Abstract
Retrospective studies in human populations indicate that protein deprivation during pregnancy and early life (early protein malnutrition, EPM) is associated with cognitive impairments, learning disabilities and may represent a risk factor for the late onset of some psychiatric disorders, fundamentally schizophrenia, a condition where the prefrontal cortex plays an important role. The purpose of this study was to analyze whether EPM affects structural aspects of the rat medial prefrontal cortex (mPFC), such as cortical volume, neuronal density and neuronal soma size, which seem altered in patients with schizophrenia. For this, a rat model of EPM (5% casein from conception to postnatal day 60) was adopted and the rat mPFC volume, total number of neurons and average neuronal volume were evaluated on postnatal day 60 (post-pubertal animals) by histo- and immunohistochemical techniques using unbiased stereological analysis. EPM did not alter the number of NeuN+ neurons in the rat mPFC. However, a very significant decrease in mPFC volume and average neuronal size was observed in malnourished rats. Although the present study does not establish causal relationships between malnutrition and schizophrenia, our results may indicate a similar structural phenomenon in these two situations.
Keywords: Malnutrition, Prefrontal cortex, Neuronal size, Neuronal density, NeuN, Stereology, Schizophrenia
1. Introduction
Undernourishment is one of the main non-genetic factors that affect the development of the central nervous system. Clinical and experimental studies reported that undernourishment induces structural and biochemical changes in the brain, causing cognitive dysfunction (Alamy and Bengelloun, 2010, Black et al., 2017). This places hunger, which still affects a large number of children throughout the world, among the most critical issues in global public health.
Despite decades of investigation, the real effects of malnutrition on brain development, behavior, and cognitive processes are not yet fully understood. In addition, the effectiveness of palliative treatments aiming to improve structural parameters altered by food insufficiency requires further investigation. Although experimental models of malnutrition are insufficient to thoroughly evaluate and compare the effects of malnutrition in humans, they enable the analysis of possible structural and behavioral changes associated with this issue (Laus et al., 2011). In this context, experimental models have been largely used to evaluate the effects of malnutrition on brain development in mice and rats.
Experimental dietary manipulations can be applied throughout the gestational period or during parts of it, and may be extended to the lactation period, or even afterwards. Another advantage of experimental models is that the brain of malnourished animals can be examined at specific developmental stages. This is important because several developmental events, such as cellular proliferation, migration, differentiation, synaptogenesis, and myelination, occur during specific critical periods. Thus, these events can be individually studied (Rice and Barone, 2000).
Among the dietary manipulations, early protein malnutrition (EPM) has been widely used. In this model, protein deficiency may affect all or parts of the pregnancy and extend after birth, partially reproducing the nutritional deficiencies commonly observed in humans (Alamy and Bengelloun, 2010). Studies using similar models indicate that EPM is associated with structural and biochemical alterations in certain brain structures such as the hippocampus. These alterations include a decreased number of neurons in the dentate gyrus and in CA1 and CA3 areas, changes in cellular differentiation, and decreased dendritic and synaptic complexity. Additionally, alterations were observed in several neurotransmitter systems (Morgane et al., 2002, Díaz-Cintra et al., 2007). Behavioral studies revealed that these biochemical and structural changes were also associated with cognitive deficits in malnourished animals (Lukoyanov and Andrade, 2000). Although malnutrition effects are well known in the hippocampus, other brain structures showed less evident modifications. Protein malnutrition did not alter the number of neurons in the locus coeruleus (King et al., 1999) or in the serotonergic raphe nuclei (King et al., 2002), indicating that cell proliferation and apoptosis in these regions were not affected. However, a decrease in the neuronal number in the locus coeruleus of undernourished rats was recently described (Pinos et al., 2006).
From a clinical perspective, studies in human populations support a link between malnutrition and an increased risk for the late onset of psychiatric conditions such as depression (Susser et al., 1996, Neugebauer et al., 1999) and schizophrenia (St Clair et al., 2005, Brown and Susser, 2008), conditions where the prefrontal cortex (PFC) reportedly seems to be involved (Harrison, 1999, Selemon and Zecevic, 2015). However, the mechanisms by which EPM could affect the PFC development remain unknown.
Furthermore, if the PFC development is structurally affected by EPM, it is still not known whether these alterations are similar to those observed in the PFC of patients with schizophrenia. To answer some of these questions, unbiased stereological methods were used to measure the impact of severe experimental EPM on mPFC volume, total neuronal number and neuronal average volume in 60-day-old rats. This age corresponds to post-pubertal animals according to functional neuroendocrine studies (Viau et al., 2005).
2. Material and methods
2.1. Subjects and nutritional treatment
Young male and female Wistar rats (Rattus norvegicus) weighing between 280 and 320 g were used. The rats were obtained from the animal facility of the Department of Anatomy (Institute of Biomedical Sciences, University of São Paulo, São Paulo, Brazil). All experimental procedures complied with the Ethical Principles for Use of Animals in Scientific Research formulated by the Brazilian College of Animal Experimentation (COBEA) and were approved by the local ethics committee (Protocol FOA-UNESP N° 2013-00738).
Food and water were available ad libitum before the experiment. Groups of one male and two females were allowed to mate for seven days in standard plastic cages, which were kept in air-conditioned rooms under controlled temperature (23–25°C) and 12 h/12 h light/dark cycle. From the first day of mating, rats had free access to one of the two isocaloric diets, which differed in casein protein content. The first diet contained adequate protein content (20% casein; control group, C), whereas the second diet had low protein content (5% casein; malnourished group, M).
Pregnancy was confirmed by presence of a sperm plug (embryonic day, E0) and the females were placed into individual cages following their respective diets. After parturition, only six male pups were kept with their dams. Finally, rat pups were separated from their mothers after weaning (postnatal day, P21), housed in groups of three animals and fed their respective diets until P60.
