Abstract
Altered stress response theoretically contributes to the etiology of cardiometabolic disease. Mindfulness may be a protective buffer against the effects of stress on health outcomes by altering how individuals evaluate and respond to stress. We engaged adolescent girls at risk for developing Type 2 diabetes in a cold-pressor test in order to determine the relationship of dispositional mindfulness to cortisol response and subjective stress, including perceived pain and unpleasantness during the stressor, and negative affect following the stressor. We also evaluated mindfulness as a moderator of psychological distress (depressive/anxiety symptoms) and stress response. Participants were 119 girls age 12–17 years with overweight/obesity, family history of diabetes, and mild-to-moderate depressive symptoms. Greater mindfulness was associated with less perceived pain and negative affect, but was unrelated to cortisol response to the stressor. Regardless of mindfulness, greater depressive/anxiety symptoms related to a more blunted cortisol response. Mindfulness might promote better distress tolerance in adolescents at risk for diabetes by altering how youth perceive and relate to acute stress, rather than through altering the physiological stress response. At all levels of mindfulness, depressive/anxiety symptoms relate to greater blunting of cortisol response. Findings contribute to emerging literature on the role of mindfulness in promoting the mental and physical health and well-being of individuals at risk for Type 2 diabetes.
Keywords: Adolescence, Anxiety, Depression, Dispositional mindfulness, Stress
Introduction
An individual’s response to stress encompasses subjective, cognitive, and emotional appraisal, as well as the body’s physiological response (Campbell & Ehlert, 2012; Ursin & Eriksen, 2004). Stress response has been proposed to contribute to the etiology of cardiometabolic disease through a host of mechanisms, including chronic over-activation of the hypothalamic-pituitary-adrenal (HPA) axis (Downs & Faulkner, 2015; Loucks et al., 2015; Pervanidou & Chrousos, 2012). The HPA axis is the critical neuroendocrine system that governs the body’s peripheral physiological response to stress (Chrousos, 2009). Over-activation of the HPA axis results in subtle, tonic elevations of the stress hormone cortisol, which promotes selective accumulation of visceral fat, insulin resistance, and a metabolic syndrome, key physiological precursors in the progression to Type 2 diabetes and cardiovascular disease (Joseph & Golden, 2017; Rosmond, 2003).
Dysegulation in stress response has been observed in adults with Type 2 diabetes (Champaneri et al., 2012; Siddiqui, Madhu, Sharma, & Desai, 2015). For instance, adults with new-onset Type 2 diabetes report greater subjective stress, have higher evening cortisol, and display a more pronounced cortisol response to an acute laboratory stressor than adults who do not have diabetes, even after controlling for differences in body mass index (BMI; Siddiqui et al., 2015). In observational studies of developing youth, subjective, perceived stress predicts more excessive gains in adolescents’ BMI over time (Tomiyama, Puterman, Epel, Rehkopf, & Laraia, 2013). Serum cortisol is associated with greater insulin resistance and worsening of insulin resistance in healthy adolescents and those at risk for Type 2 diabetes, even after accounting for body fat (Adam et al., 2010; Huybrechts et al., 2014; Prodam et al., 2013).
Adolescence is a developmental period known for increases in psychosocial stress (Steinberg, 2014). Moreover, adolescents who are at heightened risk for youth-onset (<20 years of age) Type 2 diabetes, including females from historically disadvantaged racial/ethnic groups, face particularly high levels of stress (DuBois, Burk– Braxton, Swenson, Tevendale, & Hardesty, 2002). Among girls in particular, adolescence marks a peak period for the onset of symptoms of psychopathology, such as depression, which may impair stress responding (Lewinsohn, Rohde, & Seeley, 1998). Puberty is also accompanied by a normative rise in insulin resistance, making adolescence a sensitive period for developing Type 2 diabetes (Goran, Shaibi, Weigensberg, Davis, & Cruz, 2006), and youth who experience greater psychosocial distress may be more vulnerable. Thus, understanding potentially modifiable factors that influence stress response in adolescents, particularly in females who may be at higher risk, is important to the design of effective preventative interventions.
Mindfulness has gained increasing attention as an individual attribute important for stress response (Creswell, Pacilio, Lindsay, & Brown, 2014; Kadziolka, Di Pierdomenico, & Miller, 2016; Weinstein, Brown, & Ryan, 2009) and potentially beneficial for Type 2 diabetes prevention and disease management (Medina et al., 2017). Dispositional mindfulness is the propensity to stay focused on the present moment and to observe experiences without judgment (Brown & Ryan, 2003). In healthy adolescents, dispositional mindfulness was inversely associated with pain interference, referring to the degree to which pain interferes with day-to-day life activities (Petter, Chambers, McGrath, & Dick, 2013). Among adolescents with meditation experience, a brief mindfulness manipulation more significantly reduced perceived pain during a cold-pressor stress test, compared to a distraction-based manipulation delivered before the stressor (Petter, McGrath, Chambers, & Dick, 2014).
Randomized controlled trials evaluating more prolonged (e.g., three-month) mindfulness-based interventions have shown reductions in adolescents’ psychological distress, including depression and anxiety symptoms, and reduced salivary cortisol as compared to a control group (Sibinga et al., 2013; Weigensberg et al., 2014). Complimentary research on mindfulness and stress response in adults indicates that state mindfulness is related to quicker cortisol recovery during an interpersonal laboratory stressor in healthy adults (Laurent, Hertz, Nelson, & Laurent, 2016). Similarly, a brief mindfulness manipulation experimentally induced faster decline in cortisol following a social stressor, as compared to a neutral control condition, among adults in good general health (Bergeron, Almgren-Dore, & Dandeneau, 2016). Faster cortisol recovery and decline reflect mitigation of the physiological impact of the stressor among those who are more mindful in the moment, which would purportedly have positive effects on health and well-being (Bergeron et al., 2016). In a small body of existing studies evaluating the effects of mindfulness-based interventions on cortisol in adults, the effects have been mixed (O'Leary, O'Neill, & Dockray, 2016).
In addition to the potential direct effects of mindfulness on stress response, mindfulness also has been theorized to serve as a buffer of the effects of psychological distress on stress response (Brown, Weinstein, & Creswell, 2012; Daubenmier, Hayden, Chang, & Epel, 2014). Psychological distress, including symptoms of depression and anxiety, have shown an inconsistent relationship with cortisol response. In some data, adolescents with depressive symptoms display a hypo-responsive or blunted cortisol reaction to laboratory stressors such as a cold-pressor test (Keenan et al., 2013), and this blunted cortisol response profile predicts the subsequent recurrence of elevated depressive symptoms during adolescence (Calhoun et al., 2012). However, other studies suggest that depressive and anxiety symptoms relate to hyper or prolonged cortisol response to acute stress (Lopez-Duran, Kovacs, & George, 2009).
