Abstract
In this study, we clarified the functions of three uncharacterized enzymes, XCV2724, XCV2728, and XCV2729, in Xanthomonas euvesicatoria, the causal agent of bacterial spot of tomato and pepper. The genes corresponding to the three enzymes are homologs of hypBA1, hypBA2, and hypAA from Bifidobacterium longum and are unique to Xanthomonas spp. among plant pathogenic bacteria. Functional characterization of the recombinant enzymes expressed using microbial systems revealed that they degrade the arabinofurano-oligosaccharides present on hydroxyproline (Hyp)-rich glycoproteins (HRGPs) such as extensin and solanaceous lectins in plant cell walls. These enzymes work coordinately to degrade the oligosaccharides. First, XeHypAA (XCV2728), belonging to the glycoside hydrolase (GH) 43 family, releases L-arabinose from L-arabinofuranose (Araf)-α1,3-Araf-ß1,2-Araf-ß1,2-Araf-ß-Hyp (Ara4-Hyp), cleaving its α1,3 bond; second, XeHypBA2 (XCV2729), belonging to the GH121 family, releases the disaccharide Araf-ß1,2-Araf from Araf-ß1,2-Araf-ß1,2-Araf-ß-Hyp (Ara3-Hyp); finally, XeHypBA1 (XCV2724), belonging to GH family 127, releases L-arabinose from Araf-ß-Hyp (Ara-Hyp). In summary, the main oligosaccharide structure of Ara4-Hyp on the HRGPs is degraded to Ara3-Hyp, then to Ara-Hyp, and finally to Ara monosaccharides by the action of these three enzymes. HRGPs containing oligosaccharide substrates have been reported to contribute to plant defense, and interestingly, the promoter region of the operon (xehypBA2 and xehypAA) contains the plant-inducible promoter box for binding the regulator protein HrpX involved in pathogenicity. We then analyzed the expression level of the operon gene in hrp-inducing medium and in plants and constructed gene-deletion mutants. However, although the operon was evidently upregulated by HrpX, three single-gene deletion mutants (ΔxehypBA1, ΔxehypBA2, ΔxehypAA) and even a triple-gene deletion mutant (ΔxehypBA1-BA2-AA) remained pathogenic, and had no effect on nonhost resistance, either, indicating that these three enzymes are not involved in either pathogenicity or nonhost resistance reactions. This is the first report of enzymes in plant pathogenic bacteria that catalyze the degradation of Hyp-linked-L-arabinofuranosides in plant cell walls.
Introduction
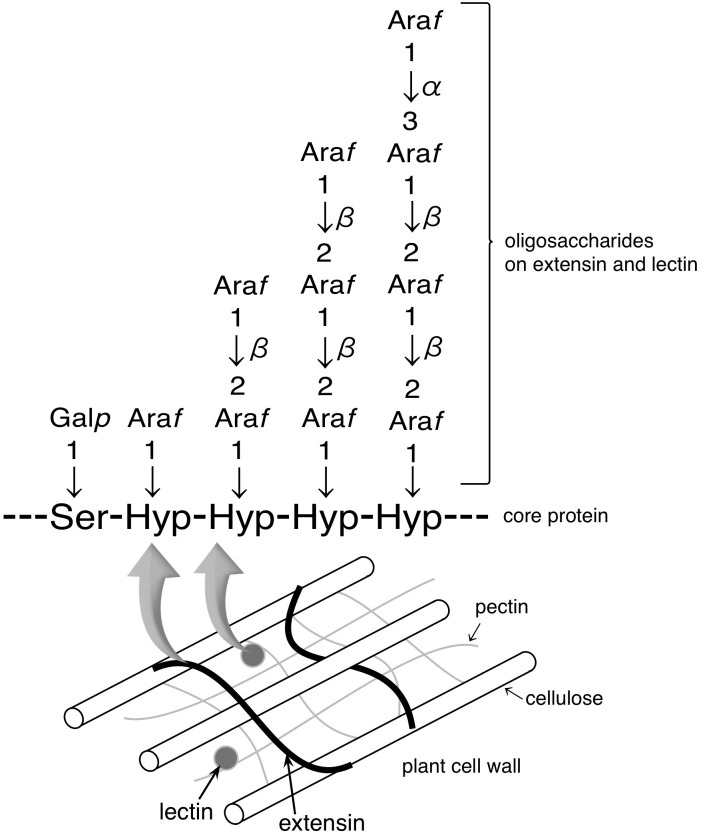
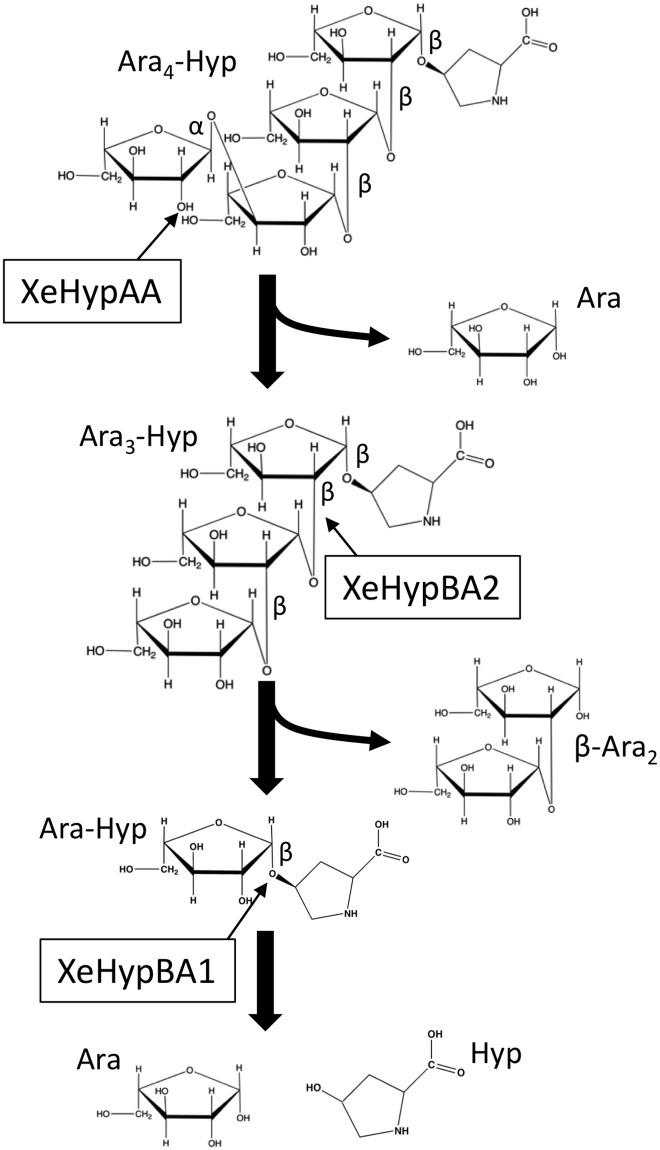
Three novel glycoside hydrolases GH 127 ß-l-arabinofuranosidase (HypBA1) [1], GH 121 ß-l-arabinobiosidase (HypBA2) [2], and GH43 α-l-arabinofuranosidase (HypAA) [unpublished data] in the gut bacterium Bifidobacterium longum were recently reported. The three enzymes degrade arabinofurano-oligosaccharides on hydroxyproline-rich glycoproteins (HRGPs) such as extensin and solanaceous lectins, thus providing the bacterium with L-arabinose as a carbon source from HRGPs that reach the intestine [1, 2]. HRGPs are found in plant cell walls, and their synthesis can be induced during plant defense against a pathogen [3–5]. Inter- and intramolecular cross-linking of extensin forms a highly linked network as a major structural component of plant cell walls and a barrier to pathogen ingress [6–9]. Lectins, which recognize and bind specific carbohydrates, can also function in plant defense signaling and responses to pathogens [10–13]. Extensin and solanaceous lectins contain repetitive serine (Ser)-hydroxyproline (Hyp)4 motifs with Hyp residues that are O-glycosylated with 1–4 arabinofuranosyl (Araf) residues with ß-l-Araf linkages (Fig 1). The structures of Ara3-Hyp and Ara4-Hyp, which are the major constituents of Hyp-linked ß-l-arabinofuranosides in dicotyledons [14–16], are Araf-ß1,2-Araf-ß1,2-Araf-ß-Hyp and Araf-α1,3-Araf-ß1,2-Araf-ß1,2-Araf-ß-Hyp, respectively. HypAA from B. longum releases L-arabinose from Ara4-Hyp by cleaving the α1,3 bond [unpublished data]. HypBA2 liberates Araf-ß1,2-Araf (ß-Ara2) from Ara3-Hyp [2]. HypBA1 releases L-arabinose from ß-Ara2, Araf-ß-Hyp (Ara-Hyp), Araf-ß1,2-Araf-ß-Hyp (Ara2-Hyp), and Ara3-Hyp [1].
