Abstract
Osteogenesis imperfecta (OI) is a genetic disorder characterized by altered bone quality and imbalanced bone remodeling, leading to skeletal fractures which are most prominent during childhood. Treatments for OI have focused on restoring pediatric bone density and architecture to recover functional strength and consequently reduce fragility. Though antiresorptive agents like bisphosphonates (BP) are currently the most common intervention for the treatment of OI, a number of studies have shown efficacy of sclerostin antibody (SclAb) in inducing gains in bone mass and reducing fragility in OI mouse models. In this study, the effects of the concurrent use of BP and SclAb were evaluated during bone growth in a mouse harboring an OI-causing Gly→Cys mutation on col1a1. A single dose of antiresorptive BP facilitated the anabolic action of SclAb by increasing availability of surfaces for new bone formation via retention of primary trabeculae that would otherwise be remodeled. Chronic effects of concurrent administration of BP and SclAb revealed that accumulating cycles conferred synergistic gains in trabecular mass and vertebral stiffness, suggesting a distinct advantage of both therapies combined. Cortical gains in mass and strength occurred through SclAb alone, independent of presence of BP. In conclusion, these preclinical results support the scientific hypothesis that minimal antiresorptive treatment can amplify the effects of SclAb during early stages of skeletal growth to further improve bone structure and rigidity, a beneficial outcome for children with OI.
Introduction
Osteogenesis imperfecta (OI) is a genetic bone disorder caused by collagen-related mutations resulting in type-dependent skeletal phenotypes ranging from subclinical to lethal severity (1). Though these phenotypes are type dependent, OI is most commonly associated with low bone mass, altered bone quality, and imbalanced bone remodeling leading to skeletal fractures and deformities like scoliosis,short stature, and bowing of the long bones (2). In the presence of OI, up-regulation of osteoclast activity causes a cellular imbalance that favors resorption, resulting in thinner bones with fewer trabeculae both of which greatly increase fracture risk during childhood (3).
Despite no current cure for the disease, treatments for pediatric OI have focused on improving bone density to promote functional strength and consequently reduce bone fragility. Currently, anti-resorptive agents from the class of bisphosphonates (BP) are the standard of care for pediatric OI. Through their high affinity for calcium ions, these potent inhibitors of bone resorption strongly bind to hydroxyapatite bone surfaces where they can later be internalized by osteoclasts to interrupt the resorption process (4). Several controlled clinical trials have established the beneficial effects of treating pediatric OI with BP, including decreased bone turnover and increased bone mineral density, particularly at sites of trabecular bone (5–11). During endochondral growth, primary trabeculae are remodeled and converted to secondary spongiosa, and extended BP treatment interrupts this process, thus retaining calcified cartilage and increasing metaphyseal mass. In support of these studies, we and others have shown that with BP treatment, significant improvements in trabecular number, but not trabecular thickness are realized (12–17). However, there remain concerns about the long-term treatment and retention of BPs in a growing skeleton (18), and there remains a clinical need for additional therapeutic strategies that can minimize BP dose while maximizing therapeutic benefit.
Recently, sclerostin antibody (SclAb) has gained interest as an anabolic approach for the treatment of OI (19–24). Sclerostin, expressed in mature osteocytes, inhibits bone formation by exerting antagonistic effects on Wnt signaling and downstream osteoblast activity (25). We have previously shown a significant anabolic response to SclAb in an OI mouse model (19–23). These studies showed that treatment with a neutralizing antibody to sclerostin stimulated bone formation in cortical and trabecular bone, resulting in significant improvements in biomechanical properties. Unlike bisphosphonates, SclAb led to significant improvements in trabecular thickness. In the present study we sought to determine whether bisphosphonates could be used to augment SclAb efficacy by inducing retention of primary trabeculae which could then serve as a substrate for the anabolic actions of SclAb to increase trabecular bone mass. We hypothesize that when combined, these two interventions would independently target different pathways of the bone remodeling cycle, leading to increases in trabecular bone mass greater than when either therapy was given alone. To accomplish this, in the present study, we test the immediate and long-term effects of treating rapidly growing Brtl/+ mice, harboring an OI-causing defect, with SclAb and BP together during growth. Our knock-in mouse model reproduces features of moderately severe Type IV OI. Treatment of Brtl/+ with alendronate alone increased trabecular bone mass through retention of calcified cartilage, with modest cortical gains that did not translate directly to biomechanical improvements (17). Conversely, treatment of Brtl/+ with Scl-Ab alone elicited significant gains in both trabecular and cortical bone mass (20). In the present study, through combination therapy, SclAb and BP induce gains in both trabecular thickness and number, leading to synergistic gains in trabecular bone stiffness, suggesting a distinct advantage to combination therapy in a growing mouse model of OI.
Materials and Methods
Animals
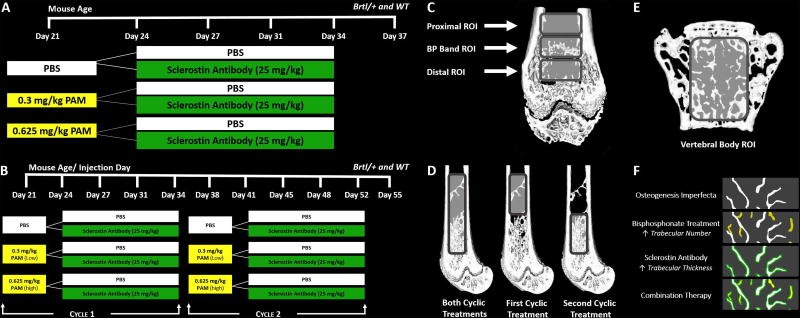
Wildtype (WT) and Brtl/+ mice with a mixed background of SV129/CD-1/C57BL/6S were derived from heterozygous Brtl/+ and WT parental strains (26). To assess the short-term effects of pamidronate (PAM) and SclAb combined, at 21 days of age, male WT and Brtl/+ mice received a single intraperitoneal injection of pamidronate (PAM) at either 0.3mg/kg or 0.625mg/kg (Sigma-Aldrich, St Louis, MO, USA) or saline control. These doses of PAM represent 10% and 20% of doses shown to induce trabecular retention for 20 days in mice of similar age (13). After a three-day latency period, mice were randomly assigned to SclAb treatment (Scl-Ab VI, 25 mg/kg, Amgen, Thousand Oaks, CA, USA) or saline groups and injected subcutaneously twice a week for two weeks (through day 34). SclAb was chosen at a dose that consistently induced an anabolic effect in our prior studies (19–23). Calcein and alizarin were administered one day before PAM and at day 35, respectively by intraperitoneal injection (30mg/kg; Sigma-Aldrich, St Louis, MO, USA) to visualize the growth pattern during treatment. Short-term treatment mice (n = 7/group) were euthanized at day 37. Similarly, to evaluate the long-term effects of PAM and SclAb combined, male WT and Brtl/+ mice received two cycles of combination therapy, repeating the treatment described above on day 38. Calcein, alizarin and calcein were administered one day before cycle 1, one day before cycle 2, and at day 53, respectively by intraperitoneal injection (30mg/kg). Mice (n = 10/group) were euthanized at day 55. A summary of the experimental design for short- and long-term assessment of combination therapy is shown in Figure 1. All protocols and procedures were approved by the University of Michigan’s Committee on Use and Care of Animals.
Fig. 1.
Combination therapy to assess the effects after A) a single cycle and B) concurrent cycles of combination therapy. Region of interest placement in distal femoral metaphysis to analyze the C) short-term, D) long-term and E) vertebral trabecular bone effects of combination therapy. F) Proposed mechanism of action for combination therapy.
Micro-computed tomography (µCT)
To determine the short and long-term effects of PAM and SclAb on trabecular and cortical bone morphology, L5 vertebra and left femora were scanned in water via µCT (eXplore Locus SP, GE Healthcare Pre-Clinical Imaging, London, ON, Canada). Scans were performed with the source set at 80kVp and 80µA using the Parker method of rotation (180 degrees plus a 20 degree fan angle), 0.5 degree increment angle, 4 frames averaged, and an acrylic beam flattener with a 0.02 Al filter were used for filtration of beam hardening artifacts. Images were reconstructed at 18 µm voxel size, and a hydroxyapatite-mimicking phantom was used to calibrate grayscale values for densitometry (27).
