Abstract
The storage and use of glycogen, the main energy reserve in the brain, is a metabolic feature of astrocytes. Glycogen synthesis is regulated by Protein Targeting to Glycogen (PTG), a member of specific glycogen-binding subunits of protein phosphatase-1 (PPP1). It positively regulates glycogen synthesis through de-phosphorylation of both glycogen synthase (activation) and glycogen phosphorylase (inactivation). In cultured astrocytes, PTG mRNA levels were previously shown to be enhanced by the neurotransmitter noradrenaline. To achieve further insight into the role of PTG in the regulation of astrocytic glycogen, its levels of expression were manipulated in primary cultures of mouse cortical astrocytes using adenovirus-mediated overexpression of tagged-PTG or siRNA to downregulate its expression. Infection of astrocytes with adenovirus led to a strong increase in PTG expression and was associated with massive glycogen accumulation (>100 fold), demonstrating that increased PTG expression is sufficient to induce glycogen synthesis and accumulation. In contrast, siRNA-mediated downregulation of PTG resulted in a 2-fold decrease in glycogen levels. Interestingly, PTG downregulation strongly impaired long-term astrocytic glycogen synthesis induced by insulin or noradrenaline. Finally, these effects of PTG downregulation on glycogen metabolism could also be observed in cultured astrocytes isolated from PTG-KO mice. Collectively, these observations point to a major role of PTG in the regulation of glycogen synthesis in astrocytes and indicate that conditions leading to changes in PTG expression will directly impact glycogen levels in this cell type.
Keywords: Glia, Noradrenaline, Insulin, Glucose metabolism
Abbreviations: PTG, Protein targeting to glycogen; GS, Glycogen synthase; GP, Glycogen phosphorylase; NA, Noradrenaline; Ins, Insulin
1. Introduction
Glycogen is the main energy reserve in the central nervous system (CNS) and is localized primarily in astrocytes (Magistretti et al., 1993a). At the physiological level, the role of glycogen as an energy reserve in energy deficiency has been well documented (see e.g. Magistretti and Allaman, 2015 and references therein). More recent evidence also points to an important role played by astrocytic glycogen in higher brain functions in mammals such as learning and memory (Boury-Jamot et al., 2016, Duran et al., 2013, Gibbs, 2016, Newman et al., 2011, Obel et al., 2012, Suzuki et al., 2011). In particular, an activity-dependent mobilization of glycogen associated with astrocytic lactate production and transfer to neurons is crucial for the establishment of long term memory in rodents in an inhibitory avoidance learning paradigm (Gao et al., 2016, Steinman et al., 2016, Suzuki et al., 2011). A direct correlate of such observations is that tight regulation of glycogen metabolism, and in particular the maintenance of a releasable glycogen pool, is crucial to sustain such physiological processes.
As in peripheral organs, cerebral glycogen mobilization and synthesis is under complex metabolic regulations involving both allosteric and covalent modifications and for which glycogen synthase (GS) and glycogen phosphorylase (GP) represent the two central elements (see e.g. Allaman and Magistretti, 2015, Obel et al., 2012, Roach et al., 2012). In particular, protein kinase-catalyzed phosphorylation inactivates GS and activates GP, resulting in glycogenolysis and generation of glucose and glucose 6-phosphate whereas dephosphorylation of both enzymes leads to inactivation of GP and activation of GS allowing synthesis of glycogen from the precursor UDP-glucose. While phosphorylation of both GS and GP involves different set of kinases, protein phosphatase-1 (PPP1) is the enzyme responsible for dephosphorylation of both GS and GP. In order to be active, PPP1 must be bound to glycogen through PPP1 glycogen-binding subunits (Ceulemans et al., 2002, Korrodi-Gregorio et al., 2014). So far, at least seven distinct glycogen-binding subunit have been identified (PPP1R3A to G) which show specific cellular and tissue distributions (Ceulemans et al., 2002). The major ones include PPP1R3A (GM) which is muscle-specific (Suzuki et al., 2001), PPP1R3B (GL) which is the primary PPP1R3 expressed in the liver (Doherty et al., 1995), and the relatively widespread-ubiquitous isoforms PPP1R3C (PPP1R5 or PTG for protein targeting to glycogen) (Printen et al., 1997) and PPP1R3D (PPP1R6) (Armstrong et al., 1997). Interestingly, the gene encoding PTG is abundantly expressed in astrocytes of the CNS (Lovatt et al., 2007, Zhang et al., 2014), and several lines of evidence suggested that PTG plays an important role in the regulation of glycogen metabolism in this cell type (Allaman et al., 2000, Allaman et al., 2003, Petit et al., 2013, Vilchez et al., 2007).
Astrocytic glycogen levels are under tight control by numerous glycogenolytic and glycogenic neuro-active substances including, noradrenaline (NA), VIP, insulin (Ins), insulin-growth factors I and II, glutamate, glucocorticoids, ATP or adenosine (see e.g. Brown, 2004, Magistretti et al., 1993a, Magistretti et al., 1993b, Obel et al., 2012). Regarding the maintenance of a releasable glycogen pool, NA and Ins represent interesting cases since both are known to promote long-term glycogen synthesis in astrocytes (Allaman et al., 2003, Dringen and Hamprecht, 1992, Hamai et al., 1999, Heni et al., 2011, Kum et al., 1992, Sorg and Magistretti, 1992). While NA and Ins glycogenic effects ultimately involve dephosphorylation and activation of GS (Allaman et al., 2004, Hamai et al., 1999), the activation cascades resulting in GS activation differ between the two agents (cAMP and PI3-kinase cascades for NA and Ins respectively). Interestingly, we have previously shown that NA, but not Ins, stimulates PTG mRNA expression concomitantly with glycogen resynthesis, suggesting PTG as the main mediator of glycogen resynthesis induced by NA.
In this study, we manipulated PTG expression in primary cultures, by up- or down-regulation, to determine its role in the regulation of glycogen content in astrocytes. Moreover, we aimed to address PTG involvement in the glycogenic action of NA and Ins.
2. Materials and methods
2.1. Reagents and antibodies
All chemicals and culture mediums, if not otherwise specified are from Sigma-Aldrich, Buchs SG, Switzerland.
2.2. Astrocytes cultures
Experiments were conducted in accordance with the Swiss Federal Guidelines for Animal Experimentation and were approved by the Cantonal Veterinary Office for Animal Experimentation (Vaud, Switzerland). Primary cultures of cerebral cortical astrocytes were prepared as previously described (Bélanger et al., 2011) from newborns (1-2 days-old) of OF1 mice (Charles River Laboratories, L'Arbresle, France) or heterozygous C57/Bl6j PTG KO mice (produced by A. A. DePaoli-Roach). For PTG KO astrocytic cultures, newborn pups from heterozygous breedings of C57/Bl6j PTG KO mice were genotyped by quantitative PCR. From each wild-type and knock-out homozygote mice, one littermate was selected per experiment and brain was dissected for astrocytic cultures. Cells were plated in 35 mm Ø dishes or glass coverslips and incubated in culture medium (Dulbecco's Modified Eagle Medium [DMEM] containing 25 mM glucose, Sigma-Aldrich, D7777) supplemented with 10% fetal calf serum, at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air. The culture medium was renewed 3–5 days after seeding and subsequently twice a week. Experiments were performed on confluent 14 days-old cultures (DIV14) with the exception of immunofluorescence experiments which were performed on non-confluent (low density plated) cultures (DIV14) to facilitate cellular localization of Flag-PTG.
