Abstract
Fear, a response to threatening stimuli and important for survival, is a behavior found throughout the animal kingdom. One critical structure involved in the expression of fear-related behavior is the periaqueductal gray (PAG) in mammals, and in the zebrafish, the griseum centrale. Here, we show in the lamprey, belonging to the oldest now living group of vertebrates, that a bilateral periventricular nucleus in the ventral mesencephalon has a similar location to that of the PAG and griseum centrale. It targets the pretectum and the substantia nigra pars compacta (SNc), expresses the dopamine D1 and D2 receptors and receives input from the pallium (cortex in mammals), hypothalamus, the raphe area and SNc. These are all hallmarks of the mammalian PAG. In addition, like in the zebrafish, there is an input from the interpeduncular nucleus. Our results thus suggest that a structure homologous to the PAG/griseum centrale was present very early in vertebrate evolution.
Keywords: Interpeduncular nucleus, Fear-related behavior, PAG, Evolution, SNc
Graphical abstract
Highlights
-
•
A homologue of the mammalian PAG is present in the lamprey.
-
•
As in the zebrafish, this structure is named griseum centrale.
-
•
The neuronal circuitry for fear-related behavior is evolutionarily conserved.
1. Introduction
Fear responses to environmental threats, is an important aspect of vertebrate behavior. Its expression typically involves fighting, fleeing, and freezing responses. These different response strategies are in turn composed of various behavioral and physiological components, shared between animals across the vertebrate phylum (Anderson and Adolphs, 2014). Already in 1872, Darwin hypothesized that even species evolutionarily distant from mammals, such as insects, may express emotions corresponding to fear or anger. The similarities in behavior and physiology suggest a similar neuronal circuitry controlling fear-related responses.
In mammals, the structures involved in the expression of fear-related behavior include the prefrontal cortex, amygdala, hypothalamus, medial habenula, and periaqueductal gray (PAG; e.g. Behbehani, 1995, Ressler et al., 2002, Gross and Canteras, 2012, Tovote et al., 2016). With regard to observable physiological and behavioral responses, the PAG has been shown to be of special importance and serve as a downstream command center (see Carrive, 1993). The PAG is located in the mesencephalon under the colliculi and surrounds the mesencephalic aqueduct (Liu and Hamilton, 1980, Behbehani, 1995). It is subdivided into functionally distinct longitudinal columns (Carrive, 1993). Dorsally situated structures have been suggested to be more involved in fight or flight responses, whereas more ventrally located subdivisions participate in freezing responses (Bandler and Carrive, 1988, Carrive, 1993, Bandler et al., 2000, Tovote et al., 2016; see also Subramanian and Holstege, 2014). In humans, distal threat elicits activity in the prefrontal cortex, whereas when the threat comes closer activity is elicited preferentially in the PAG (Mobbs et al., 2007). The switch is important since this evolutionarily conserved midbrain region controls defensive behavior including fight, flight or freezing.
The griseum centrale (GC) in the zebrafish corresponds to the mammalian PAG, and includes the dorsal tegmental nucleus and nucleus incertus. It is a longitudinally oriented column that starts in mesencephalon at the level of the interpeduncular nucleus (IPN) and extends along the ventral lining of the rhomboencephalic ventricle (Wullimann et al., 1996). The GC has been shown to receive input from the dorsal portion of IPN, a nucleus that itself is a major recipient of input from the medial habenula (Okamoto et al., 2012), and the habenula-IPN-GC pathway has been suggested to control freezing responses (Agetsuma et al., 2010).
In the present study we investigate, using neuroanatomical tracing, immunohistochemistry and in situ hybridization, whether a structure corresponding to PAG/GC is present already in the lamprey, representing the oldest group of now living vertebrates (Kumar and Hedges, 1998). Other fear-related structures, including the hypothalamus, IPN and medial habenula are all present in the lamprey, and the medial habenula has a similar organization with regard to input as in mammals and zebrafish, and has IPN as a downstream structure (Stephenson-Jones et al., 2012). Here, we identify a region in the lamprey that in location and connectivity is similar to the PAG/GC.
2. Material and methods
Experiments were performed on a total of 32 adult river lampreys (Lampetra fluviatilis). Experimental procedures were approved by the local ethical committee (Northern Stockholm Animal Review Board) and were in accordance with the NIH Guide for the Care and Use of Laboratory Animal (1996 revision). Every effort was made to minimize animal suffering and to reduce the number of animals used during the study.
2.1. Tract tracing
Animals were deeply anesthetized using tricaine methane sulfonate (MS-222; 100 mg/L; Sigma-Aldrich) diluted in fresh water. They were then transected caudally at the third gill, and the brain was exposed by removing the dorsal skin and cartilage. During dissection and injection of tracers, the head was pinned down and submerged in ice-cooled oxygenated HEPES-buffered physiological solution containing 138 mM NaCl, 2.1 mM KCl, 1.8 mM CaCl2, 1.2 mM MgCl2, 4 mM glucose, and 2 mM HEPES, pH 7.3–7.4.
