Abstract
Objective
To determine red blood cell (RBC) transfusion practice and relationships between RBC transfusion volume and mortality in infants and children treated with extracorporeal membrane oxygenation (ECMO).
Design
Secondary analysis of a multi-center prospective observational study
Setting
8 pediatric institutions within the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Collaborative Pediatric Critical Care Research Network
Patients
Patients aged < 19 years treated with ECMO at a participating center
Interventions
none
Measurements and Main Results
Clinical data and target hemoglobin or hematocrit values (if set) were recorded daily by trained bedside ECMO specialists and research coordinators. Laboratory values, including hemoglobin and hematocrit, were recorded daily using the value obtained closest to 8:00 am. RBC transfusion was recorded as total daily volume in ml/kg. Multivariable logistic regression was used to determine the relationship between RBC transfusion volume and hospital mortality, accounting for potential confounders. Average goal hematocrits varied across the cohort with a range of 27.5 – 41.3%. Overall, actual average daily hematocrit was 36.8%, and average RBC transfusion volume was 29.4 [17.4, 49.7] mL/kg/day on ECMO. On multivariable analysis, each additional 10mL/kg/day of RBC transfusion volume was independently associated with a 9% increase in odds of hospital mortality (aOR 1.09 (1.02, 1.16), p = 0.009).
Conclusions
In this multicenter cohort of pediatric ECMO patients, daily hematocrit levels were maintained at normal or near normal values and RBC transfusion burden was high. RBC transfusion volume was independently associated with odds of mortality. Future clinical studies to identify optimum RBC transfusion thresholds for pediatric ECMO are urgently needed.
Keywords: pediatric, extracorporeal membrane oxygenation, blood transfusion
Introduction
There are no prospective studies evaluating optimum red blood cell (RBC) transfusion thresholds for infants and children on ECMO support, and it is believed that RBC transfusion is often guided by institutional policies targeting normal or near-normal hematocrit values (1). Consequently, RBC transfusion burden among neonatal and pediatric ECMO patients may be exceptionally high, with single-center reports suggesting up to 30 -40 mL/kg/day (2–4). However, little is known about RBC transfusion practice across multiple centers.
The risk/benefit balance of RBC transfusion to treat mild anemia in infants and children on ECMO remains unclear. Certainly, maintaining normal or near-normal hematocrit values stands in stark contrast to current evidence-based RBC transfusion practice guidelines which recommend much lower transfusion thresholds for critically ill patients not on ECMO (5, 6). Likewise, multiple single-center reports suggest that RBC transfusion volume is independently associated with mortality (4, 7–9). However, relationships between RBC transfusion burden and clinical outcomes among neonatal and pediatric ECMO patients across multiple centers are unknown. These data are essential to inform future prospective trials to determine optimum RBC transfusion strategies for infants and children on ECMO.
The recently completed Bleeding and Thrombosis during ECMO (BATE) study which enrolled 514 consecutive pediatric ECMO patients at eight pediatric institutions within the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s Collaborative Pediatric Critical Care Research Network (CPCCRN) offers a unique opportunity to evaluate multi-center RBC transfusion epidemiology in neonatal and pediatric ECMO patients (10). As such, we conducted a secondary analysis of the BATE dataset to determine red blood cell transfusion practice and relationships between RBC transfusion burden and mortality among infants and children receiving ECMO support.
Methods
Setting and Subjects
This study is a secondary analysis of data collected for the Bleeding and Thrombosis during ECMO (BATE) study. The details of the BATE study methodology are previously published (10). In brief, BATE was a prospective observational study which included all patients less than 19 years of age treated with ECMO in a neonatal (NICU), pediatric (PICU), or cardiothoracic intensive care unit (CICU) at one of 8 participating centers within the CPCCRN between December 2012 and September 2014. The study was approved with a waiver of informed consent by the Institutional Review Boards at each of the participating hospitals and the Data Coordination Center at the University of Utah.
Clinical data and definitions
Data were recorded by trained bedside ECMO specialists and research coordinators. Baseline data included demographics, diagnoses, laboratory values, ventilator settings, vasoactive inotrope scores, and ECMO indication. Laboratory values while on ECMO, including hemoglobin and hematocrit, were collected once daily using the value obtained closest to 8:00 am. Transfusion data were collected as total daily volumes of red blood cells or whole blood. Bleeding complication in the primary study was defined as either bleeding requiring RBC transfusion or intracranial hemorrhage (10). For the purposes of the current analysis, bleeding complication was defined as bleeding requiring RBC transfusion on any study day. Intended transfusion practice was defined by hemoglobin and/or hematocrit goals as recorded by the bedside ECMO specialist. Bedside ECMO specialists were asked to record whether a goal hemoglobin or hematocrit level was set at ECMO initiation and the value of that goal. For each subsequent ECMO day, the specialist was asked to record goal hemoglobin or hematocrit values only if they had changed from previous. Actual transfusion practice was defined by the surrogate marker of average daily hematocrit values across all days of ECMO support for each individual subject because pre-transfusion hemoglobin/hematocrit values were not recorded. Transfusion practice was assessed by center and by ICU (NICU, PICU, CICU) rather than by patient diagnosis or ECMO indication because we were primarily interested in physician/ECMO team practice patterns. Acute and chronic diagnoses were identified by review of physician documentation. Multiple measures were used to assess baseline severity of illness, including: pre-ECMO pediatric risk of mortality (PRISM III) score (11), pre-ECMO arterial pH, pre-ECMO oxygenation index, and pre-ECMO vasoactive inotrope score (VIS). VIS was categorized as none (no vasoactive medications), low (VIS < 20), or high (VIS ≥ 20). Baseline immunocompromised state was defined as acute or chronic diagnosis of cancer, hematologic disorder, transplant, HIV infection, immunodeficiency, or adrenal insufficiency.
