Abstract
Signal transducer and activator of transcription 3 (STAT3) plays a prominent role in the growth and invasion of several types of solid tumors. In this study, to assess the expression status and prognostic significance of the STAT3 pathway in upper urinary tract urothelial carcinoma (UTUC), we immunohistochemically stained for STAT3 and STAT3 pathway proteins, sphingosine-1-phosphate receptor 1 (S1PR1) and interleukin-6 (IL-6), in a tissue microarray containing 99 UTUC specimens. There were no significant associations between STAT3, S1PR1, or IL-6 expression pattern and tumor grade or pT stage. However, the patients with high STAT3 tumor had a significantly higher risk of both disease progression (p = 0.009) and cancer-specific mortality (p = 0.009), but not with tumors expressing S1PR1 or IL-6. High STAT3 expression in the nucleus was also associated with a significantly higher risk of both disease progression (p = 0.003) and cancer-specific mortality (p = 0.034). Multivariate analysis revealed that high STAT3 expression in the nucleus was significantly associated with cancer-specific survival after adjustment for pathological stage, lymph node involvement, lymphovascular invasion, and tumor grade (HR = 2.136, 95% CI = 1.009–4.767, p = 0.047). Our findings indicated that STAT3 could be a cancer-promoting factor and potentially a significant prognostic factor in UTUC.
Introduction
Upper urinary tract urothelial carcinoma (UTUC) is relatively rare, accounting for approximately 5% to 10% of all urothelial tumors of the urinary tract [1–3]. Although urothelial carcinoma of the bladder and UTUC share many characteristics, practical, anatomical, biological, and molecular differences have been proven [4, 5]. Due to the lower incidence of UTUC compared with bladder cancer, little is known about the molecular markers confirmed to be useful for daily clinical decision making.
The signal transducer and activator of transcription (STAT) proteins are intracellular transcription factors that mediate various aspects of cellular immunity), proliferation, apoptosis, and differentiation) [6]. The STAT family includes seven members (STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6). Among them, STAT3 has been shown to play a prominent role in tumor growth and invasion [7]. In response to cytokines and growth factors, STAT3 is phosphorylated by receptor-associated Janus kinases (JAK), forms homo- or hetero-dimers, and translocates to the nucleus where it acts as a transcription activator [8].
S1PR1 is the one of the G-protein-coupled receptors for sphingosine-1-phosphate (S1P), a biologically active metabolite of sphingolipid [9]. S1P-S1PR1 signaling activates STAT3 [10]. IL-6 is a well-known traditional activator of STAT3 [11–13].
In this study, we evaluated the expression status of STAT3 pathway proteins, STAT3, S1PR1, and IL-6, in UTUC and analyzed their prognostic significance.
Material and methods
Patients and tissue samples
A UTUC tissue microarray (TMA) was constructed with spotted triplicate tumor samples from 99 patients who underwent radical nephroureterectomy performed with curative intent between 1997 and 2011 at Osaka General Medical Center, Osaka, Japan, as previously described [14–16]. Appropriate approval was obtained from the local institutional review board (Osaka General Medical Center Institutional Review Board, Protocol Number: 25–2014) before construction and use of the TMA, and written informed consent was obtained from all patients. Clinicopathological characteristics of the patients were obtained from medical records and follow-up data at the time of TMA production, and tissues samples were de-identified. Tumor progression was defined as the development of recurrence at the site of radical nephroureterectomy, lymph node metastasis, and/or visceral metastasis. Metachronous or synchronous lower tract recurrence (e.g., in the bladder) was not defined as tumor progression. Patients were followed up from initial diagnosis to the appearance of the event of interest or the end of the study. Patients who did not present the event of interest by the end of the study were censored from time-to-event analyses.
Immunohistochemistry
Immunohistochemical staining was performed on 5-μm sections from the UTUC TMA using a primary antibody to STAT3 (LS-B4102, Cell Signaling Technology, Danvers, MA, USA), S1PR1 (sc-25489, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and IL-6 (ab-6672, Abcam, Cambridge, UK). We used normal kidney tissue as positive controls (S1 Fig). Sections were deparaffinized, rehydrated, and subjected to heat-induced antigen retrieval with a buffer solution using a steamer for 20 min before staining, and endogenous peroxidase activity was quenched with H2O2. Sections were then incubated with the appropriate primary antibody, and the DAKO EnVision™ Kit was used according to the manufacturer’s instructions. All of the stained sections were manually scored by two researchers (K.M. and K.F.) who were blinded to sample identity.
