Opioid initiation for postsurgical and musculoskeletal pain is associated with the highest dose and duration at initiation, respectively, relative to other indications.
Keywords: Opioid analgesics, Prescribing, Drug policy
Abstract
Concerns over prescription opioids contributing to high levels of opioid use disorder and overdose have led policymakers and clinicians to seek means to reduce inappropriate and high-dose initial prescriptions. To inform such efforts, we sought to describe the clinical indications associated with opioid initiation and the characteristics of the initial prescriptions and patients through a retrospective population-based cohort study. Our cohort included Ontarians initiating prescription opioids for pain management between April 1, 2015, and March 31, 2016. We identified the apparent clinical indication for opioid initiation by linking prescription drug claims to procedural and diagnostic information on health service records on the day of, and 5 days preceding prescription. Outcomes included initial opioid type, prescription duration, and daily dose (in milligram morphine equivalents), stratified either by indication or indication cluster. Among 653,993 individuals, we successfully classified 575,512 (88.0%) people initiating opioids into 23 clinical indications in 6 clusters: dental (23.2%); postsurgical (17.4%); musculoskeletal (12.0%); trauma (11.2%); cancer/palliative care (6.5%); and other less frequent indications (17.7%). Individuals with postsurgical pain received the highest daily doses (40.5% with greater than 50 milligram morphine equivalent), and those with musculoskeletal pain received more initial prescriptions with a duration exceeding 7 days (34.2%). Opioids are initiated for a wide range of indications with varying doses and durations; yet, those who initiated opioids for postsurgical and musculoskeletal pain received the greatest doses and durations of therapy, respectively. These findings may help tailor and prioritize efforts to promote more appropriate opioid prescribing.
1. Introduction
The use of prescription opioids has increased considerably over the past 2 decades, particularly in Canada and the United States, which had the highest level of opioid consumption per capita worldwide in 2015.12,19 High levels of opioid prescribing have raised concerns among clinicians and policymakers, given the limited evidence of long-term effectiveness of this class of drugs and research finding increased risks of adverse events including mortality with long-term use.14,22
Previous research has associated long-term use of prescription opioids and dose escalation with worse outcomes.11 In turn, recently published clinical practice guidelines for chronic noncancer pain management recommended nonopioid alternatives as first-line and have suggested threshold doses for patients newly started on opioids.1,6 However, opioids are prescribed for a range of acute and chronic pain conditions, including arthritis, back pain, postsurgical pain, and dental pain.23 Studies in select populations have demonstrated that characteristics of opioid initiation and prescribing vary by clinical indication, which may reflect different anticipated needs for different indications as well as variations in training across specialties.26 Therefore, there has been an emerging recognition that policies and programs developed to address appropriate prescribing of opioids may need to be tailored to each clinical indication. This requires an understanding of the relative contribution of each clinical indication to patterns of opioid initiation.29 Previous studies investigating these indications have been limited to smaller populations.15,18,25,26
Accordingly, we set out to determine the apparent clinical indications for opioid initiation at the population level and to describe the characteristics of the initial prescriptions and patients by indication.
2. Methods
2.1. Setting
We conducted a population-based retrospective cohort study of all Ontarians who were newly dispensed an opioid between April 1, 2015, and March 31, 2016. Ontario is Canada's most populous province, with a population of 13.4 million in 2016, representing 38% of the Canadian population.27 This study was approved by the institutional review board at Sunnybrook Health Sciences Centre, Toronto, Canada.
2.2. Data sources
We identified opioid prescription characteristics from the Narcotics Monitoring System (NMS), which captures information regarding all opioids dispensed from all retail pharmacies regardless of payer. Pharmacist data entry into the NMS is mandatory for all controlled substances.20 We acquired cancer diagnoses from the Ontario Cancer Registry, and details regarding cancer treatment and palliative care from the cancer Activity Level Reporting database. We obtained hospital visit data (including diagnoses and procedures) from the Canadian Institute for Health Information's Discharge Abstract Database (CIHI DAD), emergency department visit and same day surgery data from CIHI's National Ambulatory Care Reporting System. We identified physician-billing data from the Ontario Health Insurance Plan (OHIP) Claims History Database, and used the OHIP Registered Persons Database (RPDB) to identify patients' place of residence and demographic characteristics. These databases have high levels of completeness and are regularly used in health services research.2,3,13 We linked data sets using unique encoded identifiers, and all analyses were performed at the Institute for Clinical Evaluative Sciences (ICES) using SAS software (version 9.4; SAS Institute Inc, Cary, NC).
