–1113. doi: 10.1210/mend.15.7.0658. [DOI] [PubMed] [Google Scholar]
- Ciana P, Raviscioni M, Mussi P, Vegeto E, Que I, Parker MG, Lowik C, Maggi A. In vivo imaging of transcriptionally active estrogen receptors. Nature medicine. 2003;9:82–86. doi: 10.1038/nm809. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Roig LM. Estrogen receptors in human nontarget tissues: biological and clinical implications. Endocrine reviews. 1995;16:35–62. doi: 10.1210/edrv-16-1-35. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE. Hypothalamic control of the male neonatal testosterone surge. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2016;371:20150115. doi: 10.1098/rstb.2015.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conte FA, Grumbach MM, Ito Y, Fisher CR, Simpson ER. A syndrome of female pseudohermaphrodism, hypergonadotropic hypogonadism, and multicystic ovaries associated with missense mutations in the gene encoding aromatase (P450arom) The Journal of clinical endocrinology and metabolism. 1994;78:1287–1292. doi: 10.1210/jcem.78.6.8200927. [DOI] [PubMed] [Google Scholar]
- Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors alpha and beta. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JA. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacological reviews. 2006;58:773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- Della Torre S, Mitro N, Meda C, Lolli F, Pedretti S, Barcella M, Ottobrini L, Metzger D, Caruso D, Maggi A. Short-term fasting reveals AA metabolism as a major sex discriminating factor in the liver. Cell Metabolism. 2018 doi: 10.1016/j.cmet.2018.05.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson JA. International Union of Pharmacology. LXIV. Estrogen receptors. Pharmacological reviews. 2006;58:773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- De Mees C, Laes JF, Bakker J, Smitz J, Hennuy B, Van Vooren P, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein controls female fertility and prenatal development of the gonadotropin-releasing hormone pathway through an antiestrogenic action. Molecular and cellular biology. 2006;26:2012–2018. doi: 10.1128/MCB.26.5.2012-2018.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Principe D, Ruggieri A, Pietraforte D, Villani A, Vitale C, Straface E, Malorni W. The relevance of estrogen/estrogen receptor system on the gender difference in cardiovascular risk. International journal of cardiology. 2015;187:291–298. doi: 10.1016/j.ijcard.2015.03.145. [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors alpha (ERalpha) and beta (ERbeta) on mouse reproductive phenotypes. Development. 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, et al. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS genetics. 2008;4:e1000108. doi: 10.1371/journal.pgen.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco TL, Furlow JD, Duello TM, Gorski J. Immunodetection of estrogen receptors in fetal and neonatal female mouse reproductive tracts. Endocrinology. 1991;129:1326–1332. doi: 10.1210/endo-129-3-1326. [DOI] [PubMed] [Google Scholar]
- Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert FJ, Luppa PB, Nykjaer A, et al. Role of endocytosis in cellular uptake of sex steroids. Cell. 2005;122:751–762. doi: 10.1016/j.cell.2005.06.032. [DOI] [PubMed] [Google Scholar]
- Hanley K, Rassner U, Jiang Y, Vansomphone D, Crumrine D, Komuves L, Elias PM, Feingold KR, Williams ML. Hormonal basis for the gender difference in epidermal barrier formation in the fetal rat. Acceleration by estrogen and delay by testosterone. The Journal of clinical investigation. 1996;97:2576–2584. doi: 10.1172/JCI118706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel M, Isaksson P, Schaefer O, Kaufmann U, Ciana P, Maggi A, Schleuning WD. Tissue specificity of 8-prenylnaringenin: protection from ovariectomy induced bone loss with minimal trophic effects on the uterus. The Journal of steroid biochemistry and molecular biology. 