Abstract
Background:
Perfluoroalkyl substances (PFASs) are widespread persistent organic compounds that have been suggested to affect neurodevelopment.
Objective:
We aimed to evaluate whether prenatal exposure to PFASs is associated with IQ in children.
Methods:
We studied 1,592 pregnancies enrolled in the Danish National Birth Cohort (DNBC) during 1996–2002. Sixteen PFASs were measured in maternal plasma collected in early gestation. Child IQ was assessed at 5 y of age using the Wechsler Primary and Preschool Scales of Intelligence–Revised (WPPSI-R) administered by trained psychologists. Using multivariable linear regression models, we estimated the differences in child IQ scores according to PFAS concentration [per natural-log (ng/mL) unit increase or values categorized in quartiles].
Results:
Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) were detected in all samples, and five additional PFASs were quantified in of the samples. Overall, we found no strong associations between a natural-log unit increase in each of the seven PFASs we evaluated and child IQ scores. A few positive and negative associations were found in the sex-stratified PFAS quartile analyses, but the patterns were inconsistent.
Conclusion:
Overall, we did not find consistent evidence to suggest prenatal exposure to PFASs to be associated with child IQ scores at 5 y of age in the DNBC. Some of the sex-specific observations warrant further investigation. Additional studies should examine offspring IQ at older ages and assess other functional cognitive and neuropsychiatric measures in addition to intelligence. Postnatal exposures to PFASs and mixture effects for PFASs and PFASs with other environmental pollutants should also be considered in future research. https://doi.org/10.1289/EHP2754
Introduction
Perfluoroalkyl substances (PFASs) are a group of synthetic fluorine-containing compounds that have been widely used in commercial and manufacturing products since the 1950s for the treatment of paper, clothing, carpets, food packing material, and kitchenware (Houde et al. 2006). PFASs are extremely resistant to biotransformation and environmental degradation (Houde et al. 2006). Several PFASs have been spread widely throughout the environment and are detectable in humans worldwide (Houde et al. 2006; Lau et al. 2007). Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) have been the two most frequently used PFASs and have estimated biological half-lives in humans between 3 and 5 y (Olsen et al. 2007). PFOS and PFOA have been phased out from production in the United States and in some other countries since 2000, but these compounds remain widely detectable (Bjerregaard-Olesen et al. 2016; Chu et al. 2016; Kato et al. 2011; Nøst et al. 2014). Human exposure to other short-chain and to some long-chain PFAS compounds has been found to be on the increase (Glynn et al. 2012). In addition, some new fluorinated compounds used as replacements for PFASs have also recently been detected in the biota (Chu et al. 2016; Gebbink et al. 2017; Sun et al. 2016).
PFASs cross the placental barrier and expose the fetus during the vulnerable period of development (Fei et al. 2007). Numerous studies have suggested that PFASs may interfere with thyroid hormone homeostasis in pregnant women: for example, a recent systematic review showed that three PFASs were positively correlated with thyroid stimulating hormone and negatively correlated with free thyroxine (Ballesteros et al. 2017; Wang et al. 2013, 2014; Webster et al. 2014). Thyroid hormones transferred from the mother to the embryo and fetus might be critical for normal brain development (Lazarus 1999). Severe thyroid hormone deficiency during gestation can cause cretinism and cognitive and/or mental disorders (Hong and Paneth 2008; Modesto et al. 2015; Oppenheimer and Schwartz 1997). Subclinical maternal hypothyroxinemia in pregnancy has also been linked to adverse neurodevelopmental outcomes (Andersen et al. 2014, 2017). Previous research has indicated that even a slight reduction in the amount of circulating free thyroid hormone levels in mothers ( parts per trillion) might be associated with a loss of 4 to 7 IQ points in children (Haddow et al. 1999).
To date, only two epidemiologic studies have evaluated the associations between prenatal PFAS exposure and child IQ. The C8 Health Study examined 320 children between 6 and 12 y of age from a community highly exposed to PFOA for decades via contaminated drinking water and unexpectedly found that geospatially estimated (not measured) in utero PFOA levels were associated with higher full-scale IQ (Stein et al. 2013). The C8 study did not investigate other types of PFASs. More recently, a study conducted in Taiwan with PFASs measured in third-trimester maternal serum samples reported that prenatal perfluoroundecanoic acid (PFUnDA) concentrations were inversely associated with performance IQ scores in children at 5 y of age, and at further follow-up at 8 y of age, prenatal exposure to seven types of PFASs appeared to be associated with reduction of the child’s full-scale, verbal, and performance IQ scores (Wang et al. 2015). However, the Taiwanese study included only 120 children, each of whom was examined at 5 and 8 y old; therefore, replication of these results in a larger study population is needed. Here, we investigated associations between prenatal exposure to several PFASs and IQ at 5 y of age in a sample of nearly 1,600 children from the Danish National Birth Cohort (DNBC).
Methods
Study Participants
The DNBC is a national birth cohort study that originally enrolled 101,041 pregnancies through general practitioners at the first antenatal visit (weeks 6 to 12) during 1996–2002 (Olsen et al. 2001). The DNBC conducted four computer-assisted telephone interviews (twice during pregnancy and twice postpartum), and two prenatal maternal blood samples were collected and stored (one each in the first and second trimester). For this study, we utilized additional data collected from the Lifestyle During Pregnancy Study (LDPS), a subcohort nested within the DNBC and designed with two-stage sampling with the aim of collecting additional data to evaluate potential influences of prenatal lifestyle factors, primarily alcohol intake, on neuropsychological outcomes in young children. The design and sampling scheme of the LDPS have been described elsewhere (Kesmodel et al. 2010). Briefly, 3,478 mothers and children from the DNBC were invited to participate in the LDPS between September 2003 and June 2008 when the children reached 5 y of age (age range: ). Exclusion criteria for the LDPS were nonsingleton birth, children with impaired hearing or vision to the extent that the test session could not be performed, and severe disabilities due to congenital defects. Among those invited, 1,782 (51%) agreed to participate in a 3-h extensive neuropsychological assessment conducted by trained psychologists at four testing sites located in the four largest cities in Denmark. All test procedures were standardized, and regular interrater comparisons were performed for examiners to minimize potential systematic bias from the examiner or the examination site. The final sample included 1,592 participants with a maternal blood sample collected at a median of 8 wk of gestation (interquartile range 7 to 10 wk) available for PFAS measurement. A flowchart depicting the study sample selection can be found in the Supplemental Material (see Figure S1). A comparison of study characteristics of those invited and those who participated in the LDPS is also presented in the Supplemental Material (see Table S1).
