Abstract
Objectives. To assess a community health worker (CHW) program’s impact on childhood illness treatment in rural Liberia.
Methods. We deployed CHWs in half of Rivercess County in August 2015 with the other half constituting a comparison group until July 2016. All CHWs were provided cash incentives, supply chain support, and monthly clinical supervision. We conducted stratified cluster-sample population-based surveys at baseline (March–April 2015) and follow-up (April–June 2016) and performed a difference-in-differences analysis, adjusted by inverse probability of treatment weighting, to assess changes in treatment of fever, diarrhea, and acute respiratory infection by a qualified provider.
Results. We estimated a childhood treatment difference-in-differences of 56.4 percentage points (95% confidence interval [CI] = 36.4, 76.3). At follow-up, CHWs provided 57.6% (95% CI = 42.8, 71.2) of treatment in the intervention group. The difference-in-differences diarrhea oral rehydration therapy was 22.4 percentage points (95% CI = −0.7, 45.5).
Conclusions. Implementation of a CHW program in Rivercess County, Liberia, was associated with large, statistically significant improvements treatment by a qualified provider; however, improvements in correct diarrhea treatment were lower than improvements in coverage. Findings from this study offer support for expansion of Liberia’s new National Community Health Assistant Program.
Liberia ranks among the worst nations globally in child health outcomes, with a mortality rate for those younger than 5 years estimated at 94 per 1000 live births.1 Although Liberia attained the 2012 Millennium Development Goals for child mortality in 2012,2 rural areas continue to suffer the greatest burden of mortality because of poor access and utilization of health care services.3,4 Furthermore, progress in reducing child mortality rates was interrupted by the 2014–2015 Ebola virus disease epidemic. Approximately 40% of deaths among those younger than 5 years in the region are attributable to malaria, diarrhea, and acute respiratory infections (ARIs).5 To reduce childhood mortality, many countries have implemented an integrated community case management (iCCM) strategy, which relies on community health workers (CHWs) to treat uncomplicated cases of childhood illness in the community and refer complicated cases to the nearest health facility.6 After implementing iCCM interventions, mortality reductions have been observed attributable to increased delivery of malaria, ARI, and diarrhea treatments,7–12 as well as bed net distribution.13,14
In response to Liberia’s poor maternal and child health outcomes, Last Mile Health, a nongovernmental organization, partnered with the Liberia Ministry of Health to implement a CHW program, which included an iCCM component, in 2 counties in Liberia. This program built upon Liberia’s existing “general community health volunteer” program, which included iCCM but lacked systematic supervision, supply chain systems, and monetary incentives. This demonstration project informed the development of a national-scale, government-led program called the National Community Health Assistant (CHA) Program, which uses a cadre of workers called CHAs performing similar duties as the CHWs in this study, which was launched by the Ministry of Health in 2016.
A previous evaluation of the program found significant improvements in child health outcomes with an uncontrolled before–after study design in a single implementation area.15 We expanded upon that study to assess the impact of the demonstration program after a controlled implementation in a second county. Our principal aim was to assess whether the program increased treatment of fever, diarrhea, and ARI compared with a control area during the 1-year implementation period.
METHODS
We conducted a 2-phase implementation of a CHW program in Rivercess County, Liberia. Rivercess is the poorest county in Liberia, with 71.3% of its population of about 71 000 falling within the lowest quintile of wealth in the country.1 In 2013, Rivercess had among the lowest treatment rates for children with fever, ARI, and diarrhea, and the highest proportion of women describing distance to health facility as a barrier to accessing health care.1
Implementation was phased for programmatic reasons to enable efficient use of human resources and to optimize the quality of CHW training. In August 2015, CHWs were deployed in 3 of the county’s 6 health districts, hereafter referred to as the “intervention group.” The 3 remaining health districts, hereafter referred to as the “control group,” were scheduled for implementation in October 2015 but were instead enveloped into the implementation of the National CHA Program, launched in July 2016.
Community Health Worker Program
The CHWs were recruited from the communities in which they live; only remote communities (those greater than 5 kilometers from the nearest health facility) were targeted (see Appendix A, available as a supplement to the online version of this article at http://www.ajph.org, for full details of recruitment and training). In brief, the training program contained 4 modules: community health and surveillance, child health, maternal and neonatal health, and adult health. The child health component included iCCM protocols for community treatment and management of diarrhea, ARI, and malaria, along with referral protocols for patients with clinical danger signs. These protocols were adapted from World Health Organization guidelines by clinical staff. The CHWs provided iCCM services both through active case finding of ill children and self-referral by parents. The CHWs were provided a monthly cash incentive of US $70 for approximately 20 hours of work per week. Community health worker peer supervisors conducted weekly supervision visits and clinically trained nurse supervisors conducted monthly supervision visits, both in the CHW’s home community. At the time of our follow-up survey, there were 229 CHWs, 21 peer supervisors, and 11 nurse supervisors working. Each CHW served approximately 161 people.
