Abstract
A copper chelator known as diacetylbis(N(4)-methylthiosemicarbazonato) copper II (CuATSM), has been reported to be efficacious in multiple transgenic SOD1 models of amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disorder affecting motor neurons. Here we report that we also observed CuATSM efficacy on disease onset and progression in a standardized litter-matched and gender-balanced efficacy study using B6SJL-SOD1G93A/1Gur mice. We also report improved survival trends with CuATSM treatment. In addition, we report a lack of efficacy by unmetallated ATSM in the same model using the same standardized study design. These results add to existing evidence supporting an efficacious role for copper delivery using chaperone molecules in mouse models of ALS.
Highlights
-
•
CuATSM administration slows disease onset and progression in high copy SOD1 mice.
-
•
Signs of CuATSM efficacy are more pronounced in male SOD1 mice than in female SOD1 mice.
-
•
Unmetallated ATSM administration reveals no detectable effects on disease progression in high copy SOD1 mice.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease characterized by dysfunction and degeneration of motor neurons in the brain and spinal cord (Rowland, 1998). Until now, only riluzole has been approved in the United States as a treatment for ALS aimed at slowing the progression of the disease. It has been shown to extend patient life by approximately 3 months (Miller et al., 2007). New and better treatments are necessary to address this unmet medical need.
ALS can be classified into two categories, sporadic ALS (SALS) and familial ALS (FALS). The causes and drivers of SALS are not clearly understood, however, in the case of FALS a diverse set of genes has been identified in which mutations dramatically increase lifetime risk of ALS (Robberecht and Philips, 2013). The first of the genes identified, and the most studied to date, is Cu/Zn superoxide dismutase (SOD1) (Rosen et al., 1993, Bunton-Stasyshyn et al., 2015). As many as 75 of the 154 amino acids making up SOD1 have been reported as mutated in ALS cases (Saccon et al., 2013). Many of these mutations have been shown to cause SOD1 to lose the metal ions required for its catalytic function (Cu and Zn) and to cause its misfolding. Recently, a small molecule known as dithiosemicarbazone, diacetylbis(N(4)-methylthiosemicarbazonato) copper II (CuATSM), has been reported to be efficacious in multiple transgenic SOD1 models of amyotrophic lateral sclerosis (8, 9, 10, 32).
Cu(II) dithiocarbazone complexes attracted initial interest in the 1960's because of their anti-tumor properties. Eventually, imaging studies of CuATSM demonstrated selective distribution to, or retention in, hypoxic tissues or tissues affected by oxidative stress. Today, CuATSM is commonly used in the clinic as a PET-imaging agent for labeling of hypoxic tissues (Vavere and Lewis, 2007; Dearling and Packard, 2010, Fujibayashi et al., 1997). Hypoxia by vascular insufficiency and oxidative stress have been implicated in ALS disease pathogenesis (Anand et al., 2013). Thus, it was not surprising that a CuATSM PET imaging study comparing 12 ALS patients with 9 age-matched healthy controls found greater accumulation of CuATSM in the motor cortex and right superior parietal lobule of ALS patients (Ikawa et al., 2015, Crouch, 2015).
The most remarkable effect of CuATSM treatment was demonstrated by Williams et al., who reported the induction of an average 18-month survival extension by CuATSM treatment in double transgenic SOD1-G93A mice co-expressing copper-chaperone-for-SOD (CCS) (Williams et al., 2016). This G93ASOD1xCCS double transgenic mouse typically presents with the most aggressive ALS-like phenotype in a rodent model, with a mean survival of 36 days (Son et al., 2007). In the work done by Williams et al., the G93ASOD1xCCS vehicle control cohort survived for an average of approximately 14 days (Williams et al., 2016). In these and other studies, CuATSM was shown to increase copper content in the SOD1-positive fraction in the spinal cords of treated mice (Roberts et al., 2014). Furthermore, like Cu(II)ATSM, Zn(II)ATSM treatment in SOD1 mice also resulted in improved survival and increased levels of copper content in the SOD1-positive fraction of the spinal cord. The authors suggest that this could be caused by in vivo transmetallation and subsequent delivery of copper to SOD1 (McAllum et al., 2015). Still, other results show efficacy in multiple animal models of Parkinson's disease suggesting that CuATSM could be efficacious even in neurodegeneration contexts that are less sensitive to SOD1 folding and metallation (Hung et al., 2012).