Individual differences in dispositional mindfulness have been proposed to offer one potential moderating factor that explains these discrepancies. Mindfulness involves paying moment-to-moment attention to unpleasant emotions as they arise, noticing the physiological sensations of emotions in the body, and refraining from judgmental cognitions or rumination about these sensations (Brown & Ryan, 2003; Kabat-Zinn, 2003). As such, mindfulness theoretically may shift the way in which an individual relates to and processes psychological distress, resulting in a more adaptive stress response in the face of acute challenges (Garland, Gaylord, & Fredrickson, 2011). Alternatively, lower dispositional mindfulness may render individuals more susceptible for psychological distress to affect dysfunctional stress response. Although a number of studies in adults support dispositional mindfulness as a moderator of psychological distress and stress response (Brown et al., 2012; Daubenmier et al., 2014), there is a paucity of published studies that have evaluated this possibility in adolescents.
The objective of this study was to evaluate the association of dispositional mindfulness with subjective stress, including perceived pain, unpleasantness and negative affect following a cold-pressor test, and cortisol stress response in adolescent girls at risk for Type 2 diabetes. We expected that mindfulness would be inversely related to subjective stress and cortisol response. The second aim was to evaluate dispositional mindfulness as a moderator of the association between depressive/anxiety symptoms and stress response. Based on prior data (Brown et al., 2012; Daubenmier et al., 2014), we anticipated that the relationship of depressive/anxiety symptoms to stress response would be strongest in adolescents who were lower in dispositional mindfulness.
Method
Participants
The current study represents a secondary data analysis. Participants were 119 adolescent (age 12–17 years) girls at risk for Type 2 diabetes, as determined by being overweight/obese (≥85th BMI percentile for age and sex) and having a first- or second-degree relative with Type 2 diabetes, gestational diabetes, or prediabetes. Adolescents were participating in the baseline phase of a Type 2 diabetes behavioral prevention trial (ClinicalTrials.gov: NCT01425905), and baseline data were collected from September 2011 to June 2014. Additional inclusion criteria required that girls have mild-to-moderate depressive symptoms as indicated by a score ≥16 on the Center for Epidemiologic Studies-Depression (CES-D) Scale (Radloff, 1977), which has good psychometric properties in this age group (Garrison, Addy, Jackson, McKeown, & Waller, 1991). All participants were in good general health. Girls were excluded from the study if they met criteria for Type 2 diabetes (fasting glucose level >126 mg/dL or 2-hour glucose after oral glucose administration >200 mg/dL; American Diabetes Association, 2016); had a major medical diagnosis; were participating in structured weight loss or psychotherapy; were taking medication that could affect cortisol, mood, or insulin (e.g. anti-depressants); or were pregnant.
Procedure
Participants were recruited through the National Institutes of Health (NIH) clinical trials website, local community postings, letters to physician offices, and direct mailings to homes within a 60-mile radius of Bethesda, Maryland, USA. All study procedures took place in a pediatric outpatient clinic at the NIH Mark O. Hatfield Clinical Center (Bethesda, Maryland, USA) over the course of two outpatient visits spaced, on average, two weeks apart. At an initial appointment during after-school hours, adolescents and their parents/guardians provided written assent and consent after having the study described to them in detail by a trained member of the research team; an endocrinologist or nurse practitioner conducted a medical examination and health history; and adolescents completed surveys administered electronically using Clinical Trials Database software. All adolescents returned for a separate outpatient visit that occurred following an overnight fast and included assessment of body composition, phlebotomy, and the cold-pressor stress test procedure. All procedures were approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and were carried out in compliance with the 1964 Helsinki declaration and its later amendments.
Measures
Dispositional Mindfulness
Dispositional mindfulness was measured using the 15-item Mindful Attention Awareness Scale (Brown & Ryan, 2003), a relatively brief instrument designed to be understandable by adolescents and adults, regardless of their degree of exposure to mindfulness training (Brown & Ryan, 2003). Participants read statements describing episodes of mindlessness (e.g. “I find myself doing things without paying attention”) and reported how frequently they typically had each experience on a scale of 1-almost always to 6-almost never. A total score is calculated as the sum of all items (possible range 15–90), with higher scores reflecting greater mindfulness and lower scores, less mindfulness. This measure has demonstrated adequate test-retest reliability, internal consistency, and convergent validity with alternate measures of internal state awareness (Brown & Ryan, 2003; Park, Reilly-Spong, & Gross, 2013; Visted, Vøllestad, Nielsen, & Nielsen, 2015). Internal reliability in the current sample was adequate (α=.87).
Cold-Pressor Test
In order to measure acute stress response, participants took part in a 3-minute (maximum duration) afternoon cold-pressor test administered by a trained post-baccalaureate research evaluator using manualized, standard operating procedures. At 3:00pm, girls were escorted to a quiet room for a 1-hour rest period. At 4:00pm, they were asked to submerge their hand to the wrist in a bath of water cooled with ice and maintained at a consistent temperature of 10° C (50° F), a commonly recommended set point for cold-pressor studies with children and adolescents (von Baeyer, Piira, Chambers, Trapanotto, & Zeltzer, 2005). Upon submersion, they were told to flex and relax their hand to prevent a layer of warm water from surrounding it. Adolescents were instructed to keep their hand submerged for as long as possible, but they could remove their hand at any time. They were asked to verbally rate pain and unpleasantness on a visual analog scale of 1-none at all to 100-extreme, every 15 seconds during the cold-pressor test. Maximum pain and unpleasantness were calculated as the highest rating endorsed at any point.
Immediately before and after, and again 20, 40, and 60 minutes following the cold-pressor test, participants reported state negative affect on the Positive and Negative Affect Schedule (Crawford & Henry, 2004). Adolescents indicated to what extent they felt each of 10 negative emotions in the present moment on a scale of 1-very slightly or not at all to 5-extremely, with higher scores reflecting greater negative affect. An average of all items was calculated at each time point. Negative affect response was assessed as the maximum negative affect value at any point following the cold-pressor test. This scale had adequate internal reliability in the current sample (α=.76).
Cortisol was collected at corresponding intervals with a salivary swab (Sarstedt, Newton, NC) placed under the tongue for 120 seconds, immediately before and after, and again 20, 40, and 60 minutes following the test. Peak cortisol response was evaluated as the highest cortisol value 20–60 minutes after the stress exposure, as salivary cortisol response manifests approximately 30 minutes following exposure. Peak response allows for variability in inter-individual differences in the timing of physiological reactions to stress (Lopez-Duran, Mayer, & Abelson, 2014). We also evaluated the more traditional area under the curve with respect to increase (AUCi) and with respect to ground (AUCg) as secondary measures (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003).
