Abstract
Hyperoxia during treatment for prematurity may enhance susceptibility to other risk factors for adverse brain development, such as air pollution exposure, as both of these risk factors have been linked to a variety of adverse neurodevelopmental outcomes. This study investigated the combined effects of neonatal hyperoxia followed by inhalation of concentrated ambient ultrafine particles (CAPS, <100nm in aerodynamic diameter) on learning. C57BL/6J mice were birthed into 60% oxygen until postnatal day (PND) 4 and subsequently exposed to filtered air or to CAPS using the Harvard University Concentrated Ambient Particle System (HUCAPS) from PND 4–7 and 10–13. Behavior was assessed on a fixed interval (FI) schedule of reinforcement in which reward is available only after a fixed interval of time elapses, as well as expected reductions in behavior during an extinction procedure when reward was withheld. Both produce highly comparable behavioral performance across species. Performance measures included rate of responding, response accuracy, and temporal control (quarter life). Exposure to hyperoxia or CAPS resulted in lower mean quarter life values, an effect that was further enhanced in males by combined exposure, findings consistent with delayed learning of the FI schedule. Females also initially exhibited greater reductions in quarter life values following the combined exposure to hyperoxia and CAPS and delayed reductions in response rates during extinction. Combined hyperoxia and CAPS produced greater learning deficits than either risk factor alone, consistent with enhanced neurodevelopmental toxicity, findings that could reflect a convergence of both insults on common neurobiological systems. The basis for sex differences in outcome warrants further research. This study highlights the potential for heightened risk of adverse neurodevelopment outcomes in individuals born preterm in regions with higher levels of ultrafine particle (UFP) air pollution, in accord with the multiplicity of risk factors extant in the human environment.
Keywords: ultrafine particles, prematurity, neonatal hyperoxia, air pollution, temporal control, learning
Introduction
Risk factors associated with prematurity include hyperoxia (Deulofeut et al. 2006), ischemia (Vohr et al. 2000), infection (Stoll et al. 2004), and thermoregulatory impairments (Simbruner et al. 2010), all of which can lead to cognitive dysfunction. Advances in medical care over the past two decades have increased survival and prognosis for very preterm infants (<32 weeks) (Stoll et al. 2015). Successful survival of these infants, however, raises additional concerns surrounding their unique challenges and susceptibilities. One early environmental insult of concern is exposure to air pollution, which itself is associated with risk of prematurity (Laurent et al. 2016), and as such, pre-term infants may be returning to homes in higher air pollution environments. Thus, the CNS injuries that can occur in children born prematurely may be followed by exposure to other risk factors for adverse neurodevelopment, such as air pollution.
Air pollution is considered a global health risk, and represents a heightened threat to vulnerable populations (Cohen et al. 2017). Air pollution is a heterogeneous mixture of particles, gases, and organic compounds, many of which have the potential to alter CNS development. Of particular concern with regard to the CNS is the ambient ultrafine particle (UFPs; <100nm in diameter) component of this mixture. UFPs are capable of directly translocating into the CNS via uptake into nerve terminals in the olfactory mucosa and subsequently depositing within the brain parenchyma where they can produce neuroinflammation (Elder et al. 2006; Oberdorster et al. 2004). UFPs can also exert effects via indirect mechanisms, including long-term retention in the lung, which can trigger chronic inflammation (Park et al. 2015; Sun et al. 2012) and potentially lead to systemic inflammation. Our laboratory has shown that early neonatal exposure to concentrated ultrafine ambient particles (CAPS) can produce neuropathological changes indicative of a neuroinflammatory response, including ventriculomegaly, microglial activation, pro-inflammatory cytokine changes and delayed-white matter development (Allen et al. 2014a; Allen et al. 2015), highlighting the susceptibility of the developing CNS to the consequences of air pollution.
Preterm infants undergo a unique, untimely transition from an appropriate low oxygen, hypoxic in utero environment to a high oxygen clinical environment to ensure their survival (Gao and Raj 2010; Sola 2015). During this transition, it is common for oxygen blood saturation levels to fluctuate above clinical targets in preterm infants during long-term oxygen supplementation, with infants in some facilities spending up to 50% of their time in hyperoxic conditions (Deulofeut et al. 2006; Sink et al. 2011). Neonatal hyperoxia in preterm infants is well-established as a contributor to bronchopulmonary dysplasia (BPD), a chronic respiratory disease characterized by simplified alveolar structure and restrictive airways (Domm et al. 2015). More evidence is emerging that hyperoxia also contributes to CNS deficits, as duration of oxygen support for preterm infants has been linked to decreased cortical growth (Bouyssi-Kobar et al. 2016) and increased cerebral oxygenation following birth has also been linked to poor cognitive outcomes measured using the Bayley Scales (Verhagen et al. 2015). Neonatal hyperoxia exposure in rodents decreases cortical growth (Sirinyan et al. 2006), disrupts white matter growth (Ritter et al. 2013), and leads to glial activation (Schmitz et al. 2011; Vottier et al. 2011).
The adverse consequences of a hyperoxic environment on the developing CNS could conceivably be compounded by subsequent exposure to air pollution, given its targeting of the developing CNS and the fact that like hyperoxia, it too can impair cognitive functions, as human brain development remains significant nearly into adulthood (Arnold 2009; Vertes and Bullmore 2015). Prematurity has been linked to a variety of adverse neurodevelopmental clinical outcomes including autism spectrum disorder (ASD) (Jarjour 2015), attention deficit hyperactivity disorder (ADHD) (Bhutta et al. 2002), schizophrenia (Dalman et al. 1999) and cognitive dysfunction (Soria-Pastor et al. 2008), neurodevelopmental disorders that have also been linked to early air pollution exposures. Exposures to traffic-related (Becerra et al. 2013; Volk et al. 2011) and PM2.5 (Volk et al. 2013) air pollution exposure during the perinatal period have been associated with an increased risk for ASD in Los Angeles. Elevated ambient PM10 exposure during childhood was associated with increased prevalence of ADHD in India (Siddique et al. 2011). Increased black carbon exposure of children in Boston, Massachusetts was associated with decreased scores in verbal and nonverbal memory assessment (Suglia et al. 2008). Increased PM2.5 exposure was associated with reductions in cognitive growth and working memory in a prospective cohort of schoolchildren in Barcelona, Spain (Basagana et al. 2016). The similarity of the adverse neurodevelopmental outcomes of hyperoxia and UFPs and their potential for sequential occurrence raises the possibility that these exposures could produce cumulative neurodevelopmental risk, and underscores the need to consider their combined effects. The imposition of UFP exposure post hyperoxia exposure at birth therefore embodies a more real-world environmental exposure scenario and thus is of greater translational relevance.
To understand the potential for enhanced neurodevelopmental risk, this study used a model of neonatal hyperoxia followed by CAPS exposure in mice. As CNS development in rodents at birth is equivalent to the early third-trimester in humans (Semple et al. 2013), this model effectively resembles the CNS developmental stage of preterm infants exposed to hyperoxia. Learning was assessed by examining acquisition of prototypical behavior on a fixed-interval (FI) schedule of food reward which provides access to reward contingent upon the first occurrence of a designated response that occurs after a specified fixed interval of time has elapsed; responses prior to the completion of the interval have no consequence and cannot accelerate time to reward availability. The characteristic behavioral pattern controlled by this schedule occurs across a wide range of species (Kelleher and Morse 1968) and relies on temporal control, i.e, it ultimately consists of little or no responding early in the interval followed by maximal rates of responding as the end of the interval approaches to minimize delay of reward, thus necessitating a temporal discrimination by the organism. Temporal control increases with age (Brannon et al. 2007), and is correlated with IQ (Chelonis et al. 2004). Measuring temporal behavior on the FI schedule using a quarter life measure (time during the specified interval at which 25% of the responses occurred), as well as reductions in response rate when reward was subsequently withheld during extinction, this study sought to assess whether combined neonatal hyperoxia and CAPS exposure would result in enhanced learning deficits.
