Abstract
The bulky dehydroamino acids dehydrovaline (ΔVal) and dehydroethylnorvaline (ΔEnv) can be inserted into the turn regions of β-hairpin peptides without altering their secondary structures. These residues increase proteolytic stability, with ΔVal at the (i + 1) position having the most substantial impact. Additionally, a bulky dehydroamino acid can be paired with a D-amino acid (i.e., D-Pro) to synergistically enhance resistance to proteolysis. A link between proteolytic stability and peptide structure is established by the finding that a stabilized ΔVal-containing β-hairpin is more highly folded than its Asn-containing congener.

Although peptides play crucial roles in medicine,1 their use as drugs is hampered by rapid proteolytic degradation. Several methods for stabilizing peptides to proteolysis have been devised,2–12 but additional strategies are needed if they are to realize their full potential as therapeutic agents. In seminal work, Stammer and co-workers demonstrated that incorporating a,β-dehydroamino acids (ΔAAs) such as ΔAla, Z-ΔPhe, and Z-ΔLeu into enkephalin mimics protected these peptides from proteolysis.13 Presumably, the A1,3 strain induced by the trisubstituted alkenes present in Z-ΔPhe and Z-ΔLeu rigidifies the backbones of peptides containing these residues, thereby conferring proteolytic stability by favoring folded states over more flexible random coil conformations.14 We hypothesized that bulky ΔAAs containing tetrasubstituted alkenes (e.g., ΔVal and its homologue dehydroethylnorvaline, or ΔEnv) would be particularly effective at stabilizing peptides to proteolysis due to their elevated levels of A1,3-strain caused by the presence of two allylic alkyl groups. We also reasoned that these residues would be less reactive Michael acceptors than ΔAAs possessing di- or trisubstituted alkenes, which are prone to attack by biologically relevant nucleophiles such as thiols.15 To the best of our knowledge, the impact of bulky ΔAAs on the proteolysis of peptides that contain them has not been studied previously. Prompted by these considerations and inspired by the presence of ΔVal and ΔIle in bioactive peptides such as yaku’amides,16 phomopsins,17 and myxovalargin A,18 we investigated the effect of bulky ΔAAs on proteolysis and peptide structure. Herein, we report that ΔVal and ΔEnv can impart substantial proteolytic stability to β-hairpin peptides. This result can be at least partially attributed to an increased thermodynamic preference for the folded states of the β-hairpins.