The research protocol for this study was approved by the Danish data protection agency and the University of California, Los Angeles (UCLA) Institutional Review Board.
Child and Maternal IQ Assessment
Child IQ was assessed using the Wechsler Primary and Preschool Scales of Intelligence–Revised (WPPSI-R) (Wechsler 1990) administered by 10 psychologists with special training in neuropsychological testing and study procedures. All testers were blinded to alcohol and PFAS exposure. The WPPSI-R is one of the most widely used measures of intelligence in children between 3 and 7 y old. The full WPPSI-R consists of five verbal and five performance (nonverbal) subtests. The verbal subtests were designed to measure general knowledge, language, and reasoning, and the performance subtests were designed to measure spatial, sequencing, and problem-solving skills. The LDPS used a shorter version of the WPPSI-R that included three verbal (arithmetic, information, and vocabulary) and three performance (block design, geometric design, and object assembly) subtests. The subtests were selected to make derivation of verbal IQ, performance IQ, and full-scale IQ scores possible using standard procedures. Because Danish WPPSI-R norms were not available at the time of the study, Swedish norms were used to derive scaled scores (Wechsler 1999). To ensure interrater reliability, each psychologist blindly rescored a number of subtests in WPPSI-R that had been administered by other psychologists. The interrater agreement for scoring was high (97–97.5%) (Kesmodel et al. 2010). The final composite IQ scores were restandardized to a mean of 100 and a standard deviation (SD) of 15 for statistical analysis.
Maternal IQ was assessed based on two verbal (information and vocabulary) subtests from the Wechsler Adult Intelligence Scale (WAIS) (Wechsler 1955) and on the nonverbal Raven’s Standard Progressive Matrices™ (Raven et al. 1998). The raw scores from each test were first standardized based on the results from the full sample and then weighted equally to create a combined score that was restandardized to the full IQ scale with a mean of 100 and an SD of 15.
PFAS Measurements
Details describing our analytic methods for PFASs have been described previously (Liew et al. 2014, 2015). Briefly, maternal blood samples were collected (mean 8.7 wk of gestation) and sent by mail to Statens Serum Institut in Copenhagen, where they were separated and stored in freezers at or . We used stored maternal plasma, and the samples were analyzed at the Department of Environmental Science at Aarhus University. Solid phase extraction (SPE) was used for sample extraction and purification. PFAS concentrations were measured using liquid chromatography–tandem mass spectrometry (LC-MS/MS). Measurements were performed in a random sequence by laboratory personnel blinded to exposure and outcome. We measured 16 different PFASs, but in this study, we focused on the 7 PFASs with measures above the lower limit of quantitation (LLOQ) for of the samples: PFOS, 100%; PFOA, 100%; perfluorohexane sulfonate (PFHxS), 99.9%; perfluoroheptane sulfonate (PFHpS), 99.4%; perfluorononanoic acid (PFNA), 94.2%; perfluorodecanoic acid (PFDA), 96.6%; and perfluorooctanesulfonic acid (PFOSA), 81.2%. The full panel of LLOQs and the distributions of all PFASs are presented in Table 2.
Table 2.
Detection and quantitation limits of perfluoroalkyl substances (PFASs) and plasma concentrations of maternal PFASs in the Lifestyle During Pregnancy Study–Danish National Birth Cohort (LDPS-DNBC) ().
| Number | Abbreviation | Chemical Name | Lower limit of detection (ng/mL) | Lower limit of quantitation (ng/mL) | Percent in all samples | PFAS concentrations (ng/mL) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Minimum | 25th Percentile | Median | 75th Percentile | Maximum | ||||||
| 1 | PFOS | Perfluorooctane sulfonate | 0.09 | 0.28 | 100% | 2.36 | 21.60 | 28.10 | 35.80 | 127.00 |
| 2 | PFOA | Perfluorooctanoic acid | 0.07 | 0.20 | 100% | 0.61 | 3.15 | 4.28 | 5.49 | 15.00 |
| 3 | PFHxS | Perfluorohexane sulfonate | 0.03 | 0.08 | 99.94% | 0.76 | 1.07 | 1.38 | 12.80 | |
| 4 | PFNA | Perfluorononanoic acid | 0.09 | 0.27 | 94.29% | 0.36 | 0.46 | 0.57 | 2.23 | |
| 5 | PFHpS | Perfluoroheptane sulfonate | 0.04 | 0.11 | 99.37% | 0.27 | 0.37 | 0.49 | 2.01 | |
| 6 | PFDA | Perfluorodecanoic acid | 0.03 | 0.09 | 96.55% | 0.14 | 0.17 | 0.22 | 0.90 | |
| 7 | PFOSA | Perfluorooctanesulfonic acid | 0.40 | 1.19 | 81.68% | 1.38 | 2.32 | 4.16 | 35.90 | |
| 8 | PFHpA | Perfluoroheptanoic acid | 0.02 | 0.05 | 64.64% | 0.07 | 0.12 | 3.00 | ||
| 9 | PFUnA | Perfluoroundecanoic acid | 0.05 | 0.15 | 43.78% | 0.19 | 1.34 | |||
| 10 | PFDS | Perfluorodecane sulfonate | 0.12 | 0.37 | 4.33% | 7.21 | ||||
| 11 | PFDoA | Perfluorododecanoic acid | 0.14 | 0.41 | 0.44% | 0.85 | ||||
| 12 | PFTrA | Perfluorotridecanoic acid | 0.14 | 0.41 | 0.06% | 0.49 | ||||
| 13 | PFHxA | Perfluorohexanoic acid | 0.01 | 0.03 | 0.00% | |||||
| 14 | PFPeA | Perfluoropentanoic acid | 0.06 | 0.19 | 0.00% | |||||
| 15 | PFBS | Perfluorobutane sulfonate | 0.02 | 0.07 | 0.00% | |||||
| 16 | PFTeA | perfluorotetradecanoic acid | 0.14 | 0.41 | 0.00% | |||||
Note: LLOQ, lower limit of quantitation.