Materials and Methods
Animals and Exposure Paradigm
Adult male and female C57BL/6J mice from Jackson Laboratories (Bar Harbor, ME) were bred using a scheme designed to ensure timed births as previously described (Allen et al. 2014a). Newborn C57BL6 mice were birthed and maintained at 60% oxygen conditions until neonatal day 4, then maintained under normal animal room oxygen levels (21%). For this purpose, pure oxygen was humidified with sterile distilled water to 40–70%, filtered, and passaged into the chambers before venting out of the building. Mice birthed into room air served as controls. Because adult mice are sensitive to hyperoxia, dams were rotated every 24 hours between litters exposed to room air or hyperoxia. This exposure paradigm has been described and further detailed previously (Yee et al. 2009). A total of 46 litters were exposed to room air or hyperoxia and then divided so that pups from each litter were randomly assigned to subsequent exposure to CAPS or filtered air (Air) in a counterbalanced order that precluded litter-specific effects.
Following hyperoxia exposure, mice were removed from dams and exposed to CAPS, as described previously (Allen et al. 2013; Allen et al. 2014a). Neonatal mice were exposed to filtered air or ambient UFP (<100 nm in diameter) concentrated 10–20 fold using the Harvard Ultrafine Concentrated Ambient Particle System (HUCAPS). Exposures lasted for 4 hours per day beginning at 9:00 a.m. on PND (postnatal day) 4–7 and 10–13. During these exposures, pups were housed in small mesh chambers with four pups per chamber. This high-volume ambient sampling system utilizes condensational growth of the particulate phase in conjunction with virtual impaction to provide aerosols that are enriched for sizes smaller than 200 nm in diameter, with median sizes being typically in the 70–90 nm range with concentrations of approximately 0.2–2 × 105/cm3. The gas-phase components of the ambient aerosol are not concentrated by the HUCAPS system. Particle counts were obtained using a condensation particle counter (model 3022A;TSI, Shoreview, MN), and mass concentration calculated using idealized particle density (1.5 g/cm3). PTFE filters (47 mm, 0.2 μm pore size, Pall, Port Washington, New York) were collected daily from the filtered air and HUCAPS exposure chambers for analysis of elemental composition using x-ray fluorescence (XRF). The hyperoxia and CAPS exposures generated 4 treatment groups per sex: neonatal hyperoxia with and without CAPS (designated H Air and H CAPS, respectively) and neonatal room air with and without CAPS (designated A Air and A CAPS, respectively). No more than 1–2 pups per litter/per sex were assigned to any given treatment group to preclude litter effects, and each treatment group had n=10 except for female A CAPS, where n=9. All experimental activities were approved by the University of Rochester Institutional Animal Care and Use Committee.
Locomotor Behavior
To evaluate motor activity levels, spontaneous locomotor activity was measured in photobeam chambers equipped with a transparent acrylic arena with a 48-channel infrared source, detector, and controller (Med Associates, St. Albans, VT). Locomotor behavior was assessed prior to the start of FI schedule-controlled behavior training (postnatal day 60). Locomotor activity was quantified in three 45-min sessions occurring once per day for 3 consecutive days, with the primary endpoint, ambulatory time, collected at 5 minute epochs. Ambulatory time was defined as the cumulative time in which there were successive breaks of 2×2 photobeam virtual boxes within the chamber. Habituation and average ambulatory activity in each session was explored.
Operant Behavior Apparatus and Fixed Interval Schedule
Food restriction followed locomotor assessment. To enhance and normalize motivation for a food-reinforcement, mice were placed on a food-restricted schedule for 3 days immediately prior to initiation of operant training to reach 85% of ad libitum weight. Mice were maintained at 85% ad libitum body weight throughout the operant training schedule. Behavioral testing was conducted in operant chambers (Med Associates, St. Albans, VT) housed in sound-attenuating cabinets equipped with white noise and fans for ventilation. Three levers were located horizontally across the back wall of the chamber, with a pellet dispenser for reinforcer delivery on the front (opposite) wall. Mice were initially trained to press a lever for food reward using a variable time 60s fixed ratio 1 schedule (VT60FR1), in which a reinforcer (20 mg food pellet) was delivered simultaneously with a light and sound cue on average every 60s independently of behavior; a response on the designated correct lever during this period would also trigger the light and sound cue and reinforcement delivery. Following 10 correct lever press responses or a total of 20 min on the VT60 component, the schedule was changed to a fixed ratio 1 schedule that required a lever press on the designated correct lever for each food delivery until 50 reinforcers had been delivered. After lever press training was completed in all mice, the schedule was shifted to a 60s FI schedule (FI60) examined in 30 minute sessions over a total of 36 sessions (30 consecutive intervals/session/day) to assess learning. On the FI schedule, the first lever press response on the designated correct lever after completion of a 60s interval produced food delivery and initiated the next 60s interval until 30 minutes had elapsed. Responses during the interval itself had no explicit consequence, i.e., were not consequated.
Measures of FI performance included response rate (total responses/total session time, and accuracy (total number of responses on the correct lever/total responses on all levers). Quarter life, i.e., the latency from the onset of an interval to the time at which the first one quarter of the responses in the interval occurred, was used to assess temporal control. Initially, performance on the FI schedule is characterized by uniform responding throughout the interval. However, over sessions, as temporal control is established, pausing begins to follow reinforcement delivery, and maximal responding shifts to later in the interval, with quarter life values thus initially increasing significantly over early sessions, followed by more gradual but continual increases as behavior stabilizes. A mean quarter life (MQL) value was generated across intervals in each session. To further assess temporal control of behavior, the interval was changed from 60s to 120s (FI120) for six sessions following the last session of FI60.
Extinction
To further assess learning, the FI60 schedule was reimposed for two sessions following the FI 120s schedule, after which an extinction schedule was imposed for two sessions. During extinction, all light and sound cues of the FI schedule remained intact, but no food delivery followed the correct lever press after the 60s interval. Under extinction conditions, response rates normally decline rapidly. Correct response rate was assessed across twenty-five 60s intervals in each session.
Statistical Analysis
Data were analyzed using a multi-level mixed-model approach with the “nlme” R package (Pinheiro et al. 2016). Each behavioral endpoint describes a different fundamental aspect of behavior on the FI schedule, thus each measure was analyzed independent of the others in our models. All of the behavioral analyses were stratified by sex. A random intercept and slope model was used to capture subject-level variability. The intercept component was centered and was used as a means of exploring the average response across the sessions while the slope was used to define the linear function of learning across the sessions. Hyperoxia, CAPS, and Session were designated as the fixed effects in the model and we explored the average response and learning slope differences between treatment groups. Interactions between the two factors were explored for each behavioral parameter, but if the interaction term had a p-value > 0.1, it was dropped from the model to avoid over-parameterization. Additionally, as serial autocorrelations were seen across sessions for each animal for FI60 response rates and mean quarter life value, a subject-level autoregressive residual structure (AR1) was used in those models.