Data Collection and Sampling
We collected data through 2 stratified cluster-sample population-based surveys conducted in March through April 2015 (baseline) and April through June 2016 (follow-up). We populated the survey with validated questions from the 2013 Liberia Demographic and Health Survey (see Appendix B for questionnaire). We translated the questionnaire to Liberian Vernacular English and back-translated it to American English to optimize accuracy. We trained bilingual enumerators to administer the survey in Liberian Vernacular English and Bassa, the local dialect, which lacks a written form. We collected data via Android mobile phones by using a modified version of the Open Data Kit application.16 Seven enumerators were recruited and they completed a 5-day training on questionnaire administration, the Open Data Kit application, and human participant research. We conducted data quality control by using direct observation of survey administration by supervisors and the validation relaxation technique with a purposive selection of 11 survey questions, as described previously.17
We selected survey households by using a 2-stage cluster-sample stratified by intervention and comparison areas, with communities drawn at random within each, to ensure even distribution across intervention and comparison areas.18 Communities were the primary sampling units and were selected via probability-proportional-to-size sampling. Communities were eligible for selection if they were located 5 or more kilometers from the nearest health facility. Households within the selected communities were selected to participate via a modified random walk approach.19 Within each selected household, we invited all women aged 18 to 49 years to complete the survey to collect data about their health and the health of all living and deceased children. Households and respondents who did not participate or were not available were not replaced, though nonresponse was rare (see Results). We estimated sample sizes to detect a before-to-after change of 10% in all maternal and child health outcomes for each phase of implementation and to produce precise (± < 5%) estimates of outcomes at baseline. During program implementation, we discovered that our baseline survey sampling frame was lacking households and, in some cases, entire communities. We corrected the sampling frame before the 2016 survey and used inverse probability of sampling (IPT) weighting to mitigate potential selection bias introduced by the sampling frame in 2015 (see “Data Analysis” section for full details).
Data Analysis
Our primary outcome of interest was the proportion of children with an iCCM illness, defined as diarrhea, fever, or ARI, who received treatment from a qualified health care provider. We defined a qualified provider as a hospital- or clinic-based provider, a CHW, or a general community health volunteer. We defined fever and diarrhea by maternal self-report of symptoms within the past 2 weeks.20 We defined ARI as maternal report of cough plus fast or difficult breathing in the past 2 weeks.20 In our primary analysis, we reported treatment among children with any of the 3 conditions; in secondary analyses, we reported disease-specific treatment. To assess the quality of care provided, we also reported the proportion of children with diarrhea who received oral rehydration therapy (ORT). To evaluate availability of CHW services on outcomes, we reported the proportion of care received from CHWs and the proportion of respondents who cited distance from provider as a barrier to seeking care.
Our primary exposure of interest was household residence in an implementation versus control area. We controlled for the following covariates: household wealth quintile (calculated via principal component analysis from an asset index via the Filmer–Pritchett method21), quintile of distance to nearest health facility, whether the community is accessible by motorbike, mother’s formal education (any or none), child’s age quintile, mother’s age, mother’s primary language (English or Bassa), number of illnesses per child in past 2 weeks (1 vs ≥ 2), number of children younger than 5 years in household (1 vs ≥ 2), and whether the primary economic activity of the community is agriculture or mining.
We employed a difference-in-differences analysis to compare changes in indicators in the intervention group compared with the control group between the 2 surveys. We used an intent-to-treat approach to estimate the difference in before-to-after differences for all outcomes. To account for potential compositional changes between surveys and the incomplete sampling frame at baseline, we used IPT weighting to adjust our primary analysis.22 We weighted the IPT weighting models to balance covariate levels of the full sample across all 4 intervention-by-time groups. Models incorporated the covariates listed previously, which have been found to be determinants of child health care seeking.3,15,23 (We provide fuller details and balance diagnostics in Appendix C, available as a supplement to the online version of this article at http://www.ajph.org.) We then fit linear regression models, applying the IPT weights, and estimated difference-in-differences as the coefficient on the interaction term for treatment (implementation vs control) by time. All analyses incorporated the stratified, clustered, and weighted design of the survey, and we adjusted standard errors by using Taylor series linearization and incorporated a finite population correction.