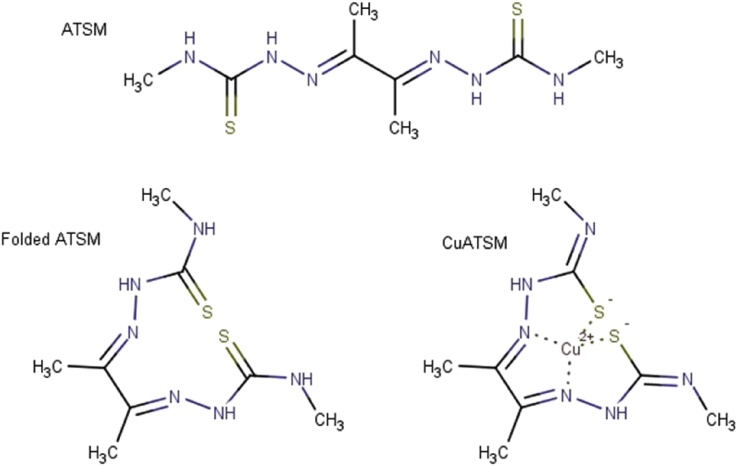
Evidence from recent studies supports the idea that ATSM, when carrying either Cu or Zn, greatly improves the symptoms of ALS in mice, presumably by safely delivering the metals in vivo and by increasing copper and zinc association with unmetallated SOD1 (Roberts et al., 2014, McAllum et al., 2015). By contrast, in a study where Zn was administered without ATSM, little benefit was shown, and disease was actually accelerated in some SOD1 mice when higher dietary Zn doses were given (Ermilova et al., 2005). This Zn dependent disease acceleration was mitigated by copper supplementation suggesting that the acceleration might have been attributable to less efficient copper absorption. Further, genetic studies aiming to shift the equilibrium of human SOD1 from immature and unmetallated SOD1 to mature and metallated SOD1 by overexpressing its copper chaperone protein also accelerated the disease in the absence of CuATSM. Together, these findings suggest that ATSM plays an important role, likely as a metal delivery molecule (See Fig. 1).
Fig. 1.
ATSM, CuATSM, and ATSM in a less likely conformation, favored only in the presence of a divalent cation.
Despite neuroprotective effects shown by CuATSM, even in neurodegeneration not mediated by mutated SOD1, the relative contribution of ATSM itself to any observed therapeutic effects has not yet been reported. In the current studies using a gender balanced and litter matched design in SOD1-G93A mice (Gurney et al., 1994), we aimed to delineate the relative contributions of ATSM and CuATSM toward observed therapeutic effects. Two parallel studies were conducted: one aiming to test a dosing regimen of 30 mg/kg/day oral CuATSM that has previously shown efficacy in B6.Cg-Tg(SOD1*G37R)29Dpr/J mice (Roberts et al., 2014), and a second testing unmetallated ATSM at 30 mg/kg/day in the same strain.
2. Materials and methods
2.1. Animals
The studies were approved by the ALSTDI Institutional Animal Care and Uses Committee (IACUC) and in accordance with the Institute for Laboratory Animal Research (ILAR) Guide for Care and Use of Laboratory animals (Guide for the Care and Us, 2010). The SOD1-G93A mouse colony was derived from the B6SJLTgN(SOD1-G93A)1Gur strain, obtained from The Jackson Laboratory (Bar Harbor, Maine) and originally produced by Gurney et al. (1994). The colony was being maintained by Biomedical Research Models, Inc. (Worcester, Massachusetts) by crossing hemizygous C57BL6-SJL sires harboring the SOD1 transgenic with non-transgenic C57BL6-SJL dams. Mice were shipped to ALSTDI at 35–45 days of age. Mice were allowed at least one week to acclimate to ALSTDI's animal facility (12-h light/dark cycle at a temperature of 18–23° C and 40–60% humidity) before being assigned to a study. In all studies described herein, male mice were singly housed while female mice were housed in pairs. Food and water were provided ad libitum. The diet used was Teklad Global diet #2918 for rodents (Harlan Laboratories, Houston, TX). Drinking water was refreshed twice weekly.
2.2. Mouse genotyping
Genotyping was performed on ear tissue samples from mice that were approximately 35 days old. 100 μL of genomic DNA was extracted from approximately 15-mg ear samples using the QIAmp Tissue DNA extraction protocol for the QIAcube HT automated liquid handler. gDNA quality and quantity was measured on a SpectraMax M5 plate reader taking readings at 260 nm and 280 nm gDNA from the ear tissue of a verified high-copy SOD1 mouse was serially diluted 2-fold to create a standard curve starting from 5 ng/uL; the concentration of the standard was verified using a SpectraMax NanoDrop. A relative quantification qPCR was used to probe for the human SOD1 gene, which is copy-number variable in the SOD1-G93A mouse model, using murine glyceraldehyde 3-phosphate dehydrogenase (Gapdh) as an endogenous control gene. The absolute concentration of the unknown samples was interpolated from the standard curve, and the resulting concentrations were used to normalize the SOD1 Ct's. The median of the resulting Ct's was assigned a relative copy number of 23; the copy numbers of the other Ct's were calculated using the fold change between each Ct and the median. Mice having fewer than 20 copies were flagged as low copy and not assigned to a study. The SOD1 primer/probe set is custom-made from Life Technologies using the following sequences: GTAAATCAGCTGTTTTCTTTGTTCAGA for the forward primer, TTCACTGGTCCATTACTTTCCTTTAA for the reverse primer, and ACTCTCTCCAACTTTG for the VIC probe. The Gapdh primer/probe set is a Life Technologies Assay-On-Demand with Assay ID # Mm00186822_cn.