Injections were made with glass micropipettes (borosilicate, outer diameter = 1.5 mm, inner diameter = 1.17 mm), pulled to a diameter of 10–20 μm. Micropipettes were then secured in a holder, which was attached to an air supply and a Narishige micromanipulator. Between 50 and 200 nL of Neurobiotin (20%; Vector Laboratories), Alexa Fluor 488- (10kD), biotin- (3kD; BDA) or rhodamine- (3kD) conjugated dextran amine (20%; MolecularProbes) in distilled water was pressure injected into the interpeduncular nucleus (IPN; n = 3), pretectum (n = 8), substantia nigra pars compacta (SNc; n = 8), pallium (n = 4) or the putative griseum centrale (n = 3). Fast Green was added to the Neurobiotin and BDA solution to aid visualization of the tracer. For the IPN injections, the brains were dissected out prior to injection, and pinned down with the ventral side up. Following injection, all brains were kept submerged in HEPES in the dark for 12–24 h to allow transport of the tracers. All brains were fixed in 4% formalin and 14% saturated picric acid in 0.1 M phosphate buffer (PB), pH 7.4, for 12–24 h, and then cryoprotected in 20% (wt/vol) sucrose in PB for 3–12 h. Transverse sections (30 μm thick) were cut on a cryostat, collected on gelatin-coated slides, and stored at −20 °C until further processing.
2.2. Immunohistochemistry
For visualization of Neurobiotin, the sections were incubated for 2–3 h at room temperature with Cy2-or Cy3-conjugated streptavidin (1:1000; Jackson ImmunoResearch). When combined with immunohistochemistry (see below) the streptavidin was added to the secondary antibody and the sections incubated for 2–3 h. For immunohistochemical detection of tyrosine hydroxylase (TH), serotonin (5-HT) or GABA, sections were incubated overnight at 4 °C with a mouse anti-tyrosine hydroxylase antibody (1:500; MAB318; Millipore), a rabbit anti-serotonin antibody (1:1000; ImmunoStar, Inc.), or a mouse monoclonal anti-GABA antibody (1:5000, mAb 3A12; RRID AB_2314450; kindly donated by Prof. Peter Streit, Brain Research Institute, University of Zurich, Switzerland), respectively. Following a thorough rinse in phosphate buffered saline (PBS) the sections were incubated with donkey anti-mouse IgG-Cy3 or donkey anti-rabbit IgG-Cy3 (1:500; Jackson ImmunoResearch). Slides were then rinsed in PBS for 3 × 10 min and cover-slipped with glycerol containing 2.5% DABCO (Sigma-Aldrich). All antisera were diluted in 1% BSA and 0.3% Triton-X 100 in 0.1 M PB. In addition, all sections were counterstained with a fluorescent Nissl stain (1:1000; MolecularProbes).
2.3. In situ hybridization
Animals (n = 6) were deeply anesthetized in MS-222 (100 mg/L; Sigma-Aldrich) diluted in fresh water and killed by decapitation. Brains were quickly removed and fixed in 4% paraformaldehyde in 0.01 M PBS overnight at 4 °C. Afterward, they were cryoprotected in 20% sucrose in 0.01 M PBS overnight, and 20-μm-thick transverse cryostat sections were obtained and immediately used for in situ hybridization. The sections were left at room temperature for 30 min, washed in 0.01 M PBS, acetylated in 0.25% acetic anhydride in 0.1 M triethanolamine, pH 8.0, for 5 min, washed in 0.01 M PBS, and prehybridized (50% formamide, 5X SSC, pH 7.0, 5X Denhardt's solution, 500 g/ml salmon sperm DNA, and 250 g/ml yeast RNA) for 2–4 h at 60 °C. DIG-labeled D2 (see Robertson et al., 2012 for details) or D1 (see Pérez-Fernández, 2013) riboprobes were prepared and added to the hybridization solution to a final concentration of 500 ng/ml and the hybridization process was carried out overnight at 60 °C. An RNase treatment (Roche Diagnostics; 20 g/ml in 2X SSC) was performed for 30 min at 37 °C after stringent washes in SSC (Applied Biosystems). After additional washes in maleic acid buffer (MABT), pH 7.5, the sections were incubated overnight at 4 °C in anti-DIG Fab fragments conjugated with alkaline phosphatase (1:2000; Roche Diagnostics) in 10% heat inactivated normal goat serum (Vector Laboratories). Several washes in MABT were performed, and the alkaline phosphatase reaction was visualized using NBT/BCIP substrate (Roche Diagnostics) in staining buffer (0.1 M Tris buffer, pH 9.5, containing 100 mM NaCl and 5 mM levamisole). The staining process was stopped with washes in PBS. Sections were subsequently dehydrated and mounted with Entellan (Merck).
2.4. Analysis
Photomicrographs were taken with an Olympus XM10 digital camera mounted on an Olympus BX51 microscope (Olympus Sweden). Illustrations were prepared in Adobe Illustrator and Adobe Photoshop CS6 (Adobe Systems). Images were only adjusted for brightness and contrast.
3. Theory
Since fear-related behaviors are found throughout the animal kingdom it suggests that the neuronal circuitry responsible for these behaviors may be evolutionarily conserved. We therefore investigated if one of the structures that plays a pivotal role in expression of emotions, the mammalian PAG and the GC of zebrafish, is also present in the phylogenetically oldest vertebrate, the lamprey.