Scoring system
TMA spots stained with each marker were evaluated for the pattern (nuclear and cytoplasmic), extent (percentage of positive cells), and intensity (0 to 3+ score) of staining. Cytoplasmic and nuclear staining was assessed for STAT3, and cytoplasmic staining was assessed for S1PR1 and IL-6 positivity. To account for the percentage of positive cells and staining intensity, an “H score” was assigned to each TMA spot as the sum of the products of the intensity (0, negative; 1, weakly positive; 2, moderately positive; and 3, strongly positive) and the extent of immunoexpression (0 to 100%), obtaining a value from 0 to 300, as previously described [14]. The final H score for each case was defined as the average score of triplicate TMA spots and was used during statistical analyses for all markers. For statistical analysis, the patients were divided into two groups according to the H score (High: H score > the median, Low: H score ≤ the median).
Statistical analysis
Statistical analyses were performed using JMP® Pro 13.2.0 (SAS Institute Inc., Cary, NC, USA). Patient characteristics were compared using the Mann-Whitney U test and χ2-test. The survival rates were determined using the Kaplan-Meier method and compared with the log-rank test. A Cox proportional hazards model was performed to determine the statistical significance of prognostic indicators in a multivariate setting. Differences were considered statistically significant when the p value was < 0.05.
Results
Table 1 shows the characteristics of the 99 patients. The patients included 60 men and 39 women with a median (range) age of 71 (48–87) years at the time of surgery and a median (range) follow-up of 47 (2–173) months after surgery. Included in these patients were 45 renal pelvic tumors and 50 ureteral tumors (4 patients with tumors at both sites), 15 low-grade urothelial carcinomas and 84 high-grade urothelial carcinomas, 37 non-muscle-invasive tumors (pTa or pT1) and 62 muscle-invasive tumors (pT2, pT3, or pT4), 44 small tumors and 38 large tumors (17 patients without the record of tumor size), 84 pN0 tumors and 12 pN+ tumors (3 patients with pNx tumors), and two pM1 tumors. These two pM patients had peritoneal dissemination and distant lymph node metastases (merentery lymph nodes), and concomitant radical metastasectomy were performed. There were two patients with positive surgical margin, and one patient had pT2 and another had pT3 tumor. During follow-up, metachronous or synchronous recurrence in the lower urinary tract was observed in 32 patients. The details of the patients information were shown in S1 Table.
Table 1. Associations between clinicopathological profile of the patients and the expression level of STAT3, S1PR1 and IL-6.
| Variable parameters | All cases |
STAT3 expression | S1PR1 expression | IL-6 expression | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low N = 50 |
High N = 49 |
P-value | Low N = 50 |
High N = 49 |
P-value | Low N = 55 |
High N- = 44 |
P-value | ||
| Age median (range) | 71 (48–87) |
71.5 (51–87) |
71 (48–84) |
0.986 | 71 (52–87) |
71 (48–85) |
0.113 | 72 (53–87) |
68 (48–85) |
0.003 |
| Gender n, (%) | 0.592 | 0.485 | 0.027 | |||||||
| male | 60 | 29 (58.0%) | 31 (63.3%) |
32 (64.0%) |
28 (57.1%) |
28 (50.9%) |
32 (72.7%) |
|||
| female | 39 | 21 (42.0%) |
18 (36.7%) |
18 (36.0%) |
21 (42.9%) |
