Highlights
-
•
Remote tissue conditioning is an emerging neuroprotective strategy.
-
•
Remote ischemic conditioning and remote photobiomodulation were tested in MPTP mice.
-
•
Both interventions protected the midbrain against MPTP insult.
-
•
Combining the interventions yielded no added benefit.
Abbreviations: CPu, caudate-putamen complex; LED, light emitting diode; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; PBM, photobiomodulation; PD, Parkinson’s disease; RIC, remote ischemic conditioning; SNc, substantia nigra pars compacta; TH, tyrosine hydroxylase
Keywords: Photobiomodulation, Remote ischemic conditioning, Neuroprotection, Mouse model, MPTP, Parkinson’s disease
Abstract
Current treatments for Parkinson’s disease (PD) are primarily symptomatic, leaving a need for treatments that mitigate disease progression. One emerging neuroprotective strategy is remote tissue conditioning, in which mild stress in a peripheral tissue (e.g. a limb) induces protection of life-critical organs such as the brain. We evaluated the potential of two remote tissue conditioning interventions – mild ischemia and photobiomodulation – in protecting the brain against the parkinsonian neurotoxin MPTP. Further, we sought to determine whether combining these two interventions provided any added benefit.
Male C57BL/6 mice (n = 10/group) were pre-conditioned with either ischemia of the leg (4 × 5 min cycles of ischemia/reperfusion), or irradiation of the dorsum with 670 nm light (50 mW/cm2, 3 min), or both interventions, immediately prior to receiving two MPTP injections 24 hours apart (50 mg/kg total). Mice were sacrificed 6 days later and brains processed for tyrosine hydroxylase immunohistochemistry.
Stereological counts of functional dopaminergic neurons in the substantia nigra pars compacta revealed that both remote ischemia and remote photobiomodulation rescued around half of the neurons that were compromised by MPTP (p < 0.001). Combining the two interventions provided no added benefit, rescuing only 40% of vulnerable neurons (p < 0.01).
The present results suggest that remote tissue conditioning, whether ischemia of a limb or photobiomodulation of the torso, induces protection of brain centers critical in PD. The lack of additional benefit when combining these two interventions suggests they may share common mechanistic pathways. Further research is needed to identify these pathways and determine the conditioning doses that yield optimal neuroprotection.
Introduction
Current treatments for Parkinson’s disease (PD) are primarily symptomatic, leaving a need for interventions that can slow or stop the tragic course of the disease. Ideally, such interventions would not only be efficacious but also safe, well-tolerated, non-invasive, easy to administer and relatively affordable.
One emerging neuroprotective strategy that meets these criteria is remote tissue conditioning (Kim et al., 2017). This refers to the induction of mild stress in a non-life-critical tissue (e.g. a limb) in order to provide protection of ‘remote’ life-critical organs (e.g. the brain), based on the physiological concept that mild, transient forms of stressful stimuli can induce systemic mechanisms that promote cellular resilience and host survival (Mattson, 2014).
One well-characterised remote conditioning strategy is remote ischemic conditioning (RIC), in which one or more cycles of non-lethal ischemia and reperfusion in a peripheral tissue protects critical organs, including the brain, from more severe insults (Hess et al., 2015). While most animal and clinical studies of RIC have focussed on protection against subsequent severe ischemia-reperfusion injury (e.g. ischemic heart disease, acute pre-renal kidney injury, ischemic stroke), there is evidence from animal studies that the beneficial effects of RIC are not confined to ischemic injuries (Brandli et al., 2016; Liu et al., 2013).
A second emerging remote conditioning strategy is photobiomodulation (PBM), the irradiation of tissue with low-intensity red to near-infrared light (600–1100 nm). While many studies have demonstrated that direct PBM of the brain mitigates neuropathology in animal models of various neurodegenerative diseases, including rodent and macaque models of PD (Darlot et al., 2016; Johnstone et al., 2016), recent discoveries suggest that PBM applied to peripheral tissues can also provide neuroprotection via an indirect mechanism. This treatment modality, termed here remote PBM, provides significant rescue of dopaminergic neurons in an MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of PD (Johnstone et al., 2014), consistent with observations of neuroprotection in other disease models (Farfara et al., 2015; Saliba et al., 2015).