4. Results
When a given brain structure has a similar location and detailed connectivity in different species belonging to different classes of vertebrates, this indicates that the structure is evolutionarily conserved. Here we investigate if a cell group in the lamprey mesencephalic tegmentum could be a homologue of the mammalian PAG and the GC of the zebrafish (Okamoto et al., 2012).
4.1. Identifying the location of a GC/PAG homologue
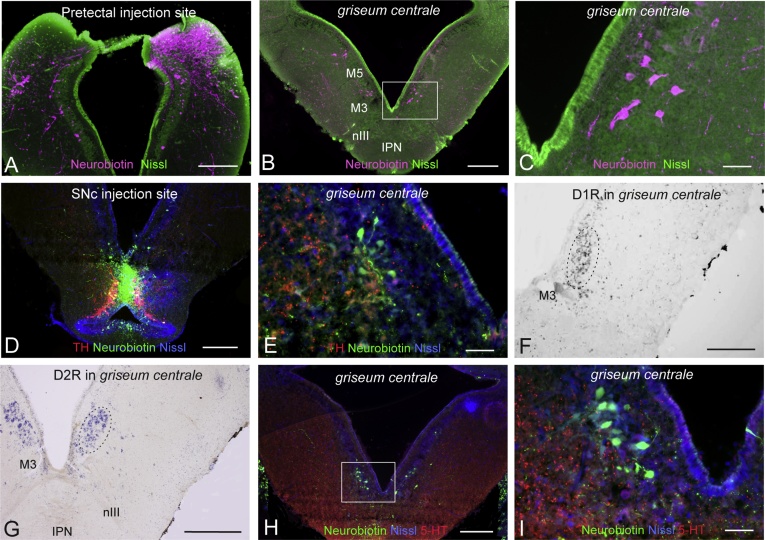
A recent study involving pretectal injections of Neurobiotin (Fig. 1A; Capantini et al., 2016) resulted in the identification of a previously uncharacterized bilateral cell cluster in the ventral portion of the mesencephalon near to the ventricular surface (Fig. 1B and C). These cells are situated ventral to the optic tectum, dorsal to the IPN, and immediately dorsal to the oculomotor nuclei, at the level of the M3 Müller cell. This cell group is clearly distinct from the retinopetal nucleus M5, and located immediately ventral to this nucleus. The location of this so far unidentified nucleus is very similar to that of the mammalian PAG and the zebrafish GC.
Fig. 1.
Identifying the PAG homologue. (A) Injection of Neurobiotin into the pretectum. (B–C) Bilaterally located retrogradely labeled cell body clusters in the periventricular area in the ventromedial mesencephalon. The squared area in B is shown in high magnification in C. (D) Injection of Neurobiotin into the SNc, confirmed by the presence of tyrosine hydroxylase (TH) immunoreactive cells (red). (E) High magnification photomicrograph of retrogradely labeled cell bodies (green) located periventricularly in the ventromedial mesencephalon with TH immunopositive fibers (red) in close proximity to the cells. (F and G) In situ hybridization of the dopamine D1 and D2 receptor transcripts showing expression of the dopamine D1 (F) and the D2 (G) receptor, respectively, in griseum centrale,. (H) Retrogradely labeled cells (green) in the griseum centrale following SNc injection (D) combined with 5-HT immunohistochemistry. (I) 5-HT immunoreactive fibers (red) in close proximity to the retrogradely labeled cells (green; high magnification photomicrograph of the squared area in H). All sections were counterstained with a fluorescent Nissl stain. 5-HT, serotonin; M1, Müller cell 1; M3, Müller cell 3; nIII, oculomotor nerve; IPN, interpeduncular nucleus; TH, tyrosine hydroxylase. Scale bars = A, B, D, F, G, H, 250 μm; C, E, I, 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Since the mammalian PAG projects to both the pretectum and SNc/VTA (Berkley and Mash, 1978, Foster et al., 1989, Omelchenko and Sesack, 2010), we investigated in the next step if the periventricular cell group also targets the dopaminergic neurons in the nucleus of the posterior tuberculum, the lamprey homologue of SNc/VTA (Pérez-Fernández et al., 2014). Neurobiotin was injected into the SNc (Fig. 1D), and the injection site was verified by the presence of tyrosine hydroxylase (TH)-expressing neurons (Fig. 1D). Following SNc injections, retrogradely labeled cell bodies were observed in the same ventromedial mesencephalic location (Fig. 1E) as after pretectal injections (Fig. 1A–C). In the following, we will refer to this cell group as the putative lamprey GC (pLGC).
4.2. Identification of afferents to the putative lamprey griseum centrale
The mammalian PAG is known to receive dopaminergic input (Mansour et al., 1990, Kitahama et al., 2000, Messanvi et al., 2013). Similarly, dopaminergic fibers were found in the pLGC following TH-immunohistochemistry (Fig. 1E). Another feature of the mammalian PAG neurons is that they express both the dopamine D1 and D2 receptor subtypes (Kitta et al., 2008, Meyer et al., 2009). To identify if a similar expression occurred in pLGC, in situ hybridization for both the D1 and D2 isoforms were performed. D1 and D2 expressing cells were observed in the same region as those projecting to the pretectum and the SNc (Fig. 1F and G; see also Pérez-Fernández et al., 2014).