Treatment of the growing skeleton with anti-resorptive agents induces formation of a BP-laden band reflecting retention of primary spongiosa in the femur (28, 29). To study the short-term combined influence of PAM and SclAb on the femur, a 0.7 mm ROI was placed at this BP-induced retention region of the distal femur. A second ROI was located distal to the band (closer to the growth plate), representing bone formed subsequent to BP injection, during SclAb treatment. A third ROI was located proximal to the band representing a site of trabecular bone formed prior to BP injection, but still under the influence of SclAb. A summary of the position of these ROIs are shown in Figure 1C. Segmentation and binarization of trabecular bone at these sites was performed using a local threshold adjusted at each site for optimal representation of trabecular microarchitecture. Trabecular bone at and distal to BP band was segmented at 1500 HU, while trabecular bone found at the region proximal to BP band was segmented at 1300 Hounsfield units.
To understand the resulting effects from concurrent cycles of combination therapy in the femur, a 4.5 mm ROI was placed at the metaphysis just proximal to the growth plate and extending through to the location of the proximal-most band of retained trabecular bone. To isolate the effects of each administered cyclic treatment, this full cycle ROI was broken down into representative regions of cycle 1 (2.7 mm) and cycle 2 (1.8 mm). Cycle 1 spanned from above the first BP band to right above the second induced BP band. Cycle 2 spanned above the second sclerotic BP band to right above the growth plate. A schematic of the placement of these three regions of interest is shown in Figure 1D. Due to the maturity of the bone and size of ROIs, a local auto threshold was used to quantify trabecular bone at these three regions (30).
While a retention-related band was observed in the femur following BP treatment, a “bone in bone” appearance was induced in the vertebral body, consistent with vertebral growth patterns and clinical observations (31). As a result, an ROI was place between the cranial and caudal endplates of the L5 vertebrae to analyze both short-term and long-term effects of combination treatment. Vertebral cortical and trabecular bone were separated through manual contouring to denote the outer and inner boundaries of the cortex and segmented by a local auto-threshold.
To measure both short-term and long-term effects of treatment on femoral cortical bone, an ROI spanning 15% of total femoral length was centered midway between the lateral third trochanter and the distal femoral growth plate in all samples. A local auto threshold was used to quantify cortical bone. Bone architecture parameters (trabecular number – TbN, trabecular thickness – TbTh, bone volume fraction – BV/TV, and cortical thickness – CTh) were analyzed using commercially available software (MicroView Advanced Bone Analysis Application, GE Healthcare Pre-Clinical Imaging, London, ON, Canada).
Bone histomorphometry
To qualitatively analyze bone formation response from short and long-term combination therapy, femurs and L2 vertebrae were embedded without decalcification in methyl methacrylate. Both tissues were sectioned longitudinally with a Reichert-Jung Polycut microtome (Reichert-Jung, Germany) at a thickness of 8 µm and mounted on gelatin–coated glass slides. An upright microscope (Nikon Eclipse Ni-U) associated with a DS-Fi2 digital camera and NIS BR software (Nikon France, Champigny-sur-Marne, France) was used to acquire calcein and alizarin fluorescent images with a 10× dry objective.
Biomechanical testing
To assess the mechanical effects of short and long-term combination therapy, L5 vertebra (short and long-term) and left femora (long-term only) were loaded to failure in compression and four-point bending, respectively, using an MTS 858 Mini-Bionix servo-hydraulic testing system (MTS Systems Corp., Eden Prairie, MN, USA). All specimens were kept hydrated in lactated ringer's solution (LRS) prior to mechanical testing. The vertebral body was vertically aligned along its loading axis with an alignment pin (attached to lower platen and extending through the spinal column) and compressed to failure at a displacement rate of 0.05 mm/second. For four-point bending, the posterior surface of the femur was oriented in tension and the mid-diaphysis was loaded to failure at a displacement rate of 0.5 mm/second. Force and vertical displacements were continuously recorded throughout each test by a 50 lb load cell (Sensotec, Columbus, OH, USA) and an external linear variable differential transducer (LVDT; Lucas Schavitts, Hampton, VA, USA), respectively. A custom- developed LABVIEW program was used to calculate the mechanical properties for both tissues.
Statistics
Regional variations in bone architecture and biomechanics among the different treatments were determined using two-way ANOVA (Graph Pad Prism 6.0) for each genotype independently. P-values <0.05 were considered significant, and significance is expressed on each plot for independent effects of PAM (vertical arrow) and SclAb (horizontal arrow) within each genotype. Data are reported through dumbbell dot plots which display group means of PAM treatment (white markers) and PAM + SclAb treatment (solid markers) for both Brtl/+ (blue and circles) and WT (black and squares). Standard deviations are shown as shadows behind each marker.
Results
PAM and SclAb contribute distinct gains in distal femoral trabecular number and thickness following a single cycle of combination therapy
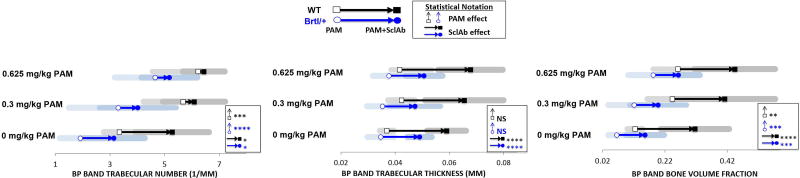
At the metaphyseal band site, where BP induces the greatest trabecular retention, PAM monotherapy induced a significant dose-dependent preservation of TbN at 0.3 mg/kg (Brtl/+ 3.297 ± 1.796/mm; WT 5.677 ± 1.446/mm) and 0.625 mg/kg (Brtl/+ 4.648 ± 1.471/mm; WT 6.225 ± 1.675/mm) compared to vehicle- treated control (Brtl/+ 1.905 ± 0.746/mm; WT 3.343 ± 0.574/mm; Fig. 2, Table S1). While PAM solely acted to stabilize TbN, SclAb monotherapy induced significant gains in both TbTh (Brtl/+ 0.049 ± 0.004 mm; WT 0.059 ± 0.007 mm) and TbN (Brtl/+ 3.143 ± 0.574 mm; WT 5.272 ± 1.349 mm) compare to untreated control (Brtl/+ 0.035 ± 0.005 mm, 1.905 ± 0.746/mm; WT 0.037 ± 0.003 mm, 3.343 ± 0.574/mm). When PAM and SclAb were administered together, PAM continued to show no effect on TbTh, while SclAb triggered consistent thickness gains of 0.049 ± 0.008 mm for Brtl/+ and 0.067 ± 0.013 mm for WT across PAM doses. Interestingly, SclAb alone induced retention of TbN comparable to that of 0.3 mg/kg PAM alone (3.143 ± 1.133/mm vs. 3.297 ± 1.796/mm). Together, combining SclAb and PAM during a single cycle of combination therapy contributed to a maximum increase in bone volume fraction of 0.263 ± 0.069 for Brtl/+ and 0.443 ± 0.130 for WT over untreated control (Brtl/+ 0.065 ± 0.027; WT 0.124 ± 0.025). Comparing regions proximal and distal to metaphyseal band, we noted that these effects were isolated to the site of concurrent drug administration (see Supplemental Fig.S1). Trabecular bone distal to BP site demonstrated minimal effect of BP as TbN was not significantly different from placebo. However, SclAb induced consistent gains in TbTh and TbN as monotherapy. At the proximal-most sites formed prior to BP intervention, BP effect was less evident, while significant gains in TbTh and BV/TV were still observed with SclAb.
Fig. 2.