Statistical Analysis
Interval variables were summarized using mean ± standard deviation or median [interquartile range]. Nominal variables were summarized with counts and percentages and tested for associations using Fisher’s exact test. Monte Carlo approximations were used to estimate p-values for Fisher’s exact test to make their computation more practical on large tables.
Univariable analyses were performed to identify factors associated with in-hospital mortality. Univariable analyses were stratified by PICU, CICU, and NICU locations and the overall cohort in order to assess for potential interactions based on ICU. A single multivariable logistic regression model was then created to assess the relationship between mean daily RBC transfusion volume and in-hospital mortality across the entire cohort. Variables were considered for inclusion in the multivariable model if they were 1) available for > 90% of the cohort and 2) associated with mortality (p < 0.10) on univariable analyses. The final model was selected using a bidirectional stepwise selection process with a criterion of p < 0.05 to enter and stay in the multivariable model. Adjusted odds ratios and the associated 95% confidence intervals are reported for all variables in the final model. Using the same methodology, a second multivariable logistic regression model was built to assess the relationship between mean daily hematocrit and in-hospital mortality. All analyses were performed using SAS 9.4 (SAS Institute; Cary, NC).
Results
All 514 subjects in the BATE study were included in these analyses. Demographic information, ECMO indication, mode of ECMO, and subject outcomes are in Table 1. As expected, respiratory indications predominated in the NICU and PICU, while cardiac indications predominated in the CICU. Across all ICUs, the majority of subjects were supported with veno-arterial (VA) ECMO. Overall, average duration of ECMO support was 7.4 ± 8.3 days, with about a quarter of subjects treated with ECMO for greater than nine days. Overall, in-hospital mortality was 45%, with the highest mortality rate among subjects treated in the CICU.
Table 1.
Description of cohort
| Location of ECMO care
|
||||
|---|---|---|---|---|
| Characteristic | PICU (N = 103) |
NICU (N = 156) |
CICU (N = 255) |
Overall (N = 514) |
| Age (months) | 25.0 [3.8, 158.8] | 0.1 [0.0, 0.1] | 2.4 [0.3, 14.5] | 0.7 [0.1, 11.5] |
| Male | 48 (46.6%) | 96 (61.5%) | 158 (62.0%) | 302 (58.8%) |
| Primary ECMO indication | ||||
| Respiratory | 66 (64.1%) | 143 (91.7%) | 28 (11.0%) | 237 (46.1%) |
| Cardiac | 28 (27.2%) | 13 (8.3%) | 166 (65.1%) | 207 (40.3%) |
| ECPR | 9 (8.7%) | 0 (0.0%) | 61 (23.9%) | 70 (13.6%) |
| VA ECMO | 62 (60.2%) | 119 (76.3%) | 250 (98%) | 431 (83.9%) |
| Acute diagnoses | ||||
| Airway abnormality | 6 (5.8%) | 2 (1.3%) | 4 (1.6%) | 12 (2.3%) |
| Cardiac Arrest | 10 (9.7%) | 4 (2.6%) | 35 (13.7%) | 49 (9.5%) |
| Cardiovascular disease (acquired) | 10 (9.7%) | 5 (3.2%) | 50 (19.6%) | 65 (12.6%) |
| Cardiovascular disease (arrhythmia) | 4 (3.9%) | 2 (1.3%) | 12 (4.7%) | 18 (3.5%) |
| Cardiovascular disease (congenital) | 20 (19.4%) | 5 (3.2%) | 168 (65.9%) | 193 (37.5%) |
| Hypoxic/anoxic injury | 2 (1.9%) | 14 (9.0%) | 1 (0.4%) | 17 (3.3%) |
| Gastrointestinal disorder | 4 (3.9%) | 2 (1.3%) | 17 (6.7%) | 23 (4.5%) |
| Pertussis or Sepsis | 28 (27.2%) | 49 (31.4%) | 14 (5.5%) | 91 (17.7%) |
| Pneumonia or bronchiolitis | 15 (14.6%) | 3 (1.9%) | 6 (2.4%) | 24 (4.7%) |
| Shock (non-septic) | 5 (4.9%) | 3 (1.9%) | 6 (2.4%) | 14 (2.7%) |
| Respiratory distress / failure | 75 (72.8%) | 20 (12.8%) | 75 (29.4%) | 170 (33.1%) |
| Neurologic condition | 5 (4.9%) | 10 (6.4%) | 2 (0.8%) | 17 (3.3%) |
| Meconium aspiration syndrome | 1 (1.0%) | 46 (29.5%) | 0 (0.0%) | 47 (9.1%) |
| Congenital diaphragmatic hernia | 3 (2.9%) | 53 (34.0%) | 0 (0.0%) | 56 (10.9%) |