Statistical Analysis
We used multivariable linear regression models to estimate the differences in child IQ scores according to prenatal PFAS exposures. Each of the PFAS compounds was first analyzed as a continuous variable [as per natural-log (ln) unit increase], and we also categorized the PFAS values into quartiles, using the lowest quartile as the reference group. Trend tests were performed using the median value of the PFAS in each quartile as a continuous variable. Moreover, we fitted generalized additive models for PFASs with or without ln-transformation to examine potential nonlinear relationships. Five degrees of freedom was set as the upper limit in the smoothing spline, and we compared model fit and visually inspected plots of the smoothed data. We also dichotomized child IQ scores below 1 SD (scores ) to indicate low IQ, and we used generalized linear models to estimate risk ratios (RRs) for low IQ following prenatal PFAS exposures.
We adjusted for potential confounders including maternal age at delivery (continuous), parity (0, 1, ), maternal IQ (continuous), socioeconomic status (SES; 3 levels derived from the mother’s and father’s education and occupation), maternal smoking during pregnancy (yes, no), maternal alcohol consumption during pregnancy (0, 1–4, drinks per week), maternal prepregnancy body mass index (BMI; , 18.5–24.9, 25.0–29.9, ), child’s sex, and gestational week of blood draw (continuous). Birth year does not predict the outcome scores in our sample; however, because PFAS exposures may vary by year, we adjusted for birth year (continuous or categorical) in additional sensitivity analyses. Additionally, we performed stratified analyses to evaluate potential effect measure modification by child’s sex (male and female), parity (nulliparous and parous) and maternal SES (high and median/low). Tests of heterogeneity were also performed by assessing the p-value of the interaction term of each PFAS and potential modifying factors in the regression models. To account for PFAS values below the LLOQ when PFASs were analyzed as continuous variables, we used multiple imputations including seven PFASs and all abovementioned covariates in the model (Lubin et al. 2004). We conducted a sensitivity analysis excluding PFAS values that were greater than three times the value of the 75th percentile to ensure that individuals with extreme exposure values did not disproportionately influence our results. The blood samples collected in the DNBC were transported to the biobank by ordinary mail that could subject them to measurement errors resulting from processing delay (Bach et al. 2015). Therefore, we excluded samples that were processed after in another sensitivity analysis.
To assess possible bias due to subject selection for the LDPS, we first compared the PFAS levels in this sample with those in a previous DNBC study that randomly sampled live-born singleton children at birth such that the study was unaffected by loss to follow-up (Liew et al. 2014). We also compared our PFAS values to those reported for participants in the U.S. NHANES survey 1999–2000 (Calafat et al. 2007). In addition, we computed inverse probability weights (IPWs) to account for subject selection in the LDPS. Sampling of LDPS from all DNBC participants was random within alcohol intake categories, and the sampling probabilities were available for adjustment and have been used in previous studies (Kesmodel et al. 2010, 2012). We estimated the probability of selective nonparticipation in the LDPS according to factors measured for all women in the DNBC at baseline. Preterm birth was negatively related and high maternal SES and organic food intake during pregnancy were positively related to willingness to participate (all ). We also included factors that were weakly associated with LDPS participation such as maternal age, season of conception, prepregnancy BMI, home size, planned pregnancy, location of birth, and having missed a telephone interview at baseline (all ). In primary analyses, we presented the results from weighted regression models that included the IPW combining the probabilities of sampling and participation. The 95% confidence intervals (CIs) were computed using robust variance estimators. Additionally, we compared the findings with the IPW model that included only the sampling probabilities without adjusting for participation probabilities. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc.).
Results
Table 1 presents the characteristics of the study sample. Table 2 shows the median and interquartile range (IQR) of maternal PFAS concentrations during pregnancy in our sample. The concentrations and distribution of PFASs in the LDPS samples were comparable to previous DNBC studies as well as to those reported in representative samples from the United States during a similar collection period (see Table S2). As expected, PFAS levels were higher in nulliparous women (see Table S3). Mothers with higher prepregnancy BMI and those who performed more poorly on IQ tests also tended to have higher PFAS levels in pregnancy.
Table 1.
Characteristics of study participants in the Lifestyle During Pregnancy Study–Danish National Birth Cohort (LDPS-DNBC) ().
| Characteristics | or Mean | Percent or standard deviation |
|---|---|---|
| Maternal age at child birth (years) | 30.8 | 4.4 |
| Maternal IQa | 100.1 | 15 |
| Gestational week of blood drawa | 8.7 | 2.5 |
| Child's sex | ||
| Female | 761 | 47.8 |
| Male | 831 | 52.2 |
| Parity | ||
| 0 | 801 | 50.3 |
| 1 | 511 | 32.1 |
| 280 | 17.6 | |
| Socioeconomic status (SES)b | ||
| High | 1,131 | 71.0 |
| Medium | 412 | 25.9 |
| Low | 43 | 2.7 |
| Missing | 6 | 0.4 |
| Maternal alcohol consumption during pregnancy | ||
| Never | 756 | 47.5 |
| 1–4 glasses per week | 655 | 41.1 |
| glasses per week | 181 | 11.4 |
| Maternal smoking during pregnancy | ||
| No | 1,097 | 68.9 |
| Yes | 495 | 31.1 |
| Maternal prepregnancy body mass index (BMI)a | ||
| 61 | 3.8 | |
| 18.5–24.9 | 1,093 | 68.7 |
| 25.0–29.9 | 292 | 18.3 |
| 116 | 7.3 | |
| Missing | 30 | 1.9 |
8 and 135 observations were missing information about maternal IQ and gestational week of blood draw, respectively.