Additionally, 5-hydroxytryptamine (5-HT) is known to have neuromodulatory effects in the mammalian PAG (Graeff, 2004). By combining immunohistochemistry for serotonin with retrograde tracing from the SNc (Fig. 1D), we observed 5-HT fibers in close appositions to pLGC cells retrogradely labeled from the SNc (Fig. 1H and I).
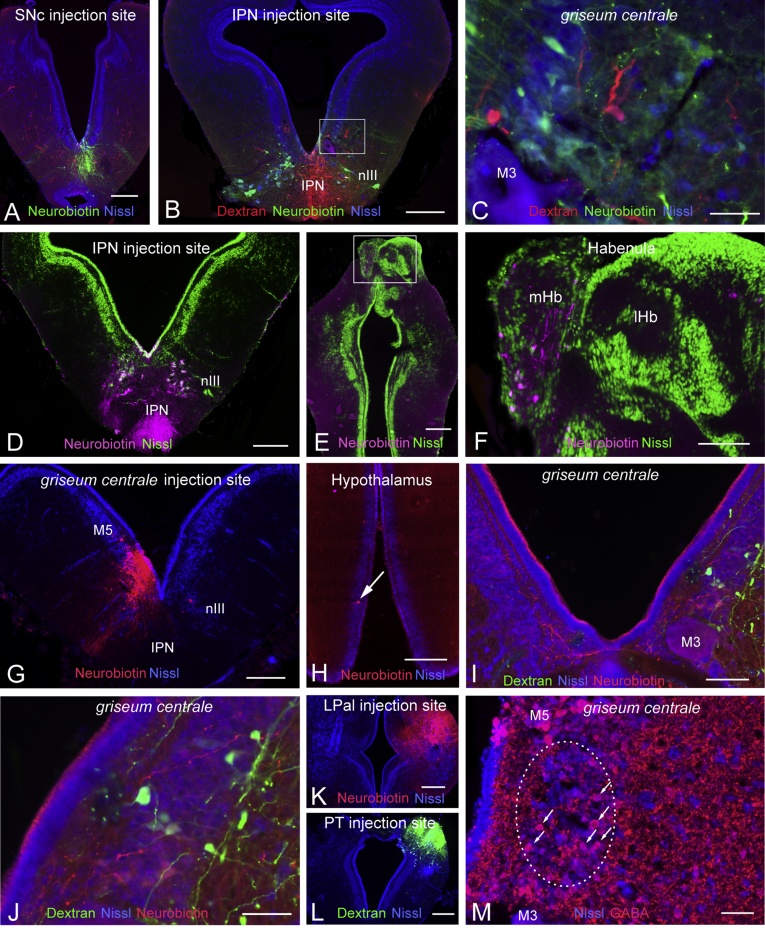
The zebrafish GC receives input from the dorsal IPN (Okamoto et al., 2012). In order to investigate whether a similar connection is present in the lamprey, dual tracer experiments were carried out with a Neurobiotin injection into the SNc (Fig. 2A), and an Alexa 488 conjugated dextran injection into the IPN (Fig. 2B). Anterogradely labeled fibers from IPN were observed in close proximity to cell bodies in pLGC, which were retrogradely labeled from the SNc injection (Fig. 2B and C). Injection of Neurobiotin into IPN (Fig. 2D) resulted in turn in retrogradely labeled cells in the medial habenula (Fig. 2E and F) as shown previously (Stephenson-Jones et al., 2012).
Fig. 2.
Identification of afferents to the griseum centrale. (A–C) Dual injections of Neurobiotin into the SNc (A) and biotin-dextran amine into the IPN (B). (B–C) Anterogradely labeled nerve fibers from IPN injection (red) in close proximity to retrogradely labeled cell bodies from SNc injection (green). (D–F) Injection of Neurobiotin into IPN (D) resulted in retrogradely labeled cells in the medial habenula (E-F; magenta). (G–H) Injection of Neurobiotin into griseum centrale (G) resulted in retrogradely labeled cells in the hypothalamus (H, arrow). (I) Pallial fibers and presumed terminals (red) in close apposition to cells in GC (green) retrogradely labeled from pretectum. Note that pallial fibers also innervated the contralateral GC. (J) Higher magnification of the photomicrograph in (I). (K) Neurobiotin injection into the lateral pallium (LPal). (L) Dextran injection into pretectum (PT). (M) GABA immunoreactive cells in the griseum centrale. All sections were counterstained with a fluorescent Nissl stain. IPN, interpeduncular nucleus; lHb, lateral habenula; M3, Müller cell 3; M5, the M5 nucleus of Schober; mHb, medial habenula nIII, oculomotor nerve. Scale bars = A, B, D, E, G, H, K, L, 250 μm; F, I 100 μm; C, I, J, M 50 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Another source of input to the mammalian PAG is from the hypothalamus. Following injection of Neurobiotin into the pLGC (Fig. 2G), retrogradely labeled cell bodies were observed in the ventral hypothalamus (Fig. 2H). PAG receives GABAergic input from the amygdala (Oka et al., 2008, Tovote et al., 2016) and we, in a recent study described a small subpopulation of GABAergic neurons in the pallium (cortex in mammals) that send descending projections to the brainstem (Capantini et al., 2016). Here we examined if pLGC receives pallial input. Fibers and thin presumed terminals originating from pallium were observed bilaterally in the area of the pLGC, in the vicinity of retrogradely labeled cells from pretectum (Fig. 2I and J), following Neurobiotin injection into the lateral pallium (Fig. 2K) and dextran injection into the pretectum (Fig. 2L), Lastly, we showed that there are GABA expressing neurons as well as terminals in the GC (Fig. 2M), which is also true of the mammalian PAG (Tovote et al., 2016).