We conducted a series of sensitivity analyses for robustness checks. First, we assessed difference-in-differences by using only standard sampling weights to assess accuracy of the sampling frames. Second, we fit logistic regression models adjusted for confounders by using the covariates in the IPT weighting model and the mining community variable, and used predictive margins to estimate difference-in-differences.24,25 Third, we fit additional IPT weighting and outcome models restricted to agricultural communities because of previous data demonstrating that these community types moderate CHW program effectiveness in Liberia.15 We used Stata version 14.2 (StataCorp LP, College Station, TX) for all analyses. We provide statistical code for all analyses and IPT-weighted model diagnostics in Appendix D (available as a supplement to the online version of this article at http://www.ajph.org).
RESULTS
Among households that were approached for participation, household response rates were 97.2% in 2015 and 98.4% in 2016 resulting in 455 and 539 surveys, respectively. Within eligible households, 82.2% of listed women participated in 2015 and 84.5% in 2016 (549 and 604 surveys); information about 97.5% of listed children was provided in 2015 and 99.3% in 2016, (340 and 492 surveys). Less than 3% of data items were missing.
Overall, the samples were similar (Table 1); however, households in the intervention areas were farther from the nearest health facility than were those in the control areas at both time points. More households in the intervention group were in mining communities and more respondents in the intervention areas completed the survey in English than in the control group. In all groups, IPT weighting produced approximate balance, as seen by decreased standardized differences from the baseline control group. We present full IPT weighting balance diagnostics and an IPT-weighted version in Appendix C, Table A (available as a supplement to the online version of this article at http://www.ajph.org).
TABLE 1—
Sample Characteristics of Children With Fever, Acute Respiratory Infection, or Diarrhea Before the Application of Inverse Probability of Treatment Weighting: Liberia, 2015–2016
| Baseline |
Follow-Up |
|||
| Respondent Characteristics (by Child) | Intervention (n = 180), % (95% CI) or Mean (95% CI) | Control (n = 160), % (95% CI) or Mean (95% CI) | Intervention (n = 190), % (95% CI) or Mean (95% CI) | Control (n = 302), % (95% CI) or Mean (95% CI) |
| Child’s household located in mining community, % | 27.8 (15.2, 45.2) | 10.0 (3.2, 27.0) | 29.5 (16.3, 47.4) | 2.3 (0.6, 8.2) |
| Child’s household reachable by motorcycle, % | 88.3 (73.8, 95.3) | 74.4 (56.2, 86.8) | 72.6 (53.2, 86.1) | 75.2 (62.4, 84.7) |
| Mother’s education (any), % | 56.1 (48.1, 63.8) | 56.9 (49.4, 64.0) | 45.8 (37.4, 54.5) | 51.7 (44.7, 58.6) |
| Mother’s survey language, % | ||||
| English | 48.3 (37.4, 59.4) | 31.9 (23.0, 42.3) | 42.1 (28.3, 57.3) | 16.9 (11.7, 23.8) |
| Bassa | 51.7 (40.6, 62.6) | 68.1 (57.7, 77.0) | 57.9 (42.7, 71.8) | 83.1 (76.2, 88.3) |
| Mother married or cohabitating, % | 86.7 (80.6, 91.0) | 84.4 (78.9, 88.7) | 90.5 (85.6, 93.9) | 92.7 (88.2, 95.6) |
| Number of illnesses per child in the past 2 wk, % | ||||
| 1 illness | 51.7 (44.0, 59.2) | 62.5 (55.8, 68.8) | 61.1 (54.4, 67.3) | 49.3 (42.1, 56.6) |
| ≥ 2 illnesses | 48.3 (40.8, 56.0) | 37.5 (31.2, 44.2) | 39.0 (32.7, 45.6) | 50.7 (43.4, 57.9) |
| Child’s household distance from facility, km, mean | 13.3 (11.5, 15.2) | 8.6 (7.6, 9.5) | 15.3 (13.0, 17.6) | 8.9 (7.9, 9.9) |
| Child’s household wealth index score relative to DHS Rural,a mean | −0.59 (−0.71, −0.47) | −0.53 (−0.67, −0.38) | −0.55 (−0.67, −0.42) | −0.50 (−0.63, −0.36) |
| Mother’s no. of children, mean | ||||
| Aged < 5 y | 1.38 (1.27, 1.49) | 1.65 (1.55, 1.75) | 1.61 (1.47, 1.75) | 1.67 (1.58, 1.75) |
| Aged < 1 y | 0.40 (0.28, 0.52) | 0.44 (0.34, 0.54) | 0.34 (0.28, 0.40) | 0.40 (0.34, 0.46) |
| Mother’s age, y, mean | 29.0 (28.2, 29.8) | 28.0 (27.0, 29.0) | 28.6 (27.7, 29.5) | 28.8 (28.0, 29.7) |
Note. CI = confidence interval. Estimates in this table incorporate sampling weights only.