Fig 1. Structure of arabino-oligosaccharides on extensin and solanaceous lecins in plant cell walls.
Hydoroxyproline (Hyp) residues are O-glycosylated with 1–4 arabinofuranosyl (Araf) residues with ß-l-arabinofuranosyl linkages. These proteins contain repetitive Ser-Hyp4 motifs.
Fujita et al. [1, 2] searched the Pfam database for homologs of the three novel genes encoding HypBA1, HypBA2 and HypAA from B. longum, and interestingly, homologs were found only in the genomes of Xanthomonas spp. among plant pathogenic bacteria. Our interest was piqued because the enzymes from B. longum work on sugar chains of HRGPs that contribute to plant defense [1, 2]. In addition, xcv2729 from X. euvesicatoria, corresponding to the homolog of hypBA2, is expressed inductively by HrpG and HrpX [17], two upstream regulators of hrp genes encoding the type III secretion system (a membrane-embedded nanomachine) that is essential for pathogenicity in Xanthomonas spp. [18, 19]. Therefore, to investigate whether these enzymes from Xanthomonas spp. have the same functions as those of B. longum and are involved in pathogenicity in this study, we cloned the homologous genes and characterized the recombinant enzymes from X. euvesicatoria (formerly X. campestris pv. vesicatoria), which causes bacterial leaf spot [20, 21]. This bacterium infects solanaceous plants, such as tomato and pepper, that contain both extensin and solanaceous lectins. This is the first report of enzymes that catalyze the degradation of Hyp-linked-L-arabinofuranosides in plant pathogenic bacteria. We also discuss why X. euvesicatoria may have these three unique enzymes.
Materials and methods
Bacterial strains, growth conditions and plasmids
The bacterial strains and plasmids used in this study are listed in Table 1. X. euvesicatoria UPB139 corresponds to strain 85–10 in the KEGG database (T00288) [20–22] and was grown at 28°C using complex nutrient-yeast-glycerol medium (NYG) [23] or hrp-inducing medium (XVM2) that provides an environment similar to the plant extracellular space [24]. Escherichia coli strains were grown at 37°C in Luria-Bertani broth (LB) [25] for all routine purposes. Brevibacillus choshinensis was grown at 30°C using TM medium [26]. Antibiotics were added to media at the following final concentrations: 100 μg/mL ampicillin, 25 μg/mL kanamycin, and 50 μg/mL neomycin.
Table 1. Bacterial strains and plasmids used in study.
| Strains/plasmids | Relevant characteristics | Reference/source |
|---|---|---|
| Xanthomonas euvesicatoria | ||
| UPB139 | Wild type isolated from tomato | [22] |
| ΔhrpX | hrpX deletion mutant of UPB139 | This study |
| ΔxehypBA1 | xehypBA1 deletion mutant of UPB139 | This study |
| ΔxehypBA2 | xehypBA2 deletion mutant of UPB139 | This study |
| ΔxehypAA | xehypAA deletion mutant of UPB139 | This study |
| ΔxehypBA2-AA | xehypBA2 and xehypAA deletion mutant of UPB139 | This study |
| ΔxehypBA1-BA2-AA | xehypBA1, xehypBA2 and xehypAA deletion mutant of UPB139 | This study |
| Escherichia coli | ||
| XL1-Blue | hsdR17, supE44, recA1, endA1, gyrA46, thi, relA1, lac/F’ {proAB+, lac Iq, lacZΔM15:: Tn10(tetr)} | Agilent |
| JM109 | ecA1, endA1, gyrA96, thi, hsdR17(rK- mK+),e14- (mcrA-), supE44, relA1, Δ (lac-proAB)/F’{traD36, proAB+, lacIq, lacZΔM15} | Takara Bio |
| BL21 (λDE3) | F-, ompT, hsdSB(rB- mB-), gal(λcI 857, ind1, Sam7, nin5, lacUV5-T7gene1), dcm(DE3) | Merck |
| S17-1 (λpir) | hsdR, recA, pro, RP4-2 (Tc::Mu; Km::Tn7) (λ pir) | Biomedal |
| Brevibacillus choshinensis | ||
| SP3 | imp, em-, spoIIAC | Takara Bio |
| Plasmids | ||
| pET-23b (+) | Expression vector, Apr, His-tag | Merck |
| pBIC3 | Expression vector, P22 promoter, P22 signal peptide, Nmr, His-tag | Takara Bio |
| pCold TF | Expression vector, trigger factor, Apr, His-tag | Takara Bio |
| pK18mobSacB | Mobilizable cloning vector; pUC-oriV, mob, sacB, Kmr | [27] |
| pET23-xcv2724 | Expression vector for XeHypBA1 in pET-23b (+) | This study |
| pBIC3-xcv2728 | Expression vector for XeHypAA in pBIC3, Nmr | This study |
| pCold-xcv2729 | Expression vector for XeHypBA2 in pCold TF, Apr | This study |
| pK18-hrpX | Deletion vector for hrpX in pK18mobSacB, Kmr | This study |
| pK18-xcv2724 | Deletion vector for xehypBA1 in pK18mobSacB, Kmr | This study |
| pK18-xcv2728 | Deletion vector for xehypAA in pK18mobSacB, Kmr | This study |
| pK18-xcv2729 | Deletion vector for xehypBA2 in pK18mobSacB, Kmr | This study |
| pK18-xcv2728-2729 | Deletion vector for xehypAA and xehypAA in pK18mobSacB, Kmr | This study |
| pK18-xcv2724-2728-2729 | Deletion vector for xehypBA1, xehypAA and xehypBA2 in pK18mobSacB, Kmr | This study |
Apr, ampicillin resistance; Nmr, neomycin resistance; Kmr, kanamycin resistance
Construction of protein expression vectors
The genomic DNA of X. euvesicatoria UPB139 was extracted using a NucleoSpin Microbial DNA kit (Takara Bio, Otsu, Japan) and used for further PCR amplification. The primers used in this work are all shown in S1 Table. A fragment of xcv2724, encoding amino acids (aa) 44 to 791, was amplified with primer set PEX1/PEX2 to eliminate the N-terminal signal peptide (N-sp). The amplified fragment was cloned into pET-23b digested with NdeI and XhoI using an In-Fusion HD Cloning kit (Takara Bio), yielding pET23-xcv2724. A fragment of xcv2728, encoding aa 25 to 528, was amplified with primer set PEX5/PEX6 without N-sp. The amplified fragment was cloned into the linearized pBIC3 using the Brevibacillus in vivo cloning (BIC) method [28, 29]; briefly, a mixture of the PCR product and the linearized plasmid is directly transferred into B. choshinensis competent cells in which the insert and the plasmid are spontaneously combined via homologous recombination. Thus, the expression vector of xcv2728 was constructed without using E. coli, resulting in pBIC3-xcv2728. A fragment of xcv2729, encoding aa 43 to 1452, was amplified with primer set EXP3/EXP4 without N-sp and cloned into pCold TF DNA digested with NdeI and XhoI using an In-Fusion HD Cloning kit (Takara Bio), yielding pCold-xcv2729. The cloned inserts were sequenced with an ABI 3100 DNA sequencer using a BigDye Terminator 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA).