For some behaviors, there is a distinct ceiling effect, as behavior increases and reaches asymptotic levels during the finite interval and the learning rate was evaluated with two separate slopes. This included response rates on the FI60 schedule, in which response rates were modeled as two slopes, Sessions 1–12, and Sessions 12–32. Additionally accuracy on the FI60 schedule was modeled as two slopes, Sessions 1–13 and Sessions 13–32. For FI60 mean quarter life, an early acquisition slope (a slope fitted across Sessions 1–14) and a late acquisition slope (a slope fitted across Sessions 14–32) in both males and females. Beyond using the slopes to assess mean quarter life, we also separately evaluated the last third of the sessions on the FI60 and FI120 schedule to evaluate their final latent stable/plateau performance, i.e. how much did the mice learn at the end. Additionally, extinction behavior is limited by a floor effect, as response rate values can only decrease to zero, as was seen following Interval 10 of the second session. Thus, two periods were evaluated (Intervals 1–10, and Intervals 11–25) for extinction session 2. To prevent inappropriate skewing of analysis due to high initial variation when starting on the fixed interval schedule, the first four sessions of FI60 were removed from analysis.
For our findings we are reporting the parameter estimates (β), standard errors (SE), along with the significance tests. The reference groups for the parameter estimates are the unexposed animals with the estimates describing the magnitude of the change in the exposed animals. The default significance test in the “nlme” R package is derived from a t-distribution using t-values calculated by dividing the estimate by the standard error. An example would be if females exposed to hyperoxia had a significant increase in their slope it could be reported as (β = 1.00, SE = 0.30, p = 0.02) with this estimate describing for every one unit change in slope (one session) females exposed to hyperoxia (H Air, H CAPS) increased by one unit more on the given behavioral outcome than the unexposed controls (A Air, A CAPS). Any potential additive (main effects only) or non-additive (interaction effects) effects on average performance were explored with a Tukey post-hoc using the “lsmeans” R package while interactions on the slope component were explored with post-hoc contrasts tests, i.e. comparing the slopes of each the four treatment groups to each other. P-values ≤0.05 were considered significant but near-significant p-values <0.1 were also explored and discussed.
Results
CAPS Exposure Levels, Elemental Analysis and Body Weights
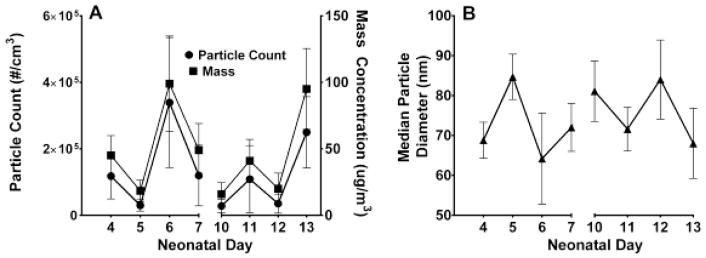
Figure 1 shows particle concentrations and average particle diameter across the period of CAPS exposures. Particle sizes remained in the UFP range, and mean exposure concentrations across treatment were approximately128,426 particles/cm3 and the average mass was 48.1 ug/m3. The average particle diameter remained ultrafine (<100um) throughout the exposures. Table 1 shows the elemental composition of the filtered air and CAPS exposures, as determined using XRF analysis. The average pup weight (litter weight divided by number of pups) following hyperoxia exposure on PND4 was 2.04 grams (g) for the hyperoxia litters and 1.99 g for room air litters. Following CAPS exposure, on PND14, the average pup weights for females was 5.95g (A Air), 6.37g (A CAPS), 5.92g (H Air), 6.06g (H CAPS) and for males 6.05g (A Air), 6.72g (A CAPS), 6.42g (H Air), 6.31g (H CAPS). No treatment-related differences were found for body-weight at P4 or P14.
Figure 1.
Exposure conditions for mice exposed to CAPS during the early neonatal period. Changes in particle count concentration and particle mass concentrations (A), and the median particle diameter across all exposure days (B).
Table 1. Metal Levels in filters from filtered air (HEPA Average) and CAPS exposure from the HUCAPS chambers.
Elemental x-ray fluorescence characterization of Teflon filters from HEPA-filtered (high-efficiency particulate arresting) and CAPS (concentrated ambient ultrafine particulates) exposure chambers. The HEPA-average was determined using three filters collected on three different days from the filtered air system. The total CAPS exposure was eight days with a Teflon filter collected each day, and the top twenty elements by average concentration in the CAPS exposure chamber as characterized by XRF are shown.
| Element | HEPA Average (ng/m3) | CAPS Average (ng/m3) | CAPS Range (ng/m3) | CAPS Days above LOD (%) |
|---|---|---|---|---|
| S | 0.006 | 2058.96 | 519.2 – 5137 | 100% |
| Eu | 0.110 | 313.14 | 372.7 – 869.5 | 50% |
| Zn | 0.026 | 297.96 | 24.35 – 939.5 | 75% |
| Sm | 0.003 | 232.21 | 305.5 – 887.1 | 50% |
| La | 0.033 | 200.56 | 200.6 – 410.4 | 63% |
| Al | 0.115 | 163.19 | 92.9 – 246.8 | 100% |
| Ce | 0.012 | 160.18 | 285.4 – 380.3 | 50% |
| Cs | 0 | 151.13 | 91.7 – 344 | 63% |
| Sc | 0.034 | 116.88 | 38.9 – 273.8 | 88% |
| Na | 0.072 | 97.81 | 155.1 – 396.6 | 38% |
| Ba | 0 | 93.88 | 136.3 – 345.1 | 38% |
| K | 0.003 | 76.68 | 22.75 – 198.45 | 100% |
| Cu | 0.039 | 69.01 | 11.6 – 138.2 | 100% |
| Si | 0.009 | 64.24 | 27.8 – 105.5 | 100% |
| Tb | 0 | 49.61 | 396.9 – 396.9 | 13% |
| Fe | 0 | 47.1 | 13.5 – 130.9 | 63% |
| Sn | 0.025 | 40.55 | 28.6 – 86.4 | 75% |
| Ca | 0 | 28.84 | 14.8 – 73.8 | 88% |
| Pb | 0.028 | 25.1 | 17 – 65.3 | 75% |
| W | 0 | 24.88 | 199.1 – 199.1 | 13% |
The range of female adult weights after food restriction was 16.8–19.5 (A Air), 15.8–19.4 (A CAPS), 16.4–19.6 (H Air) and 15.1–18.8 (H CAPS) and for males 19.3–23.4 (A Air), 19.9–23.3 (A CAPS), 20.1–23.5 (H Air), and 20.1–22.8 (H CAPS). No treatment differences were found in the adult weights.
Locomotor
Ambulatory time of mice during 3 locomotor sessions is shown in Figure 2. Data for female and male mice were analyzed separately, and average ambulatory time and activity habituation (slope) in each session were not significantly different across exposure groups for either females or males.
Figure 2.
Ambulatory times in five minute epochs across three sessions for females (A, B, C) and males (D, E, F) in all four treatment groups across three 45-min sessions. Ambulatory movement was defined as successive breaks of multiple 2 × 2 defined photobeam virtual boxes within the chamber. Data are reported as average ambulatory time for each bin ± SE.
Fixed-Interval 60s Schedule Response Rates and Accuracy
All mice learned food-rewarded lever pressing within 3 training sessions, with no treatment-related differences in the number of sessions required for training.