2005;97:299–305. doi: 10.1016/j.jsbmb.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Inamdar LS, Khodnapur BS, Nindi RS, Dasari S, Seshagiri PB. Differential expression of estrogen receptor alpha in the embryonic adrenal-kidney-gonadal complex of the oviparous lizard, Calotes versicolor (Daud.) General and comparative endocrinology. 2015;220:55–60. doi: 10.1016/j.ygcen.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Jensen EV. The contribution of "alternative approaches" to understanding steroid hormone action. Molecular endocrinology. 2005;19:1439–1442. doi: 10.1210/me.2005-0154. [DOI] [PubMed] [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kininis M, Kraus WL. A global view of transcriptional regulation by nuclear receptors: gene expression, factor localization, and DNA sequence analysis. Nuclear receptor signaling. 2008;6:e005. doi: 10.1621/nrs.06005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz DM, Hewitt SC, Ciana P, Raviscioni M, Lindzey JK, Foley J, Maggi A, DiAugustine RP, Korach KS. Requirement of estrogen receptor-alpha in insulin-like growth factor-1 (IGF-1)-induced uterine responses and in vivo evidence for IGF-1/estrogen receptor cross-talk. The Journal of biological chemistry. 2002;277:8531–8537. doi: 10.1074/jbc.M109592200. [DOI] [PubMed] [Google Scholar]
- Korach KS, Horigome T, Tomooka Y, Yamashita S, Newbold RR, McLachlan JA. Immunodetection of estrogen receptor in epithelial and stromal tissues of neonatal mouse uterus. Proceedings of the National Academy of Sciences of the United States of America. 1988;85:3334–3337. doi: 10.1073/pnas.85.10.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudwa AE, Michopoulos V, Gatewood JD, Rissman EF. Roles of estrogen receptors alpha and beta in differentiation of mouse sexual behavior. Neuroscience. 2006;138:921–928. doi: 10.1016/j.neuroscience.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Kummer S, Jeruschke S, Wegerich LV, Peters A, Lehmann P, Seibt A, Mueller F, Koleganova N, Halbenz E, Schmitt CP, et al. Estrogen receptor alpha expression in podocytes mediates protection against apoptosis in-vitro and in-vivo. PloS one. 2011;6:e27457. doi: 10.1371/journal.pone.0027457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane PH. Estrogen receptors in the kidney: lessons from genetically altered mice. Gender medicine. 2008;5(Suppl A):S11–18. doi: 10.1016/j.genm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Lapid K, Lim A, Clegg DJ, Zeve D, Graff JM. Oestrogen signalling in white adipose progenitor cells inhibits differentiation into brown adipose and smooth muscle cells. Nature communications. 2014;5:5196. doi: 10.1038/ncomms6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmen JG, Broekhof JL, Kuiper GG, Gustafsson JA, van der Saag PT, van der Burg B. Expression of estrogen receptor alpha and beta during mouse embryogenesis. Mechanisms of development. 1999;81:163–167. doi: 10.1016/s0925-4773(98)00223-8. [DOI] [PubMed] [Google Scholar]
- Lemmen JG, van den Brink CE, Legler J, van der Saag PT, van der Burg B. Detection of oestrogenic activity of steroids present during mammalian gestation using oestrogen receptor alpha- and oestrogen receptor beta-specific in vitro assays. The Journal of endocrinology. 2002;174:435–446. doi: 10.1677/joe.0.1740435. [DOI] [PubMed] [Google Scholar]
- Li Y, Birnbaumer L, Teng CT. Regulation of ERRalpha gene expression by estrogen receptor agonists and antagonists in SKBR3 breast cancer cells: differential molecular mechanisms mediated by g protein-coupled receptor GPR30/GPER-1. Molecular endocrinology. 2010;24:969–980. doi: 10.1210/me.2009-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Sladek R, Bader JA, Matthyssen A, Rossant J, Giguere V. Placental abnormalities in mouse embryos lacking the orphan nuclear receptor ERR-beta. Nature. 1997;388:778–782. doi: 10.1038/42022. [DOI] [PubMed] [Google Scholar]