4.3. Rostrocaudal extent of the putative lamprey griseum centrale
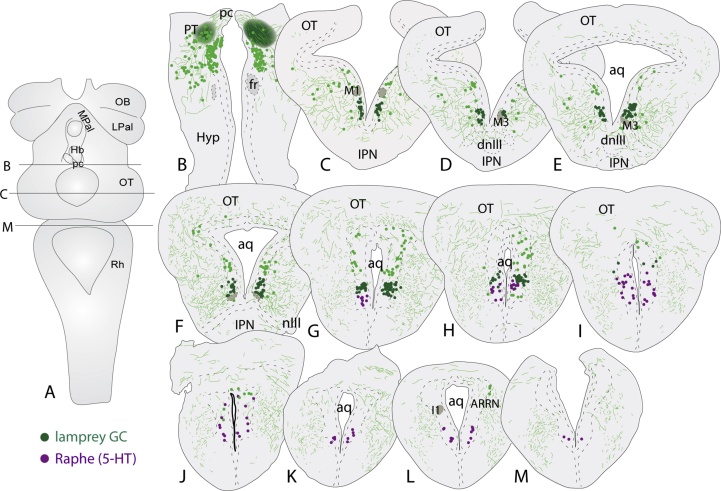
To establish the rostro-caudal extent of the pLGC, and its relation to other structures in the lamprey mesencephalon, serial sections were analyzed following the injection of Neurobiotin into the lamprey pretectum (Fig. 3A and B) and immunohistochemical staining for 5-HT. This showed that the rostral tip of the pLGC cell column begins between the M1 and M3 Müller cells and continue caudally through the mesencephalon, along the ventricular surface, to overlap with the rostral tip of the mesencephalic serotonergic raphe cell group (Fig. 3C–M). Also in rodents, the caudal PAG overlaps with the rostral dorsal raphe that extends along the caudoventral part of the central gray (see Paxinos, 2004).
Fig. 3.
Location of the lamprey griseum centrale. (A) Dorsal view of the lamprey brain indicating the level of the injection site (B), as well as the most rostral (C) and the most caudal section (M). (B) The bilateral injection sites in the pretectum. (C–M) Schematic drawing illustrating the rostrocaudal extent of griseum centrale (GC; green) and its relation to the serotonergic raphe nucleus (magenta). Aq, aqueduct; ARRN, anterior rhombencephalic reticular nucleus; dnIII, dorsal nucleus of the oculomotor nerve; Hyp, hypothalamus; I1, I1 Müller cell; IPN, interpeduncular nucleus; nIII, the oculomotor nerve; OT, optic tectum; pc, posterior commissure; PT, pretectum. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
5. Discussion
The present results suggest that the lamprey possesses a structure corresponding to the mammalian PAG and the zebrafish GC. We show a high degree of homology between mammals, zebrafish, and lamprey in terms of afferent and efferent connectivity patterns, as well as the anatomical location of PAG/GC. In lamprey, this periventricular cell group is located in the ventral mesencephalon, at the level of the oculomotor nuclei. Rostrally it extends to the level of the Müller cells M1 and M3, and caudally to the 5-HTergic raphe cell group (see Fig. 3). The lamprey pLGC is thus located in a similar area as the mammalian PAG, which stretches from the IPN and the oculomotor nucleus to the level of the dorsal raphe. Moreover, the lamprey pLGC targets the pretectum and SNc, receives input from the pallium, hypothalamus and IPN, as well as serotoninergic input from the raphe and dopaminergic input most probably from the SNc, and it expresses dopamine D1 and D2 receptors - all these features are hallmarks of the mammalian PAG, except for the input from IPN which is has been shown in the zebrafish but not investigated in mammals. The amygdala, hypothalamus, PAG and the IPN have all been shown to be involved in the processing of fear-related behaviors (Beitz, 1982, Agetsuma et al., 2010, Okamoto et al., 2012, Gross and Canteras, 2012, Subramanian and Holstege, 2014, Tovote et al., 2016). In mammals, zebrafish and lamprey, the IPN receives exclusive input from the medial habenula (Herkenham and Nauta, 1979, Bianco and Wilson, 2009, Agetsuma et al., 2010, Okamoto et al., 2012, Stephenson-Jones et al., 2012). Given these striking similarities with the zebrafish GC and the mammalian PAG, we will below refer to this structure as the lamprey griseum centrale, and not PAG since the mammalian PAG or central gray is situated around the aqueduct whereas in the lamprey, GC occupies only a limited part of periventricular region as in zebrafish.