In the present study, we evaluated the neuroprotective potential of RIC and remote PBM in an MPTP mouse model of PD. Furthermore, we assessed whether combining RIC and remote PBM yields an additive, synergistic, neutral or even antagonistic effect when compared with each single modality conditioning approach. The outcome of the combination treatment, we postulate, could offer clues as to whether the protection is mediated by similar or distinct pathways − if activating similar pathways there would presumably be no additive effect whereas if distinct mechanisms are being activated we could expect additive or synergistic neuroprotection.
Experimental procedures
Animals
All protocols were approved by the University of Sydney Animal Ethics Committee. Male C57BL/6 mice were obtained from the Animal Resource Centre (Murdoch, WA, Australia) and housed on a 12 hour light/dark cycle at 22 °C; food pellets and water were available ad libitum. Mice were 10 weeks of age at the beginning of experimentation.
Experimental design
Mice were randomized into 5 experimental groups: 1) Sham-Saline 2) Sham-MPTP 3) RIC-MPTP 4) Remote PBM-MPTP 5) Combination-MPTP (n = 10 per group; total n = 50). Mice in Groups 2-5 received two intraperitoneal injections of 25 mg/kg MPTP (total of 50 mg/kg per mouse), 24 hours apart, while mice in Group 1 received equivalent volume injections of vehicle (isotonic saline). This MPTP dosing regime has been used extensively by our team (Johnstone et al., 2014), and generally leads to ∼40% reduction in the number of functional dopaminergic cells in the substantia nigra pars compacta (SNc).
For Groups 3 and 5, immediately prior to each MPTP injection, mice were subjected to four cycles of 5 min ischemia followed by 5 min reperfusion, alternating hind limbs. Ischemia was achieved by applying a tourniquet around the inguinal fold, during which venous congestion was observed (purple discoloration) in the foot, followed by a rapid return of foot colour during reperfusion. Induction of ischemia was further confirmed by measuring a consistent decrease in distal limb temperature during constriction followed by recovery during reperfusion (Supplementary Fig. 1). Groups 1, 2 and 4 received sham treatment, which involved identical handling but without constriction.
For Groups 4 and 5, immediately prior to each MPTP injection (and after sham or RIC treatment), mice were subjected to 3 min remote PBM (670 nm, 50 mW/cm2) using a WARP 10 LED panel (Quantum Devices, Barneveld, WI, USA). Mice were restrained and the exposed dorsum was irradiated, while the head was shielded by foil to prevent transcranial irradiation. Sham treatment (Groups 1–3) involved holding mice under the LED panel but leaving the device switched off. Prior to remote PBM or sham treatment, the dorsum and hind limbs of all mice was shaved to minimize absorption of light by fur.
Tissue collection and immunohistochemistry
Six days after the final injection and treatment, mice were anesthetised by intraperitoneal injection of pentobarbitone (60 mg/kg) and perfused transcardially with 4% buffered formaldehyde. Brains were harvested, post-fixed for 24 h and cryoprotected in 30% sucrose in PBS. Serial coronal sections (60 μm thickness) were collected between approximately −3.80 mm and −2.52 mm Bregma for SNc and −1.94 mm and 0.02 mm Bregma for caudate-putamen complex (CPu). After accounting for the death of one animal during the experimental course and the loss of some tissue due to microtome malfunctions, immunohistochemistry was performed on tissue from 8–10 mice per experimental group.
Sections were briefly immersed in 1% Triton-PBS, followed by incubation in 10% normal goat serum for 1 h and washing and permeabilisation in 1% Triton-PBS for 1 h. Sections were then incubated in rabbit anti-tyrosine hydroxylase (TH) primary antibody (1:1000; T8700, Sigma-Aldrich) at 4 °C for 48 h, followed by biotinylated goat anti-rabbit secondary antibody at room temperature for 4 h and then streptavidin-peroxidase for 2 h (HistoMark Biotin Streptavidin-HRP Systems package, KPL). Bound antibody was visualized using 3,3′-diaminobenzidine tetrahydrochloride solution (Sigma-Aldrich). Sections were mounted onto gelatinised slides and air-dried overnight before dehydration in ascending alcohols, clearing in histolene and coverslipping using DPx mountant (Sigma-Aldrich).
