Abstract
OBJECTIVE.
The objective of this study was to identify features that impact the diagnostic performance of intermediate-delay washout CT for distinguishing malignant from benign adrenal lesions.
MATERIALS AND METHODS.
This retrospective study evaluated 127 pathologically proven adrenal lesions (82 malignant, 45 benign) in 126 patients who had undergone portal venous phase and intermediate-delay washout CT (1–3 minutes after portal venous phase) with or without unenhanced images. Unenhanced images were available for 103 lesions. Quantitatively, lesion CT attenuation on unenhanced (UA) and delayed (DL) images, absolute and relative percentage of enhancement washout (APEW and RPEW, respectively), descriptive CT features (lesion size, margin characteristics, heterogeneity or homogeneity, fat, calcification), patient demographics, and medical history were evaluated for association with lesion status using multiple logistic regression with stepwise model selection. Area under the ROC curve (Az) was calculated from both univariate and multivariate analyses. The predictive diagnostic performance of multivariate evaluations was ascertained through cross-validation.
RESULTS.
Az for DL, APEW, RPEW, and UA was 0.751, 0.795, 0.829, and 0.839, respectively. Multivariate analyses yielded the following significant CT quantitative features and associated Az when combined: RPEW and DL (Az = 0.861) when unenhanced images were not available and APEW and UA (Az = 0.889) when unenhanced images were available. Patient demographics and presence of a prior malignancy were additional significant factors, increasing Az to 0.903 and 0.927, respectively. The combined predictive classifier, without and with UA available, yielded 85.7% and 87.3% accuracies with cross-validation, respectively.
CONCLUSION.
When appropriately combined with other CT features, washout derived from intermediate-delay CT with or without additional clinical data has potential utility in differentiating malignant from benign adrenal lesions.
Keywords: adrenal, adrenal tumors, characterization, delayed washout CT
CT with 10- to 15-minute delayed contrast-enhanced imaging has a well-established role in the evaluation of adrenal lesions [1–8]. It leverages the observation of the differing washout characteristics of adenomas and nonadenomas after IV CT contrast media administration [1, 2, 4]. The dedicated 10- to 15-minute delay for adrenal imaging is typically undertaken at a separate, and thus additional, visit after an adrenal lesion is detected. In the event that an adrenal lesion is detected and delayed contrast-enhanced CT images are available, it is much more common to encounter CT studies that include contrast-enhanced images of shorter delay intervals, typically less than 3 minutes, which are referred to as intermediate-delay images.
Some authors have suggested that CT with intermediate-delay intervals may have some efficacy in differentiating adrenal adenomas from nonadenomas [9–13]. These studies have suggested using cutoff thresholds for quantitative CT parameters obtained, including lesion size; unenhanced and contrast-enhanced lesion attenuation values; and material wash-in, washout, or both. Some have also suggested using combinations of these parameters, but to our knowledge, neither the independent nor the multivariate performance of the parameters has been evaluated. Various descriptive radiologic features, such as the characteristics of the lesion margin and internal morphology, have also been explored as potential discriminators [14, 15].
In clinical practice, having limited patient and clinical data is not uncommon. The relative importance of these data, such as patient age, sex, or medical history, in the evaluation of adrenal lesions has not been assessed, nor has the potential utility of combining additional information that might be available.
Adrenal lesions have been reported in up to 9% of abdominal CT studies, which presents a substantial challenge in clinical practice [16–21]. Although the majority of such lesions are benign, detection of a malignant lesion has substantial implications. To reduce the risk of lesion misclassification, we used pathology as our reference standard for all lesions, which has not been commonly done in previous studies.
The objective of our retrospective study was to determine which imaging features influence the diagnostic performance of contrast-enhanced intermediate-delay CT for distinguishing malignant from benign adrenal lesions in multivariate analyses using pathology as the reference standard. We characterized the relative contributions of quantitative imaging features when considered conjointly with descriptive CT and other clinical data that might be available in clinical practice and ascertained their joint predictive performance in the diagnostic setting.
Materials and Methods
This retrospective study was approved by our institutional review board. A waiver of informed consent was granted. We searched the institutional pathology database for pathologic diagnoses between January 2001 and January 2010 that contained the word “adrenal”; this search yielded 792 cases. The search term strategy inevitably included cases in which no disease or abnormality was present; 332 such cases were found. For the remaining 460 cases, CT images obtained before the pathologic diagnoses were reviewed.
Inclusion criteria for our study were patient age of 18 years old or older at the time of CT; no clinical or biochemical evidence of functioning adrenal lesions (because those lesions typically undergo alternative focused clinical evaluations); time between pathology and preceding CT of less than 160 days; CT studies including portal venous phase and intermediate-delay (1–3 minutes after the portal venous phase) contrast-enhanced images, with or without unenhanced images; and at least one adrenal lesion greater than 1 cm and larger than twice the CT image slice thickness. In all, 127 cases (126 patients) met the inclusion criteria.
