Abstract
Objective
Psoriasis and depression may have common mechanisms, such as systemic inflammation, dysfunction of the hypothalamic-pituitary-adrenal axis, and vitamin D3 deficiency. Among men with psoriasis, this study examined whether depression severity was associated with serum concentrations of different metabolic and inflammatory markers.
Methods
The study included 85 men with psoriasis (mean age ± standard deviation [SD], 47 ± 14 years) and 65 men without psoriasis (mean age ± SD, 44 ± 13 years). In both groups, we measured the body mass index; blood pressure; and serum concentrations of lipids, uric acid, lipase, interleukins 6 and 18, cortisol, and 25-hydroxyvitamin D3. All participants completed the Beck Depression Inventory. Other variables analyzed included psoriasis duration, the Psoriasis Area Severity Index, and the percentage of body surface area affected by psoriatic lesions.
Results
Compared with controls, patients with psoriasis had significantly greater depression severity, higher body mass indices, and higher serum concentrations of total cholesterol and interleukins 6 and 18; moreover, they had significantly lower serum 25-hydroxyvitamin D3 concentrations. In patients with psoriasis, depression severity correlated positively with psoriasis duration, the Psoriasis Area Severity Index, the percentage of body surface area affected by psoriatic lesions, and interleukin-18 concentration. In patients with psoriasis, depression severity correlated negatively with 25-hydroxyvitamin D3 concentration, but it did not correlate significantly with the serum concentrations of interleukin 6 and cortisol.
Conclusions
High concentrations of interleukin 18 and low concentrations of 25-hydroxyvitamin D3 may be associated with depression severity in men with psoriasis. Thus, further studies should examine whether effective anti-inflammatory treatments or vitamin D3 supplementation can improve depression outcomes in these patients.
Introduction
Psoriasis is diagnosed more and more often; its pathogenesis is complex and involves immune dysregulation and chronic systemic inflammation [1]. Due to this complex pathogenesis, psoriasis rarely presents in isolation, but rather co-occurs with other conditions, such as psychiatric diseases, metabolic syndrome, and alcohol and tobacco dependence [1–4]. Of all conditions that co-occur with psoriasis, depression is the most common, affecting up to 80% of patients with psoriasis [5–6]. In psoriasis, depression worsens both treatment outcomes and prognosis [7]; for example, it increases the risk of psoriatic arthritis [8]. We and other investigators showed that longer psoriasis duration and greater skin lesion severity are associated with depressed mood [9–14]. However, some studies did not support these observations [15–16]. A growing body of evidence suggests that psoriasis and depression may have common mechanisms, such as systemic inflammation [17–18], dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis [19–21], and vitamin D3 deficiency [22–24]. It is hypothesised that such non-psychological factors may increase the risk of depression in patients with psoriasis, but the evidence supporting this hypothesis is lacking.
Thus, among men with psoriasis, we analyzed the relationship between depression severity and serum concentrations of interleukins (IL) 6 and 18 (both implicated in the pathogenesis of psoriasis), cortisol, and 25-hydroxyvitamin D3 [25(OH)D3].
Patients and methods
Participants
The study included 85 men with psoriasis (mean age ± standard deviation [SD], 47 ± 14 years) and 65 men without psoriasis, who served as controls (mean age ± SD, 44 ± 13 years). Because women with psoriasis have depression more often than do men with psoriasis, [25], we did not include women, to avoid potential confounding effects of sex. All participants were recruited between 2014 and 2016 at the Department of Dermatology, Venereology, and Pediatric Dermatology, Medical University of Lublin, Poland. The mean psoriasis duration was 18 ± 13 (SD) years, and the mean Psoriasis Area Severity Index (PASI) score was 17 ± 9 (SD) points. The controls received treatment due to diseases other than psoriasis, such as pigment nevi, mild acne, small filiform warts, and single fungal nail infections. The exclusion criteria were as follows: history of recent myocardial disease, renal insufficiency, severe systemic diseases with fever, psychiatric disorders, and previous or current treatment with biologicals or immunosuppressive agents. Neither the patients nor the controls received any medications that might have changed their mood. None of the patients received ultraviolet light therapy or vitamin D3 supplementation.
Ethics
The study protocol was approved by the Local Bioethics Committee of the Medical University of Lublin (decision no. KE-0254/283/2014 of 30 October 2014), and written informed consent was obtained from all individual participants included in the study.
Variables analyzed
During a routine visit to the clinic, each participant was asked to complete the Beck Depression Inventory (BDI). The BDI comprises 21 items, each scored on a four -point scale (from 0 to 3 points). The final score ranges from 0 to 63 points and is interpreted as no depression (0–11 points), mild depression (12–26 points), moderate depression (27–49 points), or severe depression (50–63 points). The BDI can be used to examine symptoms of depression over any period; typically, over the past month, which was also the case in our study. The BDI’s internal consistency is very good (Cronbach’s alpha, 0.91).
The explanatory variables were as follows: disease duration; PASI score; percentage of body surface area affected by psoriatic lesions (BSA); body mass index (BMI); systolic and diastolic blood pressure; and serum concentrations of total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides (TG), uric acid, lipase, IL-6, IL-18, cortisol, and 25(OH)D3.
