Highlights
-
•
In mice, WFA-positive PNNs express aggrecan in selective brain regions.
-
•
Appearance and glycosylation of aggrecan-positive PNNs is brain-region specific.
-
•
Density of AB1031-, Cat-315-, and Cat-316-positive PNNs is brain-region specific.
-
•
Localization of WFA-, AB1031-, Cat-315-, and Cat-316-positive molecules differ in PNNs.
Abbreviations: FrA, frontal association cortex; DLO, dorsolateral orbital cortex; LO, lateral orbital cortex; VO, ventral orbital cortex; Cg, cingulate cortex; PL, prelimbic cortex; IL, infralimbic cortex; DP, dorsal peduncular cortex; M1, primary motor cortex; M2, secondary motor cortex; MPtA, medial parietal association cortex; LPtA, lateral parietal association cortex; S1Tr, primary somatosensory cortex–trunk region; S1BF, primary somatosensory cortex–barrel field; S2, secondary somatosensory cortex; V2MM, secondary visual cortex–mediomedial area; V2ML, secondary visual cortex mediolateral area; V1M, primary visual cortex monocular area; V1B, primary visual cortex binocular area; V2L, secondary visual cortex lateral area; Au1, primary auditory cortex; AuD, secondary auditory cortex dorsal area; AuV, secondary auditory cortex ventral area; TeA, temporal association cortex; Ect, ectorhinal cortex; PRh, perirhinal cortex; DIEnt, dorsintermed entorhinal cortex; DLEnt, dorsolateral entorhinal cortex; RSD, retrosplenial dysgranular cortex; RSGc, retrosplenial granular cortex c region; RSGb, retrosplenial granular cortex b region; RSGa, retrosplenial granular cortex a region
Keywords: Aggrecan, Brain region-specific, Chondroitin sulfate proteoglycan, Extracellular matrix, Perineuronal nets, Plasticity
Abstract
Specific regions of the cerebral cortex are highly plastic in an organism’s lifetime. It is thought that perineuronal nets (PNNs) regulate plasticity, but labeling for Wisteria floribunda agglutinin (WFA), which is widely used to detect PNNs, is observed throughout the cortex. The aggrecan molecule—a PNN component—may regulate plasticity, and may also be involved in determining region-specific vulnerability to stress. To clarify cortical region-specific plasticity and vulnerability, we qualitatively analyzed aggrecan-positive and glycosylated aggrecan-positive PNNs in the mature mouse cerebral cortex. Our findings revealed the selective expression of both aggrecan-positive and glycosylated aggrecan-positive PNNs in the cortex. WFA-positive PNNs expressed aggrecan in a region-specific manner in the cortex. Furthermore, we observed variable distributions of PNNs containing WFA- and aggrecan-positive molecules. Together, our findings suggest that PNN components and their function differ depending on the cortical region, and that aggrecan molecules may be involved in determining region-specific plasticity and vulnerability in the cortex.
1. Introduction
To preserve region-specific functions, certain areas of the cortex are associated with high and low plasticity over the course of development (Craig and Commins, 2006; Kolb, 2009). One molecule that is crucial in maintaining cortical plasticity is the perineuronal net (PNN), a highly condensed extracellular matrix (ECM) molecule in the central nervous system (Sorg et al., 2016). The PNN is a mesh-like structure that surrounds the cell body, proximal dendrites, and axonal initial segment of specific neurons. Approximately 15% of neurons in the mature brain are surrounded by PNNs (Guimarães et al., 1990; McRae et al., 2007), most of which are parvalbumin (PV)-positive GABAergic interneurons, and a small portion of which are pyramidal cells (Härtig et al., 1994; Wegner et al., 2003).
Maturation of GABAergic circuity in the sensory cortex implies the onset of a critical period that is associated with cortical plasticity (Hensch and Fagiolini, 2005; Maffei and Turrigiano, 2008). The formation of PNNs around PV-positive interneurons in the sensory cortex indicates the end of this so-called critical period (Pizzorusso et al., 2002; McRae et al., 2007). In the visual cortex of the mature brain, it has been shown that treatment of PNNs with the enzyme, chondroitinase ABC, can restore plasticity (Pizzorusso et al., 2002). Similar mechanisms have been described for sensory input-dependent plasticity in other brain regions (Balmer et al., 2009; Gogolla et al., 2009).
Although the detailed function of PNNs is not clear, it is thought that their main roles involve neural plasticity, synaptic stability, and neuroprotective function (Sorg et al., 2016). The main constituents of PNNs include hyaluronic acid, tenascin-R, and the lectican family of chondroitin sulfate proteoglycans (CSPGs) (i.e., aggrecan, versican, brevican, and neurocan) (Bandtlow and Zimmermann, 2000; Yamaguchi, 2000). The plant-derived lectin, Wisteria floribunda agglutinin (WFA), has been widely used to detect PNNs through binding of N-acetylgalactosamine (Brückner et al., 1993; Schweizer et al., 1993; Seeger et al., 1994; Giamanco et al., 2010). Another method used to detect PNNs is through antibodies against aggrecan, the main PNN component (Matthews et al., 2002). These antibodies include AB1031 and Cat-315, with the former recognizing the central protein domain of the chondroitin sulfate glycosaminoglycan binding region of aggrecan (Giamanco et al., 2010; Lendvai et al., 2013), and the latter recognizing the HNK-1 carbohydrate epitope of aggrecan (Matthews et al., 2002; Dino et al., 2006; McRae et al., 2007).
While there is general agreement that PNNs regulate plasticity, WFA-labeled PNNs have been found throughout the cortex of the mature mouse (Brückner et al., 2000; Horii-Hayashi et al., 2015). Moreover, WFA-positive PNN labeling does not appear to vary over development (Horii-Hayashi et al., 2015). Considering that the plasticity of specific brain regions is highly variable over the span of an organism’s lifetime, it is unlikely that WFA-positive PNNs control plasticity. Some studies have suggested the possibility that aggrecan molecules regulate plasticity as, during postnatal development, aggrecan expression is delayed when sensory input is deprived (McRae et al., 2007; Ye and Miao, 2013; Ueno et al., 2017b). Furthermore, when mice are housed in enriched environments after experimental cerebral ischemia, Cat-315-positive PNNs decrease (Madinier et al., 2014). In fact, it has been suggested that aggrecan molecules are not ubiquitously expressed throughout the cortex (Morawski et al., 2012a, Morawski et al., 2012b; Ueno et al., 2017a). However, a quantitative analysis of aggrecan-positive PNNs in the cortex has not been conducted.
Along with their possible role in developmental plasticity, it has been suggested that aggrecan molecules are necessary for mediating the neuroprotective function of PNNs (Suttkus et al., 2014). It is well-established that certain brain regions are more susceptible to damage in neuropsychiatric disorders and neurodegenerative diseases. Interestingly, postmortem studies of patients with schizophrenia and autism show selective PNN abnormalities in the prefrontal and entorhinal cortices (Pantazopoulos et al., 2010; Mauney et al., 2013; Berretta et al., 2015). One theory explaining the cause of neuropsychiatric disorders is oxidative stress, which aggrecan-positive PNNs show resistance to (Gawryluk et al., 2011; Cabungcal et al., 2013; Suttkus et al., 2014). Indeed, in Alzheimer's disease, aggrecan-expressing PNNs are less susceptible to tau protein-induced damage (Morawski et al., 2010). It is therefore possible that aggrecan molecules are involved in neurological disorders, which target specific brain regions.
In this study, we focused on the quantitative measurement of aggrecan-positive PNNs and glycosylated aggrecan-positive PNNs in the mature mouse cortex. We examined the region-specific presence of aggrecan using three antibodies (i.e., AB1031, Cat-315, and Cat-316) that recognize different components of the aggrecan molecule (McRae et al., 2007, McRae et al., 2010; Foster et al., 2014; Madinier et al., 2014; Suttkus et al., 2014; Carstens et al., 2016; Morikawa et al., 2017). Note that Cat-316 recognizes the o-linked chondroitin sulfate epitope of aggrecan (Lander et al., 1997; Matthews et al., 2002). We believe that our findings will contribute to clarifying the state of region-selective vulnerability and plasticity in the cortex of individuals with neuropsychiatric disorders.
2. Materials and methods
2.1. Animals
Five adult male mice (C57BL/6J) were used for these experiments. Animals were purchased from Charles River Laboratories (Kanagawa, Japan), and housed in cages (3–5 animals per cage) with food and water available ad libitum under a 12 h light/dark cycle at 23–26 °C. All efforts were made to minimize the number of animals used and their suffering. All experimental protocols were performed in accordance with the U.S. National Institute of Health (NIH) Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised in 1996), and were approved by the Committee for Animal Experiments at Kawasaki Medical School Advanced Research Center and the Institutional Animal Care and Use Committee of University of Toyama.
2.2. Tissue preparation
For tissue preparation, animals were deeply anesthetized with a lethal dose of sodium pentobarbital (120 mg/kg, i.p.), and transcardially perfused, first with ice-cold phosphate buffered saline (PBS) for 2 min and then with 4% paraformaldehyde in PBS (pH 7.4) for 10 min (10 ml/min). Brains were dissected and postfixed overnight with 4% paraformaldehyde in PBS at 4 °C, and cryoprotected by immersion in 15% sucrose for 12 h followed by 30% sucrose for 20 h at 4 °C. Brains were frozen in O.C.T. Compound (Tissue-Tek; Sakuma Finetek, Tokyo, Japan) using dry ice-cold normal hexane, and serial coronal sections of 40-μm thickness were prepared using a cryostat (CM3050S; Leica Wetzlar, Germany) at −20 °C. Sections were collected in ice-cold PBS containing 0.05% sodium azide.
2.3. Immunohistochemistry
Cryostat sections were treated with 0.1% Triton X-100 in PBS at 20 °C for 15 min. After three washes in PBS, sections were incubated with 10% normal goat serum (ImmunoBioScience Corp, WA, USA) in PBS at room temperature for 1 h, washed three time in PBS, and incubated overnight at 4 °C in PBS containing biotinylated WFA (B-1355, Vector Laboratories, Funakoshi Co., Tokyo, Japan; 1:200) and primary antibodies (described below). After washing in PBS, sections were incubated with corresponding secondary antibodies (indicated below) and streptavidin-conjugated Texas Red (SA-5006, Vector Laboratories) at room temperature for 2 h. Labeled sections were rinsed again with PBS and mounted on glass slides with Vectashield medium (H-1400, Vector Laboratories). Prepared slides were either immediately imaged or stored at 4 °C.
2.4. Antibodies
The following primary antibodies were used for staining: rabbit anti-aggrecan (AB1031, Millipore, Tokyo, Japan; 1:200), mouse anti-aggrecan (Cat-315; MAB1581, Millipore, 1:1000), or mouse anti-aggrecan (Cat-316; MAB1582, Millipore, 1:10 000). The following secondary antibodies were used for visualization: Alexa Fluor 488-conjugated goat anti-rabbit IgG (ab150077, Abcam; Cambride, MA; 1:1000) or FITC-conjugated anti-mouse IgM (sc-2082, Santa Cruz, Texas, USA, 1:1000).
2.5. Microscopy imaging
For the quantification of WFA-, aggrecan-, Cat-315-, and Cat-316-positive PNNs, sections were stained as described previously and imaged using confocal microscopy (LSM700; Carl Zeiss, Oberkochen, Germany). Images (1024 × 1024 pixel) were saved as TIFF files with ZEN software (Carl Zeiss). Briefly, low magnification analysis was performed using a 10 × objective lens, and a pinhole setting corresponding to a focal plane thickness of less than 1 μm. For observing PNN morphology, samples were randomly selected, and high-magnification images using a 100× objective lens were acquired. Images from whole sections were obtained using a 10× objective lens on a fluorescence microscope (BZ-X, KEYENCE, Tokyo, Japan), and merged using the KEYENCE BZ-X Analyzer software (KEYENCE).
2.6. Quantification of labeled PNNs
Brain regions were determined in accordance with the mouse brain atlas of Paxinos and Franklin (2012). From each mouse, 12 coronal sections were selected from the intermediate frontal, intermediate parietal, and rostral occipital cortices, and processed for staining. All confocal images were acquired as TIFF files, and analyzed with the NIH ImageJ software (NIH, Bethesda, MD, USA; http://rsb.info.nih.gov/nih-image/). The number of PNNs was quantified from at least three sections per region. Stained PNNs (soma size above 60 μm2) were manually tagged and counted within the area of interest, and PNN density was calculated as cells/mm2. Slides were coded and quantified by a blinded independent observer.
2.7. Data analysis
Data are expressed as the mean ± SEM of five animals. Statistical analyses were carried out using SPSS Statistics (IBM Corp., Armonk, NY, USA). Statistical significance was determined using a one-way analysis of variance (ANOVA) with post hoc tests, and statistical significance was set at *p < .05.
3. Results
3.1. WFA-, AB1031-, Cat-315-, and Cat-316-positive PNNs in the mouse cerebral cortex
To examine PNN composition in the mouse cerebral cortex, we labeled PNNs with WFA lectin, as well as the following anti-aggrecan antibodies: AB1031, Cat-315, and Cat-316 (Fig. 1, Fig. 2, Fig. 3). Overall, we observed more WFA-positive (WFA+) PNNs in the mouse cerebral cortex than PNNs positive for AB1031, Cat-315, and Cat-316. While WFA+ PNNs appeared in many cortical areas, the expression of AB1031-, Cat-315-, and Cat-316-positive PNNs was area-specific. In general, we observed a greater number of labeled PNNs in the primary sensory cortices than in the association cortices and, as noted, the distribution of labeled PNNs was not homogenous across different areas or layers (Fig. 1, Fig. 2, Fig. 3, Fig. 4). More specifically, a laminar pattern of labeling was observed in many cortical areas, with the most prominent and conspicuous labeling occurring in the mid-cortical layers and in layer I, respectively. Taken together, these histochemical analyses revealed that the spatial expression of PNN components was region-specific.
