Abstract
Background
High N-terminal pro-brain natriuretic peptide (NT-proBNP) during COPD exacerbations is associated with worse clinical outcomes. The prognostic value of NT-proBNP measured during clinical stability has not been well characterized.
Methods
We studied SPIROMICS participants 40–80 years of age with COPD GOLD spirometric stages 1–4. The association between baseline NT-proBNP and incident COPD exacerbations within one year of follow-up was tested using zero-inflated Poisson regression models adjusted for age, gender, race, body mass index, current smoking status, smoking history, FEV1 percent predicted, COPD Assessment Test score, exacerbation history, total lung capacity on chest CT and cardiovascular disease (any of coronary artery disease, myocardial infarction or congestive heart failure).
Results
Among 1,051 participants (mean age 66.1 years, 41.4% women), mean NT-proBNP was 608.9 pg/ml. Subjects in GOLD stage D had the highest mean NT-proBNP. After one year of follow-up, 268 participants experienced one or more COPD exacerbations. One standard deviation increase in baseline NT-proBNP was associated with a 13% increase in the risk of incident exacerbations (incident risk ratio 1.13; 95% CI 1.06–1.19; p<0.0001). This association was maintained in participants with and without cardiovascular disease.
Conclusion
Baseline NT-proBNP in COPD is an independent predictor of respiratory exacerbations, even in individuals without overt cardiac disease. The impact of detection and treatment of early cardiovascular dysfunction on COPD exacerbation frequency warrants further investigation.
Keywords: chronic obstructive pulmonary disease, brain natriuretic peptides, cardiovascular disease, respiratory exacerbation
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) exacerbations are associated with poor quality of life (1), accelerated lung function decline (2), increased mortality (3) and elevated healthcare costs (4). Therefore, identification of COPD patients at increased risk of exacerbations has the potential to improve outcomes. Although a history of prior exacerbations is currently the best predictor of future events (5), the variability of exacerbation frequency both within and between COPD subjects (6) and the overall stochastic nature of these events suggest that multiple mechanisms are likely involved. Blood biomarkers, which can easily be measured during clinical stability, are attractive candidates for inclusion in exacerbation prediction models, but their role in this context has yet to be defined.
N-terminal pro-brain natriuretic peptide (NT-proBNP) and BNP are fragments of the precursor hormone pro-BNP which is synthesized in and released from ventricular myocytes in response to myocardial stretch (7). NT-proBNP is a well-established diagnostic and prognostic biomarker in congestive heart failure (CHF) (8, 9) and has also been found to be an independent predictor of death and cardiovascular events in non-ST elevation acute coronary syndrome (10), stable coronary artery disease (11), and even in asymptomatic individuals sampled from the community (12). Natriuretic peptide levels increase during acute exacerbations of COPD and return to baseline after successful treatment (13, 14). Higher NT-proBNP levels measured at the time of a COPD exacerbation have been associated with increased need for intensive care (15) and higher mortality (16–19). However, the role of NT-proBNP as a prognostic biomarker in stable COPD has not been fully investigated. NT-proBNP is a particularly interesting potential marker of risk in COPD because even in patients not experiencing an exacerbation, its levels are higher compared to healthy controls and individuals with asthma (13, 20, 21).
We hypothesized that higher NT-proBNP levels measured during clinical stability are associated with an increased risk of future COPD exacerbations. To test this association, we conducted a prospective analysis of the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) cohort.
METHODS
Participants and Study Design
SPIROMICS is a multicenter longitudinal study funded by the National Health Lung and Blood Institute (ClinicalTrials.gov identifier: NCT01969344) designed to identify different COPD subgroups and to validate intermediate outcome measures for use in therapeutic clinical trials (22). It enrolled participants 40–80 years of age between 2010 and 2015 who were either never-smokers (< 1 pack-year smoking history) with pre-bronchodilator forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) ≥ 0.7 and FVC > lower limit of normal (23) or current/former smokers (at least 20 pack-year smoking history) with and without airflow obstruction defined as post-bronchodilator FEV1/FVC < 0.7. Post-bronchodilator spirometry was obtained after each of four inhalations of albuterol 90 µg and ipratropium 18 µg. Spirometric tracings were independently reviewed to confirm that they met American Thoracic Society and European Respiratory Society standards (24). Participants were classified in stages of Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric severity (25) as well as in GOLD ABCD stages based on both 2011 and 2017 guidelines (26). In addition, extensive data was collected at the initial study visit, including demographics, comorbidities, questionnaires to assess symptoms and quality of life, cigarette smoke exposure, 6-minute walk distance and high-resolution computed tomography (CT) of the chest (22). Total lung capacity (TLC) was computed from chest CT at full inflation (VIDA Diagnostics, Iowa City, IA). The SPIROMICS protocol was approved by the institutional review boards of all participating institutions and all participants gave written informed consent.
NT-proBNP Levels
The characteristics of blood assays used in SPIROMICS have been previously reported (27). All NT-proBNP measurements described in this analysis were obtained from blood samples collected at baseline using a Myriad-RBM assay (Austin, TX).