Whole-blood samples were collected in the morning, after an overnight fast. After 20 minutes of centrifugation at 1,000 × g, supernatant was separated from the blood samples and used for testing or stored at -80°C. The total cholesterol concentration was measured with a colorimetric method with cholesterol esterase and oxidase; HDL-cholesterol concentration, with a direct enzymatic-colorimetric method with polyethylene glycol (PEG)-modified cholesterol esterase and oxidase; and TG, with an enzymatic-colorimetric method with phosphoglycerol oxidase. The serum LDL-cholesterol concentration was calculated according to the Friedewald equation. The uric acid concentration was measured with an enzymatic-colorimetric method with uricase. The lipase concentration was measured with a colorimetric method with 1,2-O-dilauryl-rac-glycero-3-glutaric acid-(6-methylresorufin) ester as a chromogenic substrate. The Cobas Integra 400 analyzer (Roche Diagnostics, Tokyo, Japan) and commercially available reagents (Roche, Tokyo, Japan) were used for all these measurements.
The serum concentration of 25(OH)D3 was measured with an electro-chemiluminescence assay and the Cobas e601 analyzer (Roche Diagnostics, Tokyo, Japan). Enzyme-linked immunosorbent assays were used to measure the serum concentrations of cortisol (Cortisol ELISA, IBL International, Hamburg, Germany), IL-6 (Human IL-6 High Sensitivity ELISA Kit, Diaclone, Besancon, France), and IL-18 (Human IL-18 ELISA Kit, MBL, Nagoya, Japan). All ELISAs were read with a spectrophotometric plate reader (Power Wave XS, Bio-Tek, Winooski, United States).
Statistical analysis
Normal distribution of continuous variables was checked with the Shapiro-Wilk test, and normally distributed variables were presented as means and SDs. The Student t-test for independent variables was used to compare continuous variables between two groups. The Pearson’s coefficient (r) was used to examine correlations between pairs of variables. The predictors of depression in patients with psoriasis were identified with an artificial neural network with MLP 7-9-1 structure and BFGS learning algorithm (SOS error function, activation function of the neurons in the hidden layer–tangent function and activation function of the neurons in the output layer–logistic function). We used analysis of variance (ANOVA) with the Tukey’s test for post hoc comparisons or the Kruskall-Wallis test to compare patients without depression, patients with mild depression, and patients with moderate depression. The Statistica 10 package (StatSoft, Tulsa, OK, United States) was used for all calculations; p < 0.05 was considered statistically significant.
Results
Physical, clinical, and laboratory characteristics of the patients and controls are presented in Table 1. Compared with the controls, patients with psoriasis had significantly higher BDI scores, BMIs, and serum concentrations of total cholesterol, IL-6, and IL-18; they had significantly lower 25(OH)D3 concentrations. The concentrations of LDL-cholesterol, HDL-cholesterol, TG, uric acid, lipase, and cortisol did not differ between the groups.
Table 1. Characteristics of men with psoriasis and controls.
| Parameter | Patients with psoriasis (n = 85) [mean ± SD] |
Controls (n = 65) [mean ± SD] |
p |
|---|---|---|---|
| Age (years) | 47 ± 14 | 44 ± 13 | NS |
| PASI | 17 ± 9 | - | - |
| Disease duration (years) | 18 ± 13 | - | - |
| BSA (%) | 26 ± 19 | - | - |
| BMI (kg/m2) | 28 ± 5 | 26 ± 3 | 0.003 |
| Systolic blood pressure (mm Hg) |
1.3 x 102 ± 0.1 x 102 | 1.3 x 102 ± 0.1 x 102 | NS |
| Diastolic blood pressure (mm Hg) | 81 ± 10 | 80 ± 8 | NS |
| Total cholesterol (mg/dl) | 2.1 x 102 ± 0.4 x 102 | 1.9 x 102 ± 0.4 x 102 | 0.01 |
| LDL-cholesterol (mg/dl) | 1.2 x 102 ±0.3 x 102 | 1.3 x 102 ± 0.4 x 102 | NS |
| HDL-cholesterol (mg/dl) | 50 ± 13 | 54 ± 15 | NS |
| TG (mg/dl) | 1.6 x 102 ± 0.8 x 102 | 1.3 x 102 ± 0.7 x 102 | NS |
| Uric acid (mg/dl) | 6.1 ± 1.6 | 5.7 ± 1.1 | NS |
| Lipase (U/l) | 35 ± 19 | 32± 16 | NS |
| Interleukin 6 (pg/ml) | 4.3 ± 4.6 | 0.700 ± 0.005 | <0.0001 |
| Interleukin 18 (pg/ml) | 2.8 x 102 ± 1.2 x 102 | 2.2 x 102 ± 1 x 102 | 0.006 |
| Cortisol (ng/ml) | 1.3 x 102 ± 0.4 x 102 | 1.3 x 102 ± 0.4 x 102 | NS |
| 25-hydroxyvitamin D3 (ng/ml) | 12 ± 5 | 22± 9 | <0.0001 |
| Beck Depression Inventory (points) | 13 ± 11 | 4.5 ± 4.4 | <0.0001 |
BMI, body mass index; BSA, body surface area affected by psoriatic lesions; NS, non-significant (p>0.05); PASI, Psoriasis Area Severity Index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; TG, triglycerides; SD, standard deviation
Among patients with psoriasis, the BDI scores correlated positively with the serum IL-18 concentration (r = 0.27, p = 0.0100), PASI scores (r = 0.46, p < 0.0001), BSAs (r = 0.46, p < 0.0001), and psoriasis duration (r = 0.43, p < 0.0001); the BDI scores correlated negatively with serum 25(OH)D3 concentrations (r = -0.23, p = 0.0200; Table 2).
