Abstract
By using a 5-day forced swimming test (FS) that we previously developed, swim immobility was induced in 3xTg Alzheimer's model mice and wild-type (WT) mice. After the initial 5-day FS, the next and last swimming session was performed at a 4-week interval, during which the immobility was reduced in 3xTg mice, but was maintained fully in WT mice. After FS, context-dependent fear learning was normally induced in WT mice, but was impaired in 3xTg mice, suggesting that FS may exaggerate cognitive deficits typical to 3xTg mice. Hippocampal long-term potentiation (LTP) at Schaffer collateral-CA1 synapses was suppressed by FS in WT mice, but not in 3xTg mice, indicating that FS modifies LTP in the WT mouse hippocampus, but not in 3xTg tissue. FS increased excitability of cingulate cortex pyramidal cells similarly in WT and 3xTg mice. Agreeing with our previous finding that expression of Homer1a protein is decreased in the cingulate cortex in harmony with FS-induced immobility, western blot showed that Homer1a expression is reduced by FS in the WT mice. In 3xTg mice, by contrast, FS failed to reduce Homer1a expression. The disrupted endurance of FS-induced immobility in 3xTg mice appears to be attributable to impaired cognition typical to this genotype. Failure of FS to alter LTP magnitude might be related to unaltered Homer1a expression after FS in 3xTg mice.
Keywords: Depression, Alzheimer's disease, Long-term potentiation, Excitability, Forced swimming, Homer1
Highlights
-
•
Four-week-long immobility was induced by forced swimming (FS) in wild-type (WT) mice.
-
•
The immobility was recovered within 4 weeks in Alzheimer's model (AD) mice.
-
•
FS reduced magnitude of long-term potentiation in WT but not AD mouse hippocampus.
-
•
FS reduced Homer1a expression in WT but not AD mouse cingulate cortex.
1. Introduction
The forced swimming (FS) test, a widely-used model for depression in rodents, was invented originally as a means by which to evaluate candidate compounds for antidepressant drugs in rats (Porsolt et al., 1977). In this test, a depression-like state represented by swim immobility or quiescent floating is induced on the first day, and its recovery by compounds is evaluated on the second day. In the later introduced mouse version of FS (Porsolt et al., 1978), both the induction and evaluation procedures are completed on one day only. More recently, however, it has frequently been questioned whether FS is a suitable model procedure for human depression (Holmes, 2003, Nestler and Hyman, 2010, Veenema et al., 2003). Changes in animal behavior induced by acute intensive insults in general may be less likely to simulate human depression than those induced by chronic succession of unpredictable mild stress (Holmes, 2003, Mineur et al., 2006, Nestler and Hyman, 2010, Willner, 1997, Willner, 2005). Such debate is related to the question on the physical characteristics of the stressor. More essentially, the swim immobility is not unanimously accepted as a manifestation of despair. Rather, the floating behavior is occasionally interpreted as active learning instead of depression-like behavior (De Pablo et al., 1989, West, 1990, Veenema et al., 2003). If mice save energy consumption by instinct, quiescent floating would be strategically advantageous in the inescapable environment rather than making useless efforts to desperately escape.
One way to approach such an argument would be to use mice defective in learning as the FS subjects, and check how much floating behavior they achieve. We previously used Homer1a-konckout (H1aKO) mice (Sun et al., 2015), which are defective at least in hippocampus-dependent contextual fear learning (Inoue et al., 2009). Homer1a is a member of the scaffold protein family Homer that link and regulate various receptors on the cell and endoplasmic reticulum membrane (Brakeman et al., 1997, Kato et al., 1997, Ango et al., 2001). In H1aKO mice, floating behavior was partially recovered in our original version of FS (Sun et al., 2011, Sun et al., 2015), in which the induction phase consisted of a 10-min-long daily swim for 5 consecutive days and the evaluation of floating was done 4 weeks later. Since no recovery is observed in wild-type (WT) mice, effects of genetic modification or antidepressant treatment during the interval could be examined. It was thus suggested that the partial recovery of the floating in H1aKO mice may be attributed to a defective learning ability, and that the floating may at least partly represent active learning. This interpretation is not necessarily straightforward, since Homer1 is implicated in major depression (Rietschel et al., 2010) and a possibly frail resilience to stress in H1aKO mice may preclude the full manifestation of cognitive ability. Therefore, the present study used 3xTg Alzheimer's model mice (Oddo et al., 2003), in which learning deficit has been well confirmed, to test if the floating behavior during FS is altered in cognitively impaired animals.
2. Experimental procedures
2.1. Animals
All the experiments were performed in accordance with the guiding principle of the Physiological Society of Japan and were approved by the Animal Care Committee of Kanazawa Medical University. Triple transgenic AD model mice (3xTg; Oddo et al., 2003) with 129/C57BL6 hybrid background, provided by Dr LaFerla (University of California, Irvine), were kept under day-night control (12:12 h), and allowed free access to food and water. Male 3xTg mice of 4–5 months of age were used. As the age-matched control, non-transgenic mice from the same hybrid background were used (wild-type).