CT
A range of CT scanners had been used in the 127 identified cases, with the following parameters: median tube current, 265 mA (range, 110–630 mA); 120 kVp in 122 cases; and median slice thickness, 2.5 mm (range, 2.5–10.0 mm). Scanner models were GE Healthcare LightSpeed 16 (n = 63), LightSpeed Plus (n = 29), LightSpeed QX/I (n = 21), LightSpeed VCT (n = 13), and other (n = 1).
Contrast-enhanced CT was obtained 60–70 seconds after IV administration of 100–150 mL of nonionic contrast agent (300 mg I/mL iohexol) at a rate of 2.0–3.0 mL/s by power injector. The mean delay interval ± SD between delayed and portal venous phase images was 125 ± 20 s (range, 60–178 s).
Quantitative CT Evaluation
ROI analyses were undertaken on the image of each scan phase that contained the maximum axial cross-sectional area of the adrenal lesion. This process was undertaken within a graphic user interface in Matlab (version 2013b, MathWorks). An ROI was drawn freehand, with an electronic cursor and mouse, around the periphery of the adrenal lesion. The edges of the mass were avoided to prevent potential partial volume artifacts. The ROI was saved and transposed onto the image of all other phases of the study containing the maximal cross-sectional area of the target lesion. Translational adjustments to the ROI were made as necessary to correct any axial misalignments in any given image, but the shape and size of the original ROI were preserved. The ROI was redrawn as necessary to ensure satisfactory alignment of the identical ROI on the images of all phases. Attenuation measurements for each pixel within each ROI, for each CT phase, were saved and exported for statistical analysis.
Absolute and relative percentage of enhancement washout (APEW and RPEW, respectively) for images with intermediate-delay scans was calculated on the basis of the pixel values of respective ROIs, as follows:
| (1) |
| (2) |
where UA, PV, and DL are the mean attenuations on unenhanced, portal venous phase contrast-enhanced, and intermediate-delay phase contrast-enhanced CT images, respectively [4].
Descriptive CT Evaluation
A radiologist with more than 5 years’ experience in abdominal CT who was blinded to the lesion status evaluated descriptive CT features of the whole adrenal lesion using soft-tissue windows (width = 400 HU, level = 50 HU) on an IntelliSpace PACS (version 4.4, Philips Healthcare). The descriptive CT features assessed were size (maximum long and short axis diameters); lesion margin (ill- or well-defined); texture on contrast-enhanced images (homogeneous or heterogeneous); calcification (present or absent); and fat (present [< −20 HU] or absent).
Demographics and Medical History
Electronic medical records were reviewed to determine patient sex and age at the time of pathology diagnosis. In particular, the records were carefully reviewed to determine any history of malignancy before CT was performed.
Statistical Analysis
Lesion characteristics and CT parameters were summarized by median and range and evaluated for association with lesion status (benignity vs malignancy) using the Mann-Whitney U test. Binary classifiers for differentiating malignant from benign adrenal lesions were identified from single parameter evaluations of the CT features (UA, APEW, and RPEW). An optimal threshold was selected for each single parameter evaluation using the Youden index [22].
Univariate logistic regression was applied to individual features obtained from evaluating four types of patient-level information: quantitative CT evaluation based on objective measurements of lesion attenuation, descriptive CT features, demographics (i.e., patient age and sex), and personal history of malignancy. Holm-Bonferroni correction was applied to control overall type I error rate at 5% among all univariate hypothesis tests conducted within each of the four information types [23].
In addition, several multivariate logistic regression models were fit sequentially to characterize the relative contributions of the quantitative CT features when considered conjointly, as well as their effects when integrated with descriptive CT features and additional patient-level information available at the time of diagnostic assessment, to confer malignancy risk. The quantitative CT features were evaluated for association with lesion status and then combined successively with the descriptive CT features, demographics, and medical history. Final models were selected using backward elimination based on the likelihood ratio test. An ROC curve was fitted to the linear predictors that resulted from estimation of each logistic regression model. For each model, the area under the ROC curve (Az) was estimated along with the DeLong 95% CI [24]. Partial effects were evaluated for significance using likelihood ratio tests.
After identifying features that exhibited evidence of association with pathologic results, we ascertained the predictive accuracy of multifeatured evaluations. A final predictive classifier was determined for each level of source information using logistic regression with weighted estimation. Gaussian density approximation was applied to the sample of multivariate continuous features and used to assign a weight to each observation that reflected its relative population frequency. The resulting weighted regression coefficients are reported in the Results section for each type of predictive classifier. Computed from the maximum accuracy threshold, diagnostic properties are reported on the basis of the full data as well as under a leave-one-lesion-out cross-validation strategy that adjusts the measure for predictive loss due to sampling variability.
With 127 lesions and 82 malignancies, our study provides at least 80% power to detect a difference of 0.129 between the Az under the null hypothesis of 0.5 and an Az under the alternative hypothesis of 0.629 using a one-sided z-test at significance level of 0.05. All statistical analyses were conducted using SAS (version 9, SAS Institute) or R software (version 3.1.2, R Development Core Team).