DHS Rural refers to the wealth index calculated for rural areas in Liberia in the 2013 Demographic Health Survey.1,20
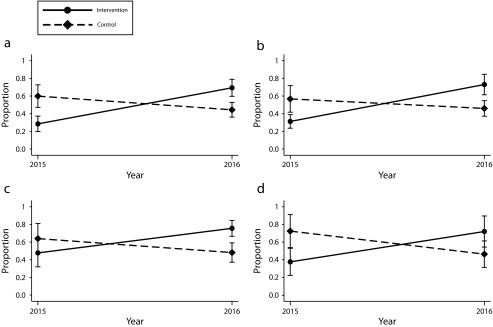
In IPT-weighted models, total iCCM treatment rates increased from 28.5% (95% confidence interval [CI] = 19.7%, 37.2%) to 69.3% (95% CI = 59.7%, 78.9%) in the intervention areas from baseline to follow-up and decreased from 59.9% (95% CI = 47.1%, 72.7%) to 44.4% (95% CI = 36.1%, 52.6%) in control areas, corresponding to a 56.4 percentage point difference-in-differences (95% CI = 36.4, 76.3; P < .001) when comparing the intervention to control areas between baseline and follow-up (Figure 1; Table 2). The disease-specific absolute difference-in-differences between intervention and control areas were 52.6 percentage points (95% CI = 30.2, 74.9; P < .001) for fever, 43.6 percentage points (95% CI = 16.4, 70.8; P = .002) for diarrhea, and 60.5 percentage points (95% CI = 27.0, 94.0; P = .001) for ARI. The difference-in-differences for treatment of diarrhea with ORT was 22.4 percentage points (95% CI = −0.7, 45.5; P = .058). The regression-adjusted and unadjusted models demonstrated similar results (Figure 1; Table 2), as did models restricted to agricultural communities (difference-in-differences 61.1; 95% CI = 39.6, 82.6; P < .001; Appendix E, Table F, available as a supplement to the online version of this article at http://www.ajph.org).
FIGURE 1—
Proportion of Children Receiving Care From a Qualified Provider for (a) Any Illness, (b) Fever, (c) Diarrhea, and (d) Acute Respiratory Infection: Liberia, 2015–2016
TABLE 2—
Absolute Difference-in-Differences in Care Seeking for Any Integrated Community Case Management Illness, for Specific Illnesses, and Receipt of Oral Rehydration Therapy Among Children With Diarrhea: Liberia, 2015–2016
| No. of Children |
IPT Model |
Regression Model (Unadjusted) |
Regression Model (Adjusted) |
|||||
| 2015 | 2016 | DID% (95% CI) | P | DID% (95% CI) | P | DID% (95% CI) | P | |
| Care seeking from a qualified provider for: | ||||||||
| Any illness | 340 | 492 | 56.4 (36.4, 76.3) | < .001 | 48.3 (32.7, 64.0) | < .001 | 49.7 (34.8, 64.6) | < .001 |
| Fever | 255 | 360 | 52.6 (30.2, 74.9) | < .001 | 44.3 (27.0, 61.7) | < .001 | 46.1 (30.0, 62.2) | < .001 |
| Diarrhea | 184 | 279 | 43.6 (16.4, 70.8) | .002 | 45.4 (24.7, 66.1) | < .001 | 51.8 (32.6, 71.1) | < .001 |
| ARI | 84 | 139 | 60.5 (27.0, 94.0) | .001 | 49.1 (20.7, 77.5) | .001 | 51.5 (23.1, 79.9) | .001 |
| ORT receipt among children with diarrhea | 74 | 157 | 22.4 (–0.7, 45.5) | .06 | 18.0 (–1.3, 37.3) | .07 | 22.6 (3.4, 41.8) | .021 |
Note. CI = confidence interval; ARI = acute respiratory infection; DID = difference in differences; IPT = inverse probability of treatment; ORT = oral rehydration therapy.