2.2. Behavior
Forced swimming was performed as described (Sun et al., 2011). Briefly, transparent acrylate cylinder (24 cm diameter, 60 cm high) were filled with 25 °C water (25 cm deep). The mice swam for 10 min daily for 5 consecutive days and 4 weeks later on day 33. Swimming trajectory was analyzed by ANY-Maze (Stoelting Co., Wood Dale, IL, USA).
Fear conditioning was performed as described (Sun et al., 2015). Briefly, a dark-colored plastic chamber (25 W × 25 D × 27 cm H) located inside a sound-proof box (66W × 54 D × 54 cm H; Panlab s.l.u., Cornella, Spain) was positioned on the gravity sensor controlled by FREEZING software (Panlab). On day 1, after a 3-min habituation, the animals were exposed to white noise (80 dB) and illumination for 28 s, then to the same tone and illumination combined with electrical shock (0.4 mA) for 2 s, which was repeated twice at the interval of 30 s. On day 2, the animals were placed in the same chamber as on day 1, initially with no tone given. Freezing was measured for 5min. One hour later, the animals were placed in a white-colored chamber of the same size to assess the baseline activity for 3 min, and then the same tone as on day 1 was given for 3 min to assess tone-dependent fear conditioning.
2.3. Electrophysiology
Slice experiments were performed as described (Sun et al., 2011, Wang et al., 2015b, Yamamoto et al., 2011). After behavioral tests, mice were sacrificed by decapitation under ether anesthesia. The brain was soaked into a medium (pH 7.4; 25 °C) containing the following (in mM): 124 NaCl, 3.3 KCl, 1.3 KH2PO4, 26 NaHCO3, 2.5 CaCl2, 2.0 MgSO4, and 10 glucose. Sections of the cingulate cortex or hippocampus were cut with a slicer at 200 μm (Zero-1, Dosaka). Slices were placed in a recording chamber on the stage of an upright microscope (Eclipse E600FN, Nikon) with a ×40 water-immersion objective (Fluor 40 × 0.80 W, Nikon). The chamber was continuously perfused with medium (25 °C) and bubbled with a mixture of 95% O2 and 5% CO2. For recording, we used patch pipettes (resistance, 4–10 MΩ) filled with a solution (pH 7.3) containing the following (in mM): 130 K-gluconate, 10 KCl, 2 MgCl2, 2 Na-ATP, 0.4 Na-GTP, 0.2 EGTA, 10 HEPES, 5 K2-Phosphocreatine, with pH adjusted to 7.2–7.3 using KOH.
Whole-cell recordings were made from layer II/III pyramidal cells that had sufficiently negative resting membrane potentials (< −55 mV) without spontaneous action potentials. Membrane potentials were recorded in the current-clamp mode (Axoclamp 200A and B, Molecular Devices) and digitized at 10 kHz (Digidata 1322 and pCLAMP10, Molecular Devices). To assess membrane excitability of recorded neurons, depolarizing currents (50–500 pA for 500 ms) were injected through the patch pipette.
For field potential recording at Schaffer collateral-CA1 synapses, recording electrodes (2–5 MΩ) filled with 2.5 M NaCl were placed in the stratum radiatum and a set of bipolar tungsten electrodes was inserted nearby. Long-term potentiation (LTP) was induced with theta-burst stimulation (TBS). The test pulse intensity was adjusted to be 50–75% of threshold for population spikes and two trains of TBS at the interval of 20 s were given to induce LTP.
2.4. Western blot
Western blot was done as described (Sun et al., 2011). Briefly, cingulate cortex tissue was homogenized in cold buffer (lithium lauryl sulfate, 2%; aprotinin, 1.7 mg/ml; phenylmethylsulfonyl fluoride, 10 mg/ml; sodium orthovanadate, 1 mM). The homogenate was centrifuged at 15,000 rpm for 15 min at 4 °C. Protein concentration was determined by the Bradford method. Protein samples (40 μg) were analyzed by SDS-PAGE with 5–15% or 10–20% Ready Gels (Bio-Rad). After blocking with a skim milk solution (5%), immunoblotting was done with goat anti-Homer1a (1:500; SC8922; Santa Cruz Biotechnology) and rabbit anti-β-actin antibodies (1:2000; IMG-5142A; Imgenex). After reaction with HRP-conjugated secondary antibodies (donkey anti-goat IgG, 1:1000. HAF109, R&D Systems; goat anti-rabbit IgG, 1:1000, catalog no. 32460, Thermo Scientific), bands were detected with a chemiluminescence substrate kit (Super-Signal West Femto kit, Thermo Scientific) and a detector (LAS-4000, FUJIFILM) and analyzed by using ImageJ software.
2.5. Data analysis
Data are expressed as averages ± SEM. For statistics, pairwise or unpaired t-test and repeated-measures or one-way ANOVA followed by Dunnett T3 test were used (SPSS, version 21; Japan IBM).