SES was created based on self-reported maternal and paternal education and occupation using three categories (high, medium, and low): higher education (four years beyond high school) or work in management were classified as high, skilled workers and middle-range education as medium, unskilled workers and unemployed as low.
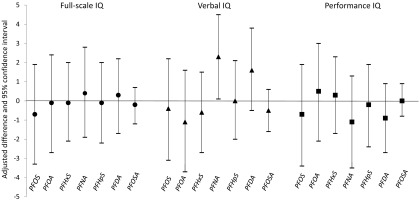
In linear regressions, we found no associations between an ln-unit (ng/mL) increase of each of the seven PFASs and child full-scale or performance IQ scores, and higher prenatal PFNA values appeared to be associated with higher verbal IQ [adjusted difference: 2.3 (95% CI: 0.1, 4.5)] (Figure 1). No associations were observed in sex-stratified analyses (see Figure S2), and none of the p-values for the sex and PFAS interaction term was . We also observed no clear differences in the results when stratifying by parity, except that the positive association between PFNA and verbal IQ was slightly more apparent among nulliparous women; however, other types of PFASs were not associated with IQ scores in both nulliparous and parous women (see Table S4). No clear differences were observed in the results when we stratified by maternal SES (see Table S5). All p-values for interaction between PFAS and parity or SES were .
Figure 1.
Adjusted difference in IQ scores for 1,592 children at 5 y old according to prenatal perfluoroalkyl substance (PFAS) levels [per natural-log unit (ng/mL) increase]. All 1,592 children were analyzed for each PFAS and for outcome scores. Multivariable linear regression modeling was used to estimate the expected difference in IQ score. Models were adjusted for maternal age at childbirth, parity, child's sex, maternal socioeconomic status, maternal IQ, maternal smoking during pregnancy, maternal alcohol consumption during pregnancy, maternal prepregnancy body mass index (BMI), and gestational week of blood draw. Note: PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFOSA, perfluorooctanesulfonic acid.
We found no associations between PFAS quartiles and all three IQ scales in the total sample (see Table S6). In girls, the second quartile of PFOA and the third quartile of PFNA were associated with higher IQ scores compared with the lowest quartile. In linear-trend tests, increasing PFNA and PFDA levels were also related to higher verbal IQ scores (p-trend 0.02 and 0.04, respectively). In boys, the second quartile of PFHxS appeared to be associated with lower IQ, but no dose–response pattern was observed (Table 3).
Table 3.
Mean differences in IQ scores in boys and girls at 5 y old according to quartiles of prenatal perfluoroalkyl substance (PFAS) levels.
| PFAS quartiles (unit in ng/mL) | Boys (), difference in scores (95% CI) | Girls (), difference in scores (95% CI) | ||||
|---|---|---|---|---|---|---|
| Full Scale IQ | Verbal IQ | Performance IQ | Full Scale IQ | Verbal IQ | Performance IQ | |
| PFOS | ||||||
| 2.36–21.60 | Reference | Reference | Reference | Reference | Reference | Reference |
| 21.61–28.10 | (, 3.3) | (, 1.9) | 0.9 (, 5.2) | 0.3 (, 4.1) | 0.4 (, 4.2) | 0.6 (, 4.4) |
| 28.11–35.80 | 1.3 (, 5.5) | (, 3.1) | 2.8 (, 7.1) | 0.9 (, 4.5) | 0.8 (, 5.2) | 0.2 (, 3.6) |
| (, 1.7) | (, 1.7) | (, 2.3) | 1.8 (, 5.7) | 1.5 (, 5.2) | 1.9 (, 5.9) | |
| p-Trend | 0.30 | 0.30 | 0.37 | 0.35 | 0.43 | 0.39 |
| PFOA | ||||||
| 0.61–3.15 | Reference | Reference | Reference | Reference | Reference | Reference |
| 3.16–4.28 | 0.2 (, 4.3) | (, 3.8) | 0.2 (, 4.4) | 4.5 (1.1, 8.0) | 4.9 (0.9, 8.9) | 3.2 (, 6.6) |
| 4.29–5.49 | (, 2.2) | (, 0.3) | (, 4.0) | 1.6 (, 5.8) | (, 4.0) | 2.6 (, 7.0) |
| 0.0 (, 4.7) | (, 3.3) | 0.6 (, 5.3) | (, 3.8) | (, 3.5) | (, 3.8) | |
| p-Trend | 0.86 | 0.40 | 0.82 | 0.57 | 0.30 | 0.77 |
| PFHxS | ||||||
| –0.76 | Reference | Reference | Reference | Reference | Reference | Reference |
| 0.77–1.07 | (, ) | (, 0.4) | (, 0.6) | 2.8 (, 6.5) | 2.1 (, 5.9) | 2.6 (, 6.4) |
| 1.08–1.38 | (, 1.6) | (, 0.9) | (, 3.1) | 2.6 (, 6.2) | 1.6 (, 5.3) | 2.7 (, 6.6) |
| (, 2.9) | (, 2.1) | (, 4.1) | (, 3.6) | (, 3.7) | (, 3.2) | |
| p-Trend | 0.58 | 0.35 | 0.96 | 0.69 | 0.73 | 0.64 |
| PFNA | ||||||
| –0.36 | Reference | Reference | Reference | Reference | Reference | Reference |
| 0.37–0.46 | 2.1 (, 6.2) | 2.3 (, 6.6) | 1.2 (, 5.3) | 1.0 (, 4.8) | 0.3 (, 4.5) | 1.0 (, 4.6) |
| 0.47–0.57 | 3.2 (, 7.2) | 3.1 (, 7.2) | 2.2 (, 6.4) | 2.8 (, 6.2) | 3.8 (0.4, 7.2) | 1.7 (, 5.2) |