Expression and purification of recombinant protein
For the expression of XeHypBA1, vector pET23-xcv2724 was transferred into E. coli BL21 (λDE3) cells, then a single colony was added to 50 mL LB containing 50 mg/mL kanamycin and grown to an OD600 of 0.6 at 37°C. The culture was then induced with 1 mM isopropyl ß-d-1-thiogalactopyranoside (IPTG) for 18 h at 15°C. For the expression of XeHypBA2, vector pCold-xcv2729 was transferred into E. coli BL21 (λDE3) cells, and the protein fused with the trigger factor chaperone was expressed as described above. For the expression of XeHypAA, vector pBIC3-xcv2728 was transferred into B. choshinensis SP3, and a single colony was grown in 50 mL TM containing 50 mg/mL neomycin at 30°C for 48 h.
Expressed proteins were purified with the MagneHis Protein Purification System (Promega, Madison, WI) or the Capturem His-Tagged Purification kit (Takara Bio) and desalted with the Zeba Spin Desalting Columns 7K MWCO (Thermo Scientific, Rockford, IL, USA). The purified proteins were confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) or Western blotting with Anti-His-tag mAb-HRP-DirecT (Medical & Biological Laboratories, Nagoya, Japan).
Substrate preparation
Extensin was extracted from carrot and Hyp-linked ß-l-arabino-oligosaccharides (ß-Ara2, Ara2-Hyp, Ara3-Hyp, and Ara4-Hyp) were prepared as described previously [2]. To simplify the assay for enzymatic activity, dansylated Hyp-linked ß-L-arabino-oligosaccharides (Ara2-Hyp-DNS, Ara3-Hyp-DNS and Ara4-Hyp-DNS) were prepared as described by Gray [30].
Enzymatic assays
A 10-μl reaction mixture for thin-layer chromatography (TLC) analysis of dansylated substrates contained 50 mM sodium acetate buffer (pH 4.5), 50 μM substrate, and 1 μL of the expressed recombinant enzyme. After 12 h at 30°C, the reaction mixtures were spotted on a Silica Gel 60 aluminum plate (Merck, Darmstadt, Germany) and developed with a 3:1:1 solvent (v/v/v) of 1-butanol/acetic acid/water and finally visualized with UV light. For orcinol-stained TLC analysis, the 100-μL reaction mixture contained 50 mM sodium acetate buffer (pH 4.5), 35 μM substrate, and 2 μL of an expressed enzyme, and the reaction was conducted at 30°C for 12 h. Spotted silica gels were developed with a 2:1:1 solvent (v/v/v) of ethyl acetate/acetic acid/water. Sugars were visualized by spraying an orcinol—sulfate reagent onto the silica gel plate [31]. For high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) analysis, oligosaccharides in the 100-μL reaction mixture were analyzed with a CarboPac PA-1 column. The column was eluted at a flow rate of 1.0 mL/min with the following gradient: 0–5 min, 100% eluent A (0.1 M NaOH); 5–30 min, 0–100% eluent B (0.5 M sodium acetate and 0.1 M NaOH); and 30–35 min, 100% eluent B.
RNA expression analysis
Total RNA from X. euvesicatoria UPB139 was extracted using the Nucleospin RNA II (MACHERY-NAGEL, Duren, Germany) according to the manufacturer’s instructions, except that the phenol—chloroform—isoamyl alcohol mixture was used only when inoculated plants were macerated in the first step. To confirm the absence of any genomic DNA contamination, extracted RNAs were directly subjected to PCR. DNA-free RNA was then converted to cDNA using the ReverTra Ace (Toyobo, Osaka, Japan) with random hexamers. Quantitative reverse-transcription PCR (qRT-PCR) was conducted in a LightCycler Nano System (Roche Diagnostics, Rotkreuz, Switzerland) with TB Green Premix Ex Taq II (Takara Bio). Cycling conditions were initial denaturation for 2 min at 95°C; 45 cycles of 95°C for 15 s, 55 °C for 15 s, and 72°C for 15 s. Relative levels of gene expression were calculated using the 2-ΔΔCt method [32]. For each amplification run, the calculated threshold cycle for each gene amplification was normalized against that of the reference gene 16S rRNA. Three technical replicates were performed each time. Primers used in this analysis are shown in S1 Table.
Generation of gene-deletion mutants
X. euvesicatoria mutants were constructed via double homologous recombination using the suicide vector pK18mobSacB [27], which harbors the sacB gene as a counterselection marker. All primers used in this experiment are shown in S1 Table. Single-gene deletion mutants of xehypBA1, xehypAA, and xehypBA2 were generated as below. For the xehypBA1 deletion, the two primer pairs, DIS1/DIS2 containing the EcoRI site and DIS3/DIS4 containing the XbaI site were used to amplify the 492-bp upstream region and the 590-bp internal region, respectively. The upstream region was first cloned into the EcoRI site of pK18mobSacB via In-Fusion HD Cloning kit (Takara Bio), while the internal region was cloned into the XbaI site in the same manner, yielding pK18-xcv2724. Similarly, for the xehypAA deletion, primer pairs DIS13/DIS14 and DIS15/DIS16 were used to amplify the 584-bp and 510-bp fragments and cloned into pK18mobSacB, yielding pK18-xcv2728. For the xehypBA2 deletion, primer pairs, DIS7/DIS8 and DIS9/DIS10 were used to amplify the 517-bp and 591-bp fragments and cloned into pK18mobSacB, yielding pK18-xcv2729. For the xehypAA-xehypBA2 double deletion, primer pairs DIS7/DIS8 and DIS15/DIS16, described above were used to construct vector pK18-xcv2728-2729. For the xehypBA1-xehypAA-xehypBA2 triple deletion, the double deletion mutant was used as a recipient and the xehypBA1 gene was deleted in the manner described above. The hrpX-deletion mutant of X. euvesicatoria was created using primer pairs DIS19/DIS20 and DIS21/DIS22 to amplify the 500-bp and 419-bp fragments, which were then cloned into pK18mobSacB, yielding pK18-hrpX.
The deletion vectors were inserted into E. coli S17-1 (λpir) cells and then introduced into X. euvesicatoria by biparental conjugation. Crystal violet (0.3% w/v) was used to select a kanamycin-resistant isolate X. euvesicatoria, discriminating it from a kanamycin-resistant E. coli for the first screening. A marker-exchanged mutant was obtained by double homologous recombination using sucrose selection (SacB) as reported previously [33]. Deletion mutants were confirmed by PCR and sequencing.
Inoculation tests
Micro-Tom plants [34] were grown from seeds in a plant growth chamber at 28°C with 16 h light/8 h dark. Plants 4–5 weeks old were dipped into a suspension of bacteria (OD600 of 0.1) containing 0.02% (v/v) surfactant Silwet L-77 and 10 mM MgCl2. Plants were then covered with plastic bags for 48 h to maintain a moist environment, grown for 10 more days in the chamber, and checked for symptoms. Leaves of other 4–5-week-old plants were infiltrated with a 100-fold dilution of a bacterial suspension grown to an OD600 of 0.1.
For assessing nonhost responses, tobacco plants (Nicotiana tabacum) were grown in a greenhouse up to the 4–5 leaf stage and transferred to a growth chamber at 25°C under a 16-h light/8-dark 3 days before inoculation. Leaves were infiltrated with a bacterial suspension (OD600 of 0.4) in 10 mM MgCl2 using a syringe. Inoculated plants were then grown in the chamber for another 3 days and checked for symptoms.