27 (49.1%) |
12 (27.3%) |
|||
| Tumor site n, (%) | 0.004 | 0.393 | 0.367 | |||||||
| Renal pelvis | 45 | 31 (62.0%) |
14 (28.6%) |
20 (40.0%) |
25 (51.0%) |
24 (43.6%) |
21 (47.7%) |
|||
| Ureter | 50 | 18 (36.0%) |
32 (65.3%) |
27 (54.0%) |
23 (46.9%) |
30 (54.6%) |
20 (45.5%) |
|||
| Both | 4 | 1 (2.0%) |
3 (6.1%) |
3 (6.0%) |
1 (2.1%) |
1 (1.8%) |
3 (6.8%) |
|||
| Tumor grade n, (%) | 0.425 | 0.149 | 0.851 | |||||||
| Low grade | 15 | 9 (18.0%) |
6 (12.2%) |
5 (10.0%) |
10 (20.4%) |
8 (14.5%) |
7 (15.9%) |
|||
| High grade | 84 | 41 (82.0%) |
43 (87.8%) |
45 (90.0%) |
39 (79.6%) |
47 (85.5%) |
37 (84.1%) |
|||
| Tumor size n, (%) | 0.557 | 0.193 | 0.706 | |||||||
| <3cm | 44 | 18 (36.0%) |
26 (53.1%) |
26 (52.0%) |
18 (36.7%) |
26 (47.3%) |
18 (40.9%) |
|||
| ≧3cm | 38 | 18 (36.0%) |
20 (40.8%) |
17 (34.0%) |
21 (42.9%) |
24 (43.6%) |
14 (31.8%) |
|||
| unknown | 17 | 14 (28.0%) |
3 (6.1%) |
7 (14.0%) |
10 (20.4%) |
5 (9.1%) |
12 (27.3%) |
|||
| Pathologic stage n, (%) | 0.593 | 0.779 | 0.814 | |||||||
| pTa | 19 | 9 (18.0%) |
10 (20.4%) |
9 (18.0%) |
10 (20.4%) |
10 (18.2%) |
9 (20.5%) |
|||
| pT1 | 18 | 12 (24.0%) |
6 (12.2%) |
10 (20.0%) |
8 (16.3%) |
10 (18.2%) |
8 (18.2%) |
|||
| pT2 | 8 | 4 (8.0%) |
4 (8.2%) |
3 (6.0%) |
5 (10.2%) |
3 (5.5%) |
5 (11.4%) |
|||
| pT3 | 48 | 23 (46.0%) |
25 (51.0%) |
26 (52.0%) |
22 (44.9%) |
28 (50.9%) |
20 (45.5%) |
|||
| pT4 | 6 | 2 (4.0%) |
4 (8.2%) |
2 (4.0%) |
4 (8.2%) |
4 (7.3%) |
2 (4.5%) |
|||
| LVI n, (%) | 0.085 | 0.744 | 0.614 | |||||||
| 0 | 59 | 34 (68.0%) |
25 (51.0%) |
29 (58.0%) |
30 (61.2%) |
34 (61.8%) |
25 (56.8%) |
|||
| 1 | 40 | 16 (32.0%) |
24 (49.0%) |
21 (42.0%) |
19 (38.8%) |
21 (38.2%) |
19 (43.2%) |
|||
| Lymph node involvement n, (%) | 0.151 | 0.782 | 0.949 | |||||||
| pN0 | 84 | 43 (86.0%) |
41 (83.7%) |
40 (80.0%) |
44 (89.8%) |
45 (81.8%) |
39 (88.6%) |
|||
| pN1-3 | 12 | 4 (8.0%) |
8 (16.3%) |
7 (14.0%) |
5 (10.2%) |
7 (12.7%) |
5 (11.4%) |
|||
| pNx | 3 | 3 (6.0%) |
0 (0%) |
3 (6.0%) |
0 (0%) |
3 (5.5%) |
0 (0%) |
|||
| H score median (range) | 41.7 (0–63.4) |
125 (66.7–265) |
150 (0–225) |
263.3 (226.7–300) |
116.7 (93.3–150) |
208.3 (153.3–300) |
||||
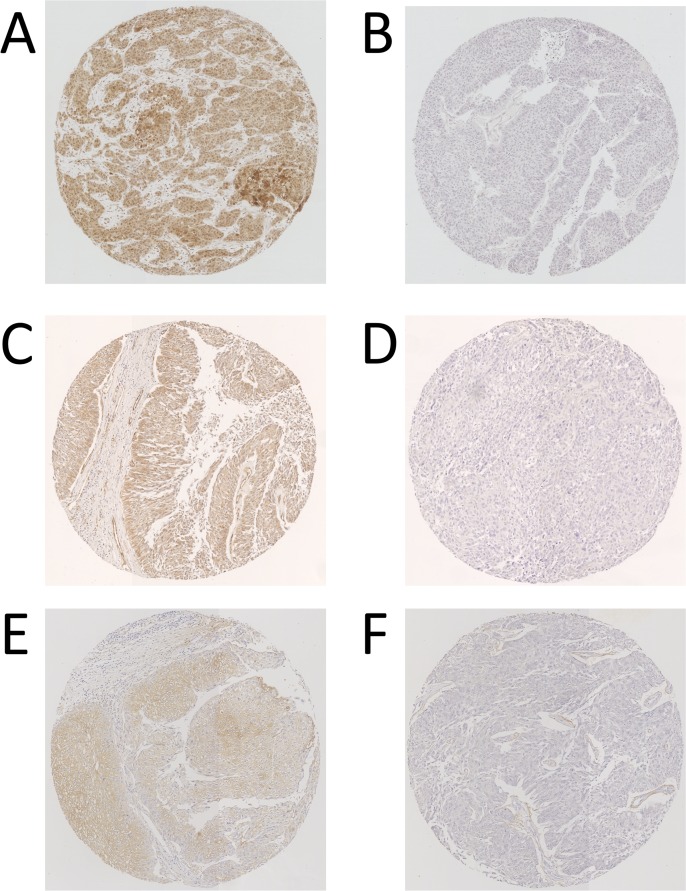
Representative patterns of immunoexpression are depicted in Fig 1. Table 1 also shows the association between the status of STAT3, S1PR1, or IL-6 expression in UTUC tissues and the clinicopathological profile. The ureteral UTUC showed significantly higher STAT3 expression (p = 0.004) than the UTUC of the renal pelvis, and men or younger-aged patients with UTUC had significantly higher IL-6 expression than women (p = 0.027) or older-aged patients with UTUC (p = 0.003). There was no significant difference in S1PR1 expression between the groups for various parameters. We analyzed the Spearman’s rank correlation coefficient of these 3 proteins expression, and it showed that S1PR1 expression weakly correlated with IL-6 expression (ρ = 0.302, p = 0.002). STAT3 expression did not correlated with both S1PR1 (ρ = 0.090, p = 0.375) and IL-6 (ρ = -0.154, p = 0.129). Because STAT3 activation has been reported to be related in chronic inflammation, we also evaluated the correlation of these markers with systemic inflammation. Neither of STAT3, S1PR1 and IL6 expression was associated with serum CRP, WBC and Neutrophil / Lymphocyte ratio (NLR) (S2 Table).
