Abstract
The inactivity of Ure2p, caused by either a ure2 mutation or the presence of the [URE3] prion, increases DAL5 transcription and thus enables Saccharomyces cerevisiae to take up ureidosuccinate (USA+). Rtg2p regulates transcription of glutamate-repressible genes by facilitation of the nuclear entry of the Rtg1 and Rtg3 proteins. We find that rtg2Δ cells take up USA even without the presence of [URE3]. Thus, the USA+ phenotype of rtg2Δ strains is not the result generation of the [URE3] prion but is a regulatory effect. Because rtg1Δ or rtg3Δ mutations or the presence of glutamate do not produce the USA+ phenotype, this is a novel function of Rtg2p. The USA+ phenotype of rtg2Δ strains depends on GLN3, is caused by overexpression of DAL5, and is blocked by mks1Δ, but not by overexpression of Ure2p. These characteristics suggest that Rtg2p acts in the upstream part of the nitrogen catabolism regulation pathway.
Keywords: retrograde signaling|DAL5|[URE3]|prion
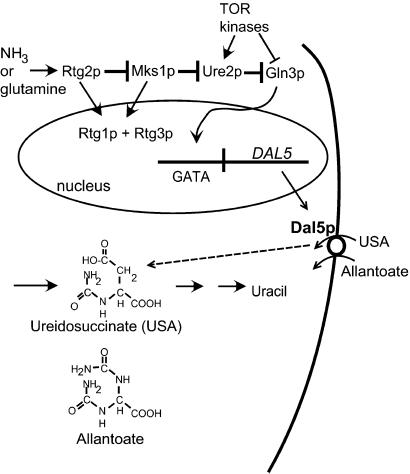
In the presence of a good nitrogen source, such as ammonia, yeast blocks the transcription of genes needed for utilization of poor nitrogen sources, such as allantoate (reviewed in refs. 1 and 2). This phenomenon is called nitrogen catabolite repression (NCR) and is mediated by the positively acting DNA-binding transcription factors Gln3p and Gat1p/Nil1p (3, 4). Under repressing conditions, Gln3p is hyperphosphorylated and is bound to the cytoplasmic phosphorylated Ure2p, whereas in derepressed conditions, Gln3p and Ure2p phosphorylation decreases, and Gln3p migrates to the nucleus to act (5–8). DAL5 encodes the permease for allantoate, a poor but usable nitrogen source for yeast (9). Regulation of DAL5 is believed to be mediated entirely through Ure2p and Gln3p (10) and can be conveniently measured by uptake of the chemically related compound, ureidosuccinate (USA), an intermediate in uracil biosynthesis (ref. 11; Fig. 1).
Figure 1.
NCR. In the presence of a good nitrogen source, such as ammonia, the cytoplasmic protein Ure2p binds to Gln3p and prevents its entrance into the nucleus. Gln3p is a DNA-binding positive transcription regulator required for the expression of multiple genes (such as DAL5) involved in transport or assimilation of poor nitrogen sources (such as allantoate). Dal5p, the allantoate transporter, also takes up the chemically similar USA, an intermediate in the uracil biosynthetic pathway. Cells blocked in aspartate transcarbamylase and supplied with USA in place of uracil on ammonia-containing medium can grow only if the NCR pathway is inactive. A ure2 mutation or the presence of the [URE3] prion results in inactivity of Ure2p and constitutive activity of Gln3p and thus the USA+ phenotype. Mks1p acts as an inhibitor of Ure2p. The role tentatively assigned to Rtg2p in NCR is based on work presented in this study.
The Mks1 protein, originally defined as a growth regulator downstream of the cAMP-A kinase system (12), also acts on nitrogen regulation upstream of Ure2p (13). Overexpression of Mks1p derepresses DAL5 by a mechanism involving Ure2p (Fig. 1). An mks1Δ strain is further unable to derepress DAL5 on a poor nitrogen source (13). Genetic experiments place Mks1p upstream of Ure2p in the NCR pathway (Fig. 1). The TOR kinases have also been implicated in NCR (6–8, 14) through their phosphorylation of Gln3p and Ure2p.
Ure2p is also of interest because the nonchromosomal gene [URE3] (15) is an altered, inactive, infectious form of the Ure2 protein (a prion) (16). The yeast prion [URE3] is a self-propagating amyloid form of Ure2p (refs. 17–19; reviewed in ref. 20). Ure2p consists of an N-terminal prion domain (residues 1–80; refs. 21and 22) and a C-terminal domain that functions in regulation of nitrogen catabolism (residues 81–354; refs. 5 and 21). In vivo, Ure2p is evenly distributed in the cytoplasm of normal cells but is aggregated in [URE3] prion-containing cells because of its amyloid state (17, 19). [URE3] requires the chaperone Hsp104 for its propagation (23) and is efficiently cured by growth in the presence of 3–5 mM guanidine (16, 24), probably because guanidine inhibits Hsp104 in vivo (25, 26).
In addition to its role in NCR, Mks1p also affects prion generation in that mks1Δ mutants have dramatically reduced rates of de novo [URE3] formation (27). Likewise, slightly elevated production of Mks1p increases the frequency with which [URE3] arises (27). The Ras–cAMP pathway negatively regulates Mks1p (12) and through it, [URE3] prion generation (27). Expressing a constitutively active allele of Ras2 nearly eliminates the de novo generation of the [URE3] prion. Deletion of MKS1 does not affect [URE3] propagation, thus the effect is specific for prion generation.