Results
Expression and purification of XeHypBA1, XeHypBA2, and XeHypAA
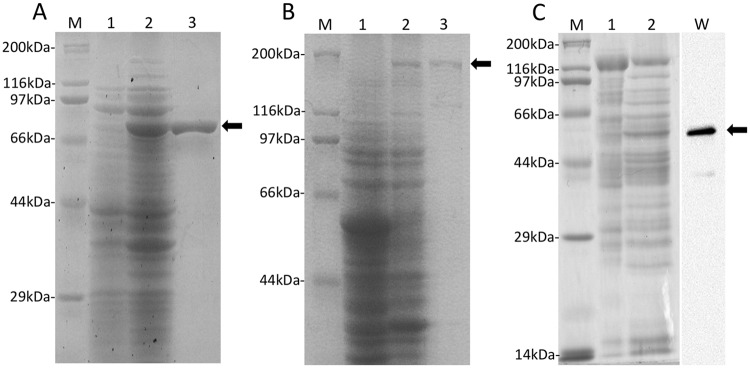
XeHypBA1 consisted of 791 aa. The recombinant protein without the signal peptide (43 aa) was expressed in E. coli BL21 (λDE3) and obtained as a soluble protein. SDS-PAGE showed that the purified recombinant XeHypBA1 protein migrated as a single band with an estimated molecular mass of 82.8 kDa (Fig 2A).
Fig 2. SDS-PAGE analysis of expressed enzymes.
XeHypBA1 (A), XeHypBA2 (B), XeHypAA (C). M, protein molecular weight marker; lane 1, soluble proteins from mock vectors; lane 2, soluble proteins from expression vectors; lane 3, His-tagged proteins; W, western blots for the purified protein. Arrows indicate purified proteins.
XeHypBA2 consisted of 1452 aa. The recombinant protein without the signal peptide (42 aa) was expressed in E. coli BL21 (λDE3) as a fusion protein, in which the protein of interest was fused with the trigger factor chaperone because a soluble protein was not obtained without a trigger that induces protein solubility. SDS-PAGE showed one band with a slightly lower molecular mass than the expected size 200 kDa (XeHypBA2, 152 kDa; and trigger factor, 48 kDa; Fig 2B).
XeHypAA consisted of 528 aa, and the recombinant protein without the signal peptide (24 aa) was expressed in B. choshinensis SP3 as a secretory protein because we could not obtain a soluble protein in E. coli even with the trigger factor. The expression level of XeHypAA in B. choshinensis was low, and the purified protein was not visible in an SDS-PAGE gel stained with Coomassie brilliant blue. Thus, we used Western blotting with His-tag antibodies to confirm expression of the target protein (Fig 2C). Western blotting showed a His-tagged protein with an estimated molecular mass of 55.3 kDa (Fig 2C).
Substrate specificity of XeHypBA1, XeHypBA2, and XeHypAA
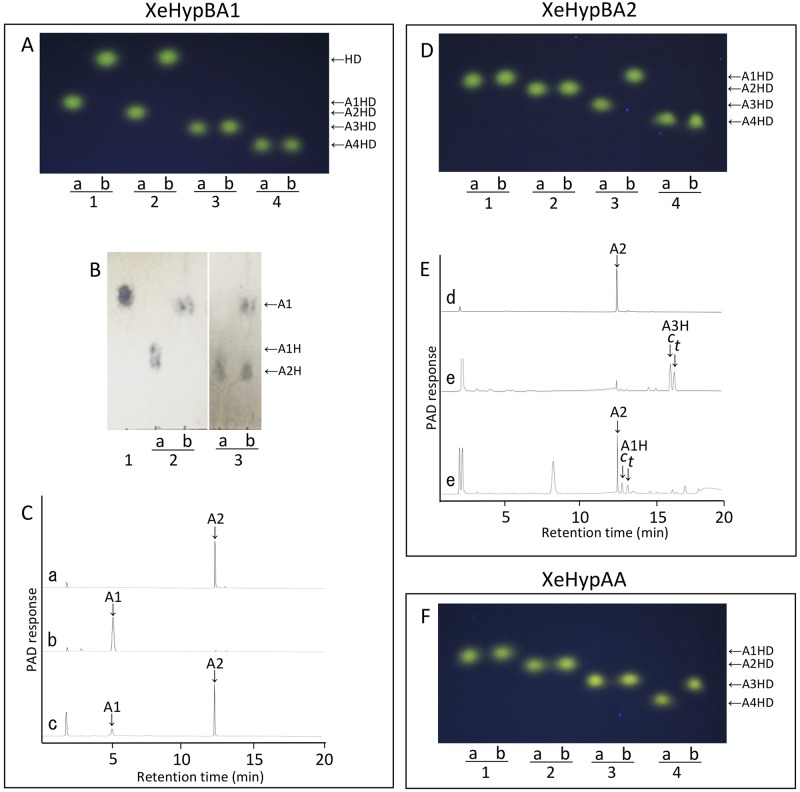
XeHypBA1 preferred Ara-Hyp as a substrate and liberated L-arabinose (Fig 3A and 3B) and was only slightly active on ß-Ara2 (Fig 3C), but did not use Ara4-Hyp and Ara3-Hyp at all. Ara2-Hyp was completely degraded in its dansylated form, but only partially when unmodified (Fig 3A and 3B). These activities differ from those of HypBA1 from B. longum, which degrade ß-Ara2, Ara3-Hyp, Ara2-Hyp, and Ara-Hyp. These results indicate that XeHypBA1 mainly acts on Ara-Hyp.
Fig 3. Substrate specificity of XeHypBA1 (A, B, C), XeHypBA2 (D, E) and XeHypAA (F).
(A, D, F) Dansylated (DNS) cis-substrates were incubated either without (lane a) or with (lane b) XeHypBA1, XeHypBA2, or XeHypAA and the reaction products were analyzed by TLC. Ara-Hyp-DNS (lane 1), Ara2-Hyp-DNS (lane 2), Ara3-Hyp-DNS (lane 3), and Ara4-Hyp-DNS (lane 4) were used as substrates. (B) TLC analysis of XeHypBA1 reaction products. l -arabinose standard (lane 1). Ara-Hyp (lane 2) and Ara2-Hyp (lane 3) were incubated either without (lane a) or with (lane b) XeHypBA1. (C, E) HPAEC-PAD analysis of XeHypBA1or XeHypBA2 reaction products. β-Ara2 standard (a, d); l-arabinose standard (b); Ara3-Hyp standard (e); β-Ara2 incubated with XeHypBA1 (c); Ara3-Hyp incubated with XeHypBA2 (e). HD, Hyp-DNS; A1HD, Ara-Hyp-DNS; A2HD, Ara2-Hyp-DNS; A3HD, Ara3-Hyp-DNS; A4HD, Ara4-Hyp-DNS. A1, L-arabinose; A1H, Ara-Hyp; A2, β-Ara2; A2H, Ara2-Hyp; A3H, Ara3-Hyp; c, cis-isomer; t, trans-isomer.
XeHypBA2 catalyzed the hydrolysis of Ara3-Hyp (Fig 3D), liberating ß-Ara2 (arabinobiose) (Fig 3E). However, Ara4-Hyp, Ara3-Hyp, and Ara-Hyp were not hydrolyzed. XeHypBA2 is thus a ß-l-arabinobiosidase that has strict substrate specificity for Ara3-Hyp. These activities were also the same as HypBA2 from B. longum.
XeHypAA specifically degraded Ara4-Hyp to Ara3-Hyp and L-arabinose (Fig 3F). The enzyme did not hydrolyze Ara3-Hyp, Ara2-Hyp, and Ara-Hyp, indicating that XeHypAA is an α1,3-specific α-l-arabinofuranosidase, which recognizes the Araf-α1,3-Araf structure of Ara4-Hyp. These activities were exactly the same as found for HypAA from B. longum.
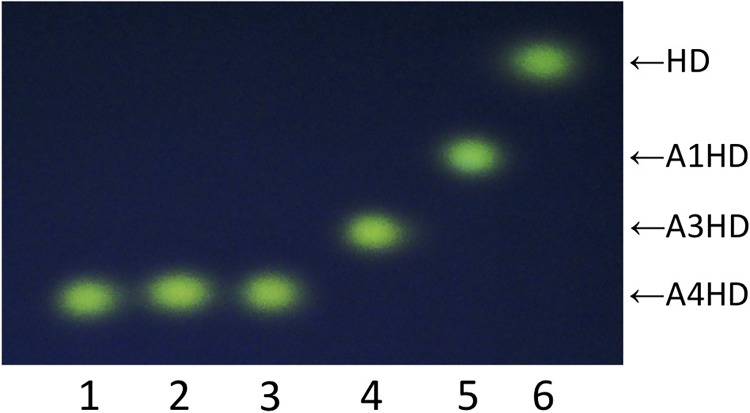
We also examined the synergistic effects of the three enzymes on the degradation of Hyp-linked arabino-oligosaccharides. Ara4-Hyp was completely degraded by combined activity of XeHypAA, XeHypBA2, and XeHypBA1 (Fig 4).