2.3. Study patients
We defined opioid users as those dispensed an eligible opioid (ie, an opioid formulation not used to treat opioid use disorder) between April 1, 2015, and March 31, 2016. We defined an individual's index date and prescription based on their first receipt of a prescription opioid in the accrual period.
We limited our analysis to prescriptions dispensed to individuals with a valid Ontario health card. We excluded individuals with opioid use before the index date, defined as any prescription for an eligible opioid between the index date and July 1, 2012, which is when all pharmacies were mandated to use the NMS. We excluded individuals currently or having previously been on treatment for opioid use disorder (defined as having been dispensed an opioid for the treatment of opioid use disorder since July 1, 2012, and before or on index date). We excluded patients who visited an emergency department or hospital for opioid toxicity (defined by ICD10 diagnosis codes T40.0-T40.4 or T40.6 in the National Ambulatory Care Reporting System or DAD databases) in the 2 years before the index date because this would reflect previous use of opioids.
Among the remaining new users of opioids, we excluded individuals dispensed an opioid formulation used only as an antitussive, thereby limiting our analysis to individuals newly initiating opioids for pain management.
2.4. Identifying the apparent clinical indication
We used a stepwise hierarchical approach to identify the most likely clinical indication for opioid initiation among individuals in the cohort. We developed the hierarchy based on the licensing college of the initial prescriber and the degree of certainty that the procedural and diagnostic information on the related health care administrative data would warrant an opioid prescription. For example, we were most certain of indications for dental pain because the index prescription was directly linked to a dentist. For all other prescriptions written by physicians, our hierarchy was informed by clinical insight as to the likelihood that the diagnosis or procedure would lead to an opioid initiation.
In the first step of the hierarchical approach, we classified individuals whose index opioid was prescribed by a dentist, then those with evidence of palliative care in the past year, followed by those with evidence of active cancer in the past year into each of these 3 indications accordingly. For those remaining, we identified the diagnostic and procedural information on their most recent health care interaction on or in the 5 days preceding the first-opioid prescription. Health care interactions included inpatient hospitalizations, emergency department visits, outpatient surgical procedures, and physician office visits.
In the second hierarchical step, we classified individuals with a recent hospitalization or procedure into procedure-based indications according to the Canadian Classification of Health Interventions (CCI) procedure code on the identified health care record. In the third hierarchical step, remaining individuals were classified into diagnosis-based indications according to the International Statistical Classification of Diseases and Related Health Problems 10th revision (ICD10) or OHIP diagnostic code recorded. We classified individuals with diagnostic codes that would not normally warrant an opioid prescription into an “Unknown” group, and did the same with individuals who had no evidence of a health care encounter in the previous 5 days. We identified a total of 23 clinical indications, which were then grouped into 6 indication clusters. We provide more details on the approach and hierarchy used to assign indications along with associated procedure and diagnostic codes in the Supplementary Appendix (available online at http://links.lww.com/PAIN/A570).
2.5. Prescription and patient characteristics
Within each indication, we defined prescription characteristics at initiation, including the daily dose dispensed in milligram morphine equivalents (MMEs), the number of days supplied, opioid formulation type (immediate-release or long-acting), and type of opioid. Within each indication, we also identified the proportion of initial prescriptions that had a potentially inappropriate initial dose (defined as daily dose exceeding 50 MME) or a potentially inappropriate duration (defined as exceeding 7 days' supply). For daily dose, the threshold of 50 MME reflects how current U.S. and Canadian chronic noncancer pain guidelines suggest clinicians avoid initiating opioids above this daily dose, likely due to associations with adverse events such as road trauma and fatal overdose.1,6,8,9 For prescription duration, the threshold of 7 days reflects how these longer prescriptions may be associated with more long-term use.6,25,26
For people dispensed 2 or more opioids on their index date, we counted the longest number of days supplied and summed the doses to calculate daily dose. Finally, we described patient demographic characteristics (including age, sex, neighbourhood income quintile, and urban/rural location of residence) by indication cluster. No formal statistical tests were performed.