Table 2. Pearson’s correlation coefficients (r) between Beck Depression Inventory scores and clinical characteristics of patients with psoriasis (n = 85).
| Variables | r | p | |
|---|---|---|---|
| Beck Depression Inventory scores | 25-hydroxyvitamin D3 (ng/ml) | -0.23 | 0.02 |
| Interleukin 6 (pg/ml) | 0.18 | NS | |
| Interleukin 18 (pg/ml) | 0.27 | 0.01 | |
| Cortisol (ng/ml) | 0.03 | NS | |
| PASI | 0.46 | <0.0001 | |
| BSA (%) | 0.46 | <0.0001 | |
| BMI (kg/m2) | 0.11 | NS | |
| Disease duration (years) | 0.43 | <0.0001 | |
BMI, body mass index; BSA, body surface area affected with psoriatic lesions; NS, non-significant (p>0.05); PASI, Psoriasis Area Severity Index
These findings were confirmed by the artificial neural network analysis, in which numerical weights for all variables that correlated significantly with BDI scores in patients with psoriasis were markedly higher than 1 (Table 3).
Table 3. Clinical and laboratory predictors of depression in patients with psoriasis–numerical weights of an artificial neural network.
| Group | Factor | Numerical weight |
|---|---|---|
| Clinical predictors | Disease duration (years) | 1.5 |
| PASI | 4.8 | |
| BSA (%) | 1.2 | |
| Laboratory predictors | Interleukin 6 (pg/ml) | 0.9 |
| Interleukin 18 (pg/ml) | 2.8 | |
| 25-hydroxyvitamin D3 (ng/ml) | 1.1 | |
| Cortisol (ng/ml) | 1.0 |
BSA, body surface area affected with psoriatic lesions; PASI, Psoriasis Area Severity Index
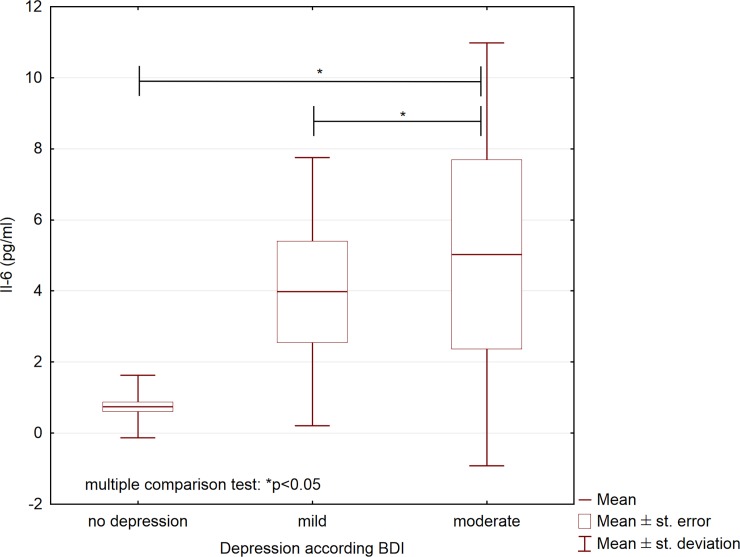
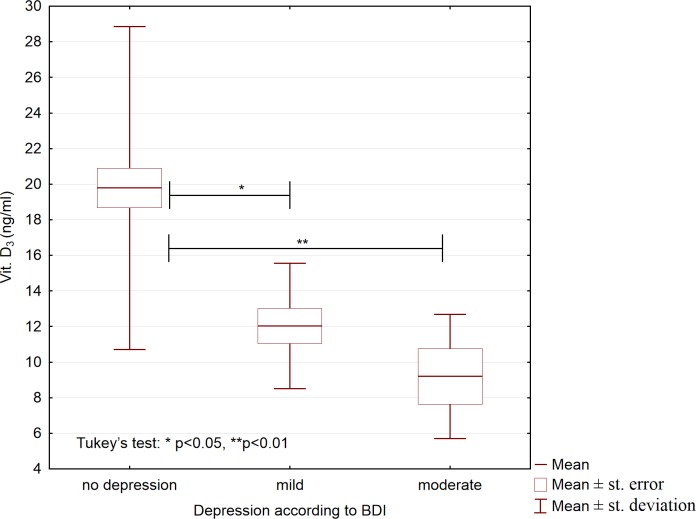
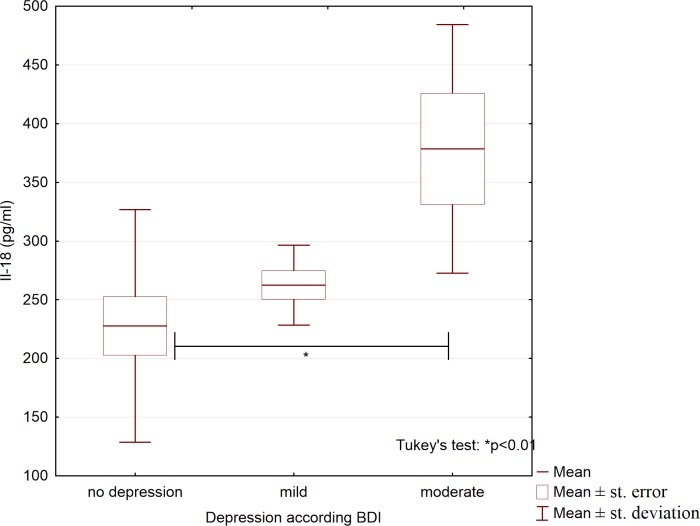
We also studied whether the analyzed variables differed between participants who differed in depression severity. Based on the BDI scores, we allocated all participants, both patients and controls, to the three following groups: no depression (BDI score, 0–11; n = 112), mild depression (BDI score, 12–26; n = 25), and moderate depression (BDI score, 27–49; n = 13); no participants had severe depression (BDI score, 50–63 points). These groups differed significantly with respect to the concentrations of IL-6, IL-18, and 25(OH)D3 (Table 4). Compared to participants with mild depression and participants without depression, participants with moderate depression had higher serum concentrations of IL-6 and IL-18 (Figs 1 and 2), and lower 25(OH)D3 concentrations (Fig 3).