COPD exacerbations
Self-reported history of COPD exacerbations in the year before study enrollment was taken from participants at the initial visit. Prospective exacerbation data was collected every three months through a structured telephone questionnaire. Exacerbations were defined as respiratory flare-up events that required health care utilization (office visit, Emergency Department visit or hospital admission) and the use of antibiotics and/or systemic corticosteroids. Severe exacerbations were defined as those necessitating a visit to the Emergency Department or hospitalization. Exacerbations were fully managed by the participants’ usual care providers, with the study investigators not providing any guidance on treatment.
Statistical Analysis
We assessed demographic, spirometric, symptom burden and comorbidity differences between participants with higher (≥ 900 pg/ml) and lower (< 900 pg/ml) baseline NT-proBNP levels, a cutoff chosen for its high accuracy in acute CHF (8). Continuous variables were described as means and standard deviations and categorical ones as percentages. We also examined the distribution of NT-proBNP across GOLD spirometry stages and GOLD ABCD stages based on 2011 and 2017 guidelines. We used two-sample t-tests to compare NT-proBNP levels between individual spirometric stages and individual ABCD stages. The association between baseline NT-proBNP and incident total COPD exacerbations within one year of follow-up was tested using multivariable zero-inflated Poisson models adjusted for age, gender, race, body mass index (BMI), current smoking status, smoking history, FEV1 percent predicted, COPD Assessment Test (CAT) score (28), prior history of COPD exacerbations, TLC measured on chest CT and cardiovascular disease (coronary artery disease [CAD], myocardial infarction [MI] and CHF). Continuous NT-proBNP was standardized and the associations were presented by change in one standard deviation of the distribution (29); an alternative model using NT-proBNP ≥ 900 pg/ml as a dichotomous variable was also tested. Additionally, we used the same covariates as above to examine the relationship between NT-proBNP and severe exacerbations (≥1) using multivariable logistic regression. We also conducted subgroups analyses according to the presence of underlying cardiovascular disease (defined as CAD, MI or CHF) and tested for interactions between NT-proBNP and cardiovascular disease. All analyses were performed in SAS 9.4 (Cary, NC, USA). A p-value < 0.05 was considered statistically significant.
RESULTS
Description of participants
We identified 1,051 ever-smoker participants with airflow obstruction (post-bronchodilator FEV1/FVC < 0.7) who had an NT-proBNP level drawn at baseline. A description of the baseline characteristics of participants stratified by NT-proBNP level is presented in Table 1. Subjects with NT-proBNP ≥ 900 pg/ml were older, more likely to be women and less likely to be African-American, had a greater smoking history and were more likely to report CAD, MI and CHF as comorbidities. Those with NT-proBNP < 900 pg/ml had lower FEV1 percent predicted and greater TLC on chest CT. Mean NT-proBNP in the entire cohort was 608.9 ± 894.1 pg/ml.
Table 1.
Baseline characteristics of study participants
| NT-proBNP < 900 pg/ml (n=849) |
NT-proBNP ≥ 900 pg/ml (n=202) |
p-value | |
|---|---|---|---|
| Demographics | |||
| Age | 65.2 ± 7.7 | 70.0 ± 6.8 | < 0.0001 |
| Female gender (%) | 334 (39.3%) | 101 (50.0%) | 0.006 |
| African-American (%) | 123 (14.5%) | 11 (5.5%) | 0.0005 |
| Smoking exposure | |||
| Smoking history (pack-years) | 52.4 ± 23.6 | 57.3 ± 26.8 | 0.009 |
| Current smoker (%) | 281 (33.1%) | 59 (29.2%) | 0.29 |
| Markers of respiratory health | |||
| Post-bronchodilator FEV1 (% predicted) | 61.4 ± 23.8 | 65.5 ± 20.1 | 0.02 |
| TLC (L) | 5.4 ± 1.3 | 5.0 ± 1.2 | < 0.0001 |
| CAT score | 14.9 ± 7.8 | 14.2 ± 7.9 | 0.28 |
| 6MWD (m) | 401.1 ± 118.5 | 384.3 ± 125.4 | 0.08 |
| ≥ 1 COPD exacerbation in past year (%) | 246 (29.0%) | 53 (26.2%) | 0.44 |
| Comorbidities | |||
| CAD (%) | 79 (9.5%) | 44 (22.0%) | < 0.0001 |
| MI (%) | 50 (6.0%) | 31 (15.5%) | < 0.0001 |
| CHF (%) | 16 (1.9%) | 13 (6.5%) | 0.0004 |
| Any of CAD, MI or CHF (%) | 109 (12.8%) | 62 (30.7%) | < 0.0001 |
| GERD (%) | 258 (30.4%) | 68 (33.7%) | 0.37 |
| Obesity (%) | 271 (31.9%) | 58 (28.7%) | 0.38 |
Data are presented as mean ± standard deviation for continuous variables and as number (percentage) for categorical variables. NT-proBNP, N-terminal pro-brain natriuretic peptide, FEV1, forced expiratory volume in one second; TLC, total lung capacity measured on chest CT; CAT, COPD Assessment Test; 6MWD, 6-minute walking distance; CAD, coronary artery disease; MI, myocardial infarction; CHF, congestive heart failure; GERD, gastroesophageal reflux disease; obesity defined as BMI > 30 kg/m2.