Results
Patients and Lesions
The study cohort consisted of 127 adrenal lesions in 126 patients (one patient had bilateral lesions). Median overall patient age was 61.4 years (range, 23–85 years). The median age of the 82 male patients was 60.4 years (range, 23–84 years), and the median age of the 44 female patients was 63.9 years (range, 27–85 years). Of the 127 lesions, 82 (65%) were malignant and 45 (35%) were benign. The malignant lesions were metastases (n = 77), lymphoma (n = 4), and unclassified malignancy (n = 1); the benign lesions were adenoma (n = 41), hyperplasia (n = 2), myelolipoma (n = 1), and neurogenic tumor (n = 1). Pathologic diagnoses were established by adrenalectomy (n = 106), biopsy (n = 11), and fine-needle aspiration (n = 10). Seventy-nine (62%) lesions were on the left side; 48 (38%) were on the right. The median time interval between CT and surgery or biopsy was 16 days (range, 1–151 days).
At the time of the index CT, the existence of a prior malignancy was known in 87 (69%) of the 127 lesions. Unenhanced images were available in 103 lesions (102 patients).
Lesion CT Characteristics and Univariate Analyses
Summaries of the quantitative CT, descriptive CT, and history of malignancy data are presented in Table S1 (which can be viewed in the AJR electronic supplement to this article, available at www.ajronline.org). For our quantitative CT parameters, UA was signaificantly higher and RPEW significantly lower for malignant than benign lesions (p < 0.0001).
Table 1 presents sensitivities, specificities, positive predictive values, negative predictive values, and accuracies based on commonly used typical (as used in 15-minute delayed protocols) and optimal cut-offs for UA, APEW, and RPEW to allow comparisons with previous work. Use of the optimal cutoffs yielded higher accuracies than corresponding typical cut-offs used with 15-minute delayed protocols.
TABLE 1:
Sensitivity, Specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and Accuracy for Malignancy With Typical Cutoff Thresholds Used in 15-Minute Delayed Protocols and Sample Optimal Values
| Parameter | Cutoff | Sensitivity | Specificity | PPV | NPV | Accuracy (95% CL) |
|---|---|---|---|---|---|---|
| Typicala | ||||||
| Unenhanced attenuation | ≥ 10 HU | 96.8 | 31.7 | 68.2 | 86.7 | 70.9 (61.1, 79.4) |
| APEW | ≤ 60 | 100.0 | 5.0 | 62.0 | 100.0 | 62.8 (52.6, 72.1) |
| RPEW | ≤ 40 | 95.1 | 25.0 | 70.3 | 73.3 | 70.6 (61.9, 78.4) |
| Optimal | ||||||
| Unenhanced attenuation | ≥ 24.02 HU | 87.1 | 75.6 | 84.4 | 79.5 | 82.5 (73.8, 89.3) |
| APEW | ≤ 22.78 | 59.7 | 87.5 | 88.1 | 58.3 | 70.6 (60.8, 79.2) |
| RPEW | ≤ 24.04 | 89.0 | 68.2 | 83.9 | 76.9 | 81.8 (73.9, 88.1) |
Note—Except where otherwise specified, values are percentages. CL = confidence limits, APEW = absolute percentage of enhancement washout, RPEW = relative percentage of enhancement washout.
Typical refers to commonly applied cutoff thresholds used for 15-minute delayed adrenal CT and are presented only for comparative purposes.
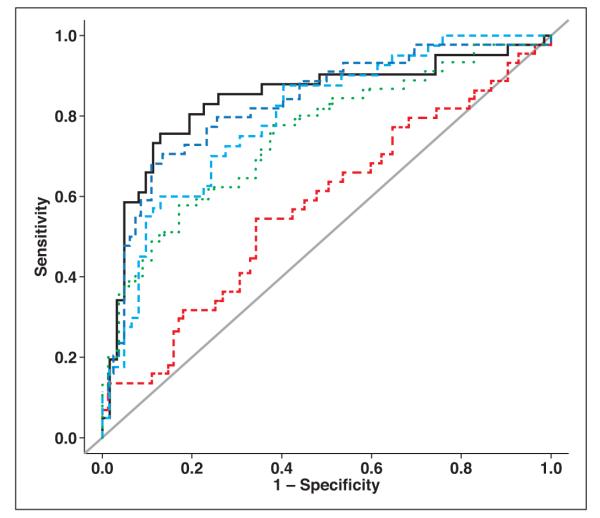
On univariate analyses, the quantitative CT parameters UA and DL were significantly associated with malignancy (p < 0.0001) (Table S2, which can be viewed in the AJR electronic supplement to this article, available at www.ajronline.org); increasing risk of malignancy was seen for larger values of UA and DL. The derived washout parameters APEW and RPEW were also significantly associated with malignancy (p < 0.0001), with higher washout decreasing risk of malignancy. In terms of ROC performance, Az for UA (0.839) was higher than for DL (0.751) and PV (0.577), and Az for RPEW (0.829) was higher than for APEW (0.795) (Table 2, Fig. 1).