Table 4. Serum concentrations of interleukins 6 and 18, 25-hydroxyvitamin D3, and cortisol in all participants (N = 150) according to depression severity.
| No depression (mean ± SD; n = 112) | Mild depression (mean ± SD; n = 25) | Moderate depression (mean ± SD; n = 13) | p | |
|---|---|---|---|---|
|
Interleukin 6 (pg/ml) |
0.74 ± 0.88 | 3.9 ± 3.8 | 5.0 ± 6.0 | 0.001 |
| Interleukin 18 (pg/ml) | 2.1 x102 ± 0.9 x 102 | 2.6 x 102 ± 0.4 x 102 | 3.8 x 102 ± 1.1 x 102 | <0.001 |
| 25-hydroxyvitamin D3 (ng/ml) | 19.7 ± 8.9 | 12.0 ± 3.5 | 9.2 ± 3.5 | <0.001 |
| Cortisol (ng/ml) | 1.2 x 102 ± 0.4 x 102 | 1.4 x 102 ± 0.5 x 102 | 1.2 x 102 ± 0.1 x 102 | 0.31 |
SD, standard deviation
Fig 1. Serum concentrations of interleukin 6 in participants with different depression severity (N = 150).
Fig 2. Serum concentrations of interleukin 18 in participants with different depression severity (N = 150).
Fig 3. Serum concentrations of 25-hydroxyvitamin D3 in participants with different depression severity (N = 150).
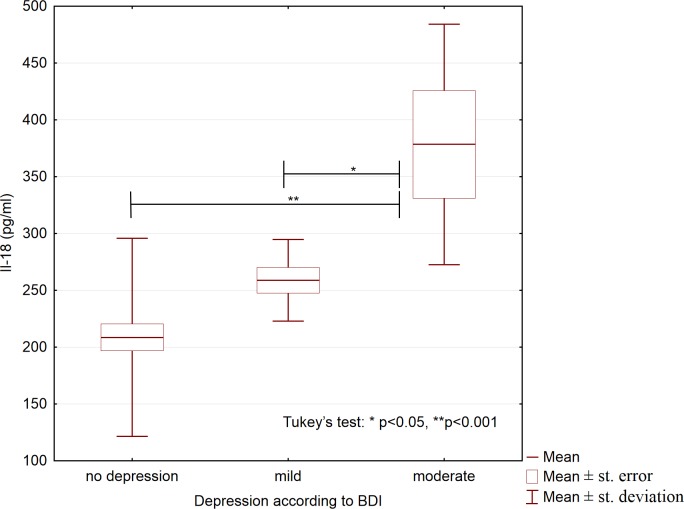
Moreover, we examined whether the variables analyzed differed between patients with psoriasis who had no depression (n = 52), mild depression (n = 20), and moderate depression (n = 13). Only the IL-18 concentration differed significantly between these groups (Table 5); compared with patients without depression, patients with moderate depression had significantly higher IL-18 concentrations (Fig 4).
Table 5. Serum concentrations of IL-6, IL-18, 25-hydroxyvitamin D3, and cortisol in patients with psoriasis (n = 85) according to depression severity.
| No depression (mean ± SD; n = 52) |
Mild depression (mean ± SD; n = 20) | Moderate depression (mean ± SD; n = 13) |
p | |
|---|---|---|---|---|
| Interleukin 6 (pg/ml) | 1.3 ± 0.8 | 3.9 ± 4.1 | 5.0 ± 6.0 | 0.25 |
| Interleukin 18 (pg/ml) | 2.3 x 102 ±1.0 x 102 | 2.7 x 102 ± 0.3 x 102 | 3.8 x 102 ± 1.1 x 102 | <0.01 |
| 25-hydroxyvitamin D3 (ng/ml) | 11.9 ± 4.2 | 12.1 ± 3.9 | 9.2 ± 3.5 | 0.6 |
| Cortisol (ng/ml) | 1.2 x 102 ± 0.3 x 102 | 1.4 x 102 ± 0.6 x 102 | 1.2 x 102 ± 0.1 x 102 | 0.38 |
SD, standard deviation
Fig 4. Serum concentrations of interleukin 18 in patients with psoriasis and different depression severity.
Discussion
In our study, compared with healthy controls, men with psoriasis had significantly higher BDI scores, body mass indices, and serum concentrations of total cholesterol and interleukins 6 and 18; moreover, they had lower serum 25(OH)D3 concentrations. Depression severity correlated negatively with the 25(OH)D3 concentration and positively with psoriasis duration, the PASI, the percentage of body surface area affected by psoriatic lesions, and the interleukin-18 concentration.
In previous studies, we and other authors [5, 6, 11] showed a strong association between psoriasis and depressed mood. However, the association of depression with the severity and duration of psoriasis remains less clear [15–16]. The co-occurrence of psoriasis and depression may result from common mechanisms of these two conditions, and not solely from the psychological effect of skin lesions on patients’ mood. Our study examined some of the potential mechanisms that link psoriasis and depression.