Fig 4. TLC analysis of reactions by activity from various combinations of XeHypBA1, XeHypBA2, and XeHypAA.
Ara4-Hyp-DNS was incubated either without any enzymes (lane 1), or with XeHypBA1 (lane 2), with XeHypBA2 (lane 3), with XeHypAA (lane 4), with XeHypBA2 and XeHypAA (lane 5), or with XeHypBA1, XeHypBA2, and XeHypAA (lane 6). HD, Hyp-DNS; A1HD, Ara-Hyp-DNS; A3HD, Ara3-Hyp-DNS; A4HD, Ara4-Hyp-DNS.
The substrate specificities of each enzyme are summarized in Table 2, and the coordinated degradation of the oligosaccharides is shown in Fig 5.
Table 2. Substrate specificity of the enzymes.
| Substrates | ||||||
|---|---|---|---|---|---|---|
| Enzymes | Classification | Ara4-Hyp | Ara3-Hyp | Ara2-Hyp | Ara-Hyp | ß-Ara2 |
| XeHypAA | α-l-arabinofuronasidase | + | - | - | - | - |
| XeHypBA2 | ß-l-arabinobiosidase | - | + | - | - | - |
| XeHypBA1 | ß-l-arabinofuronasidase | - | - | +/- | + | +/- |
+, well hydrolyzed; +/-, weakly hydrolyzed; -, not hydrolyzed
Fig 5. Schematic flow chart of hydrolysis of Ara4-Hyp by XeHypAA, XeHypBA2, and XeHypBA1.
Thin arrows indicate cleavage sites for the enzymes.
Expression analysis of xehypBA2-AA operon
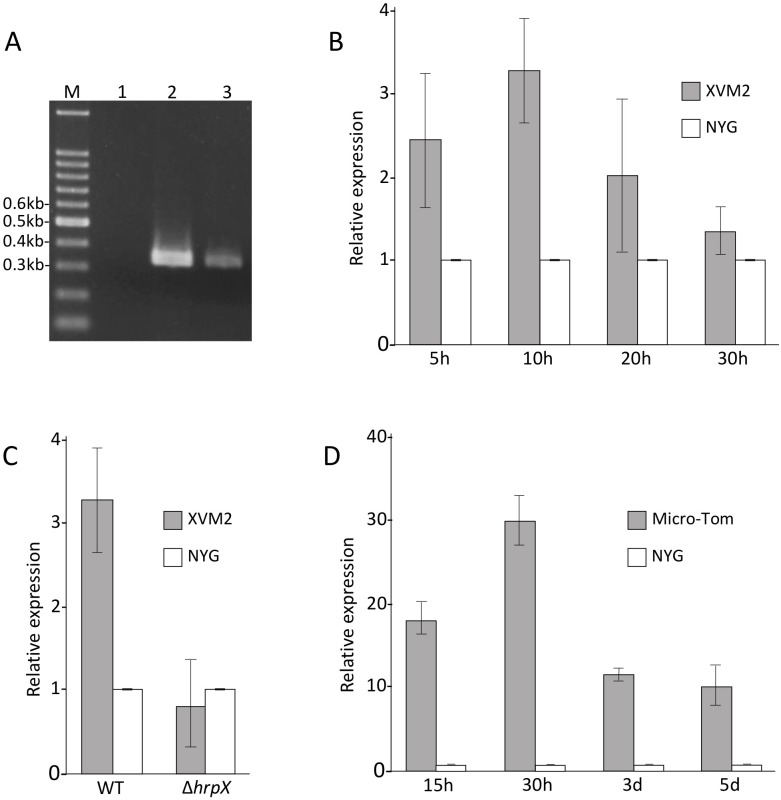
The stop codon of xehypBA2 and the start codon of xehypAA are overlapped, typical of an operon gene. Thus, to confirm whether the two genes form an operon, we conducted RT-PCR using primers EXG1 and EXG2 (S1 Table), designed to amplify a 346-bp fragment containing the junction region of the two genes. Expected fragments were obtained from cDNAs derived from the bacteria in XVM2 (hrp-inducing medium) (Fig 6A), indicating that xehypBA2 and xehypAA are transcribed into a single mRNA. No PCR product was obtained from extracted RNAs, demonstrating the absence of genomic DNA contamination in the RNAs (Fig 6A).
Fig 6. Expression analysis of xehypBA2-AA operon.
(A) RT-PCR was conducted to confirm whether the two genes, xehypBA2 and xehypAA, form an operon. A 346-bp fragment containing the junction region of the two genes was amplified using extracted mRNAs (lane 1), genomic DNAs (lane 2) and cDNAs (lane 3) derived from X. euvesicatoria UPB139 grown in XVM2 (hrp-inducing medium). M, DNA molecular weight marker. (B) Relative expression level of the operon in NYG (complete medium) and XVM2 (hrp-inducing medium) was examined by qRT-PCR. (C) Relative expression level of the operon in the wild type (WT) and a hrpX-deletion mutant (ΔhrpX) in NYG and XVM2. (D) Relative expression level of the operon in NYG and infected Micro-Tom. The expression values relative to the mean expression in NYG were calculated using the 2-ΔΔCt method. Error bars indicate standard deviation (±SD) of three independent experiments.
In the promoter region of the xehypBA2-AA operon, a conserved cis-regulatory element, PIP box with the consensus sequence TTCGCN15-TTCGC [17, 35] was found. Thus, to investigate whether the operon was regulated by HrpX, we analyzed the expression of the operon gene over time using qRT-PCR; expression in XVM2 reached a maximum at 10 h and was significantly higher than in NYG (complete medium) (Fig 6B). Next, we compared the expression level of the wild type with that of the hrpX-deletion mutant in XVM2 after a 10-h incubation. The ΔhrpX mutant was generated as shown in S1 Fig. The expression level of the gene in the wild type was significantly higher than in the ΔhrpX mutant (Fig 6C), indicating that the gene was upregulated by HrpX. We also analyzed the expression of the gene in infected plants (Micro-Tom). The expression was much higher than in NYG at all times tested (15 h–5 days), and was highest at 30 h after inoculation (Fig 6D). Obviously, the expression of the operon gene was induced in infected plants.
Pathogenicity of mutants
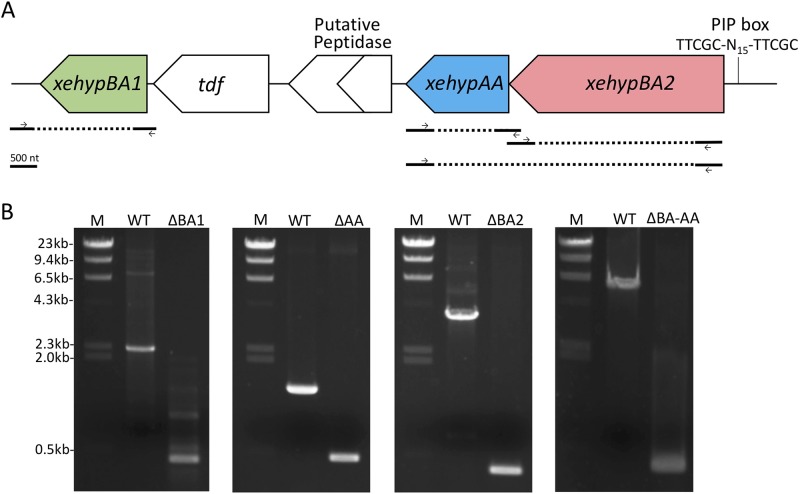
Because the xehypBA2-AA operon was regulated by HrpX, we constructed a gene-deletion mutant of the operon gene, as well as that of xehypBA1 to investigate whether these genes are involved in pathogenicity. Single-gene deletion mutants of each gene (ΔxehypBA1, ΔxehypBA2, ΔxehypAA) and a triple-gene deletion mutant of the three genes (ΔxehypBA1-BA2-AA) were created through biparental mating. The triple-gene deletion mutant was constructed from the ΔxehypBA1 mutant. The obtained mutants were confirmed by the reduced size of the PCR products (Fig 7).