NT-proBNP level by GOLD spirometry and ABCD stages
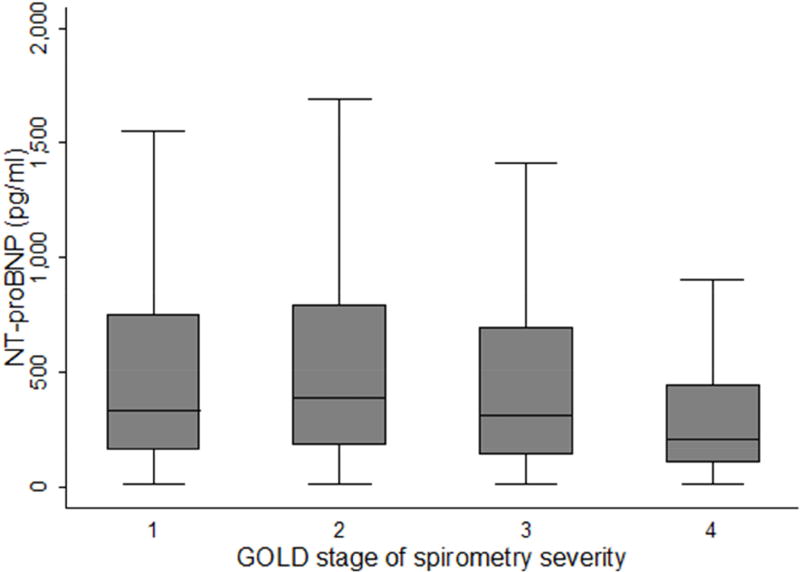
GOLD spirometry stage 4 subjects (n = 92) had the lowest mean NT-proBNP (338.6 ± 383.4 pg/ml), with a statistically significant difference found compared to subjects in GOLD spirometry stage 2 only, but not those in GOLD stages 1 or 3 (Figure 1). NT-proBNP levels by 2011 and 2017 GOLD ABCD stages are shown in Table 2. In the 2017 classification, 24 subjects moved from stage C to A and 176 subjects moved from B to D compared to the 2011 classification. There was no statistically significant difference in NT-proBNP levels between any two individual GOLD stages in the 2011 classification. In the 2017 classification, subjects in stage D had significantly higher NT pro-BNP levels compared to those in stage B (758.4 vs. 574.9 pg/ml, p=0.03)
Figure 1.
Box plots of NT-proBNP by GOLD spirometry stage
Table 2.
NT-proBNP level by GOLD stages ABCD based on 2011 and 2017 guidelines
| GOLD stages | A | B | C | D | |
|---|---|---|---|---|---|
| 2011 guidelines | n | 286 | 384 | 56 | 325 |
| NT-proBNP level | 575.4 ± 652.7 | 664.3 ± 1032.8 | 634.5 ± 692.7 | 568.6 ± 931.3 | |
| 2017 guidelines | n | 310 | 533 | 32 | 176 |
| NT-proBNP level | 580.5 ± 652.0 | 574.9 ± 728.6 | 629.1 ± 731.0 | 758.4 ± 1519.9 |
NT-proBNP levels are presented as mean ± standard deviation.
Association of NT-proBNP with COPD exacerbations
After one year of follow-up, 268 (25.5%) of the 1,051 participants experienced one or more COPD exacerbations. A higher baseline NT-proBNP was associated with an increased incidence of COPD exacerbations in multivariable analysis of the entire cohort (Table 3); for one standard deviation increase in NT-proBNP, there was a 13% increase in the risk of total exacerbations (Incidence Risk Ratio [IRR] 1.13; 95% CI 1.06–1.19; p < 0.0001). We also examined the relationship between an NT-proBNP threshold of ≥ 900 pg/ml and exacerbations in this same model, and again found a significant association between higher NT-proBNP and exacerbation risk (IRR 1.62; 95% CI 1.19–2.21; p = 0.002). Similarly, one standard deviation increment in NT-proBNP was associated with a higher incidence of severe exacerbations (those requiring Emergency Department visit or hospitalization) during one-year follow-up (IRR 1.27; 95% CI 1.06–1.52; p = 0.009). The incidence risk ratio of severe exacerbations when NT-proBNP ≥ 900 pg/ml was 1.65 (95% CI 0.89–3.06; p=0.11). Finally, we performed additional analyses in participants with and without cardiovascular disease and found that the association between NT-proBNP and incident total COPD exacerbations was maintained in both subgroups (Table 3). An interaction between NT-proBNP and cardiovascular disease was not statistically significant (p = 0.57).
Table 3.
Association of baseline NT-proBNP with COPD exacerbations during one-year follow-up in multivariable analyses
| All subjects n = 1,051 |
Subjects with cardiovascular disease n = 171 |
Subjects without cardiovascular disease n = 880 |
|
|---|---|---|---|
| IRR | 1.13 | 1.22 | 1.10 |
| 95% CI | 1.06 – 1.19 | 1.01 – 1.47 | 1.02 – 1.20 |
| p-value | < 0.0001 | 0.04 | 0.01 |
All entries represent incidence risk ratios (IRR) for exacerbations per one standard deviation increment of NT-proBNP within each group. Models are based on zero-inflated Poisson regression adjusted for demographics (age, gender, race), smoking exposure (smoking history, current smoking status), markers of lung health (FEV1 % predicted, COPD Assessment Test [CAT] score, exacerbation history at enrollment and total lung capacity on chest CT), body mass index and cardiovascular disease (coronary artery disease, myocardial infarction and congestive heart failure – included only for analysis of the entire cohort, not subgroup analyses).