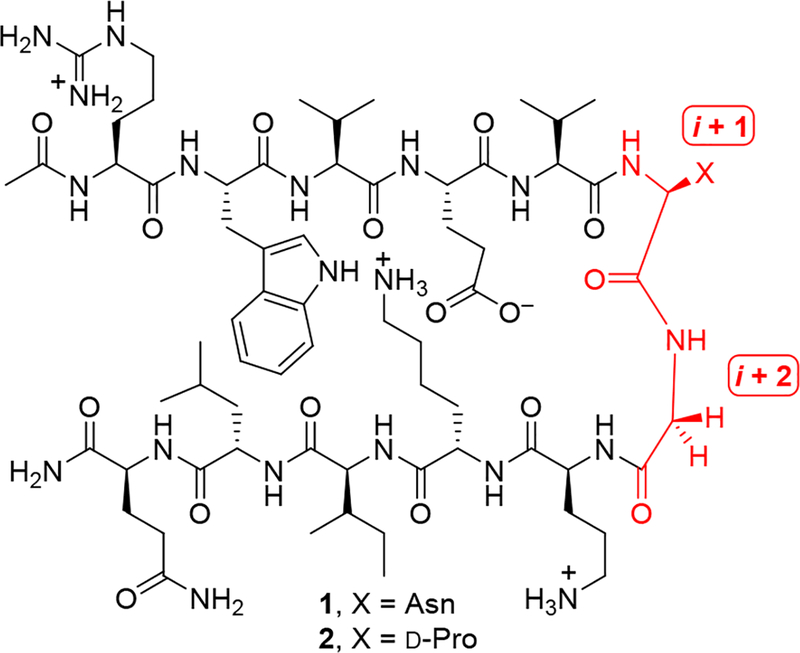
Reports describing the effect of ΔVal on peptide structure are scarce,19 and we are unaware of published studies involving ΔEnv.20 A dipeptide containing ΔVal exhibits a solution conformation that resembles a β-turn,19a and X-ray crystallography data show that ΔVal can adopt dihedral angles compatible with the (i + 1) position of a type II β-turn.19b,e Type II’ β-turns should be equally accessible given that this residue is achiral. Accordingly, we posited that β-hairpins containing ΔVal or ΔEnv in their turn regions would retain their secondary structure while possessing enhanced stability to proteolysis. We selected β-hairpins designed by Waters and co-workers21 (1 and 2, Figure 1) to test this hypothesis. Close relatives of these peptides are well-folded and somewhat resistant to proteolysis, with the D-Pro-Gly-turn-containing peptide22 analogous to 2 exhibiting the most stability.21b Thus, β-hairpins 1 and 2 presented a stringent test of the ability of bulky ΔAAs to impart rigidity and stability to peptides. Additionally, the amenability of 1 and 2 to study by NMR spectroscopy would yield structural insights that could help explain the increased proteolytic stability that we hoped to observe.
Figure 1.
Model β-hairpins designed by Waters and co-workers.
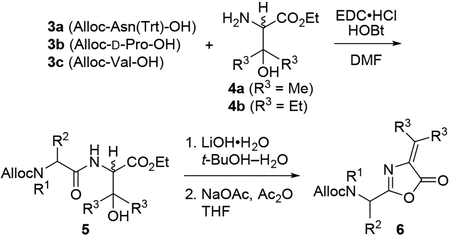
We used our recently published method for opening azlactones (i.e., oxazolones) with resin-bound amines23 to construct the targeted bulky ΔAA-containing peptides. The requisite azlactone dipeptides were synthesized as shown in Table 1. Coupling of Alloc-protected amino acids 3 with racemic β-OHVal-OEt (4a)24 or racemic β-OHEnv-OEt (4b)23 furnished dipeptides 5. In contrast to our prior work, the use of rigorously purified samples of 4b enabled EDC•HCl and HOBt to be employed in couplings instead of the more expensive COMU. Saponification was followed by a one-pot dehydration-cyclization sequence mediated by NaOAc and Ac2O.25 We originally employed Ac2O as solvent,23 but challenges in completely removing this high-boiling compound from 5 prior to the ring-opening reaction led us to devise a new procedure utilizing THF as solvent. The yields listed in Table 1 for azlactones 6 were affected by partial decomposition during SiO2 chromatography. In practice, the purity of the crude azlactones was usually sufficient for use in the ring-opening reactions.