3. Results
Among 778,803 new users, 653,993 (84.0%) met our inclusion criteria (Fig. 1). Just over half (N = 339,525, 51.9%) were women, and the median age was 48 years (Q1-Q3, 29-63 years). The vast majority (N = 644,762, 98.6%) of new opioid users received only an immediate-release prescription on their index date, and the most common opioid prescribed to these individuals was codeine-combination products (N = 343,094; 53.2%). Among all new opioid users, the median daily dose on the index prescription was 34 MME (Q1-Q3, 21-45 MME) and 156,461 (23.9%) initial prescriptions had a daily dose above 50 MME. The median prescription duration was 4 days (Q1-Q3, 3-6 days); 113,523 (17.4%) had an initial duration exceeding 7 days.
Figure 1.
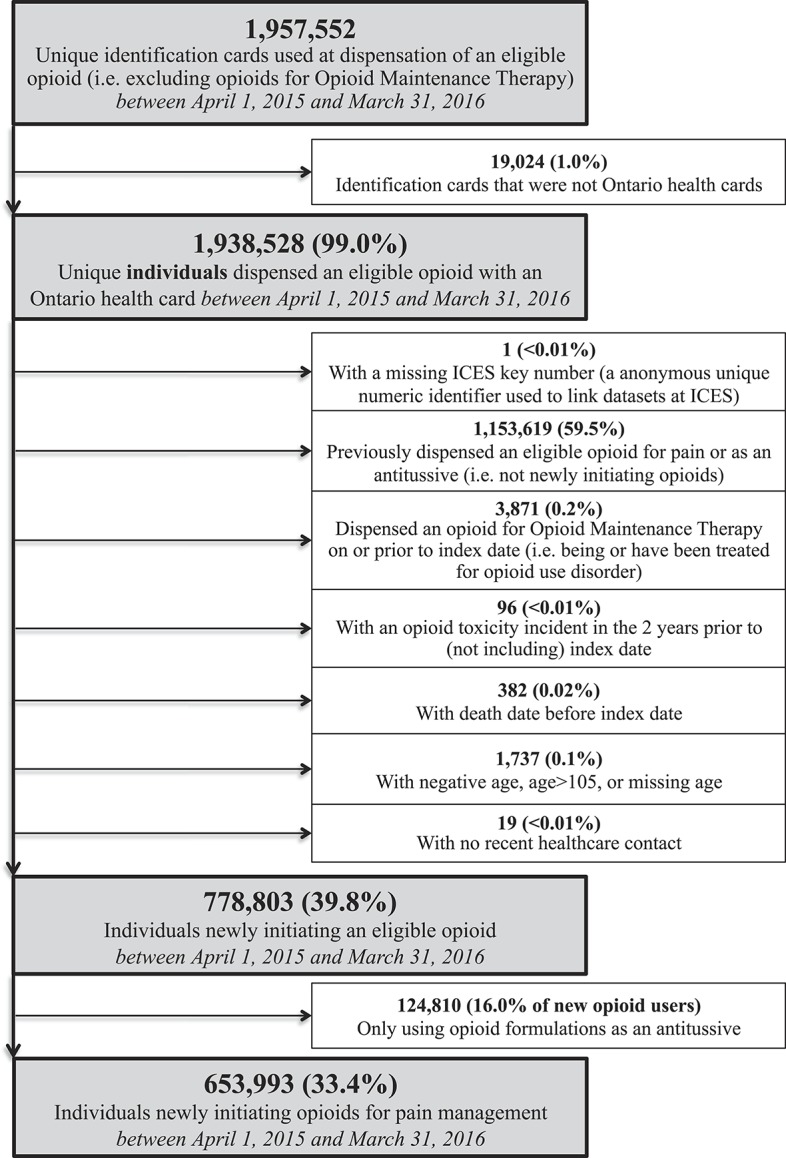
Cohort identification. This figure depicts the order in which inclusion and exclusion criteria were used to identify the study cohort of Ontarians newly initiating opioids between April 1, 2015, and March 31, 2016. ICES, Institute for Clinical Evaluative Sciences.