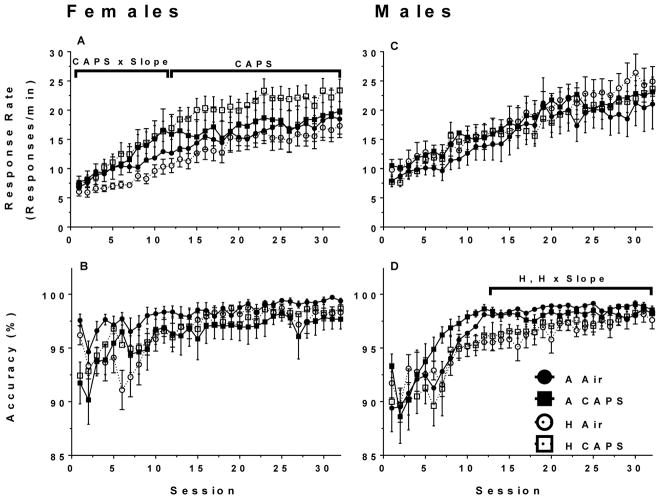
Females exposed to hyperoxia showed no significant changes in response rate slope from Sessions 1–12 (β = 0.01, SE = 0.16, p = 0.99) or Session 12–32 (β = 2.63, SE = 4.76, p = 0.58) nor any significant changes in average response rate (Figure 3A). Females exposed to CAPS had an increase in response rate slope from Sessions 1–12 (β = 0.39, SE = 0.16, p = 0.02) and average response rates were higher in females exposed to CAPS in Sessions 12–32 (β = 5.35, SE = 2.40, p = 0.03). There no significant interaction differences between CAPS and hyperoxia exposed females on average response rates or slopes. There were no significant differences between female treatment groups on accuracy.
Figure 3.
FI60 mean response rates and accuracy for females (A, B, n= 9–10/treatment group) and males (C, D, n =10/treatment group). Data are reported as mean response rate or accuracy for each session ± SE. A linear mixed-model was used to assess for hyperoxia and CAPS differences with significance being defined as p ≤ 0.05. H and CAPS indicate a significant effect of neonatal hyperoxia or CAPS exposure respectively on the average response rate or accuracy, while H x Slope or CAPS x Slope are significant effects of hyperoxia or CAPS on learning rate. The brackets indicate the time period in which the significant effect was present.
In males there were no significant effects of hyperoxia or CAPS on the response rate slope or on average response rates (Figure 3C). Males exposed to hyperoxia had a decrease in accuracy slope during the initial 13 sessions (β = −0.21, SE = 0.17, p = 0.22) though it did not approach significance (Figure 3D). Males exposed to hyperoxia had an increased slope in Sessions 13–32 (β = 0.10, SE = 0.04, p < 0.01) though average accuracy was lower in the hyperoxia-exposed males during that period (β = −1.29, SE = 0.52, p = 0.01) (Figure 3D). There was no significant effect of CAPS or interaction with hyperoxia on accuracy learning rate or average accuracy in Sessions 1–13, or Sessions 13–32.
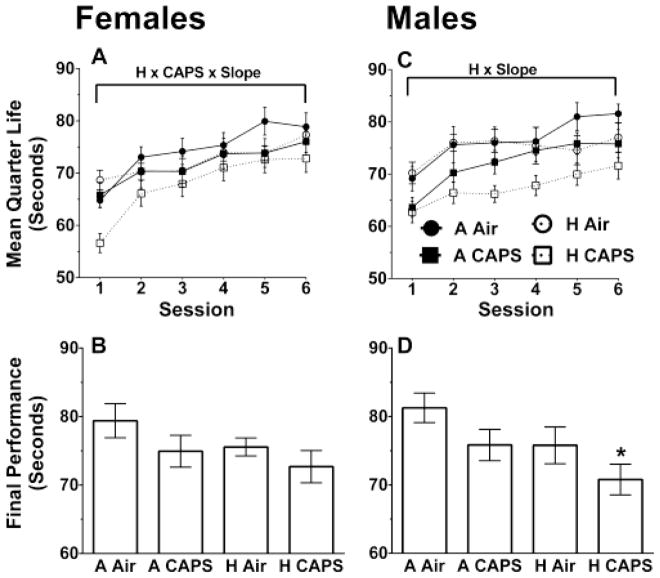
Fixed-Interval 60s Schedule Mean Quarter Life
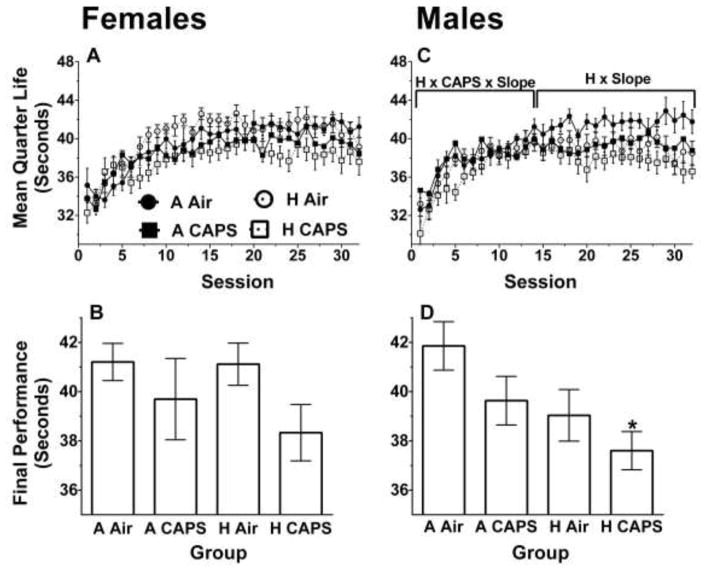
Females exposed to CAPS had a decrease in the mean quarter life slope (β = −0.19, SE = 0.11, p = 0.08) in Sessions 1–14 and hyperoxia-exposed females had a decreased slope in Sessions 14–32 (β = −0.09, SE = 0.05, p = 0.09) though both differences failed to reach statistical significance (Figure 4A). Females exposed to CAPS had a decrease in average performance in the last 10 sessions on the FI60 schedule (β = −2.16, SE = 1.07, p = 0.05) (Figure 4B). Females exposed to hyperoxia showed no significant differences in the final 10 sessions then their unexposed controls (β = −0.75, SE = 1.07, p = 0.49) and there were no significant interaction effects between hyperoxia and CAPS.
Figure 4.
FI60 mean quarter life and average over last 10 Sessions for females (A, B, n= 9–10/treatment group) and for males (C, D, n =10/treatment group). Data are reported as mean quarter life for each bin or session ± SE. A linear mixed-model was used to assess for hyperoxia and CAPS differences with significance being defined as p ≤ 0.05. H and CAPS indicate a significant effect of neonatal hyperoxia or CAPS exposure respectively on the average mean quarter life performance while H x Slope or CAPS x Slope are significant effects of hyperoxia or CAPS on learning rate. H x CAPS x Slope, indicate a significant interaction between hyperoxia, and CAPS on learning rate. The brackets indicate the period in which the significant difference was present. * indicates p ≤ 0.05 when compared to A Air alone with Tukey post-hoc.
In males there was an interaction between CAPS and hyperoxia on the mean quarter life slope for Sessions 1–14 (β = −2.16, SE = 1.07, p = 0.05) (Figure 4C). Contrast tests showed the H CAPS males having a significantly steeper slope then the A CAPS males (β = 0.31, SE = 0.14, p = 0.02) though neither was significantly different from the full control A Air. To further explore this interaction, a Tukey post-hoc was run on the first session, which showed the H CAPS males had a decreased mean quarter life compared to A CAPS males (β = −4.51, SE = 1.89, p = 0.10) though it did not approach significance and neither was significantly different from control A Air. Males exposed to hyperoxia had a decrease on the slope in Sessions 14–32 (β = −0.12, SE = 0.05, p = 0.02) with no differences in the CAPS-exposed males in Sessions 14–32 (β = −0.05, SE = 0.05, p = 0.36) (Figure 4C). No significant interactions were found between hyperoxia and CAPS on the slope in Sessions 14–32.