The functional role of GC in the lamprey's defensive behavior has yet to be established. In mammals, there are three regions, which when stimulated, will provoke a full fear response: the lateral and central amygdala, the anterior and medial hypothalamus and distinct areas of the PAG (Panksepp, 1998, Bandler et al., 2000, Ressler et al., 2002, Gross and Canteras, 2012, Kim et al., 2013, Subramanian and Holstege, 2014). A recent study in the mouse has defined a GABAergic pathway from the central nucleus of amygdala to the ventrolateral PAG that mediates freezing (Tovote et al., 2016). These authors suggest that the freezing is caused by inhibition of local GABAergic interneurons and thereby disinhibition within PAG, since light activation of the glutamatergic neurons within PAG elicits freezing. In addition, they show that there is an interaction between the pathway for freezing and that for flight. Likewise, there is a GABAergic subpopulation in the lamprey GC. Furthermore, we recently described a small subpopulation of GABAergic projection neurons in the lamprey pallium that target brainstem areas (Capantini et al., 2016) and in addition we now show that there are pallial afferents to the GC. It is, however, not at this stage possible to know if the GABAergic pallial projection neurons could represent the lamprey homologue of the central nucleus of amygdala.
In the zebrafish, the projection pattern originating from the IPN is segregated such that the GC receives most of the input from the dorsal IPN, which in turn receives its input almost exclusively from the left dorsal (medial) habenula (Okamoto et al., 2012). The projection pattern from habenula to IPN is present also in the lamprey where the left habenula (part of the medial habenula homologue) projects mainly to the rostral IPN (Stephenson-Jones et al., 2012). This medial habenula–IPN–GC projection further suggests that GC is related to fear responses also in lamprey.
The presence of a PAG homologue in the lamprey, which represents the oldest branch-off in the vertebrate phylum (Kumar and Hedges, 1998), points to an ancient origin and profound importance of this structure, in the generation of motor patterns underlying fear responses. The lamprey GC is thus likely to control an evolutionarily important set of fear-related behaviors. The experience of fear may be different in different animals. However, the motor expressions of fear are similar, and they are accompanied by similar physiological responses such as changes in respiration and heart rate. Taken together, it appears that the basic elements of the circuitry for fear-related responses had evolved already in the phylogenetically oldest group of vertebrates, the lamprey.
6. Conclusion
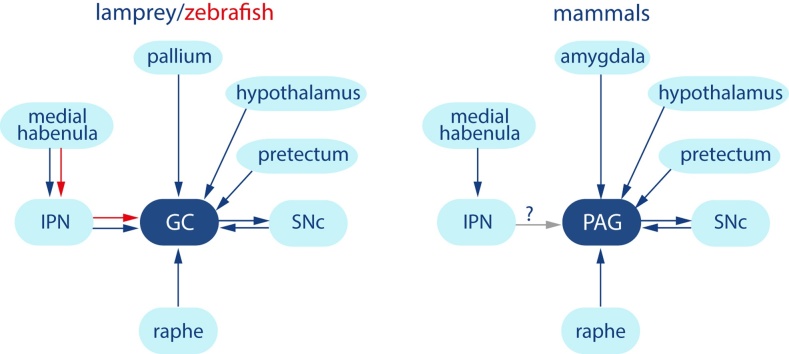
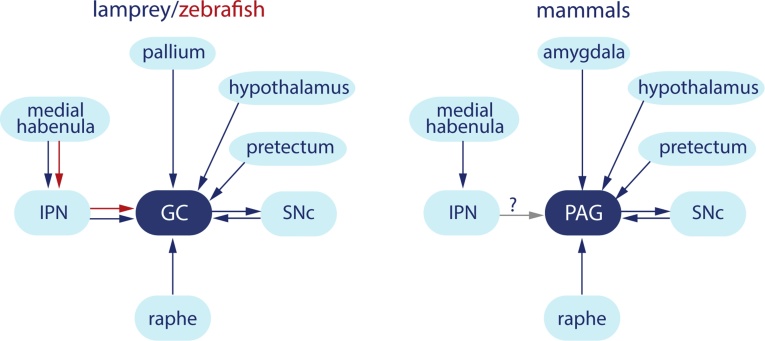
Fear-related behavior is common to all animals including humans and is vital for survival. This suggests that there is a common basic circuitry for fear-related responses. One structure that plays a pivotal role in this behavior is the mammalian PAG and the zebrafish GC. Here, we identify a structure in the lamprey that with respect to location, connectivity (Fig. 4) and expression of dopamine receptors is homologous to the PAG/GC. We thus show that also the lamprey GC is conserved as are other forebrain structures including the habenulae, basal ganglia and the pallial projection pattern (Stephenson-Jones et al., 2011, Stephenson-Jones et al., 2012, Ocana et al., 2015, Grillner and Robertson, 2016).
Fig. 4.
The connectivity pattern of the griseum centrale and PAG. Diagram comparing the afferent and efferent connectivity of the griseum centrale in lamprey and zebrafish, and the PAG in mammals. GC, griseum centrale; IPN, interpeduncular nucleus; PAG, periaqueductal gray; SNc, substantia nigra pars compacta.