We identified 23 clinical indications for initiating opioids grouped into 6 indication clusters: dental pain (N = 151,874, 23.2%), postsurgical pain (N = 113,605, 17.4%), musculoskeletal pain (N = 78,155, 12.0%), trauma-related pain (N = 73,069, 11.2%), cancer or palliative care (N = 42,832, 6.5%), and other types of pain (N = 115,977, 17.7%) (Table 1). Overall, 78,481 (12.0%) individuals could not be linked to an indication. Among these individuals, 39,803 (50.7%) had no health care record identified, whereas the remainder (49.3%) had a health care record identified that would not normally warrant opioid initiation (eg, anxiety and hypertension; see Supplementary Appendix for details; available online at http://links.lww.com/PAIN/A570).
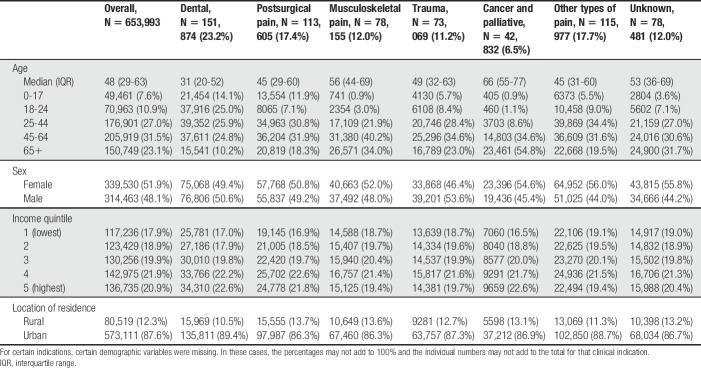
Table 1.
Patient characteristics of individuals newly initiated on opioids for pain, by major clinical indication cluster.
Individuals who initiated opioids for dental pain were typically younger (median age 31 years, Q1-Q3 20-52 years), whereas those who initiated for musculoskeletal pain (median age 56 years, Q1-Q3 44-69 years), and cancer and palliative care (median age 66 years, Q1-Q3 55-77 years) were typically older than those in the other indication clusters (range in medians from 45 to 49 years). The neighbourhood income quintile profiles of patients were fairly evenly distributed across indication clusters.
Within each indication cluster, we identified a number of highly prevalent clinical indications. Although specific dental indications could not be identified in our data, the overwhelming majority of patients initiating an opioid for dental pain received their prescription from a dentist (144,118 of 151,874 [94.9%], representing 22.0% of the entire cohort) (Table 2). Other common clinical indications were common excisions (4.2%), joint and muscle pain (7.1%), back pain (4.8%), dislocations, sprains and strains (4.0%), cancer (5.3%), and abdominal or pelvic pain (6.0%).
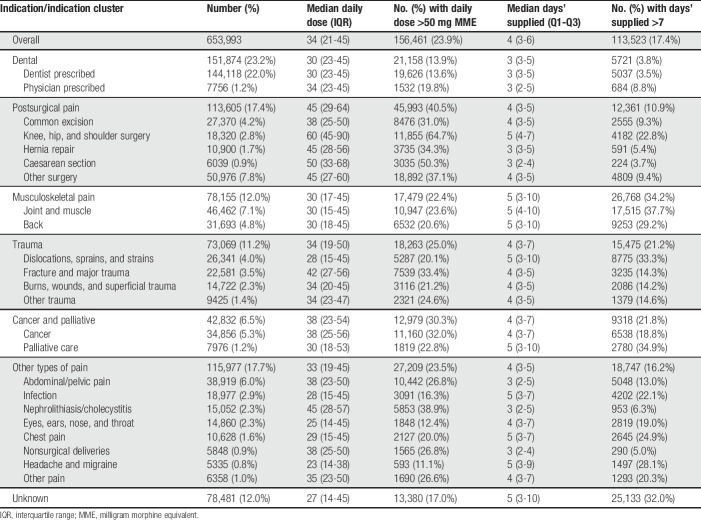
Table 2.