| (, 4.1) | 0.5 (, 5.0) | (, 3.2) | 0.7 (, 4.3) | 3.9 (0.2, 7.5) | (, 2.0) | |
| p-Trend | 0.71 | 0.96 | 0.50 | 0.56 | 0.02 | 0.42 |
| PFHpS | ||||||
| –0.27 | Reference | Reference | Reference | Reference | Reference | Reference |
| 0.28–0.37 | 2.5 (, 6.6) | 0.7 (, 4.9) | 2.9 (, 7.1) | 1.1 (, 4.4) | 1.8 (, 5.7) | 0.6 (, 3.8) |
| 0.38–0.49 | 0.8 (, 5.0) | (, 2.6) | 2.3 (, 6.6) | 1.7 (, 5.6) | 0.4 (, 4.4) | 1.6 (, 5.7) |
| (, 3.4) | (, 2.4) | (, 4.0) | 0.0 (, 4.2) | 1.5 (, 5.7) | (, 3.2) | |
| p-Trend | 0.44 | 0.27 | 0.65 | 0.98 | 0.61 | 0.68 |
| PFDA | ||||||
| –0.14 | Reference | Reference | Reference | Reference | Reference | Reference |
| 0.15–0.17 | 2.0 (, 6.4) | 1.2 (, 5.5) | 2.1 (, 6.5) | 0.1 (, 4.1) | (, 3.9) | 0.2 (, 3.9) |
| 0.18–0.22 | (, 4.4) | 1.0 (, 5.4) | (, 3.3) | (, 3.1) | 0.7 (, 4.3) | (, 2.6) |
| (, 3.7) | 1.6 (, 5.4) | (, 2.2) | 0.9 (, 4.3) | 3.7 (0.2, 7.2) | (, 2.1) | |
| p-Trend | 0.75 | 0.45 | 0.25 | 0.68 | 0.04 | 0.38 |
| PFOSA | ||||||
| –1.38 | Reference | Reference | Reference | Reference | Reference | Reference |
| 1.39–2.32 | (, 4.3) | (, 3.3) | 0.9 (, 5.2) | 0.7 (, 4.3) | (, 3.4) | 1.7 (, 5.3) |
| 2.33–4.16 | (, 4.1) | (, 2.2) | 1.5 (, 5.6) | 0.7 (, 4.3) | (, 3.7) | 1.6 (, 5.1) |
| (, 3.6) | (, 1.9) | 0.6 (, 4.9) | (, 3.5) | (, 3.5) | 0.4 (, 4.5) | |
| p-Trend | 0.65 | 0.24 | 0.92 | 0.63 | 0.63 | 0.95 |
Note: Multivariable linear regression modeling was used to estimate the mean difference in IQ scores. Models were adjusted for maternal age at childbirth, parity, maternal socioeconomic status, maternal IQ, maternal smoking during pregnancy, maternal alcohol consumption during pregnancy, maternal prepregnancy body mass index (BMI), and gestational week of blood draw. p-Trend was modeled based on the midpoint of each category. CI, confidence interval; IQ, intelligence quotient; PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFOSA, perfluorooctanesulfonic acid.
In addition, we found no association between prenatal PFAS values and low IQ scores () in the offspring (Table 4). We did not find evidence to suggest nonlinear associations between any of the PFASs and IQ scores (all p-values for the spline term were ). In addition, the results did not markedly change in an IPW model that only accounted for probabilities reflecting sampling according to maternal alcohol intake at baseline without considering factors that may have affected participation in the LDPS (see Table S7). Finally, our findings did not change in models further adjusted for birth year (see Table S8) or after excluding extreme PFAS values or blood samples with processing delays (see Table S9).
Table 4.
Risk ratio for low IQ (scores ) in children at 5 y of age according to prenatal perfluoroalkyl substance (PFAS) exposures [per natural-log unit (ng/mL) increase].
| Prenatal PFAS | Boys (), RR and 95% CI | Girls (), RR and 95% CI | ||||
|---|---|---|---|---|---|---|
| Low full-scale IQ () | Low verbal IQ () | Low performance IQ () | Low full-scale IQ () | Low verbal IQ () | Low performance IQ () | |
| Per 1 natural-log unit (ng/mL) increase | ||||||
| PFOS | 1.1 (0.6, 1.9) | 1.1 (0.6, 2.0) | 1.2 (0.7, 2.0) | 0.6 (0.3, 1.2) | 0.6 (0.3, 1.3) | 0.8 (0.4, 1.8) |
| PFOA | 1.2 (0.8, 1.7) | 1.1 (0.6, 1.8) | 1.1 (0.7, 1.6) | 0.9 (0.3, 2.4) | 0.6 (0.2, 1.6) | 1.6 (0.5, 5.0) |
| PFHxS | 1.0 (0.7, 1.5) | 1.0 (0.6, 1.6) | 0.9 (0.6, 1.4) | 1.2 (0.6, 2.1) | 1.4 (0.7, 2.6) | 1.0 (0.5, 1.9) |
| PFNA | 0.7 (0.5, 1.1) | 0.6 (0.4, 1.0) | 1.2 (0.7, 2.0) | 0.6 (0.4, 1.0) | 0.7 (0.4, 1.0) | 1.3 (0.7, 2.6) |
| PFHpS | 1.1 (0.7, 1.6) | 0.9 (0.6, 1.4) | 1.1 (0.7, 1.8) | 0.6 (0.3, 1.2) | 0.7 (0.4, 1.3) | 0.8 (0.4, 1.7) |
| PFDA | 0.9 (0.7, 1.2) | 0.8 (0.6, 1.2) | 1.1 (0.7, 1.7) | 0.7 (0.5, 1.0) | 0.7 (0.5, 1.1) | 1.1 (0.6, 1.9) |
| PFOSA | 1.0 (0.8, 1.2) | 1.0 (0.8, 1.3) | 1.0 (0.9, 1.2) | 0.9 (0.7, 1.2) | 0.9 (0.7, 1.3) | 0.9 (0.7, 1.2) |
Note: Generalized linear modeling was used to estimate the RR for low IQ. Models were adjusted for maternal age at childbirth, parity, maternal socioeconomic status, maternal IQ, maternal smoking during pregnancy, maternal alcohol consumption during pregnancy, maternal prepregnancy body mass index (BMI), and gestational week of blood draw. CI, confidence interval; PFDA, perfluorodecanoic acid; PFHpS, perfluoroheptane sulfonate; PFHxS, perfluorohexane sulfonate; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctane sulfonate; PFOSA, perfluorooctanesulfonic acid; RR, risk ratio.