- Maggi A, Ciana P. Reporter mice and drug discovery and development. Nature reviews. Drug discovery. 2005;4:249–255. doi: 10.1038/nrd1661. [DOI] [PubMed] [Google Scholar]
- Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annual review of physiology. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- Maniu A, Aberdeen GW, Lynch TJ, Nadler JL, Kim SO, Quon MJ, Pepe GJ, Albrecht ED. Estrogen deprivation in primate pregnancy leads to insulin resistance in offspring. The Journal of endocrinology. 2016;230:171–183. doi: 10.1530/JOE-15-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewicz M, Znoyko S, Stawski L, Ghatnekar A, Gilkeson G, Trojanowska M. A role for estrogen receptor-alpha and estrogen receptor-beta in collagen biosynthesis in mouse skin. The Journal of investigative dermatology. 2013;133:120–127. doi: 10.1038/jid.2012.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel CM. The free hormone hypothesis: a physiologically based mathematical model. Endocrine reviews. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- Mogi K, Takanashi H, Nagasawa M, Kikusui T. Sex differences in spatiotemporal expression of AR, ERalpha, and ERbeta mRNA in the perinatal mouse brain. Neuroscience letters. 2015;584:88–92. doi: 10.1016/j.neulet.2014.10.028. [DOI] [PubMed] [Google Scholar]
- Munger SC, Natarajan A, Looger LL, Ohler U, Capel B. Fine time course expression analysis identifies cascades of activation and repression and maps a putative regulator of mammalian sex determination. PLoS genetics. 2013;9:e1003630. doi: 10.1371/journal.pgen.1003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussi P, Liao L, Park SE, Ciana P, Maggi A, Katzenellenbogen BS, Xu J, O'Malley BW. Haploinsufficiency of the corepressor of estrogen receptor activity (REA) enhances estrogen receptor function in the mammary gland. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16716–16721. doi: 10.1073/pnas.0607768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen M, Bjornsdottir S, Hoyer PE, Byskov AG. Ontogeny of oestrogen receptor alpha in gonads and sex ducts of fetal and newborn mice. Journal of reproduction and fertility. 2000;118:195–204. doi: 10.1530/jrf.0.1180195. [DOI] [PubMed] [Google Scholar]
- Pang SF, Caggiula AR, Gay VL, Goodman RL, Pang CS. Serum concentrations of testosterone, oestrogens, luteinizing hormone and follicle-stimulating hormone in male and female rats during the critical period of neural sexual differentiation. The Journal of endocrinology. 1979;80:103–110. doi: 10.1677/joe.0.0800103. [DOI] [PubMed] [Google Scholar]
- Park CJ, Chen G, Koo Y, Lin PP, Cacioppo JA, Prohaska H, Ko CJ. Generation and characterization of an estrogen receptor alpha-iCre knock-in mouse. Genesis. 2017;55 doi: 10.1002/dvg.23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partanen AM. Epidermal growth factor and transforming growth factor-alpha in the development of epithelial-mesenchymal organs of the mouse. Current topics in developmental biology. 1990;24:31–55. [PubMed] [Google Scholar]
- Patrone C, Cassel TN, Pettersson K, Piao YS, Cheng G, Ciana P, Maggi A, Warner M, Gustafsson JA, Nord M. Regulation of postnatal lung development and homeostasis by estrogen receptor beta. Molecular and cellular biology. 2003;23:8542–8552. doi: 10.1128/MCB.23.23.8542-8552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penza M, Jeremic M, Marrazzo E, Maggi A, Ciana P, Rando G, Grigolato PG, Di Lorenzo D. The environmental chemical tributyltin chloride (TBT) shows both estrogenic and adipogenic activities in mice which might depend on the exposure dose. Toxicology and applied pharmacology. 2011;255:65–75. doi: 10.1016/j.taap.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- Rizzi N, Rebecchi M, Levandis G, Ciana P, Maggi A. Identification of novel loci for the generation of reporter mice. Nucleic acids research. 2017;45:e37. doi: 10.1093/nar/gkw1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savu L, Benassayag C, Vallette G, Christeff N, Nunez E. Mouse alpha 1-fetoprotein and albumin. A comparison of their binding properties with estrogen and fatty acid ligands. The Journal of biological chemistry. 1981;256:9414–9418. [PubMed] [Google Scholar]