2.3. Small interfering RNA (siRNA) transfection
Specific PPP1R3C (PTG) downregulation in astrocytes was achieved using Stealth Select RNAi™ (siRNA, Ref. no. PPP1R3C/MSS284999; Invitrogen, Basel, Switzerland). Control groups (Mock siRNA) were transfected with siRNA negative controls (Hi GC complex #2, Invitrogen). For each 35 mm Ø dish, 100 pmoles siRNA was transfected following a previously described protocol (Bélanger et al., 2011). Each siRNA was applied to astrocytes for 4 days, followed by Western blot analysis, quantitative PCR and glycogen determination.
2.4. Adenovirus mediated Flag-PTG overexpression
To infect astrocytes, DIV9 cortical astrocytes (around 80% of confluency) were incubated in 1 mL serum free DMEM (Sigma-Aldrich, D5030) supplemented with (final concentrations) 5 mM glucose, 44 mM NaHCO3, 0.06 g/L penicillin and 0.1 g/L streptomycin (DMEM5). 1 × 106 Pfu of adenovirus containing Flag-tagged mouse PTG (Ad-Flag-m-PPP1R3C, ADV-269217 clone) or enhanced GFP (EGFP) (Ad-GFP used as control adenovirus, cat. n° 1060) both under the control of the CMV promoter, obtained by Vector BioLabs (Philadelphia, USA), were added directly in the dishes and incubated overnight. The next day, DMEM5 medium was replaced by fresh culture medium. Experiments using adenovirus were conducted in biosafety level 2 laboratory and performed 5 days after infection. When indicated siRNAs were applied 24 hours after adenoviruses infection following the above-described protocol.
2.5. Cell treatment with noradrenaline and insulin
During all incubations, primary cultures of astrocytes were maintained at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air. 16 h before treatment, the culture medium was removed and astrocytes were incubated in serum free DMEM5. Without changing the medium, the cells were then exposed to noradrenaline (NA) or Insulin (Ins) for 6 h followed by glycogen determination. It was previously demonstrated that under these experimental conditions glucose concentration is not a limiting factor for glycogen synthesis induced by glycogenic agents (Allaman et al., 2003, Allaman et al., 2004).
2.6. Quantitative PCR (qPCR)
Gene expression analysis: RNA isolation, cDNA synthesis and qPCR amplification were performed as previously described (Bélanger et al., 2011). Forward and reverse sequences were as follows: β-Actin: 5′-GCTTCTTTGCAGCTCCTTCGT-3′ and 5′-ATATCGTCATCCATGGCGAAC-3′; Cyclophilin A: 5′-CAAATGCTGGACCAAACACAA-3′ and 5′-GCCATCCAGCCATTCAGTCT-3′;Protein phosphatase 1 regulatory subunit 3C (PPP1R3C or PTG): 5′-GGCATGACGGAACTTGTCAA-3′ and 5′-TGCCTCTCGGTCCAATGAG-3′; Glycogen Synthase 1, muscle (Gys1): 5′-GCTGGACAAGGAGGACTTCACT-3′ and 5′-TGCACACTGGTGGGAAAGAC-3′; Glycogenin (Gyg): 5′-ACACCTTCACCACCAACGTCTT-3′ and 5′-GCTCCTGAGACATGTTCCATCAT-3′; Brain Glycogen Phosphorylase (Pygb): 5′-GCTGCTCAACTGCCTACACATT-3′ and 5′-AACAGTCCTGGGCACAAAGG-3′; Protein phosphatase 1 regulatory subunit 3D (PPP1R3D or PPP1R6): 5′-GAGGAGGCCTGCTATCTGTTTTC-3′ and 5′-AATGCAGGCCACACTGTGTATC-3′.
Genotyping: Genotyping was performed using qPCR. Genomic DNA was isolated from cerebellum of C57/Bl6j pups using Dneasy blood and tissue kit (Qiagen, Hombrechtikon, Switzerland) according to the manufacturer instructions. PCR mix was composed of 10 ng of genomic DNA, 300 nM of forward and reverse primers in 10 μL of 1x SYBR-Green Fast PCR MasterMix (Applied Biosystems). Genomic DNA and LoxP sequences for forward and reverse primers were as follows: β-Actin (NC_000071.6 Chromosome 5): 5′-CCCTTCTCTTTGGCCAGCTT-3′ and 5′-ACGGCAGAAGAAAGACAATTGAG-3′; Ppia (Peptidyl-prolyl cis-trans isomerase): (NC_000077.6 Chromosome 11) 5′-GATCTGTTGACAAGAGTGCAAAGC-3′ and 5′-AGAGGGAATGAAAGGAACACTGAA-3′; PPP1R3C (PTG WT): (NC_000085.6 Chromosome 19): 5′-AAATCGCAGAGTGAGTGGAAGAG-3′ and 5′- GCAAACACGACCCGCTTCT-3′; LoxP (PTG KO): 5′-CGCGCCAAGCTGTCTAGAAT-3′ and 5′-GAACAAAGCTGGTGGCATCA-3′. Genotypes were identified from the relative genomic abundance of PTG and LoxP following normalization to β-Actin and Ppia genomic content levels.
2.7. Western blot
Protein extraction and detection were performed following a previously described procedure (Yang et al., 2014). PTG protein levels were quantified using the ratio of Flag-tagged PTG/Actin as detected by chemiluminescence. Antibodies used were: mouse anti-Flag M2 (Sigma-Aldrich, F1804) (dilution 1/1′000), mouse anti-β-Actin (Sigma-Aldrich, A5441) (dilution 1/1′000′000) and mouse horseradish peroxidase-conjugated secondary antibody (GE Healthcare, Glattburg, Switzerland).
2.8. Immunofluorescence and image acquisition
DIV14 astrocytes grown on glass coverslips were fixed with 4% paraformaldehyde for 10 min, washed 3 times with PBS and permeabilized for 10 min in phosphate-buffered saline (PBS) containing 0.1% triton X-100. Coverslips were then incubated in PBS containing 5% bovine serum albumin and 0.05% triton X-100 for 30 min to block non-specific binding before incubation with a monoclonal antibody directed against the Flag epitope (see above, 1/500 dilution) in PBS for 2 h. Glass slides were rinsed thoroughly and probed with the secondary antibody, anti-mouse Alexa Fluor® 488 (Thermo Fisher Scientific, Reinach, Switzerland) for 1 h at room temperature in PBS. After extensive washing, coverslips were finally incubated with 0.2 μg/mL Hoechst to stain nuclei, rinsed in PBS and mounted with an anti-bleaching medium (DABCO). Image acquisitions were performed as a stack of 10 images with 300 nm step size on a wide-filed microscope (Leica DM5500) with a 40 × 1.3 oil immersion objective and recorded with DFC 350FX B/W camera. Deconvolution was performed on each image to sharpen their signal.
2.9. Glycogen quantification
At the end of incubation periods, the cells were lysed and harvested for glycogen quantification as previously descried (Allaman et al., 2000). Glycogen content is expressed as nmoles of glycosyl units originating from glycogen per mg of protein.
2.10. Calcein viability assay
At the end of adenovirus exposure, astrocytic viability was determined by the calcein assay using a standard procedure (see e.g. Allaman et al., 2010).
2.11. Statistical analysis
All results are presented as the mean ± SEM and significance was considered at p ≤ 0.05 for all statistical tests. Data were analyzed for statistical significance by unpaired Student's t-test or by one-way ANOVA followed by a Bonferroni's multiple comparison test (Prism 5.0, GraphPad, San, Diego, CA).