Rtg2p is a cytoplasmic protein discovered by Butow and colleagues (28, 29) through its involvement in signaling mitochondrial impairment to the nucleus. Deleting the mitochondrial genome triggers the “retrograde signaling pathway” and results in the activation of transcription of CIT2 (peroxisomal citrate synthase), DLD3 (D-lactate dehydrogenase), and the tricarboxylic acid (TCA) cycle enzyme genes CIT1, ACO1, IDH1, and IDH2 (28, 30, 31). Rtg2p requires two basic helix–loop–helix leucine zipper proteins, Rtg1p and Rtg3p, for this transcription regulation action, and these regulatory effects are strongly inhibited by glutamate in the medium (28, 30, 32). Induction of the RTG system is accompanied by movement of the Rtg1 and Rtg3 proteins from the cytoplasm into the nucleus and the partial dephosphorylation of the Rtg3 protein (29). The relocation of Rtg1p and Rtg3p requires Rtg2p, as does the dephosphorylation of Rtg3 (29, 33). Like the nitrogen regulation system, the TOR kinases also affect the RTG system (33). The precise mechanism of Rtg2 action is not yet clear, but this protein has substantial homology to a number of bacterial exopolyphosphatases and an ATP-binding site like that in Hsp70s (34).
We isolated mutants of a [URE3] strain that remained able to take up USA after a period of growth in the presence of the prion-curing agent guanidine. We find that rtg2Δ strains are ureidosuccinate uptake (USA+), but that this phenotype was not the result of retention of the [URE3] prion. We show that Rtg2p affects nitrogen catabolite regulation, an effect of Rtg2p that seems to be independent of its action in transcriptional regulation of tricarboxylic acid (TCA) cycle enzymes through Rtg1p and Rtg3p.
Methods
Media, Strains, and Plasmids.
Rich dextrose media [yeast extract peptone adenine dextrose (YPAD)], minimal media [synthetic dextrose (SD)], and glycerol media [yeast extract/peptone/glucose (YPG)] were used as described (35). USA uptake (and indirectly Ure2p activity) was scored in two ways (15). For ura2 mutants, blocked in the production of USA, ability to grow on SD plates with 33 μg/ml of USA was scored. Alternatively, uptake of USA and secretion of excess uracil produced on SD with 100 μg/ml of USA seeded with a lawn of the ura2/ura2 diploid strain 1065 allows growth of the lawn of strain 1065 around the streak.
Plasmids pVTG11 and pVTG12 expressing green fluorescent protein (GFP) and an N-terminal Ure2p (1–65)-GFP fusion, respectively, have been described (17). Ure2p was overexpressed from the ADH1 promoter by using the LEU2 2μ and CEN plasmids, pH14 and pH67, and Ure2pΔApa was similarly overexpressed from pH438 (13, 17). pMP1, expressing RTG2 under the control of its native promoter, was constructed as follows. A 3-kb fragment was PCR-amplified from a lambda phage clone (ATCC 70008) by using oligonucleotides pRS>RTG2pro (5′-GACTCACTATAGGGCGAATTGGAGCTCCACCGCGGTGGCGGCCGCCTGCAGCAGTTATTCACCC-3′) and RTG2>pRS (5′-CAA AAG CTG GGT ACC GGG CCC CCC CTC GAG GTC GAC GGT ATC GAT GAA CAA CAA GAA GGT GCC C-3′) containing 45 bp homologous to the vector pRS315 (36) and 15 bases homologous to RTG2. Cotransformation of the 3-kb PCR fragment and XbaI- and HindIII-digested pRS315 into yeast strain 4242 generated vector pMP1 by homologous recombination. A 1.9-kb PCR fragment containing RTG2 was amplified by using oligonucleotides pADH>RTG2 (5′-TTCAAGCTATACCAAGCATACAATCAACTCCAAGCTGGATCCCAAATGTCAACACTTAGCGATAG-3′) and RTG2>pADH (5′-ACCTCTGGCGAAGAAGTCCAAAGCTTCAGCTGCTGCAGGCTCGAGTTATTCTTCATAAAATTGCACGCC-3′). The amplified fragment was cotransformed with BamHI- and NotI-digested pH14 (2μ PADH1URE2) or pH67 (CEN PADH1URE2) or pH402 (CEN PADH1HIS3) to generate the plasmids pMP2, pMP3, and pMP4.
Isolation of Guanidine-Incurable Mutants.
The [URE3] strain 4037 (MATa kar1 ura2Δ leu2 trp1Δ ade5 [URE3–1]) was transformed with a bank of yeast DNA mutagenized with a modified Tn3 transposon carrying the yeast LEU2 gene (37) and plated for Leu+ transformants. Pooled transformants were plated for single colonies on YPAD containing 5 mM guanidine hydrochloride. Colonies were regrown on YPAD without guanidine, replica-plated to −Leu, and tested for growth on USA. The USA+ Leu+ clones were restreaked on YPAD with guanidine and retested for both phenotypes. Candidate mutants were crossed with strain 4053 (MATα leu2 ura2 [ure-o]), and meiotic tetrads were analyzed.