Table 1. Synthesis of Azlactone Dipeptides.
 | ||
|---|---|---|
| Azlactone | yield of 5 (%) | yield of 6 (%) |
| 6aa | 77 | 70 |
| 6ab | 71 | 64 |
| 6ba | 81 | 72 |
| 6bb | 79 | 73 |
| 6ca | 84 | 80 |
| 6cb | 78 | 74 |
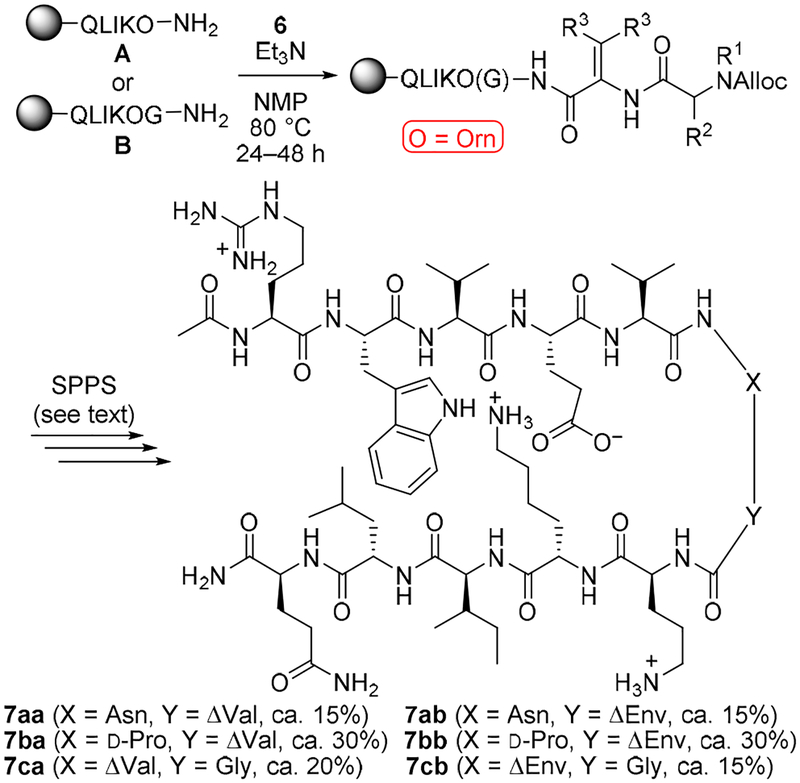
Modifying our published ring-opening procedure23 by employing Et3N instead of DMAP facilitated attachment of the bulky ΔAAs to solid-supported peptides (Scheme 1). Slow stirring was required to prevent degradation of the resin during the reaction. Pentapeptide A was used as the nucleophile to construct β-hairpins with bulky ΔAAs at the (i + 2) position (i.e., “Y” in Scheme 1), whereas hexapeptide B was enlisted to synthesize β-hairpins with Gly at (i +2) and bulky ΔAAs at (i + 1). Subsequent acetylation capped any unreacted resin-bound amines. Alloc deprotection enabled attachment of the remaining residues via standard microwave-promoted solid-phase peptide synthesis (SPPS) protocols.26 Acetylation, cleavage from the resin, and HPLC purification afforded the desired bulky ΔAA-containing peptides 7aa–7cb. By considering the resin loadings, HPLC traces of the crude peptides, and concentrations of peptide solutions utilized in NMR and proteolysis studies, we estimated the overall yields of 7aa–7cb that are given in Scheme 1. These approximations suggest that the ring-openings are fairly efficient, with the best results obtained from D-Pro-containing azlactones 6ba and 6bb and some attenuation in yield observed with the hindered Asn(Trt)-containing azlactones 6aa and 6ab.
Scheme 1.
Synthesis of Bulky ΔAA-Containing Peptides
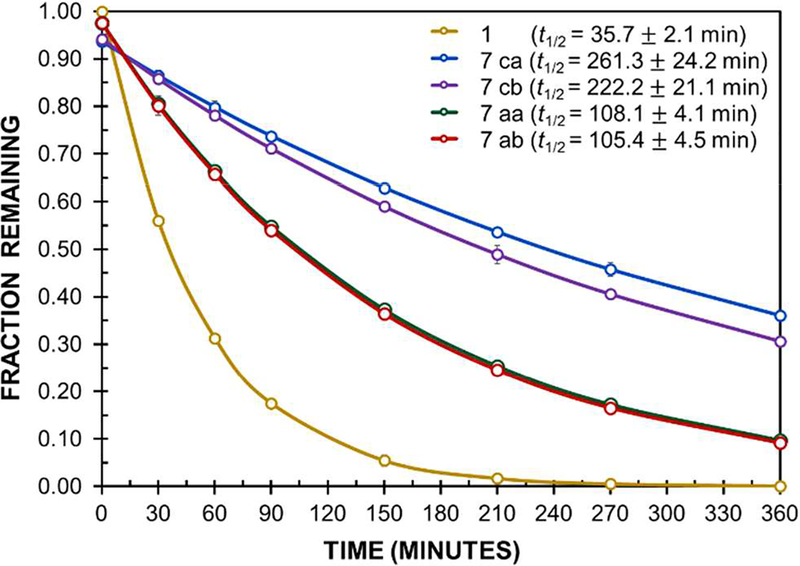
The proteolytic stabilities of 1 and its four bulky-ΔAA-containing analogues were determined by exposure to Pronase, an aggressive cocktail of nonspecific proteases from Streptomyces griseus.21b Monitoring of the proteolytic degradation by HPLC established that each analogue was substantially more stable than 1. Peptides 7ca and 7cb containing ΔVal and ΔEnv, respectively, in place of the (i + 1) Asn were ca. 6–7 times more stable to proteolysis than 1 (Figure 2). Replacement of the (i + 2) Gly with either ΔVal (7aa) or ΔEnv (7ab) also improved stability, but was less beneficial than (i + 1) substitution, resulting in a ca. threefold increase in half-life. ΔVal exhibited a slightly stronger stabilizing effect at (i + 1) than did ΔEnv, and the impact of both bulky ΔAAs at (i + 2) was identical within experimental error.