We detected a significant increase in the proportion of children receiving care from CHWs (0% to 47.9%; P < .001) from baseline to follow-up in the intervention areas (Table 3). As expected, no care from a CHW was reported in the control areas. The increase in CHW treatment in intervention areas was accompanied by reductions in treatment from drugstores and informal dispensers and a nonsignificant reduction in treatment from hospitals or clinics (P = .056); however, the net increase in CHW care exceeded the reduction from other providers. The increase in treatment by a CHW was greater among households 5 to 10 kilometers from the health facility than those in households greater than 10 kilometers from the health facility (Appendix E, Table D, available as a supplement to the online version of this article at http://www.ajph.org). In the intervention group, the proportion of women who reported distance from health care provider as a barrier to receiving care decreased from 20.8% (95% CI = 14.7, 26.8) to 7.5% (95% CI = 5.0, 10.1; P < .001). In the control group, the proportion of women who reported distance from health care provider as a barrier to receiving care was unchanged (15.0%; 95% CI = 10.1, 21.8 at baseline and 14.4%; 95% CI = 10.1, 20.1 at follow-up; P = .871).
TABLE 3—
Percentage of Sick Children Who Sought Care From Each Provider Type: Liberia, 2015–2016
| Intervention Regions |
Control Regions |
|||||
| Provider Type | 2015, % (95% CI) | 2016, % (95% CI) | P | 2015, % (95% CI) | 2016, % (95% CI) | P |
| Drugstore | 19.9 (11.4, 32.5) | 5.8 (2.3, 14.1) | .015 | 7.0 (4.3, 11.2) | 9.1 (5.1, 15.7) | .48 |
| Informal drug dispensers | 3.3 (1.2, 9.1) | 6.8 (4.1, 10.9) | .20 | 6.2 (3.2, 11.6) | 11.3 (7.5, 16.7) | .11 |
| gCHV | 2.3 (0.9, 5.9) | 0.2 (0, 1.3) | .005 | 2.7 (0.8, 8.8) | 0 | .09 |
| Hospital or clinic | 41.5 (29.7, 54.4) | 25.7 (16.9, 37.0) | .06 | 60.6 (50.5, 70.0) | 49.3 (42.9, 55.8) | .06 |
| CHW | 0 | 57.6 (42.8, 71.2) | < .001 | 0 | 0 | . . . |
| Traditional providers | 5.0 (2.9, 8.7) | 3.2 (1.4, 6.9) | .34 | 2.6 (1.0, 6.6) | 4.9 (2.9, 8.2) | .22 |
Note. CHW = community health worker; CI = confidence interval; gCHV = general community health volunteer. Estimates in this table incorporate inverse probability of sampling weights. Care could be sought from more than 1 provider.
DISCUSSION
Implementation of a CHW-based iCCM program in remote Rivercess County, Liberia, was associated with statistically significant improvements in receiving treatment of pediatric iCCM diseases after 12 months from all providers. As compared with residents in a control region, children living in the intervention area were more likely to receive treatment from qualified providers for a combination of all 3 target iCCM diseases, as well as for each condition individually, and to receive ORT for diarrhea, though the latter was not statistically significant. The change appears to be mediated by increased uptake of CHW-delivered services in intervention areas and occurred despite the intervention launching at end of the Ebola virus disease epidemic. Although previous research showed that the epidemic reduced care seeking and caused shifts in provider types,18,26 we would expect disruptions to be minimal by the summer of 2015.27
A causal interpretation of the effect of the program is supported by several pieces of evidence. First, we assessed before-to-after outcomes against a simultaneous control population in the same county. Second, the effects demonstrated were robust to multiple analytic approaches to controlling for sources of bias, including both demographic and temporal differences between the control and intervention areas. Third, the large effect sizes, in the range of 40 to 60 percentage point increases in access to care, were consistent across all 3 iCCM diseases. Fourth, we found evidence of a substantial increase in receipt of treatment from program CHWs in the intervention area only, which was accompanied by a decrease in reporting that distance from clinic was a barrier to accessing health care.
Our results show similar or larger effect size for the treatment of childhood illness when compared with previous work describing the impact of CHWs on iCCM indicators in Liberia and elsewhere.9,11 We previously observed similar increases in a different remote part of the country in an uncontrolled analysis.15 Community health worker iCCM programs have also improved child health outcomes that are associated with child mortality elsewhere.6,14 Nonetheless, studies demonstrating a direct impact of such programs on mortality are limited, arguably because of insufficient power or lack of uptake of iCCM programs.11,28,29
Our data also demonstrated that additional interventions or program strengthening will be required to achieve programmatic goals of 100% treatment coverage for childhood illnesses. Despite significant increases in treatment of iCCM conditions by a qualified provider, a substantial percentage of children (approximately 30%) still did not receive care in areas with CHWs during the intervention period. In addition, we found evidence of imperfect quality of care delivery: the difference-in-differences in use of ORT receipt was only about half the total increase reported in care by an appropriate provider for children with diarrhea. Past programs have found achieving high rates of ORT coverage to be feasible.30 The reasons for suboptimal rates of coverage and quality remain an important area of investigation for this and other iCCM programs. Standardizing reporting in the global health literature of both programmatic elements and the context in which CHW programs are implemented would be valuable to enable cross-program comparisons.31
Although we observed increased treatment rates in the intervention areas, a proportion of our observed difference-in-differences treatment was attributable to reductions in treatment in the control area. We hypothesize that this was attributable to stock-outs of iCCM commodities in the control areas during 2016, which may have reduced treatment levels in facilities. Because these stock-outs affected supplies to health facilities in both control and intervention areas, we believe the control communities represent an appropriate counterfactual scenario. Notably, the program evaluated here implemented a direct supply chain for CHWs, which enabled the maintenance of services during this period. However, the magnitude of our findings may not generalize to locations with stronger facility-based services or those in which facility stock-outs also restrict supplies to CHWs.