| Persistent pulmonary hypertension of the newborn | 2 (1.9%) | 88 (56.4%) | 0 (0.0%) | 90 (17.5%) |
| Chronic diagnoses | ||||
| Chronic lung disease | 8 (7.8%) | 2 (1.3%) | 7 (2.7%) | 17 (3.3%) |
| Congenital anomaly or chromosomal defect | 17 (16.5%) | 66 (42.3%) | 35 (13.7%) | 118 (23.0%) |
| Neurologic condition | 10 (9.7%) | 4 (2.6%) | 13 (5.1%) | 27 (5.3%) |
| Cardiovascular disease (congenital) | 15 (14.6%) | 34 (21.8%) | 48 (18.8%) | 97 (18.9%) |
| Immunocompromised | 16 (15.5%) | 7 (4.5%) | 19 (7.5%) | 42 (8.2%) |
| Duration of ECMO (days) | ||||
| < 2 | 13 (12.6%) | 12 (7.7%) | 63 (24.7%) | 88 (17.1%) |
| 2 - < 4 | 15 (14.6%) | 17 (10.9%) | 86 (33.7%) | 118 (23.0%) |
| 4 - < 9 | 36 (35.0%) | 61 (39.1%) | 76 (29.8%) | 173 (33.7%) |
| >= 9 | 39 (37.9%) | 66 (42.3%) | 30 (11.8%) | 135 (26.3%) |
| Length of ICU Stay (weeks) | 4.3 [2.0, 8.2] | 4.6 [2.3, 8.4] | 3.4 [1.9, 6.2] | 4.0 [2.0, 7.3] |
| Length of hospital stay (weeks) | 5.5 [2.5, 10.2] | 4.9 [2.3, 9.0] | 5.0 [2.3, 10.1] | 5.2 [2.3, 9.7] |
| New documented infection on at least one study day | 45 (43.7%) | 17 (10.9%) | 50 (19.6%) | 112 (21.8%) |
| In-hospital mortality | 45 (43.7%) | 55 (35.3%) | 132 (51.8%) | 232 (45.1%) |
Data are n (%) or median [interquartile range]. ECMO = extracorporeal membrane oxygenation. PICU = pediatric intensive care unit. NICU = neonatal intensive care unit. CICU = cardiac intensive care unit. VA ECMO = venoarterial ECMO. ECPR = extracorporeal cardiopulmonary resuscitation.
Supplemental data table 1 depicts how often a hemoglobin and/or a hematocrit goal was recorded by the bedside ECMO specialist on the first day of ECMO treatment. Across all centers and ICUs, an RBC transfusion threshold was set for 92% of subjects. However, the proportion of subjects with a specified threshold varied widely across units and centers, ranging from as high as 100% to as low as 23%. Hematocrit as opposed to hemoglobin values were used to define goals in the majority of cases. Goal hematocrit values varied across units and centers (Supplemental data table 2). Within most centers/ICUs, goal hematocrit values were similar for VA versus VV ECMO patients and were higher in NICU subjects compared to PICU and CICU subjects. Among PICU and CICU subjects, average goal hematocrit values ranged between 27.5 and 41.3%. These values approximate hemoglobin values of 9.2 g/dL to 13.3 g/dL, respectively. For the majority of subjects, there were no changes in goal hematocrit across the study duration. In 21.5% of subjects, the goal hematocrit was decreased on at least one study day, and in 8.8% the goal was increased.
Consistent with stated goals, average daily hematocrit values overall ranged from a median of 35.0 to 38.7% across ICUs (Table 2). Across study days, for subjects treated with ECMO in the PICU, daily mean hematocrit values remained fairly stable. The same was true for subjects treated in the CICU after ECMO Day 2. Mean daily hematocrit values for NICU subjects, by contrast, tended to decrease over time (Figure 1). Overall, average volume of whole blood or RBC transfusion was 29.4 [17.4, 49.7] mL/kg/day, with the highest transfusion burden among CICU subjects (Table 2). Whole blood transfusion was rare – representing only 0.3% of RBC-containing transfusions. Regarding potential causes of anemia, bleeding was very common. Details of bleeding events and anti-coagulation management are previously published (10). Briefly, 65.8%of patients had bleeding requiring transfusion on any study day, and over 80% of subjects received greater than 40 mL/kg total blood products on at least one study day. Average daily blood loss from laboratory samples ranged from as low as 10% of average daily RBC transfusion volume in CICU patients to as high as 26% in NICU patients.