Figure 2.
Proteolysis of 1 and analogues by Pronase.
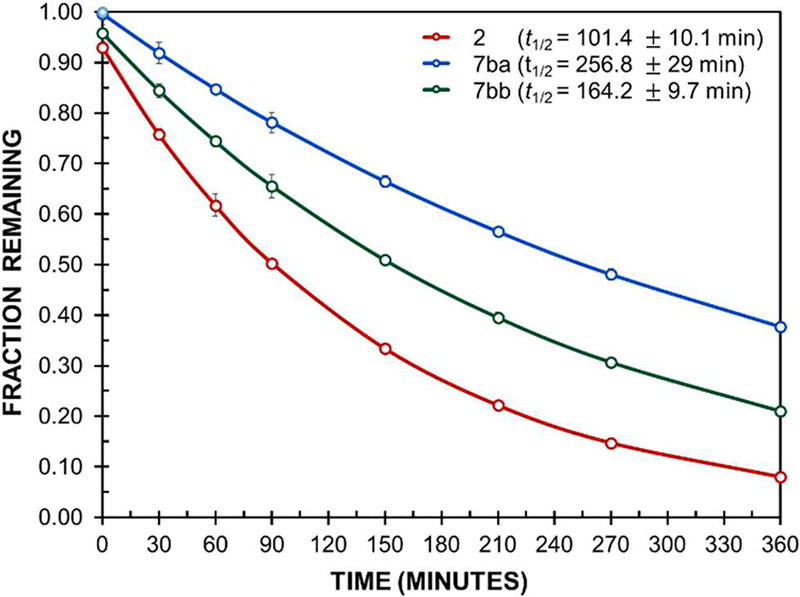
These encouraging results caused us to wonder if bulky ΔAAs could work synergistically with other proteolytically stabilizing elements without negatively impacting secondary structure. Thus, analogues of 2 pairing the (i + 1) D-Pro with either ΔVal (7ba) or ΔEnv (7bb) at (i + 2) were subjected to the Pronase proteolysis assay (Figure 3). These assays were conducted with higher concentrations of the enzymes than those shown in Figure 2 in order to permit proteolysis of these more stable peptides to occur on a reasonable timescale. While this prevents direct comparison of the two sets of assays, examination of Figure 3 indicates that (1) bulky ΔAAs can impart additional proteolytic stability to peptides that contain a D amino acid, and (2) in this case the stabilizing impact of ΔVal is more pronounced than that of ΔEnv.
Figure 3.
Proteolysis of 2 and analogues by Pronase.
NMR spectral data (1.0–3.5 mM of peptide in 10% v/v D2O/H2O buffered to pD 3.9) demonstrated that bulky ΔAA-containing peptides 7 retain the β-hairpin structures of 1 and 2. For example, their Hα protons were shifted significantly downfield (i.e., ≥0.2 ppm) relative to random coil reference values,27,28 with the exception of residues in the turn and C-terminal regions. The C-terminal regions are likely affected by fraying of the strand. Numerous cross-strand NOEs were also observed.28 The 1H NMR spectra of 1, 2, and most of the peptides 7 were characterized by one set of well-defined signals, which is indicative of either a single dominant low-energy conformation or rapid equilibration between multiple conformations. However, extra signals were present in the spectra of ΔEnv-containing peptides 7ab and 7cb. Since these compounds are homogenous according to HPLC analysis, they presumably adopt two or more conformations that interconvert slowly relative to the NMR timescale. Finally, CD spectra of 1, 2, 7ca, and 7ba all showed the minimum at 215 nm that is characteristic of a β-sheet or a β-hairpin.