DISCUSSION
In a large cohort of smokers with COPD, we found that a higher NT-proBNP level measured during clinical stability is associated with an increased risk of COPD exacerbations within one year of follow-up, regardless of the presence of underlying cardiovascular disease.
Participants with GOLD stage 4 spirometry in our cohort had the lowest baseline mean NT-proBNP with a significant difference found compared to those with stage 2, but not stages 1 or 3. Results from several smaller prior studies examining the relationship between natriuretic peptide (BNP or NT-proBNP) levels and GOLD spirometric stage are inconsistent, ranging from higher levels in GOLD stages 3 and 4 (30), no significant difference across GOLD stages (13, 31) and a trend for lower BNP levels at higher GOLD stages (14). One possibility is that, on average, hyperinflation with more severe emphysema reduces cardiac chamber size and stretch (32, 33), thereby decreasing natriuretic peptide levels in subjects with advanced COPD. Another possibility is that survival bias is contributing to lower NT-proBNP levels in individuals with severe COPD as those with high levels could be underrepresented due to death. Regardless, there is still a wide range of NT-proBNP levels in all GOLD spirometric stages; our multivariable analysis, which adjusts for FEV1 percent predicted, suggests an increased risk of exacerbations when elevated baseline NT-proBNP levels are present.
When participants were reclassified from 2011 to 2017 GOLD ABCD groups, some moved from stage C to A and others from stage D to B. These findings are consistent with prior reports comparing the distribution of COPD subjects across GOLD stages between the two classifications (34). Whereas the exacerbation risk is assessed based on the severity of airflow obstruction and/or exacerbation history in the 2011 guidelines, it is assessed based on exacerbation history alone in the 2017 guidelines. Under the 2017 classification, NT-proBNP was overall higher in stages C and D compared to stages A and B; it was also significantly higher in stage D compared to stage B. These results indicate that COPD individuals with frequent or severe exacerbations in the previous year have a high NT-proBNP level which then becomes an independent predictor of future exacerbations, even when exacerbation history is accounted for.
While a higher NT-proBNP level measured at the time of a COPD exacerbation has been found to be associated with poor short- and long-term clinical outcomes (15–19), its prognostic role when measured in stable COPD has not been well characterized previously. A study of 60 subjects with COPD showed that the time to the next exacerbation was significantly shorter in those with high baseline plasma BNP levels (13). Although this study enrolled a smaller number of participants and evaluated a different yet related outcome, its findings support the presence of an association between higher natriuretic peptide levels and subsequent COPD exacerbations. We have found a relationship between baseline NT-proBNP and exacerbations (both total and severe) in the year immediately following biomarker measurement. This extends a prior report from the SPIROMICS cohort by Keene et al. that found an association between baseline NT-proBNP and severe, but not total, exacerbations when a group of smokers both with and without airflow obstruction was examined (35).
The pathophysiologic mechanisms behind the association we found still need to be determined, but it is likely that cardiac dysfunction both in individuals with and without overt cardiovascular disease contributes to or is uncovered by acute exacerbation events (36). Cardiac comorbidities are more prevalent in subjects with COPD even after accounting for shared risk factors like age and smoking (37, 38). Compared to subjects with COPD and no ischemic heart disease, Patel and colleagues found that those with COPD and ischemic heart disease have higher NT-proBNP levels both in the stable state and during COPD exacerbations as well as a longer symptom recovery time following exacerbations (39, 40). Left ventricular diastolic dysfunction is also common in COPD (41) and can be found in as many as 90% of patients with advanced disease and no cardiovascular comorbidities (42). Brain natriuretic peptides can also be elevated in right ventricular strain and cor pulmonale. A significant positive correlation has been found between natriuretic peptide level and systolic pulmonary artery pressure in stable COPD subjects (20), even among those without congestive heart failure or a history of pulmonary embolism (30). In addition, Wells and colleagues found that an enlarged pulmonary artery (defined as the ratio of pulmonary artery to aorta diameters greater than 1 on chest CT) was independently predictive of total and severe COPD exacerbations (43). Therefore, the presence of underlying subclinical cardiac dysfunction (such as early diastolic heart failure or early pulmonary hypertension) in COPD subjects without overt cardiovascular disease could either increase the severity of an acute COPD exacerbation (thereby escalating the event to require the attention of a healthcare provider) or be the cause of the acute respiratory event itself as more than 20% of exacerbations occur in the absence of pulmonary infection or inflammation (44, 45). Elevation of NT-proBNP reflects diverse aspects of cardiopulmonary stress such as systolic dysfunction, diastolic dysfunction, right heart strain and pulmonary hypertension, which limits the utility of this biomarker when applied in isolation. It remains to be determined whether a subset of COPD patients with higher baseline NT-proBNP and no known comorbid cardiovascular disease would benefit from early evaluation (by echocardiography or cardiac stress test) and treatment of cardiac dysfunction to minimize their risk of adverse events, including respiratory exacerbations.