Chromosomal DNA was purified from one of these segregants (4770–7C), cut with EcoRI, ligated, and a junction fragment of the Tn3-LEU2 transposon and yeast DNA was amplified by using primers within the transposon. The sequence showed that the transposon inserted at base 771 from the ATG start of the RTG2 gene.
Disruption of RTG2 in Strain MP51.
Oligonucleotides RTG2 5′ (5′-CAGCGGCGAGCTCAATAAG-3′) and RTG2 3′ (5′-CTCAAACCTCACTAGACGAC-3′) were used to amplify the RTG2∷G418 insertion from strain ATCC 4004619 (38). The amplified product was used to transform the diploid [ure-o] strain MP51. Transformants were selected on YPAD + 300 μg/ml of G418 and one (MP52) was confirmed by PCR amplification of DNA sequences specific to the G418 insertion.
Cytoductions.
Transmission of [URE3] is tested by the transfer of cytoplasm (cytoduction) from a donor strain to a ρO recipient strain as described (39) by using the [URE3] donor strains 4184 and 4833-3B. These strains were also used as recipients in cytoduction experiments after curing of [URE3] by guanidine and elimination of the mitochondrial genome by ethidium. The propagation of [URE3] in rtg2∷G418 strains is tested by using a two-step cytoduction experiment described in the legend of Table 3.
Table 3.
rtg2Δ does not inhibit [URE3] propagation or curing
| Cytoduction 1
|
Guanidine treatment | Cytoduction 2 USA+/total | |
|---|---|---|---|
| Donor | Recipient | ||
| None | MP29 RTG2 [URE3] | − | 13/13 |
| None | MP27 rtg2Δ | − | 0/9 |
| None | MP28 (rtg2Δ) | − | 0/12 |
| [URE3] → | MP71 RTG2 [ure-o] | − | 6/6 |
| [URE3] → | MP71 RTG2 [ure-o] | + | 0/17 |
| [URE3] → | MP27 rtg2Δ | − | 8/8 |
| [URE3] → | MP27 rtg2Δ | + | 0/12 |
| [URE3] → | MP28 rtg2Δ | − | 10/10 |
| [URE3] → | MP28 rtg2Δ | + | 0/5 |
Propagation of [URE3] was tested by using a two-stage cytoduction experiment of the type: [URE3] → RTG+ or rtg2Δ → [ure-o]. In the first cytoduction, cytoplasm was transferred from the [URE3] donor strains 4184 or 4833-3B to ρo RTG2 and rtg2Δ strains. Haploid recipients that acquired a functional mitochondrial genome were grown either in the presence or absence of 5 mM guanidine and then used as donors in a second cytoduction to guanidine-cured ρo derivatives of 4184 or 4833-3B. The number of USA+ cytoductants/total cytoductants observed in the second cytoduction is shown. The presence of USA+ cytoductants from the second cytoduction confirms the ability of donor strains to propagate [URE3].
DAL5-lacZ Reporter Assays.
DAL5 expression in rtg2, [URE3], and [ure-o] strains was determined by using a TRP1-ARS1 plasmid (pRR29) expressing the lacZ gene from the DAL5 promoter (40). Strains carrying the plasmid were grown in minimal selective media (−Trp) to OD550 = 0.6–0.9. Cells were permeabilized in chloroform and 0.1% SDS, and β-galactosidase activity was determined by measuring the hydrolysis of O-nitrophenyl-β-d-galactopyranoside (41).
Results
USA+ Phenotype of rtg2 Mutants Is Unaffected by Growth in Guanidine.
In a search for mutants that cannot be cured of [URE3] by growth in the presence of guanidine, we transformed a [URE3] strain with a bank of yeast DNA carrying random insertions of LEU2. Transformants that remained USA+ after growth on rich medium containing 10 mM guanidine were retested and then mated with a [ure-o] strain, and meiotic segregants were examined. For isolate B2/7, most Leu+ USA+ meiotic segregants remained USA+ after growth on rich medium containing 5 mM guanidine. The LEU2 insert was linked tightly to ade5 (parental ditypes = 34, nonparental ditypes = 0, and tetratypes = 9). The insert was found at base 771 from the ATG start of the RTG2 gene, which is closely linked to ade5 (see Methods).
An rtg2∷G418 deletion mutant (ura2Δ strain 4791-6B) was crossed with the [URE3] strain 1735. All 14 rtg2∷G418 USA+ segregants remained USA+ when streaked to single colonies on YPAD medium containing 5 mM guanidine, whereas all 9 wild-type USA+ segregants were uniformly converted to USA− (cured of [URE3]) by the same treatment.
Both the original rtg2∷LEU2 mutant and the rtg2∷G418 deletion mutation were associated with a USA+ phenotype unchanged by growth on guanidine. Among possible explanations are (i) that RTG2 is necessary for curing of [URE3]; (ii) that an rtg2 mutation results in altered nitrogen regulation so that DAL5 is inappropriately expressed; or (iii) that an rtg2 mutation gives rise to de novo [URE3] formation at such high frequency that the USA+ phenotype seems incurable.
The USA+ Phenotype of rtg2Δ Mutants Arises De Novo.