Cell quantification and image analysis
For all analyses, the investigator was blinded to sample identity. The number of TH+ cells in the SNc was estimated by stereology using the optical fractionator method (StereoInvestigator, MBF Science), as described previously (Johnstone et al., 2014). To determine the density of TH+ expression in the CPu, bright-field images were obtained at 16× magnification and ImageJ used to process each image. The “Threshold Colour” was set to ‘black and white’ and colour space set to ‘HSB’ to obtain a standardized mean value of luminosity, reflecting the density of TH+ CPu terminations. The density of terminations was calculated using the ‘analyse’ function in ImageJ. Statistical comparisons of experimental groups utilised one-way ANOVA followed by Tukey’s multiple comparisons test in GraphPad Prism 6.
Results
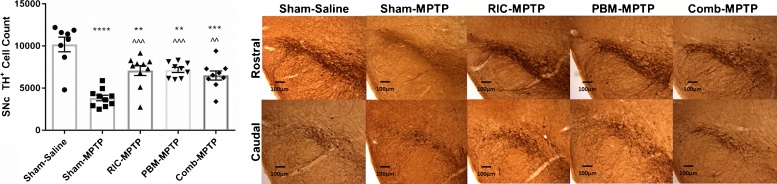
To determine whether RIC or remote PBM protect against MPTP insult, we quantified the number of cells in the substantia nigra pars compacta (SNc) positive for tyrosine hydroxylase, a marker of functional dopaminergic neurons. Representative photomicrographs of TH-labeled SNc sections and TH+ cell counts across the experimental groups are presented in Fig. 1. Overall, the variations in TH+ cell number between experimental groups were significant (ANOVA: F = 16.88, p < 0.0001). Relative to mice receiving saline injections, sham-treated mice receiving MPTP showed a 60% reduction in TH+ cell number (p < 0.0001). MPTP mice treated with either RIC or remote PBM had significantly higher TH+ cell counts (∼85% higher) than sham-treated MPTP mice (p < 0.001), but significantly lower TH+ cell counts than saline controls (p < 0.01). A combination of RIC and remote PBM provided significant neuroprotection relative to sham-treated MPTP animals (p < 0.01), but there were no additive or synergistic effects of the two treatments.
Fig. 1.
(A) Stereological counts of TH+ cells in the SNc. Data are presented as mean ± SEM. **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. Sham-Saline; ^^p < 0.01, ^^^p < 0.001 vs. Sham-MPTP. (B) Representative photomicrographs of TH labelling of the SNc. Top row: rostral SNc; bottom row: caudal SNc. Scale bar = 100 μm.
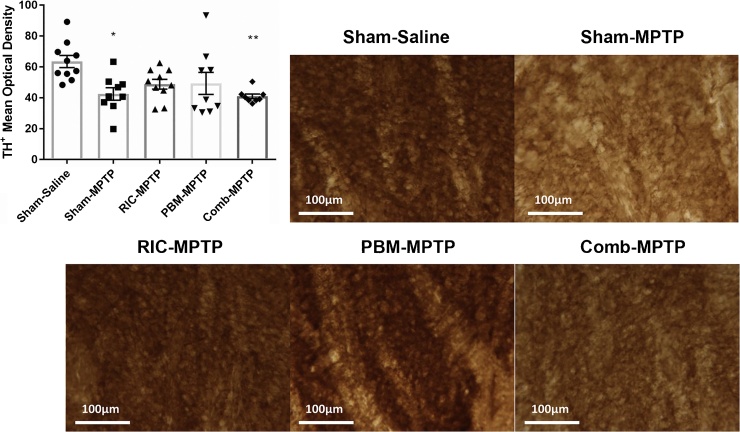
As dopaminergic neurons in the SNc project to the caudate-putamen complex (CPu) as part of the nigrostriatal pathway, we also assessed the density of TH+ terminations in the CPu. Representative photomicrographs of TH-labeled CPu sections and TH+ densities across the experimental groups are presented in Fig. 2. The variations in the group means were statistically significant (ANOVA: F = 4.216, p = 0.006), with post-hoc analysis revealing a significant difference between sham-treated MPTP and saline-injected groups (p < 0.05). While mice treated with either RIC or remote PBM had densities somewhere between, and not significantly different from, the saline and MPTP control groups, mice receiving the combination treatment had significantly lower TH+ densities than saline controls (p < 0.01).