2.2. Tissue Processing
Twelve animals at P60 (control group, n = 6; malnourished group, n = 6) were weighed, anesthetized with sodium pentobarbital (30 mg/kg, i.p.) and transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3), between 8:00 and 10:00 AM. The brains were collected, weighed, and placed in a cryoprotective solution containing 10% glycerol and 2% dimethyl sulfoxide in 0.1 M phosphate buffer (pH 7.3) at 4°C for two days. Additional cryoprotection was done using similar solution with 20% glycerol for two days. Subsequently, the brains were sectioned coronally at 50 μm thickness using a freezing microtome (SMR 2000, Leica Instruments, GMbH, Germany). Sections were collected in six different series and kept in an antifreeze solution at −60°C and one series was processed with 0.1% thionin for cytoarchitectonic reference.
2.3. NeuN immunocytochemistry
Free-floating sections were rinsed with 0.05 M Tris-buffered saline (TBS, Tris Base/NaCl, Amresco, USA, Amresco, USA) three times for 10 min and then treated with 0.3% H2O2 in 0.05 M TBS-TX (TBS + 0.5% Triton X-100, Amresco, USA) at pH 7.6 for 30 min. Then, the sections were washed in TBS-TX and subsequently incubated with 3% fetal calf serum (Vector Laboratories, USA) in TBS-TX for 1 h. Following three rinses in TBS-TX, sections were incubated for 48 h with mouse monoclonal anti-NeuN, (Millipore, clone A60, 1:2000) diluted in TBS-TX, while gently shaken at 4°C. Sections were subsequently rinsed with TBS-TX for 30 min and incubated for 2 h with biotinylated secondary goat anti-mouse antibody (1:200, Vector Laboratories). Then, they were rinsed again (3 × 10 min with TBS-TX) and incubated in avidin–biotin–peroxidase complex (Vectastain Elite ABC kit, Vector Laboratories; 1:100 dilution in TBS-TX) at room temperature for 90 min. The immunoreaction was developed using 0.02-0.05% 3,3'-diaminobenzidine tetrahydrochloride (DAB; Sigma-Aldrich, USA) and 0.1% nickel ammonium sulfate. Sections were mounted on gelatinized slides, dehydrated through a graded series of alcohol (70, 90, 100, and 100%; 1 min each), cleared in xylene (5, 10, and 30 min), and coverslipped with DPX mounting media (Sigma-Aldrich, USA). The brain sections of control and malnourished animals were processed simultaneously, using the same solutions and reaction times. In control experiments, primary antibody was omitted and no specific staining was observed.
2.4. Stereology and Statistical Analysis
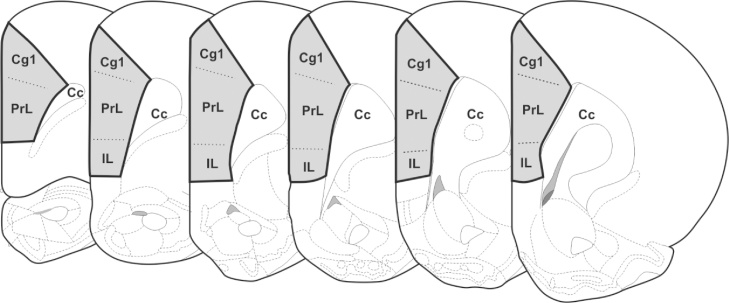
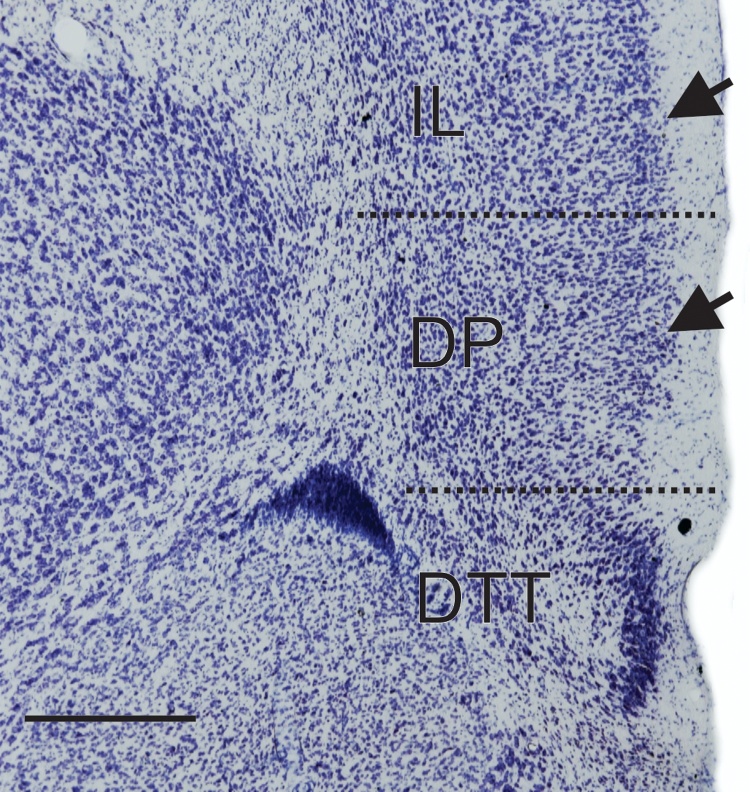
Sections were analyzed under a light microscope (Zeiss Imager Z2 microscope, Zeiss, Germany) equipped with a motorized stage and digital camera, and connected to a computer using Stereo Investigator 11.0 software (MBF Bioscience, USA). According to the parcellation proposed by Paxinos and Watson (1998), the rat mPFC was divided into prelimbic cortex (PrL), infralimbic cortex (IL), and cingulate cortex, area 1 (Cg1; Fig. 1). In order to obtain reproducible and reliable results for stereological estimation, our region of interest (ROI) included layers I–VI (the mPFC of rats has no layer IV) of the PrL, IL, and the rostral extension of Cg1, excluding the ventral portion of the dorsal peduncular cortex (DP; Fig. 1). The border between IL and DP was defined cytoarchitectonically using criteria adopted in earlier studies (Krettek and Price, 1977, Van Eden and Uylings, 1985, Zilles, 1985, Swanson, 1992, Paxinos and Watson, 1998). Ventrally, the transition between IL and DP is marked mainly by the change in layer II, which is narrow and compact in IL, but wider and loose in DP. In addition, lamination in IL is more visible than in DP (Fig. 2). Dorsally, the border between Cg1 and the dorsally located secondary motor area (M2) is less clear. To obtain a reproducible ROI, the dorsal border was determined by a line sectioning the cerebral cortex from the dorsomedial angle of the hemisphere to the adjacent white matter (Fig. 1). For consistency, the terms ROI and mPFC will be used interchangeably in this article.