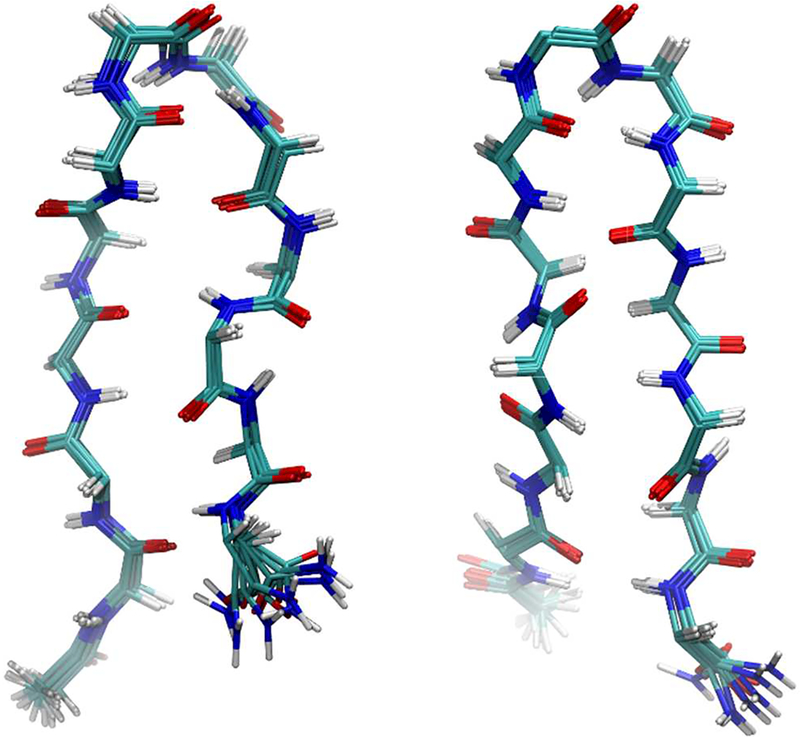
NOE-restrained structural calculations using CYANA29 revealed further insights regarding the solution structures of peptides 1, 2, and 7. Superimposing the ten lowest-energy conformations of each peptide that fit the NOE data indicated that β-hairpins were formed in all cases.28 Comparison of the structural ensembles for peptides 1 and 7ca (Figure 4) illustrates the conformational similarities between these compounds. Qualitative examination of the simulated structures suggested that peptides containing ΔVal are more rigid than those containing ΔEnv. This observation was supported by RMSD calculations28 and is consistent with the multiple conformations seen in the NMR spectra of 7ab and 7cb. Since each ΔVal-containing peptide is either more stable to Pronase exposure than its Env-containing congener or equivalently stable, it appears that proteolytic stability and rigidity of the peptide backbone are correlated for this series of β-hairpins.
Figure 4.
Solution-phase structures of 1 (left) and 7ca (right) as calculated using NOE distance restraints. Side chains have been removed for clarity.