3. Results
3.1. Behavioral study
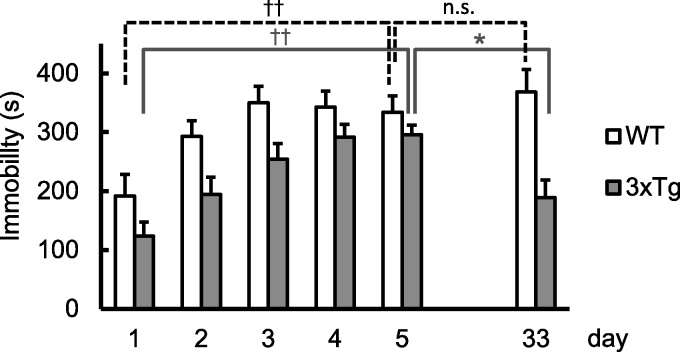
Depression-like behavior was induced by the 5-day forced swimming test that we have designed (Sun et al., 2011). In both WT and 3xTg mice, the immobility time developed during the initial 5-day phase of forced swimming (Fig. 1) and became longer on day 5 (WT, 333.9 ± 29.7 s, N = 15; 3xTg, 295.7 ± 37.2 s, N = 13) than on day 1 (WT, 191.4 ± 17.3 s, P < 0.001; 3xTg, 123.9 ± 28.0 s, P < 0.001, paired t-test). This pattern of immobility development was the same as we previously reported in WT mice (Sun et al., 2011) and in Homer1a knockout (H1aKO) mice (Sun et al., 2015). In the last swimming session on day 33, WT mice maintained the elongated immobility time (368.1 ± 19.2 s), whereas the immobility was significantly shortened in 3xTg mice (189.0 ± 39.6 s, P = 0.017 as compared to day 5 and P = 0.18 as compared to day 1). It is thus apparent that the depression-like behavior, as manifested by the immobility time, is recovered within a 4-week period in 3xTg mice, but is persistently maintained in WT mice.
Fig. 1.
Immobility induced by the 5-day paradigm of forced swimming (FS). A, Immobility as assessed by the immobility time during the 5 consecutive days and on day 33 in WT and 3xTg (Tg) mice. The immobility time (in seconds) over the 10-min swimming time was longer on day 5 than on day 1 in both groups. On day 33, it became shorter than on day 5 in the Tg group, but not in the WT group, indicating that there was recovery of the immobility time in the Tg group during the last 4 weeks. ††, P < 0.001; *, P < 0.05.
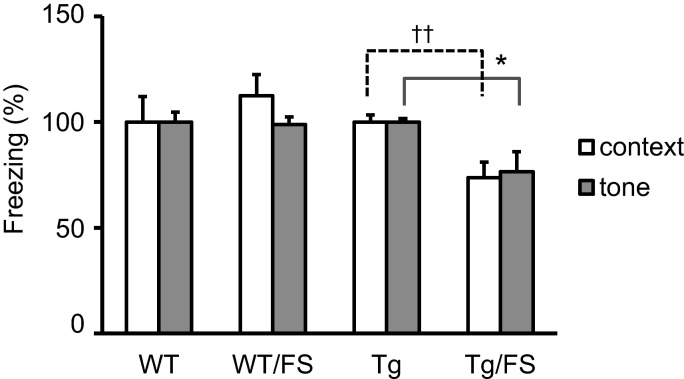
The influence of forced swimming on cognitive performance was assessed by contextual fear conditioning (Fig. 2). After the induction of depression-like behavior by the 5-day forced swimming in WT mice (WT/FS group, N = 17), context- or tone-dependent fear learning was no different than in naïve WT mice (WT group, N = 15). The freezing score in the WT/FS group (context, 1.12 ± 0.10; tone, 0.99 ± 0.04), expressed relative to the WT group, was no different than in the WT group (context, 1.00 ± 0.12; tone, 1.00 ± 0.05). In 3xTg mice (naïve Tg group, N = 13; Tg/FS groups, N = 16), however, both types of fear learning were partly impaired by the 5-day forced swimming. The freezing score in the Tg/FS group (context, 0.74 ± 0.07; tone, 0.76 ± 0.09), expressed relative to the Tg group, was reduced as compared to the Tg group (context, 1.00 ± 0.03, P = 0.003; tone, 1.00 ± 0.02, P = 0.030, t-test). These findings suggest that the depression-like state induced by FS may exaggerate cognitive deficits in 3xTg mice, whereas WT mice were allowed to elude such a potentially deleterious effect of FS.
Fig. 2.
Fear learning results. The freezing scores for the contextual fear conditioning (open bars) and tone-dependent conditioning (filled bars) in the 4 groups. The scores for the WT/FS and Tg/FS groups are expressed as percent of the data for the corresponding no-FS control (WT or Tg). In 3xTg mice (Tg) only, the freezing score was reduced by FS. ††, P < 0.001; *, P < 0.05.