TABLE 2:
Diagnostic Performance of Individual Single-Parameter CT Quantitative Features
| Parameter | Area Under the ROC Curve (95% CI) |
|---|---|
| UA | 0.839 (0.752–0.926) |
| PV | 0.577 (0.500–0.683) |
| DL | 0.751 (0.660–0.842) |
| APEW | 0.795 (0.708–0.882) |
| RPEW | 0.829 (0.751–0.907) |
Note—UA = lesion attenuation on unenhanced images, PV = lesion attenuation on portal venous phase contrast-enhanced images, DL = lesion attenuation on intermediate-delay (1–3 minute) contrast-enhanced images, APEW = absolute percentage of enhancement washout, RPEW = relative percentage of enhancement washout.
Fig. 1.
Summary ROC plots for univariate analyses of lesion attenuation on unenhanced images (black line, AUC = 0.839), portal venous phase contrast-enhanced images (red line, AUC = 0.577), and intermediate-delay (1–3 minute after portal venous phase) contrast-enhanced images (green line, AUC = 0.751); absolute percentage of enhancement washout (light blue line, AUC = 0.795); and relative percentage of enhancement washout (dark blue line, AUC = 0.829). Gray line is null line, depicting AUC of 0.5 (i.e., no diagnostic value).
Logistic regression modeling was not undertaken for parameters that had unbalanced sample sizes (namely, the descriptive CT features of lesion margin definition, fat, and calcification). For demographic features, male sex was significantly associated with malignancy (p = 0.002). History of malignancy was significantly associated with an increased risk for malignancy of adrenal lesions (p < 0.0001) (Table S2).
Multivariate Analyses
Multivariate analyses were undertaken in two groups that reflected potential clinical scenarios that might be encountered: unenhanced scans not available and unenhanced scans available.
Unenhanced images not available
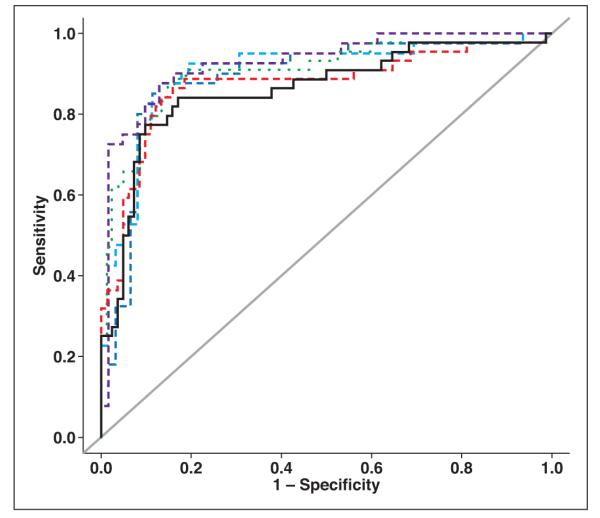
In cases for which unenhanced images were not available, our multivariate analysis of the quantitative CT features alone indicated that RPEW and DL each contributed significant partial effects in multiple regression analysis. Including these two features in the logistic model yielded an Az of 0.861 (Table 3, Fig. 2), which was higher than that obtained for the individual quantitative CT parameters in univariate analyses (Az = 0.829 and 0.751, respectively).
TABLE 3:
Multiple Regression Analyses and Associaited ROC Performance and Diagnostic Accuracy of Models
| Model, Covariate | Comparison | Odds Ratioa | p | Area Under the ROC Curve |
Sens (%) | Spec (%) | Acc (%) |
|---|---|---|---|---|---|---|---|
| Unenhanced images unavailableb | |||||||
| CT quantitative | |||||||
| DL | 1-HU increase | 1.04 (1.02, 1.06) | 0.0007 | 0.861 (0.787, 0.935) | 90.2 | 77.3 | 85.7 |
| RPEW | 1% increase | 0.93 (0.90, 0.96) | < 0.0001 | ||||
| CT quantitative and demographics | |||||||
| DL | 1-HU increase | 1.04 (1.02, 1.07) | 0.0006 | 0.873 (0.799, 0.947) | 86.6 | 84.1 | 85.7 |
| RPEW | 1% increase | 0.93 (0.89, 0.95) | < 0.0001 | ||||
| Age | 1-y increase | 1.05 (1.01, 1.10) | 0.03 | ||||
| CT quantitative, demographics, and history | |||||||
| DL | 1-HU increase | 1.03 (1.01, 1.06) | 0.007 | 0.903 (0.841, 0.965) | 89.0 | 79.5 | 85.7 |
| RPEW | 1% increase | 0.94 (0.90, 0.97) | 0.0001 | ||||
| PMH | Yes vs no | 9.26 (3.30, 28.42) | < 0.0001 | ||||
| Unenhanced images availablec | |||||||
| CT quantitative | |||||||
| UA | 1-HU increase | 1.09 (1.084, 1.194) | < 0.0001 | 0.889 (0.819, 0.959) | 88.7 | 85.0 | 87.3 |
| APEW | 1% increase | 0.95 (0.919, 0.981) | 0.002 | ||||
| CT quantitative and demographics | |||||||
| UA | 1-HU increase | 1.10 (1.05, 1.14) | < 0.0001 | 0.902 (0.836, 0.969) | 87.1 | 87.5 | 87.3 |
| APEW | 1% increase | 0.95 (0.92, 0.98) | 0.003 | ||||
| Age | 1-y increase | 1.07 (1.02, 1.14) | 0.011 | ||||
| CT quantitative, demographics, and history | |||||||
| UA | 1-HU increase | 1.09 (1.04, 1.15) | 0.0003 | 0.927 (0.873, 0.980) | 98.4 | 72.5 | 88.2 |
| APEW | 1% increase | 0.96 (0.92, 0.99) | 0.02 | ||||
| PMH | Yes vs no | 11.06 (3.42, 41.53) | 0.0001 | ||||
Note—Values in parentheses are 95% confidence limits. Sens = sensitivity, Spec = specificity, Acc = accuracy, DL = lesion attenuation on intermediate-delay (1–3 minute) contrast-enhanced images, RPEW = relative percentage of enhancement washout, PMH = history of malignancy, UA = lesion attenuation on unenhanced images, APEW = absolute percentage of enhancement washout.