Table 2.
Transfusion and Bleeding by ICU
| Location of ECMO care
|
||||
|---|---|---|---|---|
| Characteristc | PICU (N = 103) |
NICU (N = 156) |
CICU (N = 255) |
Overall (N = 514) |
| Mean daily hematocrit (%) | 35.0 [33.1, 37.5] | 38.7 [36.4, 40.1] | 36.3 [34.0, 38.8] | 36.8 [34.6, 39.3] |
| Average daily RBC transfusion volume (mL/kg) | 15.8 [10.7, 32.4] | 29.3 [21.0, 44.1] | 35.0 [21.0, 59.9] | 29.4 [17.4, 49.7] |
| Average daily platelet transfusion volume (mL/kg) | 8.3 [4.4, 14.9] | 16.1 [11.6, 21.8] | 10.9 [5.4, 19.7] | 12.1 [6.8, 20.4] |
| Average daily plasma transfusion volume (mL/kg) | 3.4 [0.9, 10.0] | 7.1 [3.0, 13.5] | 8.2 [2.7, 17.5] | 7.0 [2.1, 15.1] |
| Average daily cryoprecipitate volume (mL/kg) | 0.0 [0.0, 0.7] | 0.5 [0.0, 1.5] | 0.6 [0.0, 2.4] | 0.4 [0.0, 1.7] |
| Massive transfusion on any study day, n (%)1 | 69 (67.0%) | 143 (91.7%) | 211 (82.7%) | 423 (82.3%) |
| Average daily blood loss from lab samples (mL/kg) | 2.0 [0.6, 3.8] | 7.6 [5.1, 10.4] | 3.4 [1.2, 6.1] | 4.4 [1.5, 7.7] |
| Bleeding requiring a transfusion on any study day, n (%) | 70 (68.0%) | 83 (53.2%) | 185 (72.5%) | 338 (65.8%) |
Massive transfusion defined as receipt of >40 ml/kg total blood products in one day. ECMO = extracorporeal membrane oxygenation. PICU = pediatric intensive care unit. NICU = neonatal intensive care unit. CICU = cardiac intensive care unit. RBC = red blood cell. Data are median [interquartile range], unless otherwise specified.
Figure 1.
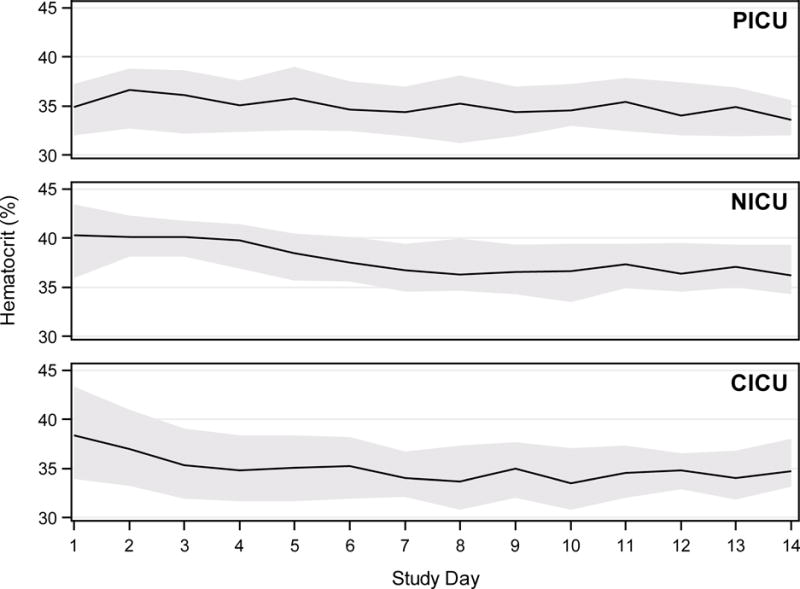
Median (lines) and 25th – 75th percentile ranges (shaded areas) of average daily hematocrit (HCT) per patient are displayed across study days up to ECMO Day 14 for subjects treated with ECMO in a pediatric intensive care unit (PICU), neonatal intensive care unit (NICU), or cardiothoracic intensive care unit (CICU).
Variables associated with mortality on univariable analyses can be found in Supplemental Data Table 3. By multivariable logistic regression, controlling for bleeding requiring transfusion on any study day, primary ECMO indication, baseline immune compromise, chronic neurologic condition, meconium aspiration syndrome, congenital diaphragmatic hernia, mean daily PaO2, and presence of hepatic or renal organ failure, higher RBC transfusion volume remained independently associated with hospital mortality (Table 3). In a separate multivariable regression model, mean daily hematocrit was not independently associated with hospital mortality (aOR 1.01 (0.94, 1.07); p=0.87).
Table 3.