Conflict of interest
The authors declare no conflict of interest.
Role of authors
SG designed the research together with BR, IO and SMS. IO, SMS and BR performed the experiments. The data were analyzed by IO, BR and SMS. SG, IO and BR wrote the manuscript. All authors have approved the final article.
Acknowledgements
We thank Dr Peter Wallén for valuable comments on the manuscript. This work was supported by the Swedish Research Council (grant numbers VR-M-K2013-62X-03026 and VR-NT-621-2013-4613), the European Union Seventh Framework Programme (FP7/2007–2013; under grant agreement no 604102, HBP) and the European Union's Horizon 2020 Research and Innovation Programme under Grant Agreement No. 720270 (HBP SGA1), the Karolinska Institutet's Research Funds, and StratNeuro, Karolinska Institutet.
Contributor Information
Ian Olson, Email: iolsodrum@gmail.com.
Shreyas M. Suryanarayana, Email: shreyas.suryanarayana@ki.se.
Brita Robertson, Email: brita.robertson@ki.se.
Sten Grillner, Email: sten.grillner@ki.se.
References
- Agetsuma M., Aizawa H., Aoki T., Nakayama R., Takahoko M., Goto M., Sassa T., Amo R., Shiraki T., Kawakami K., Hosoya T., Higashijima S., Okamoto H. The habenula is crucial for experience-dependent modification of fear responses in zebrafish. Nat. Neurosci. 2010;13:1354–1356. doi: 10.1038/nn.2654. [DOI] [PubMed] [Google Scholar]
- Anderson D.J., Adolphs R. A framework for studying emotions across species. Cell. 2014;157:187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R., Carrive P. Integrated defence reaction elicited by excitatory amino acid microinjection in the midbrain periaqueductal grey region of the unrestrained cat. Brain Res. 1988;439:95–106. doi: 10.1016/0006-8993(88)91465-5. [DOI] [PubMed] [Google Scholar]
- Bandler R., Keay K.A., Floyd N., Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res. Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Behbehani M.M. Functional characteristics of the midbrain periaqueductal gray. Prog. Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Beitz A.J. The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience. 1982;7:133–159. doi: 10.1016/0306-4522(82)90157-9. [DOI] [PubMed] [Google Scholar]
- Berkley K.J., Mash D.C. Somatic sensory projections to pretectum in cat. Brain Res. 1978;158:445–449. doi: 10.1016/0006-8993(78)90687-x. [DOI] [PubMed] [Google Scholar]
- Bianco I.H., Wilson S.W. The habenular nuclei: a conserved asymmetric relay station in the vertebrate brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:1005–1020. doi: 10.1098/rstb.2008.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capantini L., von Twickel A., Robertson B., Grillner S. The pretectal connectome in lamprey. J. Comp. Neurol. 2016 doi: 10.1002/cne.24102. [DOI] [PubMed] [Google Scholar]
- Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav. Brain Res. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Darwin C. Murray; London: 1872. The Expressions of the Emotions in Man and Animal. [Google Scholar]
- Foster G.A., Sizer A.R., Rees H., Roberts M.H. Afferent projections to the rostral anterior pretectal nucleus of the rat: a possible role in the processing of noxious stimuli. Neuroscience. 1989;29:685–694. doi: 10.1016/0306-4522(89)90141-3. [DOI] [PubMed] [Google Scholar]
- Graeff F.G. Serotonin, the periaqueductal gray and panic. Neurosci. Biobehav. Rev. 2004;28:239–259. doi: 10.1016/j.neubiorev.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Grillner S., Robertson B. The basal ganglia over 500 million years. Curr. Biol. 2016;26:R1088–R1100. doi: 10.1016/j.cub.2016.06.041. [DOI] [PubMed] [Google Scholar]
- Gross C.T., Canteras N.S. The many paths to fear. Nat. Rev. Neurosci. 2012;13:651–658. doi: 10.1038/nrn3301. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Nauta W.J. Efferent connections of the habenular nuclei in the rat. J. Comp. Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Kim E.J., Horovitz O., Pellman B.A., Tan L.M., Li Q., Richter-Levin G., Kim J.J. Dorsal periaqueductal gray-amygdala pathway conveys both innate and learned fear responses in rats. Proc. Natl. Acad. Sci. U. S. A. 2013;110:14795–14800. doi: 10.1073/pnas.1310845110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahama K., Nagatsu I., Geffard M., Maeda T. Distribution of dopamine-immunoreactive fibers in the rat brainstem. J. Chem. Neuroanat. 2000;18:1–9. doi: 10.1016/s0891-0618(99)00047-2. [DOI] [PubMed] [Google Scholar]
- Kitta T., Matsumoto M., Tanaka H., Mitsui T., Yoshioka M., Nonomura K. GABAergic mechanism mediated via D receptors in the rat periaqueductal gray participates in the micturition reflex: an in vivo microdialysis study. Eur. J. Neurosci. 2008;27:3216–3225. doi: 10.1111/j.1460-9568.2008.06276.x. [DOI] [PubMed] [Google Scholar]
- Kumar S., Hedges S.B. A molecular timescale for vertebrate evolution. Nature. 1998;392:917–920. doi: 10.1038/31927. [DOI] [PubMed] [Google Scholar]