MicroCT analysis at the site of concurrent drug administration showed that PAM induced a significant dose-dependent increase in TbN while SclAb contributed to significant gains in both TbN and TbTh. Together these independent gains led to an additive gain in bone volume fraction with increasing PAM doses. Results of Two-Way ANOVA factors: * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
Multiple cycles helps stabilize bone mass gains from combination treatment
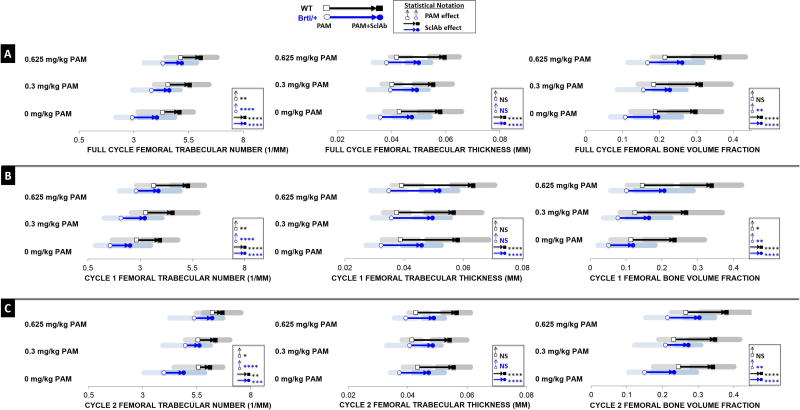
Following two cycles of combination therapy (Fig.3A, Table S2), in trabecular regions extending across the entire metaphysis, PAM continued to show no effect on TbTh, acting solely to further stabilize TbN at 0.3 mg/kg (Brtl/+ 3.813 ± 0.856/mm; WT 4.557 ± 0.624/mm) and 0.625 mg/kg (Brtl/+ 4.329 ± 0.863/mm; WT 5.128 ± 0.687/mm) compared to untreated controls (Brtl/+ 2.929 ± 0.753/mm; WT 4.318 ± 1.131/mm). SclAb continued to induce gains in TbTh (Brtl/+ 0.050 ± 0.004 mm; WT 0.057 ± 0.006 mm), as well as a significant preservation of TbN (Brtl/+ 4.899 ± 0.595/mm; WT 5.796 ± 0.823/mm) across all PAM doses. As a result of these effects, maximum gains in bone volume fraction of 0.262 ± 0.057 for Brtl/+ and 0.362 ± 0.071 for WT were observed due to cumulative effects from both drugs compared to untreated control (Brtl/+ 0.107 ± 0.039; WT 0.189 ± 0.067). To further explore the effect of dose and time, the entire metaphyseal trabecular bone ROI was subdivided into two separate proximal and distal regions. The proximal-most region represented cycle 1 (Fig.3B, Table S2), and represents the fate of the band explored in Figure 2 following a second cycle of therapy. During a typical course of therapy, bisphosphonate will interfere with the growth-associated trabecular modeling that would normally cause trabeculae to gradually disappear during growth, thus stabilizing trabecular number of time. Here (Fig. 3B, Table S2), TbN of PAM-alone animals drops below levels shown in Figure 2, confirming that the low dose of PAM was insufficient to stabilize bone mass long-term. While the low dose of PAM was sufficient to restore Brtl/+ TbN to WT levels after the first cycle, the higher dose was required for the rescue effects to sustain throughout the second cycle. The distal-most region chosen represented cycle 2 (Fig. 3C, Table S2), and shows the retention band induced by the second PAM administration in the presence or absence of SclAb. Here, progressive gains in TbN are apparent, and TbTh gains in response to SclAb following cycle 2 (Brtl/+ 29%: WT 28%) were substantially less than those observed in the proximal region of interest (Brtl/+ 53%; WT 53%), reflecting either the shorter treatment duration for this site, or a saturation effect of SclAb on trabecular thickness.
Fig. 3.
A) Entire metaphyseal trabecular bone ROI following two cycles of combination therapy showed that PAM continued to influence TbN while inducing no effects on TbTh. SclAb, however, induced gains in both TbTh and TbN. Each cyclic treatment was separately analyzed by subdividing the entire metaphyseal trabecular bone ROI into B) cycle 1 and C) cycle 2. Isolating the effects of each administered cycle confirmed that these low doses of PAM were weak in sustaining long-term trabeculae preservation. Results of Two-Way ANOVA factors: * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
Multiple treatments of PAM and SclAb significantly slows the turnover of primary spongiosa
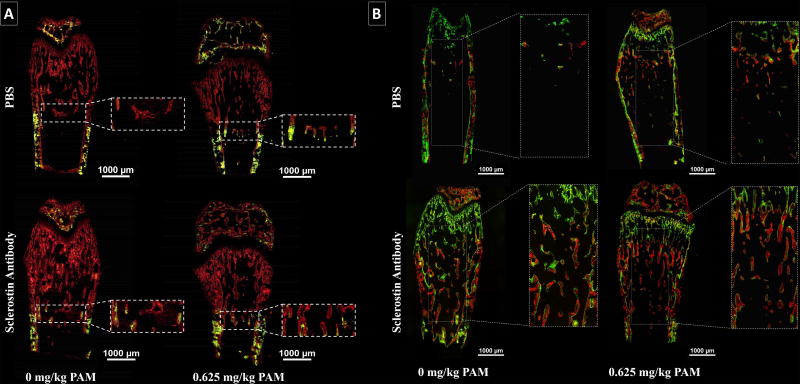
To assess the fate of bone formed during a single treatment cycle (Fig. 4A), calcein (green) was administered at the beginning of PAM (or control) and alizarin (red) at the end of SclAb (or control) treatment. Using this labeling scheme, bone formed and retained following PAM is shown in green, while bone formed subsequent to PAM is green. During growth, untreated Brtl/+ control showed a significant loss of primary trabeculae, reflected by total absence of green trabecular label (Fig. 4A, top left). PAM alone increased preservation of trabecular bone shown by retained spicules of calcein label (Fig. 4A, top right). In contrast SclAb alone (Fig 4A, bottom left) exhibited retention of calcein label with adjacent alizarin-labeled bone, indicating trabecular thickening on preserved primary spongiosa. Combined, SclAb and PAM induced an additive bone mass response at the site of concurrent drug administration (Fig 4A, bottom right). These patterns of trabecular preservation and thickening amplify under the influence of two cycles of therapy (Fig. 4B) as gains expand further down the proximal femur compared to gains seen after one cycle (Fig.4A). Significant preservation of second-cycle TbN is apparent from alizarin-labeled trabeculae in PAM-treated femora (Fig. 4B, top right), while SclAb alone led to both retention of trabeculae and thickening, indicated by alizarin-labeled trabeculae with abundant calcein label (Fig. 4B, bottom left). Robust gains in trabeculae and thickness are appreciated when both drugs were given together in multiple cycles as seen through retained alizarin-labeled trabeculae with a calcein-labeled thickness (Fig. 4B, bottom right).
Fig. 4.
Histological sections of Brtl/+ metaphysis revealed short-term (A) and long-term (B) bone formation activity from a single and concurrent cycles of combination therapy, respectively. A) At the BP-induced retention region, higher dosages of PAM were associated with greater primary trabeculae retention (yellow), while SclAb predominantly increased thickness (red). B) Consecutive cycles of combination therapy showed a greater robust effect on bone mass with higher dosages of PAM. Calcein (green) was administered at the beginning and end of concurrent treatment cycles while alizarin (red) was administered prior to the second cyclic treatment. Sections visualized represent an N = 4 per group.