2.3. Mouse survival efficacy testing
We used general survival efficacy testing methods previously described for the current studies (Scott et al., 2008). For both efficacy studies, transgene copy number was verified and mice were assigned to either drug treatment or vehicle treatment groups at 50 days of age. Groups were balanced with respect to gender (16 males, 16 females per group) and body weight within gender (mean weights at study start were within 0.3 grams for either gender between groups). Litters were evenly represented across treatment and vehicle groups within each study. Observers were blinded to treatment group identity.
Both CuATSM and ATSM were delivered as 3 mg/mL suspensions. CuATSM (American Advanced Scientific, catalog # AAS-11448) and ATSM (American Advanced Scientific, catalog # AAS-11446) were independently suspended in 0.5% Methyl Cellulose (Sigma, M0262) formulated in 0.9% Saline (Sigma, 07982) with the addition of 0.4% Tween 80 (Sigma, P8074). The drug preparations were sonicated for five minutes and mixed by inversion while in use. Formulations were produced daily.
Mice were monitored for neurological disease progression according to the protocol detailed by us in the Journal of Visualized Experiments (Hatzipetros et al., 2015). Briefly, the NeuroScore is a five point observation based scoring system. Severity of disease is rated on a scale from 0 to 4 with 0 being asymptomatic and 4 representing a moribund phenotype secondary to paralysis which manifests primarily in the hind limbs of the mice. Neurological scoring procedures and body weight measurements were completed on a bench-top in the animal holding room. End-stage mice were euthanized in a separate procedure room. Euthanasia for animals in all studies was carried out by a Preset Flow System (VetEquip) CO2 chamber. This system is designed to follow the AVMA guidelines displacement rate, 10–30% of the chamber volume per minute. End-stage was defined by an inability of the mouse to right itself within 10 s when placed its side. The observing technician was required to test the mouse by placing the animal on both sides. Failure to right itself from either side resulted in euthanasia. Kaplan-Meier curves and long-rank tests were used to analyze age at onset of paresis and survival data using GraphPad Prism 6. Using JMP v10.0.2 statistical software by SAS Institute, Inc, we generated graphs of ordinal expected NeuroScore vs. median age at Neuroscore graphs for drug-treated and vehicle-treated animals by interpolating from the y-axis the median ages at each stage of NeuroScore based disease progression.
2.4. Mouse pharmacokinetics
ATSM formulation was prepared as described above. Twenty-four mice were each treated with a single bolus of 30 mg/kg ATSM in a 10 mL/kg volume orally by gavage. Three mice were sacrificed at each timepoint: 5 min, 15 min, 30 min, 45 min, 60 min, 120 min, 240 min, and 360 min after bolus. Blood samples were harvested by cardiac puncture and collected into K+EDTA tubes. Plasma was prepared by centrifugation at 2000xg for 15 min.
For analytical chemistry standards, five microliter aliquots were prepared in 1:1 H2O:acetonitrile and added to 45 microliters of blank control plasma or blank spinal cord homogenate in a 96 deep-well plate. One hundred fifty microliters of acetonitrile containing 500 ng/mL of pyrimethamine as an internal standard and 0.1% formic acid were added to each well. The plate was agitate vigorously and next centrifuged at 500 x g for 30 min at 4° C. One hundred microliters of the supernatant from each well were pipetted into a new 96 well plate for LC-MS/MS analyses. Plasma and spinal cord samples from mice that had been treated with ATSM at the defined time points were treated similarly to the standards excepting any ATSM spiking steps.
Compartmental modeling of the data was completed using Pharsight Phoenix 64, Build 6.4.0.768, (WinNonlin 6.4). The pharmacokinetics model used was Gauss-Newton (Level and Hartley) nonlinear fitting with uniform weighting.
3. Results
3.1. CuATSM efficacy study
CuATSM efficacy against disease progression and survival endpoints was tested in the B6SJL-SOD1G93A/1Gur mouse model of ALS (SOD1 mice). 16 male and 16 female mice were assigned to receive CuATSM treatment; 16 male and 16 female mice were assigned to receive vehicle treatment. Mice within each gender in the CuATSM group were litter-matched with mice in the vehicle control group. Observers were blinded. Dosing was initiated when mice were approximately 50 days old. CuATSM-treated mice received daily oral doses of 30 mg/kg CuATSM suspended in 0.5% methylcellulose in PBS containing 0.4% Tween80, by gavage, in a volume of 10 mL/kg. Control vehicle-treated mice received daily oral doses of 0.5% methylcellulose in PBS containing 0.4% Tween80, by gavage. Of the 64 total mice that were assigned, 62 survived until they displayed end-stage ALS-like disease. Two vehicle control mice, one male and one female, died of gavage trauma and were removed from the study.