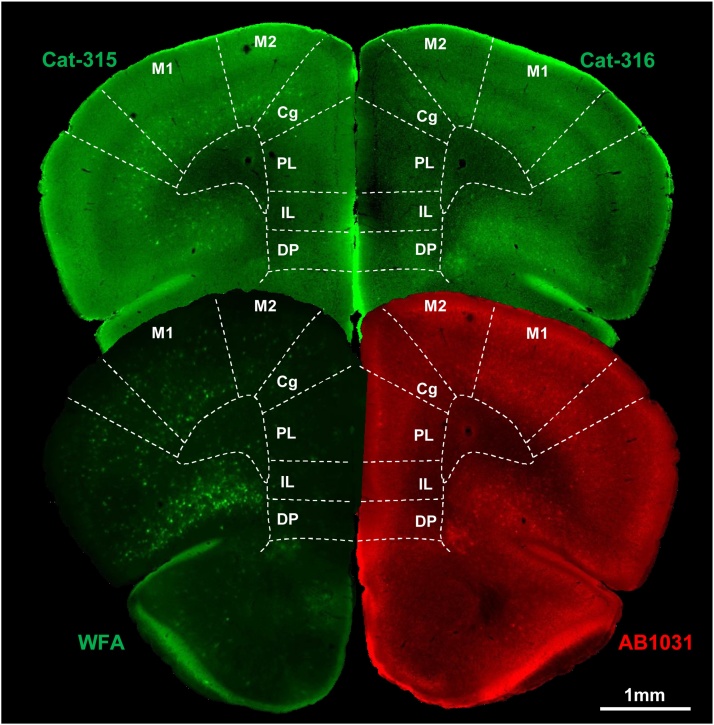
Fig. 1.
Distribution of WFA-, AB1031-, Cat-315-, and Cat-316-positive PNNs in the mouse frontal cortex.
Representative whole brain sections labeled for WFA, AB1031, Cat-315, and Cat-316. Scale bar = 1 mm.
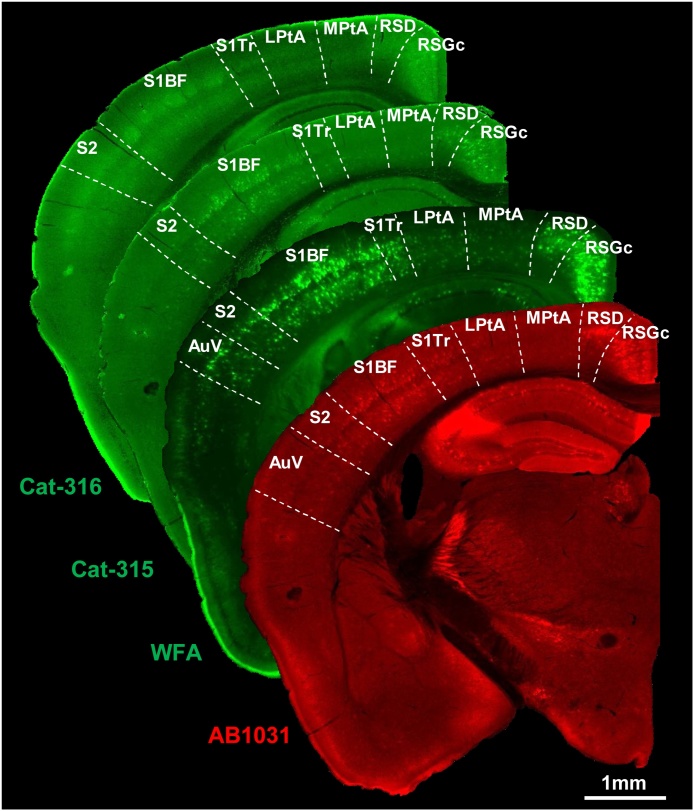
Fig. 2.
Distribution of WFA-, AB1031-, Cat-315-, and Cat-316-positive PNNs in the mouse parietal cortex.
Representative whole brain sections labeled for WFA, AB1031, Cat-315, and Cat-316. Scale bar = 1 mm.
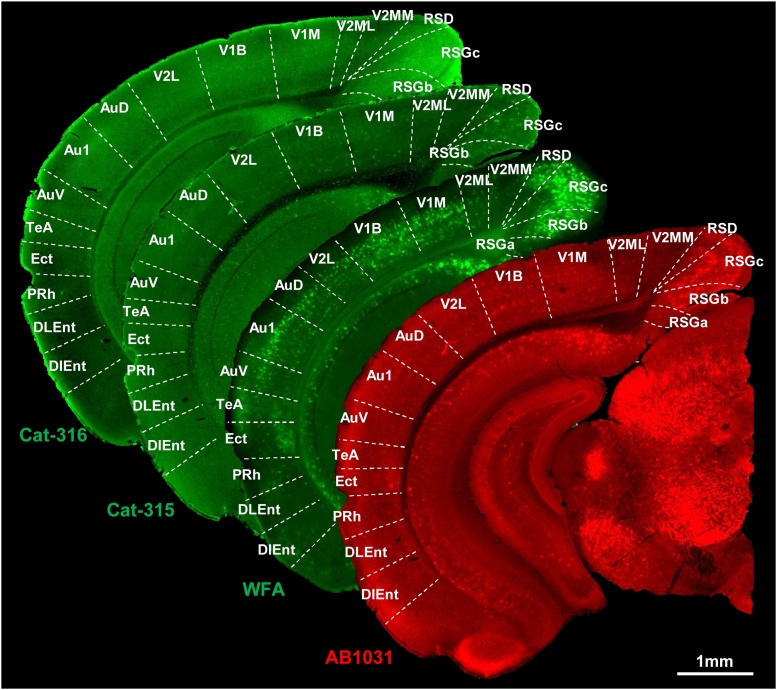
Fig. 3.
Distribution of WFA-, AB1031-, Cat-315-, and Cat-316-positive PNNs in the mouse temporal cortex.
Representative whole brain sections labeled for WFA, AB1031, Cat-315, and Cat-316. Scale bar = 1 mm.
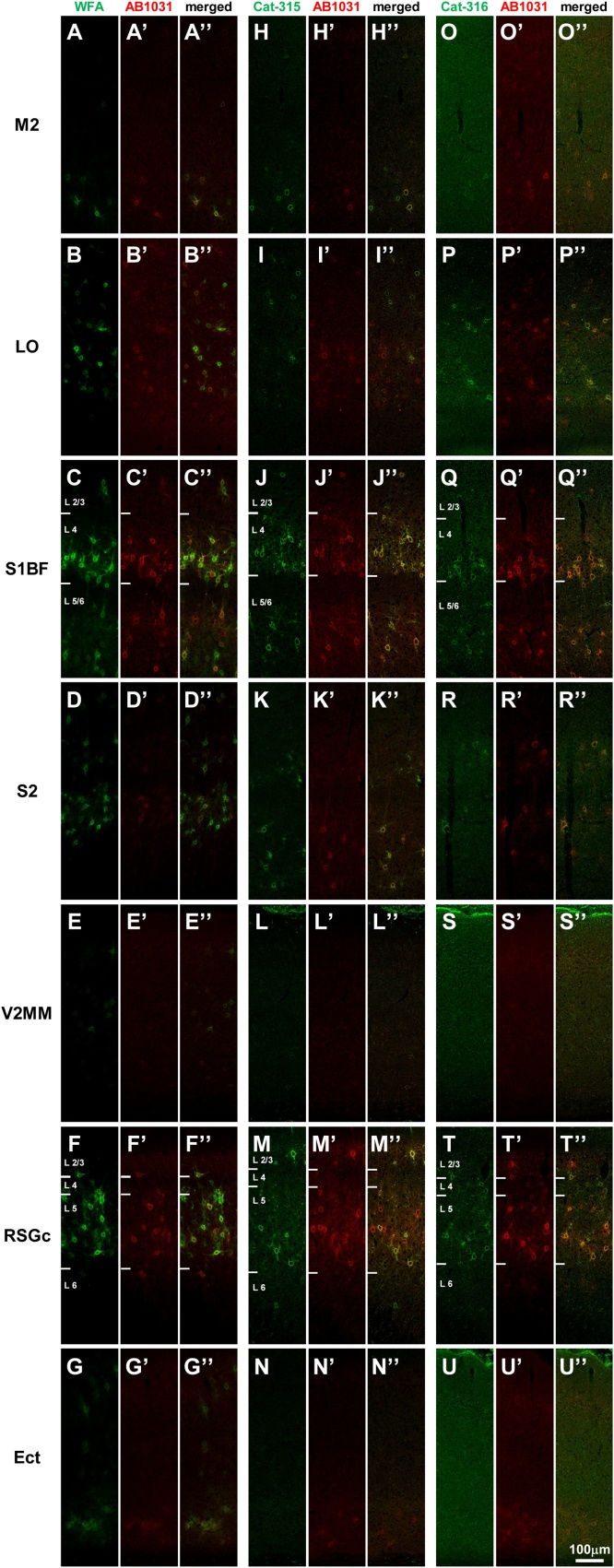
Fig. 4.
Distribution patterns of WFA-, AB1031-, Cat-315-, and Cat-316-positive PNNs in the mouse cerebral cortex.
Confocal images of WFA (A-G) and AB1031 (A’-G’) labeling, and the merged image (A”-G”). Confocal images of Cat-315 (H-N) and AB1031 (H’-N’) labeling, and the merged image (H”-N”). Confocal images of Cat-316 (O-U) and AB1031 (O’-U’) labeling, and the merged image (O”-U”). Images of M2 (A-A”, H-H”, O-O”), LO (B-B”, I-I”, P-P”), S1BF (C-C”, J-J”, Q-Q”), S2 (D-D”, K-K”, R-R”), V2MM (E-E”, L-L”, S-S”), RSGc (F-F”, M-M”, T-T”), and Ect (G-G”, N-N”, U-U”). Scale bar = 100 μm in U” (applies to A–U”).
3.2. Comparative distribution of WFA- and AB1031-positive PNNs
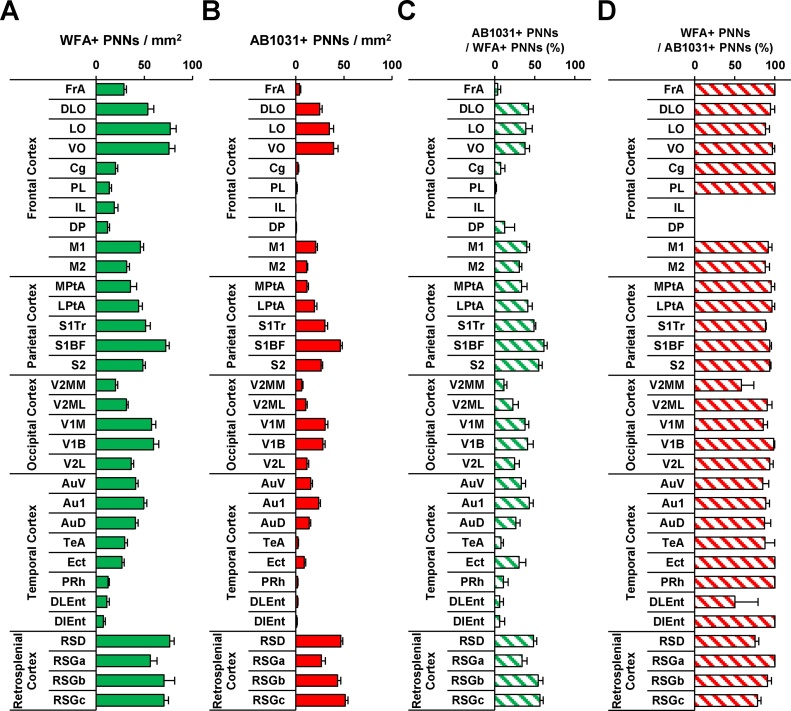
The density of WFA+ PNNs within the mouse cerebral cortex is shown in Fig. 5A. In the frontal cortex, low numbers of WFA+ PNNs were observed in the cingulate (Cg), prelimbic (PL), infralimbic (IL), and dorsal peduncular (DP) subregions. WFA+ PNNs were significantly more abundant in the motor cortex (M1/M2), as well as in the remaining subregions of the frontal cortex (i.e., dorsolateral orbital [DLO], lateral orbital [LO], and ventral orbital [VO] cortices). A high number of WFA+ PNNs was a common feature in the parietal cortex. In the primary somatosensory barrel field (S1BF), the number of WFA+ PNNs was higher than in the remaining subregions of the parietal cortex. The number of WFA+ PNNs was higher in the primary (V1 monocular [V1 M] and V1 binocular [V1B]), relative to the secondary (V2 mediomedial [V2 M], V2 medial lateral [V2ML], and V2 lateral [V2L]) visual cortices of the occipital cortex. WFA+ PNNs were highly abundant in all subregions of the auditory cortex (i.e., primary auditory [Au1], as well as secondary auditory, dorsal [AuD], and ventral [AuV] areas). The number of WFA+ PNNs in subregions of the entorhinal cortex (perirhinal [PRh], dorsolateral entorhinal [DLEnt], and dorsolateral entorhinal [DLEnt]) was significantly lower when compared to that in the auditory cortices. The number of WFA+ PNNs in the retrosplenial cortex was significantly higher than in the other cortices .