Fig. 2.
(A) Density of TH+ terminations in the dorsal CPu. Data are presented as mean ± SEM. *p < 0.05, **p < 0.01 vs. Sham-Saline. (B) Representative photomicrographs of TH labelling of the CPu. Scale bar = 100 μm.
Discussion
The present study builds on previous evidence for the neuroprotective actions of two remote tissue conditioning strategies – remote ischemic conditioning (RIC) and remote photobiomodulation (PBM). We have shown that both RIC and remote PBM provide some level of neuroprotection against MPTP insult, with each mitigating the loss of functional dopaminergic cells in the SNc by more than 50%. Combining these two remote tissue conditioning strategies yielded no additive or synergistic therapeutic effects.
The present study adds to the findings of our previous report, which demonstrated that remote PBM is neuroprotective against MPTP insult in Balb/c mice, a strain more resistant to MPTP (Johnstone et al., 2014). Extending our investigation to the more susceptible C57BL/6 mouse strain, we were able to generate a more severe MPTP lesion (∼60% loss of functional dopaminergic neurons), consistent with what has been reported previously for this strain (Jackson-Lewis and Przedborski, 2007), and confirm that remote PBM offers protection to dopaminergic neurons of the SNc.
One highly novel aspect of this study relates to the observation of RIC-induced neuroprotection against MPTP insult. To our knowledge, this is the first demonstration that RIC protects the brain against non-ischemic injury. Previous studies of another CNS tissue, the retina, support the assertion that the neuroprotective effects of RIC are not limited to mitigating ischemia-related injury, contributing evidence that RIC provides therapeutic benefits following optic nerve transection and light-induced photoreceptor degeneration (Brandli et al., 2016; Liu et al., 2013). Further support comes from a number of studies demonstrating that exercise, which parallels RIC with respect to the presence of transient ischemic in peripheral tissues, mitigates the neurological effects of MPTP insult (Koo et al., 2017; Smeyne et al., 2015; Toy et al., 2014). Together, these findings expand the possibilities for future clinical application of RIC in the context of chronic neurodegenerative diseases.
The present study confirmed the feasibility of combining RIC and remote PBM interventions but detected no augmentation of neuroprotection. This observation might provide some insights into the mechanisms by which RIC and remote PBM deliver neuroprotection. The lack of any additive effect suggests that these interventions modulate common, rather than distinct, pathways. Considerable work has already been done in trying to understand the mechanisms of RIC (Kim et al., 2017); it will be valuable in future studies to probe the same molecular mediators in the context of remote PBM to confirm the extent to which these pathways overlap. A second implication of this study relates to dosage. Both RIC and PBM exhibit a biphasic dose-response relationship, meaning dose must be carefully controlled to induce an optimal protective effect (Kim et al., 2017). As both interventions appear to condition tissues by stimulating endogenous stress response systems, it is possible that combining these treatments pushes the total stress dose to a sub-optimal level, beyond the “point of diminishing returns”. It will be interesting to test this theory by assessing whether any summation is observed when applying lower doses of RIC and remote PBM.