Males exposed to hyperoxia had a decreased average performance on the last 10 sessions (β = −2.41, SE = 0.91, p = 0.01) and males exposed to CAPS had a decreased average performance on the last 10 sessions (β = −1.83, SE = 0.91, p = 0.05) (Figure 4D). A pairwise Tukey post-hoc on the last 10 sessions showed only the H CAPS males had a significant decrease in final average performance when compared to A Air (β = −4.25, SE = 1.28, p = 0.01).
Fixed-Interval 120s Schedule Mean Quarter Life
In females, there was an interaction between CAPS and hyperoxia on the mean quarter life slope for FI120s schedule (β = −2.10, SE = 0.81, p = 0.01) (Figure 5A). Contrasts tests showed that the H CAPS females had a steeper slope then A CAPS (β = 1.10, SE = 0.58, p = 0.06) though it failed to reach significance and a significantly steeper slope then H Air (β = 1.32, SE = 0.58, p = 0.02) but H CAPS females were not different from control (β = 0.32, SE =0.57, p = 0.57). A Tukey post-hoc analysis on the first session showed the H CAPS females had a significantly decreased average performance when compared to A CAPS (β = −9.21, SE = 2.69, p < 0.01), H Air (β = −12.1, SE = 2.63, p < 0.01), and A Air (β= −8.2, SE = 2.63, p = 0.02). Although average performance on the final two sessions was lower for females exposed to hyperoxia (β = −3.1, SE = 2.10, p = 0.15) and females exposed to CAPS (β = −3.64, SE = 2.10, p = 0.09), it failed to reach statistical significance and there was no significant interaction (Figure 5B).
Figure 5.
FI120 mean quarter life and average over last 2 Sessions for females (A, B, n= 9–10/treatment group) and for males (C, D, n =10/treatment group). Data are reported as mean quarter life for each bin or session ± SE. A linear mixed-model was used to assess for hyperoxia and CAPS differences with significance being defined as p ≤ 0.05. H and CAPS indicate a significant effect of neonatal hyperoxia or CAPS exposure respectively on the average mean quarter life performance while H x Slope or CAPS x Slope are significant effects of hyperoxia or CAPS on learning rate. H x CAPS x Slope, indicate a significant interaction between hyperoxia, and CAPS on learning rate. The brackets indicate the time period in which the significant difference was present. *indicates p ≤ 0.05 when compared to A Air alone with Tukey post-hoc.
Males exposed to hyperoxia had a decreased slope on the FI120 schedule (β = −1.04, SE = 2.10, p = 0.05) and there was no significant differences for the slopes of males exposed to CAPS (β = 0.42, SE = 0.54, p = 0.43) (Figure 5C). However males exposed to CAPS had a decrease in mean quarter life performance on the first session (β = −6.49, SE = 2.09, p < 0.01). Males exposed to hyperoxia had a decreased performance on the final two sessions (β = −5.27, SE = 2.32, p = 0.03) as well as males exposed to CAPS (β = −5.22, SE = 2.32, p = 0.03) (Figure 5D). To explore cumulative effects, a Tukey post-hoc on the final two sessions revealed that only the H CAPS males had a decreased final average performance significantly different from the full controls A Air (β = −10.49, SE = 3.33, p = 0.02).
Fixed-Interval Schedule Extinction
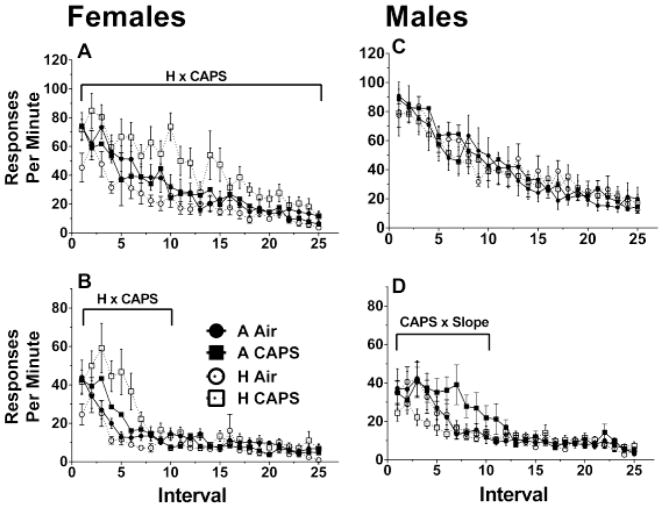
In females, there was an interaction between CAPS and hyperoxia on Session 1 on the average response rate (β = 26.19, SE = 9.09, p < 0.01) and on the slope (β = −1.17, SE = 0.56, p = 0.04) (Figure 6A). The contrasts test showed the H CAPS females had a significantly steeper slope compared to H Air females (β = −1.02, SE = 0.39, p < 0.01) though neither group was significantly different from control. Furthermore the Tukey post-hoc showed the average responses of the H CAPS females was only significantly higher than the H Air females (β = 21.72, SE = 5.56, p < 0.01) and though H CAPS females were higher than A CAPS (β = 14.49, SE = 5.72, p = 0.07) and A Air (β = 12.93, SE = 5.56, p = 0.11), the differences failed to reach statistical significance. On Session 2, no differences were found with the slopes, however there was a hyperoxia and CAPS interaction on the average response in the initial 10 intervals (β = 15.48, SE = 6.98, p = 0.03) (Figure 6B). The Tukey-posthoc on the average responses showed H CAPS had a significantly higher response rate then H Air (β = 19.68, SE = 4.87, p < 0.01), and A Air (β = 14.35, SE = 4.87, p = 0.03) though not significant when compared to A CAPS (β = 10.14, SE = 5.00, p = 0.20). No significant treatment-related effects were observed on Intervals 11–25 in Session 2.
Figure 6.
FI60 extinction correct response rate for Session 1 and Session 2 for females (A, C, n=9–10/treatment group) and males (B, D, n=10/treatment group). A linear mixed-model was used to assess for hyperoxia and CAPS differences with significance being defined as p ≤ 0.05. H and CAPS indicate a significant effect of neonatal hyperoxia or CAPS exposure respectively on average response rates while H x Slope or CAPS x Slope are significant effects of hyperoxia or CAPS on learning rate. H x CAPS indicate a significant interaction between hyperoxia, and CAPS on the average response rate. The brackets indicate the time period in which the significant difference was present.
There were no significant differences in hyperoxia or CAPS exposed males for their average response or slopes during the first extinction session or any interactions (Figure 6C). Males exposed to CAPS had a shallower slope for response rate (β = 2.60, SE = 0.91, p < 0.01) over Intervals 1–10 of Session 2 (Figure 6D). There were no significant differences in hyperoxia-exposed males or any interaction with CAPS in the initial 10 sessions or any significant treatment differences over Intervals 11–25 in Session 2.
Discussion
In this study, both hyperoxia and CAPS impaired temporal control as measured by mean quarter life slopes and final absolute values on the FI60 and FI120 schedules, indicative of slower learning of the schedule parameters. This pattern of behavioral changes occurred in a sex-specific fashion (Figure 3 and Figure 4). Mean quarter life values reflect the allocation of responses during the fixed interval. Under normal conditions, these values should increase, reflecting the shift of responses toward the end of the interval, such that the highest rates of responding are occurring at the point when reward becomes available.
On the FI60 schedule, the mean quarter lives averaged over the last 10 sessions for males appeared to be influenced by both hyperoxia and CAPS, as the H CAPS group was the only group with quarter life values significantly lower than A Air controls. This difference in males could reflect, in part the continued increase in mean quarter life values in A Air males coupled with slight reductions of values in the H CAPS males over the final 10 sessions and further separation of these values from all other treatment groups. In females, the only differences seen in the final 10 sessions of FI60 schedule were seen in CAPS-exposed females only, and no cumulative or synergistic differences seen with hyperoxia exposure.