Table 1.
| A: Fig. 5A | WFA+ PNNs / mm2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal Cortex | Parietal Cortex | Temporal Cortex | |||||||||||||
| FrA | vs | DLO | 0.0025 | VO | vs | Cg | <0.0001 | MPtA | vs | LPtA | 0.1807 | AuV | vs | Au1 | 0.0198 |
| FrA | vs | LO | <0.0001 | VO | vs | PL | <0.0001 | MPtA | vs | S1Tr | 0.0348 | AuV | vs | AuD | 0.9101 |
| FrA | vs | VO | <0.0001 | VO | vs | IL | <0.0001 | MPtA | vs | S1BF | <0.0001 | AuV | vs | TeA | 0.0024 |
| FrA | vs | Cg | 0.2428 | VO | vs | DP | <0.0001 | MPtA | vs | S2 | 0.0305 | AuV | vs | Ect | 0.0002 |
| FrA | vs | PL | 0.0443 | VO | vs | M1 | <0.0001 | LPtA | vs | S1Tr | 0.3082 | AuV | vs | PRh | <0.0001 |
| FrA | vs | IL | 0.1999 | VO | vs | M2 | <0.0001 | LPtA | vs | S1BF | <0.0001 | AuV | vs | DLEnt | <0.0001 |
| FrA | vs | DP | 0.0289 | Cg | vs | PL | 0.2048 | LPtA | vs | S2 | 0.4426 | AuV | vs | DIEnt | <0.0001 |
| FrA | vs | M1 | 0.0229 | Cg | vs | IL | 0.8325 | S1Tr | vs | S1BF | 0.0041 | Au1 | vs | AuD | 0.0148 |
| FrA | vs | M2 | 0.7034 | Cg | vs | DP | 0.1261 | S1Tr | vs | S2 | 0.6562 | Au1 | vs | TeA | <0.0001 |
| DLO | vs | LO | 0.0002 | Cg | vs | M1 | <0.0001 | S1BF | vs | S2 | <0.0001 | Au1 | vs | Ect | <0.0001 |
| DLO | vs | VO | 0.0003 | Cg | vs | M2 | 0.0246 | Occipital Cortex | Au1 | vs | PRh | <0.0001 | |||
| DLO | vs | Cg | 0.0053 | PL | vs | IL | 0.3234 | V2MM | vs | V2ML | 0.041 | Au1 | vs | DLEnt | <0.0001 |
| DLO | vs | PL | <0.0001 | PL | vs | DP | 0.7341 | V2MM | vs | V1M | <0.0001 | Au1 | vs | DIEnt | <0.0001 |
| DLO | vs | IL | <0.0001 | PL | vs | M1 | <0.0001 | V2MM | vs | V1B | <0.0001 | AuD | vs | TeA | 0.0033 |
| DLO | vs | DP | <0.0001 | PL | vs | M2 | 0.0006 | V2MM | vs | V2L | 0.0035 | AuD | vs | Ect | 0.0003 |
| DLO | vs | M1 | 0.1901 | IL | vs | DP | 0.2093 | V2ML | vs | V1M | < 0.0001 | AuD | vs | PRh | <0.0001 |
| DLO | vs | M2 | 0.0003 | IL | vs | M1 | <0.0001 | V2ML | vs | V1B | <0.0001 | AuD | vs | DLEnt | <0.0001 |
| LO | vs | VO | 0.7967 | IL | vs | M2 | 0.0201 | V2ML | vs | V2L | 0.3587 | AuD | vs | DIEnt | <0.0001 |
| LO | vs | Cg | <0.0001 | DP | vs | M1 | <0.0001 | V1M | vs | V1B | 0.6917 | TeA | vs | Ect | 0.4414 |
| LO | vs | PL | <0.0001 | DP | vs | M2 | 0.0004 | V1M | vs | V2L | 0.0001 | TeA | vs | PRh | <0.0001 |
| LO | vs | IL | <0.0001 | M1 | vs | M2 | 0.0055 | V1B | vs | V2L | <0.0001 | TeA | vs | DLEnt | <0.0001 |
| LO | vs | DP | <0.0001 | Retrosplenial Cortex | TeA | vs | DIEnt | <0.0001 | |||||||
| LO | vs | M1 | <0.0001 | RSD | vs | RSGa | 0.0464 | Ect | vs | PRh | 0.0001 | ||||
| LO | vs | M2 | <0.0001 | RSD | vs | RSGb | 0.544 | Ect | vs | DLEnt | <0.0001 | ||||
| RSD | vs | RSGc | 0.5042 | Ect | vs | DIEnt | <0.0001 | ||||||||
| RSGa | vs | RSGb | 0.1759 | PRh | vs | DLEnt | 0.7486 | ||||||||
| RSGa | vs | RSGc | 0.1609 | PRh | vs | DIEnt | 0.1735 | ||||||||
| RSGb | vs | RSGc | 0.9814 | DLEnt | vs | DIEnt | 0.2959 | ||||||||
| B: Fig. 5B | AB1031+ PNNs / mm2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal Cortex | Parietal Cortex | Temporal Cortex | |||||||||||||
| FrA | vs | DLO | <0.0001 | VO | vs | Cg | <0.0001 | MPtA | vs | LPtA | 0.012 | AuV | vs | Au1 | <0.0001 |
| FrA | vs | LO | <0.0001 | VO | vs | PL | <0.0001 | MPtA | vs | S1Tr | <0.0001 | AuV | vs | AuD | 0.3619 |
| FrA | vs | VO | <0.0001 | VO | vs | IL | <0.0001 | MPtA | vs | S1BF | <0.0001 | AuV | vs | TeA | <0.0001 |
| FrA | vs | Cg | 0.6815 | VO | vs | DP | <0.0001 | MPtA | vs | S2 | <0.0001 | AuV | vs | Ect | 0.0003 |
| FrA | vs | PL | 0,4445 | VO | vs | M1 | <0.0001 | LPtA | vs | S1Tr | 0.0017 | AuV | vs | PRh | <0.0001 |
| FrA | vs | IL | 0.3161 | VO | vs | M2 | <0.0001 | LPtA | vs | S1BF | <0.0001 | AuV | vs | DLEnt | <0.0001 |
| FrA | vs | DP | 0.3697 | Cg | vs | PL | 0.664 | LPtA | vs | S2 | 0.016 | AuV | vs | DIEnt | <0.0001 |
| FrA | vs | M1 | <0.0001 | Cg | vs | IL | 0.4662 | S1Tr | vs | S1BF | <0.0001 | Au1 | vs | AuD | <0.0001 |
| FrA | vs | M2 | 0.0399 | Cg | vs | DP | 0,5484 | S1Tr | vs | S2 | 0.2349 | Au1 | vs | TeA | <0.0001 |
| DLO | vs | LO | 0.0084 | Cg | vs | M1 | <0.0001 | S1BF | vs | S2 | <0.0001 | Au1 | vs | Ect | <0.0001 |
| DLO | vs | VO | 0.0002 | Cg | vs | M2 | 0.0026 | Occipital Cortex | Au1 | vs | PRh | <0.0001 | |||
| DLO | vs | Cg | <0.0001 | PL | vs | IL | 0.7602 | V2MM | vs | V2ML | 0.1345 | Au1 | vs | DLEnt | <0.0001 |
| DLO | vs | PL | <0.0001 | PL | vs | DP | 0.8599 | V2MM | vs | V1M | <0.0001 | Au1 | vs | DIEnt | <0.0001 |
| DLO | vs | IL | <0.0001 | PL | vs | M1 | <0.0001 | V2MM | vs | V1B | <0.0001 | AuD | vs | TeA | <0.0001 |
| DLO | vs | DP | <0.0001 | PL | vs | M2 | 0.0006 | V2MM | vs | V2L | 0.0599 | AuD | vs | Ect | 0.0069 |
| DLO | vs | M1 | 0.2827 | IL | vs | DP | 0.8999 | V2ML | vs | V1M | <0.0001 | AuD | vs | PRh | <0.0001 |
| DLO | vs | M2 | 0.0003 | IL | vs | M1 | <0.0001 | V2ML | vs | V1B | <0.0001 | AuD | vs | DLEnt | <0.0001 |
| LO | vs | VO | 0.1678 | IL | vs | M2 | 0.0002 | V2ML | vs | V2L | 0.7072 | AuD | vs | DIEnt | <0.0001 |
| LO | vs | Cg | <0.0001 | DP | vs | M1 | <0.0001 | V1M | vs | V1B | 0.4069 | TeA | vs | Ect | 0.0007 |
| LO | vs | PL | <0.0001 | DP | vs | M2 | 0.0004 | V1M | vs | V2L | <0.0001 | TeA | vs | PRh | 0.8235 |
| LO | vs | IL | <0.0001 | M1 | vs | M2 | 0.0011 | V1B | vs | V2L | <0.0001 | TeA | vs | DLEnt | 0.843 |
| LO | vs | DP | <0.0001 | Retrosplenial Cortex | TeA | vs | DIEnt | 0.5046 | |||||||
| LO | vs | M1 | <0.0001 | RSD | vs | RSGa | <0.0001 | Ect | vs | PRh | 0.0003 | ||||
| LO | vs | M2 | <0.0001 | RSD | vs | RSGb | 0.0001 | Ect | vs | DLEnt | 0.0003 | ||||
| RSD | vs | RSGc | 0.2784 | Ect | vs | DIEnt | 0.0005 | ||||||||
| RSGa | vs | RSGb | 0.0004 | PRh | vs | DLEnt | 0.9797 | ||||||||
| RSGa | vs | RSGc | <0.0001 | PRh | vs | DIEnt | 0.6527 | ||||||||
| RSGb | vs | RSGc | 0.0698 | DLEnt | vs | DIEnt | 0.6345 | ||||||||
| C: Fig. 5C | AB1031+ PNNs / WFA+ PNNs (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal Cortex | Parietal Cortex | Temporal Cortex | |||||||||||||
| FrA | vs | DLO | 0.0017 | VO | vs | Cg | 0.0003 | MPtA | vs | LPtA | 0.3152 | AuV | vs | Au1 | 0.2105 |
| FrA | vs | LO | 0.003 | VO | vs | PL | <0.0001 | MPtA | vs | S1Tr | 0.0888 | AuV | vs | AuD | 0.3723 |
| FrA | vs | VO | 0.0038 | VO | vs | IL | <0.0001 | MPtA | vs | S1BF | 0.0003 | AuV | vs | TeA | 0.0016 |
| FrA | vs | Cg | 0.7328 | VO | vs | DP | 0.0036 | MPtA | vs | S2 | 0.0039 | AuV | vs | Ect | 0.7153 |
| FrA | vs | PL | 0.8156 | VO | vs | M1 | 0.7913 | LPtA | vs | S1Tr | 0.3834 | AuV | vs | PRh | 0.0053 |
| FrA | vs | IL | 0.7461 | VO | vs | M2 | 0.3727 | LPtA | vs | S1BF | 0.0062 | AuV | vs | DLEnt | 0.0017 |
| FrA | vs | DP | 0.4426 | Cg | vs | PL | 0.3987 | LPtA | vs | S2 | 0.054 | AuV | vs | DIEnt | 0.0017 |
| FrA | vs | M1 | 0.0016 | Cg | vs | IL | 0.3506 | S1Tr | vs | S1BF | 0.143 | Au1 | vs | AuD | 0.0341 |
| FrA | vs | M2 | 0.0176 | Cg | vs | DP | 0.5331 | S1Tr | vs | S2 | 0.4715 | Au1 | vs | TeA | 0.0002 |
| DLO | vs | LO | 0.6843 | Cg | vs | M1 | <0.0001 | S1BF | vs | S2 | 0.3152 | Au1 | vs | Ect | 0.1076 |
| DLO | vs | VO | 0.6171 | Cg | vs | M2 | 0.0029 | Occipital Cortex | Au1 | vs | PRh | <0.0001 | |||
| DLO | vs | Cg | 0.0001 | PL | vs | IL | 0.8896 | V2MM | vs | V2ML | 0.1993 | Au1 | vs | DLEnt | 0.0003 |
| DLO | vs | PL | <0.0001 | PL | vs | DP | 0.1581 | V2MM | vs | V1M | 0.0025 | Au1 | vs | DIEnt | 0.0003 |
| DLO | vs | IL | <0.0001 | PL | vs | M1 | <0.0001 | V2MM | vs | V1B | 0.0008 | AuD | vs | TeA | 0.0197 |
| DLO | vs | DP | 0.0015 | PL | vs | M2 | 0.0002 | V2MM | vs | V2L | 0.1182 | AuD | vs | Ect | 0.5966 |
| DLO | vs | M1 | 0.7794 | IL | vs | DP | 0.1418 | V2ML | vs | V1M | 0.0695 | AuD | vs | PRh | 0.0518 |
| DLO | vs | M2 | 0.1815 | IL | vs | M1 | <0.0001 | V2ML | vs | V1B | 0.0307 | AuD | vs | DLEnt | 0.0182 |
| LO | vs | VO | 0.9194 | IL | vs | M2 | 0.0003 | V2ML | vs | V2L | 0.7985 | AuD | vs | DIEnt | 0.0182 |
| LO | vs | Cg | 0.0002 | DP | vs | M1 | 0.0009 | V1M | vs | V1B | 0.7043 | TeA | vs | Ect | 0.0047 |
| LO | vs | PL | 0.0001 | DP | vs | M2 | 0.0261 | V1M | vs | V2L | 0.1064 | TeA | vs | PRh | 0.6847 |
| LO | vs | IL | <0.0001 | M1 | vs | M2 | 0.2216 | V1B | vs | V2L | 0.0484 | TeA | vs | DLEnt | 0.8661 |
| LO | vs | DP | 0.0027 | Retrosplenial Cortex | TeA | vs | DIEnt | 0.8661 | |||||||
| LO | vs | M1 | 0.8744 | RSD | vs | RSGa | 0.4697 | Ect | vs | PRh | 0.0144 | ||||
| LO | vs | M2 | 0.3188 | RSD | vs | RSGb | 0.427 | Ect | vs | DLEnt | 0.0047 | ||||
| RSD | vs | RSGc | 0.2265 | Ect | vs | DIEnt | 0.0047 | ||||||||
| RSGa | vs | RSGb | 0.0104 | PRh | vs | DLEnt | 0.5817 | ||||||||
| RSGa | vs | RSGc | 0.0028 | PRh | vs | DIEnt | 0.5817 | ||||||||
| RSGb | vs | RSGc | 0.7226 | DLEnt | vs | DIEnt | 1 | ||||||||
| D: Fig. 5D | WFA+ PNNs / AB1031+ PNNs (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal Cortex | Parietal Cortex | Temporal Cortex | |||||||||||||
| FrA | vs | DLO | 0.7176 | VO | vs | Cg | 0.7809 | MPtA | vs | LPtA | 0.7934 | AuV | vs | Au1 | 0.743 |
| FrA | vs | LO | 0.4442 | VO | vs | PL | 0.8357 | MPtA | vs | S1Tr | 0.1987 | AuV | vs | AuD | 0.8496 |
| FrA | vs | VO | 0.8357 | VO | vs | IL | <0.0001 | MPtA | vs | S1BF | 0.6527 | AuV | vs | TeA | 0.8577 |
| FrA | vs | Cg | 1 | VO | vs | DP | 0.8357 | MPtA | vs | S2 | 0.6667 | AuV | vs | Ect | 0.2151 |
| FrA | vs | PL | 1 | VO | vs | M1 | 0.4318 | LPtA | vs | S1Tr | 0.1339 | AuV | vs | PRh | 0.3513 |
| FrA | vs | IL | <0.0001 | VO | vs | M2 | 0.1929 | LPtA | vs | S1BF | 0.4643 | AuV | vs | DLEnt | 0.0198 |
| FrA | vs | DP | 1 | Cg | vs | PL | 1 | LPtA | vs | S2 | 0.4762 | AuV | vs | DIEnt | 0.5572 |