Cortisol Assay
All cortisol samples collected were measured using an enzyme immunoassay (Siemens Immulite 1000; sensitivity 60 ng/dL, intra- and inter-assay CVs 5.8–11.2%). Salivary cortisol is a convenient, low subject-burden indicator of circulating free plasma cortisol and a good marker of the physiologically active form.
Depressive/Anxiety Symptoms
Depressive/anxiety symptoms were assessed using the 13-item depression/anxiety narrow-band subscale of the Youth Self-Report (Achenbach, 1991). Participants indicated to what extent each statement was true on a 3-point scale (0-never true; 1-sometimes true; and 2-very often true). A total score is derived from the sum of all items, with higher scores indicating greater depressive/anxiety symptoms. The Youth Self-Report is widely utilized in adolescents and has demonstrated adequate reliability and validity (Achenbach, 1991). The continuous total score was used in primary analyses; for graphical purposes, a dichotomous median split (<13 versus ≥13) variable also was computed. In the current project, we utilized the Youth Self-Report, as opposed to the CES-D, because it measures both types of internalizing symptoms (depression and anxiety) and had larger variability in this sample, by design. The depressive/anxiety subscale of the Youth Self-Report (current sample, α=.78) was chosen for the current study (versus the depressive/withdrawal subscale, for example) because it encompassed the range of internalizing symptoms that have been linked with altered stress response in prior studies with children and adolescents (Lopez-Duran et al., 2009).
Additional Anthropometric and Metabolic Variables
Additional anthropometric and metabolic variables were collected to characterize the sample and/or to ensure that participants met eligibility criteria. Participants underwent a medical history and physical examination conducted by an endocrinologist or nurse practitioner. Pubertal development was assessed according to Tanner stages of breast development (Marshall & Tanner, 1969). BMI (kg/m2) was calculated from participants’ weight (kg), measured using a calibrated scale (Scale Tronix, White Plains, NY), and height in triplicate by stadiometer (Scale Tronix, White Plains, NY). BMI-z was computed using the Centers for Disease Control and Prevention 2000 growth standards. Percent body fat and lean mass (kg) were obtained from dual-energy x-ray absorptiometry using a Hologic QDR-4500A or Discovery instrument (Waltham, MA). After a 10-hour overnight fast, participants provided fasting blood samples for serum insulin and glucose. Glucose was measured using a Hitachi 917 analyzer using reagents from Roche Diagnostics (Indianapolis, IN). Insulin concentrations were determined using a commercially available immunochemiluminometric assay purchased against insulin reference preparation 66/304. The insulin assay used a monoclonal anti-insulin antibody and was run on an Immulite2000 machine (Diagnostic Product Corporation, Los Angeles, CA). The cross-reactivity of the insulin assay with proinsulin was <8% and with Cpeptide was <1%, sensitivity was 2 µU/mL, and the mean inter- and intra- assay coefficients of variation were 5.8 and 3.6%. Insulin resistance was estimated using homeostasis model assessment of insulin resistance (HOMA-IR) index, calculated as: (fasting insulin [µU/mL] X fasting glucose [mmol/L])/22.5.
Data Analyses
Analyses were performed using SPSS 22.0 (IBM Corp, 2013). Outliers were adjusted to 1.5 times the interquartile range below or above the 25th or 75th percentile, resulting in satisfactory skew/kurtosis for all variables (Behrens, 1997). This strategy has been recommended by statisticians in order to minimize outliers’ influence on the distribution, minimally change the distribution overall, and avoid potential bias associated with eliminating outliers (Behrens, 1997). Missing data were handled using a listwise deletion method such that cases were not included in a particular regression model if missing data for any of the variables. Multiple linear hierarchical regression models were conducted to evaluate the study aims. The dependent variables were subjective stress response (maximum state pain, unpleasantness, and negative affect) and cortisol response (peak response, AUCg, and AUCi). In the initial model step, significant covariates (p<.05) were included; we considered collection time, age, race/ethnicity, body fat percent, lean mass, height, puberty, basal cortisol (i.e., pre-cold-pressor test cortisol), basal subjective stress response (i.e., pre-cold-pressor test pain, unpleasantness, or negative affect), and cold-pressor test duration. In the next step, dispositional mindfulness and depression/anxiety symptoms were entered as independent variables; we entered these variables simultaneously so that any significant effects of mindfulness accounted for the effects of depressive/anxiety symptoms and vice versa. The interaction of mindfulness by depressive/anxiety symptoms was added in the last step of the model. Depressive/anxiety symptoms and mindfulness were mean-centered in models and the interaction term.
Results
Descriptive information for the study cohort is presented in Table 1. One hundred nineteen adolescent girls participated. There were minimal missing data for survey measures of dispositional mindfulness (n=7) and depressive/anxiety symptoms (n=1). Complete bloodwork could not be obtained on three adolescents, leading to n=3 missing values for insulin resistance. Six adolescents had missing cold-pressor test data because they declined to take part in that procedure. Because the study was designed to recruit a sample at risk for diabetes, adolescents were highly insulin resistant, on average, as indicated by a mean HOMA-IR value exceeding 5 (Stern et al., 2005). Consistent with diurnal patterns of cortisol in the late afternoon, there was a decreasing pattern of cortisol levels during and after administration of the cold-pressor test, reflected by a negative, average AUCi for the sample as a whole (−210.3±1114.3 ng/dL), with considerable variability (Range = −2145–1575). Thus, the cold-pressor test, on average, did not elicit a cortisol increase in this sample. Thirty-one percent of adolescents had a positive AUCi. Bivariate correlations among key study variables revealed a negative association between dispositional mindfulness and depressive/anxiety symptoms (r=−.34, p<.01). Mindfulness also was inversely correlated with cold-pressor peak pain (r=−.21, p<.05) and negative affect (r=−.27, p<.01). Depressive/anxiety symptoms were negatively correlated with cold-pressor peak cortisol (r=−.20, p<.05). There was a strong positive correlation between cold-pressor pain and unpleasantness (r=.81, p<.01) and between cortisol AUCg and peak cortisol (r=.93, p<.01). No other correlations among key variables reached significance.
Table 1.