On the FI120 schedule, the additive effect of hyperoxia and CAPS in males was sustained. In females, the slopes of the mean quarter life values for H CAPS, but not for H AIR or A CAPS groups differed from those of A Air controls, suggesting an initial enhanced effect of combined hyperoxia and CAPS. The absence of this interactive effect in the final absolute mean quarter life values for females likely reflects the greater increases in the H CAPS female slope values over the final sessions, an increase that was not seen in H CAPS males. Collectively, these findings suggest that hyperoxia could unmask and enhance the neurodevelopmental consequences of ambient UFP exposures with regards to learning in a subtle sex-specific fashion. Such data are critical as cognition is an outcome that may be particularly relevant for high risk populations such as premature infants (Maxwell et al. 2017) that may sequentially experience these risk factors during development.
Beyond temporal control, this study also examined extinction behavior; i.e., the reduction in frequency of a learned behavior when that behavior is no longer reinforced (Figure 6). Interestingly, again females exhibited learning deficits, i.e., a slower reduction in response rates observed in the H CAPS group, suggesting that hyperoxia primed the vulnerability of females to a subsequent CAPS exposure, similarly to the alterations in mean quarter life values of this group of females on the FI120 schedule. These results represent a potentially disturbing scenario, where both for the learning of temporal control on the FI schedule and the breaking of the association between responding and reward during extinction, females that were exposed to hyperoxia alone were somewhat resilient to its effects, whereas it was only in the context of subsequent CAPS exposure, that a “silent” effect of hyperoxia was unmasked. Equally disconcerting were the apparent cumulative effects of hyperoxia and CAPS on acquisition of temporal control under conditions of both the FI60 and FI120 schedules of reward, with the changes in mean quarter life values of the H CAPS groups being proportional to the additive effects induced by each insult alone. The basis for the sex differences in vulnerability to hyperoxia and CAPs are as yet unknown, but greater impacts of both CAPS (Allen et al. 2014a; Allen et al. 2015) and of hyperoxia (Lingappan et al. 2016; Namba et al. 2016) have been described in males, with the latter being attributed in some studies to sex-related differences in cytochrome P-450 (CYP)1a (Lingappan et al. 2015; Macak-Safranko et al. 2011).
One of this study’s novel findings it that hyperoxia, a potential consequence of preterm birth, and postnatal air pollution exposure both affect learning on a fixed-interval schedule of reinforcement, a behavioral paradigm in which characteristic response patterns translate across many-species, including humans (Lowe et al. 1978). The temporal control and extinction deficits appear not to reflect sheer differences in motor activity, given that there were no significant differences in activity levels as measured using a locomotor assay, which suggests that these deficits represent cognitive alterations. However, the specific mechanisms of timing deficits observed in this study require further behavioral analyses to gain clarity and determine the associated neurobehavioral mechanisms, i.e. attention, motivation, and/or impulsivity. Preterm birth has been linked to cognitive delay at all stages of development in humans. Preterm birth and very low birth weight are associated with delayed academic functioning in children (Reijneveld et al. 2006), adolescents (Odd et al. 2016; Stewart et al. 1999), and adults (Strauss 2000). Increasingly studies recognize adverse effects of air pollution on cognitive development. Black carbon exposure, a surrogate for air pollution, was associated with cognitive decline in schoolchildren in Massachusetts (Suglia et al. 2008) and fine particulate matter exposure was associated with decreased verbal learning in adults (Gatto et al. 2014). Furthermore, some shared neurodevelopmental disorders associated with both preterm birth and early-life exposure to air pollution, including ASD (Allman et al. 2011), ADHD (Barkley et al. 1997), and schizophrenia (Davalos et al. 2011), all share deficits in temporal control.
Beyond failure to acquire more precise temporally-controlled behavior and the delay in response rate reductions during the extinction paradigm, our study also revealed sex-and-treatment dependent effects on other behavioral endpoints. Females exposed to CAPS alone, for example, displayed an increase in response rates on the FI60 schedule of reward. This effect is interesting given that elevated response rates on the FI schedule of reinforcement have been shown to be a surrogate for ‘impulsivity’ in both infants and children (Darcheville et al. 1992; Darcheville et al. 1993), as assessed using a delay of reward or self-control paradigm. Specifically, children with higher response rates on an FI schedule tended to likewise show greater impulsivity, i.e., preference for smaller reward after a short delay in lieu of larger reward after a longer delay. By what mechanism(s) these exposures may influence FI rates and potentially contribute to impulsivity remains unclear, but certainly changes in brain mesocorticolimbic dopamine systems are a possibility (Allen et al. 2014a; Allen et al. 2014b; Cory-Slechta et al. 1997; Evans and Cory-Slechta 2000). Additionally, hyperoxia alone increased the number of responses on the non-reinforced levers and thereby decreased accuracy in males on the FI 60 schedule of reward that could potentially be indicative of self-control deficits an inability to respond to contingencies of reinforcement and/or failure to discriminate absence of reward. Preterm boys have been shown to exhibit increased hyperactivity and attention issues within the classroom compared to preterm females (Samara et al. 2008).
One limitation of this study is that it examines only one potential risk factor from preterm birth, hyperoxia, whereas many preterm infants also experience other risk factors, potentially simultaneously, including hypoxia-ischemia, hypothermia, and infection. Future studies may want to address the potential compounding effects of air pollution exposure utilizing well-characterized animal models that address these other preterm risk factors (Hagberg et al. 2002). Another limitation is we do not have sufficient data on factors that could potentially contribute to our CAPS exposure variability including wind direction, weather data, traffic congestion, flights from nearby airport, etc. Further exploration of these factors on future exposures could provide specificity on the sources of certain constituents within the CAPS mixture. Also given the heterogeneous composition of CAPS, it is difficult to assess the underlying direct or indirect mechanisms by which CAPS and hyperoxia interact, but several of the predominant elements including zinc and aluminum have been linked to respiratory distress (Bell et al. 2014; Cakmak et al. 2014), one of the common outcomes of neonatal hyperoxia exposure. Compounded effects of CAPS and hyperoxia on pulmonary function could indirectly contribute to CNS deficits via insufficient oxygen flow to the brain or dysregulation of the body’s autonomic system via activation of vagal nerves in the lung. Brain tissues from mice used in this study are currently being processed for a full pathological analysis.
Both hyperoxia and CAPS exposure had protracted and sex-dependent effects on learning, suggesting increased risk following developmental exposures to both of these insults. By utilizing hyperoxia, a very common health risk associated with preterm birth and combining it with a realistic, real-time air pollution exposure, this study’s model provides a relevant context for assessing preterm infants’ vulnerability to air pollution. Results of the study underscore the vital need to assess the potential risk susceptibility of preterm infants in regions with high levels of UFP air pollution in particular and to assess developmental exposures to air pollution within the context of other brain and lung insults.
Highlights.
Hyperoxia and CAPS-exposed male mice showed an augmented reduction in temporal control
Hyperoxia and CAPS exposure unmasked a learning deficit in females during a schedule transition
Hyperoxia and CAPS exposed females had increased response rates on an extinction schedule
Acknowledgments
This work was funded in part by National Institutes of Health Grants R01 ES025541 (D.A. Cory-Slechta), R01 HL091968 (M. A. O’Reilly), and a pilot award from P30 ES001247 (M.A. O’Reilly and D. A. Cory-Slechta). NIH Training Grant T32 ES07026 supported K. Morris-Schaffer. NIH Center Grant P30 ES001247 supported the animal inhalation facility and the animal behavior core. The University of Rochester’s Department of Pediatrics provided financial support through the Perinatal and Pediatric Origins of Disease Program. M. Aurora was supported by the National Institutes of Environmental Health Sciences research grants DP2ES025453, R01ES024674, and P30ES023515.