Discussion
In summary, we did not find strong evidence to suggest that prenatal exposure to PFASs is associated with child IQ measured at 5 y of age within the exposure range of our samples. Although a few sex-specific exposure associations were observed, the patterns were inconsistent and could reflect chance findings given the large number of statistical tests conducted. However, some of these observations might warrant further investigation.
Intelligence is a lifelong trait that has a strong influence on educational attainment, career success, mental well-being, adult morbidity, and life expectancy (Kilgour et al. 2010). Exposure to environmental neurotoxicants even at relatively low levels can have a long-lasting impact on intelligence if it occurs during critical periods of development (Bellinger et al. 1992; Braun 2017; Jacobson and Jacobson 1996; Schwartz 1994). PFASs are widespread, and in experimental and human studies, exposures have been shown to interfere with maternal thyroid hormone function that might be essential for fetal brain development (Kjeldsen and Bonefeld-Jørgensen 2013; Long et al. 2013; Wang et al. 2013, 2014; Webster et al. 2014). However, until now, associations between prenatal PFAS exposures and child IQ have only been investigated in two small studies (Stein et al. 2013; Wang et al. 2015). The C8 Health Study found PFOA levels to be associated with improved full-scale IQ (Stein et al. 2013), whereas a Taiwanese cohort study reported that several PFASs were associated with reduction of child IQ scores at 5 and 8 y old (Wang et al. 2015). In a large sample nested within the DNBC, we found no associations or consistent patterns for prenatal exposure to seven PFASs and child IQ scores at .
Several key differences in these epidemiological studies may explain the inconsistency among the findings. First, the exposure levels and the composition of PFAS exposure mixtures differ between study populations. The C8 Health Study (Stein et al. 2013) recruited volunteers from communities highly exposed to industrial PFOA contamination of drinking water with estimated median levels of prenatal PFOA as high as 43.7 (IQR: 11.7–110.8) ng/mL. This value is about 10 times higher than the value measured in our samples from Denmark (median 4.28; IQR: ) or the values reported for the study participants in Taiwan (median 2.50; IQR: ). In the Taiwanese cohort (Wang et al. 2015), several long-chain PFASs such as PFNA, PFDeA, PFUnDA, and PFDoA were detected frequently and in higher concentrations than in the DNBC samples. Even though all three studies utilized Wechsler scales to measure IQ, child IQ scores were evaluated at different ages (from 5 to 12 y). We may expect some variability in IQ measures by age, but a recent cohort study in Cincinnati, Ohio, reported rather high correlations of repeated IQ measures performed at [intraclass correlation coefficient (ICC) ranges 0.71–0.80 for performance, verbal, and full-scale IQ] (Braun et al. 2017). Furthermore, covariates included in the regression models varied between studies; notably, the Taiwanese study did not account for maternal IQ. We included maternal IQ in the analysis because maternal IQ scores were moderately and negatively correlated with several PFAS measures in our sample and because it is a strong predictor of child IQ (Eriksen et al. 2013). Thus, it is possible that some residual confounding by maternal behaviors was not fully accounted for in the Taiwanese study.
Our study has several strengths. First, we included a large sample with prenatal PFAS measurements in maternal plasma collected in early pregnancy. These compounds have long biological half-lives, and PFAS levels measured in early gestation have been shown to reflect the exposure levels throughout the entire pregnancy period (Ehresman et al. 2007; Fei et al. 2007). Secondly, trained psychologists administered the IQ tests blinded to exposure status. A large number of potential confounders were included in the analyses. Finally, participants were selected from a well-designed longitudinal cohort, and we accounted for sampling and nonparticipation using weighted regression methods to minimize the possible influence of selection bias on our results. The PFAS levels found in this LDPS sample are comparable to those in a previous sample from the DNBC unaffected by selective dropout. We cannot rule out the possibility of residual bias due to nonparticipation in the LDPS that is unaccounted for in our IPW model, but the magnitude of such bias is likely to be small (Greene et al. 2011). Moreover, a previous LPDS paper described associations with some well-known risk factors such as parental education and maternal IQ, as well as several birth and postnatal characteristics and child IQ at , supporting the validity of the outcome measures (Eriksen et al. 2013).
Limitations of the study include that we had only a one-time measure of child IQ scores at available for analysis and that child blood samples are not available in the DNBC to study the possible influence of postnatal exposures to PFASs. Moreover, owing to data availability, we were unable to conduct multiple exposure analyses and investigate the effects of PFASs together with other environmental exposures such as other persistent or nonpersistent endocrine disruptors. We expect generalizability of our study findings to populations with similar characteristics, that is to say, Nordic or high-income populations with similar exposure ranges. Whether higher levels of PFASs could disproportionately affect IQ scores among children from low-SES families should be evaluated in future studies.
Conclusion
In summary, we did not find consistent associations between prenatal PFAS exposures and child IQ at in a large cohort nested within the DNBC. However, findings from this study alone may not rule out possible neurodevelopmental effects of early-life exposures to PFASs. Further study is also needed to examine offspring IQ scores at older ages. Alternatively, studies may be able to assess other functional cognitive measures such as school performance and educational achievement. Other brain functions related to attention and to other behaviors in addition to intelligence also need to be evaluated. In addition, early childhood exposure to PFASs should be investigated. Populations affected by higher exposure levels and potential mixture effects of PFASs should also be considered in future research.
Supplemental Material
Acknowledgments
J.O. and Z.L. conceptualized and designed the study. Z.L. performed data analyses and drafted the manuscript. R.B.’s lab performed measurements of PFASs. All authors contributed to writing the manuscript and interpretation of results.