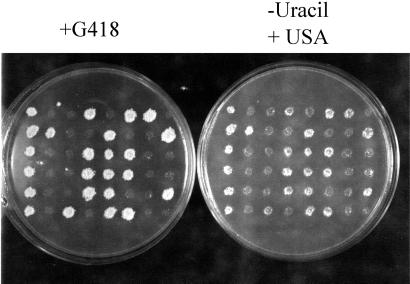
In crosses of [URE3] and rtg2 strains, all rtg2 segregants grew on USA containing media after 3 days of growth, but RTG2 segregants exhibited both USA+ and USA− phenotypes. To determine whether the USA+ phenotype is a consequence of the RTG2 deletion, we disrupted one copy of RTG2 from the [ure-o] diploid strain MP51 (see Methods) with a PCR-amplified rtg2∷G418 deletion cassette, the heterozygous diploids were sporulated, and 12 tetrads were tested for the ability to grow on USA. As shown in Fig. 2, all 24 rtg2 spores were USA+ as indicated by the cosegregation of G418 resistance and growth on USA, whereas none of the RTG2 segregants were USA+. The USA+ phenotype of rtg2 segregants may reflect either an increase in the generation of [URE3] or release of NCR caused by a metabolic effect, but does not simply reflect the stabilization of preexisting [URE3].
Figure 2.
rtg2Δ strains are USA+. (Left) The [ure-o] strains YHE861 and -867 were crossed to generate the [ure-o] diploid strain MP52. One copy of RTG2 was deleted by transformation with a PCR-amplified rtg2∷G418 cassette. (Right) The rtg2Δ/+ diploid was verified by PCR, sporulated, and the segregants were tested for growth on USA. All 24 rtg2 segregants were able to grow on USA, whereas all of the RTG2 segregants were USA−.
Next, rtg2 segregants were grown in either the presence or absence of 5 mM guanidine to determine whether the USA+ phenotype was curable. For all rtg2 strains tested, growth on 5 mM guanidine did not eliminate the USA+ phenotype (data not shown). In contrast to these results, guanidine efficiently cured [URE3] from wild-type strains.
The USA+ Phenotype of rtg2Δ Strains Is Not Caused by [URE3].
Several rtg2∷G418 USA+ segregants from MP52 (see above) were used as cytoduction donors for the transfer of cytoplasm to wild-type [ure-o] ρO recipients (Table 2). None were able to donate [URE3] to the [ure-o] recipients. Because infectivity is the defining characteristic of a prion, this proves that these rtg2∷G418 strains were USA+ for reasons other than carrying the [URE3] prion.
Table 2.
[URE3] is not present in USA+ rtg2Δ strains
| Donor | Growth on USA | Recipient | USA+/total cytoductants |
|---|---|---|---|
| MP29 RTG2 [URE3] | + | 4184 [ure-o] | 12/12 |
| MP29 RTG2 [ure-o] | − | 4184 [ure-o] | 0/17 |
| 3-1C rtg2∷G418 | + | 4184 [ure-o] | 0/7 |
| 3-4D rtg2∷G418 | + | 4833-3B [ure-o] | 0/9 |
| 3-6A rtg2∷G418 | + | 4833-3B [ure-o] | 0/13 |
| 3-12D rtg2∷G418 | + | 4184 [ure-o] | 0/10 |
RTG2 control strains and rtg2∷G418 segregants of strain MP51 were used as cytoduction donors to ρo [ure-o] derivatives of strains 4184 and 4483-3B.
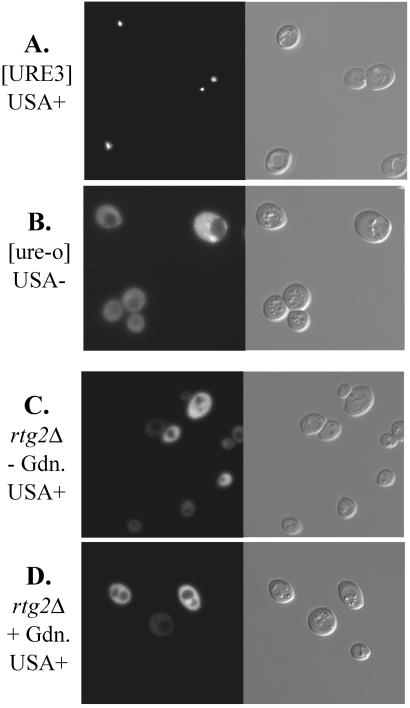
[URE3] is the result of amyloid formation by Ure2p (17–19), and as a result, Ure2p-GFP is aggregated in [URE3] prion-carrying strains (17). To test whether the rtg2 mutation was leading to aggregation of Ure2p by a mechanism that might not lead to infectivity, we examined the distribution of Ure2p1–65-GFP in RTG2+ [URE3], RTG2+ [ure-o], and rtg2 strains. Whereas the aggregation was clearly seen in the [URE3] strain, the fusion protein was evenly distributed in both [ure-o] and rtg2 cells (Fig. 3).
Figure 3.
Ure2p is not aggregated in rtg2Δ strains. Strains (A) MP29 (RTG2 [URE3]), (B) MP71 (RTG2 [ure-o], a guanidine-cured derivative of MP29), and (C and D) MP46 (rtg2) carrying pVTG12, expressing the N-terminal prion-inducing domain of Ure2p fused to GFP were examined by fluorescence microscopy as described (17). The rtg2Δ strain was treated (C) or untreated (D) with 5 mM guanidine.
rtg2Δ Does Not Preclude [URE3] Propagation or Curing.