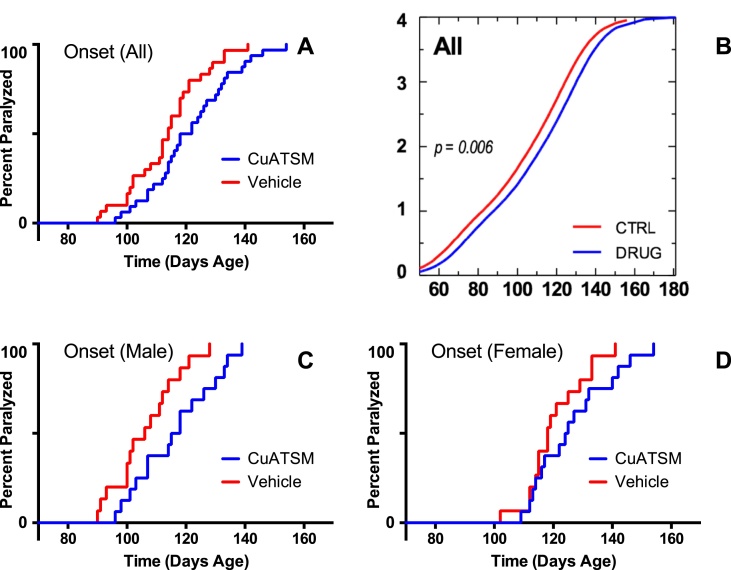
CuATSM's efficacy against ALS-related endpoints was determined by comparing performance in the CuATSM-treated group (n = 32) with performance in the vehicle-treated group (n = 30). The age at which onset of paresis occurred was delayed in Cu-ATSM-treated mice, compared to vehicle-treated mice. Overall (both genders combined), onset of paresis was delayed by 6 days in Cu-ATSM-treated mice (p = 0.01, Fig. 2a). Male mice, when analyzed separately, revealed a 10.5 day delay in onset of paresis (p < 0.01, Fig. 2C). Female mice, when analyzed separately, revealed a trend toward delay in onset of approximately 7 days (Fig. 2D), however, this effect was not statistically significant (p = 0.2, Table 1). Further, an ordinal logistic regression of NeuroScore, by median age at score (Hatzipetros et al., 2015), revealed a delay in overall neurological disease progression with a rightward shift in the CuATSM-treated cohort of approximately 7 days (p = 0.006, Fig. 2b). Thus, male mice contributed most to this overall effect.
Fig. 2.
Overall, SOD1 mice treated with CuATSM exhibited delayed onset of paresis (A), and overall neurological disease progression as measured by analysis of ordinal expected score by median age at score (B). Analyzing males separately revealed statistically significant differences (C), however, females when analyzed as a separate cohort only showed a modest trend toward delayed onset of paresis (D).
Table 1.
Summary of median age at onset results in B6SJL-SOD1G93A/1Gur mice.
| Subjects | Cohort | N | Median | Change | p valuea |
|---|---|---|---|---|---|
| All | Control | 15 | 114.0 | +6.0 | 0.01 |
| CuATSM | 16 | 120.0 | |||
| Male | Control | 15 | 106.0 | +10.5 | 0.00 |
| CuATSM | 16 | 116.5 | |||
| Female | Control | 15 | 118.0 | +6.5 | 0.20 |
| CuATSM | 16 | 124.5 |
Cox Proportional Hazard, Likelihood Ratio Test.
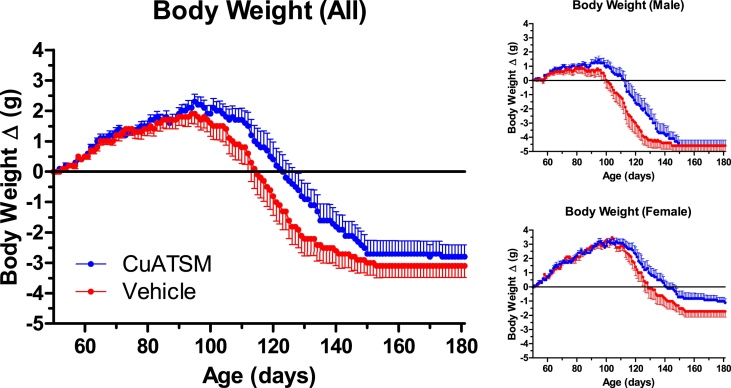
SOD1 mice assigned to this study were weighed daily. While there were no significant differences in peak body weight achieved by CuATSM-treated mice compared to vehicle control mice, the age at which CuATSM-treated male mice achieved peak body weight was delayed by 11.5 days (p = 0.02). In addition, longitudinal analysis of individual animals' body weight over time, including both male and female mice and allowing for random effects of litter, revealed that CuATSM-treated mice weighed approximately 0.7 grams more than vehicle-treated mice of the same age (p = 0.023, Fig. 3). Most of this weight increase is attributable to better weight maintenance after the mice achieved peak body weight. These results are consistent with a therapeutic mitigation of the body weight loss that normally coincides with ALS-like disease progression in SOD1 mice.
Fig. 3.
SOD1 mice in both CuATSM- and vehicle-treated cohorts gained weight after study initiation. Mice treated with CuATSM tended to maintain bodyweight better than vehicle control animals following onset of definitive disease progression.