Fig 1.
Typical patterns of immunohistochemical expression of STAT3 (A, High; B, Low), S1PR1 (C, High; D, Low), and IL-6 (E, High; F, Low) in UTUC tissue.
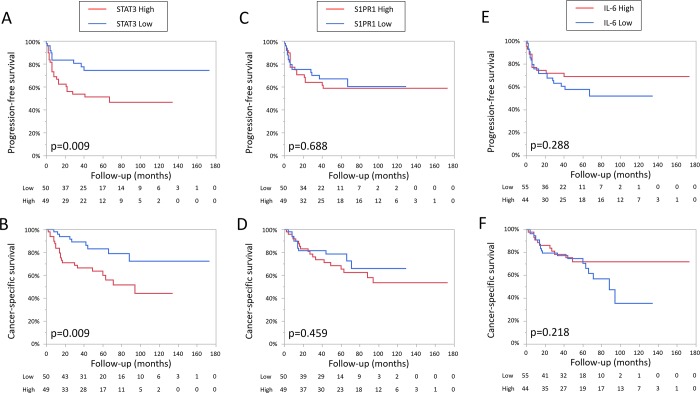
Next, we performed Kaplan-Meier analysis coupled with the log-rank test to evaluate the prognostic values of STAT3, S1PR1, and IL-6 expression in UTUC. The patients with tumors showing high STAT3 expression had a significantly higher risk of both disease progression (p = 0.009) and cancer-specific mortality (p = 0.009), but not with tumors expressing S1PR1 or IL-6 (Fig 2). In subgroup of patients with high grade tumor (n = 84), the patients with high STAT3 tumor had a significantly higher risk of both disease progression (p = 0.008) and cancer-specific mortality (p = 0.008), but not with tumors expressing high S1PR1 (p = 0.257 and p = 0.161, respectively) or high IL-6 (p = 0.395 and p = 0.250, respectively). In the subgroup of low grade tumor, there was no significant difference in prognosis regardless of the expression level of STAT3, S1PR1 and IL6.
Fig 2.
Progression-free survival and cancer-specific survival in patients with UTUC according to the expression levels of STAT3 (A, B), S1PR1 (C, D), and IL-6 (E, F).
In patients with high stage tumor (≧pT3, n = 54), the patients with high STAT3 tumor had a significantly higher risk of both disease progression (p = 0.035) and cancer-specific mortality (p = 0.012), but not with tumors expressing high S1PR1 (p = 0.193 and p = 0.159, respectively) or high IL-6 (p = 0.609 and p = 0.365, respectively). In the subgroup of advanced tumor (≥pT1), only high STAT3 tumor had a significantly higher risk of both disease progression (p = 0.002) and cancer-specific mortality (p = 0.003). In the subgroup of non-invasive tumor (pTa) subgroup there was no significant difference regardless of the expression level of STAT3, S1PR1 and IL6.
Finally, we performed univariate logistic regression analysis of variable parameters associated with patient prognosis. It showed that the STAT3 score, nuclear STAT3 score, pathological stage (≥ pT3), lymph node involvement, lymphovascular invasion, and tumor grade were associated with both progression-free survival (Table 2) and cancer-specific survival (Table 3). Multivariate analysis with the Cox proportional hazards model revealed that pathological stage (≥ pT3), lymph node involvement, and tumor grade, but not STAT3 score, were significantly associated with progression-free survival and that pathological stage (≥ pT3) and tumor grade were significantly associated with cancer-specific survival (Tables 2 and 3, multivariate model 1). We also evaluated nuclear STAT3 expression by multivariate analysis, which revealed it to be significantly associated with cancer-specific survival after adjustment for pathological stage, lymph node involvement, lymphovascular invasion, and tumor grade (hazard ratio = 2.136, 95% confidence interval = 1.009–4.767, p = 0.047, Tables 2 and 3, multivariate model 2).