| FrA | vs | M1 | 0.5717 | Cg | vs | IL | <0.0001 | S1Tr | vs | S1BF | 0.3192 | Au1 | vs | AuD | 0.8968 |
| FrA | vs | M2 | 0.4226 | Cg | vs | DP | 1 | S1Tr | vs | S2 | 0.3116 | Au1 | vs | TeA | 0.9452 |
| DLO | vs | LO | 0.4344 | Cg | vs | M1 | 0.4447 | S1BF | vs | S2 | 0.9827 | Au1 | vs | Ect | 0.3427 |
| DLO | vs | VO | 0.7516 | Cg | vs | M2 | 0.2793 | Occipital Cortex | Au1 | vs | PRh | 0.4764 | |||
| DLO | vs | Cg | 0.6325 | PL | vs | IL | <0.0001 | V2MM | vs | V2ML | 0.0055 | Au1 | vs | DLEnt | 0.0107 |
| DLO | vs | PL | 0.7176 | PL | vs | DP | 1 | V2MM | vs | V1M | 0.0084 | Au1 | vs | DIEnt | 0.6543 |
| DLO | vs | IL | <0.0001 | PL | vs | M1 | 0.5717 | V2MM | vs | V1B | 0.0002 | AuD | vs | TeA | 0.9748 |
| DLO | vs | DP | 0.7176 | PL | vs | M2 | 0.4226 | V2MM | vs | V2L | 0.0018 | AuD | vs | Ect | 0.2965 |
| DLO | vs | M1 | 0.6941 | IL | vs | DP | <0.0001 | V2ML | vs | V1M | 0.6119 | AuD | vs | PRh | 0.4286 |
| DLO | vs | M2 | 0.3822 | IL | vs | M1 | <0.0001 | V2ML | vs | V1B | 0.3963 | AuD | vs | DLEnt | 0.0152 |
| LO | vs | VO | 0.2385 | IL | vs | M2 | <0.0001 | V2ML | vs | V2L | 0.7534 | AuD | vs | DIEnt | 0.6158 |
| LO | vs | Cg | 0.3061 | DP | vs | M1 | 0.5717 | V1M | vs | V1B | 0.1226 | TeA | vs | Ect | 0.417 |
| LO | vs | PL | 0.4441 | DP | vs | M2 | 0.4226 | V1M | vs | V2L | 0.3776 | TeA | vs | PRh | 0.5048 |
| LO | vs | IL | <0.0001 | M1 | vs | M2 | 0.5765 | V1B | vs | V2L | 0.5909 | TeA | vs | DLEnt | 0.0351 |
| LO | vs | DP | 0.4442 | Retrosplenial Cortex | TeA | vs | DIEnt | 0.6481 | |||||||
| LO | vs | M1 | 0.6425 | RSD | vs | RSGa | 0.0005 | Ect | vs | PRh | 1 | ||||
| LO | vs | M2 | 0.95 | RSD | vs | RSGb | 0.0164 | Ect | vs | DLEnt | 0.0021 | ||||
| RSD | vs | RSGc | 0.6531 | Ect | vs | DIEnt | 1 | ||||||||
| RSGa | vs | RSGb | 0.1813 | PRh | vs | DLEnt | 0.0103 | ||||||||
| RSGa | vs | RSGc | 0.0015 | PRh | vs | DIEnt | 1 | ||||||||
| RSGb | vs | RSGc | 0.0432 | DLEnt | vs | DIEnt | 0.0733 | ||||||||
| E: Fig. 6A | Cat-315+ PNNs / mm2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal Cortex | Parietal Cortex | Temporal Cortex | |||||||||||||
| FrA | vs | DLO | 0.1879 | VO | vs | Cg | <0.0001 | MPtA | vs | LPtA | 0.4051 | AuV | vs | Au1 | <0.0001 |
| FrA | vs | LO | 0.0257 | VO | vs | PL | <0.0001 | MPtA | vs | S1Tr | 0.0234 | AuV | vs | AuD | 0.4884 |
| FrA | vs | VO | <0.0001 | VO | vs | IL | <0.0001 | MPtA | vs | S1BF | <0.0001 | AuV | vs | TeA | 0.0637 |
| FrA | vs | Cg | 0.6584 | VO | vs | DP | <0.0001 | MPtA | vs | S2 | 0.0161 | AuV | vs | Ect | 0.0018 |
| FrA | vs | PL | 0.2165 | VO | vs | M1 | 0.0363 | LPtA | vs | S1Tr | 0.1331 | AuV | vs | PRh | 0.0069 |
| FrA | vs | IL | 0.1719 | VO | vs | M2 | <0.0001 | LPtA | vs | S1BF | <0.0001 | AuV | vs | DLEnt | 0.3664 |
| FrA | vs | DP | 0.8083 | Cg | vs | PL | 0.0962 | LPtA | vs | S2 | 0.1106 | AuV | vs | DIEnt | 0.0018 |
| FrA | vs | M1 | <0.0001 | Cg | vs | IL | 0.0733 | S1Tr | vs | S1BF | 0.0001 | Au1 | vs | AuD | <0.0001 |
| FrA | vs | M2 | 0.0395 | Cg | vs | DP | 0.4943 | S1Tr | vs | S2 | 0.9821 | Au1 | vs | TeA | <0.0001 |
| DLO | vs | LO | 0.7698 | Cg | vs | M1 | 0.0001 | S1BF | vs | S2 | <0.0001 | Au1 | vs | Ect | <0.0001 |
| DLO | vs | VO | 0.0013 | Cg | vs | M2 | 0.1009 | Occipital Cortex | Au1 | vs | PRh | <0.0001 | |||
| DLO | vs | Cg | 0.3122 | PL | vs | IL | 0.0733 | V2MM | vs | V2ML | 0.5425 | Au1 | vs | DLEnt | <0.0001 |
| DLO | vs | PL | 0.0312 | PL | vs | DP | 0.3182 | V2MM | vs | V1M | 0.0005 | Au1 | vs | DIEnt | <0.0001 |
| DLO | vs | IL | 0.0249 | PL | vs | M1 | <0.0001 | V2MM | vs | V1B | <0.0001 | AuD | vs | TeA | 0.0141 |
| DLO | vs | DP | 0.1381 | PL | vs | M2 | 0.0015 | V2MM | vs | V2L | 0.0987 | AuD | vs | Ect | 0.0002 |
| DLO | vs | M1 | 0.0659 | IL | vs | DP | 0.2586 | V2ML | vs | V1M | 0.0028 | AuD | vs | PRh | 0.0011 |
| DLO | vs | M2 | 0.872 | IL | vs | M1 | <0.0001 | V2ML | vs | V1B | < 0.0001 | AuD | vs | DLEnt | 0.0011 |
| LO | vs | VO | <0.0001 | IL | vs | M2 | 0.001 | V2ML | vs | V2L | 0.2829 | AuD | vs | DIEnt | 0.0002 |
| LO | vs | Cg | 0.0691 | DP | vs | M1 | <0.0001 | V1M | vs | V1B | 0.1062 | TeA | vs | Ect | 0.173 |
| LO | vs | PL | 0.0008 | DP | vs | M2 | 0.0224 | V1M | vs | V2L | 0.0362 | TeA | vs | PRh | 0.3664 |
| LO | vs | IL | 0.0006 | M1 | vs | M2 | 0.0188 | V1B | vs | V2L | 0.0006 | TeA | vs | DLEnt | 0.3711 |
| LO | vs | DP | 0.0142 | Retrosplenial Cortex | TeA | vs | DIEnt | 0.173 | |||||||
| LO | vs | M1 | 0.0294 | RSD | vs | RSGa | 0.0004 | Ect | vs | PRh | 0.6371 | ||||
| LO | vs | M2 | 0.8523 | RSD | vs | RSGb | 0.5417 | Ect | vs | DLEnt | 0.6307 | ||||
| RSD | vs | RSGc | 0.0064 | Ect | vs | DIEnt | 1 | ||||||||
| RSGa | vs | RSGb | 0.0029 | PRh | vs | DLEnt | 0.9928 | ||||||||
| RSGa | vs | RSGc | <0.0001 | PRh | vs | DIEnt | 0.6371 | ||||||||
| RSGb | vs | RSGc | 0.0035 | DLEnt | vs | DIEnt | 0.6307 | ||||||||
| F: Fig. 6B | Cat-316+ PNNs / mm2 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal Cortex | Parietal Cortex | Temporal Cortex | |||||||||||||
| FrA | vs | DLO | 0.0052 | VO | vs | Cg | <0.0001 | MPtA | vs | LPtA | 0.1062 | AuV | vs | Au1 | 0.0326 |
| FrA | vs | LO | <0.0001 | VO | vs | PL | <0.0001 | MPtA | vs | S1Tr | 0.0002 | AuV | vs | AuD | 0.1075 |
| FrA | vs | VO | <0.0001 | VO | vs | IL | <0.0001 | MPtA | vs | S1BF | <0.0001 | AuV | vs | TeA | <0.0001 |
| FrA | vs | Cg | 0.7054 | VO | vs | DP | <0.0001 | MPtA | vs | S2 | 0.0018 | AuV | vs | Ect | 0.0016 |
| FrA | vs | PL | 0.6572 | VO | vs | M1 | <0.0001 | LPtA | vs | S1Tr | 0.0067 | AuV | vs | PRh | <0.0001 |
| FrA | vs | IL | 1 | VO | vs | M2 | <0.0001 | LPtA | vs | S1BF | <0.0001 | AuV | vs | DLEnt | <0.0001 |
| FrA | vs | DP | 0.8637 | Cg | vs | PL | 0.9256 | LPtA | vs | S2 | 0.0776 | AuV | vs | DIEnt | <0.0001 |
| FrA | vs | M1 | 0.0016 | Cg | vs | IL | 0.5933 | S1Tr | vs | S1BF | 0.0066 | Au1 | vs | AuD | 0.0004 |
| FrA | vs | M2 | 0.133 | Cg | vs | DP | 0.7702 | S1Tr | vs | S2 | 0.1991 | Au1 | vs | TeA | <0.0001 |
| DLO | vs | LO | 0.0004 | Cg | vs | M1 | 0.0001 | S1BF | vs | S2 | <0.0001 | Au1 | vs | Ect | <0.0001 |
| DLO | vs | VO | 0.0014 | Cg | vs | M2 | 0.1108 | Occipital Cortex | Au1 | vs | PRh | <0.0001 | |||
| DLO | vs | Cg | 0.0024 | PL | vs | IL | 0.5308 | V2MM | vs | V2ML | 0.7465 | Au1 | vs | DLEnt | <0.0001 |
| DLO | vs | PL | 0.0028 | PL | vs | DP | 0.7001 | V2MM | vs | V1M | <0.0001 | Au1 | vs | DIEnt | <0.0001 |
| DLO | vs | IL | 0.0008 | PL | vs | M1 | 0.0002 | V2MM | vs | V1B | <0.0001 | AuD | vs | TeA | 0.0029 |
| DLO | vs | DP | 0.0013 | PL | vs | M2 | 0.1323 | V2MM | vs | V2L | 0.1201 | AuD | vs | Ect | 0.078 |
| DLO | vs | M1 | 0.8093 | IL | vs | DP | 0.8083 | V2ML | vs | V1M | <0.0001 | AuD | vs | PRh | 0.0029 |
| DLO | vs | M2 | 0.0432 | IL | vs | M1 | <0.0001 | V2ML | vs | V1B | <0.0001 | AuD | vs | DLEnt | 0.0016 |
| LO | vs | VO | 0.6008 | IL | vs | M2 | 0.0361 | V2ML | vs | V2L | 0.2114 | AuD | vs | DIEnt | 0.0026 |
| LO | vs | Cg | <0.0001 | DP | vs | M1 | <0.0001 | V1M | vs | V1B | 0.8902 | TeA | vs | Ect | 0.1997 |
| LO | vs | PL | <0.0001 | DP | vs | M2 | 0.0614 | V1M | vs | V2L | <0.0001 | TeA | vs | PRh | 0.8873 |
| LO | vs | IL | <0.0001 | M1 | vs | M2 | 0.0127 | V1B | vs | V2L | <0.0001 | TeA | vs | DLEnt | 0.9586 |
| LO | vs | DP | <0.0001 | Retrosplenial Cortex | TeA | vs | DIEnt | 0.9131 | |||||||
| LO | vs | M1 | <0.0001 | RSD | vs | RSGa | 0.0668 | Ect | vs | PRh | 0.2298 | ||||
| LO | vs | M2 | <0.0001 | RSD | vs | RSGb | 0.3277 | Ect | vs | DLEnt | 0.1651 | ||||
| RSD | vs | RSGc | 0.6879 | Ect | vs | DIEnt | 0.2178 | ||||||||
| RSGa | vs | RSGb | 0.0081 | PRh | vs | DLEnt | 0.8392 | ||||||||
| RSGa | vs | RSGc | 0.0294 | PRh | vs | DIEnt | 0.9727 | ||||||||
| RSGb | vs | RSGc | 0.558 | DLEnt | vs | DIEnt | 0.8659 | ||||||||
| G: Fig. 6C | Cat-315+ PNNs / AB1031+ PNNs (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal Cortex | Parietal Cortex | Temporal Cortex | |||||||||||||
| FrA | vs | DLO | 0.9812 | VO | vs | Cg | 0.9751 | MPtA | vs | LPtA | 0.9042 | AuV | vs | Au1 | 0.0007 |
| FrA | vs | LO | 0.8194 | VO | vs | PL | 0.2426 | MPtA | vs | S1Tr | 0.6842 | AuV | vs | AuD | 0.0568 |
| FrA | vs | VO | 0.2297 | VO | vs | IL | 0.0509 | MPtA | vs | S1BF | 0.0871 | AuV | vs | TeA | 0.2413 |
| FrA | vs | Cg | 0.2951 | VO | vs | DP | 0.2426 | MPtA | vs | S2 | 0.2953 | AuV | vs | Ect | 0.0729 |
| FrA | vs | PL | 0.0749 | VO | vs | M1 | 0.168 | LPtA | vs | S1Tr | 0.5466 | AuV | vs | PRh | 0.2413 |
| FrA | vs | IL | 0.1776 | VO | vs | M2 | 0.4038 | LPtA | vs | S1BF | 0.0379 | AuV | vs | DLEnt | 0.2413 |
| FrA | vs | DP | 0.0749 | Cg | vs | PL | 0.2513 | LPtA | vs | S2 | 0.1785 | AuV | vs | DIEnt | 0.2413 |
| FrA | vs | M1 | 0.0132 | Cg | vs | IL | 0.061 | S1Tr | vs | S1BF | 0.0993 | Au1 | vs | AuD | 0.0414 |
| FrA | vs | M2 | 0.0532 | Cg | vs | DP | 0.2513 | S1Tr | vs | S2 | 0.4283 | Au1 | vs | TeA | 0.0017 |
| DLO | vs | LO | 0.89 | Cg | vs | M1 | 0.2051 | S1BF | vs | S2 | 0.31 | Au1 | vs | Ect | <0.0001 |
| DLO | vs | VO | 0.4055 | Cg | vs | M2 | 0.4331 | Occipital Cortex | Au1 | vs | PRh | 0.0017 | |||
| DLO | vs | Cg | 0.4461 | PL | vs | IL | 0.0199 | V2MM | vs | V2ML | 0.4141 | Au1 | vs | DLEnt | 0.0017 |
| DLO | vs | PL | 0.1173 | PL | vs | DP | 1 | V2MM | vs | V1M | 0.2244 | Au1 | vs | DIEnt | 0.0017 |
| DLO | vs | IL | 0.2269 | PL | vs | M1 | 0.6654 | V2MM | vs | V1B | 0.1046 | AuD | vs | TeA | 0.024 |
| DLO | vs | DP | 0.1173 | PL | vs | M2 | 0.4845 | V2MM | vs | V2L | 0.1157 | AuD | vs | Ect | 0.0009 |
| DLO | vs | M1 | 0.0755 | IL | vs | DP | 0.0199 | V2ML | vs | V1M | 0.666 | AuD | vs | PRh | 0.0241 |