Sample characteristics and descriptive information in 119 girls at risk for Type 2 diabetes
| Mean | SD | Range | |
|---|---|---|---|
| Age, years | 14.5 | 1.6 | 12–17 |
| BMI, kg/m2 | 33.0 | 6.6 | 22.9–52.2 |
| BMI z-score | 1.97 | 0.47 | 1.02–2.93 |
| Body fat, % | 42.9 | 5.8 | 30.9–58.1 |
| Lean mass, kg | 48.0 | 8.6 | 31.1–79.8 |
| Fasting insulin, mIU/Ld | 24.2 | 13.6 | 3.7–59.2 |
| Fasting glucose, mg/dLd | 89.1 | 6.8 | 73–109 |
| HOMA-IRd | 5.3 | 3.1 | 0.7–12.8 |
| Dispositional mindfulnessb | 58.5 | 12.6 | 28–85 |
| Depressive/anxiety symptomsa | 8.6 | 4.2 | 1–17 |
| Pre-test basal afternoon cortisol, ng/dLc | 78.9 | 27.7 | 60–140 |
| Cold-pressor test maximum painc | 65.1 | 33.3 | 0–100 |
| Cold-pressor test maximum unpleasantnessc | 72.1 | 33.2 | 0–100 |
| Peak cortisol response, ng/dLc | 83.4 | 34.6 | 60–163 |
| Cortisol AUCgc | 4899.8 | 1389.8 | 3900–7950 |
| Cortisol AUCic | −210.3 | 1114.3 | −2145–1575 |
| Cold-pressor test negative affect responsec | 12.9 | 2.6 | 10–18.5 |
|
|
|||
| No. (%) | |||
|
|
|||
| Race/ethnicity | |||
| Non-Hispanic Black | 74 (62.2) | ||
| White | 19 (16.0) | ||
| Hispanic | 13 (10.9) | ||
| Asian | 4 (3.4) | ||
| Multiple races | 9 (7.6) | ||
| Late Puberty (Tanner 5) | 84 (70.6) | ||
| Obesity, BMI ≥95th percentile | 87 (73.1) | ||
BMI=body mass index; HOMA-IR=homeostasis model assessment of insulin resistance; AUCg=area under the curve with respect to ground; AUCi=area under the curve with respect to increase.
n=1 missing value;
n=7 missing values;
n=6 missing values;
n=3 missing values.
Table 2 displays the results of models predicting peak pain, unpleasantness, and negative affect in response to the cold-pressor test. The results of the regression model predicting peak pain ratings indicated that the predictors entered at step 1 accounted for 8% of the variance (p=.08). After accounting for initial pain rating, duration of the cold-pressor test, and depressive/anxiety symptoms, dispositional mindfulness was significantly, inversely related to pain (p=.02). Adolescents who were higher on dispositional mindfulness reported lower perceived pain during the cold-pressor test. Results of the regression model predicting peak negative affect indicated that, taken together, predictors accounted for 41.6% of the variance (p<.001). A similar pattern was observed for negative affect, with dispositional mindfulness having a significant main effect (p=.01) on negative affect response to the cold-pressor test, accounting for pre-test negative affect rating and depressive/anxiety symptoms. Adolescents who were higher on dispositional mindfulness reported lower negative affect in response to the cold-pressor test. Finally, results of the regression model predicting peak unpleasantness indicated that the predictors accounted for 4.6% of the variance (p=.42). The association between dispositional mindfulness and unpleasantness did not reach significance (p=.057). Depressive/anxiety symptoms were not associated with subjective stress response (all p’s>.78), and the interaction of mindfulness with depressive/anxiety symptoms was non-significant in the prediction of pain, unpleasantness, and negative affect (all p’s>.50).
Table 2.
Hierarchical multiple linear regression analyses evaluating the main and moderating effects of dispositional mindfulness and depressive/anxiety symptoms on pain, unpleasantness, and negative affect in response to a cold-pressor test
| Maximum Paina | Maximum Unpleasantnessb | Negative Affect Responsec | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Predictors | B | SE | β | B | SE | β | B | SE | β |
| Step 1 | |||||||||
| Mindfulness | −.64 | .27 | −.24* | −.52 | .27 | −.20^ | −.04 | .02 | −.21** |
| Depressive/anxiety | .02 | .81 | .00 | .14 | .80 | .02 | −.01 | .05 | −.02 |
| Model R2 | .08^ | .05 | .42*** | ||||||
| ΔR2 | .06* | .04 | .04* | ||||||
| Step 2 | |||||||||
| Mindfulness × Depression/anxiety | .03 | .06 | .05 | .00 | .06 | .00 | .00 | .00 | −.02 |
| Model R2 | .08 | .05 | .42 | ||||||
| ΔR2 | .00 | .00 | .00 | ||||||
B=Unstandardized estimate; SE=standard error; β=standardized estimate; Model R2=total variance explained in the dependent variable; ΔR2=variance explained in the dependent variable by the set of variables added at each step.
In addition to variables displayed (mindfulness, depression/anxiety, and mindfulness × depression/anxiety), estimates were adjusted for acold-pressor test start time and pre-cold-pressor test pain;
pre-cold-pressor test unpleasantness and cold-pressor test duration;
pre-cold-pressor test negative affect.
p<.001,
p<.01,
p<.05,
p<.10.
Table 3 presents the models predicting cortisol response. In the prediction of peak cortisol response, accounting for cold-pressor test start time and pre-test cortisol, depressive/anxiety symptoms were significantly and inversely related to cortisol response (p=.01) above and beyond the effect of dispositional mindfulness. There was neither a main effect of mindfulness (p=.36) nor an interactional effect of mindfulness (p=.19) on the association between depressive/anxiety symptoms and cortisol response. Regardless of level of dispositional mindfulness, adolescents with greater depressive/anxiety symptoms had reduced peak cortisol response to the cold-pressor test.
Table 3.
Hierarchical multiple linear regression analyses evaluating the main and moderating effects of dispositional mindfulness on cortisol response to a cold-pressor stress test
| Peak Cortisol Responsea | Cortisol AUCgb | Cortisol AUCib | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| Predictors | B | SE | β | B | SE | β | B | SE | β |
| Step 1 | |||||||||
| Mindfulness | −.23 | .25 | −.08 | 7.26 | 11.7 | −.06 | 2.13 | 9.78 | .02 |
| Depression/anxiety | −1.85* | .74 | −.22* | −74.00* | 33.6 | −.22* | −12.90 | 28.00 | −.05 |
| Model R2 | .30*** | .11** | .01 | ||||||
| ΔR 2 | .04* | .04^ | .00 | ||||||
| Step 2 | |||||||||
| Mindfulness × Depression/anxiety | −.07 | .06 | −.11 | −3.61 | 2.58 | −.13 | −4.02^ | 2.14 | .19^ |
| Model R2 | .31*** | .13** | .04^ | ||||||
| ΔR 2 | .01 | .02 | .04^ | ||||||
B=Unstandardized estimate; SE=standard error; β=standardized estimate; Model R2=total variance explained in the dependent variable; ΔR2=variance explained in the dependent variable by the set of variables added at each step. AUCg=area under the curve with respect to ground; AUCi=area under the curve with respect to increase.