3. Results
3.1. PTG overexpression leads to massive glycogen accumulation in cultured astrocytes
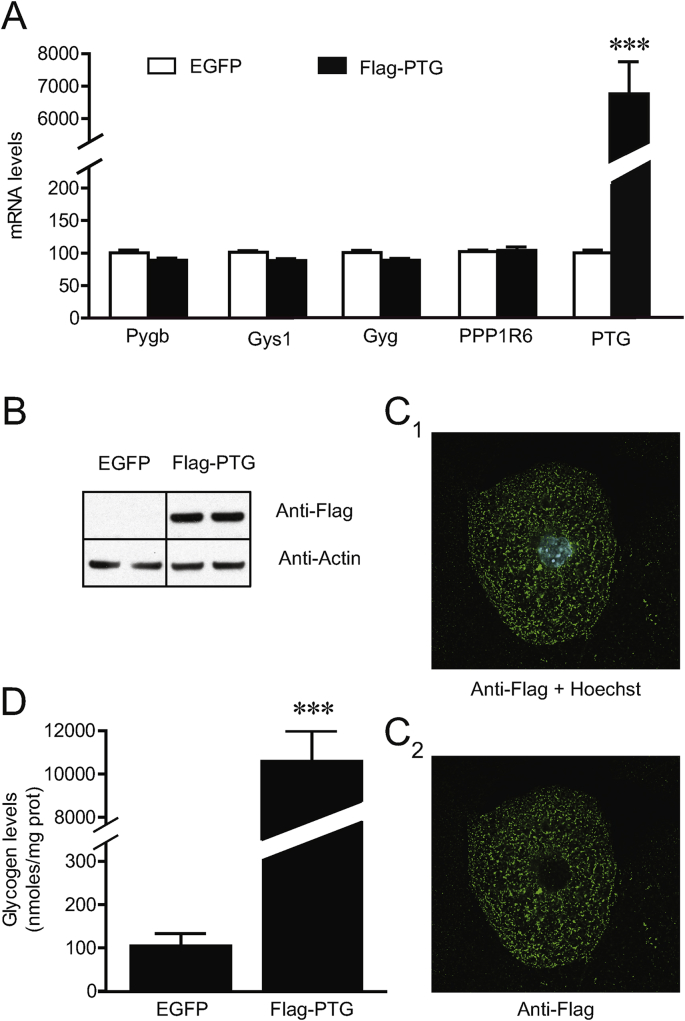
As a first step to investigate the importance of PTG in controlling astrocytic glycogen content, the impact of increased PTG expression was assessed in primary cultures of mouse astrocytes using adenovirus-mediated overexpression of a Flag-tagged PTG construct. PTG tagging was chosen to monitor PTG protein expression levels since the commercially available PTG antibodies did not produce a specific signal in our hands. Five days following culture infection, adenovirus-mediated overexpression of PTG resulted in a 67-fold increase in PTG mRNA levels, as compared to control cultures infected with an adenovirus overexpressing the EGFP protein, whereas mRNA expression of other important genes related to astrocytic glycogen metabolism were not significantly affected. Tested genes included the brain isoform of glycogen phosphorylase (Pygb), muscle isoform of glycogen synthase (Gys1), glycogenin (Gyg), as well as another PPP1 glycogen binding subunit, PPP1R3D (or PPP1R6) (Fig. 1A).
Fig. 1.
PTG overexpression increases glycogen content in cultured astrocytes. Primary cultures of mouse astrocytes were infected with adenovirus containing Flag-tagged mouse PTG (Flag-PTG), or enhanced GFP (EGFP) as control, for five days. (A) mRNA expression levels of genes related to astrocytic glycogen metabolism. Data are means ± SEM and expressed as percentage of the EGFP values for each gene (n = 12–14, 4–5 independent experiments). (B) Representative Western blot of Flag-PTG protein detection (actin as a loading control). Similar results were obtained in 5 independent experiments. (C) Representative immunostaining of Flag-PTG, with (C1) and without (C2) nuclear staining with Hoechst. No Flag-PTG staining was observed in the nuclei. Similar results were obtained in 2 independent experiments. (D) Glycogen content. Data are means ± SEM and are expressed as nmoles of glycosyl units per mg of proteins (n = 12, 4 independent experiments). ***P ≤ 0.001 vs respective EGFP conditions with ANOVA followed by Bonferroni's post hoc test (A), or unpaired t-test (D). Pygb, brain glycogen phosphorylase; Gys1, muscle glycogen synthase 1; Gyg, glycogenin; PPP1R6 (or PPP1R3D), Protein phosphatase 1 regulatory subunit 3D; PTG (or PPP1R3C), Protein phosphatase 1 regulatory subunit 3C.
PTG mRNA correlated with increased protein levels as determined by Western blot analysis and immunohistochemistry using an antibody directed against the Flag (Fig. 1B and C). At the subcellular level, the exogenous Flag-tagged PTG showed a punctate pattern that was homogeneously distributed in the cytoplasm, which is consistent with previously reported glycogen and glycogen synthase distribution patterns in cultured astrocytes (Vilchez et al., 2007).
To examine the physiological consequence of Flag-tagged PTG overexpression, we measured astrocyte glycogen content. As shown in Fig. 1D, cells overexpressing PTG present a remarkable glycogen accumulation (more than 100-fold increase), demonstrating that increased PTG expression was sufficient to drive glycogen metabolism into a synthetic mode in this cell type.
Of note, control experiments demonstrated the lack of cellular toxicity (as measured with the cell viability calcein assay) of adenovirus-mediated PTG overexpression and the associated glycogen accumulation in cultured astrocytes in our experimental conditions (not shown).
3.2. PTG downregulation decreases the glycogenic ability in astrocytes
As a mirror experiment to PTG overexpression, the impact of PTG downregulation on glycogen content was tested in cultured astrocytes using short interfering RNA (siRNA) directed against PTG. PTG silencing efficiency and specificity were assessed by quantitative PCR and Western blot after four days of siRNA treatment (Fig. 2A and B). As shown in Fig. 2A, PTG mRNA levels were decreased by 60.0 ± 2.2% in PTG siRNA transfected astrocytes compared to control mock siRNA treated cells, while mRNA expression levels of other astrocytic glycogen-related genes remained unaltered. Efficiency of siRNA-mediated downregulation of PTG protein was demonstrated in cultured astrocytes overexpressing Flag-tagged PTG (see above) for which a 75.0 ± 4.6% reduction in protein expression following siRNA co-treatment was estimated (Fig. 2B, left and middle panels); this effect was associated with a strong reduction in glycogen accumulation (63.5 ± 2.6% decrease; Fig. 2B, right panel). Basal astrocytic glycogen content when PTG expression was downregulated resulted in a 50.7 ± 10.4% decrease compared to control values (mean ± SEM; n = 19–21, 7 independent experiments; Student's t-test: p ≤ 0.0001), further establishing the importance of PTG in the regulation of basal glycogen levels in astrocytes.
Fig. 2.
Effects of RNA interference against PTG on glycogen metabolism regulation. Cultured astrocytes were transfected with siRNA against PTG or a negative control siRNA (mock siRNA) for four days. (A) mRNA expression levels of genes related to astrocytic glycogen metabolism. Data are means ± SEM and expressed as percentage of the mock siRNA values for each gene (n = 6–7, 2–3 independent experiments). ***P ≤ 0.001 vs mock siRNA condition with ANOVA followed by Bonferroni's post hoc test. (B) Downregulation of adenovirus-mediated overexpression of Flag-PTG protein by PTG siRNA. (left panel) Representative Western blot for Flag-PTG protein detection (actin as a loading control). (middle panel) Quantification of Western blots as shown on the left panel following normalization by actin content (n = 10, 5 independent experiments). (right panel) Glycogen content (n = 12, 4 independent experiments). Data are means ± SEM expressed as percentage of respective mock siRNA control conditions. ***p ≤ 0.001 vs mock siRNA with unpaired t-test. (C) Glycogen content following noradrenaline (NA, 100 μM) and Insulin (Ins, 100 nM) treatments for 6 h (n = 10–12, 4 independent experiments). Data are means ± SEM and expressed as percentage of the mock siRNA untreated condition (Ctr). ***p ≤ 0.001 vs Ctr and ###p ≤ 0.001 vs respective mock siRNA conditions with ANOVA followed by Bonferroni's post-hoc test for NA conditions comparison (including Ctrl and NA, in mock and PTG siRNA conditions), respectively Ins conditions comparison (including Ctrl and Ins, in mock and PTG siRNA conditions).