The Danish National Research Foundation established the Danish Epidemiology Science Centre, which initiated and created the Danish National Birth Cohort. The cohort is furthermore a result of a major grant from this Foundation. Additional support for the Danish National Birth Cohort was obtained from the Pharmacy Foundation, the Egmont Foundation, the March of Dimes Birth Defects Foundation and the Augustinus Foundation. This work was a part of the FETOTOX project supported by the Danish Strategic Research Council (grant no. 10-092818). ZL is supported by the National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS) Pathway to Independence Award (grant no. K99ES026729).
References
- Andersen SL, Andersen S, Liew Z, Vestergaard P, Olsen J. 2017. Maternal thyroid function in early pregnancy and neuropsychological performance of the child at 5 years of age. J Clin Endocrinol Metab. 103(2):660–670, PMID: 29220528, 10.1210/jc.2017-02171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Laurberg P, Wu CS, Olsen J. 2014. Attention deficit hyperactivity disorder and autism spectrum disorder in children born to mothers with thyroid dysfunction: a Danish nationwide cohort study. BJOG 121(11):1365–1374, PMID: 24605987, 10.1111/1471-0528.12681. [DOI] [PubMed] [Google Scholar]
- Bach CC, Henriksen TB, Bossi R, Bech BH, Fuglsang J, Olsen J, et al. 2015. Perfluoroalkyl acid concentrations in blood samples subjected to transportation and processing delay. PLoS One 10(9):e0137768, PMID: 26356420, 10.1371/journal.pone.0137768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F, Lopez-Espinosa MJ. 2017. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: a systematic review of epidemiologic studies. Environ Int 99:15–28, PMID: 27884404, 10.1016/j.envint.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Stiles KM, Needleman HL. 1992. Low-level lead exposure, intelligence and academic achievement: a long-term follow-up study. Pediatrics 90(6):855–861, PMID: 1437425. [PubMed] [Google Scholar]
- Bjerregaard-Olesen C, Bach CC, Long M, Ghisari M, Bossi R, Bech BH, et al. 2016. Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008-2013. Environ Int 91:14–21, PMID: 26891270, 10.1016/j.envint.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Braun JM. 2017. Early-life exposure to EDCs: role in childhood obesity and neurodevelopment. Nat Rev Endocrinol 13(3):161–173, PMID: 27857130, 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Stacy SL, Erar B, Papandonatos GD, Bellinger DC, et al. 2017. Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior. Neurotoxicology 62:192–199, PMID: 28736150, 10.1016/j.neuro.2017.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. 2007. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES). Environ Sci Technol 41(7):2237–2242, PMID: 17438769. [DOI] [PubMed] [Google Scholar]
- Chu S, Letcher RJ, McGoldrick DJ, Backus SM. 2016. A new fluorinated surfactant contaminant in biota: perfluorobutane sulfonamide in several fish species. Environ Sci Technol 50(2):669–675, PMID: 26649981, 10.1021/acs.est.5b05058. [DOI] [PubMed] [Google Scholar]
- Ehresman DJ, Froehlich JW, Olsen GW, Chang SC, Butenhoff JL. 2007. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ Res 103(2):176–184, PMID: 16893538, 10.1016/j.envres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Eriksen HL, Kesmodel US, Underbjerg M, Kilburn TR, Bertrand J, Mortensen EL. 2013. Predictors of intelligence at the age of 5: family, pregnancy and birth characteristics, postnatal influences, and postnatal growth. PLoS One 8(11):e79200, PMID: 24236109, 10.1371/journal.pone.0079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. 2007. Perfluorinated chemicals and fetal growth: a study within the Danish national birth cohort. Environ Health Perspect 115(11):1677–1682, PMID: 18008003, 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink WA, van Asseldonk L, van Leeuwen SPJ. 2017. Presence of emerging per- and polyfluoroalkyl substances (PFASs) in river and drinking water near a fluorochemical production plant in Netherlands. Environ Sci Technol 51(19):11057–11065, PMID: 28853567, 10.1021/acs.est.7b02488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A, Berger U, Bignert A, Ullah S, Aune M, Lignell S, et al. 2012. Perfluorinated alkyl acids in blood serum from primiparous women in Sweden: serial sampling during pregnancy and nursing, and temporal trends 1996–2010. Environ Sci Technol 46(16):9071–9079, PMID: 22770559, 10.1021/es301168c. [DOI] [PubMed] [Google Scholar]
- Greene N, Greenland S, Olsen J, Nohr EA. 2011. Estimating bias from loss to follow-up in the Danish national birth cohort. Epidemiology 22(6):815–822, PMID: 21918455, 10.1097/EDE.0b013e31822939fd. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341(8):549–555, PMID: 10451459, 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Hong T, Paneth N. 2008. Maternal and infant thyroid disorders and cerebral palsy. Semin Perinatol 32(6):438–445, PMID: 19007683, 10.1053/j.semperi.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. 2006. Biological monitoring of polyfluoroalkyl substances: a review. Environ Sci Technol 40(11):3463–3473, PMID: 16786681. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. 1996. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med 335(11):783–789, PMID: 8703183, 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. 2011. Trends in exposure to polyfluoroalkyl chemicals in the U.S. population: 1999-2008. Environ Sci Technol 45(19):8037–8045, PMID: 21469664, 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- Kesmodel US, Eriksen HL, Underbjerg M, Kilburn TR, Støvring H, Wimberley T, et al. 2012. The effect of alcohol binge drinking in early pregnancy on general intelligence in children. BJOG 119(10):1222–1231, PMID: 22712770, 10.1111/j.1471-0528.2012.03395.x. [DOI] [PubMed] [Google Scholar]