Multivariable model of mortality
| In-hospital mortality (N = 514) |
||
|---|---|---|
| Characteristic | Adjusted Odds ratio (95% CI) |
P-value |
| Average daily RBC transfusion volume (10 mL/kg) | 1.09 (1.02, 1.16) | 0.009 |
| Bleeding requiring a transfusion on any study day | 1.23 (0.79, 1.92) | 0.366 |
| Primary ECMO indication | 0.042 | |
| Respiratory | Reference | |
| Cardiac | 1.48 (0.87, 2.49) | |
| ECPR | 2.40 (1.21, 4.76) | |
| Mean daily partial pressure of oxygen in arterial blood (10 mmHg) | 1.04 (1.02, 1.07) | 0.002 |
| Hepatic organ failure on at least one study day | 2.46 (1.60, 3.78) | <.001 |
| Renal organ failure on at least one study day | 2.49 (1.61, 3.85) | <.001 |
| Immunocompromised | 2.83 (1.29, 6.20) | 0.010 |
| Chronic neurologic condition | 0.20 (0.04, 0.97) | 0.046 |
| Meconium aspiration syndrome | 0.15 (0.04, 0.55) | 0.004 |
| Congenital diaphragmatic hernia | 2.82 (1.42, 5.60) | 0.003 |
RBC = red blood cell. ECMO = extracorporeal membrane oxygenation. ECPR = extracorporeal cardiopulmonary resuscitation. Additional variables which were considered for inclusion in the multivariable model include: weight, pre-ECMO PRISM III score, baseline immunocompromised state, ICU location of ECMO care, mode of ECMO, hospital LOS prior to ECMO, ICU LOS prior to ECMO, massive transfusion on any study day, mean daily central venous oxygen saturation (SvO2), mean daily partial pressure of oxygen in arterial blood, duration of ECMO, mean daily ECMO flow rate, hepatic organ failure on at least one study day, renal organ failure on at least one study day, thrombotic event, and diagnoses of: cardiac arrest, acquired cardiovascular disease, congenital cardiovascular disease, pertussis or sepsis, neurologic condition, meconium aspiration syndrome, congenital diaphragmatic hernia, persistent pulmonary hypertension of the newborn, and congenital anomaly or chromosomal defect.
Discussion
In the largest cohort to date to evaluate RBC transfusion practice in pediatric ECMO patients, RBC transfusion burden was high – roughly the equivalent of replacing the child’s entire circulating blood volume for every three days on ECMO support. At the same time, the majority of subjects’ hematocrit values were maintained at levels much higher than current standard of care for critically ill children not receiving ECMO support.
Our findings are consistent with previous single center reports suggesting RBC transfusion burdens as high as 30 – 40 mL/kg/day among infants and children on ECMO (2–4). Some of the high RBC transfusion burden is likely due to bleeding, as evidenced by the majority of subjects having bleeding requiring transfusion on at least one study day. Blood loss due to laboratory sampling may also contribute to transfusion burden, particularly for neonates. However, some of the transfusion burden also likely stems from RBC transfusion to treat mild anemia. While there was significant variation in RBC transfusion practice across centers, most institutions transfused to maintain hematocrit values greater than 30% (roughly equivalent to a hemoglobin of 10 g/dL).
Current evidence-based guidelines recommend against routine RBC transfusion for hemoglobin levels greater than 7 g/dL (approximate hematocrit of 21%) in hospitalized patients, including hemodynamically stable critically ill patients (5). These recommendations stem from multiple randomized controlled trials of restrictive versus liberal transfusion strategies in critically ill adults and children which have failed to identify benefit to liberal RBC transfusion (5, 6, 12–16). These studies, however, excluded patients requiring ECMO support. In fact no prior study has been conducted to establish optimum RBC transfusion thresholds for pediatric ECMO patients. Thus, it is not surprising that practice variability exists.
It is unclear whether the benefits of RBC transfusion to support near-normal hematocrit while on ECMO outweigh the risks. The primary goal of RBC transfusion in the intensive care unit is to augment oxygen carrying capacity in order to support adequate tissue oxygenation. ECMO patients may receive little benefit in this regard from RBC transfusion to treat mild anemia. In a prospective observational study of 45 children receiving ECMO support, Fiser et al. found that RBC transfusion failed to enhance tissue oxygenation, as measured by central venous oxygen saturation (SVO2) and near infrared spectroscopy, when used to treat mild anemia in children on ECMO (17). While the majority of transfusions were given to patients with an adequate SVO2 of > 70%, even in those with a low pre-transfusion SVO2 of < 70% post-transfusion SVO2 only increased in 16% of subjects. These data suggest that even with evidence of oxygen debt, RBC transfusion may not consistently improve tissue oxygenation. On the other hand, RBC transfusion may have benefits outside of oxygen-carrying capacity. For instance, pre-clinical and limited clinical evidence suggest that anemia may contribute to platelet dysfunction, although this has not been evaluated in the ECMO population (18, 19). If treating anemia helps preserve platelet function on ECMO, RBC transfusion could help avoid bleeding complications, although relationships between anemia and bleeding on ECMO at this time are unknown. In the current analysis, lower daily hematocrit was not independently associated with mortality; supporting the hypothesis that at least mild permissive anemia is likely safe in pediatric ECMO. Likewise, limited adult data suggest that outcomes following the institution of restrictive RBC transfusion protocols for ECMO patients are similar to historical controls - again suggesting that permissive anemia may be safe (20, 21).