Fig 7. Isolation of gene-deletion mutants.
(A) Genetic map of xehypBA1, xehypBA2, and xehypAA of X. euvesicatoria. Solid lines below the map represent fragments used to construct mutants. Dashed lines indicate the deletion regions. Arrows indicate primers used to confirm the gene deletion. PIP box, plant-inducible promoter box. (B) Confirmation of gene-deletion mutants by PCR. M, DNA molecular weight marker; WT, wild type; ΔBA1, ΔxehypBA1; ΔBA2, ΔxehypBA2; ΔBA-AA, ΔxehypBA1-BA2-AA (constructed from ΔxehypBA1).
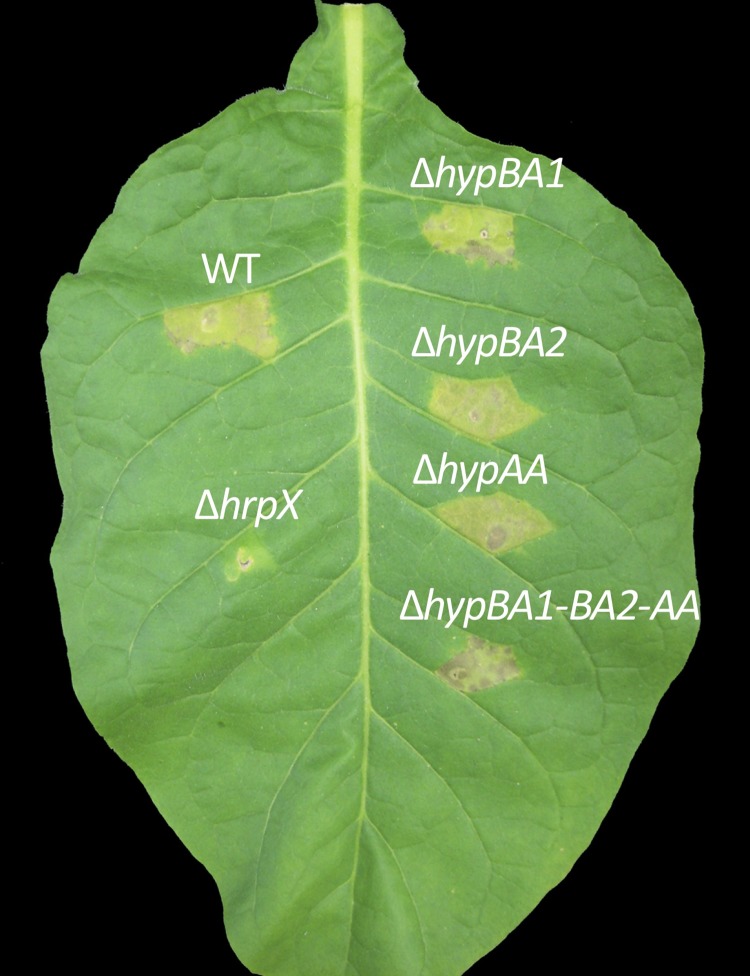
Micro-Tom plants were inoculated with one of the various mutants using the dipping method (see Materials and methods), and plants were examined for symptoms 12 days later. Single-gene deletion mutants, and even the triple-gene deletion mutant, induced the same symptoms on leaves as on the wild type (Fig 8). The double-gene deletion mutant (ΔxehypBA2-AA) also maintained pathogenicity (data not shown). The hrpX-deletion mutant (ΔhrpX) did not cause any symptoms (Fig 8). These results indicate that the three genes are not involved in the pathogenicity of X. euvesicatoria.
Fig 8. Pathogenicity test of gene-deletion mutants.
Micro-Tom plants were dip-inoculated with the wild type (WT) X. euvesicatoria or the respective mutants and examined for symptoms after 12 days.
The influence of the respective gene-deletion mutants on nonhost responses was tested by inoculating N. tabacum plants. All the mutants except for ΔhrpX induced chlorotic reactions in the same manner as the wild type (Fig 9), indicating that the three genes have no effect on nonhost resistance.
Fig 9. Nonhost responses in tobacco (N. tabacum) 96 h after inoculation with gene-deletion mutants.
Tobacco plants at the 4–5-leaf stage were infiltrated via a syringe with the wild-type X. euvesicatoria (WT) or the respective mutants at an OD600 of 0.4 in 10 mM MgCl2.
Discussion
Because the genes in B. longum that encode the enzymes to degrade arabino-oligosaccharides on HRGPs are found only in Xanthomonas spp. among plant pathogenic bacteria [1, 2], we cloned and characterized the homologous genes xehypBA1, xehypBA2, and xehypAA derived from X. euvesicatoria that infects tomato and pepper to better understand the role of these enzymes in pathogenicity of the bacterium.
The gene xehypBA1 is the homolog of hypBA1 from B. longum. HypBA1, assigned to the new GH family 127, is a novel ß-l-arabinofuranosidase that liberates L-arabinose from ß-Ara2, Ara-Hyp, Ara2-Hyp, and Ara3-Hyp [1]. However, XeHypBA1 liberated L-arabinose specifically from Ara-Hyp and slightly from ß-Ara2 and Ara2-Hyp; the enzyme likely works better on Ara2-Hyp-DNS than Ara2-Hyp (unmodified form) because the conformation of Ara2-Hyp-DNS may provide a better fit with the enzyme than that of Ara2-Hyp (Fig 3A, 3B and 3C), but the cause is unclear. HypBA1 from B. longum is an intracellular protein; B. longum takes up ß-Ara2 and degrades the disaccharide in the cells. XeHpyBA1, on the other hand, is an extracellular protein and can work directly on the arabino-oligosaccharides on HRGPs (data not shown). These results indicate that XeHpyBA1 is quite different from HypBA1.
The gene xehypBA2 is the homolog of hypBA2 from B. longum. Because the recombinant protein of XeHypBA2 was not expressed in the pET system as a soluble protein, we used the pCold TF system that expresses a fusion protein with the trigger factor chaperon as a soluble protein. In the SDS-PAGE analysis, the fusion protein migrated at a slightly lower molecular mass than expected (Fig 2B), probably because the fusion protein was not completely unfolded by the SDS even after extensive heating due to the complexity of the protein structure. HypBA2 from B. longum, assigned to the new HG family 121, is a novel ß-l-arabinobiosidase that has strict substrate specificity for Ara3-Hyp [2]. It is an extracellular protein with a membrane-anchoring region (MAR) at its C-terminal region, suggesting that the enzyme may be on the bacterial surface and that it liberates ß-Ara2 from HRGPs adjacent to the cells. XeHypBA2 also liberates ß-Ara2 only from Ara3-Hyp, not from Ara2-Hyp and Ara4-Hyp, so it also is highly specific for the structure of Ara3-Hyp (Fig 3D and 3E). It is also an extracellular protein, but it has no MAR, indicating that the secreted enzyme freely degrades Ara3-Hyp on HRGPs.
The gene xehypAA is the homolog of hypAA from B. longum. The recombinant protein of XeHypAA was not expressed either in the pET system or even in the pCold TF system. We then used the Brevibacillus expression system, which is well-suited for heterologous protein expression. HypAA from B. longum, containing a GH43 domain, is an α1,3-specific α-l-arabinofuranosidase, which recognizes the Araf-α1,3-Araf structure of Ara4-Hyp [unpublished data]. XeHypAA also specifically degraded Ara4-Hyp to Ara3-Hyp and L-arabinose, recognizing the α1,3 bond of arabinose that is only on Ara4-Hyp (Fig 3F). HypAA is a secretory enzyme with MAR at its C-terminal region like HypBA2, but XeHypAA has no MAR. XeHypAA also may freely degrade Ara4-Hyp on HRGPs.