The impact of siRNA-mediated PTG downregulation was then assessed on the glycogenic actions of both noradrenaline (NA) and insulin (Ins), two well characterized regulators of glycogen synthesis in astrocytes (Hamai et al., 1999, Heni et al., 2011, Sorg and Magistretti, 1992). Considering that increased PTG expression was previously shown to be associated with the glycogenic action of NA, but not Ins, one might have assumed that PTG downregulation would specifically impair NA-induced glycogen synthesis (Allaman et al., 2000). Indeed, a strong reduction in the fold increase of NA-stimulated glycogen synthesis following PTG downregulation could be observed (23.3 and 6.1 fold increase in mock and PTG siRNA conditions, respectively; Fig. 2C). Surprisingly however, Ins-stimulated glycogen synthesis was also found to be significantly reduced by PTG downregulation (5.3 and 2.3 fold increase in mock and PTG siRNA conditions, respectively; Fig. 2C). These findings suggest that PTG is a key element in the glycogenic actions of both NA and Ins in astrocytes.
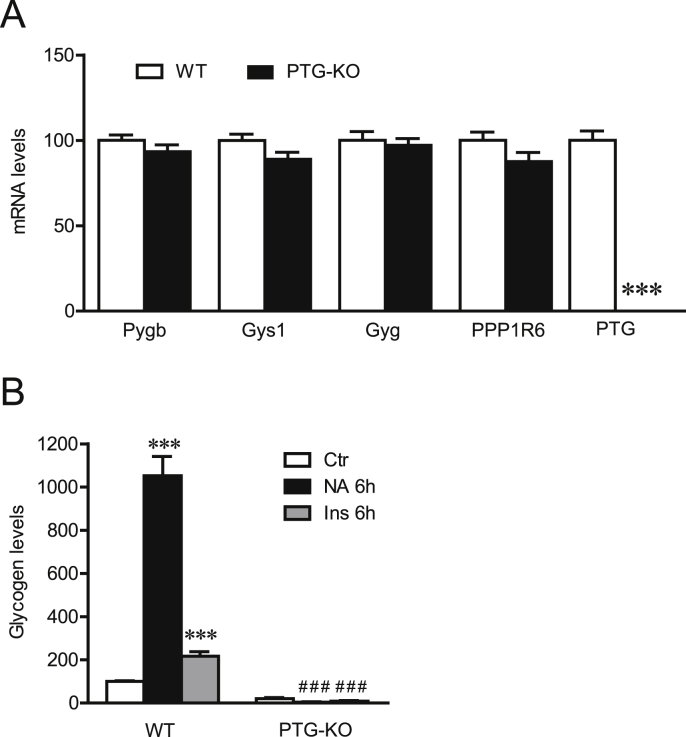
We next determined the impact of a complete loss of function of PTG in the regulation of glycogen metabolism using knock-out mice. Astrocytic cultures prepared from PTG KO mice similarly to siRNA PTG treated cultures displayed no compensatory changes in mRNA expression levels of Pygb, Gys1, Gyg or PPP1R6 (Fig. 3A) but exhibited a robust reduction of their glycogen pool size, i.e. a 80.3 ± 5.4% decrease compared to wild-type (WT) control littermate cultures (mean ± SEM; n = 12, 4 independent experiments; Student's t-test: p ≤ 0.0001). Most importantly, we observed that in PTG KO cultures, NA and Ins were unable to promote glycogen synthesis. These observations further confirm the results obtained by PTG downregulation by siRNA and suggest that PTG is an essential element for the glycogenic actions of both neurochemical and humoral factors (Fig. 3B).
Fig. 3.
Glycogen metabolism regulation in astrocytic PTG KO cultures. Cultured astrocytes were isolated from PTG KO mice (PTG-KO) or wild-type (WT) littermates. (A) mRNA expression levels of genes related to astrocytic glycogen metabolism. Data are means ± SEM and expressed as percentage of the WT values for each gene (n = 12, 4 independent experiments). ***P ≤ 0.001 vs WT condition with ANOVA followed by Bonferroni's post hoc test. (B) Glycogen content following NA (100 μM) and Ins (100 nM) treatments for 6 h (n = 11–12, 4 independent experiments). Data are means ± SEM and expressed as percentage of the WT untreated condition (Ctr). ***p ≤ 0.001 vs Ctr and ###p ≤ 0.001 vs respective mock siRNA conditions with ANOVA followed by Bonferroni's post-hoc test for NA conditions comparison (including Ctrl and NA, in mock and PTG siRNA conditions), respectively Ins conditions comparison (including Ctrl and Ins, in mock and PTG siRNA conditions).
4. Discussion
PPP1-targeting subunits are key regulators of glycogen synthesis in mammalian cells. Here, we report that one of its members, PTG, plays a major role in glycogen metabolism regulation in astrocytes under both basal and glycogen synthesis stimulating conditions. By manipulating PTG expression levels in primary astrocyte cultures either by adenovirus-mediated overexpression of PTG or siRNA-mediated downregulation of its expression as well as using PTG KO cultured astrocytes, a direct correlation between PTG proteins levels in astrocyte and glycogen synthesis could be established.
PPP1-glycogen targeting regulatory subunits (PPP1R3s) are scaffolding protein which co-localize the catalytic subunit of PPP1 (PPP1c) with specific glycogen-metabolic enzymes, enabling dephosphorylation of GS and GP hence fine-tuning glycogen synthesis flux. As PTG is highly expressed in astrocytes (Lovatt et al., 2007, Zhang et al., 2014) it represented a possible candidate as a regulator of glycogen synthesis in this cell type. Indeed, our results show that manipulating PTG levels (up or down) in astrocytes had a consistent and direct effect on glycogen content: i) overexpression of PTG led to a 100 fold increase in glycogen ii) the effects of PTG expression on protein levels and glycogen accumulation were counteracted by siRNA treatment to the same extent (75 and 63% decrease respectively) iii) downregulation of endogenous PTG expression by siRNA led to a 2 fold decrease in glycogen iv) ablation of PTG expression (PTG KO astrocytes) led to a 80% decrease in glycogen. No alteration or compensatory changes of expression of other genes involved in astrocytic glycogen metabolism was observed at the transcriptional levels (i.e. Pygb, Gys1, Gyg, PPP1R6), reinforcing the notion that the observed changes in glycogen metabolism were directly produced by changes in PTG expression.
These observations, i.e the close correlation between PTG expression and glycogen levels, are fully consistent with other reports showing that PTG overexpression leads to glycogen accumulation (i.e. associated with increased GS activity and decreased GP activity) in different cell types in cultures, such as rat hepatocytes, human muscle cells, 3T3-L1 adipocytes (Berman et al., 1998, Gasa et al., 2000, Greenberg et al., 2006, Lerin et al., 2000, Montori-Grau et al., 2011), whereas siRNA-mediated downregulation of PTG strongly reduces basal glycogen levels (Greenberg et al., 2006). Similarly, in vivo targeted overexpression of PTG in beta-cells of the pancreas, adipose tissue or liver also results in glycogen accumulation (Jurczak et al., 2007, Mir-Coll et al., 2016, O'Doherty et al., 2000) while heterozygous or homozygous PTG deletion in mice have decreased glycogen tissue levels in muscles, liver, adipose or brain tissues (Crosson et al., 2003, Turnbull et al., 2011, Turnbull et al., 2014). Altogether, these results demonstrate that PTG expression levels are key determinants for glycogen synthesis and accumulation in astrocytes as in other PTG-expressing cell types. Interestingly, in PTG KO mice the decrease in glycogen levels in the brain (70%) is more pronounced than in muscle (30%) further supporting the notion that PTG expression is a prevalent regulator of glycogen synthesis in brain both in vitro and in vivo (Turnbull et al., 2011).