Frequency and characteristics of initial opioid prescriptions for pain, by major and minor clinical indication.
The characteristics of initial prescriptions varied considerably by indication. Patients initiating for dental pain received prescriptions with a lower daily dose (median 30 MME, Q1-Q3 of 23-45) and shorter durations (median 3 days, Q1-Q3 of 3-5) relative to other indications (Table 2). Individuals initiating opioids after knee, hip, or shoulder surgery received higher daily doses (median 60 MME, Q1-Q3 of 45-90; 64.7% with initial daily dose above 50 MME), as did those initiating following caesarean section (median 50 MME, Q1-Q3 of 33-68; 50.3% with initial daily dose above 50 MME), relative to other indications. Clinical indications with a higher-than-average proportion of potentially risky prescription durations were joint and muscle pain (37.7% with durations >7 days); dislocations, sprains, and strains (33.3% with durations >7 days); palliative care (34.9% with durations >7 days); back pain (29.2% with durations >7 days); and headaches and migraines (28.1% with durations >7 days).
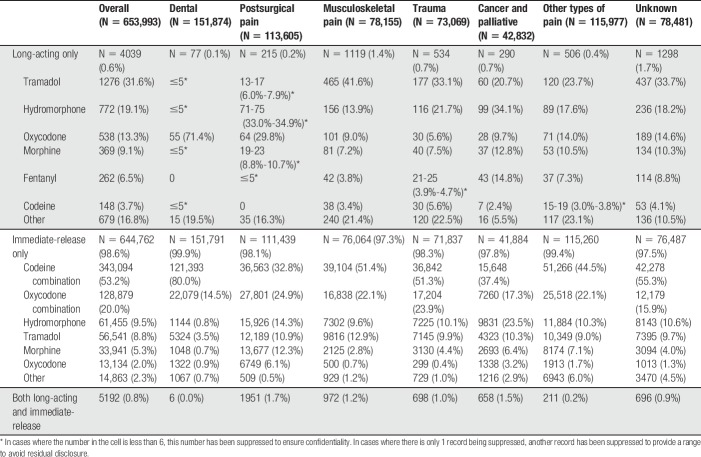
Finally, the type of opioids prescribed at initiation varied, with immediate-release codeine-combination products being the most common across all clinical indications (Table 3). Immediate-release hydromorphone prescribing occurred most commonly among individuals initiating opioids for cancer and palliative care (9,831, 23.5%), whereas morphine prescribing occurred more often among those initiating postsurgery (13,677, 12.3%).
Table 3.
Opioid types and formulations dispensed to individuals newly initiating opioids for pain, by major clinical indication cluster.
4. Discussion
In this population-based study of 653,993 new users of prescription opioids, we found wide diversity of the apparent clinical indications for which people initiate opioids for pain management. Dental pain accounted for nearly one-quarter of all new opioid prescriptions, which were generally of short duration and low dose. By contrast, 1 in 6 new opioid users were treated for postsurgical pain, and these patients generally started on higher doses (over 40% were prescribed more than 50 MME, and at least 25% were prescribed 90 MME or more). Finally, although just 1 in 10 patients initiated opioids for back, joint, or muscle pain, these patients generally received longer durations of therapy, with more than one-third receiving initial prescription durations of greater than 7 days.