Fig. 1.
Schematic drawing of coronal brain sections containing ROI (shaded area). ROI include layers I–VI of prelimbic (PrL) and infralimbic (IL) areas, as well as anterior extension of cingulate cortex, area 1(Cg1), between anterior appearance and corpus callosum decussation (Cc; Bregma 3.7 mm and 1.6 mm, respectively). External contour of each section was used to determine brain volume (BrV) (adapted from Paxinos and Watson, 1998).
Fig. 2.
Coronal section through the mPFC in Nissl-stained section showing ventral border of ROI adopted in this study. The limits between areas IL and DP are marked mainly by neuronal density in layer II, which is narrow and compact in IL, but wider and loose in DP (arrows). In addition, lamination in IL is more visible than in DP. (IL, infralimbic area; DP, dorsal peduncular cortex; DTT, dorsal tenia tecta. Scale bar = 1 mm).
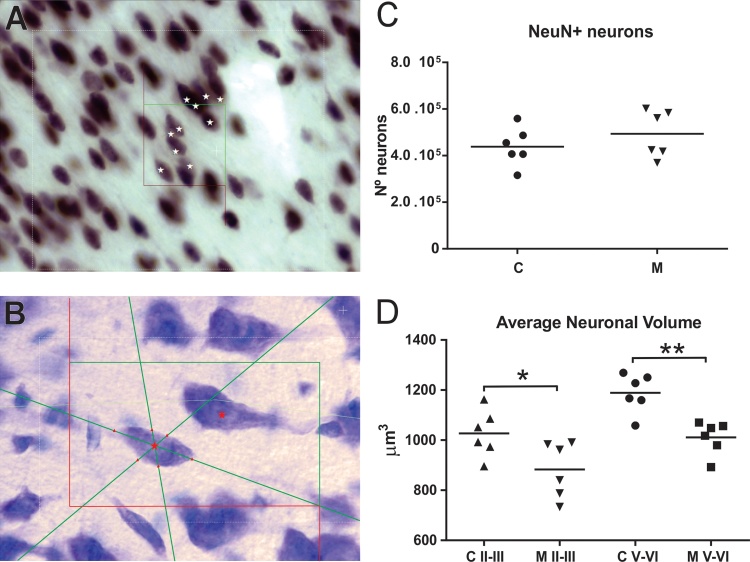
For unbiased stereological analysis, every sixth section was selected, 300 μm apart, between the first appearance and the decussation of the corpus callosum (Bregma 3.70 mm and 1.20 mm, respectively) resulting in the analysis of six sections per brain (Fig. 1). The total number of neurons (NeuN+ neurons) was estimated using the optical fractionator method (West et al., 1991) running Stereo Investigator software (MBF Bioscience, USA). Briefly, the ROI was outlined using a 1.25 × objective. Following contour delimitation, the optical fractionator analysis was conducted at high magnification (40 × objective; Fig. 3A ). In order to count a minimum of 300 NeuN+ neurons in each animal, 15 frames with an area of 50 μm2 in each ROI and an optical dissector height of 15 μm were used. To evaluate the precision of estimates (coefficients of error, CE), the Gundersen CE (Gundersen et al., 1999) was used, where m = 1. All CE values were less than or equal to 0.08.
Fig. 3.
In A, screenshot of NeuN stained section under Optical Fractionator analysis. “*” indicates countable NeuN+ neurons within counting frame. In C, data from mean number of NeuN-positive neurons in mPFC of control (C) and malnourished (M) rats. No statistically significant differences were observed between control and malnourished groups. In B, screenshot showing two Nissl stained neurons (within counting frame) using Nucleator probe. Here, a six-ray configuration was used to obtain individual neuronal volume. In D, data from average neuronal volume in layers II–III and V–VI in mPFC of control (C) and malnourished (M) rats. Statistical differences were observed when control and malnourished groups were compared, both in layers II–III (*p < 0.05) and V–VI (**p < 0.01).
The average neuronal volume was estimated in Nissl stained sections, using the Nucleator method (Gundersen et al., 1988) running the Stereo Investigator software at high magnification (100 × objective). The neurons were chosen following a systematic uniform random sampling scheme (Fig. 3B). Layers II–III and V–VI were analyzed separately. Neurons were distinguished from glia based on morphological characteristics, such as larger size and presence of a clearly defined nucleus and nucleolus and heavily stained cytoplasm, as described in previous studies (Schumann and Amaral, 2006, Markham et al., 2007, Chareyron et al., 2011). No attempts were made to differentiate pyramidal and nonpyramidal neurons.
PFC volume and total brain volume (PFCV and BrV respectively in Table I) were calculated based on the cortical region corresponding to the ROI, and the external delimitation of the whole section, respectively, in control and malnourished rats using the Cavalieri estimator (Michel and Cruz-Orive, 1988), with the Stereo Investigator software. The same sections were used for estimation of volume and neuronal numbers.