3.2. Electrophysiological experiments
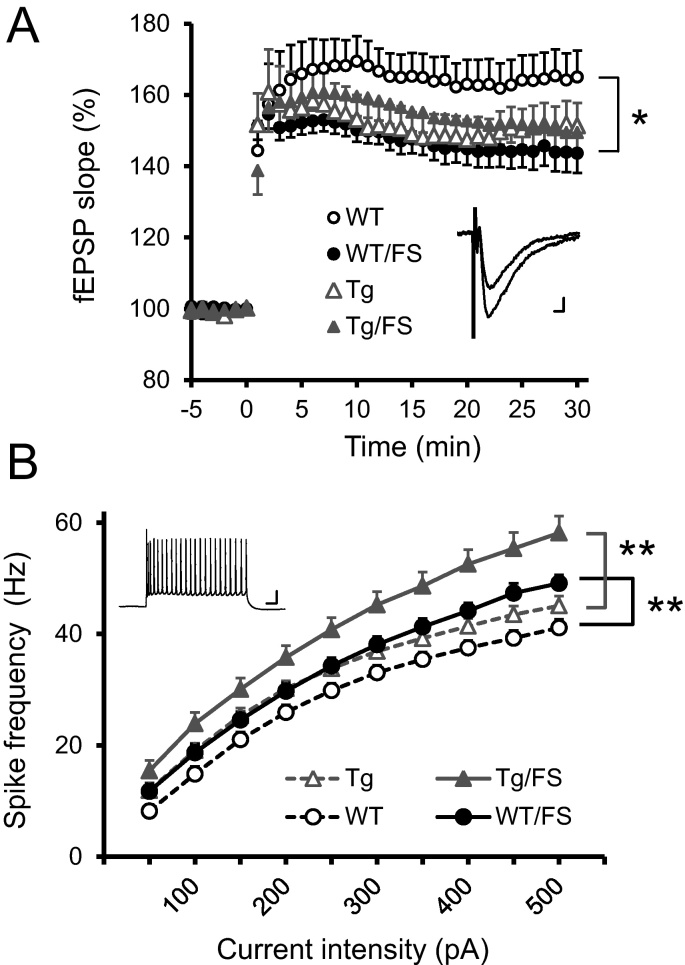
In an attempt to seek the underpinnings of the recovery of depression-like behavior in 3xTg mice that we showed here, long-term potentiation (LTP) was examined at Schaffer collateral-CA1 synapses in hippocampal slices obtained from naïve mice and those subjected to 5-day FS (Fig. 3A). With our protocol, LTP was induced in hippocampal slices obtained from 3xTg mice of 4-to-6 months of age, but was smaller in magnitude than in WT tissue, as we previously described (Wang et al., 2015b). The slope of field excitatory postsynaptic potential (fEPSP) at 30 min post-tetanus, expressed as a percent of the baseline immediately before the tetanus, was smaller in the WT/FS group (143.6 ± 5.5%, N = 20 slices) than in the WT group (165.0 ± 7.4%, N = 20 slices, P = 0.025, t-test). In 3xTg mice, by contrast, the magnitude of LTP at 30 min post-tetanus in the Tg/FS group (149.5 ± 6.5%, N = 23 slices) was no different than in the Tg group (151.4 ± 6.3%, N = 22 slices). These results indicate that LTP was suppressed in the depression-like state induced by FS in WT mice, but not in 3xTg mice. It is suggested that the effect of FS on LTP is persistent in terms of the change in magnitude in the WT mouse hippocampus, but was not maintained in 3xTg tissue.
Fig. 3.
Electrophysiological results. (A) Assessment of hippocampal long-term potentiation. The time course of CA1 fEPSPs is illustrated. The induction protocol (TBS) was delivered at time 0. In WT mice, LTP magnitude was reduced after FS. In 3xTg, FS failed to alter LTP magnitude. *, P < 0.05. Inset, specimen recording of fEPSPs recorded immediately before and 30 min after TBS in a slice obtained from the Tg/FS group. Scale bars; 3 ms, 0.1 mV. (B) Assessment of excitability in cingulate cortex pyramidal cells. Frequencies of action potentials elicited by injecting depolarizing currents of various intensities are plotted. The frequency is significantly higher in WT/FS group neurons than in WT group neurons, indicating that FS induced hyperexcitability in WT mice. The same is true in 3xTg mice. Inset, specimen recording of spikes evoked in a neuron obtained from a Tg/FS mouse by an injection of 500 ms-long current (500 pA). The majority of error bars are too short to be clearly seen. Scale bars; 20 mV, 50 ms. **, P < 0.005.
Consistent with cortical hyperexcitability in human depressives (Bajbouj et al., 2006), our previous study (Sun et al., 2011) demonstrated hyperexcitability in pyramidal cells in the cingulate cortex of mice subjected to FS. To examine whether cingulate cortex neurons exhibited hyperexcitability in the present FS mouse as well, we measured the frequency of action potentials evoked by injection of depolarizing currents of various intensities. As shown in the frequency profile (Fig. 3B), the excitability was increased by FS both in WT and 3xTg mice. There was a significant interaction between the current intensity and the group (P < 0.001, F(4.696) = 7.195 with Greenhouse-Geisser correction, repeated measures ANOVA), indicating that the dependence of the spike frequency on the current intensity differ among the 4 groups. The same statistical analysis revealed a significant interaction between the WT and WT/FS groups (P = 0.004, F(1.768) = 6.199) and between the 3xTg and 3xTg/FS groups (P = 0.001, F(1.344) = 12.879), suggesting that FS increased cingulate cortex excitability both in the WT and 3xTg groups. The frequency of spikes elicited by 500 pA differed among the 4 groups (P < 0.001, F(3, 106) = 13.662, one-way ANOVA): 41.2 ± 1.6 Hz in the WT group (N = 30 cells), 49.2 ± 1.6 Hz in the WT/FS group (N = 34 cells), 45.0 ± 1.8 Hz in the 3xTg group (N = 24 cells) and 58.2 ± 3.0 Hz in the 3xTg/FS group (N = 22 cells). Multiple comparison confirmed that FS increased the frequency both in the WT (P = 0.003, Dunnett T3 test) and 3xTg groups (P = 0.003).