Regarding transfusion risks, given their severity of underlying organ dysfunction, ECMO patients may face particularly high risks of complications such as transfusion-related immunomodulation, transfusion-related respiratory dysfunction, and thrombotic complications (22–26). In the current analysis, each additional 10 mL/kg/day of RBC transfusion volume was independently associated with a 9% increase in odds of mortality. These findings are consistent with previous reports suggesting associations between RBC transfusion volume and mortality in ECMO patients (4, 7–9). A single-center retrospective study of 132 adults supported on ECMO for a variety of indications identified RBC transfusion volume as an independent predictor of mortality, accounting for important covariates including serious bleeding events (8). Among children, a previous single-center retrospective study reported a 24% increase in adjusted odds of death for each additional 10 mL/kg/day of RBC transfusion volume among 203 infants and children supported with ECMO for non-cardiac indications (4). Relationships between RBC transfusion volume and mortality remained significant after accounting for potential confounders including patient weight, ECMO mode, diagnosis, and hemorrhagic complications. Unlike the current analysis, relationships between RBC transfusion volume and mortality were not significant in the combined cohort including cardiac and non-cardiac indications. This may have been due to more significant bleeding complications in the cardiac cohort as evidenced by a very high RBC transfusion burden of 105 mL/kg/day on ECMO. Taken together, these data suggest that excess RBC transfusion may be harmful for ECMO patients. However, because of likely residual confounding in retrospective analyses, one cannot ascribe causality to these relationships. It is notable, therefore that in a small pre-post analysis of neonates supported with ECMO for refractory hypoxemic respiratory failure, a protocol change from a target hematocrit of 40% to 35% was significantly associated with decreases in RBC transfusion volume and shorter ECMO durations (27). While mortality differences were not observed, it is likely that the study was underpowered to detect such a difference. In adults, Cahill et al. recently published a single center pre-post analysis of a comprehensive transfusion protocol in adult cardiac ECMO patients. The protocol included an RBC transfusion threshold of 8 g/dL and was associated with significantly reduced RBC transfusion burden and improved survival in the post-protocol era (28). Similarly, in a single-center report of 38 adults supported with ECMO for acute respiratory distress syndrome, a blood conservation protocol using an RBC transfusion threshold of 7 g/dL was associated with lower RBC transfusion burden and fewer bleeding events compared to historical controls (20). Taken together, these data suggest that more restrictive RBC transfusion thresholds may be beneficial for ECMO patients, though optimum thresholds for pediatric ECMO remain unknown. Given the exceptionally high RBC transfusion burden while on ECMO and the potential associated harm, additional prospective studies to determine and evaluate best RBC transfusion practice in neonatal and pediatric ECMO are urgently needed.
Our study has several limitations. Firstly, because pre-transfusion hematocrit values were not recorded, we are unable to determine what values prompted transfusion. However, daily hematocrit goals were recorded for most subjects such that intended practice could be assessed. Additional factors which may have prompted RBC transfusion outside of stated goals are unknown. However, overall mean daily hematocrit values were similar to stated goals suggesting that usual care likely guided RBC transfusion. Secondly, because bleeding was defined subjectively as bleeding requiring transfusion rather than a more objective measure, it is likely that at least some of the relationship between RBC transfusion volume and mortality may be due to differences in bleeding volume. Similarly, plasma hemoglobin values were not recorded for all centers and therefore data on hemolysis as another potential cause for anemia were not available. Residual confounding due to bleeding or hemolysis may explain the observed increase in odds of mortality related to RBC transfusion burden without apparent benefit to lower hematocrit values. Given the limitations of the dataset, we are unable to definitively separate the effects of bleeding or other causes of anemia from those of RBC transfusion. Future prospective trials are necessary to answer this important question. Thirdly, data about RBC product characteristics – including pre-storage leukoreduction, storage duration, irradiation, or other special processing were not recorded. This is important because the risk/benefit balance of RBC transfusion may differ based on the individual products used (29–32). We view this as a highly relevant topic for ongoing study. Additionally, pediatric ECMO represents a highly heterogeneous cohort and it may be that relationships between RBC transfusion volume and mortality differ among ECMO subpopulations. While one of the largest multicenter prospective observational studies to date in pediatric ECMO, the sample size was still insufficient to allow robust multivariable analyses of subpopulations, such as by ECMO indication, while accounting for other center-related differences. Similarly, the sample size was inadequate to permit multi-level modeling to account for center-level, ICU-level, and patient-level factors. We view this as an important area for future study. Lastly, while mortality rates in pediatric ECMO remain high, additional outcome measures are also important and may differentially relate to RBC transfusion practice. Particularly, neurodevelopmental outcomes, ECMO duration, and duration of organ dysfunction among survivors are highly relevant outcome measures to consider in the design of subsequent studies evaluating RBC transfusion practice in pediatric ECMO.