- Liu R.P., Hamilton B.L. Neurons of the periaqueductal gray matter as revealed by Golgi study. J. Comp. Neurol. 1980;189:403–418. doi: 10.1002/cne.901890212. [DOI] [PubMed] [Google Scholar]
- Mansour A., Meador-Woodruff J.H., Bunzow J.R., Civelli O., Akil H., Watson S.J. Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J. Neurosci. 1990;10:2587–2600. doi: 10.1523/JNEUROSCI.10-08-02587.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messanvi F., Eggens-Meijer E., Roozendaal B., van der Want J.J. A discrete dopaminergic projection from the incertohypothalamic A13 cell group to the dorsolateral periaqueductal gray in rat. Front. Neuroanat. 2013;7:41. doi: 10.3389/fnana.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer P.J., Morgan M.M., Kozell L.B., Ingram S.L. Contribution of dopamine receptors to periaqueductal gray-mediated antinociception. Psychopharmacol. (Berl.) 2009;204:531–540. doi: 10.1007/s00213-009-1482-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Petrovic P., Marchant J.L., Hassabis D., Weiskopf N., Seymour B., Dolan R.J., Frith C.D. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocana F.M., Suryanarayana S.M., Saitoh K., Kardamakis A.A., Capantini L., Robertson B., Grillner S. The lamprey pallium provides a blueprint of the mammalian motor projections from cortex. Curr. Biol. 2015;25:413–423. doi: 10.1016/j.cub.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Oka T., Tsumori T., Yokota S., Yasui Y. Neuroanatomical and neurochemical organization of projections from the central amygdaloid nucleus to the nucleus retroambiguus via the periaqueductal gray in the rat. Neurosci. Res. 2008;62:286–298. doi: 10.1016/j.neures.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Agetsuma M., Aizawa H. Genetic dissection of the zebrafish habenula, a possible switching board for selection of behavioral strategy to cope with fear and anxiety. Dev. Neurobiol. 2012;72:386–394. doi: 10.1002/dneu.20913. [DOI] [PubMed] [Google Scholar]
- Omelchenko N., Sesack S.R. Periaqueductal gray afferents synapse onto dopamine and GABA neurons in the rat ventral tegmental area. J. Neurosci. Res. 2010;88:981–991. doi: 10.1002/jnr.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G. second ed. Academic Press; 2004. The Rat Nervous System. [Google Scholar]
- Panksepp J. The source of fear and anxiety in the brain. In: Panksepp J., editor. Affective Neuroscience. Oxford University Press; New York: 1998. pp. 206–222. [Google Scholar]
- Pérez-Fernández J. Neurolam Group, Dept. of Functional Biology and Health Science, University of Vigo; Spain: 2013. Characterization of Y and Dopamine Receptors in Lamprey by Using in Situ Hybridization: an Evolutionary Approach. PhD thesis. [Google Scholar]
- Pérez-Fernández J., Stephenson-Jones M., Suryanarayana S.M., Robertson B., Grillner S. Evolutionarily conserved organization of the dopaminergic system in lamprey: SNc/VTA afferent and efferent connectivity and D2 receptor expression. J. Comp. Neurol. 2014;522:3775–3794. doi: 10.1002/cne.23639. [DOI] [PubMed] [Google Scholar]
- Ressler K.J., Paschall G., Zhou X.L., Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J. Neurosci. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B., Huerta-Ocampo I., Ericsson J., Stephenson-Jones M., Pérez-Fernández J., Bolam J.P., Diaz-Heijtz R., Grillner S. The dopamine D2 receptor gene in lamprey, its expression in the striatum and cellular effects of D2 receptor activation. PLoS One. 2012;7:e35642. doi: 10.1371/journal.pone.0035642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson-Jones M., Samuelsson E., Ericsson J., Robertson B., Grillner S. Evolutionary conservation of the basal ganglia as a common vertebrate mechanism for action selection. Curr. Biol. 2011;21:1081–1091. doi: 10.1016/j.cub.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Stephenson-Jones M., Floros O., Robertson B., Grillner S. Evolutionary conservation of the habenular nuclei and their circuitry controlling the dopamine and 5-hydroxytryptophan (5-HT) systems. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E164–E173. doi: 10.1073/pnas.1119348109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian H.H., Holstege G. The midbrain periaqueductal gray changes the eupneic respiratory rhythm into a breathing pattern necessary for survival of the individual and of the species. Prog. Brain Res. 2014;212:351–384. doi: 10.1016/B978-0-444-63488-7.00017-3. [DOI] [PubMed] [Google Scholar]
- Tovote P., Esposito M.S., Botta P., Chaudun F., Fadok J.P., Markovic M., Wolff S.B., Ramakrishnan C., Fenno L., Deisseroth K., Herry C., Arber S., Luthi A. Midbrain circuits for defensive behaviour. Nature. 2016;534:206–212. doi: 10.1038/nature17996. [DOI] [PubMed] [Google Scholar]
- Wullimann M.F., Rupp B., Reichert H. Birkhäuser; Basel: 1996. Neuroanatomy of the Zebrafish Brain: a Topographic Atlas. [Google Scholar]