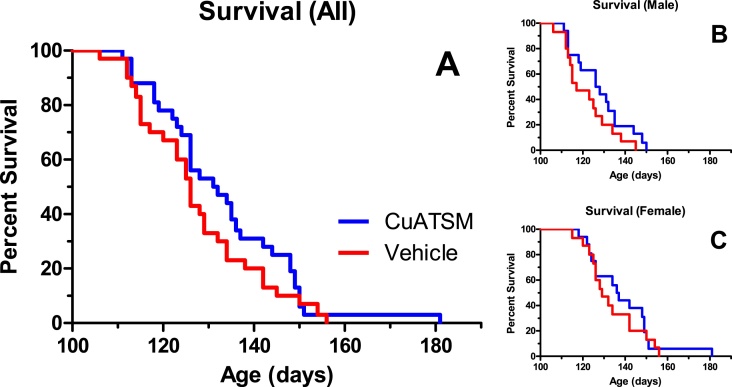
While there were trends toward extended lifespan in mice treated with CuATSM compared to vehicle-treated control mice, the effects were not statistically significant. Male CuATSM-treated mice tended to live 10 days longer than male vehicle-treated littermates (p = 0.07). Overall, median age at death was about 6 days older in CuATSM-treated mice than in vehicle-treated control mice. However, this effect was not statistically significant (p = 0.15, Fig. 4).
Fig. 4.
While there were no significant effects on survival, there was a trend toward improved survival in all mice (5.5 days, p = 0.15), with median lifespans of CuATSM-treated mice in both genders being longer than their vehicle-treated littermates.
3.2. ATSM efficacy study
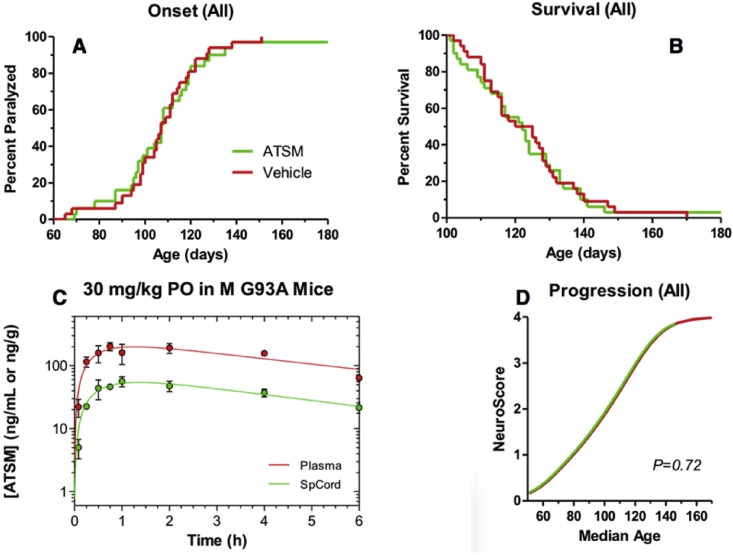
Because ATSM had not previously been tested in animal models of ALS, we sought to better understand its single-dose pharmacokinetics and spinal cord distribution. We learned that ATSM was both orally absorbed and did distribute to the spinal cord in SOD1 mice (spinal cord AUC about 30% of plasma AUC, Fig. 5C). ATSM was then tested for its efficacy against disease progression and survival endpoints in the B6SJL-SOD1G93A/1Gur mouse model of ALS (SOD1 mice) using the same study design that was applied to CuATSM, but substituting ATSM treatment for CuATSM treatment. Thirty-two mice were assigned to be treated with 30 mg/kg/day ATSM orally with drug suspended in the same vehicle used for the CuATSM study. Thirty-two mice were also assigned to a vehicle only cohort. The cohorts were litter matched and gender balanced. One male ATSM-treated mouse died at 63 days of age due to gavage trauma. One female ATSM-treated mouse was right-censored in onset and survival time-to-event analyses because neither endpoint was reached by the time of study termination at 180 days of age. There was no evidence based on multiple genotyping assays that this mouse had a reduced transgene copy number.
Fig. 5.
ATSM treatment did not delay onset (A), improve survival (B), or affect overall neurological disease progression (D), despite attaining meaningful plasma and spinal cord exposure levels (C).
The remaining 30 ATSM treated mice and 32 vehicle treated mice were analyzed for efficacy against ALS related endpoints. Overall, ATSM-treated mice exhibited no change in the time of onset of paresis compared to vehicle-treated mice (Fig. 5A). Male mice tended to have earlier onset of paresis, but this trend was not statistically significant (p = 0.22). Unlike CuATSM-treated mice, ATSM-treated mice showed a pattern of overall disease progression, evident in ordinal logistic regression of NeuroScore vs. median age at score (Hatzipetros et al., 2015) that was virtually superimposable on the progression shown by the litter-matched vehicle control cohort (Fig. 5D). In addition, there were no statistically significant differences in either peak body weight or the age at which peak body weight was attained when ATSM-treated mice were compared to vehicle control mice. Finally, unlike CuATSM treatment, ATSM treatment showed no trend toward prolonging the lifespan of SOD1 mice. Median age at death was virtually equivalent in ATSM-treated mice and vehicle-treated control mice (Fig. 5B).