Our study has a number of strengths such as its large and well characterized cohort, the inclusion of participants with a wide range of severity of airflow obstruction and its adjustment for relevant clinical confounders including factors known to affect NT-proBNP levels such as age, gender and BMI (46). We also acknowledge several limitations. First, our participants were all evaluated in academic medical centers and may not have the same characteristics or outcomes as subjects sampled from the general population. Second, natriuretic peptide levels can be elevated in renal impairment and objective measures of kidney function were not available for inclusion in our analysis. Third, since comorbidities were self-reported by participants, they could have been subject to information bias. However, multiple epidemiologic studies have showed that self-report of cardiovascular conditions is consistently reliable when compared against medical records (47, 48). Fourth, a COPD exacerbation was defined as a respiratory event that specifically required treatment with antibiotics and/or corticosteroids as determined by each participant’s usual care provider. Since diagnostic error on the part of the treating clinician is possible, this definition can potentially result in the misdiagnosis of heart failure exacerbations or other mimics but has the advantage of reflecting real-life clinical decisions and practice.
In summary, higher NT-proBNP measured during clinical stability in subjects with COPD is an independent predictor of respiratory exacerbations within one year of follow-up. Investigating the contribution and interplay of cardiac (elevated pulmonary artery pressure, left ventricular filling impairment, transient coronary ischemia) and pulmonary (emphysema severity, static and dynamic hyperinflation) variables in COPD will enhance our understanding of exacerbation events in susceptible individuals. NT-proBNP can help to risk stratify patients and may contribute to personalize care in the outpatient setting. Whether evaluation and treatment of early cardiac dysfunction decreases respiratory exacerbation frequency and severity in subjects with COPD warrants further study.
Highlights.
-
■
NT-proBNP level in stable COPD is highest in GOLD stage D
-
■
NT-proBNP in stable COPD is an independent predictor of respiratory exacerbations
-
■
This association is maintained even in the absence of cardiovascular disease
Acknowledgments
The authors thank the SPIROMICS participants and participating physicians, investigators and staff for making this research possible. More information about the study and how to access SPIROMICS data is at www.spiromics.org. We would like to acknowledge the following current and former investigators of the SPIROMICS sites and reading centers: Neil E Alexis, PhD; Wayne H Anderson, PhD; R Graham Barr, MD, DrPH; Eugene R Bleecker, MD; Richard C Boucher, MD; Russell P Bowler, MD, PhD; Elizabeth E Carretta, MPH; Stephanie A Christenson, MD; Alejandro P Comellas, MD; Christopher B Cooper, MD, PhD; David J Couper, PhD; Gerard J Criner, MD; Ronald G Crystal, MD; Jeffrey L Curtis, MD; Claire M Doerschuk, MD; Mark T Dransfield, MD; Christine M Freeman, PhD; MeiLan K Han, MD, MS; Nadia N Hansel, MD, MPH; Annette T Hastie, PhD; Eric A Hoffman, PhD; Robert J Kaner, MD; Richard E Kanner, MD; Eric C Kleerup, MD; Jerry A Krishnan, MD, PhD; Lisa M LaVange, PhD; Stephen C Lazarus, MD; Fernando J Martinez, MD, MS; Deborah A Meyers, PhD; Wendy C Moore, MD; John D Newell Jr, MD; Laura Paulin, MD, MHS; Stephen Peters, MD, PhD; Elizabeth C Oelsner, MD, MPH; Wanda K O’Neal, PhD; Victor E Ortega, MD, PhD; Robert Paine, III, MD; Nirupama Putcha, MD, MHS; Stephen I. Rennard, MD; Donald P Tashkin, MD; Mary Beth Scholand, MD; J Michael Wells, MD; Robert A Wise, MD; and Prescott G Woodruff, MD, MPH. The project officers from the Lung Division of the National Heart, Lung, and Blood Institute were Lisa Postow, PhD, and Thomas Croxton, PhD, MD.
FINANCIAL SUPPORT
SPIROMICS was supported by contracts from the National Institutes of Health / National Heart, Lung, and Blood Institute (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), and supplemented by contributions made through the Foundation for the NIH and the COPD Foundation from AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc..; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; Nycomed GmbH; Takeda Pharmaceutical Company; Novartis Pharmaceuticals Corporation; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; and Sunovion.
This analysis was also supported by National Institutes of Health grants R01HL122438, R01HL126838, T32HL007749 and K24HL138188.
JLC reports research support from the NIH, NHLBI, MedImmune, and the Department of Veterans Affairs.
RGB reports a grant from the Alpha-1 Foundation, royalties from UpToDate and travel support from the COPD Foundation.
SPB is supported by NIH grant K23HL133438.
ERB reports grants from the NHLBI, research support paid to the institution from Amgen, AstraZeneca-MedImmune, Boehringer-Ingelheim, Genentech/Roche, GlaxoSmithKline, Janssen/Johnson & Johnson, Novartis, Pfizer, Sanofi-Regneron and Teva. He reports consulting for Amgen, AstraZeneca-MedImmune, Boehringer-Ingelheim, Genentech/Roche, GlaxoSmithKline, KNoff, Novartis, Sanofi/Regeneron.
CBC reports grants from Equinox Health Clubs, personal fees from Equinox Health Clubs, grants from Amgen, personal fees from PulmonX, personal fees from Boehringer Ingelheim, personal fees from GlaxoSmithKline, grants from Spiration, personal fees from Spiration, outside the submitted work. CBC also works part-time on scientific engagement for the GlaxoSmithKline Global Respiratory Franchise.