When we examined the synergistic effects among various combinations of the three enzymes on the degradation of Ara4-Hyp (Fig 4), they coordinately degraded Ara4-Hyp (Fig 5). Ara4-Hyp and Ara3-Hyp are the major Hyp-linked l-arabinofuranosides in dicotyledons. In particular, Ara4-Hyp accounts for 33–75% of the total Hyp residues in plant cell walls [16]. Because XeHypBA2 cannot directly hydrolyze Ara4-Hyp, XeHypAA is required for further degradation. Thus, the operon construction of the two genes, xehypBA2 and xehypAA, is quite reasonable.
With regard to the involvement of HRGPs of extensin and lectins in plant defense [3–9], Brown et al. [36] reported that, in the pepper–X. campestris interaction, restriction of bacterial colony development was linked to the formation of an amorphous papillae-like matrix containing HRGPs around bacterial cells. Because the enzymes from X. euvesicatoria can remove the arabinofurano-oligosaccharides on HRGPs, we expected that deglycosylated HRGPs would be unstable and degradable by proteases, leading to a reduction in plant resistance. As a matter of fact, in the promoter region of the operon gene (xehypBA2-AA), there is a PIP box, which binds with the global-regulator HrpX to regulate pathogenicity-related genes. In the qRT-PCR for the operon gene using a hrpX-deletion mutant, the gene was evidently regulated by HrpX (Fig 6C). Koebnik et al. [17] also reported that expression of xcv2729 (xehypBA2) was dependent on HrpG and HrpX. Furthermore, the gene was upregulated in inoculated plants (Fig 6D). In the qRT-PCR of xehypBA1, surprisingly, the gene was also upregulated in infected plants, even though there were no PIP box-like sequences in the promoter region (data not shown). These data strongly suggest that the genes are involved in pathogenicity. Thus, we created in-frame deletion mutants for each enzyme gene. However, in contrast to expectations, the single-gene deletion mutants and even the triple-gene mutant remained pathogenic (Fig 8). We also investigated whether the mutants exert an influence on the resistance reactions of a nonhost plant because of the possibility that the monosaccharides (Ara) or disaccharides (ß-Ara2) freed by the enzymes might act as an elicitor. However, the inoculated tobacco plants (N. tabacum) developed chlorosis (not fast cell death) in the areas surrounded by veins (Fig 9), typical resistance reactions of N. tabacum against X. euvesicatoria [37]. These results indicate that the enzymes are not involved in either pathogenicity or nonhost resistance reactions.
B. longum uses these enzymes to degrade the arabinofurano-oligosaccharides on HRGPs that reach the intestine and thus frees L-arabinose for use as their carbohydrate source [1]. X. euvesicatoria cannot utilize L-arabinose as a carbon source [38], and as described above, the enzymes are not involved in pathogenicity. So why does X. euvesicatoria have these enzymes? Besides their presence in X. euvesicatoria, homologs of hypBA2 and hypAA are conserved in only two species of Bifidobacterium (B. longum and B. pseudocatenulatum), but not in other intestinal bacteria such as Bacteroides, Salmonella, Clostridium, and Escherichia [2]. The homologs are also found in some actinomycetes such as Streptosporangium roseum, Actinosynnema mirum, and Micromonospora aurantiaca [2]. On the other hand, in Xanthomonas spp., homologs are conserved in 12 of 13 species deposited in public databases but not in other plant pathogenic bacteria such as Pseudomonas, Erwinia, Pectobacterium, and Burkholderia. In sum, the genes are distributed among some species in several genera of bacteria, but are well conserved in the species of Xanthomonas. Therefore, these genes might have been transferred horizontally from Xanthomonas to bifidobacteria or actinomycetes. If Xanthomonas spp. had acquired these genes originally, what role did they originally play? Initially, these enzymes may have contributed to pathogenicity, but plants may have overcome it, and in response the bacteria evolved other pathogenicity factors to cause disease. In other words, these enzymes may now be useless for Xanthomonas spp. We also deleted the homologous genes from X. campestris pv. campestris that infects cruciferous plants, and the deletion did not alter its pathogenicity (data not shown), just as we found for X. euvesicatoria. Recently, the full genome sequence of X. phaseoli that infects common bean, Phaseolus vulgaris has become available, and the homolog of xehypBA2 in X. phaseoli was annotated as a pseudogene (XppCFBP6546P_19765) [39]. A putative peptidase gene located adjacent to xehypAA from X. euvesicatoria is predicted to encode a prolyl oligopeptidase to hydrolyze proline-containing peptides (Fig 7A). The enzyme may be able to degrade HRGPs rich in hydroxyproline. However, a frameshift mutation has been caused by a single nucleotide deletion, creating a stop codon in the internal region, indicating that the gene has also become a pseudogene. These facts indicate that the genes encoding enzymes related to degradation of arabino-oligosaccharides on HRGPs may be in the process of disappearing in Xanthomonas spp.
Supporting information
(A) Genetic map of hrpX of Xanthomonas euvesicatoria. Solid lines below the map represent fragments used to construct mutants. Dashed line indicates the deletion region. Arrows indicate primers used to confirm the gene deletion. (B) Confirmation of gene deletion by PCR. M, DNA molecular weight marker; WT, wild type. (C) Confirmation of pathogenicity loss of ΔhrpX mutant 10 d after infiltration of Micro-Tom leaves with wild type (WT) or ΔhrpX mutant (no symptoms).
(TIF)
(DOCX)
Acknowledgments
We thank Dr. Kenichi Tsuchiya (Kyushu University) for providing X. euvesicatoria UPB139. This research was supported by Grant-in-Aids for Scientific Research (No. 23780042 and 25450061) from Japan Society for the Promotion of Science.
Data Availability
All relevant data are within the paper and its Supporting Information files. All sequence data are available form the KEGG database (T00288) and DOI: 10.1128/JB.187.21.7254-7266.2005.
Funding Statement
This research was supported by Grant-in-Aids for Scientific Research (No. 23780042 and 25450061) from Japan Society for the Promotion of Science, on data collection and analysis.