To calculate neuronal density (ND) in the mPFC (number of NeuN-positive neurons per mm3 of mPFC), the formula ND = N/V was used, where N was the number of neurons obtained using the optical fractionator, and V was the individual PFCV obtained using the Cavalieri method.
Data are presented as mean ± standard deviation. Differences between control and malnourished animals were analyzed using the Student́s unpaired t test. For all statistical tests, p < 0.05 indicated a significant difference between malnourished and control groups.
3. Results
3.1. Body and brain weight
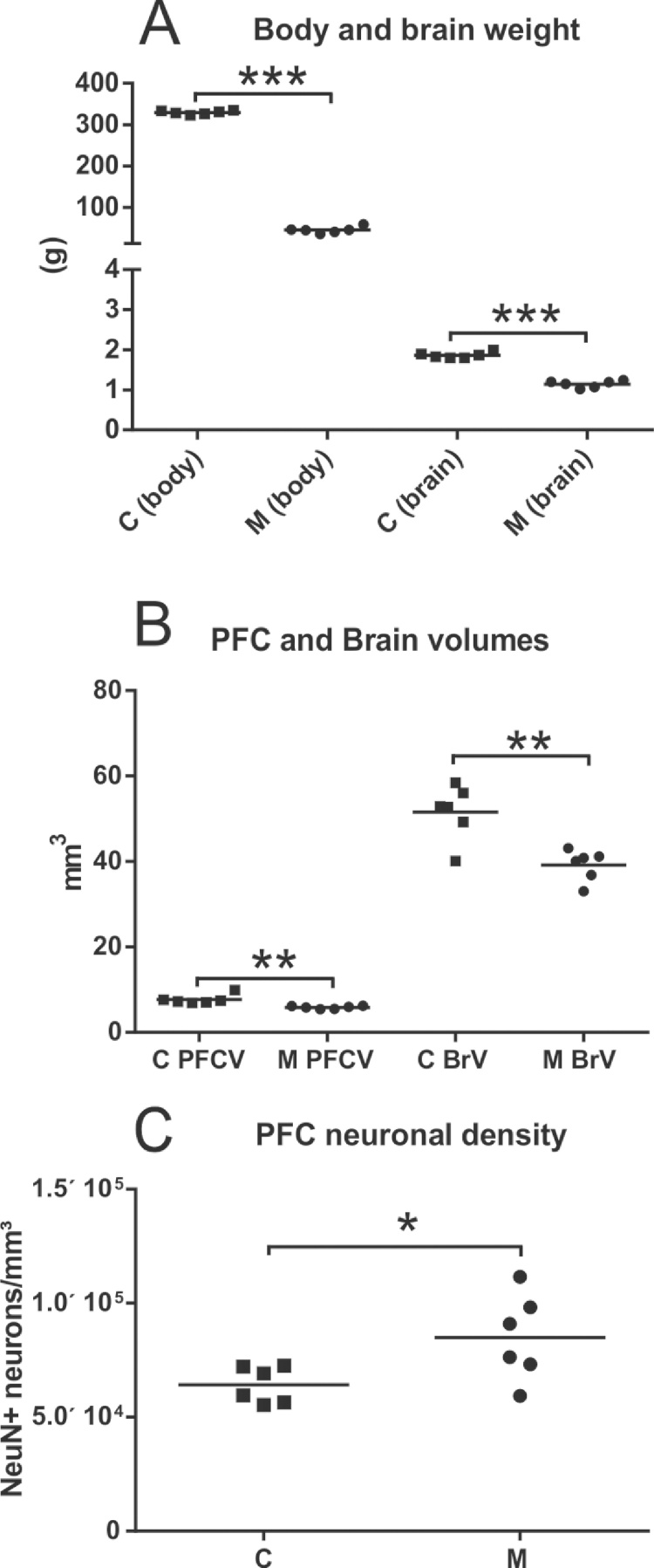
Our results showed that the EPM model adopted in this study led to a very significant decrease in body (C, 329.2 g ± 4.38; M, 44.91 g ± 7.76, p < 0.001) and brain weight (C, 1.86 g ± 0.07; M, 1.14 g ± 0.08, p < 0.001) of malnourished rats (Table 1). Body and brain weights of malnourished animals were 86% and 38% lower at P60 than the respective control values, respectively (Fig. 4A).
Table 1.
General results.
| Control (C) | Malnourished (M) | |
|---|---|---|
| BW (g) | 329.2 ± 4.38 | 44.91 ± 7.76 ** |
| BrW (g) | 1.86 ± 0.07 | 1.14 ± 0.08 *** |
| PFCV (mm3) | 7.68 ± 1.12 | 5.84 ± 0.35 ** |
| BrV (mm3) | 51.55 ± 6.43 | 39.18 ± 3.64 ** |
| ANV II–III (μm3) | 1,027 ± 93.51 | 883 ± 110.6 * |
| ANV V–VI (μm3) | 1,189 ± 77.44 | 1,027 ± 93.51** |
| NN | 438,439 ± 82,800 | 493,544 ± 100,503 |
| ND (n/mm3) | 64,199 ± 7,794 | 84,937 ± 18,964* |
BW, body weight; BrW, brain weight; PFCV, prefrontal cortex volume; BrV, brain volume (see Methods); ANV, average neuronal volume; II–III, cortical layers II and III; V–VI, cortical layers V and VI; NN, neuronal number; ND, neuronal density; *p < 0.05; ** p < 0.01; ***p < 0.001.
Fig. 4.
In A, data from body and brain weight of control (C) and malnourished (M) rats. As expected due to intensity of protein deprivation adopted in our nutritional model, there is a very significant decrease in both body and encephalic weight in malnourished animals. This decrease was also observed in mPFC and brain volumes (B). In C, due to volumetric reduction of mPFC, but preservation of neuronal number, there was neuronal density increase in malnourished rats. (*p < 0.05; **p < 0.01; ***p < 0.001).