3.3. Western blot analysis
Our previous study (Sun et al., 2011) showed that expression of Homer1a protein, which is the shorter variant of the adaptor protein Homer1 implicated in human depression (Rietschel et al., 2010), is decreased in the cingulate cortex in harmony with induction of depression-like state by FS, and is recovered with its behavioral alleviation. We therefore carried out western blot to compare the FS-associated changes of Homer1a expression in the cingulate cortex of WT and 3xTg mice. The density of the band at 30 kD, the thicker of the two bands representing Homer1a, was first measured by image J, then standardized by actin data from the same animal, averaged over the group, and finally expressed relative to the density in the WT group (Fig. 4). Homer1a expression is lower in the WT/FS group (0.67 ± 0.11 relative to WT data, N = 3) than in the WT group (1.00 ± 0.04, N = 3, P = 0.047, t-test). In 3xTg mice, by contrast, FS failed to reduce Homer1a expression (Tg, 0.97 ± 0.16 relative to WT data, N = 3; Tg/FS, 0.84 ± 0.12, N = 3).
Fig. 4.
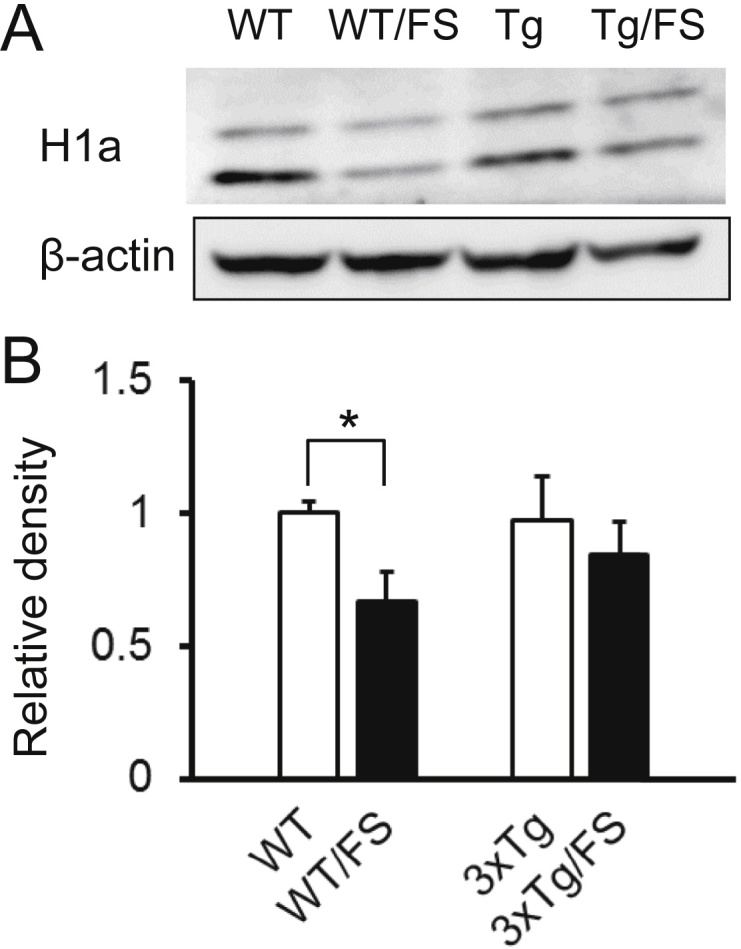
Expression of Homer1a protein in cingulate cortex. (A) Western blot of samples obtained from the WT, WT/FS, 3xTg (Tg), and Tg/FS groups. (B) Quantification of Homer1a protein expression. The density of the thicker Homer1a band at 30 kD was first standardized relative to β-actin, then averaged (N = 3), and finally expressed relative to the corresponding no-FS control group (WT or Tg). *, P < 0.05.
4. Discussion
FS is a widely-used model for depression in rodents. Swim immobility in the form of floating is the hallmark of depression-like behavior induced by FS. However, there has been argument that swim immobility may represent active coping or cognitive learning rather than manifestation of despair. We set out to test whether the swim immobility in FS has a cognitive component by using 3xTg AD model mice that are defective in various categories of memory and learning. The results showed that swim immobility is attributable at least partly to active learning.