Findings from this study will inform the Liberia National CHA Program, which intends to recruit, train, and support more than 4000 CHAs and 400 supervisors by 2020 across all 15 Liberian counties to provide health care services to the roughly 1.2 million people (29% of the country’s population) living more than 5 kilometers from a health facility. This study provides necessary empiric data on the potential benefit of and challenges to implementation of a CHW program in remote settings. This study shows that the presence of CHWs increased the overall treatment rates of the area, rather than simply providing a lower-cost substitution. Our study also highlights the need for extra effort to ensure complete coverage and high-quality care in the midst of a nationwide scale-up of services and suggests that the changes made between Liberia’s former general community health volunteer program and the new CHA program may have led to increased success—specifically, a strengthened supply chain, regular supportive supervision, and provision of monthly monetary incentives. By contrast, the general community health volunteer program mainly focused on training. At scale, although the monthly incentive substantially increases the cost of the program, it is also expected to stimulate significant economic activity by creating 4000 jobs, leading to a positive return on investment.32
Limitations
Our study had several limitations. First, community mapping for the 2015 sampling frame was incomplete, which challenged the comparability of the baseline and follow-up samples. We used 2 approaches to improve balance between groups and time points: (1) IPT-weighted modeling and (2) regression adjustment. Results were similar with both approaches. Furthermore, we did not assess trends in our control and intervention areas before our baseline measurement. In addition, we note 2 potential sources of confounding: (1) differential population compositional shifts between intervention and control populations and (2) unmeasured simultaneous interventions. We attempted to address compositional shifts in demographics and wealth and differences in preexisting trends through use of IPT weighting, regression adjustment, and restricted analyses.22 After we applied IPT weights, no covariates had sufficiently different before-to-after differences between the intervention and control areas to explain the observed effect on childhood treatment (discussed in Appendix C, available as a supplement to the online version of this article at http://www.ajph.org). However, IPT weighting only corrects shifts in measured confounders, so unmeasured confounders may remain. We believe simultaneous interventions were unlikely because of the lack of other organizations working in the county and the close partnership with the Ministry of Health through which such interventions would have been identified.
Our study is subject to standard limitations of field surveys, including data-recording errors, recall errors, and respondent miscomprehension. We have previously used our mobile data entry application to assess data-recording error rates and found them to be rare (1.6%).17 To minimize survey comprehension and external validity issues, we used standardized Demographic and Health Survey questions, adapted through translation and back-translation. We expect any existing data quality issues to occur at random, thus biasing estimates toward a null finding. In addition, we employed a modified random walk approach to identify households, which is expected to produce a representative sample in rural areas, but could contribute to selection bias.19
We were limited in collection of additional quality-of-care indicators. Standardized vaccination services were disrupted by stoppages during the Ebola virus disease epidemic and by mass campaigns after it, limiting estimation of the effect of CHW activities on vaccine uptake during the observation period. The epidemic also precluded use of malaria rapid diagnostic tests because of Ebola contraction risks, limiting accurate report of malaria.33 Of note, maternal report of correct ARI treatment has recently been found to be of limited validity in comparable settings.34,35 As a consequence, ORT was the only validated indicator available for measuring quality of services in our study.
Although we collected data on early childhood mortality rates in both surveys, we were underpowered to detect mortality differences in the timeframe observed. However, because the effects of iCCM treatment on mortality are well-established in sub-Saharan Africa,29 we hypothesize that mortality reductions could be detected at greater scale and over longer time periods. This will be the subject of future analyses conducted along with the national program scale-up.
Finally, because of differences between the program studied here and the National CHA Program, it is unclear whether these results will be replicated at scale; this will be addressed over the coming years through several ongoing studies.
Conclusions
We demonstrated significant improvements in childhood illness treatment from qualified providers after implementation of a comprehensive CHW program including an iCCM component in rural Liberia. These results offer support for continued expansion of CHW services as part of the national CHA program across the country. Important future steps will include a broader evaluation of the effectiveness of this government-led program at national scale, as well as investigation into causes of imperfect treatment rates seen in our program.