Previous studies have generally not been designed to characterize the indication for opioid initiation at population level using robust, linked health administrative databases.15,18,25,26 Four American studies, in particular, are based on prescriptions written, not those dispensed, and were limited to smaller populations (ie, a specific clinic or insurance provider) and could not account for access to opioids from other sources.15,18,25,26 Our study confirms most findings from the literature (apart from 1 previous study that identified chronic noncancer pain as the most common pain indication)25; we found that acute indications (ie, dental, postsurgical, and trauma-related pain) accounted for the majority of all opioid initiations,15 that opioid initiation was more commonly attributed to joint and muscle pain than back pain,26 and that dental pain was a significant contributor to new opioid exposure.18 However, our study also highlights the broad set of indications for which opioids are initiated, and provides important information on other significant contributors to new opioid exposure. In particular, we found that postsurgical pain accounts for nearly 1 in 6 new opioid starts, which tend to be of short duration at relatively higher doses and to older adults. Although the contribution of prescriptions for dental pain in a primary care setting has previously been identified,18 our finding of the significant contribution of prescriptions by dentists is particularly novel and important. Although the dose and duration of these prescriptions are relatively limited, the concentration of dental prescriptions among younger patients—a group at potentially greater risk of prescription opioid misuse, illicit use, and recreational experimentation—10,30 highlights the importance of policy efforts to ensure that dentist prescribing remains appropriate and necessary.16,17,21
The U.S. and Canadian clinical guidelines for opioids in chronic non-cancer pain published in 2016 and 2017, respectively, suggest that clinicians should avoid initiating opioids at daily doses above 50 MME.1,6 Our study found that, in the period immediately before the guidelines were published, nearly one-quarter of Ontarians newly initiating opioids received a daily dose exceeding this threshold, and that this was even higher in certain indications such as knee, hip, and shoulder surgeries, and caesarean sections. Moreover, at least one-quarter of individuals initiating opioids for knee, hip, and shoulder surgeries received an initial daily dose equal to or exceeding 90 MME, which guidelines now recommend avoiding even after initiation. Given that these higher doses have been associated with more adverse events, including overdose deaths, depression, road trauma, and falls,7,9 our findings suggest that improvements to safe opioid prescribing could be achieved by focusing on dose initiation patterns among surgeons. In addition to high daily doses being a concern, recent evidence suggests prescribing less than 7 days of opioid at initiation, ideally less than or equal to 3 days, as these shorter prescription durations are associated with less long-term use.6,25,26 In this study, we found that although opioids initiated postsurgery were typically of higher dose, they tended to have shorter durations, with only 1 in 10 individuals receiving more than a 7-day supply of drug. By contrast, more than one-third of people initiating opioids for musculoskeletal pain received an initial prescription duration exceeding 7 days' supply, which may contribute to the high degree of sustained opioid use among people initiated for musculoskeletal pain.24 In addition, these differences in patterns by clinical indication may reflect variations in intended duration of opioid use, but could also suggest opportunities for improved opioid initiation practices in some populations. Finally, although rare, our findings also highlight that opioids are used to treat conditions such as headaches and migraines, for which opioid-related harms have been reported to outweigh any evidence of benefit.4,28
Our study has several limitations. First, given the retrospective study design and data sources, we cannot confirm whether the diagnosis or procedure codes identified before the index prescription are responsible for opioid initiation. However, the use of similar approaches to identify opioid indications in studies with limited population samples,24,26 and the proximity of the health care encounters to opioid dispensing, increases our confidence in our approach for defining indications associated with opioid initiation. Second, we only have historical dispensing data from July 2012 onwards, so cannot ascertain more remote opioid prescriptions. In some instances, the identified initial prescription may not be an individual's first-opioid exposure. Nevertheless, because it was patients' first use of a prescription opioid in at least 2.5 years, any previously acquired tolerance would have been lost.31 Third, we cannot account for people recently moving into the province because there is often a lag period for them to gain access to the health care system.5 Fourth, we could not identify a pain indication among 12% of patients in our cohort. Although our algorithm was designed to capture all potential pain indications where suitable information was captured in our databases, some physicians may list a generic diagnosis on the billing record, which would not provide us with the detailed information required to allocate a pain indication. Therefore, in these cases, we are unable to appropriately allocate these individuals to an indication group. Notably, half of these individuals had no evidence of a physician encounter in the preceding 5 days, thus filled prescriptions that were at least 5 days old. Finally, some patients may have had multiple indications and we could only identify 1 indication because our approach to identifying indications was hierarchical. However, we developed a hierarchy intended to classify indications according to the most appropriate indication for opioid use.
As we aim to optimize opioid prescribing, patients' first-opioid prescriptions are critically important. Across all clinical indications, a high percentage of people received daily doses above 50 MME and prescription lengths over 7 days, which have been associated with potential adverse events and long-term opioid use. Given this, our findings highlight the need to prioritize certain indications in the promotion of more appropriate opioid prescribing. Knowing the nature of opioid prescribing postsurgically and for musculoskeletal pain, particular attention may be warranted to determine the appropriateness and safety of opioid use in these indications. Future efforts to inform such resource allocation efforts ought to consider which specific pain indications are associated with downstream risks of sustained use, opioid use disorder, and opioid toxicity.