NA and Ins are two well established regulators of glycogen metabolism in astrocytes involving the activation of different metabolic pathways. For instance, NA exerts a biphasic effect on glycogen levels (Allaman et al., 2003, Sorg and Magistretti, 1991, Sorg and Magistretti, 1992) i.e. a rapid glycogen mobilization induced by this neurotransmitter (involving both alpha- and beta-adrenergic receptors) followed by a delayed and massive glycogen resynthesis (taking place hours after stimulation), therefore coupling glycogen mobilization to the subsequent replenishment of glycogen reserves. Importantly, this long term glycogen resynthesis, which critically depends upon the activation of the cAMP signaling cascade (hence activation of beta-adrenergic receptors) and synthesis of new proteins, is accompanied by dephosphorylation and activation of GS which occurs concomitantly with increased PTG mRNA levels (Allaman et al., 2000, Allaman et al., 2004, Sorg and Magistretti, 1992). As for NA, other cAMP cascade activators, such as VIP and adenosine, reproduce the effect of NA on glycogen synthesis and PTG mRNA suggesting a common mechanism of action on astrocytic glycogen metabolism (Allaman et al., 2000, Allaman et al., 2003, Sorg and Magistretti, 1992). Ins also promotes long-term glycogen synthesis in astrocytes but in contrast to NA its effect is unidirectional (i.e. no glycogenolysis is elicited) (Hamai et al., 1999, Heni et al., 2011, Sorg and Magistretti, 1992). Of note, Ins-stimulated glycogen synthesis is time-dependent occurring within 2 h and maintained over time (Sorg and Magistretti, 1992). This long-term effect is, as for NA, protein synthesis dependent which contrasts with the rapid, within minutes, activation of GS induced by Ins in vivo in peripheral tissues (see e.g. Crosson et al., 2003). The glycogenic action of Ins in astrocytes involves the activation of GS through a PI3-kinase-dependent pathway and has not been associated with changes in PTG expression (Allaman et al., 2000, Hamai et al., 1999, Heni et al., 2011).
The different glycogenic activation profiles of NA and Ins on GS activation and PTG mRNA expression suggested a different mechanism for regulation of GS. Glycogen synthesis induced by NA was strongly reduced by PTG siRNA treatment in astrocytes and fully abolished in PTG KO astrocytes, establishing that PTG is a necessary mediator to account for glycogen synthesis induced by NA. However, our results also demonstrated that Ins effects on glycogen synthesis are reduced in astrocytes in which PTG was downregulated and was fully prevented in PTG KO astrocytes. Other glycogen-binding subunits such as PPP1R3A and 3B are not expressed in astrocytes or in brain (Doherty et al., 1995, Lanner et al., 2001, Zhang et al., 2014) suggesting that Ins action in astrocytes, as opposed to peripheral tissues, may rely more on PTG.
PTG KO astrocytes, as well as brains of PTG KO mice (Turnbull et al., 2011, Turnbull et al., 2014), still contain significant glycogen levels, even though strongly reduced, implying the existence of additional and active PPP1R3s-dependent glycogen regulatory mechanisms to PTG. Interestingly, the reduction in glycogen content measured in astrocytes (80%, this study) is similar to the one observed in brains of PTG KO mice (70%) (Turnbull et al., 2011), further indicating a close parallel between glycogen metabolism in cultured astrocytes and brain glycogen metabolism. Here we demonstrate that PPP1R6, another glycogen-binding subunit which is known to be expressed in brain (Armstrong et al., 1997), is also expressed in cultured astrocytes and that its transcriptional expression levels are not altered by PTG overexpression, silencing or in KO mice. Nevertheless, PPP1R6, or other putative targeting subunits expressed in cultured astrocytes, were unable to counteract the drastic effects of PTG silencing on basal and NA- and Ins-stimulated glycogen synthesis in astrocytes. While the exact role played by PPP1R6 on astrocytic glycogen metabolism remains to be fully established, these observations indicate a lack of redundancy between PPP1R6 and PTG functions. PPP1R6 was recently shown to be regulated by 14-3-3 proteins which interact with PPP1R6 through a consensus motif for 14-3-3 protein binding that is not present in other glycogenic subunits including PTG, arguing in favor of such view (Rubio-Villena et al., 2015). Of note, as PTG KO mice were fully viable (Turnbull et al., 2011, Turnbull et al., 2014) this indicates that the control of glycogen metabolism by PPP1R6, and possibly by other recently identified glycogen-binding subunits expressed in brain such as PPP1R3F (Kelsall et al., 2011) and 3G (Zhang et al., 2014), can sustain normal brain functions in the absence of PTG. However, brain functions could be affected not only by glycogen levels but also by glycogen turnover (see e.g. Obel et al., 2012), which could be altered by the absence of PTG.
While most of the characterization of NA and Ins glycogenic effects were obtained from studies performed on in vitro or ex vivo preparations there are strong indications that similar regulation of glycogen metabolism by NA and Ins may also operate in vivo. For instance, astrocytes express both alpha- and beta-noradrenergic receptors and can therefore respond to noradrenergic stimulation, hence modifying their glycogen metabolism (Stone and Ariano, 1989). In particular, a role in beta-adrenergic-induced glycogen mobilization in learning and memory in chick has been well documented (Gibbs, 2016). For Ins, Ins receptors are widely distributed throughout the CNS (Havrankova et al., 1978) and are also found in astrocytes (Heni et al., 2011, Zhu et al., 1990). A general consensus is that little or no Ins is produced in the brain; however, Ins can enter the brain via circumventricular regions that lack a tight blood-brain barrier or via a receptor-mediated active transport system (Banks, 2004, Baskin et al., 1987, Weindl and Sofroniew, 1981).
Altogether, these observations indicate that in the absence of PTG, glycogen synthesis induced by NA and Ins is impaired and that the amplitude of the effects of NA (as well as VIP and adenosine) and Ins on glycogen synthesis are directly correlated to PTG expression levels.
Expression of PTG is known to be regulated by different factors. As previously stated, in astrocytes activators of the cAMP pathways, such as NA, VIP or adenosine, upregulate PTG mRNA expression in astrocytes (Allaman et al., 2000, Allaman et al., 2003), through a transcriptional mechanism most probably involving the activation of C/EBP transcription factors (Allaman et al., 2000, Cardinaux and Magistretti, 1996). Glucose was shown to regulate PTG expression in hepatocytes through a MondoA-dependent mechanism (Petrie et al., 2013). In addition, PTG expression was also shown to be controlled by Per2, BMAL-1, FoxA2, SREBP, Hif1alpha transcriptional regulators (Cheng et al., 2006, Lu et al., 2014, Shen et al., 2010, Zani et al., 2013). Interestingly, Ins induced PTG mRNA expression in cultured hepatocytes (Petrie et al., 2013) but not in cultured astrocytes (Allaman et al., 2000), highlighting the existence of tissues specific regulation of PTG. Such factors may directly participate in the regulation of PTG expression in different conditions and therefore impact cerebral glycogen metabolism.