In our study, compared to controls, patients with psoriasis had significantly higher BMIs and concentrations of total cholesterol; moreover, they had significantly higher concentrations of pro-inflammatory cytokines IL-6 and IL-18. These cytokines are implicated in the pathogenesis of psoriatic skin lesions. For example, overproduction of IL-6 in the skin stimulates T helper cells (Th)17 and inhibits the differentiation of regulatory T cells [25–26]. This, in turn, results in an increased production of pro-inflammatory cytokines, such as IL-17 and interferon (IFN) γ, which leads to keratinocyte proliferation and formation of psoriatic skin lesions. IL-18 is synthesized primarily by macrophages, but also by other immune cells and keratinocytes [27]. In the pathogenesis of psoriasis, IL-18 has two effects. First, IL-18 increases the production of IFN-γ, which promotes Th1-mediated responses. Second, IL-18 is involved in the recruitment and adhesion of immune cells to inflammatory sites [27]. Notably, patients with psoriasis have higher IL-18 concentrations in plasma than do healthy controls [28].
In line with previous studies, we found that patients with psoriasis had lower serum concentrations of 25(OH)D3 than did controls [29]. However, because people with high BMIs tend to have low 25(OH)D3 concentrations [30], the higher BMIs of patients with psoriasis in our study, compared with controls, can partly explain the difference in 25-(OH)D3 concentrations between these two groups.
We also studied the association of clinical and laboratory variables to depression in patients with psoriasis. Similarly to previous studies [9–10,12–14], we showed that psoriasis severity, measured with disease duration, PASI, and BSA, correlated with depression severity. Moreover, we studied whether both psoriasis and depression were associated with inflammation. Previous studies showed that patients with depression and no clinically important inflammatory conditions had high concentrations of IL-6 and IL-18 [17,31–32]. Higher concentrations of IL-6 and IL-18 were also independently associated with depressive disorders in patients after stroke [33], and depression severity correlated positively with serum concentrations of IL-6 and IL-18 in hemodialyzed patients [34]. Similarly, in our study, we found a statistically significant correlation between BDI scores and serum IL-18 concentrations in patients with psoriasis. Although the IL-6 concentration did not correlate with BDI scores in our patients, it tended to be higher (non-significantly) in patients with moderate depression than in patients who had only mild depression or no depression. The average patient in our study had mild depression (mean BDI score, 13±11), whereas most previous studies that showed increased serum IL-6 concentrations included patients with severe depression. We suppose that studies involving patients with psoriasis and severe depression could show that serum IL-6 concentrations and BDI scores are significantly correlated.
Our findings support the view that increased concentrations of pro-inflammatory cytokines may worsen depression. Some investigators suggested that depression might be caused by inflammation within the central nervous system (CNS) and by neurodegeneration [35]. Previous studies showed that inflammation, which can involve increased IL-6 concentrations, was associated with depression [36–37]. Moreover, treatments with agents against pro-inflammatory cytokines reduced depression in patients with chronic inflammatory conditions [38]. In patients with rheumatoid arthritis [39] and inflammatory bowel disease [40], anxiety and depression were associated with IL-17 concentrations. Activation of Th1 and Th17 cells, which leads to an increased production of IL-2, IFN γ, and IL-17, was also observed in depression [41–42]. Because IL-17 is implicated in the development of psoriatic lesions, it would be worthwhile examining the association between IL-17 and depression in psoriasis.
Evolutionarily, the association between inflammation and depression may be explained by the phenomenon of "sickness behavior". For example, a recuperating animal will withdraw from its environment to conserve energy needed for recovery [43]. On a biochemical level, pro-inflammatory cytokines disrupt kynurenine metabolism, and kynurenine concentrations in the blood were increased in people who attempted suicide [44]. Also, aberrant kynurenine metabolism generates neurotoxic quinolinic acid [43]. Moreover, plasma IL-18 concentrations have also been linked to the availability of μ-opioid receptor in patients with major depression [45].
In animal models, pro-inflammatory cytokines promoted the development of mental disorders by increasing the activity of the HPA axis [46]. Notably, hyperactivity of the HPA axis is one of the most consistent biological findings in patients with depression [47]. Based on these and similar observations [19, 20], we expected that depression severity would be related to cortisol concentrations in patients with psoriasis; however, this association was not significant in our study. The lack of an association between depression severity and serum cortisol concentrations might be because, in contrast to patients with depression, most patients with psoriasis have decreased serum concentrations of cortisol, which is due to disorders within the HPA axis and an impaired response to stress [20]. However, in our study, patients with psoriasis and controls had similar serum cortisol concentrations.
In our study, in patients with psoriasis, BDI scores correlated negatively with serum concentrations of 25(OH)D3. Vitamin D3 deficiency is implicated in the pathogenesis of both psoriasis and depression. Many studies showed that adequate systemic concentrations of vitamin-D3 metabolites enable normal differentiation and growth of keratinocytes [24, 29, 48]. In patients with psoriasis, low concentrations of 25(OH)D3 were associated with decreased counts of circulating T regulatory cells [48]. Thus, 25(OH)D3 may act as an immunomodulator and prevent excessive Th1 and Th17 responses, which are important in the pathogenesis of psoriasis. Notably, vitamin D3 supplementation improves skin lesions in some conditions [24]. In psoriasis, ultraviolet-B treatment alone or combined with vitamin D3 supplementation reduced psoriatic skin lesions and increased 25(OH)D3 concentration in serum [49–51].