In addition to variables displayed (mindfulness, depression/anxiety, and mindfulness × depression/anxiety), estimates were adjusted for acold-pressor test start time and pre-cold-pressor test cortisol;
cold-pressor test start time.
p<.001,
p<.01,
p<.05,
p<.10.
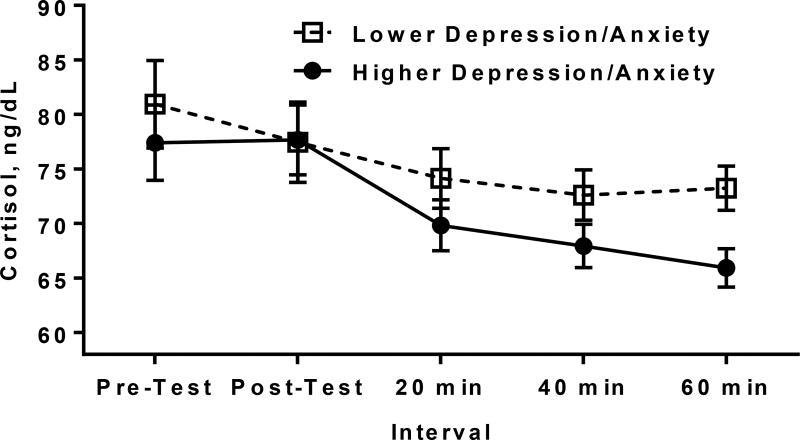
To help in the interpretation of this finding, Figure 1 illustrates for descriptive purposes the average cortisol values over time for girls who were lower or higher in depressive/anxiety symptoms, based upon a median split of the Youth Self-Report. Despite no difference in pre-cold-pressor test basal cortisol (p=.51), girls with relatively higher depressive/anxiety symptoms showed a steeper cortisol decline following cold-pressor test as compared to girls with relatively lower depressive/anxiety symptoms, with a significant difference in cortisol emerging 60 minutes following the stressor between these groups (p=.007). A parallel pattern was observed for the secondary outcome of cortisol AUCg. There was no effect of dispositional mindfulness, depressive/anxiety symptoms, or their interaction on cortisol AUCi (all p’s>.05).
Figure 1.
Cortisol response to a cold-pressor stress test among adolescent girls with lower versus higher depressive/anxiety symptoms based upon a median split of the Youth Self-Report. For descriptive purposes, values depicted are average cortisol levels observed at each time point, adjusting for cold-pressor test start time.
Discussion
In the current study, we investigated how dispositional mindfulness related to stress response among adolescent girls at risk for Type 2 diabetes who also had heterogeneous, mild-to-moderate depressive symptoms. Dispositional mindfulness was not related to any cortisol measurement, but instead was associated with significantly less perceived pain during an acute laboratory stressor and less negative affect following the stressor. By contrast, those with higher depressive/anxiety symptoms had a significantly more blunted peak cortisol response to an acute laboratory stressor, regardless of their level of dispositional mindfulness.
As predicted, dispositional mindfulness was inversely related to subjective pain and negative affect in response to acute stress. Specifically, there was a significant negative association between dispositional mindfulness and adolescents’ ratings of pain during the cold-pressor test and their negative affect response following the test, even after accounting for depressive/anxiety symptoms. These findings are in line with prior research in community samples of adolescents and adults establishing a link between dispositional mindfulness and reduced pain and negative affect in response to stress (Brown et al., 2012; Petter et al., 2013). Mindfulness has been posited to buffer the experience of suffering during conditions of stress and discomfort through reducing automatic, negative habitual responding to a stressor and promoting more positive engagement of self-directed strategies for coping (Brown & Ryan, 2003; Hanley & Garland, 2014; Ryan & Deci, 2000). Although more data are needed on mindfulness and stress response in adolescents, the current results highlight the potential importance of this construct for subjective stress and pain management in adolescents who are heightened risk for cardiometabolic disease.
In contrast to our hypotheses and prior data in adults (Brown et al., 2012; Daubenmier et al., 2014), dispositional mindfulness did not play a moderating role in the relationship between depressive/anxiety symptoms and stress response. One possible explanation for these null findings is that the linkage between depressive/anxiety symptoms and cortisol response among adolescents at risk for Type 2 diabetes reflects a biological vulnerability that simply does not differ based upon individual differences in dispositional mindfulness. The majority (70%) of participants showed no increase in cortisol response to a cold-pressor stress test. Thus, an alternative explanation is that the lack of findings for moderation are attributable to the low rate of cortisol response to the laboratory stressor.
Depressive/anxiety symptoms were negatively related to peak cortisol response to the cold-pressor test, despite no significant relationship of depressive/anxiety symptoms to basal afternoon cortisol. These results indicate that, while most of the sample did not display a significant change in cortisol response during a laboratory stressor, adolescents with relatively higher levels of depression/anxiety symptoms had the most blunted response. These findings are consistent with a hypo-active cortisol stress response and flatter diurnal patterns observed in both depressed adolescents and in adolescents with overweight/obesity (Calhoun et al., 2012; Keenan et al., 2013; Ruttle et al., 2013). Attenuation of the cortisol stress response could be reflective of the notion that depressive/anxiety symptoms reduce mobilization of resources to stress, resulting in additional symptoms like fatigue, which theoretically may further contribute over time to weight gain and other attributes of a metabolic syndrome (Pervanidou & Chrousos, 2011, 2012). Alternatively, a lesser cortisol response could also be indicative of variations in diurnal cortisol rhythm, as opposed to stress response. Indeed, flatter diurnal cortisol slopes have been associated with heightened BMI and the presence of Type 2 diabetes in adults, as well as worsened glycemia in adults with Type 2 diabetes, consistent with the possibility that dysregulated cortisol is linked to excess BMI and increased insulin resistance (Hackett, Kivimaki, Kumari, & Steptoe, 2016). However, given that the majority of adolescents did not show the expected increase in cortisol following the stressor, interpretation of these findings remains tentative.
The findings from this study add to a growing literature documenting the relationship between trait mindfulness and perceptions of distress, and extends it to include adolescent girls at risk for Type 2 diabetes. This may have important implications for how mindfulness-based stress reduction interventions function to relieve symptoms of distress among adolescents who experience precursors to diabetes. Prior studies among adults at risk for Type 2 diabetes have shown modest effects of mindfulness-based stress reduction on improving weight loss and cardiometabolic symptoms (Miller, Kristeller, Headings, Nagaraja, & Miser, 2012; Rosenzweig et al., 2007), although findings have been mixed (Loucks et al., 2015). Our findings suggest that utilizing mindfulness-based stress reduction for diabetes prevention may provide an additional benefit by improving how adolescent girls perceive and respond to challenges, which may impact the trajectory of both their mental and physical health outcomes. Such findings would be in line with the preponderance of research showing that mindfulness can promote more positive responses to stress (e.g. engaging self-regulation; Kadziolka et al., 2016) and reduce negative evaluations of stressful encounters (e.g., Creswell et al., 2014).