We showed previously that none of several rtg2∷G418 strains carried [URE3], indicating that the USA+ phenotype was a regulatory effect of the rtg2 mutation. Into several of these strains, [URE3] was introduced by cytoduction from wild-type [URE3] strains (Table 3). These cytoductant rtg2∷G418 strains were then either grown on guanidine or the same medium without guanidine. After this growth period, these cells were used as cytoduction donors to a [ure-o] strain to determine whether they carried [URE3] (Table 3). In each case, the rtg2∷G418 cells carried [URE3] after it was introduced from a [URE3] donor, as shown by the fact that they could donate it to a [ure-o] strain. However, if the rtg2∷G418 [URE3] strain was grown on medium containing 5 mM guanidine, [URE3] was cured as shown by the fact that it was no longer transmitted to the [ure-o] strain. Thus, rtg2 cells are USA+ despite the absence of [URE3]—a regulatory effect of the rtg2 mutation. rtg2∷G418 cells can carry [URE3], however, and it can be efficiently cured from such a strain.
Although Mks1p affects regulation of nitrogen catabolism, it also affects [URE3] prion generation (27). Because Mks1p and Rtg2p are involved in regulating both the retrograde signaling pathway and nitrogen catabolism (refs. 13 and 42 and this study), it seemed possible that the rtg2Δ mutation, like mild overproduction of Mks1p (27), might increase the frequency with which [URE3] arises. Because rtg2Δ strains are already USA+, it was necessary to test this issue indirectly, relying on the dominance of [URE3] and the recessive nature of the rtg2Δ mutation. Wild-type and rtg2Δ segregants from MP52 were grown and mated with RTG2 [ure-o] strains YHE859 and YHE929, and the diploids formed were plated on USA medium to determine the frequency of [URE3]. The rtg2Δ segregants averaged 18 USA+ colonies per 106 diploids cells plated, whereas the RTG2 segregants averaged 20 USA+ colonies per 106 cells plated. Thus, there is no indication that rtg2Δ increases the generation of [URE3]. Likewise, overproduction of Rtg2p from pMP4 did not inhibit the induction of [URE3] by overexpression of Ure2pΔApa from pH438 in strain 4848-3B (MATa ura2 his3 leu2 trp1). Ure2pΔApa is a mutant protein lacking 8 residues in its C-terminal domain, which is particularly active in inducing the appearance of [URE3] (21).
USA+ Phenotype of rtg2 Mutants Is the Result of a Novel Regulatory Action.
Rtg2p acts with Rtg1p and Rtg3p to elevate the expression of genes such as CIT2 and DLD3 when mitochondrial function is impaired (28, 31, 32). Moreover, glutamate is a potent inhibitor of expression of the RTG-regulated genes, an effect that mimics mutation of an RTG gene (30). We constructed various rtg1∷G418 and rtg3∷G418 strains with and without [URE3]. Those carrying [URE3] were efficiently cured by growth on guanidine and became USA−. Those without [URE3] were uniformly USA−. We also found that the presence of 0.1 or 0.2% glutamate in the medium likewise does not produce a USA+ phenotype, although it has been shown to inhibit expression of the RTG-controlled genes. Elimination of the mitochondrial genome, which activates the RTG system, does not affect growth of wild-type or [URE3] strains on USA in the presence of ammonia.
DAL5 Expression Is Increased in rtg2Δ Strains.
To verify that the uptake of USA by rtg2 mutants was caused by activation of DAL5 expression, we measured β-galactosidase activity from a plasmid carrying lacZ driven by the DAL5 promoter (pRR29; ref. 40) in wild-type [ure-o], [URE3], and rtg2 [ure-o] strains (Table 4). We found that DAL5 expression was elevated 12-fold in the rtg2 strain compared with the wild-type strain, explaining the USA+ phenotype. [URE3] strains show a greater increase in DAL5 expression than do rtg2Δ mutants, and indeed rtg2Δ strains grow more slowly on USA than do [URE3] cells.
Table 4.
DAL5 expression is increased in rtg2Δ strains
| Strain | β-Galactosidase activity (OD420/20 min/OD550) |
|---|---|
| 4149 [ure-o] | 0.01 |
| 4188 [URE3] | 0.44 |
| 2-7A RTG2 | 0.01 |
| 2-7C rtg2Δ | 0.12 |
gln3Δ and mks1Δ Are Epistatic to rtg2Δ.
DAL5 expression normally depends on Gln3p (43), and we found that the USA+ phenotype of rtg2 strains likewise is eliminated in rtg2 gln3 double mutants, indicating that Rtg2p acts upstream of Gln3p in controlling DAL5 (Table 5).
Table 5.
gln3 and mks1 are epistatic to rtg2
| Strain | Genotype | Growth on USA |
|---|---|---|
| 4-9B | GLN3 rtg2Δ | + |
| 4-10C | gln3Δ RTG2 | − |
| 4-5A | gln3Δ rtg2Δ | − |
| 4852-1C | MKS1 rtg2Δ | + |
| 4852-1D | mks1Δ RTG2 | − |
| 4852-1A | mks1Δ rtg2Δ | − |
Deletion of MKS1 makes cells unable to derepress DAL5 in response to a poor nitrogen source (13). Similarly, mks1Δ rtg2Δ double mutants were consistently USA− (e.g., Table 5), indicating that the mks1Δ mutation is epistatic to the rtg2Δ mutation, and suggesting that Rtg2p acts upstream of Mks1p in the NCR pathway.