Vitamin D3 deficiency may cause behavioral disorders in animals [52]. Similarly, absolute and relative deficiency of vitamin D3 may increase the risk of mood disorders in people [53]. Vitamin D3 deficiency is also associated with increased concentrations of inflammatory markers [23], which is consistent with the previously mentioned observations on the place of pro-inflammatory cytokines in the pathogenesis of depression [17, 54]. Vitamin D might be implicated in the development of depression, because vitamin D receptors are found in the CNS [55, 56], including the structures involved in mood control, such as the hippocampus and prefrontal cortex [57]. Moreover, in the brain, vitamin D is involved in the synthesis and release of serotonin, and thus vitamin D deficiency may disturb the function of serotoninergic brain systems, which could cause depression [58]. The negative correlation between serum 25(OH)D3 concentrations and depression severity in our patients with psoriasis supports previous observations, which indicate that vitamin D3 deficiency is implicated in the pathogenesis of both depression and psoriasis.
The skin is essential to the metabolism of vitamin D. First, the skin produces more than 95% of systemic vitamin D3. Moreover, the classical pathway of vitamin D3 activation to 1,25(OH)2D3 and 25(OH)D3, which involves the liver and kidneys or peripheral tissues, occurs also locally in the skin [59]. Importantly, the skin produces other biologically active metabolites of vitamin D3 via non-classical pathways (e.g. via CYP11A1) [59]. Thus, the non-classical pathways of vitamin D3 activation might be important in the pathogenesis of both psoriasis and depression [60].
Our study had some limitations. First, the study was cross-sectional, and thus we were unable to determine the exact sequence of events leading to inflammation, vitamin D3 deficiency, and development of psoriasis and depression. Second, the study included patients from a tertiary center only, and therefore the results cannot be generalized to the whole population of patients with psoriasis. Third, we analyzed few serum markers, and further studies should examine whether other factors, such as activation of oxidative and nitrosative stress pathways and other cytokines implicated in the pathogenesis of psoriasis (e.g., TNF-α, IL-17A, IL-12, IL-23), are associated with the development of depression in patients with psoriasis.
Conclusions
Depression in patients with psoriasis is not only a psychological reaction to a chronic condition, but common mechanisms of depression and psoriasis, in particular inflammation and vitamin D3 deficiency, might be responsible for the co-occurrence of these two diseases. Our findings encourage further studies that should examine whether effective anti-inflammatory treatments or vitamin D3 supplementation can improve depression and psoriatic lesions in patients with psoriasis.
Acknowledgments
We thank Dr. Konrad Janowski, for his advice; Dr. Malgorzata Kowal, for helping with ELISAs; and Dr. Szymon Bruzewicz (SciencePro) and Proper Medical Writing sp. z o.o. (www.propermedicalwriting.com) for the assistance in writing this manuscript.
Data Availability
All relevant data are within the paper.
Funding Statement
The study was supported from the Medical University of Lublin funds (grants no. DS 168 and DS 460/2016) (https://www.umlub.pl), and from the Polish National Science Center funds (grant no. UMO-2011/01/N/NZ6/01762) (https://ncn.gov.pl/?language=en). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Parisi R, Symmons DP, Griffiths CE, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133: 377–85. 10.1038/jid.2012.339 [DOI] [PubMed] [Google Scholar]
- 2.Bohm D, Stock Gissendanner S, Bangemann K, Snitjer I, Werfel T, Weyergraf A, et al. Perceived relationships between severity of psoriasis symptoms, gender, stigmatization and quality of life. J Eur Acad Dermatol Venereol 2013;27: 220–6. 10.1111/j.1468-3083.2012.04451.x [DOI] [PubMed] [Google Scholar]
- 3.Golpour M, Hosseini SH, Khademloo M, Ghasemi M, Ebadi A, Koohkan F, et al. Depression and anxiety disorders among patients with psoriasis: a hospital-based case-control study. Dermatol Res Pract. 2012;381905: 16 10.1155/2012/381905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strohal R, Kirby B, Puig L, Girolomoni G, Kragballe K, Luger T, et al. Psoriasis beyond the skin: an expert group consensus on the management of psoriatic arthritis and common co-morbidities in patients with moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol. 2014;28: 1661–9. 10.1111/jdv.12350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korman AM, Hill D, Alikhan A, Feldman SR. Impact and management of depression in psoriasis patients. Expert Opin Pharmacother. 2016;17: 147–52. 10.1517/14656566.2016.1128894 [DOI] [PubMed] [Google Scholar]
- 6.Lakshmy S, Balasundaram S, Sarkar S, Audhya M, Subramaniam E. A cross-sectional study of prevalence and implications of depression and anxiety in psoriasis. Indian J Psychol Med. 2015;37: 434–40. 10.4103/0253-7176.168587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connor CJ, Liu V, Fiedorowicz JG. Exploring the physiological link between psoriasis and mood disorders. Dermatol Res Pract. 2015;2015: 409637 10.1155/2015/409637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewinson RT, Vallerand IA, Lowerison MW, Parsons LM, Frolkis AD, Kaplan GG, et al. Depression Is Associated with an Increased Risk of Psoriatic Arthritis among Patients with Psoriasis: A Population-Based Study. J Invest Dermatol. 2017;137: 828–35. 10.1016/j.jid.2016.11.032 [DOI] [PubMed] [Google Scholar]