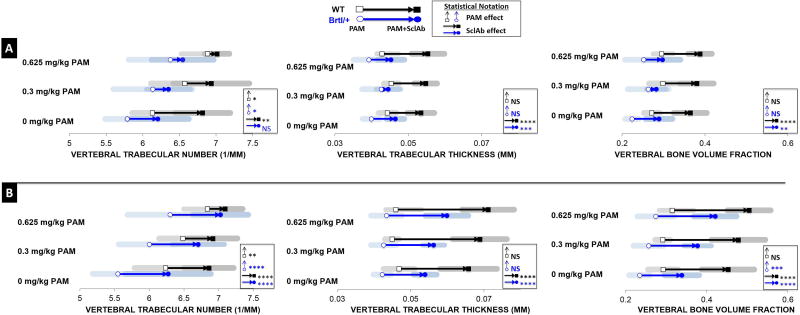
Multiple cycles of combination therapy induce synergistic gains in vertebral trabecular bone mass
Microstructural analysis of the lumbar vertebra demonstrated similar findings, albeit to a lesser extent, as seen in the femoral metaphysis, confirming consistency of effects at axial and appendicular sites. Under the influence of a single treatment cycle (Fig.5A, Table S3), PAM monotherapy significantly stabilized TbN at 0.3 mg/kg (Brtl/+ 6.137 ± 0.536/mm; WT 6.573 ± 0.459/mm) and 0.625 mg/kg (Brtl/+ 6.376 ± 0.569/mm; WT 6.886 ± 0.363/mm) over placebo control (Brtl/+ 5.787 ± 0.300/mm; WT 6.130 ± 0.271/mm), with no effect on TbTh. SclAb monotherapy produced gains in both TbN (Brtl/+ 6.208 ± 0.420/mm; WT 6.816 ± 0.376/mm) and TbTh (Brtl/+ 0.046 ± 0.002 mm; WT 0.053 ± 0.004 mm). As found in the femur, when SclAb was administered with PAM, little additional gains were observed in TbN. Higher doses of PAM were required to see additional trabecular thickening in Brtl/+, while WT showed an average 0.055 mm increase in TbTh with the two drugs combined. Despite these observations during a single cycle of combination therapy, overall gains in BV/TV were comparable whether drugs were given combined (Brtl/+ 0.298 0.043; WT 0.388 ± 0.029) or SclAb alone (Brtl/+ 0.289 ± 0.032; WT 0.365 ± 0.040). Lumbar vertebrae benefited strongly from two treatment cycles (Fig. 5B), triggering a maximum synergistic response on BV/TV (Brtl/+ 0.421 ± 0.053; WT 0.504 ± 0.053) over placebo (Brtl/+ 0.235 ± 0.026; WT 0.293 ± 0.037) compared to 0.625 mg/kg PAM (Brtl/+ 0.275 ± 0.046; WT 0.315 ± 0.033) and SclAb (Brtl/+ 0.339 ± 0.043; WT 0.452 ± 0.065) monotherapy. PAM continued to increase bone mass through TbN at 0.3 mg/kg (Brtl/+ 6.002 ± 0.444/mm; WT 6.487 ± 0.346/mm) and at 0.625 mg/kg (Brtl/+ 6.300 ± 0.614/mm; WT 6.838 ± 0.340/mm) with no effect on TbTh. SclAb induced strong gains in both TbN (Brtl/+ 6.867/mm; WT 6.889/mm) and TbTh (Brtl/+ 0.058 ± 0.004mm; WT 0.070 ± 0.007 mm) across all PAM dosages, contributing substantially to the overall synergistic gains in lumbar vertebrae trabecular bone mass.
Fig. 5.
A) Microstructural properties of the vertebral trabecular bone reveal overall preservation of TbN with PAM and increased TbTh with SclAb after a single cycle of combination therapy. B) With multiple treatment cycles, the response on TbN and TbTh was highly amplified leading to synergistic gains on bone mass. Results of Two-Way ANOVA factors: * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
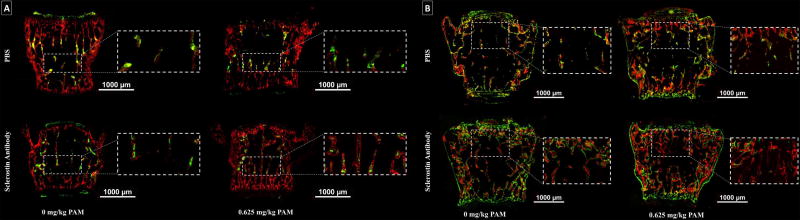
Substantial Preservation of TbN and Increased TbTh in Vertebral Body
To evaluate the response of newly formed bone under the effects of a single treatment cycle (Fig. 6A), calcein (green) was administered prior to PAM treatment and alizarin (red) after SclAb treatment. Brtl/+ control showed few primary trabeculae labeled with calcein, which was stabilized with a single dose of PAM. SclAb showed preservation of calcein labeled bone with alizarin labeled perimeters suggesting trabecular thickening. These observations confirm the antiresorptive and anabolic effects of both treatments, which led to further additive gains in bone mass. Newly formed bone under the effects of multiple treatment cycles (Fig. 6B), was labeled with calcein (green) at the beginning of treatment, alizarin (red) between cyclic treatments and again with calcein (green) at the end of treatment. Labeling of control samples showed considerable loss of primary trabeculae. PAM alone promoted the retention of primary trabeculae, noted by considerable alizarin-labeled bone adjacent to the growth plates. SclAb alone triggered both trabecular retention and thickness gains. Combination of both drugs administered cyclically induced a robust trabecular response which extended to the periosteal bone surface.
Fig. 6.
Histological coronal sections of Brtl/+ revealed bone formation activity from a single (A) and multiple (B) cycles of combination therapy. A) Under a single treatment cycle, higher dosages of PAM were associated to greater primary trabeculae retention (green), while SclAb predominantly increased thickness and connectivity between growth plates (red). B) Multiple treatment cycles showed an evident trabecular retention response from both PAM and SclAb; however, SclAb also induced gains in trabecular thickness (green). Together a robust trabecular response was triggered leading to uniform trabecular bone distribution between growth plates. Sections visualized represent an N = 4 per group.
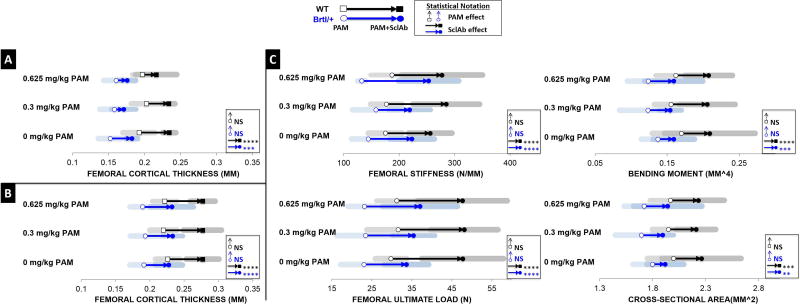
PAM intervention does not interfere with SclAb ability to improve femoral rigidity
PAM effects were isolated to the lumbar vertebrae and femoral trabecular bone, with no apparent effects on femoral diaphyseal cortical bone structure or biomechanical properties. Rather, gains in cortical thickness were solely attributed to SclAb since our administered PAM treatment used doses significantly lower than those used in human studies. After a single cycle of combination therapy (Fig. 7A, Table S4), SclAb monotherapy induced greater gains in femoral CTh (Brtl/+ 0.182 ± 0.008 mm; WT 0.234 ± 0.009 mm) than when combined with 0.3 mg/kg PAM (Brtl/+ 0.171 ± 0.017 mm; WT 0.234 ± 0.009 mm) or 0.625 mg/kg PAM (Brtl/+ 0.176 ± 0.012 mm; WT 0.216 ± 0.028 mm) compared to placebo (Brtl/+ 0.152 ± 0.016 mm; WT 0.192 ± 0.022 mm). However, this effect was transient, as after two cycles (Fig. 7B), consistent CTh gains of 0.231 ± 0.023 mm in Brtl/+ and 0.277 ± 0.023 mm in WT were observed across all PAM doses. As a result of these CTh changes, SclAb consistently improved femoral rigidity through gains in stiffness (Brtl/+ 231.768 ± 44.278 N/mm; WT 272.632 ± 57.425 N/mm) and ultimate load (Brtl/+ 35.379 ± 6.741 N; WT 47.816 ± 9.937 N) across all PAM doses, driven by gains in bending moment (Brtl/+ 0.158 ± 0.045 mm4; WT 0.207 ± 0.028 mm4) and cross-sectional area (Brtl/+ 1.919 ± 0.200 mm2; WT 2.234 ± 0.267 mm2).
Fig. 7.