Table 2. Logistic regression analysis of variables associated with progression-free survival.
| Variable | Progression-Free Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||||
| Model 1 | Model 2 | ||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| STAT3 score | 2.473 | 1.240–5.257 | 0.010 | 1.309 | 0.341–1.625 | 0.491 | |||
| Nuclear STAT3 score | 2.753 | 1.380–5.853 | 0.004 | 2.010 | 0.990–4.337 | 0.053 | |||
| age | 1.030 | 0.993–1.073 | 0.118 | ||||||
| ≧pT3 | 13.471 | 4.797–56.228 | <0.001 | 9.605 | 3.212–41.452 | <0.001 | 9.891 | 3.321–42.627 | <0.001 |
| LVI | 5.855 | 2.879–12.854 | <0.001 | 1.818 | 0.782–4.416 | 0.166 | 1.936 | 0.859–4.567 | 0.111 |
| pN stage | 5.692 | 2.585–11.619 | <0.001 | 3.439 | 1.448–7.909 | 0.006 | 3.300 | 1.396–7.535 | 0.008 |
| Tumor grade | 8.222 | 1.770–146.235 | 0.003 | 9.668 | 1.929–176.738 | 0.002 | 9.538 | 1.990–171.488 | 0.002 |
| Tumor size | 1.891 | 0.921–3.976 | 0.083 | ||||||
| Location (ureter vs pelvis) | 1.062 | 0.535–2.139 | 0.863 | ||||||
Table 3. Logistic regression analysis of variables associated with cancer-specific survival.
| Variable | Cancer-Specific Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||||
| Model 1 | Model 2 | ||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| STAT3 score | 2.725 | 1.285–6.272 | 0.008 | 1.891 | 0.863–4.491 | 0.113 | |||
| Nuclear STAT3 score | 2.186 | 1.056–4.753 | 0.035 | 2.136 | 1.009–4.767 | 0.047 | |||
| age | 1.031 | 0.990–1.077 | 0.147 | ||||||
| ≧pT3 | 36.061 | 7.683–643.242 | <0.001 | 23.382 | 4.551–428.839 | <0.001 | 23.387 | 4.618–427.152 | <0.001 |
| LVI | 6.621 | 2.981–16.726 | <0.001 | 1.893 | 0.762–5.191 | 0.173 | 2.309 | 0.949–6.217 | 0.066 |
| pN stage | 3.269 | 1.362–7.080 | 0.010 | 1.202 | 0.481–2.784 | 0.680 | 1.090 | 0.428–2.567 | 0.849 |
| Tumor grade | 7.569 | 1.614–134.996 | 0.005 | 5.865 | 1.194–106.061 | 0.025 | 8.061 | 1.691–144.539 | 0.004 |
| Tumor size | 1.366 | 0.619–3.043 | 0.437 | ||||||
| Location (ureter vs pelvis) | 1.194 | 0.574–2.531 | 0.634 | ||||||
We also performed logistic regression analysis in high grade or advanced (≥pT1) tumor subgroup (S3 and S4 Tables). In the subgroup of high grade tumor, STAT3 and nuclear STAT3 were significantly associated with both progression-free survival and cancer-specific survival in univariate analysis but not in multivariate analysis. In the subgroup of advanced (≥pT1) tumor, STAT3 and pSTAT3 were significantly associated with both progression-free survival and cancer-specific survival in univariate analysis. In multivariate analysis there was a trend toward significance in STAT3 (p = 0.090) and nuclear STAT3 (p = 0.079) for cancer-specific survival but not for progression-free survival.
Discussion
The relation between inflammation and cancer progression has been well established. STAT3 is the major inflammation-promoting transcription factor shown to play important roles in cancer progression in various types of tumors [7, 8]. Although several studies have reported STAT3 as an important factor in the development of bladder cancer [17], the relation between STAT3 and UTUC progression remains unclear. In this study, we evaluated the relation between the expression of STAT3 pathway proteins, STAT3, S1PR1, and IL-6, and the prognosis of UTUC. There were no significant differences in the expression levels of STAT3 pathway proteins between low-grade versus high-grade UTUCs or non-muscle-invasive versus muscle-invasive UTUCs. However, our study showed that high expression of STAT3 was associated with poor prognosis, indicating that STAT3 was a cancer-promoting factor in UTUC.