While glycogen metabolism is primarily associated with astrocytes it is well established that its use does not only benefit astrocytes but also the surrounding neurons. For instance, glycogen mobilization preserves neuronal viability in energy delivery failure conditions, such as in hypoxia or glucose deprivation, in a variety of models (see e.g. Magistretti and Allaman, 2015 and references therein). More recent evidence demonstrated that glycogen is important to sustain higher brain functions such as learning and memory in different experimental paradigms (Boury-Jamot et al., 2016, Duran et al., 2013, Gao et al., 2016, Gibbs, 2016, Newman et al., 2011, Obel et al., 2012, Suzuki et al., 2011). Learning and memory abilities as well as the establishment of long-term potentiation in vivo are altered by pharmacological blockade of glycogen mobilization in rodents or in a brain-specific glycogen synthase knockout (GYS1Nestin-KO) mouse model that lack glycogen (Boury-Jamot et al., 2016, Duran et al., 2013, Newman et al., 2011, Suzuki et al., 2011). Determination of the mechanisms involved in the latter effects demonstrated that they critically dependent on the transfer of glycogen-derived astrocytic lactate to neurons (Boury-Jamot et al., 2016, Newman et al., 2011, Suzuki et al., 2011). This implies that the maintenance of a readily releasable glycogen pool is of importance to sustain astrocytic-dependent neuronal plasticity. The present work highlights PTG as a master regulator of astrocytic glycogen placing PTG, as well as PTG regulation, as a central element in glycogen regulation in vivo. Interestingly, changes in cerebral PTG mRNA expression have been demonstrated in different behavioral conditions known to involve glycogen mobilization, such as sleep deprivation and learning and memory (Petit et al., 2002, Petit et al., 2010, Tadi et al., 2015). A strong reduction of PTG mRNA expression, which was coupled to an altered glycogen metabolism, was also observed in cultured astrocytes isolated from GCLM KO mice, a mouse model of schizophrenia (Lavoie et al., 2011). As a whole these observations argue in favor of a critical role of PTG in the control of cerebral glycogen metabolism, hence astrocytic functions, in both physiological and pathological conditions, such as psychiatric disorders. Undoubtedly, detailed phenotypical and behavioral characterization of PTG KO mice will contribute to the understanding of the exact role by PTG in these processes.
Conflict of interest
The authors have no conflict of interests.
Acknowledgments
The authors would like to thank Elena Gasparotto for expert technical assistance, Dr. Sylvain Lengacher for initial work on PTG adenoviral overexpression and Dr. Gabriele Grenningloh for critical reading of the manuscript. This work was supported by grants from Swiss National Science Foundation (FNRS) (grant numbers 31003A_130821 and 310030B_148169) and from the Panacée and Préfargier Foundations to PJM. The PTG knockout mice were supported by NIH grants DK27221 and NS056454 to PJR.
Contributor Information
E. Ruchti, Email: Evelyne.ruchti@epfl.ch.
P.J. Roach, Email: proach@iu.edu.
A.A. DePaoli-Roach, Email: adepaoli@iu.edu.
P.J. Magistretti, Email: pierre.magistretti@kaust.edu.sa, pierre.magistretti@epfl.ch.
I. Allaman, Email: igor.allaman@epfl.ch.
References
- Allaman I., Gavillet M., Belanger M., Laroche T., Viertl D., Lashuel H.A., Magistretti P.J. Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: impact on neuronal viability. J. Neurosci. 2010;30:3326–3338. doi: 10.1523/JNEUROSCI.5098-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaman I., Lengacher S., Magistretti P.J., Pellerin L. A2B receptor activation promotes glycogen synthesis in astrocytes through modulation of gene expression. Am. J. Physiol. Cell Physiol. 2003;284:C696–C704. doi: 10.1152/ajpcell.00202.2002. [DOI] [PubMed] [Google Scholar]
- Allaman I., Magistretti P.J. Elsevier; 2015. Glial Glycogen Metabolism, Reference Module in Biomedical Sciences. [Google Scholar]
- Allaman I., Pellerin L., Magistretti P.J. Protein targeting to glycogen mRNA expression is stimulated by noradrenaline in mouse cortical astrocytes. Glia. 2000;30:382–391. [PubMed] [Google Scholar]
- Allaman I., Pellerin L., Magistretti P.J. Glucocorticoids modulate neurotransmitter-induced glycogen metabolism in cultured cortical astrocytes. J. Neurochem. 2004;88:900–908. doi: 10.1046/j.1471-4159.2003.02235.x. [DOI] [PubMed] [Google Scholar]
- Armstrong C.G., Browne G.J., Cohen P., Cohen P.T. PPP1R6, a novel member of the family of glycogen-targetting subunits of protein phosphatase 1. FEBS Lett. 1997;418:210–214. doi: 10.1016/s0014-5793(97)01385-9. [DOI] [PubMed] [Google Scholar]
- Banks W.A. The source of cerebral insulin. Eur. J. Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Baskin D.G., Figlewicz D.P., Woods S.C., Porte D., Jr., Dorsa D.M. Insulin in the brain. Annu. Rev. Physiol. 1987;49:335–347. doi: 10.1146/annurev.ph.49.030187.002003. [DOI] [PubMed] [Google Scholar]
- Bélanger M., Yang J., Petit J.M., Laroche T., Magistretti P.J., Allaman I. Role of the glyoxalase system in astrocyte-mediated neuroprotection. J. Neurosci. 2011;31:18338–18352. doi: 10.1523/JNEUROSCI.1249-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman H.K., O'Doherty R.M., Anderson P., Newgard C.B. Overexpression of protein targeting to glycogen (PTG) in rat hepatocytes causes profound activation of glycogen synthesis independent of normal hormone- and substrate-mediated regulatory mechanisms. J. Biol. Chem. 1998;273:26421–26425. doi: 10.1074/jbc.273.41.26421. [DOI] [PubMed] [Google Scholar]
- Boury-Jamot B., Carrard A., Martin J.L., Halfon O., Magistretti P.J., Boutrel B. Disrupting astrocyte-neuron lactate transfer persistently reduces conditioned responses to cocaine. Mol. Psychiatry. 2016;21:1070–1076. doi: 10.1038/mp.2015.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.M. Brain glycogen re-awakened. J. Neurochem. 2004;89:537–552. doi: 10.1111/j.1471-4159.2004.02421.x. [DOI] [PubMed] [Google Scholar]
- Cardinaux J.R., Magistretti P.J. Vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, and noradrenaline induce the transcription factors CCAAT/enhancer binding protein (C/EBP)-beta and C/EBP delta in mouse cortical astrocytes: involvement in cAMP-regulated glycogen metabolism. J. Neurosci. 1996;16:919–929. doi: 10.1523/JNEUROSCI.16-03-00919.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans H., Stalmans W., Bollen M. Regulator-driven functional diversification of protein phosphatase-1 in eukaryotic evolution. Bioessays. 2002;24:371–381. doi: 10.1002/bies.10069. [DOI] [PubMed] [Google Scholar]
- Cheng A., Zhang M., Crosson S.M., Bao Z.Q., Saltiel A.R. Regulation of the mouse protein targeting to glycogen (PTG) promoter by the FoxA2 forkhead protein and by 3',5'-cyclic adenosine 5'-monophosphate in H4IIE hepatoma cells. Endocrinology. 2006;147:3606–3612. doi: 10.1210/en.2005-1513. [DOI] [PubMed] [Google Scholar]