3.2. PFC volume
Stereological analysis using the Cavalieri method showed that EPM led to a significant decrease in both PFCV and BrV. PFCV in malnourished animals was 20% lower (p < 0.01) than the respective control values (C, 7.68 mm3 ± 1,12; M, 5.84 mm3 ± 0.35) A similar reduction was observed in the BrV of malnourished rats, which was 24% (p < 0.01) lower than in control animals (C, 51.55 mm3 ± 6.43; M, 39.18 mm3 ± 3.64; Fig. 4B)
3.3. Average neuronal volume
Stereological analysis using the nucleator method in Nissl stained sections revealed that EPM led to a significant decrease in the average neuronal volume (Fig. 3D). This decrease was observed in layers II–III (C, 1,027 μm3 ± 93.51; M, 883 μm3 ± 110.6, p < 0.05) and it was even more intense in layers V–VI (C, 1,189 μm3 ± 77.44; M, 1,027 μm3 ± 93.51, p < 0.001).
3.4. Number of NeuN-positive neurons and neuronal density
Stereological analysis using the optical fractionator method revealed that EPM did not significantly alter the number of NeuN+ neurons in the rat mPFC (C, 438,439 ± 82,800; M, 493,544 ± 100,503, p > 0.05; Fig. 3C).
Neuronal density (ND) in the mPFC was calculated using the individual number of NeuN-positive neurons matched with the individual mPFC volume (see Methods). The average ND in control and malnourished rats was significantly different, being 24.4% higher in malnourished rats than in control rats (C, 64,199 ± 7,794; M, 84,937 ± 18,964, p < 0.05) (Fig. 4C).
4. Discussion
4.1. Methodological considerations and limitations
In this study, the estimation of the number of neurons, neuronal volume and cortical volume was performed using stereological tools that require a standardization of the region of interest (ROI) in order to perform consistent measurements on all animals. Our ROI includes a significant part of the medial wall of the frontal lobe, from the frontal pole to the genu of the corpus callosum. This region incorporates areas that are part of the so-called mPFC, although the delimitation and architectonic parcellation of this region has given rise considerable controversy among authors (Krettek and Price, 1977, Van Eden and Uylings, 1985, Zilles, 1985, Swanson, 1992, Paxinos and Watson, 1998, Uylings et al., 2003).
To obtain reproducible boundaries, we used the subcortical white matter as the internal limit of the ROI. Ventrally, the delimitation was facilitated by the clear architectonic differences between areas IL and DP (see Methods, Fig. 2).
Dorsally, the architectonic transition between the dorsal and medial regions of the PFC is less evident, which has led to discrepancies among the authors regarding the denominations and boundaries between the areas that form this region (for review, see Uylings et al., 2003). To avoid this problem and to obtain a reproducible ROI, the dorsal border was determined by a line sectioning the cerebral cortex from the dorsomedial angle of the hemisphere to the adjacent white matter (Fig. 1). Although arbitrary, this border partially corresponds to those established in some studies between areas Cg1 and M2 (Paxinos and Watson, 1998), ACd–FR2 (Van Eden and Uylings, 1985).
These limits create a ROI that can be reproduced in all animals facilitating further comparative studies. Hence our results, while indicating the actual differences between malnourished animals and their controls, correspond only to this topographically defined part of the mPFC.
4.2. Body and brain weight
Although the effects of protein malnutrition on body and brain weight have been previously described in the literature, ponderal indexes reveal the real impact of different dietary manipulations. Low dietary protein levels (5% casein) during the entire prenatal and postnatal periods (E0-P60) without nutritional supplementation led to a dramatic reduction in body weight at P60 (86%) using our EPM model. This reduction was more significant than that described in other studies using different dietary protocols (for revision, Alamy and Bengelloun, 2010).
Brain weight reduction of the malnourished animals (38% at P60) was expected considering the severity of the protein deprivation. The results are consistent with previous studies, although it is difficult to compare them directly due to the different experimental methodologies employed (Alamy and Bengelloun, 2010).
It is important to note that the reduction in brain weight of our malnourished rats was relatively smaller than the reduction observed in body weight. This might be related to brain sparing mechanisms already described in experimental models, which indicate that the intrauterine brain weight is maintained at a normal level despite fetal weight variations (Morrison, 2008).
4.3. PFC volume
In addition to the reduction of body and brain weight, a very significant decrease (p < 0.01) in the PFCV of malnourished animals was observed. This decrease was in parallel with the BrV decrease (p < 0.01), indicating that the PFCV decrease is probably non-specific. Our results are in general agreement with those obtained by other authors using different models of prenatal-postnatal protein deprivation (Levitsky and Strupp, 1995, Alamy and Bengelloun, 2010). For example, Thomas et al., 1979 found that although cerebral and cerebellar weights significantly decrease in undernourished rats (analyzed at P30), the “volume proportion” of the cortex remains unchanged, suggesting an allometric effect.
4.4. Neuronal number and neuronal size
One of the main findings in this study was that the significant volume reduction observed in the PFC of malnourished rats is not associated with a reduction in neuronal number, but with a reduction in the neuronal soma size. As this neuronal body reduction is probably followed by a reduction in its dendritic tree, the additional reduction in neuropil volume may contribute even more with the volume reduction observed both in the PFC and in the brain as a whole. This cortical volume reduction would also justify the neuronal density increase observed in malnourished rats.
Our results on the normality observed for the number of neurons corroborates previous studies. In fact, while pioneering studies in the 1960s and 1970s based on the analysis of DNA content suggested a reduction in the number of neurons in the cerebral cortex of malnourished animals, subsequent studies did not confirm this reduction (Saissi and Saissi, 1973, Díaz-Cintra et al., 1990, Bedi, 1994, Levitsky and Strupp, 1995).