| DLO | vs | M2 | 0.1616 | IL | vs | M1 | 0.0092 | V2ML | vs | V1B | 0.3777 | AuD | vs | DLEnt | 0.0241 |
| LO | vs | VO | 0.327 | IL | vs | M2 | 0.0194 | V2ML | vs | V2L | 0.39 | AuD | vs | DIEnt | 0.024 |
| LO | vs | Cg | 0.3966 | DP | vs | M1 | 0.6654 | V1M | vs | V1B | 0.6482 | TeA | vs | Ect | 1 |
| LO | vs | PL | 0.0952 | DP | vs | M2 | 0.4845 | V1M | vs | V2L | 0.6501 | TeA | vs | PRh | 1 |
| LO | vs | IL | 0.1432 | M1 | vs | M2 | 0.6196 | V1B | vs | V2L | 0.9859 | TeA | vs | DLEnt | 1 |
| LO | vs | DP | 0.0952 | Retrosplenial Cortex | TeA | vs | DIEnt | 1 | |||||||
| LO | vs | M1 | 0.0226 | RSD | vs | RSGa | 0.1749 | Ect | vs | PRh | 1 | ||||
| LO | vs | M2 | 0.0828 | RSD | vs | RSGb | 0.9902 | Ect | vs | DLEnt | 1 | ||||
| RSD | vs | RSGc | 0.2489 | Ect | vs | DIEnt | 1 | ||||||||
| RSGa | vs | RSGb | 0.217 | PRh | vs | DLEnt | 1 | ||||||||
| RSGa | vs | RSGc | 0.0268 | PRh | vs | DIEnt | 1 | ||||||||
| RSGb | vs | RSGc | 0.2908 | DLEnt | vs | DIEnt | 1 | ||||||||
| H: Fig. 6D | Cat-316+ PNNs / AB1031+ PNNs (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frontal Cortex | Parietal Cortex | Temporal Cortex | |||||||||||||
| FrA | vs | DLO | 0.0895 | VO | vs | Cg | 0.8069 | MPtA | vs | LPtA | 0.0605 | AuV | vs | Au1 | 0.4166 |
| FrA | vs | LO | 0.0049 | VO | vs | PL | 0.7294 | MPtA | vs | S1Tr | 0.0202 | AuV | vs | AuD | 0.9726 |
| FrA | vs | VO | 0.006 | VO | vs | IL | 0.006 | MPtA | vs | S1BF | 0.0047 | AuV | vs | TeA | 0.4279 |
| FrA | vs | Cg | 0.0123 | VO | vs | DP | 0.5185 | MPtA | vs | S2 | 0.0224 | AuV | vs | Ect | 0.5732 |
| FrA | vs | PL | 0.0213 | VO | vs | M1 | 0.5917 | LPtA | vs | S1Tr | 0.4731 | AuV | vs | PRh | 0.0919 |
| FrA | vs | IL | 1 | VO | vs | M2 | 0.0406 | LPtA | vs | S1BF | 0.2617 | AuV | vs | DLEnt | 0.1222 |
| FrA | vs | DP | 0.009 | Cg | vs | PL | 0.9031 | LPtA | vs | S2 | 0.6397 | AuV | vs | DIEnt | 0.9931 |
| FrA | vs | M1 | 0.011 | Cg | vs | IL | 0.0123 | S1Tr | vs | S1BF | 0.7671 | Au1 | vs | AuD | 0.3976 |
| FrA | vs | M2 | 0.0759 | Cg | vs | DP | 0.4443 | S1Tr | vs | S2 | 0.7631 | Au1 | vs | TeA | 0.1511 |
| DLO | vs | LO | 0.1512 | Cg | vs | M1 | 0.8351 | S1BF | vs | S2 | 0.5057 | Au1 | vs | Ect | 0.1863 |
| DLO | vs | VO | 0.182 | Cg | vs | M2 | 0.1223 | Occipital Cortex | Au1 | vs | PRh | 0.0195 | |||
| DLO | vs | Cg | 0.2983 | PL | vs | IL | 0.0213 | V2MM | vs | V2ML | 0.0309 | Au1 | vs | DLEnt | 0.0316 |
| DLO | vs | PL | 0.399 | PL | vs | DP | 0.4176 | V2MM | vs | V1M | 0.5469 | Au1 | vs | DIEnt | 0.6565 |
| DLO | vs | IL | 0.0895 | PL | vs | M1 | 0.9647 | V2MM | vs | V1B | 0.879 | AuD | vs | TeA | 0.4441 |
| DLO | vs | DP | 0.1406 | PL | vs | M2 | 0.223 | V2MM | vs | V2L | 0.3267 | AuD | vs | Ect | 0.5955 |
| DLO | vs | M1 | 0.3241 | IL | vs | DP | 0.009 | V2ML | vs | V1M | 0.0703 | AuD | vs | PRh | 0.0974 |
| DLO | vs | M2 | 0.8428 | IL | vs | M1 | 0.011 | V2ML | vs | V1B | 0.0258 | AuD | vs | DLEnt | 0.1286 |
| LO | vs | VO | 0.887 | IL | vs | M2 | 0.0759 | V2ML | vs | V2L | 0.1518 | AuD | vs | DIEnt | 0.9921 |
| LO | vs | Cg | 0.7135 | DP | vs | M1 | 0.3423 | V1M | vs | V1B | 0.6139 | TeA | vs | Ect | 0.7614 |
| LO | vs | PL | 0.6307 | DP | vs | M2 | 0.0727 | V1M | vs | V2L | 0.6659 | TeA | vs | PRh | 0.4698 |
| LO | vs | IL | 0.0049 | M1 | vs | M2 | 0.1013 | V1B | vs | V2L | 0.3527 | TeA | vs | DLEnt | 0.4987 |
| LO | vs | DP | 0.5724 | Retrosplenial Cortex | TeA | vs | DIEnt | 0.6316 | |||||||
| LO | vs | M1 | 0.4947 | RSD | vs | RSGa | 0.894 | Ect | vs | PRh | 0.2529 | ||||
| LO | vs | M2 | 0.0297 | RSD | vs | RSGb | 0.5058 | Ect | vs | DLEnt | 0.2927 | ||||
| RSD | vs | RSGc | 0.3893 | Ect | vs | DIEnt | 0.7614 | ||||||||
| RSGa | vs | RSGb | 0.5933 | PRh | vs | DLEnt | 1 | ||||||||
| RSGa | vs | RSGc | 0.4648 | PRh | vs | DIEnt | 0.3255 | ||||||||
| RSGb | vs | RSGc | 0.8415 | DLEnt | vs | DIEnt | 0.3408 | ||||||||
Fig. 5.
Quantitative analyses of WFA- and AB1031-positive PNNs in the mouse cerebral cortex.
Quantified number of WFA+ PNNs in the mouse cerebral cortex (A). Quantified number of AB1031+ PNNs in the mouse cerebral cortex (B). The proportion of WFA+ PNNs co-localized with AB1031 (C), and the proportion of AB1031+ PNNs co-localized with WFA (D) in the mouse cerebral cortex. Data are expressed as the mean ± SEM. The respective P values are listed in Table 1.
Further, we quantified AB1031-positive (AB1031+) PNNs in the mouse cerebral cortex (Fig. 5B). The density of AB1031+ PNNs showed significantly different variations throughout the mouse cortical regions examined. In many regions (i.e., frontal association [FrA], Cg, PL, IL, DP, temporal association [TeA], ectorhinal [Ect], PRh, DLEnt, and DIEnt), AB1031+ PNNs were very low compared with other cortices. In the frontal cortex, a high number of AB1031+ PNNs was observed in the DLO, LO, and VO subregions. In the remaining subregions of the frontal cortex (primary and secondary motor areas [M1 and M2, respectively]), the density of AB1031+ PNNs was high compared to that in the frontal cortex subregions, FrA, Cg, PL, IL, and DP. In the S1BF subregion, the number of AB1031+ PNNs was higher than in the remaining subregions of the parietal cortex (i.e., medial parietal association [MPtA], lateral parietal association [LPtA], somatosensory 1, trunk region [S1Tr], and somatosensory 2 [S2] areas). In the temporal cortex, subregions of the auditory cortex (i.e., AuV, Au1, and AuD) exhibited high AB1031+ PNN densities, followed by lower densities in the entorhinal cortex subregions (i.e., TeA, Ect, PRh, DIEnt, and DLEnt). Finally, the number of AB1031+ PNNs in subregions of the retrosplenial cortex was significantly higher than in other cortices.
3.3. Co-localization of WFA+ and AB1031+ PNNs
Further, we determined the percentage of overlapping WFA+ and AB1031+ PNNs in the mouse cerebral cortex (Fig. 5C), and determined this value to be below 50% in many regions. More specifically, the percentage of co-localized WFA+ and AB1031+ PNNs in the FrA, Cg, PL, IL, and DP subregions of the frontal cortex was lower than in the remaining subregions (i.e., DLO, LO, VO, M1, and M2). Approximately 10% of WFA+ PNNs in the FrA, Cg, PL, IL, and DP subregions of the frontal cortex were co-localized with AB1031. In the parietal cortex, the percentage of WFA+ PNNs co-localized with AB1031 was high, while WFA-AB1031 co-localization in MPtA/LPtA subregions of the parietal association cortex was low compared to the S1Tr/S1BF/S2 somatosensory cortex subregions. In the V1M/V1B subregions of the occipital cortex, the percentage of WFA-AB1031-positive PNNs was high compared to that in the V2MM/V2ML/V2L subregions of the secondary visual cortex. In the temporal cortex, the Au1/AuD/AuV subregions of the auditory cortex exhibited high percentages of WFA-AB1031 co-localization, followed by lower percentages in the TeA/PRh/DLEnt/DIEnt subregions of the entorhinal cortex. In all subregions of the retrosplenial cortex, about 40% of WFA+ PNNs co-localized with AB1031. Taken together, our findings revealed that AB1031-WFA co-localization was high (∼80%) in the PNNs of most regions in the cerebral cortex (Fig. 5D). Note that IL/DP subregions of the frontal cortex were excluded from analysis since the number of AB1031+ PNNs was significantly low in these areas.
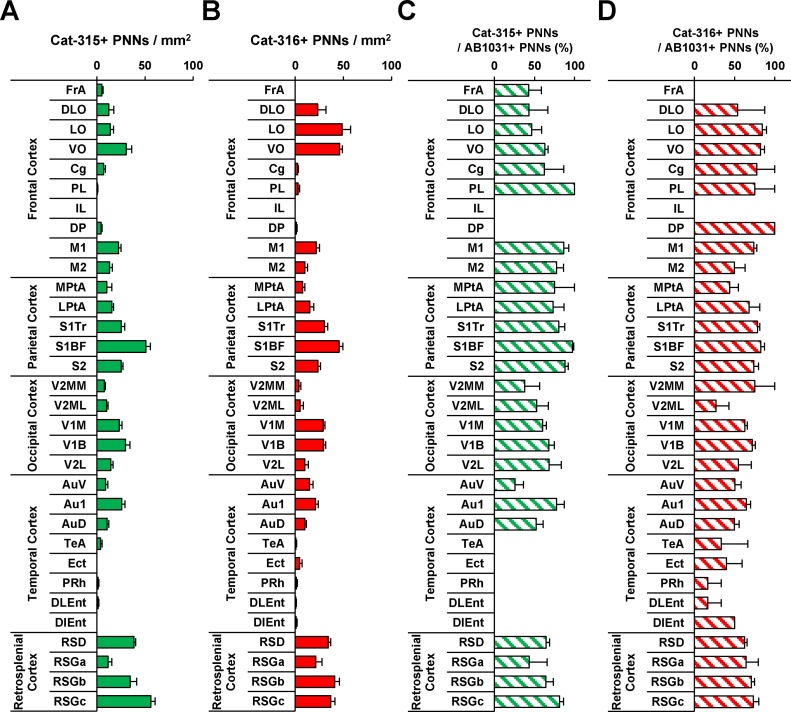
3.4. Comparative distribution of Cat-315- and Cat-316-positive PNNs
Further, we quantified the amount of PNNs co-labeled for Cat-315 and Cat-316 in the mouse cerebral cortex (Fig. 6A,B). The density of both Cat-315- and Cat-316-positive PNNs showed significantly different variations throughout the various cortical regions examined. In many areas (i.e., FrA, Cg, PL, IL, DP, TeA, Ect, PRh, DLEnt, and DIEnt), the number of both Cat-315- and Cat-316-positive PNNs was relatively low.
Fig. 6.
Quantitative analyses of Cat-315- and Cat-316-positive PNNs in the mouse cerebral cortex.
Quantified number of Cat-315-positive PNNs in the mouse cerebral cortex (A). Quantified number of Cat-316-positive PNNs in the mouse cerebral cortex (B).
The proportion of AB1031+ PNNs co-localized with Cat-315 (C), and the proportion of AB1031+ PNNs co-localized with Cat-316 (D) in the mouse cerebral cortex. Data are expressed as the mean ± SEM. The respective P values are listed in Table 1.
Cat-315 labeled many PNNs in the VO (Fig. 6A) and M1/M2 subregions of the motor cortex. In contrast, the density of Cat-315-positive PNNs was relatively low in the remaining subregions of the frontal cortex (i.e., FrA, Cg, PL, IL, and DP). In the parietal cortex, the Cat-315-positive PNN density was high, while in the MPtA/LPtA subregions of the parietal association cortex, Cat-315-positive PNN expression was low compared to the S1Tr/S1BF/S2 subregions of the somatosensory cortex. In the occipital cortex, low Cat-315-positive PNN density was observed in secondary (i.e., V2MM, V2ML, and V2L) compared to primary (i.e., V1M and V1B) subregions of the visual cortex. In the temporal cortex, Au1 exhibited high Cat-315-positive PNN density, followed by lower densities in AuV and AuD. The remaining subregions of the temporal cortex exhibited much lower Cat-315-positive PNNs than the other cortices. High numbers of Cat-315-positive PNNs were seen in the retrosplenial dysgranular cortex (RSD), as well as the b and c regions of the retrosplenial granular cortex (RSGb and RSGc, respectively). Cat-315-positive PNN density was lower in the remaining subregion of the retrosplenial cortex (i.e., RSGa).