A) Femoral cortical analysis revealed SclAb influence on CTh varies in the presence of PAM after a single treatment cycle. B) Following subsequent cycles of combination therapy, CTh nearly doubled solely in response to SclAb. C) Functionally, multiple cycles PAM and SclAb led to additive gains in femoral stiffness and ultimate load with progressive PAM doses. Results of Two-Way ANOVA factors: * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
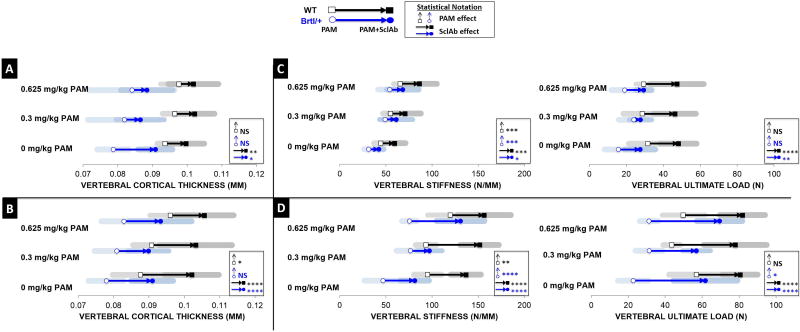
PAM and SclAb synergistically improve Brtl/+ vertebral stiffness over monotherapy effects
Similar to the cortical effects seen in the femur, CTh gains in the vertebral body were influenced by SclAb, not PAM. A single cycle of combination therapy (Fig. 8A, Table S5) resulted in lower gains in CTh at 0.3 mg/kg (Brtl/+ 0.086 ± 0.007 mm; WT 0.102 ± 0.006 mm) and 0.625 mg/kg (Brtl/+ 0.088 ± 0.007 mm; WT 0.102 ± 0.007 mm) than SclAb monotherapy (Brtl/+ 0.091 ± 0.005mm; WT 0.100 ± 0.005 mm). However, with multiple cycles (Fig. 8B), greater gains in CTh were achieved at 0.3 mg/kg (Brtl/+ 0.090 ± 0.006 mm; WT 0.103 ± 0.010 mm) and 0.625 mg/kg (Brtl/+ 0.093 ± 0.009 mm; WT 0.106 ± 0.008 mm). Functionally, over a single cycle of therapy (Fig. 8C), PAM dose-dependently improved vertebral stiffness and SclAb amplified this response (Brtl/+ 29%, WT 32%). Improvements in ultimate load were attributed to SclAb with gains of 48% in Brtl/+ and 58% in WT. Over two cycles of combination treatment (Fig. 8D), maximum gains in vertebral stiffness were observed when both PAM (Brtl/+ 75.650 ± 8.031 N/mm; WT 119.729 ± 24.514 N/mm) and SclAb (Brtl/+ 81.307 ± 15.531 N/mm; WT 136.688 ± 16.367 N/mm) were administered alone. When administered together in sequential cycles, WT showed maximum additive gains (156.340 ± 28.962 N/mm) compared to each treatment alone. Remarkably, a synergistic response was observed in Brtl/+, with a maximum stiffness increase of 130.945 ± 25.920 N/mm compared to placebo control (46.974 ± 20.181 N/mm).
Fig. 8.
A) Vertebral cortical analysis revealed that after a single cycle, SclAb triggered greater gains in CTh than when combined with PAM. B) Following two cycles, however, consistent gains in CTh were observed across all PAM dosages. C) Functionally, following a single combination cycle, PAM effect on trabecular preservation helped improve vertebral stiffness while SclAb amplified these effects. Significant improvements in ultimate load were solely attributed to SclAb. D) Both drugs improved ultimate load through an additive response, however, induced a synergistic effect on vertebral stiffness following multiple cycles of combination treatment. Results of Two-Way ANOVA factors: * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
Discussion
The results in this study demonstrate Brtl–IV mice with a genetic knock-in for moderately severe Type IV OI responded favorably to repeated cycles of a single dose of antiresorptive PAM in combination with anabolic SclAb. The immediate effects of this combination therapy showed that both interventions stimulated gains in bone mass through different means, leading to overall improvements in biomechanical function. While a preservation of TbN was observed through PAM, SclAb led to gains in both TbN and TbTh, as well as a significant cortical bone response. When this combination therapy was administered twice cyclically, the resulting gains in trabecular bone mass were dependent on anatomic site; cumulative for long bones and synergistic for the vertebral body. These findings may have a strong clinical utility to increase bone mass in OI patients—particularly those with excessive high bone turnover leading to severe trabecular osteopenia that might make them more resistant to anabolic therapy alone.
Antiresorptive bisphosphonates continue to be the most common intervention used in pediatric OI. Numerous studies have shown a favorable bone response to bisphosphonate therapy in both preclinical studies using OI mouse models (12, 17, 32, 33) and clinical studies of children with OI (7, 9, 33–37). By their antiresorptive activity, BP have been proposed to decrease the high bone turnover characteristic of OI, increase metaphyseal bone mass by increasing trabecular number, and increase cortical bone mass by inhibiting endosteal resorption. However, concern for long half-life (38), microdamage accumulation (39), and delayed healing(40) have led to attempts to minimize treatment dose (18). Recently, SclAb has gained interest as a promising anabolic approach for the treatment of OI. In contrast to bisphosphonate, SclAb has been shown to increase trabecular bone mass in OI models through trabecular thickening, and increase cortical bone mass through periosteal apposition (19, 20, 22). Furthermore, in a growing model, periosteal apposition will occur as a result of growth which contributes to further bone mass gains as PAM has not been shown to affect non-remodeling surfaces. Therefore, we hypothesized that a combination of these two agents may lead to additive if not synergistic structural and functional gains. When given together, we observed that BPs induce retention of primary trabeculae which can serve as a substrate for the subsequent anabolic response of SclAb, while cortical bone mass gains resulted from SclAb alone.
Combination effects have been explored with BP following SclAb in order to preserve gains in bone mass following cessation of drug clinically (41) and in OI models (23). Alternatively, SclAb following BP has been explored using ovariectomized rats in which SclAb induced gains in bone mass regardless of prior BP exposure (42). This study was performed in aged, osteoporotic rats, which have considerably different growth plate dynamics than in the present study. In another OI mouse model, SclAb was combined with zoledronic acid (43) where no synergistic effects were observed. Rather, the treatment effect from zoledronic acid alone led to >300% gains in proximal tibial trabecular bone volume fraction, with no additional gains observed when zoledronic acid was combined with SclAb. In the present combination study, we likely avoided this saturation response with lower doses of bisphosphonate triggering more modest trabecular preservation, allowing SclAb to synergistically increase bone mass by increasing trabecular thickness.
The present study differs from these other combination therapy studies as we aimed to explore low doses of PAM which, if given alone, would be insufficient to generate long-term preservation of trabecular bone. Indeed, when tracking the fate of the trabecular bone generated by the first cycle of pamidronate, we observed a lack of long-term anti-resorptive effects, particularly in the vertebrae. Yet when administered together with SclAb, both drugs combined led to cumulative gains on bone mass. At the site of concurrent treatment, PAM induced a sclerotic metaphyseal band consistent with previous observations of BP treatment stabilizing primary spongiosa near the growth plate (44). Treatment with PAM alone showed a significant effect on bone volume fraction for both Brtl/+ and WT when compared to untreated controls, solely due to a significant dose-dependent preservation of TbN with no concurrent effect on TbTh. Similar gains in bone volume were triggered with SclAb monotherapy, however, these were attributed to an effect on both TbN and TbTh. Gains in TbTh likely resulted from a direct anabolic effect on existing trabecular bone. However, gains in TbN could have resulted from either stabilization of thin trabeculae destined for remodeling via increased bone formation on those surfaces, or through a mild antiresorptive effect, since SclAb has been shown to have both anabolic and anti-catabolic actions (45). Indeed, SclAb alone preserved TbN to levels equivalent to low dose PAM, suggesting a similar potency of antiresorptive action between the two drugs. When given together, extended gains in trabecular bone mass were attributed to SclAb-induced trabecular thickening at sites formed following PAM exposure and retention of trabecular number. These observations were confirmed through histomorphometry. Observations at sites distal and proximal to the metaphyseal band helped verify that PAM bone response was constrained to its induced sclerotic band, while SclAb led to trabecular thickening away from these sites.
The same trabecular response was observed in the vertebral body for both Brtl/+ and WT, to a slightly greater extent, as was observed in the femoral metaphysis. These findings are consistent with other BP studies that show a variable bisphosphonate binding and bone density response between axial vs. appendicular, and cortical vs. trabecular sites (29, 46). Following two cycles of combination therapy, a synergistic effect on bone mass was observed in the vertebral body which was not observed in the femoral metaphysis which, instead showed an additive effect of the two interventions. Histomorphometry of two cycles of combination therapy demonstrated thickened bands of retained primary spongiosa emerging from both growth plates of the vertebra consistent observations in other pediatric BP studies (31, 47, 48).
In addition to trabecular effects, SclAb induced an anabolic response in femoral and vertebral cortical bone, as gains observed were solely attributed to SclAb, even when administered in combination with PAM. This increase in cortical thickness correlates with previous findings where growing Brtl/+ mice treated with SclAb showed cortical thickness gains in the femoral midshaft over a similar treatment duration (20). Furthermore, progressive gains in cortical thickness were observed comparing one vs. two cycles of SclAb monotherapy. While clinical studies of children with OI have shown slight changes in cortical bone from PAM treatment (17, 49–51), in the present study, PAM had no effect on cortical thickness, likely due to the low treatment duration and doses used in the present study compared to those in clinical trials. Low doses of PAM were deliberately chosen for this study in order to minimize the anti-resorptive effect and not mask the potential combination effects hypothesized from both drugs together.