References
- 1.Fujita K, Takashi Y, Obuchi E, Kitahara K, Suganuma T. Characterization of a novel β-L-arabinofuranosidase in Bifidobacterium longum. J Biol Chem. 2014; 289: 5240–5249. 10.1074/jbc.M113.528711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujita K, Sakamoto S, Ono Y, Wakao M, Suda Y, Kitahara K, et al. Molecular cloning and characterization of a β-L-arabinobiosidase in Bifidobacterium longum that belongs to a novel glycoside hydrolase family. J Biol Chem. 2011; 286: 5143–5150. 10.1074/jbc.M110.190512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kieliszewski MJ, Lamport DTA. Extensin: repetitive motifs, functional sites, post- translational codes, and phylogeny. Plant J. 1994; 5:157–172. [DOI] [PubMed] [Google Scholar]
- 4.Showalter A.M. (1993). Structure and function of plant cell wall proteins. Plant Cell. 1993; 5: 9–23. 10.1105/tpc.5.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Damme EJM, Barre A, Rougé P, Peumans WJ. Potato lectin: an updated model of a unique chimeric plant protein. Plant J. 2003; 37: 34–45. [DOI] [PubMed] [Google Scholar]
- 6.Esquerré-Tugayé MT, Lafitte C, Mazau D, Toppan A, Touzé A. (1979) Cell surfaces in plant—microorganism interactions. II. Evidencefor the accumulation of hydroxyproline-rich glycoproteins in the cell wallof diseased plants as a defence mechanism. Plant Physiol. 1979; 64: 320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nejat N, Vadamalai G, Dickinson M. Expression patterns of genes involved in the defense and stress response of Spiroplasma citri infected Madagascar Periwinkle Catharanthus roseus. Int J Mol Sci. 2012; 13: 2301e2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Showalter AM, Bell JN, Cramer CL, Bailey JA, Varner JE, Lamb CJ. Accumulation of hydroxyproline-rich glycoproteinmRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci USA. 1985; 82: 6551–6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei G, Shirsat AH. Extensin over-expression in Arabidopsis limits pathogen invasiveness. Mol Plant Pathol. 2006; 7: 579–592. 10.1111/j.1364-3703.2006.00363.x [DOI] [PubMed] [Google Scholar]
- 10.Coelho LCBB, Silva PMS, Lima VLM, Pontual EV, Paiva PMG, Napoleao TH, et al. Lectins, interconnecting proteins with biotechnological/pharmacological and therapeutic applications. Evid Based Complement Alternat Med. 2017; 2017: 1594074 10.1155/2017/1594074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deepak S, Shailasree S, Sujeeth N, Kini RK, Shetty HS, Mithöfer A. Purification and characterization of proline/hydroxyproline-rich glycoprotein from pearl millet coleoptiles inoculated with downy mildew pathogen Sclerospora graminicola. Phytochemistry. 2007; 68: 298–305. 10.1016/j.phytochem.2006.10.024 [DOI] [PubMed] [Google Scholar]
- 12.Hwang IS, Hwang BK. The pepper mannose-binding lectin gene CaMBL1 is required to regulate cell death and defense responses to microbial pathogens. Plant Physiol. 2011; 155: 447–463. 10.1104/pp.110.164848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peumans WJ, Van Damme EJ. Lectins as plant defense proteins. Plant Physiol. 1995; 109: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akiyama Y, Mori M, Kato K. 13C-NMR analysis of hydroxyproline arabinosides from Nicotiana tabacum. Agric Biol Chem. 1980; 44: 2487–2489. [Google Scholar]
- 15.Ashford D, Desai NN, Allen AK, Neuberger A, O’Neill MA, Selvendran RR. Structural studies of the carbohydrate moieties of lectins from potato (Solanum tuberosum) tubers and thorn-apple (Datura stramonium) seeds. Biochem J. 1982; 201: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamport DTA, Miller DH. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1971; 48: 454–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koebnik R, Krüger A, Thieme F, Urban A, Bonas U. Specific binding of the Xanthomonas campestris pv. vesicatoria AraC-type transcriptional activator HrpX to plant-inducible promoter boxes: J Bacteriol. 2006; 188: 7652–7660. 10.1128/JB.00795-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wengelnik K, Rossier O, Bonas U. Mutations in the regulatory gene hrpG of Xanthomonas campestris pv. vesicatoria result in constitutive expression of all hrp genes. J Bacteriol. 1999; 181: 6828–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wengelnik K, Van den Ackerveken G, Bonas U. HrpG, a key hrp regulatory protein of Xanthomonas campestris pv. vesicatoria is homologous to two-component response regulators. Mol Plant Microbe Interact. 1996; 9: 704–712. [DOI] [PubMed] [Google Scholar]
- 20.Moretti C, Amatulli MT, Buonaurio R. PCR-based assay for the detection of Xanthomonas euvesicatoria causing pepper and tomato bacterial spot. Lett Appl Microbiol. 2009; 49: 466–471. 10.1111/j.1472-765X.2009.02690.x [DOI] [PubMed] [Google Scholar]
- 21.Thieme F, Koebnik R, Bekel T, Berger C, Boch J, Büttner D, et al. Insights into genome plasticity and pathogenicity of the plant pathogenic bacterium Xanthomonas campestris pv. vesicatoria revealed by the complete genome sequence. J Bacteriol. 2005; 187: 7254–7266. 10.1128/JB.187.21.7254-7266.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuchiya K, D’Ursel CCM, Horita M, Nozu Y. Relation of Japanese Xanthomonas campestris pv. vesicatoria with worldwide strains revealed with three specific monoclonal antibodies. J Gen Plant Pathol. 2003; 69: 310–315. [Google Scholar]
- 23.Daniels MJ, Barber CE, Turner PC, Sawczyc MK, Byrde RJ, Fielding AH. Cloning of genes involved in pathogenicityof Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J. 1984; 3: 3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wengelnik K, Marie C, Russel M, Bonas U. Expression and localization of HrpA1, a protein of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J Bacteriol. 1996; 178: 1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JH. Experiments in molecular genetics. New York: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 26.Mizukami M, Tokunaga H, Onishi H, Ueno Y, Hanagata H, Miyazaki N, et al. Highly efficient production of VHH antibody fragments in Brevibacillus choshinensis expression system. Protein Expr Purif. 2015; 105: 23–32. 10.1016/j.pep.2014.09.017 [DOI] [PubMed] [Google Scholar]
- 27.Schäfer A, Tauch A, Jager W, Kalinowski J, Thierbach G, Puhler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994; 145: 69–73. [DOI] [PubMed] [Google Scholar]
- 28.Onishi H, Mizukami M, Hanagata H, Tokunaga M, Arakawa T, Miyauchi A. Efficient production of anti-fluorescein and anti-lysozyme as single-chain antibody fragments (scFv) by Brevibacillus expression system, Protein Expr Purif. 2013; 91: 184–191. 10.1016/j.pep.2013.08.005 [DOI] [PubMed] [Google Scholar]
- 29.Tokunaga M, Mizukami M, Yamasaki K, Tokunaga H, Onishi H, Hanagata H, et al. Secretory production of single-chain antibody (scFv) in Brevibacillus choshinensis using novel fusion partner. Appl Microbiol Biotechnol. 2013; 97: 8569–8580. 10.1007/s00253-013-4695-2 [DOI] [PubMed] [Google Scholar]
- 30.Gray WR. Dansyl chloride procedure In: Colowick SP, Kaplan NO, editors. Methods in enzymology, Vol. 11 New York: Academic Press; 1967. pp. 139–150. [Google Scholar]
- 31.Holmes EW, O’Brien JS. Separation of glycoprotein-derived oligosaccharides by thin-layer chromatography. Anal Biochem. 1979; 93: 167–170. [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods. 2001; 25: 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 33.Kaniga K, Delor I, Cornelis GR. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991; 109: 137–141. [DOI] [PubMed] [Google Scholar]
- 34.Scott JW, Harbaugh BK. Micro-Tom: a miniature dwarf tomato. Florida Agr Expt Sta Circ. 1989; 370: 1–6. [Google Scholar]
- 35.Fenselau S, Bonas U. Sequence and expression analysis of the hrpB pathogenicity operon of Xanthomonas campestris pv. vesicatoria which encodes eight proteins with similarity to components of the Hrp, Ysc, Spa, and Fli secretion systems. Mol Plant Microbe Interact. 1995; 8: 845–854. [DOI] [PubMed] [Google Scholar]
- 36.Brown I, Mansfield J, Bonas U. hrp genes in Xanthomonas campestris pv. vesicatoria determine ability to suppress papilla deposition in pepper mesophyll cells. Mol Plant Microb Interact. 1995; 8: 825–836. [Google Scholar]
- 37.Adlung N, Prochaska H, Thieme S, Banik A, Blüher D, John P, et al. Non-host resistance induced by the Xanthomonas effector XopQ is widespread within the genus Nicotiana and functionally depends on EDS1. Front Plant Sci. 2016; 7: 1796 10.3389/fpls.2016.01796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stoyanova M, Vancheva T, Moncheva P, Bogatzevska N. Differentiation of Xanthomonas spp. causing bacterial spot in Bulgaria based on biolog system. Int J Microbiol. 2014; 2014: 495476 10.1155/2014/495476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruh M, Briand M, Bonneau S, Jacques MA, Chen NWG. Xanthomonas adaptation to common bean is associated with horizontal transfers of genes encoding TAL effectors. BMC Genom. 2017; 18: 670 10.1186/s12864-017-4087-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Genetic map of hrpX of Xanthomonas euvesicatoria. Solid lines below the map represent fragments used to construct mutants. Dashed line indicates the deletion region. Arrows indicate primers used to confirm the gene deletion. (B) Confirmation of gene deletion by PCR. M, DNA molecular weight marker; WT, wild type. (C) Confirmation of pathogenicity loss of ΔhrpX mutant 10 d after infiltration of Micro-Tom leaves with wild type (WT) or ΔhrpX mutant (no symptoms).
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. All sequence data are available form the KEGG database (T00288) and DOI: 10.1128/JB.187.21.7254-7266.2005.