Limitations
Study strengths include the examination of an important issue using well-validated psychological and physiological measures, in a sample with a good representation of racially/ethnically disadvantaged groups (e.g., African Americans) at heightened risk of developing Type 2 diabetes (Dabelea et al., 2014). While the findings are relevant for highly insulin resistant girls with overweight/obesity, a family history of diabetes, and mild-to-moderate depressive symptoms, the results may not be generalizable to boys, to adults, or to individuals without the selective characteristics of the study sample. Likewise, because all adolescents had mild-to-moderate depressive symptoms on the CES-D, but were not clinically depressed, variability in depressive/anxiety symptoms was also somewhat restricted and generalizability is cautioned to adolescents without depressive symptoms, as well as to those with major depressive disorder or an anxiety disorder. In addition, we utilized cross-sectional data, which limits interpretation of the direction of influence between variables. This was also a secondary data analysis; post-hoc power analysis indicated that we had adequate (≥80%) power to detect small-to-moderate effects. Future investigations would benefit from examining larger and more heterogeneous samples longitudinally.
Although the current study followed recommended guidelines for administering the cold-pressor test stressor to youth (von Baeyer et al., 2005), only 30% of the sample had a cortisol response. Although we thoroughly trained evaluators and used standardized operating procedures, we were not able to utilize a single evaluator for all testing sessions, introducing the possibility that some variability may have been attributable to the tester. It is possible that the temperature of 10° C was not perceived as distressing to a majority of the participants. Adult studies typically have used a colder temperature and/or have modified the cold-pressor test to include a socially-evaluative component to enhance stress, limiting comparison between this study and adult work. Furthermore, compared to individuals who are lean, individuals with excess adiposity have differences in body heat transfer and release that may lessen their sensitivity to cold temperatures (Savastano et al., 2009). It is also possible that the cold-pressor test is not adequate to induce a cortisol increase among children and adolescents, as similar patterns of non-response have been observed in other studies with similar samples (Keenan et al., 2013). More research is necessary to evaluate the association of mindfulness to cortisol response to alternative stressors that may evoke a more potent cortisol response.
There are many self-report questionnaires that claim to measure mindfulness, each conceptualizing mindfulness differently and assessing different facets of the construct (Baer, Smith, Hopkins, Krietemeyer, & Toney, 2006). The current study utilized the Mindful Attention Awareness Scale (MAAS), which assessed the propensity for individuals to maintain mindful awareness in everyday life (Brown & Ryan, 2003). This measure has been critiqued for inadequately capturing the complex nature of mindfulness as it has been described in Buddhist writings and by practitioners of mindfulness-based interventions (Grossman, 2011). Trait mindfulness in the current study, measured using the MAAS, may more accurately reflect “acting with awareness,” which encompasses only one dimension of a multifaceted conception of mindfulness which includes nonjudgmental acceptance (Baer et al., 2006; Van Dam, Earleywine, & Borders, 2010). Moreover, questionnaire measures of self-perceptions of psychological traits are subject to reporting bias, and may not capture the moment-to-moment unfolding of mindful attention which may be better assessed using third-person methods, such as breath counting (Levinson, Stoll, Kindy, Merry, & Davidson, 2014). Future studies may further elucidate the relationship between mindfulness and stress response by measuring the transitory state of mindfulness throughout the course of a stressful encounter and/or by examining how specific dimensions of mindfulness, such as non-reactivity and non-judgment (Baer et al., 2006), relate to adaptive psychological and physiological responding to stress.
The relationships among psychological characteristics, stress response, and biomarkers of Type 2 diabetes disease progression are complex. In the current study, relatively greater mindfulness, even after accounting for depressive/anxiety symptoms, uniquely related to reduced sensitivity to pain and negative emotions in the face of an unpleasant challenge. Dispositional mindfulness was not related to cortisol response, nor did mindfulness moderate the association between psychological distress and stress response. Instead, at all levels of dispositional mindfulness, depressive/anxiety symptoms were associated with a more blunted cortisol response. Differences in cortisol output could reflect dysregulation of the HPA axis, which may contribute to worsening insulin resistance in the future. Taken together, these findings suggest that dispositional mindfulness may be relevant for subjective stress tolerance and support an association of depressive/anxiety symptoms with HPA axis dysregulation in adolescent girls at risk for Type 2 diabetes with mild-to-moderate depressive symptoms.
Acknowledgments
Funding: The project was supported by the National Institute of Child Health and Human Development (NICHD) K99/R00HD069516, 1ZIAHD000641; with supplemental funding from the NIH Bench to Bedside Program, the Office of Disease Prevention, NIH, and the Office of Behavioral and Social Sciences Research.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
Author Contributions
AS: conducted and interpreted the data analyses and wrote the paper. NRK: executed the study, collaborated with the design and writing of the study. RMR: executed the study, collaborated in the writing and editing of the final manuscript. KAT: collaborated in the writing and editing of the final manuscript. OG: collaborated in the writing and editing of the final manuscript. APD: collaborated in the writing and editing of the final manuscript. SMB: executed the study, collaborated in the writing and editing of the final manuscript. KYC: collaborated in the writing and editing of the final manuscript. MT: designed and executed the study, collaborated in the writing and editing of the final manuscript. JAY: designed and executed the study, collaborated in the writing and editing of the final manuscript. LBS: designed and executed the study, conducted and interpreted data analyses, assisted with writing the paper.