STAT3 is phosphorylated by upstream signals and translocates to the nucleus in which it acts as a transcription activator [8]. We also evaluated phopho-Stat3 (pSTAT3) activation. However there was no difference in prognosis between high pSTAT3 and low pSTAT3 (data not shown). This may be due to the condition of the formalin fixation. The time to the fixation could have been varied in each cases. Since phosphorylation of protein is not stable, pSTAT3 might have been affected by these conditions. We alternately evaluated STAT3 expression in the nucleus and found that the patients with high STAT3 expression in the tumor nucleus were at significantly higher risk of both disease progression (p = 0.003) and cancer-specific mortality than the other patients (p = 0.034, Fig 3).
Fig 3.
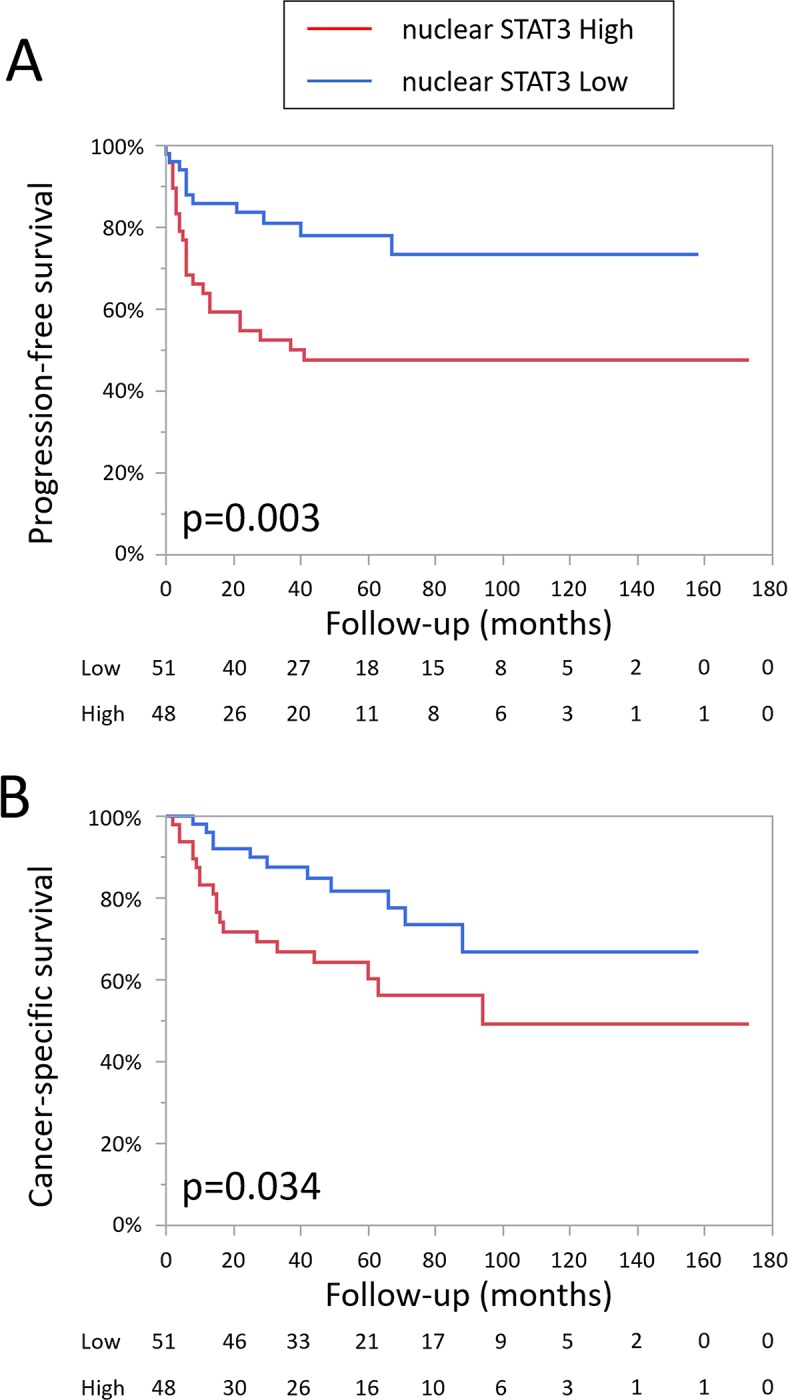
Progression-free survival (A) and cancer-specific survival (B) in patients with UTUC according to the expression level of STAT3 in the nucleus.
We also focused on two proteins, S1PR1 and IL-6, upstream of the STAT3 pathway. S1PR1 expression in non-muscle-invasive bladder cancer was reported to be associated with poor prognosis [18]. IL-6 is the most well-known traditional activator of STAT3 [10–12], and positive staining of IL-6 in bladder cancer was reported to be significantly correlated with higher clinical stage and higher recurrence rate [19]. Although our study showed no association between the expression of S1PR1 or IL-6 and UTUC progression, this might be due to differences in the epidemiologic molecular and clinical characteristics between UTUC and bladder cancer. Another reason could be that STAT3 is regulated by many other factors such as cytokines, G-protein-coupled receptors, and Toll-like receptors [8].