- Crosson S.M., Khan A., Printen J., Pessin J.E., Saltiel A.R. PTG gene deletion causes impaired glycogen synthesis and developmental insulin resistance. J. Clin. Invest. 2003;111:1423–1432. doi: 10.1172/JCI17975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M.J., Moorhead G., Morrice N., Cohen P., Cohen P.T. Amino acid sequence and expression of the hepatic glycogen-binding (GL)-subunit of protein phosphatase-1. FEBS Lett. 1995;375:294–298. doi: 10.1016/0014-5793(95)01184-g. [DOI] [PubMed] [Google Scholar]
- Dringen R., Hamprecht B. Glucose, insulin, and insulin-like growth factor I regulate the glycogen content of astroglia-rich primary cultures. J. Neurochem. 1992;58:511–517. doi: 10.1111/j.1471-4159.1992.tb09750.x. [DOI] [PubMed] [Google Scholar]
- Duran J., Saez I., Gruart A., Guinovart J.J., Delgado-Garcia J.M. Impairment in long-term memory formation and learning-dependent synaptic plasticity in mice lacking glycogen synthase in the brain. J. Cereb. Blood Flow. Metab. 2013;33:550–556. doi: 10.1038/jcbfm.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao V., Suzuki A., Magistretti P.J., Lengacher S., Pollonini G., Steinman M.Q., Alberini C.M. Astrocytic beta2-adrenergic receptors mediate hippocampal long-term memory consolidation. Proc. Natl. Acad. Sci. U. S. A. 2016;113:8526–8531. doi: 10.1073/pnas.1605063113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasa R., Jensen P.B., Berman H.K., Brady M.J., DePaoli-Roach A.A., Newgard C.B. Distinctive regulatory and metabolic properties of glycogen targeting subunits of protein phosphatase-1 (PTG, GL, GM/RGl) expressed in hepatocytes. J. Biol. Chem. 2000;275:26396–26403. doi: 10.1074/jbc.M002427200. [DOI] [PubMed] [Google Scholar]
- Gibbs M.E. Role of glycogenolysis in memory and learning: regulation by noradrenaline, serotonin and ATP. Front. Integr. Neurosci. 2016;9:70. doi: 10.3389/fnint.2015.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg C.C., Danos A.M., Brady M.J. Central role for protein targeting to glycogen in the maintenance of cellular glycogen stores in 3T3-L1 Adipocytes. Mol. Cell Biol. 2006;26:334–342. doi: 10.1128/MCB.26.1.334-342.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamai M., Minokoshi Y., Shimazu T. L-Glutamate and insulin enhance glycogen synthesis in cultured astrocytes from the rat brain through different intracellular mechanisms. J. Neurochem. 1999;73:400–407. doi: 10.1046/j.1471-4159.1999.0730400.x. [DOI] [PubMed] [Google Scholar]
- Havrankova J., Roth J., Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- Heni M., Hennige A.M., Peter A., Siegel-Axel D., Ordelheide A.M., Krebs N., Machicao F., Fritsche A., Haring H.U., Staiger H. Insulin promotes glycogen storage and cell proliferation in primary human astrocytes. PLoS ONE. 2011;6:e21594. doi: 10.1371/journal.pone.0021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurczak M.J., Danos A.M., Rehrmann V.R., Allison M.B., Greenberg C.C., Brady M.J. Transgenic overexpression of protein targeting to glycogen markedly increases adipocytic glycogen storage in mice. Am. J. Physiol. Endocrinol. Metab. 2007;292:E952–E963. doi: 10.1152/ajpendo.00559.2006. [DOI] [PubMed] [Google Scholar]
- Kelsall I.R., Voss M., Munro S., Cuthbertson D.J., Cohen P.T. R3F, a novel membrane-associated glycogen targeting subunit of protein phosphatase 1 regulates glycogen synthase in astrocytoma cells in response to glucose and extracellular signals. J. Neurochem. 2011;118:596–610. doi: 10.1111/j.1471-4159.2011.07345.x. [DOI] [PubMed] [Google Scholar]
- Korrodi-Gregorio L., Esteves S.L., Fardilha M. Protein phosphatase 1 catalytic isoforms: specificity toward interacting proteins. Transl. Res. 2014;164:366–391. doi: 10.1016/j.trsl.2014.07.001. [DOI] [PubMed] [Google Scholar]
- Kum W., Zhu S.Q., Ho S.K., Young J.D., Cockram C.S. Effect of insulin on glucose and glycogen metabolism and leucine incorporation into protein in cultured mouse astrocytes. Glia. 1992;6:264–268. doi: 10.1002/glia.440060404. [DOI] [PubMed] [Google Scholar]
- Lanner C., Suzuki Y., Bi C., Zhang H., Cooper L.D., Bowker-Kinley M.M., DePaoli-Roach A.A. Gene structure and expression of the targeting subunit, RGL, of the muscle-specific glycogen-associated type 1 protein phosphatase, PP1G. Arch. Biochem. Biophys. 2001;388:135–145. doi: 10.1006/abbi.2001.2283. [DOI] [PubMed] [Google Scholar]
- Lavoie S., Allaman I., Petit J.M., Do K.Q., Magistretti P.J. Altered glycogen metabolism in cultured astrocytes from mice with chronic glutathione deficit; relevance for neuroenergetics in schizophrenia. PLoS ONE. 2011;6:e22875. doi: 10.1371/journal.pone.0022875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerin C., Montell E., Berman H.K., Newgard C.B., Gomez-Foix A.M. Overexpression of protein targeting to glycogen in cultured human muscle cells stimulates glycogen synthesis independent of glycogen and glucose 6-phosphate levels. J. Biol. Chem. 2000;275:39991–39995. doi: 10.1074/jbc.M006251200. [DOI] [PubMed] [Google Scholar]
- Lovatt D., Sonnewald U., Waagepetersen H.S., Schousboe A., He W., Lin J.H., Han X., Takano T., Wang S., Sim F.J., Goldman S.A., Nedergaard M. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J. Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Bridges D., Yang Y., Fisher K., Cheng A., Chang L., Meng Z.X., Lin J.D., Downes M., Yu R.T., Liddle C., Evans R.M., Saltiel A.R. Metabolic crosstalk: molecular links between glycogen and lipid metabolism in obesity. Diabetes. 2014;63:2935–2948. doi: 10.2337/db13-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti P.J., Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- Magistretti P.J., Sorg O., Martin J.L. Regulation of glycogen metabolism in astrocytes: physiological, pharmacological, and pathological aspects. In: Murphy S., editor. Astrocytes: Pharmacology and Function. Academic Press; San Diego, CA: 1993. pp. 243–265. [Google Scholar]
- Magistretti P.J., Sorg O., Yu N., Martin J.L., Pellerin L. Neurotransmitters regulate energy metabolism in astrocytes: implications for the metabolic trafficking between neural cells. Dev. Neurosci. 1993;15:306–312. doi: 10.1159/000111349. [DOI] [PubMed] [Google Scholar]
- Mir-Coll J., Duran J., Slebe F., Garcia-Rocha M., Gomis R., Gasa R., Guinovart J.J. Genetic models rule out a major role of beta cell glycogen in the control of glucose homeostasis. Diabetologia. 2016;59:1012–1020. doi: 10.1007/s00125-016-3871-1. [DOI] [PubMed] [Google Scholar]