In the LO/VO subregions of the frontal cortex, the density of Cat-316-positive PNNs was high compared to that in the remaining subregions (Fig. 6B). The FrA, Cg, PL, IL, and DP subregions exhibited lower Cat-316-positive PNN density relative to the DLO, LO, VO, M1, and M2 subregions of the frontal cortex. In the parietal cortex, low Cat-316-positive PNN density was observed in the MPtA/LPtA subregions of the association cortex. In the S1Tr, S1BF, and S2 subregions of the somatosensory cortex, the density of Cat-316-positive PNNs was high relative to that observed in the MPtA/LPtA subregions. In the occipital cortex, the density of Cat-316-positive PNNs in the primary visual cortex (i.e., V1M and V1B) was high compared to that observed in the secondary visual cortex (i.e., V2MM, V2ML, and V2L). In the temporal cortex, the Au1/AuD/AuV subregions of the auditory cortex exhibited high Cat-316-positive PNN densities, followed by lower densities in the TeA/Ect/PRh/DLEnt/DIEnt subregions of the entorhinal cortex. High numbers of Cat-316-positive PNNs were also seen in the RSD, RSGb, and RSGc subregions, while the remaining subregion of the retrosplenial cortex (i.e., RSGa) exhibited fewer Cat-316-positive PNNs.
3.5. Co-localization of Cat-315, Cat-316, and AB1031
In order to examine the relationship of aggrecan components in PNNs of the cerebral cortex, we carried out a quantitative analysis of AB1031 and Cat-315 co-localization, as well as of AB1031 and Cat-316 co-localization (Fig. 6C and D). Regions that exhibited low levels of AB1031 and Cat-315 labeling were excluded from the analysis (i.e., IL, DP, TeA, Ect, PRh, DLEnt, and DIEnt; Figs. 5B and 6A,C). In most other regions, AB1031+ PNNs also labeled with Cat-315 (Fig. 6C). This double-labeling was highest in all subregions of the parietal and retrosplenial cortices, and was also high in the PL subregion of the frontal cortex, as well as in the M1/M2 subregions of the motor cortex (∼70%). In the remaining subregions of the frontal cortex (i.e., FrA, DLO, VO, and Cg), about half of the AB1031+ PNNs were positive for Cat-315. In the V2MM/V2ML subregions of the occipital cortex, a low percentage of AB1031+ PNNs co-localized with Cat-315. In the temporal cortex, Au1 exhibited a higher percentage of AB1031-Cat-315 co-localization relative to the remaining auditory cortex subregions (i.e., AuV and AuD).
Regarding the evaluation of PNNs double-labeled for AB1031 and Cat-316, regions which exhibited little individual labeling of AB1031 and Cat-316 (i.e., FrA and IL) were excluded from the analysis (Figs. 5B and 6B,D). In the frontal cortex, the percentage of AB1031+ PNNs that also labeled for Cat-316 was about 70%. In the MPtA subregion of the parietal cortex, about 50% of AB1031+ PNNs co-localized with Cat-316, while double labeling was higher (∼70%) in the remaining subregions (i.e., LPtA, S1Tr, S1BF, and S2). The percentage of AB1031+ PNNs co-localized with Cat-316 in the V2ML subregion of the occipital cortex was lower than in the remaining subregions (i.e., V2MM, V1M, V1B, and V2L). The PRh/DLEnt subregions of the temporal cortex exhibited a lower number of AB1031+ PNNs co-localized with Cat-316 than the remaining subregions of the temporal cortex. Finally, the percentage of AB1031+ PNNs that also labeled with Cat-316 was similar throughout all subregions of the retrosplenial cortex.
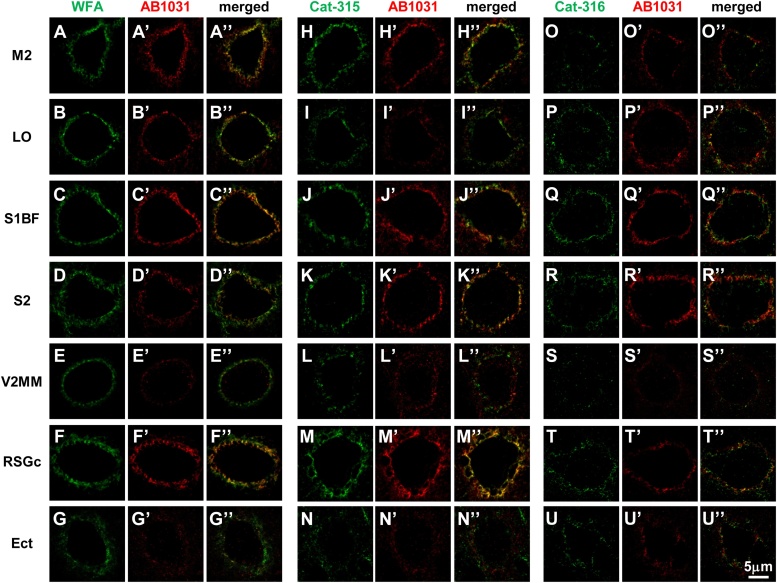
3.6. Perisomatic distribution patterns of WFA-, AB1031-, Cat-315-, and Cat-316-positive PNN components
To address the perisomatic distribution of WFA-, AB1031-, Cat-315-, and Cat-316-positive PNN components in the mouse cerebral cortex, we conducted high-magnification image analyses of stained sections. Using this approach, we found labeled components displayed a punctate distribution pattern around the soma (Fig. 7). Further, we found that AB1031+ puncta appeared close to, but did not overlap with, WFA+ molecules (Fig. 7A–G). Puncta co-labeled for Cat-315 and Cat-316 were also observed in PNNs (Fig. 7H–N, O–U) and, interestingly, these overlapped with PNN components positive for AB1031 (Fig. 7H”–N”, O”–U”).
Fig. 7.
Distribution patterns of WFA-, AB1031-, Cat-315-, and Cat-316-positive molecules in PNNs of the mouse cerebral cortex.
High-magnification confocal images of WFA (A-G) and AB1031 (A’-G’) labeling, and the merged image (A”-G”). High-magnification confocal images of Cat-315 (H-N) and AB1031 (H’-N’) labeling, and the merged image (H”-N”). High-magnification confocal images of Cat-316 (O-U) and AB1031 (O’-U’) labeling, and the merged image (O”-U”). Images of M2 (A-A”, H-H”, O-O”), LO (B-B”, I-I”, P-P”), S1BF (C-C”, J-J”, Q-Q”), S2 (D-D”, K-K”, R-R”), V2MM (E-E”, L-L”, S-S”), RSGc (F-F”, M-M”, T-T”), and Ect (G-G”, N-N”, U-U”) are shown. Scale bar = 5 μm in U” (applies to A–U”).
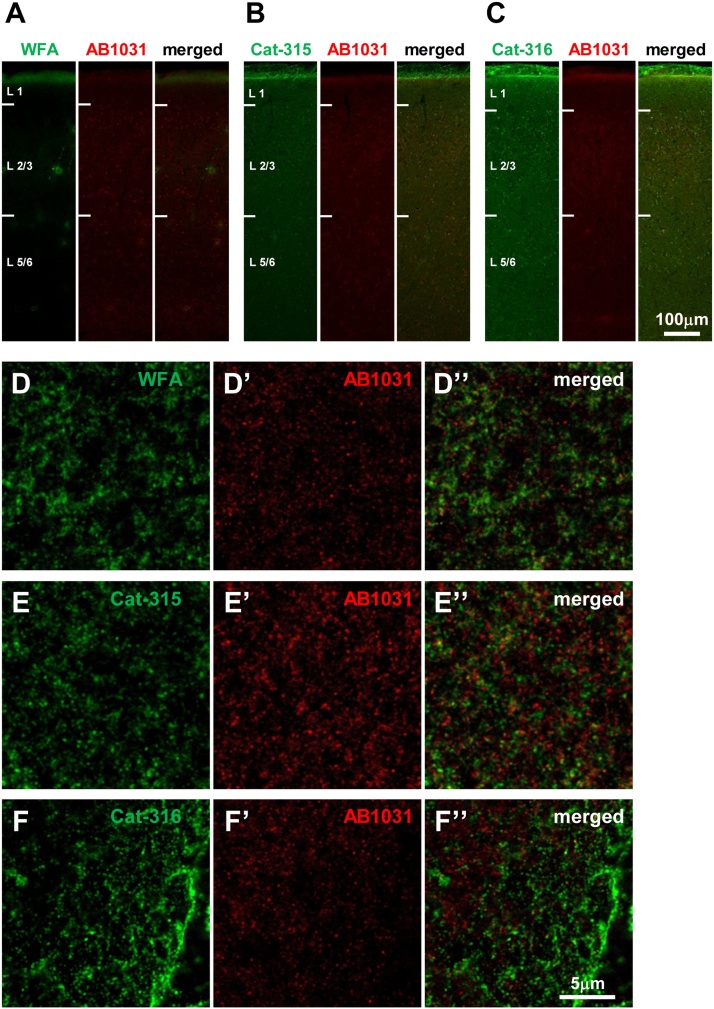
3.7. WFA+ molecules in the upper level of layer 1 of the PRh, DLEnt, and DIEnt
Since we observed intense WFA and Cat-316 reactivity in layer 1 of PRh/DLEnt/DIEnt subregions in the temporal cortex (Fig. 3), we decided to further analyze the composition of the labeled PNN components in this area. Low magnification views of WFA, AB1031, Cat-315, and Cat-316 reactivity within layer 1 showed similar associations between positively labeled components (Fig. 8A–C). High magnification images revealed AB1031-, Cat-315-, and Cat-316-positive molecules scattered throughout the upper level of layer 1 (Fig. 8D–F and D’–F’), and that WFA+ molecules did not co-localize with AB1031+ molecules in this area (Fig. 8D–D”). Moreover, AB1031+ molecules did not co-localize with either Cat-315+ or Cat-316+ molecules (Fig. 8E–E” and F–F”).
Fig. 8.
Distribution of WFA-, AB1031-, Cat-315-, and Cat-316-positive molecules in the mouse entorhinal cortex.
Representative confocal images showing double labeling of WFA and AB1031 (A), Cat-315 and AB1031 (B), and Cat-316 and AB1031 (C) in the mouse entorhinal cortex. High magnification images of WFA (D’) and AB1031 (D’) labeling, and the merged image (D”), in the upper region of L1 in the DLEnt. High magnification images of Cat-315 (E) and AB1031 (E’) labeling, and the merged image (E”), in the upper region of L1 in the DLEnt. High magnification images of Cat-316 (F) and AB1031 (F’) labeling, and the merged image (F”), in the upper region of L1 in the DLEnt. Scale bar = 100 μm in C (applies to A−C) and 5 μm in F” (applies to D–F”).
4. Discussion
This study was the first to quantitatively show region-specific distribution of PNNs in the mouse cortex through antibodies that recognize different aggrecan epitopes. Although WFA+ PNNs were present throughout the cortex, the existence of AB1031-, Cat-315-, and Cat-316-positive PNNs was disparate and region-specific. Together, our findings revealed 1) varied, brain region-specific aggrecan expression, 2) varied brain region-specific expression of aggrecan-positive PNN components, and 3) the existence of WFA+ PNNs without aggrecan in the mature mouse cortex.
Previous studies have reported the existence of region-selective WFA+ PNN expression throughout the mature rodent cortex (Brückner et al., 2000, Brückner et al., 2003; Alpár et al., 2006; Horii-Hayashi et al., 2015). However, until now, WFA+ PNN densities in the various cortical regions had not been clarified. Our study revealed heterogeneous WFA+ PNN densities across the cortex, suggesting diverging, region-specific PNN functions.
A widely held theory is that WFA+ PNNs control plasticity. However, the expression of WFA+ PNNs has been observed throughout the cortex. As only certain cortical regions (e.g., the frontal cortex) are highly plastic over the course of an organism’s lifetime (Sadato et al., 2004; Canto et al., 2008; Chapman et al., 2008; Jung et al., 2008; Kolb, 2009), it is unlikely that WFA+ PNN regulate plasticity in all cortices. Thus, WFA+ PNNs must have brain region-specific functions. Detection of PNNs using WFA is widely used but, as demonstrated in recent studies, PNN components are brain region- and cell type-specific (Berretta et al., 2015; Dauth et al., 2016; Ueno et al., 2017a). It is therefore plausible that the functions of WFA+ PNNs in each cortical region may differ depending on its components.
In the current study, we observed that almost all AB1031+ PNNs were also positive for WFA. However, in some regions of the mouse cortex, WFA+ PNNs could be detected in the absence of AB1031-, Cat-315-, and Cat-316-positive components. This is concurrent with previous findings, which have shown that aggrecan protein and aggrecan mRNA are not ubiquitously expressed in all cortical regions (Morawski et al., 2012a, Morawski et al., 2012b). Several reports have suggested the possibility that aggrecan is involved in plasticity (McRae et al., 2007; Nakamura et al., 2009; Ye and Miao, 2013; Ueno et al., 2017b). Aggrecan expression in PNNs signals the end of the critical period, and is dependent on sensory input (Sur et al., 1988; Hockfield et al., 1990). In the absence of such input, the formation of both Cat-315- and Cat-316-positive, but not WFA+, PNNs decrease (McRae et al., 2007; Lander et al., 1997; Ueno et al., 2017a, Ueno et al., 2017b, Ueno et al., 2017c). This is because PNNs in the sensory cortex are formed during postnatal development, and AB1031- and Cat-315-positive PNNs are formed after WFA+ PNNs (Ueno et al., 2017a, Ueno et al., 2017b, Ueno et al., 2017c; Ye and Miao, 2013). In addition, WFA+ PNNs that do not have the Cat-315 epitope increase with age (Karetko-Sysa et al., 2014). Together, this suggests that neural plasticity is lost following the expression of aggrecan in WFA+ PNNs.
Previous reports have suggested that to access the backbone of the aggrecan molecule for staining with the AB1031 antibody, the sections must be treated with Chondroitinase ABC to remove the GAG chains (Härtig et al., 2016; Giamanco et al., 2010). Chondroitinase ABC treatment removes the GAG chain recognized by WFA. When this enzyme treatment is performed, double staining of WFA and AB1031 cannot be performed. However, it is also known that AB1031+ aggrecan can be sufficiently stained without this enzyme treatment (Carstens et al., 2016; Dauth et al., 2016; Miyata and Kitagawa, 2016; Yamada and Jinno, 2017). For these reasons, we performed double immunostaining without enzyme treatment in the present study. In order to compare the distributions of AB1031+ and WFA+ molecules in more detail, it is necessary to develop new experimental methods.