Because STAT3 activation by the NFkB-IL-6 signaling pathway has been reported to be one of the mechanisms of carcinogenesis in chronic inflammation, we evaluated the correlation of these markers with systemic inflammation. In result, neither of STAT3, S1PR1 and IL6 expression was correlated with serum CRP, WBC and neutrophil / lymphocyte ratio (NLR). (S1 Table). However, these markers may be correlated with cancer-related immunity (Tumor-associated macrophages or regulatory T cells, etc), and further studies will be necessary to evaluate it.
Several studies reported the prognostic factors of various tissue-based molecular markers that are related to cell adhesion (metalloproteinase-9, E-cadherin, ParvB, Snail, b-catenin), cell signaling (EGFR, EMP3, HER2, PI3K/AKT, IGFBP, mTOR), angiogenesis (hypoxia-inducible factor-1), cell proliferation (Ki67, p27,cyclin D, NF-κB, Aurora-A), cell transport (GRP78), apoptosis (bcl-2, survivin) [20], immune response (NFATc1 [21]), and transcription factor (GATA3 [22]). Our study was the first report to show STAT3 as the prognostic factor of UTUC.
Recently it has been suggested that UTUC can be divided into four molecular subtypes with distinct clinical behaviors [23]. Although STAT3 was not associated with tumor stage nor tumor grade, STAT3 expression was the significant prognostic factor. For these reasons, we speculated that STAT3 expression might be associated with some molecular subtypes of urothelial carcinoma with worse prognosis such as basal-like type.
This study has a limitation. Because we used TMA samples instead of whole tumor sections, the staining heterogeneity could affect the evaluation of the expression levels. However, several studies have shown that multiple TMA spots adequately represent the expression of an entire section in the assessment of immunohistochemical markers [24].
Conclusion
STAT3 expression was significantly associated with UTUC progression, and nuclear expression of STAT3 was the significant prognostic factor of UTUC-specific survival. Our study indicated that STAT3 could potentially be a new therapeutic target, but further studies will be necessary to fully determine its biological significance in the development and progression of UTUC.
Supporting information
(TIF)
(XLSX)
(DOCX)
Logistic regression analysis of variables associated with progression-free survival (A) and cancer-specific survival (B) in high grade tumor subgroup.
(DOCX)
Logistic regression analysis of variables associated with progression-free survival (A) and cancer-specific survival (B) in advanced (≥pT1) tumor subgroup.
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Kim HS, Jeong CW, Kwak C, Kim HH, Ku JH. Association between demographic factors and prognosis in urothelial carcinoma of the upper urinary tract: a systematic review and meta-analysis. Oncotarget 2017; 31; 8(5):7464–7476. 10.18632/oncotarget.10708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyazaki J, Nishiyama H. Epidemiology of urothelial carcinoma. Int J Urol 2017; 10.1111/iju.13376 [DOI] [PubMed] [Google Scholar]
- 3.Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973–2005. BJU Int 2011; 107:1059–1064. 10.1111/j.1464-410X.2010.09675.x [DOI] [PubMed] [Google Scholar]
- 4.Colin P, Koenig P, Ouzzane A, Berthon N, Villers A, Biserte J, Rouprêt M. Environmental factors involved in carcinogenesis of urothelial cell carcinomas of the upper urinary tract. BJU Int 2009; 104:1436–1440. 10.1111/j.1464-410X.2009.08838.x [DOI] [PubMed] [Google Scholar]
- 5.Green DA, Rink M, Xylinas E, Matin SF, Stenzl A, Roupret M, Karakiewicz PI, Scherr DS, Shariat SF. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol 2013; 189(4):1214–1221. 10.1016/j.juro.2012.05.079 [DOI] [PubMed] [Google Scholar]
- 6.Seif F, Khoshmirsafa M, Aazami H, Mohsenzadegan M, Sedighi G, Bahar M () The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun Signal 2017; 15(1):23 10.1186/s12964-017-0177-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer 2009; 9(11):798–809. 10.1038/nrc2734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu H, Lee H, Herrmann A, Buettner R, Jove R. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 2014; 14(11):736–746. 10.1038/nrc3818 [DOI] [PubMed] [Google Scholar]
- 9.Spiegel S, Milstien SSphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem 2002; 277(29):25851–25854. 10.1074/jbc.R200007200 [DOI] [PubMed] [Google Scholar]