- Tait S, Tassinari R, Maranghi F, Mantovani A. Bisphenol A affects placental layers morphology and angiogenesis during early pregnancy phase in mice. Journal of applied toxicology : JAT. 2015;35:1278–1291. doi: 10.1002/jat.3176. [DOI] [PubMed] [Google Scholar]
- Takeyama J, Suzuki T, Inoue S, Kaneko C, Nagura H, Harada N, Sasano H. Expression and cellular localization of estrogen receptors alpha and beta in the human fetus. The Journal of clinical endocrinology and metabolism. 2001;86:2258–2262. doi: 10.1210/jcem.86.5.7447. [DOI] [PubMed] [Google Scholar]
- Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. doi: 10.1126/science.1086185. [DOI] [PubMed] [Google Scholar]
- Thuresson-Klein A, Moawad AH, Hedqvist P. Estrogen stimulates formation of lamellar bodies and release of surfactant in the rat fetal lung. American journal of obstetrics and gynecology. 1985;151:506–514. doi: 10.1016/0002-9378(85)90279-0. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Zahniser DJ, Baum MJ. Sexual dimorphism in the preoptic/anterior hypothalamic area of ferrets: effects of adult exposure to sex steroids. Brain research. 1986;364:249–257. doi: 10.1016/0006-8993(86)90837-1. [DOI] [PubMed] [Google Scholar]
- Toft D, Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proceedings of the National Academy of Sciences of the United States of America. 1966;55:1574–1581. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annual review of biochemistry. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Ueberschlag-Pitiot V, Stantzou A, Messeant J, Lemaitre M, Owens DJ, Noirez P, Roy P, Agbulut O, Metzger D, Ferry A. Gonad-related factors promote muscle performance gain during postnatal development in male and female mice. American journal of physiology. Endocrinology and metabolism. 2017;313:E12–E25. doi: 10.1152/ajpendo.00446.2016. [DOI] [PubMed] [Google Scholar]
- Vantaggiato C, Dell'Omo G, Ramachandran B, Manni I, Radaelli E, Scanziani E, Piaggio G, Maggi A, Ciana P. Bioluminescence imaging of estrogen receptor activity during breast cancer progression. Am J Nucl Med Mol Imaging. 2016;6(1):32–41. [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Caruana G, Bertram JF, McInnes KJ. Sexual dimorphism in mouse metanephroi exposed to 17 beta-estradiol in vitro. Nephron. Experimental nephrology. 2009;111:e42–50. doi: 10.1159/000191104. [DOI] [PubMed] [Google Scholar]
- Walker VR, Korach KS. Estrogen receptor knockout mice as a model for endocrine research. ILAR journal. 2004;45:455–461. doi: 10.1093/ilar.45.4.455. [DOI] [PubMed] [Google Scholar]
- Yuchi Y, Cai Y, Legein B, De Groef S, Leuckx G, Coppens V, Van Overmeire E, Staels W, De Leu N, Martens G, et al. Estrogen Receptor alpha Regulates beta-Cell Formation During Pancreas Development and Following Injury. Diabetes. 2015;64:3218–3228. doi: 10.2337/db14-1798. [DOI] [PubMed] [Google Scholar]
- Zoller AL, Kersh GJ. Estrogen induces thymic atrophy by eliminating early thymic progenitors and inhibiting proliferation of beta-selected thymocytes. Journal of immunology. 2006;176:7371–7378. doi: 10.4049/jimmunol.176.12.7371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Developmental changes in ER activation suggest the germ-layer origin of the tissues. Luciferase activity was measured in tissue lysates (enzymatic assay) at E16.5, E18.5 and P1. Bars represent the mean ± SEM (n = 6–20). The square brackets are meant to encompass all the tissues significantly different with regard to the skin (#) and hindbrain (*). The statistics of the comparisons with the forebrain are shown with circles (°).
°p < 0.05, °°p < 0.01 and °°°p < 0.001 versus forebrain, ***p < 0.001 versus hindbrain, ###p < 0.001 versus skin calculated by one-way ANOVA followed by Bonferroni post hoc test.