Fraction folded values for 1 and its most proteolytically stable analogue 7ca were determined using 1H NMR spectroscopy.21,30,31 Specifically, the Hα chemical shifts of Val3, Val5, Orn8, and Ile10, each of which are engaged in cross-strand hydrogen bonding, were compared to chemical shift values obtained from fully folded (i.e., cyclic) and random coil control peptides.21 This method showed 1 and 7ca to be 77% and 90% folded, respectively. Similar fraction folded values of 73% for 1 and 94% for 7ca were calculated by measuring the amount of chemical shift separation between the diastereotopic Gly7 α-hydrogens of these peptides.21 Conversion of these values into differences in free energy suggests that insertion of ΔVal at the (i + 1) position of a β-turn can increase the preference for the folded state by ca. 0.6–1.0 kcal/mol. Thus, the substantially improved resistance to proteolysis of 7ca relative to 1 can at least partially be attributed to the impact of the bulky ΔAAs on the equilibrium between the folded and unfolded states. The observed shift of the equilibrium towards the folded state could be accomplished by either stabilizing the folded state or by destabilizing the random coil conformations. Although further study is required to determine which scenario is operative, we suggest that the bulky ΔAAs are destabilizing many of the random coil conformations of β-hairpins 7 through their inherently high levels of A1,3 strain.
In conclusion, we have found that the bulky dehydroamino acids dehydrovaline and dehydroethylnorvaline can be inserted into the turn regions of β-hairpins without perturbing secondary structure. These residues enhance proteolytic stability, with more pronounced effects seen at the (i + 1) versus the (i + 2) position and with ΔVal versus ΔEnv. A bulky ΔAA can also be paired with a D-amino acid (i.e., D-Pro) to synergistically increase resistance to proteolysis. In one case, the greater proteolytic stability of a ΔVal-containing β-hairpin (7ca) relative to its parent peptide (1) was correlated with a stronger thermodynamic preference for the folded state versus unfolded states. This work adds to the growing number of uses for dehydroamino acids.32 Studies of the impact of bulky ΔAAs on other secondary structures and on the proteolytic stability and efficacy of bioactive peptides are in progress.
Supplementary Material
ACKNOWLEDGMENT
We thank the National Institutes of Health (SLC: R15GM114789) and Brigham Young University (Simmons Center for Cancer Research Fellowship to A.J., Undergraduate Research Awards to D.W.K. and K.G.I.W.) for financial support.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures, compound characterization data, simulated structures, NMR spectra, and HPLC traces (PDF)
notes
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Fosgerau K; Hoffmann T Drug Discovery Today 2015, 20, 122. [DOI] [PubMed] [Google Scholar]; (b) Kaspar AA; Reichert JM Drug Discovery Today 2013, 18, 807. [DOI] [PubMed] [Google Scholar]; (c) Craik DJ; Fairlie DP; Liras S; Price D Chem. Biol. Drug Des 2013, 81, 136. [DOI] [PubMed] [Google Scholar]
- (2).(a) Peptoids: Sun J; Zuckermann RN ACS Nano 2013, 7, 4715. [DOI] [PubMed] [Google Scholar]; (b) Fowler SA; Blackwell HE Org. Biomol. Chem 2009, 7, 1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) β- and α/β-Peptides: Horne WS; Gellman SH Acc. Chem. Res 2008, 41, 1399. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Seebach D; Gardiner J Acc. Chem. Res 2008, 41, 1366. [DOI] [PubMed] [Google Scholar]; (c) Cheng RP; Gellman SH; DeGrado WF Chem. Rev 2001, 101, 3219. [DOI] [PubMed] [Google Scholar]