MTD has received grants from NIH and the Department of Defense, consulting fees from AstraZeneca, Boerhinger Ingelheim, Genentech, GlaxoSmithKline, and PneumRx/BTG and contracted clinical trial funding from AstraZeneca, Boerhinger Ingelheim, GlaxoSmithKline, Yungjin, PneumRx/BTG, Pulmonx, Novartis, and Boston Scientific.
JMW is supported by NIH/NHLBI grant K08HL123940 and has received grant support and consulting fees from GlaxoSmithKline, AstraZeneca, Gilead, Quintiles, Mylan, and the Cystic Fibrosis Foundation.
EAH reports research support from the NIH. He also reports he is the founder and shareholder of VIDA Diagnostics.
VEO reports receiving funding from the National Institutes of Health (NIH) National Heart, Lung, and Blood Insitute (NHLBI) in the form of a K08 training award, NIH HL118128. He also reports consultancy fees from CSL Behring.
RPB reports consulting for Boehringer Ingelheim, AstraZeneca and GlaxoSmithKline.
PGW reports consulting for Genentech, Roche, AstraZeneca, Sanofi, Jannsen, Neostem and Novartis. He also reports research support from Medimmune.
FJM reports consulting for Forest, Janssens, GSK, Nycomed/Takeda, Amgen, AstraZeneca, Boehringer Ingelheim, Ikaria/Bellerophon, Genentech, Novartis, Pearl, Pfizer, Roche, Sunovion, Theravance, Axon, CME Incite, California Society for Allergy and Immunology, Annenberg, Integritas, InThought, Miller Medical, National Association for Continuing Education, Paradigm, Peer Voice, UpToDate, Haymarket Communications, Western Society of Allergy and Immunology, Informa, Bioscale, Unity Biotechnology, ConCert, Lucid, Methodist Hospital, Prime, WebMD, Kadmon, Veracyte, American Thoracic Society, Academic CME, Falco, National Association for Continuing Education, Johnson & Johnson, Clarion, Continuing Education, Potomac, Afferent and Adept. He also reports research support from the NIH.
MKH reports reports grants from NIH, grants from Foundation for the NIH and grants from COPD Foundation, during the conduct of the study. She also reports consulting for Boehringer Ingelheim, GlaxoSmithKline, Novartis and AstraZeneca, royalties from UpToDate and research support from Novartis.
ABBREVIATIONS LIST
- BMI
Body mass index
- BNP
Brain natriuretic peptide
- CAD
Coronary artery disease
- CAT
COPD assessment test
- CHF
Congestive heart failure
- COPD
Chronic obstructive pulmonary disease
- CT
Computed tomography
- FEV1
Forced expiratory volume in one second
- FVC
Forced vital capacity
- GERD
Gastroesophageal reflux disease
- GOLD
Global initiative for chronic Obstructive Lung Disease
- MI
Myocardial infarction
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- TLC
Total lung capacity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
WWL, MX, SM, NNH, REK, RB, SPP, JAK, DJC and CHM have no conflicts of interest.
References
- 1.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 2.Dransfield MT, Kunisaki KM, Strand MJ, Anzueto A, Bhatt SP, Bowler RP, Criner GJ, Curtis JL, Hanania NA, Nath H, Putcha N, Roark SE, Wan ES, Washko GR, Wells JM, Wendt CH, Make BJ Investigators CO. Acute Exacerbations and Lung Function Loss in Smokers with and without Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2017;195:324–330. doi: 10.1164/rccm.201605-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khakban A, Sin DD, FitzGerald JM, Ng R, Zafari Z, McManus B, Hollander Z, Marra CA, Sadatsafavi M. Ten-Year Trends in Direct Costs of COPD: A Population-Based Study. Chest. 2015;148:640–646. doi: 10.1378/chest.15-0721. [DOI] [PubMed] [Google Scholar]
- 5.Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA Evaluation of CLtIPSEI. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 6.Han MK, Quibrera PM, Carretta EE, Barr RG, Bleecker ER, Bowler RP, Cooper CB, Comellas A, Couper DJ, Curtis JL, Criner G, Dransfield MT, Hansel NN, Hoffman EA, Kanner RE, Krishnan JA, Martinez CH, Pirozzi CB, O'Neal WK, Rennard S, Tashkin DP, Wedzicha JA, Woodruff P, Paine R, 3rd, Martinez FJ investigators S. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017 doi: 10.1016/S2213-2600(17)30207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–322. doi: 10.1016/S0140-6736(03)13976-1. [DOI] [PubMed] [Google Scholar]
- 8.Januzzi JL, Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU, Lloyd-Jones DM, Brown DF, Foran-Melanson S, Sluss PM, Lee-Lewandrowski E, Lewandrowski KB. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95:948–954. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann F, Packer M, Coats AJ, Fowler MB, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Anker SD, Amann-Zalan I, Hoersch S, Katus HA. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial. Circulation. 2004;110:1780–1786. doi: 10.1161/01.CIR.0000143059.68996.A7. [DOI] [PubMed] [Google Scholar]
- 10.Heeschen C, Hamm CW, Mitrovic V, Lantelme NH, White HD Platelet Receptor Inhibition in Ischemic Syndrome Management I. N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation. 2004;110:3206–3212. doi: 10.1161/01.CIR.0000147611.92021.2B. [DOI] [PubMed] [Google Scholar]