In recent years, it has been shown that the expression of a chondroitin sulfate group (recognized by Cat-316) in PNNs is involved in cortical plasticity (Matthews et al., 2002; Dino et al., 2006; Miyata et al., 2012). Moreover, the HNK-1 epitope (Dino et al., 2006) (recognized by Cat-315) has also been reported to relate to synaptic plasticity (Senn et al., 2002). Both chondroitin sulfate and HNK-1 are widely present in the central nervous system as ECM molecules (Kleene and Schachner, 2004). CSPGs and other ECM proteins inhibit plasticity and suppress the reformation of both axons and synapses (Gilbert et al., 2005; Silver and Silver, 2014). During CNS trauma, CSPG expression (including aggrecan) increases around the site of injury and suppresses axonal reorganization (Oohira et al., 1994; McKeon et al., 1999; Moon et al., 2002, Jones et al., 2003). In vitro, these functions are dependent on CSPG (versican, neurocan, brevican, and aggrecan) core proteins, which, after chondroitinase ABC treatment, have no inhibitory function (Snow et al., 1990; Maeda and Noda, 1996; Margolis et al., 1996; Yamada et al., 1997; Schmalfeldt et al., 2000). These reports indicate that the inhibitory functions of CSPGs and other ECM proteins are dependent on chondroitin sulfate chains rather than on CSPG core proteins. It is therefore possible that WFA+ PNNs that contain aggrecan expressing a chondroitin sulfate group control synaptic plasticity. Our findings suggest that plasticity may be high in areas where aggrecan expression is low. Indeed, we found that areas with low aggrecan corresponded to the prefrontal, entorhinal, and secondary sensory cortices, which have been shown to be highly plastic over the course of an organism’s lifetime.
Compared to other lecticans, aggrecan is expressed on many PNNs (Matsui et al., 1998; Dauth et al., 2016), albeit in smaller amounts (Dauth et al., 2016). Generally, PNNs are thought to always contain aggrecan (Galtrey et al., 2008; Giamanco et al., 2010). Indeed, a previous study reported no WFA reactivity in primary cultured neurons of aggrecan-deficient mice, suggesting that aggrecan is essential for the formation of PNNs (Giamanco and Matthews, 2012). Conversely, some studies, including the current investigation, have reported the existence of WFA+ PNNs that do not contain aggrecan (Morawski et al., 2012a, Morawski et al., 2012b; Karetko-Sysa et al., 2014; Ueno et al., 2017a), suggesting that aggrecan may not be an essential component in the PNNs of specific brain regions. Interestingly, it is indicated that absence of aggrecan has no effect on the expression patterns of other PNN markers, hyaluronan proteoglycan link protein 1, tenascin-R, hyaluronan, and brevican (Giamanco et al., 2010). It is reasonable to suggest that the constituent elements of PNN are brain-region specific.
It is difficult to clarify the distribution of the various PNN components using low magnification analysis. Therefore, until recent years, each component was considered to be consistent in its localization. Our previous study indicated divergent distributions of WFA- and Cat-315-positive molecules (Ueno et al., 2017b). High-magnification analysis in the current study corroborated these findings, revealing varied localization of WFA-, AB1031-, Cat-315-, and Cat-316-positive molecules. Even in the rat cortex, the distribution of aggrecan-positive PNNs is different depending on the antibody used to detect them (i.e., Cat-315 or AB1031) (Madinier et al., 2014). Lectin WFA and the monoclonal antibodies, AB1031, Cat-315, and Cat-316, recognize different aggrecan epitopes; thus, divergent distributions are reasonable. Moreover, it is possible that varied PNN component distributions are due to each CSPG being secreted from different cell types. For example, aggrecan seems to be secreted from PV-positive interneurons (Lander et al., 1998), while brevican has been shown to be secreted from neurons and glia (Carulli et al., 2006). Regardless of the etiology for the distribution heterogeneity, these findings suggest that the various PNN components may have different functions, and that there is no single lectin or antibody that is capable of detecting all of the PNNs in the cortex.
While the detailed functions of PNNs remain unclear, ECM proteins are widely expressed in the central nervous system, particularly in the brainstem and cerebellum. Hence, it is assumed that these proteins must possess a protective function. Among the ECM family of proteins, aggrecan, tenascin-R, and the link proteins of PNNs are known to play neuroprotective roles (Morawski et al., 2012a, Morawski et al., 2012b; Suttkus et al., 2014). Consistent with this, overexpression of aggrecan in the cortex is associated with less susceptibility to tau protein-induced cytotoxicity in Alzheimer’s disease (Morawski et al., 2010). In addition, PNNs protect neurons from oxidative stress (Cabungcal et al., 2013; Ueno et al., 2017c), and PNN-rich brain regions are less susceptible to trauma (Harris et al., 2009). It has also been shown that PNNs and CSPGs are decreased in the entorhinal cortex of patients with schizophrenia (Pantazopoulos et al., 2010). Similarly, a decrease in prefrontal cortex PNNs has also been observed in this patient population (Mauney et al., 2013). These reports indicate that CSPGs, such as PNNs, may be one of the underlying etiologies of schizophrenia, although it remains unclear why a decrease in PNNs does not appear in the sensory cortex of patients with schizophrenia. Interestingly, our current study revealed that aggrecan was either not present or expressed in very low levels in areas of the association cortex, where human studies have revealed decreased PNNs in patients with schizophrenia (i.e., the entorhinal and prefrontal subregions). Thus, PNNs in these brain areas may have little to no neuroprotective function.
It should be noted that there is some debate as to what WFA recognizes, as recent studies have questioned the assumption that the plant-derived lectin binds to the N-acetylgalactosamine of aggrecan (Karetko-Sysa et al., 2011; Miyata and Kitagawa, 2017; Ueno et al., 2017b). In our previous study, we found an accumulation of WFA+ molecules in the upper region of the temporal cortex. In previous studies, WFA-recognition molecules have been clearly shown, but no report has focused on them yet (Brückner et al., 2003; Horii-Hayashi et al., 2015; Yang et al., 2015). In the current study, AB1031-, Cat-315-, and Cat-316-positive molecules were observed to exist in this region, but they appeared uniform in each layer of the cortex. Layer 1 is special, as it contains no pyramidal cells. Instead, this layer comprises the axons of pyramidal cells in layers 2–6, as well as those of inhibitory interneurons and neurons from other cortical regions (Felleman and Van Essen, 1991; Vogt, 1991). Condensed WFA+ molecules are likely present because of some unknown role; thus, future studies should evaluate layer 1 of the temporal cortex, as it likely holds the key to clarifying which molecule is recognized by WFA, as well as its function.
5. Conclusions
The present clarified the region-specific expression of aggrecan components in the mature mouse cortex. Specifically showing divergent localizations of WFA-, AB1031-, Cat-315-, and Cat-316-positive PNN molecules. These results indicate that plasticity and neuroprotective function may differ depending on the cortical region, and provide useful insight for the development of methods to treat neuropsychiatric disorders and neurodegenerative diseases.
Author contributions
Study concept and design: H.U., M.O., and T.K. Data acquisition: H.U., K.F., K.T., and S.S. Data analysis and interpretation: H.U., K.F., K.T., and S.S. Drafting of the manuscript: H.U., M.O., and T.K. Critical revision of the manuscript for important intellectual content: S.M., N.K., K.W., S.A., and T.I. Statistical analyses: H.U and S.S. Study supervision: M.O. and T.I.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare they have no competing financial interests.
Acknowledgements
We thank Kawasaki Medical School Central Research Institute for making instruments available to support this study. We thank Y. Koshidaka and M. Adachi for the technical assistance. The authors would also like to thank Editage (www.editage.jp) for English language editing.
Contributor Information
Hiroshi Ueno, Email: dhe422007@s.okayama-u.ac.jp.
Kazuki Fujii, Email: kfujii@cts.u-toyama.ac.jp.
Shunsuke Suemitsu, Email: ssue@med.kawasaki-m.ac.jp.
Shinji Murakami, Email: muraka@med.kawasaki-m.ac.jp.
Naoya Kitamura, Email: n-kitamura@med.kawasaki-m.ac.jp.
Kenta Wani, Email: kenta99101@yahoo.co.jp.
Shozo Aoki, Email: shoaoki@med.kawasaki-m.ac.jp.
Motoi Okamoto, Email: mokamoto@md.okayama-u.ac.jp.
Takeshi Ishihara, Email: t-ishihara@med.kawasaki-m.ac.jp.
Keizo Takao, Email: takao@cts.u-toyama.ac.jp.
References
- Alpár A., Gärtner U., Härtig W., Brückner G. Distribution of pyramidal cells associated with perineuronal nets in the neocortex of rat. Brain Res. 2006;1120:13–22. doi: 10.1016/j.brainres.2006.08.069. [DOI] [PubMed] [Google Scholar]
- Balmer T.S., Carels V.M., Frisch J.L., Nick T.A. Modulation of perineuronal nets and parvalbumin with developmental song learning. J. Neurosci. 2009;29:12878–12885. doi: 10.1523/JNEUROSCI.2974-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandtlow C.E., Zimmermann D.R. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol. Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- Berretta S., Pantazopoulos H., Markota M., Brown C., Batzianouli E.T. Losing the sugar coating: potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr. Res. 2015;167:18–27. doi: 10.1016/j.schres.2014.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brückner G., Brauer K., Härtig W., Wolff J.R., Rickmann M.J., Derouiche A., Delpech B., Girard N., Oertel W.H., Reichenbach A. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8:183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- Brückner G., Grosche J., Schmidt S., Härtig W., Margolis R.U., Delpech B., Seidenbecher C.I., Czaniera R., Schachner M. Postnatal development of perineuronal nets in wild-type mice and in a mutant deficient in tenascin-R. J. Comp. Neurol. 2000;428:616–629. doi: 10.1002/1096-9861(20001225)428:4<616::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Brückner G., Grosche J., Hartlage-Rübsamen M., Schmidt S., Schachner M. Region and lamina-specific distribution of extracellular matrix proteoglycans, hyaluronan and tenascin-R in the mouse hippocampal formation. J. Chem. Neuroanat. 2003;26:37–50. doi: 10.1016/s0891-0618(03)00036-x. [DOI] [PubMed] [Google Scholar]
- Cabungcal J.H., Steullet P., Morishita H., Kraftsik R., Cuenod M., Hensch T.K., Do K.Q. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9130–9135. doi: 10.1073/pnas.1300454110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C.B., Wouterlood F.G., Witter M.P. What does the anatomical organization of the entorhinal cortex tell us? Neural. Plast. 2008;2008:381243. doi: 10.1155/2008/381243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens K.E., Phillips M.L., Pozzo-Miller L., Weinberg R.J., Dudek S.M. Perineuronal nets suppress plasticity of excitatory synapses on CA2 pyramidal neurons. J. Neurosci. 2016;36:6312–6320. doi: 10.1523/JNEUROSCI.0245-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli D., Rhodes K.E., Brown D.J., Bonnert T.P., Pollack S.J., Oliver K., Strata P., Fawcett J.W. Composition of perineuronal nets in the adult rat cerebellum and the cellular origin of their components. J. Comp. Neurol. 2006;494:559–577. doi: 10.1002/cne.20822. [DOI] [PubMed] [Google Scholar]
- Chapman C.A., Jones R.S., Jung M. Neuronal plasticity in the entorhinal cortex. Neural. Plast. 2008;2008:314785. doi: 10.1155/2008/314785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig S., Commins S. The subiculum to entorhinal cortex projection is capable of sustaining both short- and long-term plastic changes. Behav. Brain Res. 2006;174:281–288. doi: 10.1016/j.bbr.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Dauth S., Grevesse T., Pantazopoulos H., Campbell P.H., Maoz B.M., Berretta S., Parker K.K. Extracellular matrix protein expression is brain region dependent. J. Comp. Neurol. 2016;524:1309–1336. doi: 10.1002/cne.23965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dino M.R., Harroch S., Hockfield S., Matthews R.T. Monoclonal antibody Cat-315 detects a glycoform of receptor protein tyrosine phosphatase beta/phosphacan early in CNS development that localizes to extrasynaptic sites prior to synapse formation. Neuroscience. 2006;142:1055–1069. doi: 10.1016/j.neuroscience.2006.07.054. [DOI] [PubMed] [Google Scholar]
- Felleman D.J., Van Essen D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Foster N.L., Mellott J.G., Schofield B.R. Perineuronal nets and GABAergic cells in the inferior colliculus of guinea pigs. Front. Neuroanat. 2014;7:53. doi: 10.3389/fnana.2013.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtrey C.M., Kwok J.C., Carulli D., Rhodes K.E., Fawcett J.W. Distribution and synthesis of extracellular matrix proteoglycans hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur. J. Neurosci. 2008;27:1373–1390. doi: 10.1111/j.1460-9568.2008.06108.x. [DOI] [PubMed] [Google Scholar]
- Gawryluk J.W., Wang J.F., Andreazza A.C., Shao L., Young L.T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 2011;14:123–130. doi: 10.1017/S1461145710000805. [DOI] [PubMed] [Google Scholar]
- Giamanco K.A., Matthews R.T. Deconstructing the perineuronal net: cellular contributions and molecular composition of the neuronal extracellular matrix. Neuroscience. 2012;218:367–384. doi: 10.1016/j.neuroscience.2012.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamanco K.A., Morawski M., Matthews R.T. Perineuronal net formation and structure in aggrecan knockout mice. Neuroscience. 2010;170:1314–1327. doi: 10.1016/j.neuroscience.2010.08.032. [DOI] [PubMed] [Google Scholar]
- Gilbert R.J., McKeon R.J., Darr A., Calabro A., Hascall V.C., Bellamkonda R.V. CS-4,6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Mol. Cell Neurosci. 2005;29:545–558. doi: 10.1016/j.mcn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Gogolla N., Caroni P., Lüthi A., Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Guimarães A., Zaremba S., Hockfield S. Molecular and morphological changes in the cat lateral geniculate nucleus and visual cortex induced by visual deprivation are revealed by monoclonal antibodies Cat-304 and Cat-301. J. Neurosci. 1990;10:3014–3024. doi: 10.1523/JNEUROSCI.10-09-03014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtig W., Brauer K., Bigl V., Brückner G. Chondroitin sulfate proteoglycan-immunoreactivity of lectin-labeled perineuronal nets around parvalbumin-containing neurons. Brain Res. 1994;635:307–311. doi: 10.1016/0006-8993(94)91452-4. [DOI] [PubMed] [Google Scholar]