References
- Achenbach TM. Manual for the Youth Self-Report and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Adam TC, Hasson RE, Ventura EE, Toledo-Corral C, Le KA, Mahurkar S, Goran MI. Cortisol is negatively associated with insulin sensitivity in overweight Latino youth. Journal of Clinical Endocrinology and Metabolism. 2010;95:4729–4735. doi: 10.1210/jc.2010-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in Diabetes-2016. Diabetes Care. 2016;39(Supplement 1):S1–S112. doi: 10.2337/dc16-S001. doi:doiI. [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Behrens JT. Principles and procedures of exploratory data analysis. Psychological Methods. 1997;2:131–160. doi: 10.1037/1082-989X.2.2.131. [DOI] [Google Scholar]
- Bergeron CM, Almgren-Dore I, Dandeneau S. "Letting go" (implicitly): Priming mindfulness mitigates the effects of a moderate social stressor. Frontiers in Psychology. 2016;7:872. doi: 10.3389/fpsyg.2016.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: Mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology. 2003;84:822–848. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Brown KW, Weinstein N, Creswell JD. Trait mindfulness modulates neuroendocrine and affective responses to social evaluative threat. Psychoneuroendocrinology. 2012;37:2037–2041. doi: 10.1016/j.psyneuen.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun CD, Franklin JC, Adelman CB, Guerry JD, Hastings PD, Nock MK, Prinstein MJ. Biological and cognitive responses to an in vivo interpersonal stressor: Longitudinal associations with adolescent depression. International Journal of Cognitive Therapy. 2012;5:283–299. doi: 10.1521/ijct.2012.5.3.283. [DOI] [Google Scholar]
- Campbell J, Ehlert U. Acute psychosocial stress: Does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology. 2012;37:1111–1134. doi: 10.1016/j.psyneuen.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, Diez Roux A, Golden SH. Diurnal salivary cortisol and urinary catecholamines are associated with diabetes mellitus: The Multi-Ethnic Study of Atherosclerosis. Metabolism: Clinical and Experimental. 2012;61:986–995. doi: 10.1016/j.metabol.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. Stress and disorders of the stress system. Nature Reviews in Endocrinology. 2009;5:374–381. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Henry JD. The Positive and Negative Affect Schedule (PANAS): Construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43:245–265. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Pacilio LE, Lindsay EK, Brown KW. Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology. 2014;44:1–12. doi: 10.1016/j.psyneuen.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, J D, Hamman RF. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. Journal of the American Medical Association. 2014;311:1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubenmier J, Hayden D, Chang V, Epel E. It's not what you think, it's how you relate to it: Dispositional mindfulness moderates the relationship between psychological distress and the cortisol awakening response. Psychoneuroendocrinology. 2014;48:11–18. doi: 10.1016/j.psyneuen.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs CA, Faulkner MS. Toxic stress, inflammation and symptomatology of chronic complications in diabetes. World Journal of Diabetes. 2015;6:554–565. doi: 10.4239/wjd.v6.i4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois DL, Burk–Braxton C, Swenson LP, Tevendale HD, Hardesty JL. Race and gender influences on adjustment in early adolescence: Investigation of an integrative model. Child Development. 2002;73:1573–1592. doi: 10.1111/1467-8624.00491. [DOI] [PubMed] [Google Scholar]
- Garland EL, Gaylord SA, Fredrickson BL. Positive reappraisal mediates the stress-reductive effects of mindfulness: An upward spiral process. Mindfulness. 2011;2:59–67. doi: 10.1007/s12671-011-0043-8. [DOI] [Google Scholar]
- Garrison CZ, Addy CL, Jackson KL, McKeown RE, Waller JL. The CES-D as a screen for depression and other psychiatric disorders in adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:636–641. doi: 10.1097/00004583-199107000-00017. [DOI] [PubMed] [Google Scholar]
- Goran MI, Shaibi GQ, Weigensberg MJ, Davis JN, Cruz ML. Deterioration of insulin sensitivity and beta-cell function in overweight Hispanic children during pubertal transition: a longitudinal assessment. International Journal of Pediatric Obesity. 2006;1:139–145. doi: 10.1080/17477160600780423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P. Defining mindfulness by how poorly I think I pay attention during everyday awareness and other intractable problems for psychology's (re)invention of mindfulness: comment on Brown et al. (2011) Psychological Assessment. 2011;23:1034–1040. doi: 10.1037/a0022713. discussion 1041-1036. [DOI] [PubMed] [Google Scholar]
- Hackett RA, Kivimaki M, Kumari M, Steptoe A. Diurnal cortisol patterns, future diabetes, and impaired glucose metabolism in the Whitehall II Cohort Study. Journal of Clinical Endocrinology and Metabolism. 2016;101:619–625. doi: 10.1210/jc.2015-2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley AW, Garland EL. Dispositional mindfulness co-varies with self-reported positive reappraisal. Personality and Individual Differences. 2014;66:146–152. doi: 10.1016/j.paid.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huybrechts I, De Vriendt T, Breidenassel C, Rogiers J, Vanaelst B, Cuenca-Garcia M, Group HS. Mechanisms of stress, energy homeostasis and insulin resistance in European adolescents--the HELENA study. Nutrition, Metabolism, and Cardiovascular Disease. 2014;24:1082–1089. doi: 10.1016/j.numecd.2014.04.014. [DOI] [PubMed] [Google Scholar]
- Joseph JJ, Golden SH. Cortisol dysregulation: The bidirectional link between stress, depression, and type 2 diabetes mellitus. Annals of the New York Academy of Sciences. 2017;1391:20–34. doi: 10.1111/nyas.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat-Zinn J. Mindfulness-based interventions in context: Past, present, and future. Clinical Psychology Science and Practice. 2003;10:144–156. doi: 10.1093/clipsy/bpg016. [DOI] [Google Scholar]
- Kadziolka MJ, Di Pierdomenico E, Miller CJ. Trait-like mindfulness promotes healthy self-regulation of stress. Mindfulness. 2016;7:236–245. doi:doi.org/10.1007/s12671-015-0437-0. [Google Scholar]
- Keenan K, Hipwell A, Babinski D, Bortner J, Henneberger A, Hinze A, Sapotichne B. Examining the developmental interface of cortisol and depression symptoms in young adolescent girls. Psychoneuroendocrinology. 2013;38:2291–2299. doi: 10.1016/j.psyneuen.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Hertz R, Nelson B, Laurent SM. Mindfulness during romantic conflict moderates the impact of negative partner behaviors on cortisol responses. Hormones and Behavior. 2016;79:45–51. doi: 10.1016/j.yhbeh.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Levinson DB, Stoll EL, Kindy SD, Merry HL, Davidson RJ. A mind you can count on: validating breath counting as a behavioral measure of mindfulness. Frontiers in Psychology. 2014;5:1202. doi: 10.3389/fpsyg.2014.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Rohde P, Seeley JR. Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clinical Psychology Review. 1998;18:765–794. doi: 10.1016/s0272-7358(98)00010-5. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology. 2009;34:1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duran NL, Mayer SE, Abelson JL. Modeling neuroendocrine stress reactivity in salivary cortisol: adjusting for peak latency variability. Stress. 2014;17:285–295. doi: 10.3109/10253890.2014.915517. [DOI] [PubMed] [Google Scholar]