- (4).(a) Stapled peptides:Cromm PM; Spiegel J; Grossmann TN ACS Chem. Biol 2015, 10, 1362. [DOI] [PubMed] [Google Scholar]; (b) Walensky LD; Bird GH J. Med. Chem 2014, 57, 6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Hydrogen bond surrogates: Patgiri A; Jochim AL; Arora PS Acc. Chem. Res. 2008, 41, 1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) γ-AApeptides: Teng P; Shi Y; Sang P; Cai J Chem.— Eur. J 2016, 22, 5458. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shi Y; Teng P; Sang P; She F; Wei L; Cai J Acc. Chem. Res 2016, 49, 428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).D-Peptides: Weinstock MT; Francis JN; Redman JS; Kay MS Biopolymers (Pept. Sci.) 2012, 98, 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).(a) β-Turn mimics: Lubell WD; Khashper A Org. Biomol. Chem 2014, 12, 5052. [DOI] [PubMed] [Google Scholar]; (b) MacDonald M; Aubé J Curr. Org. Chem 2001, 5, 417. [Google Scholar]
- (9).α,α-Disubstituted amino acids: Toniolo C; Formaggio F; Kaptein B; Broxterman QB Synlett 2006, 1295. [Google Scholar]
- (10).(a) PEGylation: Nischan N; Hackenberger CPR J. Org. Chem 2014, 79, 10727. [DOI] [PubMed] [Google Scholar]; (b) Harris JM; Chess RB Nat. Rev. Drug Discovery 2003, 2, 214. [DOI] [PubMed] [Google Scholar]
- (11).(a) Conjugation to albumin or antibody fragments: Elsadek B; Kratz F J. Controlled Release 2012, 157, 4. [DOI] [PubMed] [Google Scholar]; (b) Pollaro L; Heinis C Med. Chem. Commun 2010, 1, 319. [Google Scholar]
- (12).Anchoring to plasma membranes: O’Banion CP; Nguyen LT; Wang Q; Priestman MA; Holly SP; Parise LV; Lawrence DS Angew. Chem., Int. Ed. 2016, 55, 1030. [DOI] [PubMed] [Google Scholar]
- (13).(a) English ML; Stammer CH Biochem. Biophys. Res. Commun 1978, 83, 1464. [DOI] [PubMed] [Google Scholar]; (b) English ML; Stammer CH Biochem. Biophys. Res. Commun 1978, 85, 780. [DOI] [PubMed] [Google Scholar]; (c) Shimohigashi Y; Chen H3C; Stammer CH Peptides 1982, 3, 985. [DOI] [PubMed] [Google Scholar]; (d) Shimohigashi Y; Stammer CH J. Chem. Soc., Perkin Trans 1 1983, 803. [Google Scholar]
- (14).(a) Daniel RM; Cowan DA; Morgan HW; Curran MP Biochem. J 1982, 207, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Imoto T; Yamada H; Ueda T J. Mol. Biol. 1986, 190, 647. [DOI] [PubMed] [Google Scholar]; (c) Parsell DA; Sauer RT J. Biol. Chem 1989, 264, 7590. [PubMed] [Google Scholar]; (d) Klink TA; Raines RT J. Biol. Chem 2000, 275, 17463. [DOI] [PubMed] [Google Scholar]; (e) Ahmad S; Kumar V; Ramanand KB; Rao NM Protein Sci. 2012, 21, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).(a) Yang X; van der Donk WA ACS Chem. Biol 2015, 10, 1234. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chatterjee C; Paul M; Xie L; van der Donk WA Chem. Rev 2005, 105, 633. [DOI] [PubMed] [Google Scholar]
- (16).(a) Ueoka R; Ise Y; Ohtsuka S; Okada S; Yamori T; Matsunaga SJ Am. Chem. Soc 2010, 132, 17692. [DOI] [PubMed] [Google Scholar]; (b) Kuranaga T; Sesoko Y; Sakata K; Maeda N; Hsayata A; Inoue MJ Am. Chem. Soc 2013, 135, 5467. [DOI] [PubMed] [Google Scholar]; (c) Kuranaga T; Mutoh H; Sessoko Y; Goto T; Matsunaga S; Inoue MJ Am. Chem. Soc 2015, 137, 9443. [DOI] [PubMed] [Google Scholar]