The unchanged number of neurons observed in our study in the PFC of malnourished rats indicates a clearly different result compared to other brain regions, particularly the hippocampus. It has been shown that protein malnutrition during development causes significant morphological and biochemical alterations in hippocampal structures such as in granule cells of the dentate gyrus and in pyramidal neuron populations in the CA1 and CA3 areas. These alterations include reduction of cell numbers, abnormal cellular differentiation, and a decrease in synaptic and dendritic complexity (Díaz-Cintra et al., 1990, Díaz-Cintra et al., 1991, Díaz-Cintra et al., 1994). These structural changes are correlated with deficits in learning and memory functions, where the hippocampus plays a significant role (Morgane et al., 2002).
Different responses in the PFC and hippocampus upon malnutrition might be explained with the different patterns of proliferation, migration, and differentiation observed in these structures, a fact already noticed in other brain regions (Giuffrida et al., 1980). In addition, several neuropathologies, such as neurodegenerative disorders, often occur in a specific brain region, and several studies suggest that in response to the same injury the regional reactivity of neurons (Pinato et al., 2015) and glial cells (Kipp et al., 2008) can be different.
Although our model of protein deprivation does not seem to change the number of neurons in the rat PFC, our results indicate a significant decrease in average neuronal volume, mainly in infragranular layers (V–VI). Changes in the size of neurons or its nucleus is a phenomenon described both clinically and experimentally. In rats, a decreased neuronal nucleus size was observed in regions of the entorhinal cortex after short-term ethanol intoxication (Ibañez et al., 1992) or hyperammonemia-induced diet (Insausti et al., 1997). Similar results were observed in the cingulate cortex and locus coeruleus of rats subjected to chronic administration of Impiramine (Smiałowska et al., 1988). In humans, decreased neuronal size was described in the cerebral cortex of patients with late-life depression (Khundakar et al., 2009), in the cingulate cortex of patients with major depressive disorders (Cotter et al., 2001) and in the PFC of schizophrenic patients (Rajkowska et al., 1998). In these cases, dimensional changes seem to fundamentally affect the population of large pyramidal neurons. Coincidentally, in our results the most significant decrease in average neuronal volume was observed in cortical layers V–VI, which are also the layers that concentrate larger neurons.
The reason for the decreased neuronal size in malnourished rat is not fully understood yet. Some studies indicate that protein inadequacy during neurodevelopment can potentially disrupt normal mechanisms of neuronal migration, development of dendritic tree and synaptogenesis (Georgieff, 2007). Reduction in dendritic tree and neuropil may decrease metabolic needs, leading to lower perikaryal volume. Taken together, these events may contribute to the significant reduction in cortical volume observed in the malnourished rats in our study.
Some data in the rat mPFC induced by the malnourished model adopted in our study, such as cortical volume decrease, neuronal density increase and neuronal volume average decrease are similar to clinical studies in patients with schizophrenia and other psychiatric disorders (Selemon et al., 1998, Harrison, 1999, Glausier and Lewis, 2013). In fact, several studies have shown that schizophrenic patients exhibit encephalic volumetric changes, with enlargement of ventricular system and decreased cortical volume fundamentally in the PFC and temporal lobe (Harrison, 1999, Honea et al., 2005, Rimol et al., 2012). In addition, postmortem studies have shown an increase in the neuronal density of the prefrontal and occipital cortices in patients with schizophrenia (Selemon et al., 1998, Konopaske et al., 2014).
These similarities can be relevant, as epidemiological studies indicate that EPM may increase the risk for the late onset of certain mental and psychiatric conditions such as schizophrenia (Tarantino et al., 2012, Selemon and Zecevic, 2015), in which the PFC plays a fundamental role. This correlation has been well documented in humans exposed to severe malnutrition during war (Dutch hunger winter, 1944-1945) or adverse weather conditions (Chinese Famine, 1959-1961). In both cases, the prevalence of schizophrenia increased in the surviving population during adulthood (Susser et al., 1996, Neugebauer et al., 1999, St Clair et al., 2005). The possible mechanisms by which malnutrition is associated with schizophrenia are not clear, but both genetic and epigenetic factors, as well as developmental alterations due to the absence of key micro- and macronutrients may be involved (Harper and Brown, 2012).
5. Conclusion
Our study shows that protein malnutrition during gestation and early life significantly decreases average neuronal volume and increases the neuronal density of rat mPFC at P60. This increase was due to a volume reduction of the mPFC, probably related to a reduction of neuropil, but not to a change in the number of neurons.
Considering the association between nutritional deprivation and some mental disorders, and that some of our results are similar to those described in schizophrenic patients, our findings suggest that EPM models are not only important to understand the impact of malnutrition on the central nervous system, but also to analyze possible neurodevelopmental changes that are associated with some neuropsychiatric conditions.
Conflict of interest
This work is free of conflict of interest.
Role of authors
RJC-R and EAL designed the study. RJC-R, CAC and LP drafted the manuscript. RJC-R, LLL, IRP, JOBR, TFP and EE, carried out the histological and immunohistochemical processing, microscopic and stereological analysis, data collection, and preparation of figures. LP and LMGC contributed to microscopic analysis and additional experimental procedures. All authors read and approved the final manuscript.
Funding
This work was supported by grants from FAPESP (2011/21509-3; 2012/11307-7; 2012/13433-0; 2012/03067-6).