Western blots of extracts of wild-type and isogenic rtg2Δ mutants show no change in the size or amount of Ure2p (data not shown), suggesting that it is the activity of Ure2p that is altered rather than its amount. Overproduction of Rtg2p from pMP2 (2 μm, LEU2, ADH1 promoter) did not affect the USA+ phenotype of ure2Δ strain 4111, consistent with our suggestion that Rtg2p acts upstream of Ure2p. Also, overproduction of Rtg2p did not cure [URE3] (data not shown). However, overproduction of Ure2p from the ADH1 promoter on either low- or high-copy plasmids (pH67 or pH14, respectively; ref. 17) did not prevent the USA+ phenotype of an rtg2Δ mutant (data not shown).
Discussion
A frequent theme in regulation is the interaction of components of what had been thought to be different regulatory pathways. Rtg2p was originally defined in studies by Butow and colleagues (28, 29) as a mediator of the retrograde signaling pathway. Rtg2p acts with Rtg1p and Rtg3p to alter expression of genes in intermediary metabolism in response to disabled mitochondrial metabolism. Although the role of Rtg2p is not completely clear, it is known to be a cytoplasmic protein that facilitates the nuclear entry of the DNA-binding transcription factors, Rtg1p and Rtg3p. The homology of Rtg2p with bacterial exopolyphosphatases suggests a possible role in either altering a signaling nucleotide, degrading cellular polyphosphate, or removing a phosphate group from Rtg1p or Rtg3p.
In searching for genes affecting guanidine-curing of the [URE3] prion, we found that rtg2 mutants have a USA+ phenotype (like [URE3] strains) but do not affect prion replication, generation, or curing. The rtg2Δ mutation leads to derepression of DAL5, and this activity requires Gln3p, as with the derepression seen in the presence of a poor nitrogen source. That rtg2Δ mks1Δ strains were USA− suggests that Mks1p is downstream of Rtg2p in the NCR cascade. This finding would suggest the pathway: NH3 → Rtg2p –| Mks1p –| Ure2p –| Gln3p → DAL5. Two experiments that might have confirmed this pathway gave unexpected results. (i) Overexpression of Ure2p did not eliminate the USA+ phenotype of rtg2Δ strains, and (ii) a strain overproducing Rtg2p was USA+ on proline media. Neither of these experiments clearly rules out our tentative model; the overproduced Rtg2p may be completely inactive in the absence of a good nitrogen source, and the overproduced Ure2p may not overcome the hyperactive Mks1p in the rtg2Δ strain.
Although the rtg2Δ mutation activates the nitrogen-repressed gene DAL5, rtg1Δ or rtg3Δ mutations do not do so. This result is in contrast to the action of Rtg2p in the retrograde signaling pathway, which requires both Rtg1 and Rtg3. Moreover, glutamate inhibits the induction of the retrograde pathway, and thus mimics an rtg2Δ mutation. But whereas the rtg2Δ mutation allows growth on USA, the presence of glutamate does not promote growth of a wild type on USA and in fact blocks the growth of a [URE3] or ure2 strain. Thus, the effect on NCR is a novel activity of Rtg2p. Although the overall effect of Rtg2p is to facilitate the nuclear entry of Rtg1p and Rtg3p, its effect on Gln3p is apparently the opposite. It is the absence of Rtg2p that results in Gln3p entry.
Shamji et al. (42) showed by microarray methods that Mks1p is necessary for the induction by rapamycin of genes controlled by the RTG system, such as CIT2 and DLD3. In contrast, our data show that rtg2Δ strains are derepressed for genes previously shown to be regulated by Mks1p. Together, these results indicate that Rtg2p and Mks1p act together to regulate a wider array of genes. Rtg1p and Rtg3p are not involved in NCR, whereas Ure2p and Gln3p are apparently not involved in the retrograde signaling pathway. Thus, these are downstream factors that carry out the specific instructions of Mks1p and Rtg2p. However, it remains unclear how Rtg2p-Mks1p transmits the nitrogen supply signal to Ure2p-Gln3p and the mitochondrial functional status signal to Rtg1p-Rtg3p.
It is not entirely surprising that the NCR and retrograde signal transduction pathways are connected in this way. The RTG pathway seems to be designed to maintain glutamate levels in the absence of mitochondrial function, and glutamate is a potent repressor of the RTG pathway activity. Glutamate is also a primary intermediate in the nitrogen catabolism pathways and a good repressor of catabolism of many poor nitrogen sources. Aigle (24) found that although [URE3] or ure2 strains would arise on USA media containing either ammonia or glutamate as nitrogen source, they arose far more rarely on media containing both. This glutamate effect is the opposite of that expected from glutamate inhibition of the RTG system, which should mimic an rtg2 mutation and cause the USA+ phenotype. Thus, glutamate has distinct actions on the two systems, but both involve Rtg2p and Mks1p in a more complex mechanism that remains to be resolved.
Table 1.