- (17).Culvenor CCJ; Edgar JA; Mackay MF; Gorst-Allman CP; Marasas WFO; Steyn PS; Vleggaar R; Wessels PL Tetrahedron 1989, 45, 2351. [Google Scholar]
- (18).(a) Irschik H, Gerth K, Kemmer T, Steinmetz H, Reichenbach H J. Antibiot 1983, 36, 6. [DOI] [PubMed] [Google Scholar]; (b) Irschik H, Reichenbach HJ Antibiot 1985, 38, 1237. [DOI] [PubMed] [Google Scholar]; (c) Steinmetz H; Irschik H; Reichenbach H; Höfle G Chem. Pept. Proteins 1989, 4, 13. [Google Scholar]
- (19).(a) Pietrzyński G; Rzeszotarska B; Kubica Z Int. J. Peptide Protein Res 1992, 40, 524. [PubMed] [Google Scholar]; (b) Vijayaraghavan R; Kumar P; Dey S; Singh TP Acta Crystallogr., Sect. C 2001, 57, 1220. [DOI] [PubMed] [Google Scholar]; (c) Vijayaraghavan R; Kumar P; Dey S; Singh TP J. Pept. Res 2003, 62, 63. [DOI] [PubMed] [Google Scholar]; (d) Makker J; Dey S; Mukherjee S; Vijayaraghavan R; Kumar P; Singh TP J. Mol. Struct 2003, 654, 119. [Google Scholar]; (e) Siodłak D; Rzeszotarska B; Broda MA; Kozioł AE; Kołodziejczyk E Acta Biochim. Pol 2004, 51, 145. [PubMed] [Google Scholar]; (f) Broda MA; Siodłak D; Rzeszotarska B J. Pept. Sci 2005, 11, 546. [DOI] [PubMed] [Google Scholar]; (g) Siodłak D; Grondys J; Lis T; Bujak M; Broda; Rzeszotarska B J. Pept. Sci 2010, 16, 496. [DOI] [PubMed] [Google Scholar]; (h) Siodłak D; Bujak M; Staś M J. Mol. Struct 2013, 1047, 229. [Google Scholar]
- (20).For a review on bulky ΔAAs, see: Jiang JA; Ma Z; Castle, S. L. Tetrahedron 2015, 71, 5431. [Google Scholar]
- (21).(a) Tatko CD; Waters ML J. Am. Chem. Soc. 2004, 126, 2028. [DOI] [PubMed] [Google Scholar]; (b) Cline LL; Waters ML Biopolymers (Pept. Sci.) 2009, 92, 502. [DOI] [PubMed] [Google Scholar]
- (22).Stanger HE; Gellman SH J. Am. Chem. Soc. 1998, 120, 4236. [Google Scholar]
- (23).Jiang J; Luo S; Castle SL Tetrahedron Lett. 2015, 56, 3311. [Google Scholar]
- (24).Ma Z; Naylor BC; Loertscher BM; Hafen DD; Li JM; Castle SL J. Org. Chem. 2012, 77, 1208. [DOI] [PubMed] [Google Scholar]
- (25).Abdel-Motaleb RM; Bakeer HM; Tamam GH; Arafa WA A. J. Heterocycl. Chem. 2012, 49, 1071. [Google Scholar]
- (26).Murray JK; Gellman SH Org. Lett. 2005, 7, 1517. [DOI] [PubMed] [Google Scholar]
- (27).(a) Wishart DS; Sykes BD; Richards FM J. Mol. Biol. 1991, 222, 311. [DOI] [PubMed] [Google Scholar]; (b) Wishart DS; Sykes BD; Richards FM Biochemistry 1992, 31, 1647. [DOI] [PubMed] [Google Scholar]
- (28).See Supporting Information for details. [Google Scholar]
- (29).Güntert P; Mumenthaler C; Wüthrich KJ Mol. Biol. 1997, 273, 283. [DOI] [PubMed] [Google Scholar]
- (30).(a) Syud FA; Espinosa JF; Gellman SH J. Am. Chem. Soc. 1999, 121, 11577. [Google Scholar]; (b) Sarnowski MP; Kang CW; Elbatrawi YM; Wojtas L; Del Valle JR Angew. Chem., Int. Ed 2017, 56, 2083. [DOI] [PubMed] [Google Scholar]
- (31).The multiple conformations in the NMR spectra of 7ab and 7cb precluded calculation of fraction folded values for these peptides. Additionally, the absence of Gly in 7aa, 7ba, and 7bb eliminates one of the two methods of obtaining fraction folded data for these compounds. Accordingly, 7ca and its parent 1 were the only peptides in this study for which the fraction folded could be reliably determined.
- (32).Le DN; Riedel J; Kozlyuk N; Martin RW; Dong VM Org. Lett. 2017, 19, 114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.