References
- Alamy M., Bengelloun W.A. Malnutrition and brain development: an analysis of the effects of inadequate diet during different stages of life in rat. Neurosci. Biobehav. Rev. 2010;36(6):1463–1480. doi: 10.1016/j.neubiorev.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Bedi K.S. Undernutrition of rats during early life does not affect the total number of cortical neurons. J. Comp. Neurol. 1994;342(4):596–602. doi: 10.1002/cne.903420407. [DOI] [PubMed] [Google Scholar]
- Black M.M., Walker S.P., Fernald L.C.H., Andersen C.T., DiGirolamo A.M., Lu C., McCoy D.C., Fink G., Shawar Y.R., Shiffman J., Devercelli A.E., Wodon Q.T., Vargas-Barón E., Grantham-McGregor S. Early childhood development coming of age: science through the life course. Lancet. 2017;389:77–90. doi: 10.1016/S0140-6736(16)31389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.S., Susser E.S. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr. Bull. 2008;34:1054–1063. doi: 10.1093/schbul/sbn096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron L., Lavenex P.B., Amaral D.G., Lavenex P. Stereological analysis of the rat and monkey amygdala. J. Comp. Neurol. 2011;519:3218–3239. doi: 10.1002/cne.22677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D., Mackay D., Landau S., Kerwin R., Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch. Gen. Psychiatry. 2001;58:545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Díaz-Cintra S., Cintra L., Ortega A., Kemper T., Morgane P.J. Effects of protein deprivation on pyramidal cells of the visual cortex in rats of three age groups. J. Comp. Neurol. 1990;292:117–126. doi: 10.1002/cne.902920108. [DOI] [PubMed] [Google Scholar]
- Díaz-Cintra S., Cintra L., Galván A., Aguilar A., Kemper T., Morgane P. Effects of prenatal protein deprivation on postnatal development of granule cells in the fascia dentata. J. Comp. Neurol. 1991;310:356–364. doi: 10.1002/cne.903100306. [DOI] [PubMed] [Google Scholar]
- Díaz-Cintra S., García-Ruiz M., Corkidi G., Cintra L. Effects of prenatal malnutrition and postnatal nutritional rehabilitation on CA3 hippocampal pyramidal cells in rats of four ages. Brain Res. 1994;662:117–126. doi: 10.1016/0006-8993(94)90803-6. [DOI] [PubMed] [Google Scholar]
- Díaz-Cintra S., González-Maciel A., Morales M.A., Aguilar A., Cintra L., Prado-Alcalá R.A. Protein malnutrition differentially alters the number of glutamic acid Decarboxylase-67 interneurons in dentate gyrus and CA1-3 subfields of the dorsal hippocampus. Exp. Neurol. 2007;208:47–53. doi: 10.1016/j.expneurol.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Georgieff M.K. Nutrition and the developing brain: nutrient priorities and measurement. Am. J. Clin. Nutr. 2007;85(Suppl):614–620. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- Giuffrida A.M., Hamberger A., Serra I., Geremia E. Effects of undernutrition on nucleic acid synthesis in neuronal and glial cells from different regions of developing rat brain. Nutr. Metab. 1980;24:189–198. doi: 10.1159/000176341. [DOI] [PubMed] [Google Scholar]
- Glausier J.R., Lewis D.A. Dendritic spine pathology in schizophrenia. Neuroscience. 2013;251:90–107. doi: 10.1016/j.neuroscience.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen H.J., Bagge r P., Bendtsen T.F., Evans S.M., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J.R., Pakkenberg B. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. Review. [DOI] [PubMed] [Google Scholar]
- Gundersen H.J., Jensen E.B., Kiêu K., Nielsen J. The efficiency of systematic sampling in stereology reconsidered. J. Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Harper K.N., Brown A.S. Prenatal nutrition and the etiology of schizophrenia. In: Brown A.S., Patterson M.D., Paul M.D.H., editors. The origins of schizophrenia. Columbia University Press; New York: 2012. pp. 58–95. [Google Scholar]
- Harrison P.J. The neuropathology of schizophrenia: A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Honea R., Crow T.J., Passingham D., Mackay C.E. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am. J. Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Ibañez J., Herrero M.T., Insausti R., Belzunegui T., Gonzalo L.M. Short-term ethanol intoxication in rat: Effect on the entorhinal cortex. Neurosci. Lett. 1992;138:199–201. doi: 10.1016/0304-3940(92)90914-s. [DOI] [PubMed] [Google Scholar]
- Insausti A.M., Gaztelu J.M., Gonzalo L.M., Romero-Vives M., Barrenechea C., Felipo V., Insausti R. Diet induced hyperammonemia decreases neuronal nuclear size in rat entorhinal cortex. Neurosci. Lett. 1997;231:179–181. doi: 10.1016/s0304-3940(97)00560-0. [DOI] [PubMed] [Google Scholar]
- Khundakar A., Morris C., Oakley A., McMeekin W., Thomas A.J. Morphometric analysis of neuronal and glial cell pathology in the dorsolateral prefrontal cortex in late-life depression. Br. J. Psychiatry. 2009;195:163–169. doi: 10.1192/bjp.bp.108.052688. [DOI] [PubMed] [Google Scholar]
- King R.S., Kemper T.L., DeBassio W.A., Blatt G.J., Ramzan M., Rosene D.L., Galler J.R. Effect of prenatal protein malnutrition on birthdates and number of neurons in the rat locus coeruleus. Nutr. Neurosci. 1999;2:267–276. doi: 10.1080/1028415X.1999.11747283. [DOI] [PubMed] [Google Scholar]
- King R.S., Kemper T.L., DeBassio W.A., Ramzan M., Blatt G.J., Rosene D.L., Galler J.R. Birthdates and number of neurons in the serotonergic raphe nuclei in the rat with prenatal protein malnutrition. Nutr. Neurosci. 2002;5:391–397. doi: 10.1080/1028415021000055934. [DOI] [PubMed] [Google Scholar]
- Kipp M., Norkute A., Johann S., Lorenz L., Braun A., Hieble A., Gingele S., Pott F., Richter J., Beyer C. Brain-region-specific astroglial responses in vitro after LPS exposure. J. Mol. Neurosci. 2008;35:235–243. doi: 10.1007/s12031-008-9057-7. [DOI] [PubMed] [Google Scholar]