ACKNOWLEDGMENTS
Direct Relief and UBS Optimus Foundation provided programmatic evaluation funds to Last Mile Health for the survey on which this analysis was conducted (no grant identification numbers). M. J. Siedner receives research support from the National Institutes of Health (K23 MH099916). J. D. Kraemer receives salary support from Last Mile Health.
We thank the Liberia Ministry of Health and the County Health Teams of Rivercess County for their partnership. We thank Thomas Griffiths for coordinating data collection efforts for this study.
Note. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
HUMAN PARTICIPANT PROTECTION
Approval for the surveys was obtained from the institutional review boards of Partners Healthcare, Georgetown University, and the Liberia Institute for Biomedical Research. All participants gave verbal informed consent to participate.
Footnotes
See also Bradley, p. 1129.
REFERENCES
- 1.Liberia Institute of Statistics and Geo-Information Services. Liberia Demographic and Health Survey 2013. 2013:480. Available at: http://dhsprogram.com/publications/publication-fr291-dhs-final-reports.cfm. Accessed November 23, 2015.
- 2. MDG Report 2015: Assessing progress in Africa toward the Millennium Development Goals. Addis Ababa, Ethiopia: United Nations Economic Commission for Africa; 2015.
- 3.Kenny A, Basu G, Ballard M et al. Remoteness and maternal and child health service utilization in rural Liberia: a population-based survey. J Glob Health. 2015;5(2):020401. doi: 10.7189/jogh.05.020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruk ME, Rockers P, Varpilah S, Macauley R. Which doctor? Determinants of utilization of formal and informal health care in post conflict Liberia. Med Care. 2011;49(6):585–591. doi: 10.1097/MLR.0b013e31820f0dd4. [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Oza S, Hogan D et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 6.Perry HB, Zulliger R, Rogers MM. Community health workers in low-, middle-, and high- income countries: an overview of their history, recent evolution, and current effectiveness. Annu Rev Public Health. 2014;35(1):399–421. doi: 10.1146/annurev-publhealth-032013-182354. [DOI] [PubMed] [Google Scholar]
- 7.Victora CG, Bryce J, Fontaine O, Monasch R. Reducing deaths from diarrhoea through oral rehydration therapy. Bull World Health Organ. 2000;78(10):1246–1255. [PMC free article] [PubMed] [Google Scholar]
- 8.Baqui AH, Black RE, El Arifeen S et al. Effect of zinc supplementation started during diarrhoea on morbidity and mortality in Bangladeshi children: community randomised trial. BMJ. 2002;325(7372):1059. doi: 10.1136/bmj.325.7372.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delacollette C, Van der Stuyft P, Molima K. Using community health workers for malaria control: experience in Zaire. Bull World Heal Organ. 1996;74(4):423–430. [PMC free article] [PubMed] [Google Scholar]
- 10.Sazawal S, Black RE. Effect of pneumonia case management on mortality in neonates, infants, and preschool children: a meta-analysis of community-based trials. Lancet Infect Dis. 2003;3(9):547–556. doi: 10.1016/s1473-3099(03)00737-0. [DOI] [PubMed] [Google Scholar]
- 11.Das JK, Lassi ZS, Salam RA, Bhutta ZA. Effect of community based interventions on childhood diarrhea and pneumonia: uptake of treatment modalities and impact on mortality. BMC Public Health. 2013;13(suppl 3):S29. doi: 10.1186/1471-2458-13-S3-S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theodoratou E, Al-Jilaihawi S, Woodward F et al. The effect of case management on childhood pneumonia mortality in developing countries. Int J Epidemiol. 2010;39(suppl 1):i155–i171. doi: 10.1093/ije/dyq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004;(2):CD000363. doi: 10.1002/14651858.CD000363.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Nyqvist MB, Guariso A, Svensson J, Yanagizawa-Drott D. Reducing child mortality in the last mile: a randomized social entrepreneurship intervention in Uganda. 2017. Available at: https://yanagizawadrott.com/wp-content/uploads/2017/08/livinggoods_april2017.pdf. Accessed April 30, 2018.
- 15.Luckow PW, Kenny A, White E et al. Implementation research on community health workers’ provision of maternal and child health services in rural Liberia. Bull World Health Organ. 2017;95(2):113–120. doi: 10.2471/BLT.16.175513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenny A, Gordon N. ODK-Liberia. 2017. Available at: http://github.com/Last-Mile-Health/ODK-Liberia. Accessed February 13, 2018.