- Montori-Grau M., Guitart M., Garcia-Martinez C., Orozco A., Gomez-Foix A.M. Differential pattern of glycogen accumulation after protein phosphatase 1 glycogen-targeting subunit PPP1R6 overexpression, compared to PPP1R3C and PPP1R3A, in skeletal muscle cells. BMC Biochem. 2011;12:57. doi: 10.1186/1471-2091-12-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman L.A., Korol D.L., Gold P.E. Lactate produced by glycogenolysis in astrocytes regulates memory processing. PLoS ONE. 2011;6:e28427. doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty R.M., Jensen P.B., Anderson P., Jones J.G., Berman H.K., Kearney D., Newgard C.B. Activation of direct and indirect pathways of glycogen synthesis by hepatic overexpression of protein targeting to glycogen. J. Clin. Invest. 2000;105:479–488. doi: 10.1172/JCI8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obel L.F., Muller M.S., Walls A.B., Sickmann H.M., Bak L.K., Waagepetersen H.S., Schousboe A. Brain glycogen-new perspectives on its metabolic function and regulation at the subcellular level. Front. Neuroenerg. 2012;4:3. doi: 10.3389/fnene.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J.M., Gyger J., Burlet-Godinot S., Fiumelli H., Martin J.L., Magistretti P.J. Genes involved in the astrocyte-neuron lactate shuttle (ANLS) are specifically regulated in cortical astrocytes following sleep deprivation in mice. Sleep. 2013;36:1445–1458. doi: 10.5665/sleep.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J.M., Tobler I., Allaman I., Borbely A.A., Magistretti P.J. Sleep deprivation modulates brain mRNAs encoding genes of glycogen metabolism. Eur. J. Neurosci. 2002;16:1163–1167. doi: 10.1046/j.1460-9568.2002.02145.x. [DOI] [PubMed] [Google Scholar]
- Petit J.M., Tobler I., Kopp C., Morgenthaler F., Borbely A.A., Magistretti P.J. Metabolic response of the cerebral cortex following gentle sleep deprivation and modafinil administration. Sleep. 2010;33:901–908. doi: 10.1093/sleep/33.7.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie J.L., Al-Oanzi Z.H., Arden C., Tudhope S.J., Mann J., Kieswich J., Yaqoob M.M., Towle H.C., Agius L. Glucose induces protein targeting to glycogen in hepatocytes by fructose 2,6-bisphosphate-mediated recruitment of MondoA to the promoter. Mol. Cell Biol. 2013;33:725–738. doi: 10.1128/MCB.01576-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printen J.A., Brady M.J., Saltiel A.R. PTG, a protein phosphatase 1-binding protein with a role in glycogen metabolism. Science. 1997;275:1475–1478. doi: 10.1126/science.275.5305.1475. [DOI] [PubMed] [Google Scholar]
- Roach P.J., DePaoli-Roach A.A., Hurley T.D., Tagliabracci V.S. Glycogen and its metabolism: some new developments and old themes. Biochem. J. 2012;441:763–787. doi: 10.1042/BJ20111416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Villena C., Sanz P., Garcia-Gimeno M.A. Structure-function analysis of PPP1R3D, a protein phosphatase 1 targeting subunit, reveals a binding motif for 14-3-3 proteins which regulates its glycogenic properties. PLoS ONE. 2015;10:e0131476. doi: 10.1371/journal.pone.0131476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G.M., Zhang F.L., Liu X.L., Zhang J.W. Hypoxia-inducible factor 1-mediated regulation of PPP1R3C promotes glycogen accumulation in human MCF-7 cells under hypoxia. FEBS Lett. 2010;584:4366–4372. doi: 10.1016/j.febslet.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Sorg O., Magistretti P.J. Characterization of the glycogenolysis elicited by vasoactive intestinal peptide, noradrenaline and adenosine in primary cultures of mouse cerebral cortical astrocytes. Brain Res. 1991;563:227–233. doi: 10.1016/0006-8993(91)91538-c. [DOI] [PubMed] [Google Scholar]
- Sorg O., Magistretti P.J. Vasoactive intestinal peptide and noradrenaline exert long-term control on glycogen levels in astrocytes: blockade by protein synthesis inhibition. J. Neurosci. 1992;12:4923–4931. doi: 10.1523/JNEUROSCI.12-12-04923.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman M.Q., Gao V., Alberini C.M. The role of lactate-mediated metabolic coupling between astrocytes and neurons in long-term memory formation. Front. Integr. Neurosci. 2016;10:10. doi: 10.3389/fnint.2016.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone E.A., Ariano M.A. Are glial cells targets of the central noradrenergic system? A review of the evidence. Brain Res. Brain Res. Rev. 1989;14:297–309. doi: 10.1016/0165-0173(89)90015-5. [DOI] [PubMed] [Google Scholar]
- Suzuki A., Stern S.A., Bozdagi O., Huntley G.W., Walker R.H., Magistretti P.J., Alberini C.M. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell. 2011;144:810–823. doi: 10.1016/j.cell.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Lanner C., Kim J.H., Vilardo P.G., Zhang H., Yang J., Cooper L.D., Steele M., Kennedy A., Bock C.B., Scrimgeour A., Lawrence J.C., Jr., DePaoli-Roach A.A. Insulin control of glycogen metabolism in knockout mice lacking the muscle-specific protein phosphatase PP1G/RGL. Mol. Cell Biol. 2001;21:2683–2694. doi: 10.1128/MCB.21.8.2683-2694.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadi M., Allaman I., Lengacher S., Grenningloh G., Magistretti P.J. Learning-induced gene expression in the hippocampus reveals a role of neuron-astrocyte metabolic coupling in long term memory. PLoS ONE. 2015;10:e0141568. doi: 10.1371/journal.pone.0141568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull J., DePaoli-Roach A.A., Zhao X., Cortez M.A., Pencea N., Tiberia E., Piliguian M., Roach P.J., Wang P., Ackerley C.A., Minassian B.A. PTG depletion removes lafora bodies and rescues the fatal epilepsy of Lafora Disease. PLoS Genet. 2011;7:e1002037. doi: 10.1371/journal.pgen.1002037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull J., Epp J.R., Goldsmith D., Zhao X., Pencea N., Wang P., Frankland P.W., Ackerley C.A., Minassian B.A. PTG protein depletion rescues malin-deficient Lafora disease in mouse. Ann. Neurol. 2014;75:442–446. doi: 10.1002/ana.24104. [DOI] [PubMed] [Google Scholar]
- Vilchez D., Ros S., Cifuentes D., Pujadas L., Valles J., Garcia-Fojeda B., Criado-Garcia O., Fernandez-Sanchez E., Medrano-Fernandez I., Dominguez J., Garcia-Rocha M., Soriano E., Rodriguez de Cordoba S., Guinovart J.J. Mechanism suppressing glycogen synthesis in neurons and its demise in progressive myoclonus epilepsy. Nat. Neurosci. 2007;10:1407–1413. doi: 10.1038/nn1998. [DOI] [PubMed] [Google Scholar]
- Weindl A., Sofroniew M.V. Relation of neuropeptides to mammalian circumventricular organs. Adv. Biochem. Psychopharmacol. 1981;28:303–320. [PubMed] [Google Scholar]
- Yang J., Ruchti E., Petit J.M., Jourdain P., Grenningloh G., Allaman I., Magistretti P.J. Lactate promotes plasticity gene expression by potentiating NMDA signaling in neurons. Proc. Natl. Acad. Sci. U.S.A. 2014;111:12228–12233. doi: 10.1073/pnas.1322912111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zani F., Breasson L., Becattini B., Vukolic A., Montani J.P., Albrecht U., Provenzani A., Ripperger J.A., Solinas G. PER2 promotes glucose storage to liver glycogen during feeding and acute fasting by inducing Gys2 PTG and GL expression. Mol. Metab. 2013;2:292–305. doi: 10.1016/j.molmet.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Chen K., Sloan S.A., Bennett M.L., Scholze A.R., O'Keeffe S., Phatnani H.P., Guarnieri P., Caneda C., Ruderisch N., Deng S., Liddelow S.A., Zhang C., Daneman R., Maniatis T., Barres B.A., Wu J.Q. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J. Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S.Q., Kum W., Ho S.K., Young J.D., Cockram C.S. Structure-function relationships of insulin receptor interactions in cultured mouse astrocytes. Brain Res. 1990;529:329–332. doi: 10.1016/0006-8993(90)90846-4. [DOI] [PubMed] [Google Scholar]