- Härtig W., Appel S., Suttkus A., Grosche J., Michalski D. Abolished perineuronal nets and altered parvalbumin-immunoreactivity in the nucleus reticularis thalami of wildtype and 3xTg mice after experimental stroke. Neuroscience. 2016;337:66–87. doi: 10.1016/j.neuroscience.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Harris N.G., Carmichael S.T., Hovda D.A., Sutton R.L. Traumatic brain injury results in disparate regions of chondroitin sulfate proteoglycan expression that are temporally limited. J. Neurosci. Res. 2009;87:2937–2950. doi: 10.1002/jnr.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch T.K., Fagiolini M. Excitatory-inhibitory balance and critical period plasticity in developing visual cortex. Prog. Brain Res. 2005;147:115–124. doi: 10.1016/S0079-6123(04)47009-5. [DOI] [PubMed] [Google Scholar]
- Hockfield S., Kalb R.G., Zaremba S., Fryer H. Expression of neural proteoglycans correlates with the acquisition of mature neuronal properties in the mammalian brain. Cold Spring Harb. Symp. Quant. Biol. 1990;55:505–514. doi: 10.1101/sqb.1990.055.01.049. [DOI] [PubMed] [Google Scholar]
- Horii-Hayashi N., Sasagawa T., Matsunaga W., Nishi M. Development and structural variety of the chondroitin sulfate proteoglycans-contained extracellular matrix in the mouse brain. Neural. Plast. 2015;2015:256389. doi: 10.1155/2015/256389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L.L., Sajed D., Tuszynski M.H. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J. Neurosci. 2003;23:9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M.W., Baeg E.H., Kim M.J., Kim Y.B., Kim J.J. Plasticity and memory in the prefrontal cortex. Rev. Neurosci. 2008;19:29–46. doi: 10.1515/revneuro.2008.19.1.29. [DOI] [PubMed] [Google Scholar]
- Karetko-Sysa M., Skangiel-Kramska J., Nowicka D. Disturbance of perineuronal nets in the perilesional area after photothrombosis is not associated with neuronal death. Exp. Neurol. 2011;231:113–126. doi: 10.1016/j.expneurol.2011.05.022. [DOI] [PubMed] [Google Scholar]
- Karetko-Sysa M., Skangiel-Kramska J., Nowicka D. Aging somatosensory cortex displays increased density of WFA-binding perineuronal nets associated with GAD-negative neurons. Neuroscience. 2014;277:734–746. doi: 10.1016/j.neuroscience.2014.07.049. [DOI] [PubMed] [Google Scholar]
- Kleene R., Schachner M. Glycans and neural cell interactions. Nat. Rev. Neurosci. 2004;5:195–208. doi: 10.1038/nrn1349. [DOI] [PubMed] [Google Scholar]
- Kolb B. Brain and behavioural plasticity in the developing brain: neuroscience and public policy. Paediatr. Child Health. 2009;14:651–652. doi: 10.1093/pch/14.10.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander C., Kind P., Maleski M., Hockfield S. A family of activity-dependent neuronal cell-surface chondroitin sulfate proteoglycans in cat visual cortex. J. Neurosci. 1997;17:1928–1939. doi: 10.1523/JNEUROSCI.17-06-01928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander C., Zhang H., Hockfield S. Neurons produce a neuronal cell surface-associated chondroitin sulfate proteoglycan. J. Neurosci. 1998;18:174–183. doi: 10.1523/JNEUROSCI.18-01-00174.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendvai D., Morawski M., Négyessy L., Gáti G., Jäger C., Baksa G., Glasz T., Attems J., Tanila H., Arendt T., Harkany T., Alpár A. Neurochemical mapping of the human hippocampus reveals perisynaptic matrix around functional synapses in Alzheimer's disease. Acta Neuropathol. 2013;125:215–229. doi: 10.1007/s00401-012-1042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madinier A., Quattromani M.J., Sjölund C., Ruscher K., Wieloch T. Enriched housing enhances recovery of limb placement ability and reduces aggrecan-containing perineuronal nets in the rat somatosensory cortex after experimental stroke. PLoS One. 2014;9:e93121. doi: 10.1371/journal.pone.0093121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N., Noda M. 6B4 proteoglycan/phosphacan is a repulsive substratum but promotes morphological differentiation of cortical neurons. Development. 1996;122:647–658. doi: 10.1242/dev.122.2.647. [DOI] [PubMed] [Google Scholar]
- Maffei A., Turrigiano G. The age of plasticity: developmental regulation of synaptic plasticity in neocortical microcircuits. Prog. Brain Res. 2008;169:211–223. doi: 10.1016/S0079-6123(07)00012-X. [DOI] [PubMed] [Google Scholar]
- Margolis R.K., Rauch U., Maurel P., Margolis R.U. Neurocan and phosphacan: two major nervous tissue-specific chondroitin sulfate proteoglycans. Perspect. Dev. Neurobiol. 1996;3:273–290. [PubMed] [Google Scholar]
- Matsui F., Nishizuka M., Yasuda Y., Aono S., Watanabe E., Oohira A. Occurrence of a N-terminal proteolytic fragment of neurocan, not a C-terminal half, in a perineuronal net in the adult rat cerebrum. Brain Res. 1998;790:45–51. doi: 10.1016/s0006-8993(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Matthews R.T., Kelly G.M., Zerillo C.A., Gray G., Tiemeyer M., Hockfield S. Aggrecan glycoforms contribute to the molecular heterogeneity of perineuronal nets. J. Neurosci. 2002;22:7536–7547. doi: 10.1523/JNEUROSCI.22-17-07536.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauney S.A., Athanas K.M., Pantazopoulos H., Shaskan N., Passeri E., Berretta S., Woo T.U.W. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol. Psychiatry. 2013;15:427–435. doi: 10.1016/j.biopsych.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon R.J., Jurynec M.J., Buck C.R. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J. Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae P.A., Rocco M.M., Kelly G., Brumberg J.C., Matthews R.T. Sensory deprivation alters aggrecan and perineuronal net expression in the mouse barrel cortex. J. Neurosci. 2007;16:5405–5413. doi: 10.1523/JNEUROSCI.5425-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae P.A., Baranov E., Sarode S., Brooks-Kayal A.R., Porter B.E. Aggrecan expression, a component of the inhibitory interneuron perineuronal net, is altered following an early-life seizure. Neurobiol. Dis. 2010;39:439–448. doi: 10.1016/j.nbd.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Kitagawa H. Chondroitin 6-sulfation regulates perineuronal net formation by controlling the stability of aggrecan. Neural Plast. 2016 doi: 10.1155/2016/1305801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S., Kitagawa H. Formation and remodeling of the brain extracellular matrix in neural plasticity: roles of chondroitin sulfate and hyaluronan. Biochim. Biophys. Acta. 2017;1861:2420–2434. doi: 10.1016/j.bbagen.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Miyata S., Komatsu Y., Yoshimura Y., Taya C., Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat. Neurosci. 2012;15:414–422. doi: 10.1038/nn.3023. [DOI] [PubMed] [Google Scholar]
- Moon L.D.F., Asher R.A., Rhodes K.E., Fawcett J.W. Relationship between sprouting axons, proteoglycans and glial cells following unilateral nigrostriatal axotomy in the adult rat. Neuroscience. 2002;109:101–117. doi: 10.1016/s0306-4522(01)00457-2. [DOI] [PubMed] [Google Scholar]
- Morawski M., Brückner G., Jäger C., Seeger G., Arendt T. Neurons associated with aggrecan-based perineuronal nets are protected against tau pathology in subcortical regions in Alzheimer's disease. Neuroscience. 2010;169:1347–1363. doi: 10.1016/j.neuroscience.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Morawski M., Brückner G., Arendt T., Matthews R.T. Aggrecan: beyond cartilage and into the brain. Int. J. Biochem. Cell Biol. 2012;44:690–693. doi: 10.1016/j.biocel.2012.01.010. [DOI] [PubMed] [Google Scholar]
- Morawski M., Brückner G., Jäger C., Seeger G., Matthews R.T., Arendt T. Involvement of perineuronal and perisynaptic extracellular matrix in Alzheimer's disease neuropathology. Brain Pathol. 2012;22:547–561. doi: 10.1111/j.1750-3639.2011.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S., Ikegaya Y., Narita M., Tamura H. Activation of perineuronal net-expressing excitatory neurons during associative memory encoding and retrieval. Sci. Rep. 2017;7:46024. doi: 10.1038/srep46024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Nakano K., Morita S., Nakashima T., Oohira A., Miyata S. Expression of chondroitin sulfate proteoglycans in barrel field of mouse and rat somatosensory cortex. Brain. Res. 2009;1252:117–129. doi: 10.1016/j.brainres.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Oohira A., Matsui F., Watanabe E., Kushima Y., Maeda N. Developmentally regulated expression of a brain specific species of chondroitin sulfate proteoglycan, neurocan, identified with a monoclonal antibody IG2 in the rat cerebrum. Neuroscience. 1994;60:145–157. doi: 10.1016/0306-4522(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H., Woo T.U.W., Lim M.P., Lange N., Berretta S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch. Gen. Psychiatry. 2010;67:155–166. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G., Franklin K.B.J. 4th ed. Academic Press; 2012. Paxinos and Franklin's the Mouse Brain in Stereotaxic Coordinates. [Google Scholar]
- Pizzorusso T., Medini P., Berardi N., Chierzi S., Fawcett J.W., Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;8:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Sadato N., Yamada H., Okada T., Yoshida M., Hasegawa T., Matsuki K.I., Yonekura Y., Itoh H. Age-dependent plasticity in the superior temporal sulcus in deaf humans: a functional MRI study. BMC Neurosci. 2004;5:56. doi: 10.1186/1471-2202-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalfeldt M., Bandtlow C.E., Dours-Zimmermann M.T., Winterhalter K.H., Zimmermann D.R. Brain derived versican V2 is a potent inhibitor of axonal growth. J. Cell Sci. 2000;113:807–816. doi: 10.1242/jcs.113.5.807. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Streit W.J., Müller C.M. Postnatal development and localization of an N-acetylgalactosamine containing glycoconjugate associated with nonpyramidal neurons in cat visual cortex. J. Comp. Neurol. 1993;329:313–327. doi: 10.1002/cne.903290303. [DOI] [PubMed] [Google Scholar]
- Seeger G., Brauer K., Härtig W., Brückner G. Mapping of perineuronal nets in the rat brain stained by colloidal iron hydroxide histochemistry and lectin cytochemistry. Neuroscience. 1994;58:371–388. doi: 10.1016/0306-4522(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Senn C., Kutsche M., Saghatelyan A., Bösl M.R., Löhler J., Bartsch U., Morellini F., Schachner M. Mice deficient for the HNK-1 sulfotransferase show alterations in synaptic efficacy and spatial learning and memory. Mol. Cell Neurosci. 2002;20:712–729. doi: 10.1006/mcne.2002.1142. [DOI] [PubMed] [Google Scholar]
- Silver D.J., Silver J. Contributions of chondroitin sulfate proteoglycans to neurodevelopment, injury, and cancer. Curr. Opin. Neurobiol. 2014;27:171–178. doi: 10.1016/j.conb.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow D.M., Lemmon V., Carrino D.A., Caplan A.I., Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp. Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Sorg B.A., Berretta S., Blacktop J.M., Fawcett J.W., Kitagawa H., Kwok J.C., Miquel M. Casting a wide net: role of perineuronal nets in neural plasticity. J. Neurosci. 2016;36:11459–11468. doi: 10.1523/JNEUROSCI.2351-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur M., Frost D.O., Hockfield S. Expression of a surface-associated antigen on Y-cells in the cat lateral geniculate nucleus is regulated by visual experience. J. Neurosci. 1988;8:874–882. doi: 10.1523/JNEUROSCI.08-03-00874.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttkus A., Rohn S., Weigel S., Glöckner P., Arendt T., Morawski M. Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress. Cell Death. Dis. 2014;13:e1119. doi: 10.1038/cddis.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H., Suemitsu S., Okamoto M., Matsumoto Y., Ishihara T. Parvalbumin neurons and perineuronal nets in the mouse prefrontal cortex. Neuroscience. 2017;343:115–127. doi: 10.1016/j.neuroscience.2016.11.035. [DOI] [PubMed] [Google Scholar]
- Ueno H., Suemitsu S., Okamoto M., Matsumoto Y., Ishihara T. Sensory experience-dependent formation of perineuronal nets and expression of Cat-315 immunoreactive components in the mouse somatosensory cortex. Neuroscience. 2017;355:161–174. doi: 10.1016/j.neuroscience.2017.04.041. [DOI] [PubMed] [Google Scholar]
- Ueno H., Suemitsu S., Murakami S., Kitamura N., Wani K., Okamoto M., Matsumoto Y., Ishihara T. Region-specific impairments in parvalbumin interneurons in social isolation-reared mice. Neuroscience. 2017;359:196–208. doi: 10.1016/j.neuroscience.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Vogt B.A. The role of layer I in cortical function. Cereb. Cort. 1991:49–79. [Google Scholar]
- Wegner F., Härtig W., Bringmann A., Grosche J., Wohlfarth K., Zuschratter W., Brückner G. Diffuse perineuronal nets and modified pyramidal cells immunoreactive for glutamate and the GABA(A) receptor alpha1 subunit form a unique entity in rat cerebral cortex. Exp. Neurol. 2003;184:705–714. doi: 10.1016/S0014-4886(03)00313-3. [DOI] [PubMed] [Google Scholar]
- Yamada J., Jinno S. Molecular heterogeneity of aggrecan-based perineuronal nets around five subclasses of parvalbumin-expressing neurons in the mouse hippocampus. J. Comp. Neurol. 2017;525:1234–1249. doi: 10.1002/cne.24132. [DOI] [PubMed] [Google Scholar]
- Yamada H., Fredette B., Shitara K., Hagihara K., Miura R., Ranscht B., Stallcup W.B., Yamaguchi Y. The brain chondroitin sulfate proteoglycan brevican associates with astrocytes ensheathing cerebellar glomeruli and inhibits neurite outgrowth from granule neurons. J. Neurosci. 1997;17:7784–7795. doi: 10.1523/JNEUROSCI.17-20-07784.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol. Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Cacquevel M., Saksida L.M., Bussey T.J., Schneider B.L., Aebischer P., Melani R., Pizzorusso T., Fawcett J.W., Spillantini M.G. Perineuronal net digestion with chondroitinase restores memory in mice with tau pathology. Exp. Neurol. 2015;265:48–58. doi: 10.1016/j.expneurol.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q., Miao Q.L. Experience-dependent development of perineuronal nets and chondroitin sulfate proteoglycan receptors in mouse visual cortex. Matrix Biol. 2013;32:352–363. doi: 10.1016/j.matbio.2013.04.001. [DOI] [PubMed] [Google Scholar]