Malignant or benign.
n = 127 lesions.
n = 103 lesions.
Fig. 2.
Summary ROC plots for multivariate analyses. In cases for which unenhanced images were not available, using quantitative CT data only (black line) yielded AUC of 0.861; using quantitative CT and demographic data (red line) yielded AUC of 0.873; and using quantitative CT data, demographic data, and history of malignancy (green line) yielded AUC of 0.903. In cases for which unenhanced images were available, using quantitative CT data only (dark blue line) yielded AUC of 0.889; using quantitative CT and demographic data (light blue line) yielded AUC of 0.902; and using quantitative CT data, demographic data, and history of malignancy (purple line) yielded AUC of 0.927. Gray line is null line, depicting AUC of 0.5 (i.e., no diagnostic value).
The addition of descriptive CT features did not contribute significant partial effects. The addition of demographic features yielded age as an additional significant feature and increased Az to 0.873. The further addition of history of malignancy displaced age but increased Az even further to 0.903 (Table 3).
Unenhanced images available
In cases for which unenhanced images were available, our analysis of the quantitative CT features alone indicated that APEW and UA each contributed significant partial effects in multiple regression analysis, yielding an Az of 0.889 (Table 3, Fig. 2), which was higher than that obtained for any individual feature in univariate analyses.
As was the case when unenhanced images were not available, the addition of descriptive CT features did not contribute significant partial effects. However, the addition into the analysis of demographic information again yielded age as an additional significant feature and increased Az to 0.902. The sequential addition of patient history also displaced age but increased Az further to 0.927 (Table 3).
Predictive Power
Our analyses were implemented using a conventional hypothesis testing framework. Associated accuracies based on optimized thresholds were 85.7% when unenhanced images were not available and 87.3–88.2% when they were (Table 3).
Cross-validation was used to assess the robustness of these findings and account for sampling variability. The resultant classifiers attained commensurate predictive accuracies under cross-validation of 84.9–85.7% when unenhanced images were not available and 85.3–87.3% when they were.
Table 4 presents the regression coefficients for the predictive classifiers. Using equation 3, the estimated probability (π) of malignancy for a lesion can be obtained from the available predictor variables (X1, X2, X3, X4) and the regression coefficients (a, b, c, d, e) provided in Table 4:
| (3) |
where exp is the exponential function. When unenhanced images were unavailable, predictor variables consisted of DL (in HU; X1), RPEW (in %; X2), age (in years; X3), and history of malignancy (1 = yes, 0 = no; X4). When unenhanced images were available, predictor variables were UA (in HU; X1), APEW (in %; X2), age (in years; X3), and history of malignancy (1 = yes, 0 = no; X4). Appendix 1 provides examples of how to use the formulas when unenhanced images are not available as well as when they are.
TABLE 4:
Regression Coefficients for Predicting Lesion Status
| Model | Intercept | DL | UA | RPEW | APEW | Age | History of Malignancy |
|---|---|---|---|---|---|---|---|
| Unenhanced images unavailable | |||||||
| CT quantitative | −0.5356 | 0.0381 | NA | −0.0721 | NA | 0 | 0 |
| CT quantitative and demographics | −3.4911 | 0.0415 | NA | −0.0768 | NA | 0.0471 | 0 |
| CT quantitative, demographics, and history | −1.5972 | 0.0324 | NA | −0.0672 | NA | 0 | 2.2261 |
| Unenhanced images available | |||||||
| CT quantitative | −0.6347 | NA | 0.0908 | NA | −0.0490 | 0 | 0 |
| CT quantitative and demographics | −5.0515 | NA | 0.0988 | NA | −0.0514 | 0.0706 | 0 |
| CT quantitative, demographics, and history | −1.9621 | NA | 0.0861 | NA | −0.0442 | 0 | 2.4033 |
Note—Intercept, DL and UA, RPEW and APEW, age, and history of malignancy correspond to variables a, b, c, d, and e in Equation 3, respectively. DL = lesion attenuation on intermediate-delay (1–3 minute) contrast-enhanced images, RPEW = relative percentage of enhancement washout, APEW = absolute percentage of enhancement washout, NA = not applicable.