Strains of Saccharomyces cerevisiae
| Strain | Genotype | Source |
|---|---|---|
| YHE861 | MATa ura2 his3 | H. K. Edskes |
| YHE867 | MATα ura2 trp1 | H. K. Edskes |
| ATCC no. | MATa ura3Δ his3Δ leu3Δ | ATCC |
| 4004619 | met15Δ rtg2∷G418 | |
| ATCC no. | MATa ura3Δ his3Δ leu2Δ met15Δ | ATCC |
| 4000173 | gln3∷G418 | |
| MP27 | MATα ura2 leu2 met15 rtg2∷G418 | This study |
| MP28 | MATα ura2 leu2 met15 rtg2∷G418 | This study |
| MP29 | MATα ura2 leu2 met15 [URE3] | This study |
| MP46 | MATa ura2 leu2 met15 rtg2∷G418 | This study |
| MP51 | MATa/MATα ura2/ura2 his3/++/trp1 | YHE861 × YHE867 |
| MP52 | MATa/MATα ura2/ura2 his3/++/trp1 rtg2∷G418 | MP51 |
| 1735 | MATα his-ura2 [URE3] | |
| 4146 | MATα ura2 leu2 mks1∷G418 | |
| 4149 | MATa ura2 leu2 trp1 | |
| 4184 | MATα kar1 ura2 arg1 [URE3] | |
| 4188 | MATa ura2 leu2 trp1 [URE3] | |
| 4239 | MATα ura2 leu2 met15 rtg2∷G418 | |
| 4241 | MATα ura2 met15 rtg2∷G418 | |
| 2-7A | MATα ura2 leu2 trp1 | This study |
| 2-7C | MATa ura2 leu2 his3 trp1 rtg2∷G418 | This study |
| 2-17B | MATα ura2 leu2 trp1 rtg2∷G418 | This study |
| 4-9B | MATα ura2 leu2 met15 rtg2∷G418 | 4000173 × 2-17B |
| 4-10C | MATα ura2 leu2 trp1 gln3∷G418 | 4000173 × 2-17B |
| 4-5A | MATα ura2 leu2 his3 gln3∷G418 rtg2∷G418 | 4000173 × 2-17B |
| 4791-6B | MATa met15 ura2 leu2 rtg2∷G418 | 4004619 × 3920 |
| 4833-3B | MATa kar1 ura2 arg1 [URE3] | |
| 4852-1C | MATa ura2 rtg2∷G418 | 4241 × 4146 |
| 4852-1D | MATa ura2 mks1∷G418 | 4241 × 4146 |
| 4852-1A | MATα ura2 leu2 met15 mks1∷G418 rtg2∷G418 | 4241 × 4146 |
Acknowledgments
We thank Herman Edskes (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health) and Daniel Masison for their thoughtful reading of the manuscript.
Abbreviations
- NCR
nitrogen catabolite repression
- USA
ureidosuccinate
- GFP
green fluorescent protein
- YPAD
yeast extract peptone adenine dextrose
- USA+
ureidosuccinate uptake (a phenotype)
References
- 1.Cooper T G. In: The Molecular Biology of the Yeast Saccharomyces: Metabolism and Gene Expression. Strathern J N, Jones E W, Broach J R, editors. Vol. 2. Plainview, NY: Cold Spring Harbor Lab.; 1982. pp. 39–99. [Google Scholar]
- 2.Magasanik B. In: The Molecular and Cellular Biology of the Yeast Saccharomyces. Jones E W, Pringle J R, Broach J R, editors. Vol. 2. Plainview, NY: Cold Spring Harbor Lab.; 1992. pp. 283–317. [Google Scholar]
- 3.Mitchell A P, Magasanik B. Mol Cell Biol. 1984;4:2758–2766. doi: 10.1128/mcb.4.12.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coffman J A, Rai R, Cunningham T, Svetlov V, Cooper T G. Mol Cell Biol. 1996;16:847–858. doi: 10.1128/mcb.16.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coschigano P W, Magasanik B. Mol Cell Biol. 1991;11:822–832. doi: 10.1128/mcb.11.2.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cardenas M E, Cutler N S, Lorenz M C, Di Como C J, Heitman J. Genes Dev. 1999;13:3271–3279. doi: 10.1101/gad.13.24.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardwick J S, Kuruvilla F G, Tong J K, Shamji A F, Schreiber S L. Proc Natl Acad Sci USA. 1999;96:14866–14870. doi: 10.1073/pnas.96.26.14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck T, Hall M N. Nature (London) 1999;402:689–692. doi: 10.1038/45287. [DOI] [PubMed] [Google Scholar]
- 9.Rai R, Genbauffe F, Lea H Z, Cooper T G. J Bacteriol. 1987;169:3521–3524. doi: 10.1128/jb.169.8.3521-3524.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper T G. In: Mycota III. Marzluf G, Bambri R, editors. Berlin: Springer; 1996. pp. 139–169. [Google Scholar]
- 11.Turoscy V, Cooper T G. J Bacteriol. 1987;169:2598–2600. doi: 10.1128/jb.169.6.2598-2600.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuura A, Anraku Y. Mol Gen Genet. 1993;238:6–16. doi: 10.1007/BF00279524. [DOI] [PubMed] [Google Scholar]
- 13.Edskes H K, Hanover J A, Wickner R B. Genetics. 1999;153:585–594. doi: 10.1093/genetics/153.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertram P G, Choi J H, Carvalho J, Ai W, Zeng C, Chan T F, Zheng X F. J Biol Chem. 2000;275:35727–35733. doi: 10.1074/jbc.M004235200. [DOI] [PubMed] [Google Scholar]