- Konopaske G.T., Lange N., Coyle J.T., Benes F.M. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry. 2014;71:1323–1331. doi: 10.1001/jamapsychiatry.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettek J.E., Price J.L. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J. Comp. Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Laus M.F., Vales L.D., Costa T.M., Almeida S.S. Early postnatal protein-calorie malnutrition and cognition: a review of human and animal studies. Int. J. Environ. Res. Public Health. 2011;8:590–612. doi: 10.3390/ijerph8020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitsky D.A., Strupp B.J. Malnutrition and the brain: changing concepts, changing concerns. J. Nutr. 1995;125:2212–2220. doi: 10.1093/jn/125.suppl_8.2212S. [DOI] [PubMed] [Google Scholar]
- Lukoyanov N.V., Andrade J.P. Behavioral effects of protein deprivation and rehabilitation in adult rats: relevance to morphological alterations in the hippocampal formation. Behav. Brain Res. 2000;112:85–97. doi: 10.1016/s0166-4328(00)00164-9. [DOI] [PubMed] [Google Scholar]
- Markham J.A., Morris J.R., Juraska J.M. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Michel R.P., Cruz-Orive L.M. Application of the Cavalieri principle and vertical sections method to lung: estimation of volume and pleural surface area. J. Microsc. 1988;150:117–136. doi: 10.1111/j.1365-2818.1988.tb04603.x. [DOI] [PubMed] [Google Scholar]
- Morgane J.P., Mokler D.J., Galler J.R. Effect of prenatal protein malnutrition on the hippocampal formation. Neurosci. Biobehav. Rev. 2002;26:471–483. doi: 10.1016/s0149-7634(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Morrison J.L. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin. Exp. Pharmacol. Physiol. 2008;35:730–743. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- Neugebauer R., Hoek H.W., Susser E. Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. JAMA. 1999;282:455–462. doi: 10.1001/jama.282.5.455. [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. 4th ed. Academic Press; San Diego: 1998. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- Pinato L., da Silveira Cruz-Machado S., Franco D.G., Campos L.M., Cecon E., Fernandes P.A., Bittencourt J.C., Markus R.P. Selective protection of the cerebellum against intracerebroventricular LPS is mediated by local melatonin synthesis. Brain Struct. Funct. 2015;220:827–840. doi: 10.1007/s00429-013-0686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinos H., Collado P., Salas M., Perez- Torrero E. Early undernutrition decreases the number of neurons in the locus coeruleus of rats. Nutr. Neurosci. 2006;9:233–239. doi: 10.1080/10284150600937873. [DOI] [PubMed] [Google Scholar]
- Rajkowska G., Selemon L.D., Goldman-Rakic P.S. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch. Gen. Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Rice D., Barone S. Jr. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ. Health Perspect. 2000;108(Suppl. 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol L.M., Nesvag R., Hagler D.J., Jr., Bergmann O., Fennema-Notestine C., Hartberg C.B., Haukvik U.K., Lange E., Pung C.J., Server A., Melle I., Andreassen O.A., Agartz I., Dale A.M. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol. Psychiatry. 2012;71:552–560. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Saissi F., Saissi B. Differential effects of protein-calorie restriction and subsequent repletion on neuronal and nonneuronal components of cerebral cortex in newborn rats. J. Nutr. 1973;103:1625–1633. doi: 10.1093/jn/103.11.1625. [DOI] [PubMed] [Google Scholar]
- Schumann C.M., Amaral D.G. Stereological analysis of amygdala neuron number in autism. J. Neurosci. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon L.D., Rajkowska G., Goldman-Rakic P.S. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: Application of a 3-dimensional, stereologic counting method. J. Comp. Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- Selemon L.D., Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl. Psychiatry. 2015;5 doi: 10.1038/tp.2015.115. e623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smiałowska M., Bal-Klara A., Smiałowski A. Chronic imipramine diminishes the nuclear size of neurons in the locus coeruleus and cingular cortex but not in the hippocampus of the rat brain. Neuroscience. 1988;26:803–807. doi: 10.1016/0306-4522(88)90100-5. [DOI] [PubMed] [Google Scholar]
- St Clair D., Xu M., Wang P., Yu Y., Fang Y., Zhang F., Zheng X., Gu N., Feng G., Sham P., He L. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA. 2005;294:557–562. doi: 10.1001/jama.294.5.557. [DOI] [PubMed] [Google Scholar]
- Susser E., Neugebauer R., Hoek H.W., Brown A.S., Lin S., Labovitz D., Gorman J.M. Schizophrenia after prenatal famine. Further evidence. Arch. Gen. Psychiatry. 1996;53:25–31. doi: 10.1001/archpsyc.1996.01830010027005. [DOI] [PubMed] [Google Scholar]
- Swanson L.W. Elsevier; Amsterdam: 1992. Brain maps: structure of the rat brain. 240 pp. [Google Scholar]
- Tarantino L.M., Reyes T.M., Palmer A.A. Animal models of prenatal protein malnutrition relevant for schizophrenia. In: Brown A.S., Patterson M.D., Paul H., editors. The origins of schizophrenia. Columbia University Press; New York: 2012. pp. 300–334. [Google Scholar]
- Thomas Y.M., Bedi K.S., Davies C.A., Dobbing J. A stereological analysis of the neuronal and synaptic content of the frontal and cerebellar cortex of weanling rats undernourished. Early Hum Dev. 1979;3:109–126. doi: 10.1016/0378-3782(79)90001-x. [DOI] [PubMed] [Google Scholar]
- Uylings H.B., Groenewegen H.J., Kolb B. Do rats have a prefrontal cortex? Behav. Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Van Eden C.G., Uylings H.B. Cytoarchitectonic development of the prefrontal cortex in the rat. J. Comp. Neurol. 1985;241:253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- Viau V., Bingham B., Davis J., Lee P., Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- West M.J., Slomianka L., Gundersen H.J. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat. Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Zilles K. Springer-Verlag; Berlin: 1985. The cortex of the rat. A stereotaxic atlas. [Google Scholar]