In conclusion, in this multi-center cohort of pediatric and neonatal ECMO patients, RBC transfusion practice varied significantly across institutions and overall RBC transfusion burden was high. While RBC transfusion volume was independently associated with hospital mortality, these analyses are likely confounded by bleeding or other causes of anemia. A lower daily hematocrit was not associated with mortality, suggesting that more restrictive RBC transfusion strategies may be safe in pediatric ECMO. Optimum RBC transfusion strategies for pediatric ECMO remain unknown – representing a vitally important research priority.
Supplementary Material
Acknowledgments
Funding: This project is supported, in part, by the following cooperative agreements from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services: U10HD050096, U10HD049981, U10HD049983, U10HD050012, U10HD063108, U10HD063106, U10HD063114 and U01HD049934. JAM is supported by K08HL123925.
Footnotes
Copyright form disclosure: Drs. Muszynski, Reeder, Berg, Shanley, Newth, Pollack, Wessel, Carcillo, Harrison, Meert, Dean, Jenkins, Tamburro, and Dalton received support for article research from the National Institutes of Health (NIH). Drs. Reeder, Shanley, Pollack, Wessel, Harrison, and Meert’s institutions received funding from the NIH. Drs. Berg, Carcillo, and Dean’s institutions received funding from the National Institute of Child Health and Human Development. Dr. Shanley received funding from International Pediatric Research Foundation, Raynes McCarty Law Firm, Tanoury, Nauts, and McKinney & Garbarino, PLLC. Dr. Newth received funding from Philips Research North America. Drs. Jenkins and Tamburro disclosed government work. Dr. Tamburro’s institution received funding from United States Food and Drug Administration Office of Orphan Products Development and ONY Inc. (provided surfactant free of charge for the previously mentioned US FDA trial). Dr. Dalton received funding from Innovative ECMO Concepts Inc (consultant), Maquet Inc (speaker bureau and consultant), and rEVO Biologics, and she disclosed off-label product use of ECMO related equipment. Dr. Hall disclosed that he does not have any potential conflicts of interest.
The authors declare no competing interests.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.ELSO Anticoagulation Guideline. Patient Care Practice Guidelines 2014. [cited 2016 06/29/2016]Available from: http://www.elso.org/Portals/0/Files/elsoanticoagulationguideline8-2014-table-contents.pdf.
- 2.Henriquez-Henriquez M, Kattan J, Chang M, et al. Blood component usage during extracorporeal membrane oxygenation: experience in 98 patients at a Latin-American tertiary hospital. Int J Artif Organs. 2014;37(3):233–240. doi: 10.5301/ijao.5000311. [DOI] [PubMed] [Google Scholar]
- 3.Jackson HT, Oyetunji TA, Thomas A, et al. The impact of leukoreduced red blood cell transfusion on mortality of neonates undergoing extracorporeal membrane oxygenation. The J Surg Res. 2014;192(1):6–11. doi: 10.1016/j.jss.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 4.Smith A, Hardison D, Bridges B, et al. Red blood cell transfusion volume and mortality among patients receiving extracorporeal membrane oxygenation. Perfusion. 2013;28(1):54–60. doi: 10.1177/0267659112457969. [DOI] [PubMed] [Google Scholar]
- 5.Carson JL, Guyatt G, Heddle NM, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. 2016;316(19):2025–2035. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 6.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356(16):1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 7.Loforte A, Marinelli G, Musumeci F, et al. Extracorporeal membrane oxygenation support in refractory cardiogenic shock: treatment strategies and analysis of risk factors. Artificial Organs. 2014;38(7):E129–141. doi: 10.1111/aor.12317. [DOI] [PubMed] [Google Scholar]
- 8.Mazzeffi M, Greenwood J, Tanaka K, et al. Bleeding, Transfusion, and Mortality on Extracorporeal Life Support: ECLS Working Group on Thrombosis and Hemostasis. Ann Thorac Surg. 2016;101(2):682–689. doi: 10.1016/j.athoracsur.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 9.Kumar TK, Zurakowski D, Dalton H, et al. Extracorporeal membrane oxygenation in postcardiotomy patients: factors influencing outcome. J Thorac Cardiovasc Surg. 2010;140(2):330–336 e332. doi: 10.1016/j.jtcvs.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 10.Dalton HJ, Reeder R, Garcia-Filion P, et al. Factors Associated with Bleeding and Thrombosis in Children Receiving Extracorporeal Membrane Oxygenation (ECMO) Am J Respir Crit Care Med. 2017;196(6):762–771. doi: 10.1164/rccm.201609-1945OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Carson JL, Carless PA, Hebert PC. Outcomes using lower vs higher hemoglobin thresholds for red blood cell transfusion. JAMA. 2013;309(1):83–84. doi: 10.1001/jama.2012.50429. [DOI] [PubMed] [Google Scholar]