- 15.Lacroute F. J Bacteriol. 1971;106:519–522. doi: 10.1128/jb.106.2.519-522.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wickner R B. Science. 1994;264:566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 17.Edskes H K, Gray V T, Wickner R B. Proc Natl Acad Sci USA. 1999;96:1498–1503. doi: 10.1073/pnas.96.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor K L, Cheng N, Williams R W, Steven A C, Wickner R B. Science. 1999;283:1339–1343. doi: 10.1126/science.283.5406.1339. [DOI] [PubMed] [Google Scholar]
- 19.Speransky V, Taylor K L, Edskes H K, Wickner R B, Steven A. J Cell Biol. 2001;153:1327–1335. doi: 10.1083/jcb.153.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wickner R B, Taylor K L, Edskes H K, Maddelein M-L, Moriyama H, Roberts B T. J Struct Biol. 2000;130:310–322. doi: 10.1006/jsbi.2000.4250. [DOI] [PubMed] [Google Scholar]
- 21.Masison D C, Wickner R B. Science. 1995;270:93–95. doi: 10.1126/science.270.5233.93. [DOI] [PubMed] [Google Scholar]
- 22.Maddelein M-L, Wickner R B. Mol Cell Biol. 1999;19:4516–4524. doi: 10.1128/mcb.19.6.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moriyama H, Edskes H K, Wickner R B. Mol Cell Biol. 2000;20:8916–8922. doi: 10.1128/mcb.20.23.8916-8922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aigle M. Contribution à l'Étude de Hérédité Non-chromosomique de Saccharomyces cerevisiae: Facteur [URE3] et Plasmides Hybrides. Strasbourg, France: L'Universite Louis Pasteur de Strasbourg; 1979. [Google Scholar]
- 25.Jung G, Masison D C. Curr Microbiol. 2001;43:7–10. doi: 10.1007/s002840010251. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira P C, Ness F, Edwards S R, Cox B S, Tuite M F. Mol Microbiol. 2001;40:1357–1369. doi: 10.1046/j.1365-2958.2001.02478.x. [DOI] [PubMed] [Google Scholar]
- 27.Edskes H K, Wickner R B. Proc Natl Acad Sci USA. 2000;97:6625–6629. doi: 10.1073/pnas.120168697. . (First Published May 23, 2000; 10.1073/pnas.120168697) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao X, Butow R A. Cell. 1993;72:61–71. doi: 10.1016/0092-8674(93)90050-z. [DOI] [PubMed] [Google Scholar]
- 29.Sekito T, Thornton J, Butow R A. Mol Biol Cell. 2000;11:2103–2115. doi: 10.1091/mbc.11.6.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, Butow R A. Mol Biol Cell. 1999;19:6720–6728. doi: 10.1128/mcb.19.10.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chelstowska A, Liu Z, Jia Y, Amberg D, Butow R A. Yeast. 1999;15:1377–1391. doi: 10.1002/(SICI)1097-0061(19990930)15:13<1377::AID-YEA473>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 32.Jia Y, Rothermel B, Thornton J, Butow R A. Mol Biol Cell. 1997;17:1110–1117. doi: 10.1128/mcb.17.3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komeili A, Wedaman K P, O'Shea E K, Powers T. J Cell Biol. 2000;151:863–878. doi: 10.1083/jcb.151.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koonin E V. Trends Biochem Sci. 1994;19:156–157. doi: 10.1016/0968-0004(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 35.Sherman F. In: Guide to Yeast Genetics and Molecular Biology. Guthrie C, Fink G R, editors. Vol. 194. San Diego: Academic; 1991. pp. 3–21. [Google Scholar]
- 36.Sikorski R S, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burns N, Grimwade B, Ross-Macdonald P B, Choi E Y, Finberg K, Roeder G S, Snyder M. Genes Dev. 1994;8:1087–1105. doi: 10.1101/gad.8.9.1087. [DOI] [PubMed] [Google Scholar]
- 38.Winzeler E A, Shoemaker D D, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke J D, Bussey H, et al. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- 39.Ridley S P, Sommer S S, Wickner R B. Mol Cell Biol. 1984;4:761–770. doi: 10.1128/mcb.4.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rai R, Genbauffe F S, Sumrada R A, Cooper T G. Mol Cell Biol. 1989;9:602–608. doi: 10.1128/mcb.9.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guarente L. In: Recombinant DNA, Part C. Wu R, Grossman L, Moldave K, editors. Vol. 101. New York: Academic; 1983. pp. 181–191. [Google Scholar]
- 42.Shamji A F, Kuruvilla F G, Schreiber S L. Curr Biol. 2000;10:1574–1581. doi: 10.1016/s0960-9822(00)00866-6. [DOI] [PubMed] [Google Scholar]
- 43.Cooper T G, Ferguson D, Rai R, Bysani N. J Bacteriol. 1990;172:1014–1018. doi: 10.1128/jb.172.2.1014-1018.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]