One limitation of the present study was the timing of the intervention, with remote tissue conditioning applied immediately prior to MPTP insult. Many studies in the RIC field apply the intervention 24 hours before insult; it will be informative in future studies to confirm that earlier pre-conditioning also provides neuroprotection against MPTP insult. Perhaps more importantly, it remains to be seen whether commencing intervention after the onset of parkinsonian pathology (i.e. post-conditioning) will provide therapeutic benefit; however, there is evidence to support this possibility. For example, RIC has been studied as a post-conditioning therapy in patients presenting after the onset of myocardial infarction, with evidence for clinical benefits and reduced level of infarcts as detected by cardiac enzyme tests (Crimi et al., 2013). Although studies on neuroprotective RIC post-conditioning are currently scarce, there is increasing evidence of its efficacy in pre-clinical models (Gao et al., 2017; Hu et al., 2018). Meanwhile, transcranial PBM shows a broad therapeutic time window in the MPTP mouse model, with neuroprotection and behavioral improvements achieved irrespective of whether PBM is administered before, concurrent with or after MPTP insult (Reinhart et al., 2016). In any case, it seems self-evident that neuroprotective interventions with the potential to slow or halt PD progression will be most effective when initiated as early in the disease course as possible, making it critical to develop reliable biomarkers of early disease stages.
In conclusion, our study demonstrates that RIC and remote PBM both induce protection of dopaminergic neurons in the SNc, although combining these two interventions provided no additive or synergistic effects. With efficacy now demonstrated, studies are required to better understand the mechanism of remote tissue conditioning and identify the mediators (e.g. circulating cellular and humoral effectors) of this phenomenon. Nonetheless, the current findings highlight the potential for RIC or remote PBM as a disease-modifying intervention in the setting of chronic neurodegeneration, and in particular in parkinsonian disorders. Future research should build on these initial findings to establish whether remote tissue conditioning provides benefit to the brain when commenced after the onset of neurodegeneration, a scenario that is more representative of the clinical reality.
Acknowledgements
This work was supported by seed funding from Parkinson’s NSW and the Sir Zelman Cowen Universities Fund. DMJ was supported by an Early Career Fellowship from the National Health & Medical Research Council (NHMRC) of Australia (APP1052392). We thank Sharon Spana for expert technical assistance.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ibror.2018.01.001.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Brandli A., Johnstone D.M., Stone J. Remote ischemic preconditioning protects retinal photoreceptors: evidence from a rat model of light-induced photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 2016;57:5302–5313. doi: 10.1167/iovs.16-19361. [DOI] [PubMed] [Google Scholar]
- Crimi G., Pica S., Raineri C., Bramucci E., De Ferrari G.M., Klersy C., Ferlini M., Marinoni B., Repetto A., Romeo M., Rosti V., Massa M., Raisaro A., Leonardi S., Rubartelli P., Oltrona Visconti L., Ferrario M. Remote ischemic post-conditioning of the lower limb during primary percutaneous coronary intervention safely reduces enzymatic infarct size in anterior myocardial infarction: a randomized controlled trial. JACC Cardiovasc. Interv. 2013;6:1055–1063. doi: 10.1016/j.jcin.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Darlot F., Moro C., El Massri N., Chabrol C., Johnstone D.M., Reinhart F., Agay D., Torres N., Bekha D., Auboiroux V., Costecalde T., Peoples C.L., Anastascio H.D., Shaw V.E., Stone J., Mitrofanis J., Benabid A.L. Near-infrared light is neuroprotective in a monkey model of Parkinson disease. Ann. Neurol. 2016;79:59–75. doi: 10.1002/ana.24542. [DOI] [PubMed] [Google Scholar]