- Loucks EB, Schuman-Olivier Z, Britton WB, Fresco DM, Desbordes G, Brewer JA, Fulwiler C. Mindfulness and cardiovascular disease risk: State of the evidence, plausible mechanisms, and theoretical framework. Current Cardiology Reports. 2015;17:112. doi: 10.1007/s11886-015-0668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disorders of Childhood. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina WL, Wilson D, de Salvo VL, Vannucchi B, de Souza EL, Lucena L, Demarzo MM. Effects of mindfulness on diabetes mellitus: Rationale and overview. Current Diabetes Reviews. 2017;13:141–147. doi: 10.2174/1573399812666160607074817. doi:doi. [DOI] [PubMed] [Google Scholar]
- Miller CK, Kristeller JL, Headings A, Nagaraja H, Miser WF. Comparative effectiveness of a mindful eating intervention to a diabetes self-management intervention among adults with type 2 diabetes: A pilot study. Journal of the Academy of Nutrition and Dietetics. 2012;112:1835–1842. doi: 10.1016/j.jand.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary K, O'Neill S, Dockray S. A systematic review of the effects of mindfulness interventions on cortisol. Journal of Health Psychology. 2016;21:2108–2121. doi: 10.1177/1359105315569095. [DOI] [PubMed] [Google Scholar]
- Park T, Reilly-Spong M, Gross CR. Mindfulness: A systematic review of instruments to measure an emergent patient-reported outcome (PRO) Quality of Life Research. 2013;22:2639–2659. doi: 10.1007/s11136-013-0395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pervanidou P, Chrousos GP. Stress and obesity/metabolic syndrome in childhood and adolescence. International Journal of Pediatric Obesity. 2011;6:21–28. doi: 10.3109/17477166.2011.615996. [DOI] [PubMed] [Google Scholar]
- Pervanidou P, Chrousos GP. Metabolic consequences of stress during childhood and adolescence. Metabolism: Clinical and Experimental. 2012;61:611–619. doi: 10.1016/j.metabol.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Petter M, Chambers CT, McGrath PJ, Dick BD. The role of trait mindfulness in the pain experience of adolescents. Journal of Pain. 2013;14:1709–1718. doi: 10.1016/j.jpain.2013.08.015. [DOI] [PubMed] [Google Scholar]
- Petter M, McGrath PJ, Chambers CT, Dick BD. The effects of mindful attention and state mindfulness on acute experimental pain among adolescents. Journal of Pediatric Psychology. 2014;39:521–531. doi: 10.1093/jpepsy/jsu007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodam F, Ricotti R, Agarla V, Parlamento S, Genoni G, Balossini C, Bellone S. High-end normal adrenocorticotropic hormone and cortisol levels are associated with specific cardiovascular risk factors in pediatric obesity: A cross-sectional study. BMC Medicine. 2013;11:44. doi: 10.1186/1741-7015-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/S0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychology Measures. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Rosenzweig S, Reibel DK, Greeson JM, Edman JS, Jasser SA, McMearty KD, Goldstein BJ. Mindfulness-based stress reduction is associated with improved glycemic control in type 2 diabetes mellitus: A pilot study. Alternative Therapies in Health and Medicine. 2007;13:36–38. [PubMed] [Google Scholar]
- Rosmond R. Stress induced disturbances of the HPA axis: A pathway to Type 2 diabetes? Medical Science Monitor. 2003;9:RA35–39. [PubMed] [Google Scholar]
- Ruttle PL, Javaras KN, Klein MH, Armstrong JM, Burk LR, Essex MJ. Concurrent and longitudinal associations between diurnal cortisol and body mass index across adolescence. Journal of Adolescent Health. 2013;52:731–737. doi: 10.1016/j.jadohealth.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. American Psychologist. 2000;55:68–78. doi: 10.1037/0003-066X.55.1.68. [DOI] [PubMed] [Google Scholar]
- Savastano DM, Gorbach AM, Eden HS, Brady SM, Reynolds JC, Yanovski JA. Adiposity and human regional body temperature. American Journal of Clinical Nutrition. 2009;90:1124–1131. doi: 10.3945/ajcn.2009.27567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibinga EMS, Perry-Parrish C, Chung S-e, Johnson SB, Smith M, Ellen JM. School-based mindfulness instruction for urban male youth: A small randomized controlled trial. Preventive Medicine. 2013;57:799–801. doi: 10.1016/j.ypmed.2013.08.027. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, Madhu SV, Sharma SB, Desai NG. Endocrine stress responses and risk of type 2 diabetes mellitus. Stress. 2015;18:498–506. doi: 10.3109/10253890.2015.1067677. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Age of opportunity: Lessons from the new science of adolescence. New York, NY: Mariner Books; 2014. [Google Scholar]
- Stern SE, Williams K, Ferrannini E, DeFronzo RA, Bogardus C, Stern MP. Identification of individuals with insulin resistance using routine clinical measurements. Diabetes. 2005;54:333–339. doi: 10.2337/diabetes.54.2.333. [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, Puterman E, Epel ES, Rehkopf DH, Laraia BA. Chronic psychological stress and racial disparities in body mass index change between Black and White girls aged 10–19. Annals of Behavioral Medicine. 2013;45:3–12. doi: 10.1007/s12160-012-9398-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin H, Eriksen HR. The cognitive activation theory of stress. Psychoneuroendocrinology. 2004;29:567–592. doi: 10.1016/S0306-4530(03)00091-X. [DOI] [PubMed] [Google Scholar]
- Van Dam NT, Earleywine M, Borders A. Measuring mindfulness? An Item Response Theory analysis of the Mindful Attention Awareness Scale. Personality and Individual Differences. 2010;49:805–810. [Google Scholar]
- Visted E, Vøllestad J, Nielsen MB, Nielsen GH. The impact of group-based mindfulness training on self-reported mindfulness: A systematic review and meta-analysis. Mindfulness. 2015;6:501–522. doi: 10.1007/s12671-014-0283-5. [DOI] [Google Scholar]
- von Baeyer CL, Piira T, Chambers CT, Trapanotto M, Zeltzer LK. Guidelines for the cold pressor task as an experimental pain stimulus for use with children. Journal of Pain. 2005;6:218–227. doi: 10.1016/j.jpain.2005.01.349. [DOI] [PubMed] [Google Scholar]
- Weigensberg MJ, Lane CJ, Avila Q, Konersman K, Ventura E, Adam T, Spruijt-Metz D. Imagine HEALTH: Results from a randomized pilot lifestyle intervention for obese Latino adolescents using Interactive Guided ImagerySM. BMC Complementary and Alternative Medicine. 2014;14:28. doi: 10.1186/1472-6882-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein N, Brown KW, Ryan RM. A multi-method examination of the effects of mindfulness on stress attribution, coping, and emotional well-being. Journal of Reseach on Personality. 2009;43:374–385. doi: 10.1016/j.jrp.2008.12.008. [DOI] [Google Scholar]