Two cycles of PAM monotherapy showed no effect on femoral stiffness or load in either Brtl/+ and WT. SclAb, on the other hand, improved both stiffness and load as a result of the strong anabolic effect on cortical bone. When both drugs were administered concurrently, SclAb continued to be the sole contributor to these improvements in whole bone mechanical properties.
Similarly, in the vertebral body, Brtl/+ ultimate load was only improved by SclAb, yet both PAM and SclAb led to strong gains in vertebral stiffness. In fact, following two cycles of combined treatment, Brtl/+ stiffness improved synergistically when compared to combined effects from monotherapy of either drug. This finding may have important implications for treatment of spinal deformities in OI. Deformation of the spine arises from both anatomical and functional factors resulting from the OI phenotype. Depending on the severity of the deformity or fracture frequency, prophylactic interventions like BP treatment can potentially reduce fracture incidence. Combining SclAb with PAM stabilized vertebral trabecular bone resulting in significant gains in vertebral stiffness. SclAb alone improved maximum load, likely due to additional gains in cortical thickness that were not evident following PAM. These findings confirm other studies that have shown that CTh is a primary determinant feature for compressive strength of the whole vertebral body (52–54).
This study has several limitations. Since OI is a disease of many mutations, the results of this study may not extend to all types of OI. Additionally, the Brtl/+ mouse fragility phenotype is of moderate severity, and the mouse does not suffer spontaneous fractures. In addition, the study was only performed in male mice and it is now well established that gender can affect the outcome of drug treatments. Furthermore, bisphosphonate action when given after closure of the growth plate during skeletal maturity will not yield the same findings as observed in this study since turnover of primary spongiosa will be nonexistent. In many of our outcomes, treatment with our lowest PAM dose alone improved bone mass to WT control levels, effectively rescuing the phenotype, while our higher PAM dose induced gains in bone mass greater than WT baseline. While our goal was not to directly titrate our treatment outcomes to match untreated WT levels, we believe that further adjustment of treatment doses and/or duration will vary depending on clinical severity to best represent a “rescue” response.
SclAb has been proposed as a novel anabolic intervention to treat the low bone mass and fragility phenotype present in patients with OI. The results in this study extend our previous observations of single-drug therapy in Brtl/+ (17, 20) and show the benefits of combining SclAb with antiresorptive treatment during growth. In severe cases of OI, where extreme low bone mass may result from excessive resorption of primary trabeculae during endochondral growth, SclAb alone might not be enough to significantly improve trabecular bone volume due to lack of bone upon which to exert an anabolic action. Thus, in cases of extreme low bone mass, concurrent anti-resorptive and anabolic therapy might further improve bone formation outcomes. Importantly, these results suggest that modest doses of bisphosphonate, which would otherwise not induce a sustained therapeutic effect, may be sufficient to preserve trabecular architecture enough to permit an anabolic action of SclAb on bone which would otherwise be remodeled. Together, these preclinical results support the scientific premise that antiresorptive and anabolic combination therapy during early stages of skeletal growth can help induce greater gains in bone mass in OI than either drug alone. The present data provides key pre-clinical results to support a treatment plan to maximize combination therapy in OI, or other diseases associated with low bone mass during pediatric growth and development.
Supplementary Material
Table. S1: MicroCT analysis at the site of concurrent drug administration showed that PAM induced a significant dose-dependent increase in TbN while SclAb contributed to significant gains in both TbN and TbTh. Together these independent gains led to an additive gain in bone volume fraction with increasing PAM doses. The mean and standard deviation (mean ± SD) of each bone parameter is reported.
Table. S2. Entire metaphyseal trabecular bone ROI following two cycles of combination therapy showed that PAM continued to influence TbN while inducing no effects on TbTh. SclAb, however, induced gains in both TbTh and TbN. Each cyclic treatment was separately analyzed by subdividing the entire metaphyseal trabecular bone ROI into their respective cycles. Isolating the effects of each administered cycle confirmed that these low doses of PAM were weak in sustaining long-term trabeculae preservation. The mean and standard deviation (mean ± SD) of each bone parameter is reported.
Table. S3. Microstructural properties of the vertebral trabecular bone reveal overall preservation of TbN with PAM and increased TbTh with SclAb after a single cycle of combination therapy. With multiple treatment cycles, the response on TbN and TbTh was highly amplified leading to synergistic gains on bone mass. The mean and standard deviation (mean ± SD) of each bone parameter is reported.
Table. S4. Femoral cortical analysis revealed SclAb influence on CTh varies in the presence of PAM after a single treatment cycle. Following subsequent cycles of combination therapy, CTh nearly doubled solely in response to SclAb. Functionally, multiple cycles PAM and SclAb led to an additive gains in femoral stiffness and ultimate load with progressive PAM doses. The mean and standard deviation (mean ± SD) of each bone parameter is reported.
Table. S5. Vertebral cortical analysis revealed that after a single cycle, SclAb triggered greater gains in CTh than when combined with PAM. Following two cycles, however, consistent gains in CTh were observed across all PAM dosages. Functionally, following a single combination cycle, PAM effect on trabecular preservation helped improve vertebral stiffness while SclAb amplified these effects. Significant improvements in ultimate load were solely attributed to SclAb. Both drugs improved ultimate load through an additive response, however, induced a synergistic effect on vertebral stiffness following multiple cycles of combination treatment. The mean and standard deviation (mean ± SD) of each bone parameter is reported.
Fig. S1. A) Sites of bone formed following PAM cessation showed that gains in bone volume fraction were attributed solely to SclAb-induced trabecular thickening, with an average increase of 40% for Brtl/+ and 55% for WT across PAM doses. Gains in TbN were attributed solely to SclAb, not PAM, reflecting treatment site specificity and a mild anti-resorptive effect of the drug. B) Region proximal to metaphyseal band, representing trabecular bone formed prior to BP injection but under the influence of SclAb, showed a significant effect on bone volume primarily due to an average trabecular thickening of 21% for Brtl/+ and 40% for WT.
Acknowledgments
The authors thank Bonnie Nolan and Carol Whitinger for their contributions. SclAb was provided by Amgen and UCB Pharma. Financial support from NIH to KMK (R01AR062522) is gratefully acknowledged. Additional research support was received from NIH (P30AR069620). Study designed and conducted by DO, RS and KMK. Data collected by DO and RS. Data analyzed and interpreted by DO, RS, MSC, JCM, and KMK. Manuscript was written by DO and KMK and revised and approved by all authors. KMK takes responsibility for the integrity of the data analysis.