- Kesmodel US, Underbjerg M, Kilburn TR, Bakketeig L, Mortensen EL, Landrø NI, et al. 2010. Lifestyle during pregnancy: neurodevelopmental effects at 5 years of age. The design and implementation of a prospective follow-up study. Scand J Public Health 38(2):208–219, PMID: 20064917, 10.1177/1403494809357093. [DOI] [PubMed] [Google Scholar]
- Kilgour AH, Starr JM, Whalley LJ. 2010. Associations between childhood intelligence (IQ), adult morbidity and mortality. Maturitas 65(2):98–105, PMID: 19879703, 10.1016/j.maturitas.2009.09.021. [DOI] [PubMed] [Google Scholar]
- Kjeldsen LS, Bonefeld-Jørgensen EC. 2013. Perfluorinated compounds affect the function of sex hormone receptors. Environ Sci Pollut Res Int 20(11):8031–8044, PMID: 23764977, 10.1007/s11356-013-1753-3. [DOI] [PubMed] [Google Scholar]
- Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. 2007. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99(2):366–394, PMID: 17519394, 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- Lazarus JH. 1999. Thyroid hormone and intellectual development: a clinician's view. Thyroid 9(7):659–660, PMID: 10447010, 10.1089/thy.1999.9.659. [DOI] [PubMed] [Google Scholar]
- Liew Z, Ritz B, Bonefeld-Jørgensen EC, Henriksen TB, Nohr EA, Bech BH, et al. 2014. Prenatal exposure to perfluoroalkyl substances and the risk of congenital cerebral palsy in children. Am J Epidemiol 180(6):574–581, PMID: 25139206, 10.1093/aje/kwu179. [DOI] [PubMed] [Google Scholar]
- Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, et al. 2015. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case–control study in the Danish National Birth Cohort. Environ Health Perspect 123(4):367–373, PMID: 25616253, 10.1289/ehp.1408412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M, Ghisari M, Bonefeld-Jørgensen EC. 2013. Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor. Environ Sci Pollut Res Int 20(11):8045–8056, PMID: 23539207, 10.1007/s11356-013-1628-7. [DOI] [PubMed] [Google Scholar]
- Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. 2004. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect 112(17):1691–1696, PMID: 15579415, 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesto T, Tiemeier H, Peeters RP, Jaddoe VW, Hofman A, Verhulst FC, et al. 2015. Maternal mild thyroid hormone insufficiency in early pregnancy and attention-deficit/hyperactivity disorder symptoms in children. JAMA Pediatr 169(9):838–845, PMID: 26146876, 10.1001/jamapediatrics.2015.0498. [DOI] [PubMed] [Google Scholar]
- Nøst TH, Vestergren R, Berg V, Nieboer E, Odland JØ, Sandanger TM. 2014. Repeated measurements of per- and polyfluoroalkyl substances (PFASs) from 1979 to 2007 in males from northern Norway: assessing time trends, compound correlations and relations to age/birth cohort. Environ Int 67:43–53, PMID: 24657493, 10.1016/j.envint.2014.02.011. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J, Melbye M, Olsen SF, Sørensen TIA, Aaby P, Andersen AMN, et al. 2001. The Danish National Birth Cohort – its background, structure and aim. Scand J Public Health 29(4):300–307, PMID: 11775787, 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- Oppenheimer JH, Schwartz HL. 1997. Molecular basis of thyroid hormone-dependent brain development. Endocr Rev 18(4):462–475, PMID: 9267760, 10.1210/edrv.18.4.0309. [DOI] [PubMed] [Google Scholar]
- Raven J, Raven JC, Court J. 1998. Manual for Raven’s Progressive Matrices and Vocabulary Scales. Oxford: Oxford Psychologists Press, Ltd. [Google Scholar]
- Schwartz J. 1994. Low-level lead exposure and children’s IQ: a meta-analysis and search for a threshold. Environ Res 65(1):42–55, PMID: 8162884, 10.1006/enrs.1994.1020. [DOI] [PubMed] [Google Scholar]
- Stein CR, Savitz DA, Bellinger DC. 2013. Perfluorooctanoate and neuropsychological outcomes in children. Epidemiology 24(4):590–599, PMID: 23680941, 10.1097/EDE.0b013e3182944432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Arevalo E, Strynar M, Lindstrom A, Richardson M, Kearns B, et al. 2016. Legacy and emerging perfluoroalkyl substances are important drinking water contaminants in the Cape Fear river watershed of North Carolina. Environ Sci Technol Lett 3(12):415–419, 10.1021/acs.estlett.6b00398. [DOI] [Google Scholar]
- Wang Y, Rogan WJ, Chen HY, Chen PC, Su PH, Chen HY, et al. 2015. Prenatal exposure to perfluroalkyl substances and children's IQ: The Taiwan Maternal and Infant Cohort Study. Int J Hyg Environ Health 218(7):639–644, PMID: 26205657, 10.1016/j.ijheh.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rogan WJ, Chen PC, Lien GW, Chen HY, Tseng YC, et al. 2014. Association between maternal serum perfluoroalkyl substances during pregnancy and maternal and cord thyroid hormones: Taiwan Maternal and Infant Cohort Study. Environ Health Perspect 122(5):529–534, PMID: 24577800, 10.1289/ehp.1306925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Starling AP, Haug LS, Eggesbo M, Becher G, Thomsen C, et al. 2013. Association between perfluoroalkyl substances and thyroid stimulating hormone among pregnant women: a cross-sectional study. Environ Health 12:76, PMID: 24010716, 10.1186/1476-069X-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster GM, Venners SA, Mattman A, Martin JW. 2014. Associations between perfluoroalkyl acids (PFASs) and maternal thyroid hormones in early pregnancy: a population-based cohort study. Environ Res 133:338–347, PMID: 25019470, 10.1016/j.envres.2014.06.012. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1955. Manual for the Wechsler Adult Intelligence Scale. New York, NY: The Psychological Corporation. [Google Scholar]
- Wechsler D. 1990. Manual for the Wechsler Preschool and Primary Scale of Intelligence–Revised. Kent, UK: The Psychological Corporation. [Google Scholar]
- Wechsler D. 1999. Manual for the Wechsler Preschool and Primary Scale of Intelligence–Revised. Swedish Edition. Stockholm, Sweden: Psykologiforlaget AB. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.