Discussion
Our results indicate that intermediate-delay CT can contribute to the diagnostic performance of CT in differentiating malignant and benign adrenal lesions. Furthermore, our multivariate analyses identified appropriate individual CT features and clinical data that can be combined to improve diagnostic performance.
In abdominal imaging, the most commonly available images in CT are portal venous phase images. Our results indicate that images from this phase in isolation present little opportunity to differentiate malignancy from benignity, with an Az of only 0.577. In clinical practice, some delayed phase images may also be available, which present the opportunity to assess washout, conventionally described in the form of percentage of enhancement washout.
Our multivariate analyses of quantitative CT parameters derived solely from contrast-enhanced images (i.e., when unenhanced images are not available) showed that RPEW and DL were significant covariates and that their combination increased diagnostic performance (Az = 0.861) above that of the individual contributing quantitative CT parameters (Az = 0.829 and 0.751, respectively). When unenhanced images were available, our multivariate analyses found a similar pattern of significant covariates, APEW and UA, with concomitant improvement in diagnostic performance (Az = 0.889 with APEW and UA combined, compared with the individual covariates, 0.795 and 0.839, respectively). Consistently, although single measures of intermediate-delay washout (RPEW and APEW) provide some opportunity for lesion differentiation, their diagnostic performance can be improved by appropriate combination with other quantitative CT parameters that are already available.
Knowledge of patient demographics and history of malignancy further improved diagnostic performance. When they were available, their sequential addition to the models increased diagnostic performance whether unenhanced images were available or not (Az = 0.927 and 0.903, respectively). Of note, the sequential addition of the described features maintained the significance of quantitative CT features, which reinforces the robustness of these factors in the modeling. Descriptive CT factors did not contribute significant partial effects on our multivariate analyses in either scenario.
Az was higher for each corresponding combination of factors when unenhanced images were available than when they were not. Indeed, unenhanced CT in isolation yielded an Az of 0.839, which outperformed the highest single quantitative CT feature using washout data, namely, RPEW (Az = 0.829). However, in clinical practice, availability of unenhanced images is relatively uncommon. In the absence of unenhanced images, quantitative features of intermediate-delay CT, when appropriately combined, yielded diagnostic performance comparable with that of UA alone (Az = 0.861, with RPEW and DL combined).
A few authors have explored a variety of methods for combining parameters, typically by applying combinations of thresholds to individual parameters [9–11]. To allow comparisons, we have presented our results using simple cutoff thresholds for our quantitative CT parameters, both typical (as applied to 15-minute delayed protocols) and optimal. We recognize that 15-minute thresholds are unlikely to be directly relevant to intermediate-delay images; they have been presented simply for comparative purposes. The optimized thresholds yield higher accuracies than the typically applied thresholds. Different thresholds might be derived in different populations and depending on whether sensitivity or specificity is favored. Such threshold approaches do not assess the independent contributions of various CT parameters when they are considered conjointly with additional factors that confer malignancy risk, as has been undertaken in our multivariate analyses. The latter is more discriminating and powerful.
Unlike previous studies in this arena, we have described the predictive capabilities of our models according to the various levels of available data based on cross-validated measures of predictive accuracy, which adjust for population sampling variability inducing robustness to artifacts that can arise with outliers. We found predictive accuracies in the range of 84.9–87.3%. In addition, we provide formulas and regression coefficients for estimating the probability of malignancy of an adrenal lesion on the basis of CT data according to the availability of unenhanced images (Table 4).
We recognize and acknowledge several limitations in our study. This study was retrospective with inevitable resultant potential biases. The patients included in our study necessarily had a pathologic diagnosis (namely, surgery or biopsy), which was arguably a selection bias. In many clinical settings, lesions with low attenuation values (e.g., < 10 HU) on unenhanced CT would not proceed to tissue diagnoses; our data and findings are thus likely to be skewed toward lesions with higher unenhanced attenuation values.