- Farfara D., Tuby H., Trudler D., Doron-Mandel E., Maltz L., Vassar R.J., Frenkel D., Oron U. Low-level laser therapy ameliorates disease progression in a mouse model of Alzheimer's disease. J. Mol. Neurosci. 2015;55:430–436. doi: 10.1007/s12031-014-0354-z. [DOI] [PubMed] [Google Scholar]
- Gao X., Liu Y., Xie Y., Wang Y., Qi S. Remote ischemic postconditioning confers neuroprotective effects via inhibition of the BID-mediated mitochondrial apoptotic pathway. Mol. Med. Rep. 2017;16:515–522. doi: 10.3892/mmr.2017.6652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess D.C., Blauenfeldt R.A., Andersen G., Hougaard K.D., Hoda M.N., Ding Y., Ji X. Remote ischaemic conditioning-a new paradigm of self-protection in the brain. Nat. Rev. Neurol. 2015;11:698–710. doi: 10.1038/nrneurol.2015.223. [DOI] [PubMed] [Google Scholar]
- Hu X., Lv T., Yang S.F., Zhang X.H., Miao Y.F. Limb remote ischemic postconditioning reduces injury and improves longterm behavioral recovery in rats following subarachnoid hemorrhage: possible involvement of the autophagic process. Mol. Med. Rep. 2018;17:21–30. doi: 10.3892/mmr.2017.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson-Lewis V., Przedborski S. Protocol for the MPTP mouse model of Parkinson's disease. Nat. Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Johnstone D.M., el Massri N., Moro C., Spana S., Wang X.S., Torres N., Chabrol C., De Jaeger X., Reinhart F., Purushothuman S., Benabid A.L., Stone J., Mitrofanis J. Indirect application of near infrared light induces neuroprotection in a mouse model of parkinsonism – an abscopal neuroprotective effect. Neuroscience. 2014;274:93–101. doi: 10.1016/j.neuroscience.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Johnstone D.M., Moro C., Stone J., Benabid A.L., Mitrofanis J. Turning on Lights to stop neurodegeneration: the potential of near infrared light therapy in Alzheimer's and Parkinson's disease. Front. Neurosci. 2016;9:500. doi: 10.3389/fnins.2015.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B., Brandli A., Mitrofanis J., Stone J., Purushothuman S., Johnstone D.M. Remote tissue conditioning – an emerging approach for inducing body-wide protection against diseases of ageing. Ageing Res. Rev. 2017;37:69–78. doi: 10.1016/j.arr.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Koo J.H., Jang Y.C., Hwang D.J., Um H.S., Lee N.H., Jung J.H., Cho J.Y. Treadmill exercise produces neuroprotective effects in a murine model of Parkinson's disease by regulating the TLR2/MyD88/NF-kappaB signaling pathway. Neuroscience. 2017;356:102–113. doi: 10.1016/j.neuroscience.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Liu X., Sha O., Cho E.Y. Remote ischemic postconditioning promotes the survival of retinal ganglion cells after optic nerve injury. J. Mol. Neurosci. 2013;51:639–646. doi: 10.1007/s12031-013-0036-2. [DOI] [PubMed] [Google Scholar]
- Mattson M.P. Challenging oneself intermittently to improve health. Dose Response. 2014;12:600–618. doi: 10.2203/dose-response.14-028.Mattson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart F., El Massri N., Johnstone D.M., Stone J., Mitrofanis J., Benabid A.L., Moro C. Near-infrared light (670 nm) reduces MPTP-induced parkinsonism within a broad therapeutic time window. Exp. Brain Res. 2016;234:1787–1794. doi: 10.1007/s00221-016-4578-8. [DOI] [PubMed] [Google Scholar]
- Saliba A., Du Y., Liu H., Patel S., Roberts R., Berkowitz B.A., Kern T.S. Photobiomodulation mitigates diabetes-induced retinopathy by direct and indirect mechanisms: evidence from intervention studies in pigmented mice. PLoS One. 2015;10:e0139003. doi: 10.1371/journal.pone.0139003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne M., Sladen P., Jiao Y., Dragatsis I., Smeyne R.J. HIF1alpha is necessary for exercise-induced neuroprotection while HIF2alpha is needed for dopaminergic neuron survival in the substantia nigra pars compacta. Neuroscience. 2015;295:23–38. doi: 10.1016/j.neuroscience.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toy W.A., Petzinger G.M., Leyshon B.J., Akopian G.K., Walsh J.P., Hoffman M.V., Vuckovic M.G., Jakowec M.W. Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. Neurobiol. Dis. 2014;63:201–209. doi: 10.1016/j.nbd.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.