4. Discussion
Previous reports showed that CuATSM is efficacious in various transgenic mouse models of mutant human SOD1 mediated familial ALS ((Soon et al., 2011, McAllum et al., 2013, Roberts et al., 2014, Hilton et al., 2017); Table 2). These mouse strains have included B6.Cg-Tg(SOD1*G37R)29Dpr/J, B6SJL-Tg(SOD1*G93A)1Gur, [B6SJL-Tg(SOD1*G93A)dl1Gur x B6SJL-Tg(Prnp-CCS)17Jlel/J] double transgenics, and B6.Cg-Tn(SOD1*G93A)dl1Gur/J mice. The strains have varied severities of disease (McGoldrick et al., 2013). The CCSxSOD1 double transgenic mice manifest copper-specific mechanisms of disease susceptibility and thus possess a higher potential to exhibit favorable phenotypic changes when treated with copper-targeting therapeutics. Further, the dosage, frequency of treatment, and age at treatment initiation all influence the potential for efficacy against survival endpoints. While the current study did not reveal any statistically significant differences in the survival endpoint, it must be considered that the study was conducted in the most aggressive high copy transgenic mouse model of ALS, a model not responsive to potentiation by overexpression of human Copper-Chaperone-for-SOD. Additionally, of the published efficacious CuATSM dosing regimens, we used the one producing the lowest CuATSM exposure. In the current study, we tested the drug in an aggressive mouse model, without the mechanistic synergy of CCS transgenic expression, and with a lower drug exposure. Thus, it is not surprising that we only observed a trend toward improved survival for our CuATSM treatment regimen.
Table 2.
Summary of published survival results using CuATSM therapeutically in SOD1 mouse models of ALS.
| Mouse Model | CuATSM Dosing Regimen | Median Age at Death |
||||
|---|---|---|---|---|---|---|
| Vehicle Control | Treatment | % Life Extension | p value | Reference | ||
| Published Results | ||||||
| B6SJLTg(SOD1*G93A)dl1Gur x B6SJL-Tg(Prnp-CCS)17Jlel/J | 30 mg/kg 2x daily prenatally to mothers, then starting at p5 | 14 days | 660 days | >4000% | <0.0001 | Williams et al., 2016 |
| B6SJL-Tg(SOD1*G93A)1Gur | 100 mg/kg 2x daily starting at p50 | 133 days | 155 days | 16.5% | <0.0001 | Williams et al., 2016 |
| B6.Cg-Tg(SOD1*G37R)29Dpr/J | 30 mg/kg/day starting at p40 | ≈180 days based on publication figure | ≈210 days based on publication figure | ≈17%, though reports 12% | 0.006 | Roberts et al., 2014 |
| B6SJLTg(SOD1*G93A)dl1Gur | 30 mg/kg/day starting at p140 | 263 days | 300 days | 14% | 0.001 | Soon et al., 2011 |
| B6SJL-Tg(SOD1*G93A)1Gur | 100 mg/kg/day starting at p50 | 130 days | 141 days | 8% | <0.0001 | Hilton et al., 2017 |
| Current Report | ||||||
| B6SJL-Tg(SOD1*G93A)1Gur | 30 mg/kg/day starting at p50 | 125 | 132 | 5% | 0.15 | Current report |
| B6SJL-Tg(SOD1*G93A)1Gur (male only) | 30 mg/kg/day starting at p50 | 117 | 127 | 9% | 0.07 | Current report |
| B6SJL-Tg(SOD1*G93A)1Gur (female only) | 30 mg/kg/day starting at p50 | 129 | 137 | 6% | 0.60 | Current report |
Despite the muted survival endpoint results, efficacy by CuATSM was clearly revealed by neurological disease progression indicators captured using the NeuroScore system. Overall, NeuroScore analysis indicated a statistically significant 7 day right shift (slowing) in disease progression and “time-to-event” analysis showed a statistically significant delay in onset of paresis. These results support further preclinical and clinical evaluation of CuATSM in ALS.
In contrast, our evaluation of unmetallated ATSM, prepared without copper or any other cation, revealed no signs of efficacy. The study, whose design and execution were informed by a report of ZnATSM efficacy (McAllum et al., 2015), was undertaken to shed light on whether ATSM alone could be protective independently of its function as a cation-carrier. We note that by delivering empty ATSM, we were not precluding the use of the drug as a cation-carrier. It is possible that empty ATSM acted as a chelator of various cations available in vivo in circulation or in tissue. It is also plausible that any cations captured by ATSM would be subsequently redistributed to other tissue compartments in the mice treated. Despite these considerations, it is clear that these plausible actions netted neither measurable improvement nor worsening of disease in the SOD1 mouse model of ALS.
Taken together, these results support the idea that CuATSM is therapeutic in several mouse models of neurodegeneration and that the efficacy is copper or zinc dependent, possibly more specifically copper dependent. While some data support the idea that CuATSM acts in SOD1 mouse models of ALS by potentiating appropriate metallation of SOD1, it has also been proposed that CuATSM could be efficacious in neurodegeneration by SOD1-independent mechanisms including scavenging of peroxynitrite (Hung et al., 2012, Williams et al., 2016). Peroxynitrite is a source of 3-nitrotyrosine (3NT), a marker that is significantly increased in spinal cords of SOD1 mice (Ferrante et al., 1997) and in both sporadic and familial human ALS cases (Beal et al., 1997). This marker of oxidative damage was recently used in clinical trials of edaravone that ultimately led to the drug's approval as an ALS treatment in Japan and South Korea (Abe et al., 2014). This putative mechanism of action highlights the possibility that CuATSM could be efficacious in sporadic ALS or other neurodegenerative conditions like Parkinson's disease by way of peroxynitrite scavenging (Hung et al., 2012, Ikawa et al., 2015, Abe et al., 2014).