In the present study, the persistence of swim immobility was accompanied by a reduced expression of H1a protein in the WT group, which agrees with our previous results (Sun et al., 2011). In 3xTg mice, by contrast, H1a expression was not altered, and concomitantly swim immobility attributable partly to learning was decreased. Hippocampus-dependent contextual fear learning was also impaired in 3xTg mice. Hippocampal LTP magnitude was smaller in 3xTg mice than in WT mice before FS and was not altered by FS, whereas it was reduced by FS in WT mice. Taken together, these findings suggest that H1a expression may be related to the occurrence of cognitive learning, namely swim immobility and contextual fear learning, and also to induction of a model of leaning, namely LTP. By contrast, pyramidal cell excitability was increased by FS in the WT and 3xTg groups alike. This excitability increase was accompanied by reduction of H1a expression in WT mice as previously reported (Sun et al., 2011), but not in 3xTg mice as revealed here.
In the present experiments, recovery of swim immobility was observed in 3xTg mice, but not in WT mice. Immobility in FS could be interpreted two-fold. It may be the manifestation of despair, representing depression-like behavior (Porsolt et al., 1977). Alternatively, this could be attributed to cognitive failure, representing impairment of adaptive learning (De Pablo et al., 1989, West, 1990, Campus et al., 2015), since mice are likely to learn that floating would minimize useless energy consumption caused by a desperate escape attempt from the inescapable pool. By using the same 5-day FS procedure, we previously reported a similar recovery of immobility in H1a-KO mice. These mice were shown to be defective in contextual fear learning (Inoue et al., 2009) in agreement with the report that H1a plays crucial roles in fear learning (Mahan et al., 2012). An activity-independent form of hetero-synaptic plasticity is also impaired in cultured cortical neurons obtained from mice of much the same genotype (Hu et al., 2010). Such defective aspects of learning and learning-related cellular activities in H1a-KO mice may have contributed to the recovery of immobility once acquired. This interpretation has been supported by the present finding that the immobility was recovered in 3xTg mice as well, in which impaired learning has been proven in a diverse array of behavioral tests: novel object recognition (NOR) (Zhang et al., 2012), radial arm maze (Stover et al., 2015), inhibitory avoidance (Billings et al., 2005) Morris water maze (MWM) (Billings et al., 2005), Barnes maze (Clinton et al., 2010), trace eyeblink conditioning (Wang et al., 2010), Y maze (Carroll et al., 2007), and water T maze (Filali et al., 2012). In accordance, our laboratory also confirmed memory deficits of 3xTg mice at the age of 5–9 months in MWM (Wang et al., 2015b) and NOR (Wang et al., 2015a). Thus, not just in H1a-KO mice but also in 3xTg mice, learning deficits in various ranges of cognitive test paradigms are associated with the failure to maintain the FS-induced immobility for 4 weeks. The possibility thus arises that the fading of immobility in 3xTg mice may be attributable to a cognitive failure. In other words, the 4-week-long maintenance of immobility may depend at least partly on a cognitive component.
The present results suggested that reduction of H1a expression may play a key role in sustaining the immobility for 4 weeks after the initial 5-day period. In accordance, our recent study has shown that the immobility was not maintained for 4 weeks in H1a-KO mice (Sun et al., 2015). This would be well understandable, assuming that the persisted maintenance of immobility was an active learning process that depends on H1a signaling. As far as contextual fear memory is concerned, some aspect of cognition is indeed impaired in H1a-KO mice (Inoue et al., 2009). Alternatively, the failure to maintain immobility may have resulted from a defective stress coping attributed to decrease of H1a expression in 3xTg mice after FS, given that H1a-KO mice are reported to be inferior to WT mice in coping with very mild chronic stress induced by restriction to a narrow space (Shui et al., 2015). Both in human major depression and in FS-induced immobility in mice, interaction of AMPA receptors and PDZ-binding domain proteins plays a critical role (Freudenberg et al., 2013), suggesting that the model immobility and human depression may share at least one mechanism. Given that H1a could also interact on glutamate receptors and PDZ domain (Brakeman et al., 1997), lack of H1a expression may well prevent the maintenance of FS-induced immobility. However, such dysregulation of glutamate receptor functioning would be also relevant to failure of synaptic plasticity and therefore impairment of cognitive behavior in general.
We showed that contextual fear learning is suppressed after FS in 3xTg but not WT mice. An obvious interpretation would be that FS-induced stress interfered with maintenance of contextual memory in 3xTg mice only, suggesting a vicious interaction between stress and cognitive processes in 3xTg mice. It has indeed been well known that intraneuronal accumulation of Aβ and vulnerability to stress appear to interact on each other in a positive feedback manner. It is reported that 3xTg mice are more vulnerable than WT mice to chronic psychosocial stress and exhibit elevated intraneuronal Aβ after stress (Rothman et al., 2012). Intraneuronal Aβ accumulation enhances fear and anxiety in 3xTg mice (España et al., 2010). Cumulative stress is shown to exacerbate AD pathology by accelerating tau phosphorylation in rats (Sotiropoulos et al., 2011).
Acknowledgements
We express our gratitude to Dr S. Kawahara (Toyama University, Toyama, Japan) for advice and to Mr H. Adachi, Mr S. Muramoto, and Ms K. Yamada for assistance. This study was supported by Grants-in-Aid for Scientific Research (22500360 and 17H02223) from the Japan Society for the Promotion of Science, and Senryaku Projects (H2010-14, H2014-15 and H2015-16) from Kanazawa Medical University. XL, YS, FW and NK performed experiments; XL, FW, RY and NK analyzed data; NK wrote the paper.