- 17.Kenny A, Gordon N, Griffiths T, Kraemer JD, Siedner MJ. Validation relaxation: a quality assurance strategy for electronic data collection. J Med Internet Res. 2017;19(8):e297. doi: 10.2196/jmir.7813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ly J, Sathananthan V, Griffiths T et al. Facility-based delivery during the Ebola virus disease epidemic in rural Liberia: analysis from a cross-sectional, population-based household survey. PLoS Med. 2016;13(8):e1002096. doi: 10.1371/journal.pmed.1002096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett S, Woods T, Liyanage W, Smith D. A simplified general method for cluster-sample surveys of health in developing countries. World Health Stat Q. 1991;44(3):98–106. [PubMed] [Google Scholar]
- 20.Rutstein SO, Rojas G. Guide to DHS Statistics. US Agency for International Development. 2006. Available at: https://dhsprogram.com/pubs/pdf/DHSG1/Guide_to_DHS_Statistics_29Oct2012_DHSG1.pdf. Accessed November 23, 2015.
- 21.Filmer D, Pritchett L. Estimating wealth effects without expenditure data—or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 22.Stuart EA, Huskamp HA, Duckworth K et al. Using propensity scores in difference-in-differences models to estimate the effects of a policy change. Health Serv Outcomes Res Methodol. 2014;14(4):166–182. doi: 10.1007/s10742-014-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kentoffio K, Kraemer JD, Griffiths T et al. Charting health system reconstruction in post-war Liberia: a comparison of rural vs. remote healthcare utilization. BMC Health Serv Res. 2016;16:478. doi: 10.1186/s12913-016-1709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kraemer JD. Helmet laws, helmet use, and bicycle ridership. J Adolesc Health. 2016;59(3):338–344. doi: 10.1016/j.jadohealth.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 25.Powers EA. Interpreting logit regressions with interaction terms: an application to the management turnover literature. J Corp Finance. 2005;11(3):504–522. [Google Scholar]
- 26.Dunbar N, Richards E, Woldeyohannes D et al. Knockdown and recovery of malaria diagnosis and treatment in Liberia during and after the 2014 Ebola outbreak. Public Health Action. 2017;7(1):76–81. doi: 10.5588/pha.16.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morse B, Grépin KA, Blair RA, Tsai L. Patterns of demand for non-Ebola health services during and after the Ebola outbreak: panel survey evidence from Monrovia, Liberia. BMJ Glob Health. 2016;1(1):e000007. doi: 10.1136/bmjgh-2015-000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amouzou A, Hazel E, Shaw B et al. Effects of the integrated community case management of childhood illness strategy on child mortality in Ethiopia: a cluster randomized trial. Am J Trop Med Hyg. 2016;94(3):596–604. doi: 10.4269/ajtmh.15-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amouzou A, Morris S, Moulton LH, Mukanga D. Assessing the impact of integrated community case management (iCCM) programs on child mortality: review of early results and lessons learned in sub–Saharan Africa. J Glob Health. 2014;4(2):020411. doi: 10.7189/jogh.04.020411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chowdhury AMR, Cash RA. A Simple Solution: Teaching Millions to Treat Diarrhoea at Home. Dhaka, Bangladesh: University Press Limited; 1996. [Google Scholar]
- 31.Lewin S, Munabi-Babigumira S, Glenton C et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database Syst Rev. 2010;(3):CD004015. doi: 10.1002/14651858.CD004015.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dahn B, Woldemariam AT, Perry H Strengthening primary health care through community health workers: investment case and financing recommendations. World Health Organization. 2015:58. Available at: http://www.who.int/hrh/news/2015/CHW-Financing-FINAL-July-15-2015.pdf?ua=1. Accessed April 24, 2018.
- 33.Eisele TP, Silumbe K, Yukich J et al. Measuring coverage in MNCH: accuracy of measuring diagnosis and treatment of childhood malaria from household surveys in Zambia. PLoS Med. 2013;10(5):e1001417. doi: 10.1371/journal.pmed.1001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hazir T, Begum K, El Arifeen S et al. Measuring coverage in MNCH: a prospective validation study in Pakistan and Bangladesh on measuring correct treatment of childhood pneumonia. PLoS Med. 2013;10(5):e1001422. doi: 10.1371/journal.pmed.1001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell H, el Arifeen S, Hazir T et al. Measuring coverage in MNCH: challenges in monitoring the proportion of young children with pneumonia who receive antibiotic treatment. PLoS Med. 2013;10(5):e1001421. doi: 10.1371/journal.pmed.1001421. [DOI] [PMC free article] [PubMed] [Google Scholar]