However, in many clinical settings, unenhanced images are not available, and our predictive formulas derived for this common scenario can be applied. Our patients had surgery or biopsy for clinical indications, foregoing conventional 15-minute-delay CT or application of unenhanced CT threshold criteria (in which, for example, unenhanced attenuation below 10 HU would have obviated tissue sampling). Of note, in our cohort, lesions with unenhanced attenuation less than 10 HU proved to be malignant in two of 15 patients. Overall, our study benefitted from the fact that all diagnoses were established and confirmed, without which we would not have been able to accurately classify our lesions. Second, the study spanned several years and inevitably used a range of CT scanners and CT protocols, but it enabled review of a substantial number of pathologically confirmed lesions. We recognize that different scanners and acquisition protocols have potential effects on attenuation measurements [25–29]. We are unable to comment on whether longer contrast-enhanced delay intervals might have been more efficacious, because our study had no formal comparison with such a cohort of patients.
In conclusion, when appropriately combined with other CT features, washout data derived from intermediate-delay CT can assist in differentiating malignant from benign adrenal lesions with or without additional clinical data. We present models in the form of equations that provide predictive probabilities of malignancy or benignity of adrenal lesions using available CT attenuation data, demographic information, and history of malignancy.
Supplementary Material
Acknowledgments
Funded in part by the Cancer Center Support grant and National Institutes of Health / National Cancer Institute grant P30CA016672 and the John S. Dunn, Sr., Distinguished Chair in Diagnostic Imaging.
C. S. Ng has received research funding from GE Healthcare.
APPENDIX 1: Examples of the Predictive Probability Formulas
Equation 3 allows calculation of the estimated probability (π) of observing a malignant lesion, using linear combination of the regression coefficients provided in Table 4 and observed predictor variables:
Example 1: Unenhanced Images Not Available
Assume a 60-year-old patient with a history of malignancy and an adrenal lesion who underwent portal venous phase CT with an intermediate delay (1–3 minute) scan (but no unenhanced CT). The lesion has attenuation of 150 HU on portal venous phase images (PV) and 90 HU on intermediate-delay images (DL).
The relative percentage of enhancement washout (RPEW) is calculated as [(PV – DL) / (PV)] × 100 = [(150 – 90) / (150)] = 40%.
Using the parameters DL (in HU) as X1, RPEW (in %) as X2, AGE (in years) as X3, PMH (1 = yes, 0 = no) as X4, and the relevant regression coefficients in the upper half of Table 4,
| (4) |
(i.e., this adrenal lesion has a 70.1% probability of malignancy [29.9% probability of benignity]).
Example 2: Unenhanced Images Available
Assume a patient with identical characteristics to the patient in Example 1 except that this patient also underwent unenhanced CT, with unenhanced attenuation (UA) of 12 HU.
The absolute percentage of enhancement washout (APEW) is calculated as [(PV – DL) / (PV – UA)] × 100 = [(150 – 90)/(150 – 12)] = 43.5%. Using the parameters UA (in HU) as X1, APEW (in %) as X2, AGE (in years) as X3, PMH (1 = yes, 0 = no) as X4, and the relevant regression coefficients in the lower half of Table 4,
| (5) |
(i.e., this adrenal lesion has a 66.0% probability of benignity [34.0% probability of malignancy]).
References
- 1.Korobkin M, Brodeur FJ, Francis IR, Quint LE, Dunnick NR, Goodsitt M. Delayed enhanced CT for differentiation of benign from malignant adrenal masses. Radiology 1996; 200:737–742 [DOI] [PubMed] [Google Scholar]
- 2.Boland GW, Hahn PF, Pena C, Mueller PR. Adrenal masses: characterization with delayed contrast-enhanced CT. Radiology 1997; 202:693–696 [DOI] [PubMed] [Google Scholar]
- 3.Szolar DH, Kammerhuber F. Quantitative CT evaluation of adrenal gland masses: a step forward in the differentiation between adenomas and nonadenomas? Radiology 1997; 202:517–521 [DOI] [PubMed] [Google Scholar]
- 4.Szolar DH, Kammerhuber FH. Adrenal adenomas and nonadenomas: assessment of washout at delayed contrast-enhanced CT. Radiology 1998; 207:369–375 [DOI] [PubMed] [Google Scholar]
- 5.Peña CS, Boland GW, Hahn PF, Lee MJ, Mueller PR. Characterization of indeterminate (lipid-poor) adrenal masses: use of washout characteristics at contrast-enhanced CT. Radiology 2000; 217:798–802 [DOI] [PubMed] [Google Scholar]
- 6.Caoili EM, Korobkin M, Francis IR, Cohan RH, Dunnick NR. Delayed enhanced CT of lipid-poor adrenal adenomas. AJR 2000; 175:1411–1415 [DOI] [PubMed] [Google Scholar]