Footnotes
Financial Interest Declaration: The authors declare they have no actual or potential competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JL, Conrad K, Oberdörster G, Johnston CJ, Sleezer B, Cory-Slechta DA. Developmental exposure to concentrated ambient particles and preference for immediate reward in mice. Environmental Health Perspectives. 2013;121:32–38. doi: 10.1289/ehp.1205505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, Liu X, Pelkowski S, Palmer B, Conrad K, Oberdörster G, et al. Early postnatal exposure to ultrafine particulate matter air pollution: Persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environmental Health Perspectives. 2014a;122:939–945. doi: 10.1289/ehp.1307984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, Liu X, Weston D, Conrad K, Oberdorster G, Cory-Slechta DA. Consequences of developmental exposure to concentrated ambient ultrafine particle air pollution combined with the adult paraquat and maneb model of the parkinson’s disease phenotype in male mice. Neurotoxicology. 2014b;41:80–88. doi: 10.1016/j.neuro.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, Oberdorster G, Morris-Schafer K, Wong C, Klocke C, Sobolewski M, et al. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2015 doi: 10.1016/j.neuro.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman MJ, DeLeon IG, Wearden JH. Psychophysical assessment of timing in individuals with autism. American journal on intellectual and developmental disabilities. 2011;116:165–178. doi: 10.1352/1944-7558-116.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Koplowitz S, Anderson T, McMurray MB. Sense of time in children with adhd: Effects of duration, distraction, and stimulant medication. J Int Neuropsychol Soc. 1997;3:359–369. [PubMed] [Google Scholar]
- Basagana X, Esnaola M, Rivas I, Amato F, Alvarez-Pedrerol M, Forns J, et al. Neurodevelopmental deceleration by urban fine particles from different emission sources: A longitudinal observational study. Environ Health Perspect. 2016;124:1630–1636. doi: 10.1289/EHP209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra TA, Wilhelm M, Olsen J, Cockburn M, Ritz B. Ambient air pollution and autism in los angeles county, california. Environmental Health Perspectives. 2013;121:380–386. doi: 10.1289/ehp.1205827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell ML, Ebisu K, Leaderer BP, Gent JF, Lee HJ, Koutrakis P, et al. Associations of pm(2).(5) constituents and sources with hospital admissions: Analysis of four counties in connecticut and massachusetts (USA) for persons >/= 65 years of age. Environ Health Perspect. 2014;122:138–144. doi: 10.1289/ehp.1306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KS. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. JAMA. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- Bouyssi-Kobar M, du Plessis AJ, McCarter R, Brossard-Racine M, Murnick J, Tinkleman L, et al. Third trimester brain growth in preterm infants compared with in utero healthy fetuses. Pediatrics. 2016 doi: 10.1542/peds.2016-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon EM, Suanda S, Libertus K. Temporal discrimination increases in precision over development and parallels the development of numerosity discrimination. Developmental science. 2007;10:770–777. doi: 10.1111/j.1467-7687.2007.00635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak S, Dales R, Kauri LM, Mahmud M, Van Ryswyk K, Vanos J, et al. Metal composition of fine particulate air pollution and acute changes in cardiorespiratory physiology. Environmental Pollution. 2014;189:208–214. doi: 10.1016/j.envpol.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, Flake RA, Baldwin RL, Blake DJ, Paule MG. Developmental aspects of timing behavior in children. Neurotoxicology and teratology. 2004;26:461–476. doi: 10.1016/j.ntt.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the global burden of diseases study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta DA, Pazmino R, Bare C. The critical role of nucleus accumbens dopamine systems in the mediation of fixed interval schedule-controlled operant behavior. Brain Research. 1997;764:253–256. doi: 10.1016/s0006-8993(97)00591-x. [DOI] [PubMed] [Google Scholar]
- Dalman C, Allebeck P, Cullberg J, Grunewald C, Koster M. Obstetric complications and the risk of schizophrenia: A longitudinal study of a national birth cohort. Arch Gen Psychiatry. 1999;56:234–240. doi: 10.1001/archpsyc.56.3.234. [DOI] [PubMed] [Google Scholar]
- Darcheville J, Riviere V, Wearden J. Fixed-interval performance and self-control in children. Journal of the experimental analysis of behavior. 1992;57:187–199. doi: 10.1901/jeab.1992.57-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcheville JC, Rivière V, Wearden JH. Fixed-interval performance and self-control in infants. Journal of the Experimental Analysis of Behavior. 1993;60:239–254. doi: 10.1901/jeab.1993.60-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos DB, Rojas DC, Tregellas JR. Temporal processing in schizophrenia: Effects of task-difficulty on behavioral discrimination and neuronal responses. Schizophrenia Research. 2011;127:123–130. doi: 10.1016/j.schres.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deulofeut R, Critz A, Adams-Chapman I, Sola A. Avoiding hyperoxia in infants < or = 1250 g is associated with improved short- and long-term outcomes. Journal of perinatology: official journal of the California Perinatal Association. 2006;26:700–705. doi: 10.1038/sj.jp.7211608. [DOI] [PubMed] [Google Scholar]
- Domm W, Misra RS, O’Reilly MA. Affect of early life oxygen exposure on proper lung development and response to respiratory viral infections. Front Med (Lausanne) 2015;2:55. doi: 10.3389/fmed.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder A, Gelein R, Silva V, Feikert T, Opanashuk L, Carter J, et al. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environmental Health Perspectives. 2006;114:1172–1178. doi: 10.1289/ehp.9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SB, Cory-Slechta DA. Prefrontal cortical manipulations alter the effects of intra-ventral striatal dopamine antagonists on fixed-interval performance in the rat. Behav Brain Res. 2000;107:45–58. doi: 10.1016/s0166-4328(99)00108-4. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raj JU. Regulation of the pulmonary circulation in the fetus and newborn. Physiol Rev. 2010;90:1291–1335. doi: 10.1152/physrev.00032.2009. [DOI] [PubMed] [Google Scholar]
- Gatto NM, Henderson VW, Hodis HN, St John JA, Lurmann F, Chen J-C, et al. Components of air pollution and cognitive function in middle-aged and older adults in los angeles. NeuroToxicology. 2014;40:1–7. doi: 10.1016/j.neuro.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Peebles D, Mallard C. Models of white matter injury: Comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Developmental Disabilities Research Reviews. 2002;8:30–38. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- Jarjour IT. Neurodevelopmental outcome after extreme prematurity: A review of the literature. Pediatric Neurology. 2015;52:143–152. doi: 10.1016/j.pediatrneurol.2014.10.027. [DOI] [PubMed] [Google Scholar]
- Kelleher RT, Morse WH. Proceedings of the Reviews of Physiology Biochemistry and Experimental Pharmacology. Vol. 60. Berlin, Heidelberg: Springer Berlin Heidelberg; 1968. Determinants of the specificity of behavioral effects of drugs; pp. 1–56. [DOI] [PubMed] [Google Scholar]