References
- 1.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2016;387(10028):1657–71. doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363(9418):1377–85. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 3.Roughley PJ, Rauch F, Glorieux FH. Osteogenesis imperfecta--clinical and molecular diversity. European cells & materials. 2003;5:41–7. doi: 10.22203/ecm.v005a04. discussion 7. [DOI] [PubMed] [Google Scholar]
- 4.Russell RG, Croucher PI, Rogers MJ. Bisphosphonates: pharmacology, mechanisms of action and clinical uses. Osteoporos Int. 1999;9(Suppl 2):S66–80. doi: 10.1007/pl00004164. [DOI] [PubMed] [Google Scholar]
- 5.Land C, Rauch F, Travers R, Glorieux FH. Osteogenesis imperfecta type VI in childhood and adolescence: effects of cyclical intravenous pamidronate treatment. Bone. 2007;40(3):638–44. doi: 10.1016/j.bone.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Castillo H, Samson-Fang L. Effects of bisphosphonates in children with osteogenesis imperfecta: an AACPDM systematic review. Dev Med Child Neurol. 2009;51(1):17–29. doi: 10.1111/j.1469-8749.2008.03222.x. [DOI] [PubMed] [Google Scholar]
- 7.Rauch F, Munns CF, Land C, Cheung M, Glorieux FH. Risedronate in the treatment of mild pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Bone Miner Res. 2009;24(7):1282–9. doi: 10.1359/jbmr.090213. [DOI] [PubMed] [Google Scholar]
- 8.Salehpour S, Tavakkoli S. Cyclic pamidronate therapy in children with osteogenesis imperfecta. J Pediatr Endocrinol Metab. 2010;23(1–2):73–80. doi: 10.1515/jpem.2010.23.1-2.73. [DOI] [PubMed] [Google Scholar]
- 9.Bishop N, Harrison R, Ahmed F, Shaw N, Eastell R, Campbell M, et al. A randomized, controlled dose-ranging study of risedronate in children with moderate and severe osteogenesis imperfecta. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25(1):32–40. doi: 10.1359/jbmr.090712. [DOI] [PubMed] [Google Scholar]
- 10.Bishop N, Adami S, Ahmed SF, Anton J, Arundel P, Burren CP, et al. Risedronate in children with osteogenesis imperfecta: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9902):1424–32. doi: 10.1016/S0140-6736(13)61091-0. [DOI] [PubMed] [Google Scholar]
- 11.Palomo T, Andrade MC, Peters BS, Reis FA, Carvalhaes JT, Glorieux FH, et al. Evaluation of a Modified Pamidronate Protocol for the Treatment of Osteogenesis Imperfecta. Calcified tissue international. 2016;98(1):42–8. doi: 10.1007/s00223-015-0061-y. [DOI] [PubMed] [Google Scholar]
- 12.Camacho NP, Raggio CL, Doty SB, Root L, Zraick V, Ilg WA, et al. A controlled study of the effects of alendronate in a growing mouse model of osteogenesis imperfecta. Calcif Tissue Int. 2001;69(2):94–101. doi: 10.1007/s002230010045. [DOI] [PubMed] [Google Scholar]
- 13.Zhu ED, Louis L, Brooks DJ, Bouxsein ML, Demay MB. Effect of bisphosphonates on the rapidly growing male murine skeleton. Endocrinology. 2014;155(4):1188–96. doi: 10.1210/en.2013-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans KD, Sheppard LE, Rao SH, Martin RB, Oberbauer AM. Pamidronate alters the growth plate in the oim mouse model for osteogenesis imperfecta. Int J Biomed Sci. 2009;5(4):345–52. [PMC free article] [PubMed] [Google Scholar]
- 15.Rao SH, Evans KD, Oberbauer AM, Martin RB. Bisphosphonate treatment in the oim mouse model alters bone modeling during growth. J Biomech. 2008;41(16):3371–6. doi: 10.1016/j.jbiomech.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy EA, Raggio CL, Hossack MD, Miller EA, Jain S, Boskey AL, et al. Alendronate treatment for infants with osteogenesis imperfecta: demonstration of efficacy in a mouse model. Pediatr Res. 2002;52(5):660–70. doi: 10.1203/00006450-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Uveges TE, Kozloff KM, Ty JM, Ledgard F, Raggio CL, Gronowicz G, et al. Alendronate treatment of the brtl osteogenesis imperfecta mouse improves femoral geometry and load response before fracture but decreases predicted material properties and has detrimental effects on osteoblasts and bone formation. J Bone Miner Res. 2009;24(5):849–59. doi: 10.1359/JBMR.081238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasanwala RF, Sanghrajka A, Bishop NJ, Hogler W. Recurrent Proximal Femur Fractures in a Teenager With Osteogenesis Imperfecta on Continuous Bisphosphonate Therapy: Are We Overtreating? J Bone Miner Res. 2016;31(7):1449–54. doi: 10.1002/jbmr.2805. [DOI] [PubMed] [Google Scholar]
- 19.Sinder BP, White LE, Salemi JD, Ominsky MS, Caird MS, Marini JC, et al. Adult Brtl/+ mouse model of osteogenesis imperfecta demonstrates anabolic response to sclerostin antibody treatment with increased bone mass and strength. Osteoporos Int. 2014;25(8):2097–107. doi: 10.1007/s00198-014-2737-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinder BP, Salemi JD, Ominsky MS, Caird MS, Marini JC, Kozloff KM. Rapidly growing Brtl/+ mouse model of osteogenesis imperfecta improves bone mass and strength with sclerostin antibody treatment. Bone. 2015;71:115–23. doi: 10.1016/j.bone.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinder BP, Lloyd WR, Salemi JD, Marini JC, Caird MS, Morris MD, et al. Effect of anti-sclerostin therapy and osteogenesis imperfecta on tissue-level properties in growing and adult mice while controlling for tissue age. Bone. 2016;84:222–9. doi: 10.1016/j.bone.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinder BP, Eddy MM, Ominsky MS, Caird MS, Marini JC, Kozloff KM. Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J Bone Miner Res. 2013;28(1):73–80. doi: 10.1002/jbmr.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perosky JE, Khoury BM, Jenks TN, Ward FS, Cortright K, Meyer B, et al. Single dose of bisphosphonate preserves gains in bone mass following cessation of sclerostin antibody in Brtl/+ osteogenesis imperfecta model. Bone. 2016;93:79–85. doi: 10.1016/j.bone.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glorieux FH, Devogelaer JP, Durigova M, Goemaere S, Hemsley S, Jakob F, et al. BPS804 Anti-Sclerostin Antibody in Adults With Moderate Osteogenesis Imperfecta: Results of a Randomized Phase 2a Trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2017;32(7):1496–504. doi: 10.1002/jbmr.3143. [DOI] [PubMed] [Google Scholar]
- 25.Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. Faseb j. 2005;19(13):1842–4. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 26.Forlino A, Porter FD, Lee EJ, Westphal H, Marini JC. Use of the Cre/lox recombination system to develop a non-lethal knock-in murine model for osteogenesis imperfecta with an alpha1(I) G349C substitution. Variability in phenotype in BrtlIV mice. J Biol Chem. 1999;274(53):37923–31. doi: 10.1074/jbc.274.53.37923. [DOI] [PubMed] [Google Scholar]
- 27.Meganck JA, Kozloff KM, Thornton MM, Broski SM, Goldstein SA. Beam hardening artifacts in micro-computed tomography scanning can be reduced by X-ray beam filtration and the resulting images can be used to accurately measure BMD. Bone. 2009;45(6):1104–16. doi: 10.1016/j.bone.2009.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339(14):947–52. doi: 10.1056/NEJM199810013391402. [DOI] [PubMed] [Google Scholar]
- 29.Kozloff KM, Volakis LI, Marini JC, Caird MS. Near-infrared fluorescent probe traces bisphosphonate delivery and retention in vivo. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25(8):1748–58. doi: 10.1002/jbmr.66. [DOI] [PubMed] [Google Scholar]
- 30.Otsu N. A Threshold Selection Method from Gray-Level Histograms. IEEE Transactions on Systems, Man and Cybernetics. 1979;9(1):62–6. [Google Scholar]
- 31.Chakraborty PP, Biswas SN, Patra S, Santra G. “Zebra Stripe” Sign and “Bone in Bone” Sign in Cyclical Bisphosphonate Therapy. Journal of Clinical and Diagnostic Research : JCDR. 2017;11(2):RJ01–RJ2. doi: 10.7860/JCDR/2017/24349.9177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boskey AL, Marino J, Spevak L, Pleshko N, Doty S, Carter EM, et al. Are Changes in Composition in Response to Treatment of a Mouse Model of Osteogenesis Imperfecta Sex-dependent? Clin Orthop Relat Res. 2015;473(8):2587–98. doi: 10.1007/s11999-015-4268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hald JD, Evangelou E, Langdahl BL, Ralston SH. Bisphosphonates for the prevention of fractures in osteogenesis imperfecta: meta-analysis of placebo-controlled trials. J Bone Miner Res. 2015;30(5):929–33. doi: 10.1002/jbmr.2410. [DOI] [PubMed] [Google Scholar]
- 34.Gatti D, Antoniazzi F, Prizzi R, Braga V, Rossini M, Tato L, et al. Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. J Bone Miner Res. 2005;20(5):758–63. doi: 10.1359/JBMR.041232. [DOI] [PubMed] [Google Scholar]