- 10.Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, Jove R, Yu H. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med 2010; 16(12):1421–1428. 10.1038/nm.2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catlett-Falcone R, Landowski TH, Oshiro MM, Turkson J, Levitzki A, Savino R, Ciliberto G, Moscinski L, Fernández-Luna JL, Nuñez G, Dalton WS, Jove R Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity 1999; 10:105–115. [DOI] [PubMed] [Google Scholar]
- 12.Zhong Z., Wen Z. & Darnell J. E. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science 1994; 264:95–98. [DOI] [PubMed] [Google Scholar]
- 13.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signaling and its regulation. Biochem J 2003; 374:1–20. 10.1042/BJ20030407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munari E, Fujita K, Faraj S, Chaux A, Gonzalez-Roibon N, Hicks J, Meeker A, Nonomura N, Netto GJ. Dysregulation of mammalian target of rapamycin pathway in upper tract urothelial carcinoma. Hum Pathol 2013; 4(12):2668–2676. [DOI] [PubMed] [Google Scholar]
- 15.Kashiwagi E, Fujita K, Yamaguchi S, Fushimi H, Ide H, Inoue S, Mizushima T, Reis LO, Sharma R, Netto GJ, Nonomura N, Miyamoto H. Expression of steroid hormone receptors and its prognostic significance in urothelial carcinoma of the upper urinary tract. Cancer Biol Ther 2016; 17(11):1188–1196. 10.1080/15384047.2016.1235667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita K, Ujike T, Nagahara A, Uemura M, Tanigawa G, Shimazu K, Fushimi H, Yamaguchi S, Nonomura N. Endoglin expression in upper urinary tract urothelial carcinoma is associated with intravesical recurrence after radical nephroureterectomy. Int J Urol 2015; 22(5):463–467. 10.1111/iju.12719 [DOI] [PubMed] [Google Scholar]
- 17.Degoricija M, Situm M, Korać J, Miljković A, Matić K, Paradžik M, Marinović Terzić I, Jerončić A, Tomić S, Terzić J. High NF-κB and STAT3 activity in human urothelial carcinoma: a pilot study. World J Urol 2014; 32(6):1469–1475. 10.1007/s00345-014-1237-1 [DOI] [PubMed] [Google Scholar]
- 18.Go H, Kim PJ, Jeon YK, Cho YM, Kim K, Park BH, Ku JY. Sphingosine-1-phosphate receptor 1 (S1PR1) expression in non-muscle invasive urothelial carcinoma: Association with poor clinical outcome and potential therapeutic target. Eur J Cancer 2015; 51(14):1937–1945. 10.1016/j.ejca.2015.07.021 [DOI] [PubMed] [Google Scholar]
- 19.Chen MF, Lin PY, Wu CF, Chen WC, Wu CT. IL-6 expression regulates tumorigenicity and correlates with prognosis in bladder cancer. PLoS One 2016; 11(5):e0155774 10.1371/journal.pone.0155774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, Bagrodia A, Cha EK, Coleman JA. Prognostic genetic signatures in upper tract urothelial carcinoma. Curr Urol Rep 2016; 17(2):12 10.1007/s11934-015-0566-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawahara T, Inoue S, Fujita K, Mizushima T, Ide H, Yamaguchi S, Fushimi H, Nonomura N, Miyamoto H. NFATc1 expression as a prognosticator in urothelial carcinoma of the upper urinary tract. Transl Oncol 2017; 10(3):318–323. 10.1016/j.tranon.2017.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue S, Mizushima T, Fujita K, Meliti A, Ide H, Yamaguchi S, Fushimi H, Netto GJ, Nonomura N, Miyamoto H. GATA3 immunohistochemistry in urothelial carcinoma of the upper urinary tract as a urothelial marker and a prognosticator. Hum Pathol 2017; 64:83–90. 10.1016/j.humpath.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 23.Moss TJ, Qi Y, Xi L, Peng B, Kim TB, Ezzedine NE, Mosqueda ME, Guo CC, Czerniak BA, Ittmann M, Wheeler DA, Lerner SP, Matin SF. Comprehensive Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur Urol 2017; 72(4):641–649. 10.1016/j.eururo.2017.05.048 [DOI] [PubMed] [Google Scholar]
- 24.Camp RL, Neumeister V, Rimm DL. A decade of tissue microarrays: progress in the discovery and validation of cancer biomarkers. J Clin Oncol 2008; 26:5630–5637. 10.1200/JCO.2008.17.3567 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(XLSX)
(DOCX)
Logistic regression analysis of variables associated with progression-free survival (A) and cancer-specific survival (B) in high grade tumor subgroup.
(DOCX)
Logistic regression analysis of variables associated with progression-free survival (A) and cancer-specific survival (B) in advanced (≥pT1) tumor subgroup.
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.