- 11.Kragelund C, Gronning B, Kober L, Hildebrandt P, Steffensen R. N-terminal pro-B-type natriuretic peptide and long-term mortality in stable coronary heart disease. N Engl J Med. 2005;352:666–675. doi: 10.1056/NEJMoa042330. [DOI] [PubMed] [Google Scholar]
- 12.Linssen GC, Bakker SJ, Voors AA, Gansevoort RT, Hillege HL, de Jong PE, van Veldhuisen DJ, Gans RO, de Zeeuw D. N-terminal pro-B-type natriuretic peptide is an independent predictor of cardiovascular morbidity and mortality in the general population. Eur Heart J. 2010;31:120–127. doi: 10.1093/eurheartj/ehp420. [DOI] [PubMed] [Google Scholar]
- 13.Inoue Y, Kawayama T, Iwanaga T, Aizawa H. High plasma brain natriuretic peptide levels in stable COPD without pulmonary hypertension or cor pulmonale. Intern Med. 2009;48:503–512. doi: 10.2169/internalmedicine.48.1701. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura K, Nishimura T, Onishi K, Oga T, Hasegawa Y, Jones PW. Changes in plasma levels of B-type natriuretic peptide with acute exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:155–162. doi: 10.2147/COPD.S55143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stolz D, Breidthardt T, Christ-Crain M, Bingisser R, Miedinger D, Leuppi J, Mueller B, Tamm M, Mueller C. Use of B-type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008;133:1088–1094. doi: 10.1378/chest.07-1959. [DOI] [PubMed] [Google Scholar]
- 16.Medina AM, Marteles MS, Saiz EB, Martinez SS, Laiglesia FR, Rodriguez JA, Perez-Calvo JI. Prognostic utility of NT-proBNP in acute exacerbations of chronic pulmonary diseases. Eur J Intern Med. 2011;22:167–171. doi: 10.1016/j.ejim.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Marcun R, Sustic A, Brguljan PM, Kadivec S, Farkas J, Kosnik M, Coats AJ, Anker SD, Lainscak M. Cardiac biomarkers predict outcome after hospitalisation for an acute exacerbation of chronic obstructive pulmonary disease. Int J Cardiol. 2012;161:156–159. doi: 10.1016/j.ijcard.2012.05.044. [DOI] [PubMed] [Google Scholar]
- 18.Hoiseth AD, Omland T, Hagve TA, Brekke PH, Soyseth V. NT-proBNP independently predicts long term mortality after acute exacerbation of COPD - a prospective cohort study. Respir Res. 2012;13:97. doi: 10.1186/1465-9921-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang CL, Robinson SC, Mills GD, Sullivan GD, Karalus NC, McLachlan JD, Hancox RJ. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax. 2011;66:764–768. doi: 10.1136/thx.2010.155333. [DOI] [PubMed] [Google Scholar]
- 20.Bozkanat E, Tozkoparan E, Baysan O, Deniz O, Ciftci F, Yokusoglu M. The significance of elevated brain natriuretic peptide levels in chronic obstructive pulmonary disease. J Int Med Res. 2005;33:537–544. doi: 10.1177/147323000503300509. [DOI] [PubMed] [Google Scholar]
- 21.van Gestel YR, Goei D, Hoeks SE, Sin DD, Flu WJ, Stam H, Mertens FW, Bax JJ, van Domburg RT, Poldermans D. Predictive value of NT-proBNP in vascular surgery patients with COPD and normal left ventricular systolic function. COPD. 2010;7:70–75. doi: 10.3109/15412550903499472. [DOI] [PubMed] [Google Scholar]
- 22.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, Kanner R, Kleerup E, Martinez FJ, Woodruff PG, Rennard S, Group SR. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. American journal of respiratory and critical care medicine. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 24.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J, Force AET. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 25.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DM, Lopez Varela MV, Nishimura M, Roche N, Rodriguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agusti A. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report. GOLD Executive Summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 26.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 27.O'Neal WK, Anderson W, Basta PV, Carretta EE, Doerschuk CM, Barr RG, Bleecker ER, Christenson SA, Curtis JL, Han MK, Hansel NN, Kanner RE, Kleerup EC, Martinez FJ, Miller BE, Peters SP, Rennard SI, Scholand MB, Tal-Singer R, Woodruff PG, Couper DJ, Davis SM Investigators S. Comparison of serum, EDTA plasma and P100 plasma for luminex-based biomarker multiplex assays in patients with chronic obstructive pulmonary disease in the SPIROMICS study. J Transl Med. 2014;12:9. doi: 10.1186/1479-5876-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34:648–654. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 29.Laukkanen JA, Kurl S, Ala-Kopsala M, Vuolteenaho O, Ruskoaho H, Nyyssonen K, Salonen JT. Plasma N-terminal fragments of natriuretic propeptides predict the risk of cardiovascular events and mortality in middle-aged men. Eur Heart J. 2006;27:1230–1237. doi: 10.1093/eurheartj/ehi878. [DOI] [PubMed] [Google Scholar]