- 13.Cholette JM, Rubenstein JS, Alfieris GM, et al. Children with single-ventricle physiology do not benefit from higher hemoglobin levels post cavopulmonary connection: results of a prospective, randomized, controlled trial of a restrictive versus liberal red-cell transfusion strategy. Pediatr Crit Care Med. 2011;12(1):39–45. doi: 10.1097/PCC.0b013e3181e329db. [DOI] [PubMed] [Google Scholar]
- 14.Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 15.Holst LB, Petersen MW, Haase N, et al. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ. 2015;350:h1354. doi: 10.1136/bmj.h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouette J, Trottier H, Ducruet T, et al. Red blood cell transfusion threshold in postsurgical pediatric intensive care patients: a randomized clinical trial. Ann Surg. 2010;251(3):421–427. doi: 10.1097/SLA.0b013e3181c5dc2e. [DOI] [PubMed] [Google Scholar]
- 17.Fiser RT, Irby K, Ward RM, et al. RBC transfusion in pediatric patients supported with extracorporeal membrane oxygenation: is there an impact on tissue oxygenation? Pediatr Crit Care Med. 2014;15(9):806–813. doi: 10.1097/PCC.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 18.Mokhtar GM, Ibrahim WE, Kassim NA, et al. Alterations of platelet functions in children and adolescents with iron-deficiency anemia and response to therapy. Platelets. 2015;26(5):448–452. doi: 10.3109/09537104.2014.931570. [DOI] [PubMed] [Google Scholar]
- 19.Popov VM, Vladareanu AM, Bumbea H, et al. Hemorrhagic risk due to platelet dysfunction in myelodysplastic patients, correlations with anemia severity and iron overload. Blood Coagul Fibrinolysis. 2015;26(7):743–749. doi: 10.1097/MBC.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 20.Agerstrand CL, Burkart KM, Abrams DC, et al. Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg. 2015;99(2):590–595. doi: 10.1016/j.athoracsur.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 21.Voelker MT, Busch T, Bercker S, et al. Restrictive transfusion practice during extracorporeal membrane oxygenation therapy for severe acute respiratory distress syndrome. Artificial Organs. 2015;39(4):374–378. doi: 10.1111/aor.12385. [DOI] [PubMed] [Google Scholar]
- 22.Biro E, Sturk-Maquelin KN, Vogel GM, et al. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemostasis. 2003;1(12):2561–2568. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 23.Gao Y, Lv L, Liu S, et al. Elevated levels of thrombin-generating microparticles in stored red blood cells. Vox Sang. 2013;105(1):11–17. doi: 10.1111/vox.12014. [DOI] [PubMed] [Google Scholar]
- 24.Kleiber N, Lefebvre E, Gauvin F, et al. Respiratory Dysfunction Associated With RBC Transfusion in Critically Ill Children: A Prospective Cohort Study. Pediatr Crit Care Med. 2015;16(4):325–334. doi: 10.1097/PCC.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 25.Muszynski JA, Spinella PC, Cholette JM, et al. Transfusion-related immunomodulation: review of the literature and implications for pediatric critical illness. Transfusion. 2017;57(1):195–206. doi: 10.1111/trf.13855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters AL, Van De Weerdt EK, Goudswaard EJ, et al. Reporting transfusion-related acute lung injury by clinical and preclinical disciplines. Blood Transfus. 2017:1–8. doi: 10.2450/2017.0266-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawyer AA, Wise L, Ghosh S, et al. Comparison of transfusion thresholds during neonatal extracorporeal membrane oxygenation. Transfusion. 2017;57(9):2115–2120. doi: 10.1111/trf.14151. [DOI] [PubMed] [Google Scholar]
- 28.Cahill CM, Blumberg N, Schmidt AE, et al. Implementation of a Standardized Transfusion Protocol for Cardiac Patients Treated With Venoarterial Extracorporeal Membrane Oxygenation Is Associated With Decreased Blood Component Utilization and May Improve Clinical Outcome. Anesth Analg. 2017 doi: 10.1213/ANE.0000000000002238. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Bakkour S, Acker JP, Chafets DM, et al. Manufacturing method affects mitochondrial DNA release and extracellular vesicle composition in stored red blood cells. Vox Sang. 2016;111(1):22–32. doi: 10.1111/vox.12390. [DOI] [PubMed] [Google Scholar]
- 30.Hansen AL, Kurach JD, Turner TR, et al. The effect of processing method on the in vitro characteristics of red blood cell products. Vox Sang. 2015;108(4):350–358. doi: 10.1111/vox.12233. [DOI] [PubMed] [Google Scholar]
- 31.Heddle NM, Arnold DM, Acker JP, et al. Red blood cell processing methods and in-hospital mortality: a transfusion registry cohort study. Lancet Haematol. 2016;3(5):e246–254. doi: 10.1016/S2352-3026(16)00020-X. [DOI] [PubMed] [Google Scholar]
- 32.Jordan A, Chen D, Yi QL, et al. Assessing the influence of component processing and donor characteristics on quality of red cell concentrates using quality control data. Vox Sang. 2016;111(1):8–15. doi: 10.1111/vox.12378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