- 9.Jensen P, Ahlehoff O, Egeberg A, Gislason G, Hansen PR, Skov L. Psoriasis and new-onset depression: a Danish Nationwide Cohort Study. Acta Derm Venereol. 2016;96: 39–42. 10.2340/00015555-2183 [DOI] [PubMed] [Google Scholar]
- 10.Kim GE, Seidler E, Kimball AB. Effect of age at diagnosis on chronic quality of life and long-term outcomes of individuals with psoriasis. Pediatr Dermatol. 2015;32: 656–62. 10.1111/pde.12416 [DOI] [PubMed] [Google Scholar]
- 11.Pietrzak D, Pietrzak A, Krasowska D, Makara-Studzińska M, Madej A, Baranowska M, et al. Depressiveness, measured with Beck Depression Inventory, in patients with psoriasis. J Affect Disord. 2017;209: 229–34. 10.1016/j.jad.2016.11.045 [DOI] [PubMed] [Google Scholar]
- 12.Pujol RM, Puig L, Dauden E, Sanchez-Carazo JL, Toribio J, Vanaclocha F, et al. Mental health self-assessment in patients with moderate to severe psoriasis: an observational, multicenter study of 1164 patients in Spain (the VACAP Study). Actas Dermosifiliogr. 2013;104: 897–903. 10.1016/j.ad.2013.04.014 [DOI] [PubMed] [Google Scholar]
- 13.Remrod C, Sjostrom K, Svensson A. Psychological differences between early- and late-onset psoriasis: a study of personality traits, anxiety and depression in psoriasis. Br J Dermatol. 2013;169: 344–50. 10.1111/bjd.12371 [DOI] [PubMed] [Google Scholar]
- 14.Tee SI, Lim ZV, Theng CT, Chan KL, Giam YC. A prospective cross-sectional study of anxiety and depression in patients with psoriasis in Singapore. J Eur Acad Dermatol Venereol. 2016; 30:1159–64. 10.1111/jdv.13615 [DOI] [PubMed] [Google Scholar]
- 15.Akay A, Pekcanlar A, Bozdag KE, Altintas L, Karaman A. Assessment of depression in subjects with psoriasis vulgaris and lichen planus. J Eur Acad Dermatol Venereol. 2002;16: 347–52. [DOI] [PubMed] [Google Scholar]
- 16.Cohen BE, Martires KJ, Ho RS. Psoriasis and the risk of depression in the US population: National Health and Nutrition Examination Survey 2009–2012. JAMA Dermatol. 2016;152: 73–9. 10.1001/jamadermatol.2015.3605 [DOI] [PubMed] [Google Scholar]
- 17.Al-Hakeim HK, Al-Rammahi DA, Al-Dujaili AH. IL-6, IL-18, sIL-2R, and TNFalpha proinflammatory markers in depression and schizophrenia patients who are free of overt inflammation. J Affect Disord. 2015;182: 106–14. 10.1016/j.jad.2015.04.044 [DOI] [PubMed] [Google Scholar]
- 18.Granata M, Skarmoutsou E, Trovato C, Rossi GA, Mazzarino MC, D'Amico F. Obesity, type 1 diabetes, and psoriasis: an autoimmune triple flip. Pathobiology. 2017; 84: 71–79. 10.1159/000447777 [DOI] [PubMed] [Google Scholar]
- 19.Cowen PJ. Not fade away: the HPA axis and depression. Psychol Med. 2010;40: 1–4. 10.1017/S0033291709005558 [DOI] [PubMed] [Google Scholar]
- 20.Evers AW, Verhoeven EW, Kraaimaat FW, de Jong EM, de Brouwer SJ, Schalkwijk J, et al. How stress gets under the skin: cortisol and stress reactivity in psoriasis. Br J Dermatol. 2010;163: 986–91. 10.1111/j.1365-2133.2010.09984.x [DOI] [PubMed] [Google Scholar]
- 21.Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35: 722–9. 10.1016/j.pnpbp.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hock AD. Review: Vitamin D3 deficiency results in dysfunctions of immunity with severe fatigue and depression in a variety of diseases. In Vivo. 2014;28: 133–45. [PubMed] [Google Scholar]
- 23.Okereke OI, Singh A. The role of vitamin D in the prevention of late-life depression. J Affect Disord. 2016;198: 1–14. 10.1016/j.jad.2016.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wadhwa B, Relhan V, Goel K, Kochhar AM, Garg VK. Vitamin D and skin diseases: A review. Indian J Dermatol Venereol Leprol. 2015;81: 344–55. 10.4103/0378-6323.159928 [DOI] [PubMed] [Google Scholar]
- 25.Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci. USA 1989;86: 6367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman WA, Levine AD, Massari JV, Sugiyama H, McCormick TS, Cooper KD. IL-6 signaling in psoriasis prevents immune suppression by regulatory T cells. J Immunol. 2009;183: 3170–6. 10.4049/jimmunol.0803721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pietrzak A, Zalewska A, Chodorowska G, Krasowska D, Michalak-Stoma A, Nockowski P, et al. Cytokines and anticytokines in psoriasis. Clin Chim Acta. 2008;394: 7–21. 10.1016/j.cca.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 28.Flisiak I, Klepacki A, Chodynicka B. Plasma and scales levels of interleukin 18 in comparison with other possible clinical and laboratory biomarkers of psoriasis activity. Biomarkers. 2006;11: 2,194–200. 10.1080/13547500600565735 [DOI] [PubMed] [Google Scholar]
- 29.Soleymani T, Hung T, Soung J. The role of vitamin D in psoriasis: a review. Int J Dermatol. 2015;54: 383–92. 10.1111/ijd.12790 [DOI] [PubMed] [Google Scholar]
- 30.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring). 2012;20:1444–1448. 10.1038/oby.2011.404 [DOI] [PubMed] [Google Scholar]
- 31.Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35: 804–17. 10.1016/j.neubiorev.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 32.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67: 446–57. 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- 33.Kang HJ, Bae KY, Kim SW, Kim JT, Park MS, Cho KH, et al. Effects of interleukin-6, interleukin-18, and statin use, evaluated at acute stroke, on post-stroke depression during 1-year follow-up. Psychoneuroendocrinology. 2016;72: 156–60. 10.1016/j.psyneuen.2016.07.001 [DOI] [PubMed] [Google Scholar]