- 30.Chi SY, Kim EY, Ban HJ, Oh IJ, Kwon YS, Kim KS, Kim YI, Kim YC, Lim SC. Plasma N-terminal pro-brain natriuretic peptide: a prognostic marker in patients with chronic obstructive pulmonary disease. Lung. 2012;190:271–276. doi: 10.1007/s00408-011-9363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rutten FH, Cramer MJ, Zuithoff NP, Lammers JW, Verweij W, Grobbee DE, Hoes AW. Comparison of B-type natriuretic peptide assays for identifying heart failure in stable elderly patients with a clinical diagnosis of chronic obstructive pulmonary disease. Eur J Heart Fail. 2007;9:651–659. doi: 10.1016/j.ejheart.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 32.Watz H, Waschki B, Meyer T, Kretschmar G, Kirsten A, Claussen M, Magnussen H. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest. 2010;138:32–38. doi: 10.1378/chest.09-2810. [DOI] [PubMed] [Google Scholar]
- 33.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, Jiang R, Kawut SM, Kronmal RA, Lima JA, Shahar E, Smith LJ, Watson KE. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–227. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabrera Lopez C, Casanova Macario C, Marin Trigo JM, de-Torres JP, Sicilia Torres R, Gonzalez JM, Polverino F, Divo M, Pinto Plata V, Zulueta JJ, Celli B. Comparison of the 2017 and 2015 Global Initiative for Chronic Obstructive Lung Disease Reports. Impact on Grouping and Outcomes. Am J Respir Crit Care Med. 2018;197:463–469. doi: 10.1164/rccm.201707-1363OC. [DOI] [PubMed] [Google Scholar]
- 35.Keene JD, Jacobson S, Kechris K, Kinney GL, Foreman MG, Doerschuk CM, Make BJ, Curtis JL, Rennard SI, Barr RG, Bleecker ER, Kanner RE, Kleerup EC, Hansel NN, Woodruff PG, Han MK, Paine R, 3rd, Martinez FJ, Bowler RP, O'Neal WK Copdgene, dagger SId. Biomarkers Predictive of Exacerbations in the SPIROMICS and COPDGene Cohorts. American journal of respiratory and critical care medicine. 2017;195:473–481. doi: 10.1164/rccm.201607-1330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sabit R, Bolton CE, Fraser AG, Edwards JM, Edwards PH, Ionescu AA, Cockcroft JR, Shale DJ. Subclinical left and right ventricular dysfunction in patients with COPD. Respir Med. 2010;104:1171–1178. doi: 10.1016/j.rmed.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 37.Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3:631–639. doi: 10.1016/S2213-2600(15)00241-6. [DOI] [PubMed] [Google Scholar]
- 38.Roversi S, Fabbri LM, Sin DD, Hawkins NM, Agusti A. Chronic Obstructive Pulmonary Disease and Cardiac Diseases. An Urgent Need for Integrated Care. American journal of respiratory and critical care medicine. 2016;194:1319–1336. doi: 10.1164/rccm.201604-0690SO. [DOI] [PubMed] [Google Scholar]
- 39.Patel ARC, Donaldson GC, Mackay AJ, Wedzicha JA, Hurst JR. The impact of ischemic heart disease on symptoms, health status, and exacerbations in patients with COPD. Chest. 2012;141:851–857. doi: 10.1378/chest.11-0853. [DOI] [PubMed] [Google Scholar]
- 40.Patel AR, Kowlessar BS, Donaldson GC, Mackay AJ, Singh R, George SN, Garcha DS, Wedzicha JA, Hurst JR. Cardiovascular risk, myocardial injury, and exacerbations of chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2013;188:1091–1099. doi: 10.1164/rccm.201306-1170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Funk GC, Lang I, Schenk P, Valipour A, Hartl S, Burghuber OC. Left ventricular diastolic dysfunction in patients with COPD in the presence and absence of elevated pulmonary arterial pressure. Chest. 2008;133:1354–1359. doi: 10.1378/chest.07-2685. [DOI] [PubMed] [Google Scholar]
- 42.Lopez-Sanchez M, Munoz-Esquerre M, Huertas D, Gonzalez-Costello J, Ribas J, Manresa F, Dorca J, Santos S. High Prevalence of Left Ventricle Diastolic Dysfunction in Severe COPD Associated with A Low Exercise Capacity: A Cross-Sectional Study. PLoS One. 2013;8:e68034. doi: 10.1371/journal.pone.0068034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells JM, Washko GR, Han MK, Abbas N, Nath H, Mamary AJ, Regan E, Bailey WC, Martinez FJ, Westfall E, Beaty TH, Curran-Everett D, Curtis JL, Hokanson JE, Lynch DA, Make BJ, Crapo JD, Silverman EK, Bowler RP, Dransfield MT Investigators CO, Investigators ES. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367:913–921. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. American journal of respiratory and critical care medicine. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 45.Beghe B, Verduri A, Roca M, Fabbri LM. Exacerbation of respiratory symptoms in COPD patients may not be exacerbations of COPD. Eur Respir J. 2013;41:993–995. doi: 10.1183/09031936.00180812. [DOI] [PubMed] [Google Scholar]
- 46.Daniels LB, Maisel AS. Natriuretic peptides. J Am Coll Cardiol. 2007;50:2357–2368. doi: 10.1016/j.jacc.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 47.Tretli S, Lund-Larsen PG, Foss OP. Reliability of questionnaire information on cardiovascular disease and diabetes: cardiovascular disease study in Finnmark county. J Epidemiol Community Health. 1982;36:269–273. doi: 10.1136/jech.36.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bush TL, Miller SR, Golden AL, Hale WE. Self-report and medical record report agreement of selected medical conditions in the elderly. Am J Public Health. 1989;79:1554–1556. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]