- Laurent O, Hu J, Li L, Kleeman MJ, Bartell SM, Cockburn M, et al. A statewide nested case-control study of preterm birth and air pollution by source and composition: California, 2001–2008. Environ Health Perspect. 2016 doi: 10.1289/ehp.1510133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappan K, Jiang W, Wang L, Couroucli XI, Moorthy B. Sex-specific differences in hyperoxic lung injury in mice: Role of cytochrome p450 (cyp)1a. Toxicology. 2015;331:14–23. doi: 10.1016/j.tox.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappan K, Jiang W, Wang L, Moorthy B. Sex-specific differences in neonatal hyperoxic lung injury. Am J Physiol Lung Cell Mol Physiol. 2016;311:L481–493. doi: 10.1152/ajplung.00047.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CF, Harzem P, Bagshaw M. Species differences in temporal control of behavior ii: Human performance. Journal of the Experimental Analysis of Behavior. 1978;29:351–361. doi: 10.1901/jeab.1978.29-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macak-Safranko Z, Sobocanec S, Saric A, Balog T, Sverko V, Kusic B, et al. Cytochrome p450 gender-related differences in response to hyperoxia in young cba mice. Exp Toxicol Pathol. 2011;63:345–350. doi: 10.1016/j.etp.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Namba F, Ogawa R, Ito M, Watanabe T, Dennery PA, Tamura M. Sex-related differences in long-term pulmonary outcomes of neonatal hyperoxia in mice. Exp Lung Res. 2016;42:57–65. doi: 10.3109/01902148.2016.1141264. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. Translocation of inhaled ultrafine particles to the brain. Inhalation toxicology. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- Odd D, Evans D, Emond A. Preterm birth, age at school entry and long term educational achievement. PLOS ONE. 2016;11:e0155157. doi: 10.1371/journal.pone.0155157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Oh SY, Lee SJ, Lee K, Kim Y, Lee BS, et al. Chronic pulmonary accumulation of iron oxide nanoparticles induced th1-type immune response stimulating the function of antigen-presenting cells. Environ Res. 2015;143:138–147. doi: 10.1016/j.envres.2015.09.030. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D. {nlme}: Linear and nonlinear mixed effects models. 2016 R package version 3.1–125. [Google Scholar]
- Reijneveld SA, de Kleine MJK, van Baar AL, Kollée LAA, Verhaak CM, Verhulst FC, et al. Behavioural and emotional problems in very preterm and very low birthweight infants at age 5 years. Archives of disease in childhood Fetal and neonatal edition. 2006;91:F423–F428. doi: 10.1136/adc.2006.093674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter J, Schmitz T, Chew L-J, Bührer C, Möbius W, Zonouzi M, et al. Neonatal hyperoxia exposure disrupts axon–oligodendrocyte integrity in the subcortical white matter. The Journal of Neuroscience. 2013;33:8990–9002. doi: 10.1523/JNEUROSCI.5528-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samara M, Marlow N, Wolke D. Pervasive behavior problems at 6 years of age in a total-population sample of children born at≤ 25 weeks of gestation. Pediatrics. 2008;122:562–573. doi: 10.1542/peds.2007-3231. [DOI] [PubMed] [Google Scholar]
- Schmitz T, Ritter J, Mueller S, Felderhoff-Mueser U, Chew L-J, Gallo V. Cellular changes underlying hyperoxia-induced delay of white matter development. The Journal of Neuroscience. 2011;31:4327–4344. doi: 10.1523/JNEUROSCI.3942-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Progress in neurobiology. 2013;0:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique S, Banerjee M, Ray MR, Lahiri T. Attention-deficit hyperactivity disorder in children chronically exposed to high level of vehicular pollution. Eur J Pediatr. 2011;170:923–929. doi: 10.1007/s00431-010-1379-0. [DOI] [PubMed] [Google Scholar]
- Simbruner G, Mittal RA, Rohlmann F, Muche R. Systemic hypothermia after neonatal encephalopathy: Outcomes of neo. Neuro Network rct Pediatrics. 2010;126:e771–e778. doi: 10.1542/peds.2009-2441. [DOI] [PubMed] [Google Scholar]
- Sink DW, Hope SA, Hagadorn JI. Nurse:Patient ratio and achievement of oxygen saturation goals in premature infants. Archives of disease in childhood Fetal and neonatal edition. 2011;96:F93–98. doi: 10.1136/adc.2009.178616. [DOI] [PubMed] [Google Scholar]
- Sirinyan M, Sennlaub F, Dorfman A, Sapieha P, Gobeil F, Jr, Hardy P, et al. Hyperoxic exposure leads to nitrative stress and ensuing microvascular degeneration and diminished brain mass and function in the immature subject. Stroke. 2006;37:2807–2815. doi: 10.1161/01.STR.0000245082.19294.ff. [DOI] [PubMed] [Google Scholar]
- Sola A. Oxygen saturation in the newborn and the importance of avoiding hyperoxia-induced damage. NeoReviews. 2015;16:e393–e405. [Google Scholar]
- Soria-Pastor S, Gimenez M, Narberhaus A, Falcon C, Botet F, Bargallo N, et al. Patterns of cerebral white matter damage and cognitive impairment in adolescents born very preterm. International Journal of Developmental Neuroscience. 2008;26:647–654. doi: 10.1016/j.ijdevneu.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Rifkin L, Amess PN, Kirkbride V, Townsend JP, Miller DH, et al. Brain structure and neurocognitive and behavioural function in adolescents who were born very preterm. The Lancet. 1999;353:1653–1657. doi: 10.1016/s0140-6736(98)07130-x. [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. Jama. 2015;314:1039–1051. doi: 10.1001/jama.2015.10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss RS. Adult functional outcome of those born small for gestational age: Twenty-six–year follow-up of the 1970 british birth cohort. Jama. 2000;283:625–632. doi: 10.1001/jama.283.5.625. [DOI] [PubMed] [Google Scholar]
- Suglia SF, Gryparis A, Wright RO, Schwartz J, Wright RJ. Association of black carbon with cognition among children in a prospective birth cohort study. American Journal of Epidemiology. 2008;167:280–286. doi: 10.1093/aje/kwm308. [DOI] [PubMed] [Google Scholar]
- Sun Q, Tan D, Ze Y, Sang X, Liu X, Gui S, et al. Pulmotoxicological effects caused by long-term titanium dioxide nanoparticles exposure in mice. Journal of hazardous materials. 2012;235–236:47–53. doi: 10.1016/j.jhazmat.2012.05.072. [DOI] [PubMed] [Google Scholar]
- Verhagen EA, Braeckel KN, Veere CN, Groen H, Dijk PH, Hulzebos CV, et al. Cerebral oxygenation is associated with neurodevelopmental outcome of preterm children at age 2 to 3 years. Developmental Medicine & Child Neurology. 2015;57:449–455. doi: 10.1111/dmcn.12622. [DOI] [PubMed] [Google Scholar]
- Vertes PE, Bullmore ET. Annual research review: Growth connectomics--the organization and reorganization of brain networks during normal and abnormal development. J Child Psychol Psychiatry. 2015;56:299–320. doi: 10.1111/jcpp.12365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the national institute of child health and human development neonatal research network, 1993–1994. Pediatrics. 2000;105:1216–1226. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential proximity to freeways and autism in the charge study. Environmental Health Perspectives. 2011;119:873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Lurmann F, Penfold B, Hertz-Picciotto I, McConnell R. Traffic related air pollution, particulate matter, and autism. JAMA psychiatry. 2013;70:71–77. doi: 10.1001/jamapsychiatry.2013.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vottier G, Pham H, Pansiot J, Biran V, Gressens P, Charriaut-Marlangue C, et al. Deleterious effect of hyperoxia at birth on white matter damage in the newborn rat. Developmental Neuroscience. 2011;33:261–269. doi: 10.1159/000327245. [DOI] [PubMed] [Google Scholar]
- Yee M, Chess PR, McGrath-Morrow SA, Wang Z, Gelein R, Zhou R, et al. Neonatal oxygen adversely affects lung function in adult mice without altering surfactant composition or activity. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2009;297:L641–L649. doi: 10.1152/ajplung.00023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]