- 7.Park SW, Kim TN, Yoon JH, et al. The washout rate on the delayed CT image as a diagnostic tool for adrenal adenoma verified by pathology: a multicenter study. Int Urol Nephrol 2012; 44:1397–1402 [DOI] [PubMed] [Google Scholar]
- 8.Taffel M, Haji-Momenian S, Nikolaidis P, Miller FH. Adrenal imaging: a comprehensive review. Radiol Clin North Am 2012; 50:219–243 [DOI] [PubMed] [Google Scholar]
- 9.Park BK, Kim CK, Kim B. Adrenal incidentaloma detected on triphasic helical CT: evaluation with modified relative percentage of enhancement washout values. Br J Radiol 2008; 81:526–530 [DOI] [PubMed] [Google Scholar]
- 10.Kamiyama T, Fukukura Y, Yoneyama T, Takumi K, Nakajo M. Distinguishing adrenal adenomas from nonadenomas: combined use of diagnostic parameters of unenhanced and short 5-minute dynamic enhanced CT protocol. Radiology 2009; 250:474–481 [DOI] [PubMed] [Google Scholar]
- 11.Foti G, Faccioli N, Mantovani W, Malleo G, Manfredi R, Mucelli RP. Incidental adrenal lesions: Accuracy of quadriphasic contrast enhanced computed tomography in distinguishing adenomas from nonadenomas. Eur J Radiol 2012; 81:1742–1750 [DOI] [PubMed] [Google Scholar]
- 12.Angelelli G, Mancini ME, Moschetta M, Pedote P, Pignataro P, Scardapane A. MDCT in the differentiation of adrenal masses: comparison between different scan delays for the evaluation of intralesional washout. Scientific World Journal 2013; 2013:957–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumagae Y, Fukukura Y, Takumi K, et al. Distinguishing adrenal adenomas from non-adenomas on dynamic enhanced CT: a comparison of 5 and 10 min delays after intravenous contrast medium injection. Clin Radiol 2013; 68:696–703 [DOI] [PubMed] [Google Scholar]
- 14.Gufler H, Eichner G, Grossmann A, et al. Differentiation of adrenal adenomas from metastases with unenhanced computed tomography. J Comput Assist Tomogr 2004; 28:818–822 [DOI] [PubMed] [Google Scholar]
- 15.Song JH, Grand DJ, Beland MD, Chang KJ, Machan JT, Mayo-Smith WW. Morphologic features of 211 adrenal masses at initial contrast-enhanced CT: can we differentiate benign from malignant lesions using imaging features alone? AJR 2013; 201:1248–1253 [DOI] [PubMed] [Google Scholar]
- 16.Dunnick NR, Korobkin M, Francis I. Adrenal radiology: distinguishing benign from malignant adrenal masses. AJR 1996; 167:861–867 [DOI] [PubMed] [Google Scholar]
- 17.[No authors listed]. NIH state-of-the-science statement on management of the clinically inapparent adrenal mass (“incidentaloma”). NIH Consens State Sci Statements 2002; 19:1–25 [PubMed] [Google Scholar]
- 18.Grumbach MM, Biller BMK, Braunstein GD, et al. Management of the clinically inapparent adrenal mass (“incidentaloma”). Ann Intern Med 2003; 138:424–429 [DOI] [PubMed] [Google Scholar]
- 19.Choyke PL; ACR Committee on Appropriateness Criteria. ACR Appropriateness Criteria on incidentally discovered adrenal mass. J Am Coll Radiol 2006; 3:498–504 [DOI] [PubMed] [Google Scholar]
- 20.Young WF Jr. Clinical practice: the incidentally discovered adrenal mass. N Engl J Med 2007; 356:601–610 [DOI] [PubMed] [Google Scholar]
- 21.Mazzaglia PJ. Radiographic evaluation of nonfunctioning adrenal neoplasms. Surg Clin North Am 2014; 94:625–642 [DOI] [PubMed] [Google Scholar]
- 22.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–35 [DOI] [PubMed] [Google Scholar]
- 23.Proschan MA, Waclawiw MA. Practical guidelines for multiplicity adjustment in clinical trials. Control Clin Trials 2000; 21:527–539 [DOI] [PubMed] [Google Scholar]
- 24.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44:837–845 [PubMed] [Google Scholar]
- 25.Bae KT, Fuangtharnthip P, Prasad SR, Joe BN, Heiken JP. Adrenal masses: CT characterization with histogram analysis method. Radiology 2003; 228:735–742 [DOI] [PubMed] [Google Scholar]
- 26.Stadler A, Schima W, Prager G, et al. CT density measurements for characterization of adrenal tumors ex vivo: variability among three CT scanners. AJR 2004; 182:671–675 [DOI] [PubMed] [Google Scholar]
- 27.Jhaveri KS, Wong F, Ghai S, Haider MA. Comparison of CT histogram analysis and chemical shift MRI in the characterization of indeterminate adrenal nodules. AJR 2006; 187:1303–1308 [DOI] [PubMed] [Google Scholar]
- 28.Hammarstedt L, Thilander-Klang A, Muth A, Wangberg B, Oden A, Hellstrom M. Adrenal lesions: variability in attenuation over time, between scanners, and between observers. Acta Radiol 2013; 54:817–826 [DOI] [PubMed] [Google Scholar]
- 29.Lamba R, McGahan JP, Corwin MT, et al. CT Hounsfield numbers of soft tissues on unenhanced abdominal CT scans: variability between two different manufacturers’ MDCT scanners. AJR 2014; 203:1013–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.