- 35.Letocha AD, Cintas HL, Troendle JF, Reynolds JC, Cann CE, Chernoff EJ, et al. Controlled trial of pamidronate in children with types III and IV osteogenesis imperfecta confirms vertebral gains but not short-term functional improvement. J Bone Miner Res. 2005;20(6):977–86. doi: 10.1359/JBMR.050109. [DOI] [PubMed] [Google Scholar]
- 36.Sakkers R, Kok D, Engelbert R, van Dongen A, Jansen M, Pruijs H, et al. Skeletal effects and functional outcome with olpadronate in children with osteogenesis imperfecta: a 2-year randomised placebo-controlled study. Lancet. 2004;363(9419):1427–31. doi: 10.1016/S0140-6736(04)16101-1. [DOI] [PubMed] [Google Scholar]
- 37.Ward LM, Rauch F, Whyte MP, D'Astous J, Gates PE, Grogan D, et al. Alendronate for the treatment of pediatric osteogenesis imperfecta: a randomized placebo-controlled study. J Clin Endocrinol Metab. 2011;96(2):355–64. doi: 10.1210/jc.2010-0636. [DOI] [PubMed] [Google Scholar]
- 38.Papapoulos SE, Cremers SCLM. Prolonged Bisphosphonate Release after Treatment in Children. New England Journal of Medicine. 2007;356(10):1075–6. doi: 10.1056/NEJMc062792. [DOI] [PubMed] [Google Scholar]
- 39.Komatsubara S, Mori S, Mashiba T, Li J, Nonaka K, Kaji Y, et al. Suppressed bone turnover by long-term bisphosphonate treatment accumulates microdamage but maintains intrinsic material properties in cortical bone of dog rib. J Bone Miner Res. 2004;19(6):999–1005. doi: 10.1359/JBMR.040126. [DOI] [PubMed] [Google Scholar]
- 40.Anam EA, Rauch F, Glorieux FH, Fassier F, Hamdy R. Osteotomy Healing in Children With Osteogenesis Imperfecta Receiving Bisphosphonate Treatment. J Bone Miner Res. 2015;30(8):1362–8. doi: 10.1002/jbmr.2486. [DOI] [PubMed] [Google Scholar]
- 41.Recknor CP, Recker RR, Benson CT, Robins DA, Chiang AY, Alam J, et al. The Effect of Discontinuing Treatment With Blosozumab: Follow-up Results of a Phase 2 Randomized Clinical Trial in Postmenopausal Women With Low Bone Mineral Density. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30(9):1717–25. doi: 10.1002/jbmr.2489. [DOI] [PubMed] [Google Scholar]
- 42.Li X, Ominsky MS, Warmington KS, Niu QT, Asuncion FJ, Barrero M, et al. Increased bone formation and bone mass induced by sclerostin antibody is not affected by pretreatment or cotreatment with alendronate in osteopenic, ovariectomized rats. Endocrinology. 2011;152(9):3312–22. doi: 10.1210/en.2011-0252. [DOI] [PubMed] [Google Scholar]
- 43.Little DG, Peacock L, Mikulec K, Kneissel M, Kramer I, Cheng TL, et al. Combination sclerostin antibody and zoledronic acid treatment outperforms either treatment alone in a mouse model of osteogenesis imperfecta. Bone. 2017;101:96–103. doi: 10.1016/j.bone.2017.04.016. [DOI] [PubMed] [Google Scholar]
- 44.Ishizuka M, Tsuji S, Hirabayashi M, Kaneko K. Characteristic Bands Manifesting as Zebra Lines on Radiographs in Osteogenesis Imperfecta. The Indian Journal of Pediatrics. 2017;84(4):336-. doi: 10.1007/s12098-017-2316-2. [DOI] [PubMed] [Google Scholar]
- 45.Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab Treatment in Postmenopausal Women with Osteoporosis. New England Journal of Medicine. 2016;375(16):1532–43. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 46.Wen D, Qing L, Harrison G, Golub E, Akintoye SO. Anatomic site variability in rat skeletal uptake and desorption of fluorescently labeled bisphosphonate. Oral diseases. 2011;17(4):427–32. doi: 10.1111/j.1601-0825.2010.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papakonstantinou O, Sakalidou M, Atsali E, Bizimi V, Mendrinou M, Alexopoulou E. Radiographic and MR Imaging Findings of the Spine after Bisphosphonate Treatment, in a Child with Idiopathic Juvenile Osteoporosis. Case Reports in Radiology. 2015;2015:727510. doi: 10.1155/2015/727510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boyce AM, Tosi LL, Paul SM. Bisphosphonate Treatment for Children With Disabling Conditions. PM & R : the journal of injury, function, and rehabilitation. 2014;6(5):427–36. doi: 10.1016/j.pmrj.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rauch F, Travers R, Plotkin H, Glorieux FH. The effects of intravenous pamidronate on the bone tissue of children and adolescents with osteogenesis imperfecta. The Journal of clinical investigation. 2002;110(9):1293–9. doi: 10.1172/JCI15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Szalay EA. Bisphosphonate use in children with pediatric osteoporosis and other bone conditions. Journal of pediatric rehabilitation medicine. 2014;7(2):125–32. doi: 10.3233/PRM-140281. [DOI] [PubMed] [Google Scholar]
- 51.Glorieux FH. Experience with bisphosphonates in osteogenesis imperfecta. Pediatrics. 2007;119(Suppl 2):S163–5. doi: 10.1542/peds.2006-2023I. [DOI] [PubMed] [Google Scholar]
- 52.Vesterby A, Mosekilde L, Gundersen HJG, Melsen F, Mosekilde L, Holme K, et al. Biologically meaningful determinants of the in vitro strength of lumbar vertebrae. Bone. 12(3):219–24. doi: 10.1016/8756-3282(91)90044-j. [DOI] [PubMed] [Google Scholar]
- 53.Wegrzyn J, Roux J-P, Arlot ME, Boutroy S, Vilayphiou N, Guyen O, et al. Determinants of the Mechanical Behavior of Human Lumbar Vertebrae After Simulated Mild Fracture. Journal of Bone and Mineral Research. 2011;26(4):739–46. doi: 10.1002/jbmr.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ritzel H, Amling M, Pösl M, Hahn M, Delling G. The Thickness of Human Vertebral Cortical Bone and its Changes in Aging and Osteoporosis: A Histomorphometric Analysis of the Complete Spinal Column from Thirty-Seven Autopsy Specimens. Journal of Bone and Mineral Research. 1997;12(1):89–95. doi: 10.1359/jbmr.1997.12.1.89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table. S1: MicroCT analysis at the site of concurrent drug administration showed that PAM induced a significant dose-dependent increase in TbN while SclAb contributed to significant gains in both TbN and TbTh. Together these independent gains led to an additive gain in bone volume fraction with increasing PAM doses. The mean and standard deviation (mean ± SD) of each bone parameter is reported.
Table. S2. Entire metaphyseal trabecular bone ROI following two cycles of combination therapy showed that PAM continued to influence TbN while inducing no effects on TbTh. SclAb, however, induced gains in both TbTh and TbN. Each cyclic treatment was separately analyzed by subdividing the entire metaphyseal trabecular bone ROI into their respective cycles. Isolating the effects of each administered cycle confirmed that these low doses of PAM were weak in sustaining long-term trabeculae preservation. The mean and standard deviation (mean ± SD) of each bone parameter is reported.
Table. S3. Microstructural properties of the vertebral trabecular bone reveal overall preservation of TbN with PAM and increased TbTh with SclAb after a single cycle of combination therapy. With multiple treatment cycles, the response on TbN and TbTh was highly amplified leading to synergistic gains on bone mass. The mean and standard deviation (mean ± SD) of each bone parameter is reported.
Table. S4. Femoral cortical analysis revealed SclAb influence on CTh varies in the presence of PAM after a single treatment cycle. Following subsequent cycles of combination therapy, CTh nearly doubled solely in response to SclAb. Functionally, multiple cycles PAM and SclAb led to an additive gains in femoral stiffness and ultimate load with progressive PAM doses. The mean and standard deviation (mean ± SD) of each bone parameter is reported.
Table. S5. Vertebral cortical analysis revealed that after a single cycle, SclAb triggered greater gains in CTh than when combined with PAM. Following two cycles, however, consistent gains in CTh were observed across all PAM dosages. Functionally, following a single combination cycle, PAM effect on trabecular preservation helped improve vertebral stiffness while SclAb amplified these effects. Significant improvements in ultimate load were solely attributed to SclAb. Both drugs improved ultimate load through an additive response, however, induced a synergistic effect on vertebral stiffness following multiple cycles of combination treatment. The mean and standard deviation (mean ± SD) of each bone parameter is reported.
Fig. S1. A) Sites of bone formed following PAM cessation showed that gains in bone volume fraction were attributed solely to SclAb-induced trabecular thickening, with an average increase of 40% for Brtl/+ and 55% for WT across PAM doses. Gains in TbN were attributed solely to SclAb, not PAM, reflecting treatment site specificity and a mild anti-resorptive effect of the drug. B) Region proximal to metaphyseal band, representing trabecular bone formed prior to BP injection but under the influence of SclAb, showed a significant effect on bone volume primarily due to an average trabecular thickening of 21% for Brtl/+ and 40% for WT.