- 34.Zhao C, Ma H, Yang L, Xiao Y. Long-term bicycle riding ameliorates the depression of the patients undergoing hemodialysis by affecting the levels of interleukin-6 and interleukin-18. Neuropsychiatr Dis Treat. 2016;13: 91–100. 10.2147/NDT.S124630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maes M, Yirmyia R, Noraberg J, Brene S, Hibbeln J, Perini G, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metab Brain Dis. 2009;24: 27–53. 10.1007/s11011-008-9118-1 [DOI] [PubMed] [Google Scholar]
- 36.Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med. 2009;39: 413–23. 10.1017/S0033291708003723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71: 171–86. 10.1097/PSY.0b013e3181907c1b [DOI] [PubMed] [Google Scholar]
- 38.Kappelmann N, Lewis G, Dantzer R, Jones PB, Khandaker GM. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol Psychiatry. 2016; October 18 10.1038/mp.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, Ho RC, Mak A. The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis. 2012;15: 183–7. 10.1111/j.1756-185X.2011.01673.x [DOI] [PubMed] [Google Scholar]
- 40.Martin-Subero M, Anderson G, Kanchanatawan B, Berk, Maes M. Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut–brain pathways. CNS Spectr. 2016;21: 184–98. 10.1017/S1092852915000449 [DOI] [PubMed] [Google Scholar]
- 41.Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35: 664–75. 10.1016/j.pnpbp.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 42.Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry. 2013;73: 622–30. 10.1016/j.biopsych.2012.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koo J, Marangell LB, Nakamura M, Armstrong A, Jeon C, Bhutani T et al. Depression and Suicidality in Psoriasis: Review of the Literature Including the Cytokine Theory of Depression. J Eur Acad Dermatol Venereol. 2017;31: 1999–2009. 10.1111/jdv.14460 [DOI] [PubMed] [Google Scholar]
- 44.Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, et al. Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav Immun. 2011;25: 1272–8. 10.1016/j.bbi.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Prossin AR, Koch AE, Campbell PL, McInnis MG, Zalcman SS, Zubieta J-K. Association of plasma interleukin-18 levels with emotion regulation and μ-opioid neurotransmitter function in major depression and healthy volunteers. Biol Psychiatry. 2011;69: 808–12. 10.1016/j.biopsych.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 46.Raber J, O'Shea RD, Bloom FE, Campbell IL. Modulation of hypothalamic-pituitary-adrenal function by transgenic expression of interleukin-6 in the CNS of mice. J Neurosci. 1997;17: 9473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pariante CM, Lightman. The HPA axis in major depression: classical theories and new developments. Trends Neurosci SL. 2008;31: 464–8. 10.1016/j.tins.2008.06.006 [DOI] [PubMed] [Google Scholar]
- 48.Mattozzi C, Paolino G, Salvi M, Macaluso L, Luci C, Morrone S et al. Peripheral blood regulatory T cell measurements correlate with serum vitamin D level in patients with psoriasis. Eur Rev Med Pharmacol Sci. 2016;20: 1675–9. [PubMed] [Google Scholar]
- 49.Osmancevic A, Landin-Wilhelmsen K, Larko O, Krogstad AL. Vitamin D status in psoriasis patients during different treatments with phototherapy. J Photochem Photobiol B. 2010;101: 117–123. 10.1016/j.jphotobiol.2010.05.008 [DOI] [PubMed] [Google Scholar]
- 50.Ala-Houhala MJ, Karppinen T, Vähävihu K, Kautiainen H, Dombrowski Y, Snellman E et al. Narrow-band ultraviolet B treatment boosts serum 25-hydroxyvitamin D in patients with psoriasis on oral vitamin D supplementation. Acta Derm Venereol. 2014;94: 146–51. 10.2340/00015555-1685 [DOI] [PubMed] [Google Scholar]
- 51.Slominski AT, Zmijewski MA, Plonka PM, Szaflarski JP, Paus R. How ultraviolet light touches the brain and endocrine system through skin, and why. Endocrinology. 2018. March 12 [Epub ahead of print]. 10.1210/en.2017-03230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22: 982–1001. 10.1096/fj.07-9326rev [DOI] [PubMed] [Google Scholar]
- 53.Parker GB, Brotchie H, Graham RK. Vitamin D and depression. J Affect Disord. 2017;208: 56–61. 10.1016/j.jad.2016.08.082 [DOI] [PubMed] [Google Scholar]
- 54.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16: 22–34. 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29: 21–30. 10.1016/j.jchemneu.2004.08.006 [DOI] [PubMed] [Google Scholar]
- 56.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13: 100–5. [DOI] [PubMed] [Google Scholar]
- 57.Langub MC, Herman JP, Malluche HH, Koszewski NJ. Evidence of functional vitamin D receptors in rat hippocampus. Neuroscience. 2001;104: 49–56. [DOI] [PubMed] [Google Scholar]
- 58.Patrick RP, Ames BN. Vitamin D and the omega-3 fatty acids control serotonin synthesis and action, part 2: relevance for ADHD, bipolar disorder, schizophrenia, and impulsive behavior. FASEB J. 2015;29: 2207–22. 10.1096/fj.14-268342 [DOI] [PubMed] [Google Scholar]
- 59.Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EK et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep. 2015;5: 14875 10.1038/srep14875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol. 2014;144 Pt A:28–39. 10.1016/j.jsbmb.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.