References
- Ango F., Prézeau L., Muller T., Tu J.C., Xiao B., Worley P.F., Pin J.P., Bockaert J., Fagni L. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- Bajbouj M., Lisanby S.H., Lang U.E., Danker-Hopfe H., Heuser I., Neu P. Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol. Psychiatry. 2006;59:395–400. doi: 10.1016/j.biopsych.2005.07.036. [DOI] [PubMed] [Google Scholar]
- Billings L.M., Oddo S., Green K.N., McGaugh J.L., LaFerla F.M. Intraneuronal Aβ causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Brakeman P.R., Lanahan A.A., O'Brien R., Roche K., Barnes C.A., Huganir R.L., Worley P.F. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Campus P., Colelli V., Orsini C., Sarra D., Cabib S. Evidence for the involvement of extinction-associated inhibitory learning in the forced swimming test. Behav. Brain Res. 2015;278:348–355. doi: 10.1016/j.bbr.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Carroll J.C., Rosario E.R., Chang L., Stanczyk F.Z., Oddo S., LaFerla F.M., Pike C.J. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J. Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton L.K., Blurton-Jones M., Myczek K., Trojanowski J.Q., LaFerla F.M. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. J. Neurosci. 2010;30:7281–7289. doi: 10.1523/JNEUROSCI.0490-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pablo J.M., Parra A., Segovia S., Guillamón A. Learned immobility explains the behavior of rats in the forced swimming test. Physiol. Behav. 1989;46:229–237. doi: 10.1016/0031-9384(89)90261-8. [DOI] [PubMed] [Google Scholar]
- España J., Giménez-Llort L., Valero J., Miñano A., Rábano A., Rodriguez-Alvarez J., LaFerla F.M., Saura C.A. Intraneuronal beta-amyloid accumulation in the amygdala enhances fear and anxiety in Alzheimer's disease transgenic mice. Biol. Psychiatry. 2010;67:513–521. doi: 10.1016/j.biopsych.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Filali M., Lalonde R., Theriault P., Julien C., Calon F., Planel E. Cognitive and non-cognitive behaviors in the triple transgenic mouse model of Alzheimer's disease expressing mutated APP, PS1, and Mapt (3xTg-AD) Behav. Brain Res. 2012;234:334–342. doi: 10.1016/j.bbr.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Freudenberg F., Marx V., Mack V., Layer L.E., Klugmann M., Seeburg P.H., Sprengel R., Celikel T. GluA1 and its PDZ-interaction: a role in experience-dependent behavioral plasticity in the forced swim test. Neurobiol. Dis. 2013;52:160–167. doi: 10.1016/j.nbd.2012.12.003. [DOI] [PubMed] [Google Scholar]
- Holmes P.V. Rodent models of depression: reexamining validity without anthropomorphic inference. Crit. Rev. Neurobiol. 2003;15:143–174. doi: 10.1615/critrevneurobiol.v15.i2.30. [DOI] [PubMed] [Google Scholar]
- Hu J.H., Park J.M., Park S., Xiao B., Dehoff M.H., Kim S., Hayashi T., Schwarz M.K., Huganir R.L., Seeburg P.H., Linden D.J., Worley P.F. Homeostatic scaling requires group I mGluR activation mediated by Homer1a. Neuron. 2010;68:1128–1142. doi: 10.1016/j.neuron.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N., Nakao H., Migishima R., Hino T., Matsui M., Hayashi F., Nakao K., Manabe T., Aiba A., Inokuchi K. Requirement of the immediate early gene vesl-1S/homer-1a for fear memory formation. Mol. Brain. 2009;5:2–7. doi: 10.1186/1756-6606-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A., Ozawa F., Saitoh Y., Hirai K., Inokuchi K. Vesl, a gene encoding VASP/Ena family related protein, is upregulated during seizure, long-term potentiation and synaptogenesis. FEBS Lett. 1997;412:183–189. doi: 10.1016/s0014-5793(97)00775-8. [DOI] [PubMed] [Google Scholar]
- Mahan A.L., Mou L., Shah N., Hu J.H., Worley P.F., Ressler K.J. Epigenetic modulation of Homer1a transcription regulation in amygdala and hippocampus with pavlovian fear conditioning. J. Neurosci. 2012;32:4651–4659. doi: 10.1523/JNEUROSCI.3308-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur Y.S., Belzung C., Crusio W.E. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav. Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Nestler E.J., Hyman S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S., Caccamo A., Shepherd J.D., Murphy M.P., Golde T.E., Kayed R., Metherate R., Mattson M.P., Akbari Y., LaFerla F.M. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Le Pichon M., Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Porsolt R.D., Bertin A., Jalfre M. “Behavioural despair” in rats and mice: strain differences and the effects of imipramine. Eur. J. Pharmacol. 1978;51:291–294. doi: 10.1016/0014-2999(78)90414-4. [DOI] [PubMed] [Google Scholar]