Overall, it is encouraging that our independent laboratories at the ALS Therapy Development Institute replicated the finding of CuATSM efficacy in an aggressive mouse model of ALS. Previously published reports describing efficacy of a variety of potential therapeutics in SOD1 mice have not been repeatable in our labs using rigorously controlled testing methods, despite more than 15 years of testing (Scott et al., 2008, Vieira et al., 2014, Vieira et al., 2015, Gill et al., 2009). Thus, CuATSM treatment is an interesting and important exception. A Phase I clinical trial of CuATSM in people with ALS is enrolling and underway in Australia. We look forward to further preclinical and clinical investigation with CuATSM and molecules with similar properties.
Acknowledgements
First, we would like to acknowledge and thank Matt Ferola and Carlos Maya for their diligent and compassionate care of the animals involved in these studies. They provide a clean and safe environment for the mice which contributes to an atmosphere that respects the contributions of the animals and produces the most interpretable results. Next, we would like to thank all of our many supporters who provided funding for these not-for-profit studies. Many of the supporters are people living with ALS, their caretakers, immediate and extended family members, and their friends. Without their generous support these studies would not have been possible. Specifically, we would like to thank Augie's Quest and Corey's Crusade for inspiring and funding these studies.
Contributor Information
Fernando G. Vieira, Email: fvieira@als.net.
Theo Hatzipetros, Email: thatzipetros@als.net.
Kenneth Thompson, Email: ktompson@als.net.
Andy J. Moreno, Email: amoreno@als.net.
Joshua D. Kidd, Email: jkidd@als.net.
Valerie R. Tassinari, Email: vtassinari@als.net.
Beth Levine, Email: blevine@als.net.
Steven Perrin, Email: sperrin@als.net.
Alan Gill, Email: agill@als.net.
References
- Abe K., Itoyama Y., Sobue G., Tsuji S., Aoki M. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. Front. Degener. 2014;15(7–8):610–617. doi: 10.3109/21678421.2014.959024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Thakur K., Gupta P.K. ALS and oxidative stress: the neurovascular scenario. Oxid. Med. Cell Longev. 2013;2013:635831. doi: 10.1155/2013/635831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal M.F., Ferrante R.J., Browne S.E., Matthews R.T. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. 1997;442:644–654. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- Bunton-Stasyshyn R.K., Saccon R.A., Fratta P., Fisher E.M. SOD1 function and its implications for amyotrophic lateral sclerosis pathology: new and renascent themes. Neuroscientist. 2015;21(5):519–529. doi: 10.1177/1073858414561795. [DOI] [PubMed] [Google Scholar]
- Crouch P.J. Comment: Cu-ATSM to treat and image amyotrophic lateral sclerosis. Neurology. 2015;84(20):2038. doi: 10.1212/WNL.0000000000001600. [DOI] [PubMed] [Google Scholar]
- Dearling J.L.J., Packard A.B. Some thoughts on the mechanism of cellular trapping of Cu(II)-ATSM. Nucl. Med. Biol. 2010;37(3):237–243. doi: 10.1016/j.nucmedbio.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Ermilova I.P., Ermilov V.B., Levy M., Ho E., Pereira C. Protection by dietary zinc in ALS mutant G93A SOD transgenic mice. Neurosci. Lett. 2005;379(1):42–46. doi: 10.1016/j.neulet.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Ferrante R.J., Shinobu L.A., Schulz J.B., Matthews R.T., Thomas C.E. Increased 3nitrotyrosine and oxidative damage in mice with human Cu/Zn superoxide dismutase mutation. Ann. Neurol. 1997;42:326–334. doi: 10.1002/ana.410420309. [DOI] [PubMed] [Google Scholar]
- Fujibayashi Y., Taniuchi H., Yonekura Y., Ohtani H., Konishi J. Copper-62-ATSM: a new hypoxia imaging agent with high membrane permeability and low redox potential. J. Nucl. Med. 1997;38:1155–1160. [PubMed] [Google Scholar]
- Gill A., Kidd J., Vieira F., Thompson K., Perrin S. No benefit from chronic lithium dosing in a sibling-matched, gender balanced, investigator-blinded tiral using a standard mouse model of familial ALS. PLoS One. 2009;4(8):e6489. doi: 10.1371/journal.pone.0006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guide for the Care and Use of Laboratory Animals. eighth ed. Institute for Laboratory Animal Research (U.S.)-National (U.S.) – National Academies Press; 2010. [Google Scholar]
- Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1774. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Hatzipetros T., Kidd J.D., Moreno A.J., Thompson K., Gill A. A quick phenotypic neurological scoring system for evaluating disease progression in the SOD1-G93A mouse model of ALS. J. Vis. Exp. 2015;104:e53257. doi: 10.3791/53257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton J.B., Mercer S.W., Nastasia K.H.L., Faux N.G., Buncic G. CuII(atsm) improves neurological phenotype and survival of SOD1G93A mice and selectively increases enzymatically active SOD1 in the spinal cord. Sci. Rep. 2017;7:42292. doi: 10.1038/srep42292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung L.W., Villemagne V.L., Cheng L., Sherratt N.A., Ayton S. The hypoxia imaging agent CuII(atsm) is neuroprotective and improves motor and cognitive functions in multiple animal models of Parkinson's disease. J. Exp. Med. 2012;209(4):837–854. doi: 10.1084/jem.20112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa M., Okazawa H., Tsujikawa T., Matsunaga A., Yamamura O. Increased oxidative stress is related to disease severity in the ALS motor cortex. Neurology. 2015;84(20):2033–2039. doi: 10.1212/WNL.0000000000001588. [DOI] [PubMed] [Google Scholar]
- McAllum E.J., Lim N.K., Hickey J.L., Paterson B.M., Donnelly P.S. Therapeutic effects of CuII(atsm) in the SOD1-G37R mouse model of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2013;14(7–8):586–590. doi: 10.3109/21678421.2013.824000. [DOI] [PubMed] [Google Scholar]
- McAllum E.J., Roberts B.R., Hickey J.L., Dang T.N., Grubman A. ZnII(atsm) is protective in amyotrophic lateral sclerosis model mice via a copper delivery mechanism. Neurobiol. Dis. 2015;81:20–24. doi: 10.1016/j.nbd.2015.02.023. [DOI] [PubMed] [Google Scholar]
- McGoldrick P., Joyce P.I., Fisher E.M., Greensmith L. Rodent models of amyotrophic lateral sclerosis. Biochim. Biophys. Acta. 2013;1832(9):1421–1436. doi: 10.1016/j.bbadis.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Miller R.G., Mitchel J.D., Lyon M., Moore D.H. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst. Rev. 2007;24(1):CD001447. doi: 10.1002/14651858.CD001447.pub2. [DOI] [PubMed] [Google Scholar]
- Robberecht W., Philips T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013;14(4):248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- Roberts B.R., Lim N.K., McAllum E.J., Donnelly P.S., Hare D.J. Oral treatment with Cu(II)(atsm) increase mutant SOD1 in vivo but protects motor neurons and improves the phenotype of a transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2014;34(23):8021–8031. doi: 10.1523/JNEUROSCI.4196-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rowland L.P. Diagnosis of amyotrophic lateral sclerosis. J. Neurol. Sci. 1998;160(Suppl. 1):S6–S24. doi: 10.1016/s0022-510x(98)00193-2. [DOI] [PubMed] [Google Scholar]
- Saccon R.A., Bunton-Stasyshyn R.K., Fisher E.M., Fratta P. Is SOD1 loss of function involved in amyotrophic lateral sclerosis? Brain. 2013;136(Pt 8):2342–2358. doi: 10.1093/brain/awt097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott S., Kranz J.E., Cole J., Lincecum J.M., Kelly N. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph. Lateral Scler. 2008;9(1):4–15. doi: 10.1080/17482960701856300. [DOI] [PubMed] [Google Scholar]
- Son M., Puttaparthi K., Kawamata H., Rajendran B., Boyer P.J. Overexpression of CCS in G93A-SOD1 mice leads to accelerated neurological deficits with severe mitochondrial pathology. Proc. Natl. Acad. Sci. 2007;104(14):6072–6077. doi: 10.1073/pnas.0610923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soon C.P., Donnelly P.S., Turner B.J., Hung L.W., Crouch P.J. Diacetylbis(N(4)-methythiosemicarbazonato) copper(II) (CuII(atsm)) protects against peroxynitrite induced nitrosative damage and prolongs survival in amyotrophic lateral sclerosis mouse model. J. Neurosci. 2011;286(51):44035–44044. doi: 10.1074/jbc.M111.274407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavere A.L., Lewis J.S. Cu-ATSM: a radiopharmaceutical for the PET imagine of hypoxia. Dalton Trans. 2007;43:4893–4902. doi: 10.1039/b705989b. [DOI] [PubMed] [Google Scholar]
- Vieira F.G., LaDow E., Moreno A., Kidd J.D., Levine B. Dexpramipexole is ineffective in two models of ALS related neurodegeneration. PLoS One. 2014;9(12):e91608. doi: 10.1371/journal.pone.0091608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira F.G., Ping Q., Moreno A.J., Kidd J.D., Thompson K. Guanabenz treatment accelerates disease in a mutant SOD1 mouse model of ALS. PLoS One. 2015;10(8):e0135570. doi: 10.1371/journal.pone.0135570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.R., Trias E., Beilby P.R., Lopez N.I., Labut E.M. Copper delivery to the CNS by Cu-ATSM effectively treats motor neuron disease in SOD1-G93A mice co-expressing the Copper-Chaperone-for-SOD. Neurobiol. Dis. 2016;89:1–9. doi: 10.1016/j.nbd.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]