- Rietschel M., Mattheisen M., Frank J., Treutlein J., Degenhardt F., Breuer R., Steffens M., Mier D., Esslinger C., Walter H., Kirsch P., Erk S., Schnell K., Herms S., Wichmann H.E., Schreiber S., Jöckel K.H., Strohmaier J., Roeske D., Haenisch B., Gross M., Hoefels S., Lucae S., Binder E.B., Wienker T.F., Schulze T.G., Schmäl C., Zimmer A., Juraeva D., Brors B., Bettecken T., Meyer-Lindenberg A., Müller-Myhsok B., Maier W., Nöthen M.M., Cichon S. Genome-wide association-, replication-, and neuroimaging study implicates HOMER1 in the etiology of major depression. Biol. Psychiatry. 2010;68:578–585. doi: 10.1016/j.biopsych.2010.05.038. [DOI] [PubMed] [Google Scholar]
- Rothman S.M., Herdener N., Camandola S., Texel S.J., Mughal M.R., Cong W.N., Martin B., Mattson M.P. 3xTgAD mice exhibit altered behavior and elevated Aβ after chronic mild social stress. Neurobiol. Aging. 2012;33 doi: 10.1016/j.neurobiolaging.2011.07.005. 830.e1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shui Y., Wang L., Luo X., Uchiumi O., Yamamoto R., Sugai T., Kato N. Homer1a disruption increases vulnerability to predictable subtle stress normally subthreshold for behavioral change. Brain Res. 2015;1605:70–75. doi: 10.1016/j.brainres.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos I., Catania C., Pinto L.G., Silva R., Pollerberg G.E., Takashima A., Sousa N., Almeida O.F. Stress acts cumulatively to precipitate Alzheimer's disease-like tau pathology and cognitive deficits. J. Neurosci. 2011;31:7840–7847. doi: 10.1523/JNEUROSCI.0730-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover K.R., Campbell M.A., Van Winssen C.M., Brown R.E. Early detection of cognitive deficits in the 3xTg-AD mouse model of Alzheimer's disease. Behav. Brain Res. 2015;289:29–38. doi: 10.1016/j.bbr.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Sun P., Wang F., Wang L., Zhang Y., Yamamoto R., Sugai T., Zhang Q., Wang Z., Kato N. Increase in cortical pyramidal cell excitability accompanies depression-like behavior in mice: a transcranial magnetic stimulation study. J. Neurosci. 2011;31:16464–16472. doi: 10.1523/JNEUROSCI.1542-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P., Zhang Q., Zhang Y., Wang F., Chen R., Yamamoto R., Kato N. Homer1a-dependent recovery from depression-like behavior by photic stimulation in mice. Physiol. Behav. 2015;147:334–341. doi: 10.1016/j.physbeh.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Veenema A.H., Meijer O.C., de Kloet E.R., Koolhaas J.M. Genetic selection for coping style predicts stressor susceptibility. J. Neuroendocrinol. 2003;15:256–267. doi: 10.1046/j.1365-2826.2003.00986.x. [DOI] [PubMed] [Google Scholar]
- Wang J.M., Singh C., Liu L., Irwin R.W., Chen S., Chung E.J., Thompson R.F., Brinton R.D. Allopregnanolone reverses neurogenic and cognitive deficits in mouse model of Alzheimer's disease. Proc. Natl. Acad. Sci. U. S. A. 2010;107:6498–6503. doi: 10.1073/pnas.1001422107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Kang H., Li Y., Shui Y., Yamamoto R., Sugai T., Kato N. Cognitive recovery by chronic activation of the large-conductance calcium-activated potassium channel in a mouse model of Alzheimer's disease. Neuropharmacology. 2015;92:8–15. doi: 10.1016/j.neuropharm.2014.12.033. [DOI] [PubMed] [Google Scholar]
- Wang F., Zhang Y., Wang L., Sun P., Luo X., Ishigaki Y., Sugai T., Yamamoto R., Kato N. Improvement of spatial learning by facilitating large-conductance calcium-activated potassium channel with transcranial magnetic stimulation in Alzheimer's disease model mice. Neuropharmacology. 2015;97:210–219. doi: 10.1016/j.neuropharm.2015.05.027. [DOI] [PubMed] [Google Scholar]
- West A.P. Neurobehavioral studies of forced swimming: the role of learning and memory in the forced swim test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1990;14:863–877. doi: 10.1016/0278-5846(90)90073-p. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacol. Berl. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural–neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Ueta Y., Wang L., Yamamoto R., Inoue N., Inokuchi K., Aiba A., Yonekura H., Kato N. Suppression of a neocortical potassium channel activity by intracellular amyloid-β and its rescue with Homer1a. J. Neurosci. 2011;31:11100–11109. doi: 10.1523/JNEUROSCI.6752-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Xue G., Wang S., Zhang L., Shi C., Xie X. Novel object recognition as a facile behavior test for evaluating drug effects in AβPP/PS1 Alzheimer's disease mouse model. J. Alzheimers Dis. 2012;31:801–812. doi: 10.3233/JAD-2012-120151. [DOI] [PubMed] [Google Scholar]