Conflict of interest statement
M.M. Mamdani has received honoraria from Boehringer Ingelheim, Pfizer, Mristol-Myers Squibb, and Bayer. D.N. Juurlink has received payment for lectures and medicolegal opinions regarding the safety and effectiveness of analgesics, including opioids. He is a member of Physicians for Responsible Opioid Prescribing, a volunteer organization that seeks to reduce opioid-related harm through more cautious prescribing practices. No other authors have any conflicts of interest to declare.
This study was funded by a grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC) as well as the Ontario Strategy for Patient-Orientated Research (SPOR) Support Unit, which is supported by the Canadian Institutes of Health Research and the Province of Ontario. This study was also supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES, the SPOR Unit or the Ontario MOHLTC is intended or should be inferred.
Acknowledgments
The authors thank Brogan Inc, Ottawa, for use of their Drug Product and Therapeutic Class Database. Parts of this material are based on data and information compiled and provided by CIHI. However, the analyses, conclusions, opinions, and statements expressed in the material are those of the authors, and not necessarily those of CIHI. Parts of this material are based on data and information provided by Cancer Care Ontario (CCO). The opinions, results, view, and conclusions reported in this article are those of the authors and do not necessarily reflect those of CCO. No endorsement by CCO is intended or should be inferred.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/A570.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
References
- [1].Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang L, Couban RJ, Agoritsas T, Akl EA, Carrasco-Labra A, Cooper L, Cull C, da Costa BR, Frank JW, Grant G, Iorio A, Persaud N, Stern S, Tugwell P, Vandvik PO, Guyatt GH. Guideline for opioid therapy and chronic noncancer pain. CMAJ 2017;189:E659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Canadian Institute for Health Information. The CIHI data quality framework. Ottawa: CIHI, 2009. [Google Scholar]
- [3].Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ 2013;346:f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Casucci G, Cevoli S. Controversies in migraine treatment: opioids should be avoided. Neurol Sci 2013;34(suppl 1):S125–128. [DOI] [PubMed] [Google Scholar]
- [5].Clarke J. “Difficulty accessing health care services in Canada.” Health at a Glance. Ottawa, ON, Canada: Statistics Canada catalogue no. 82-624-X; 2016. [Google Scholar]
- [6].Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep 2016;65:1–49. [DOI] [PubMed] [Google Scholar]
- [7].Dunn KM, Saunders KW, Rutter CM, Banta-Green CJ, Merrill JO, Sullivan MD, Weisner CM, Silverberg MJ, Campbell CI, Psaty BM, Von Korff M. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med 2010;152:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011;171:686–91. [DOI] [PubMed] [Google Scholar]
- [9].Gomes T, Redelmeier DA, Juurlink DN, Dhalla IA, Camacho X, Mamdani MM. Opioid dose and risk of road trauma in Canada: a population-based study. JAMA Intern Med 2013;173:196–201. [DOI] [PubMed] [Google Scholar]
- [10].Hedden SL, Kennet J, Lipari R, Medley G, Tice P, Copello EAP, Kroutil LA. Center for Behavioural Health Statistics and Quality. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Rockville, MD: (HHS Publication No. SMA 15–4927, NSDUH Series H=50) 2015. [Google Scholar]
- [11].Henry SG, Wilsey BL, Melnikow J, Iosif AM. Dose escalation during the first year of long-term opioid therapy for chronic pain. Pain Med 2015;16:733–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].International Narcotics Control Board. Opioid consumption maps. Madison: Board of Regents of the University of Wisconsin System, 2015. [Google Scholar]
- [13].Kaplovitch E, Gomes T, Camacho X, Dhalla IA, Mamdani MM, Juurlink DN. Sex differences in dose escalation and overdose death during chronic opioid therapy: a population-based cohort study. PLoS One 2015;10:e0134550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li L, Setoguchi S, Cabral H, Jick S. Opioid use for noncancer pain and risk of fracture in adults: a nested case-control study using the general practice research database. Am J Epidemiol 2013;178:559–69. [DOI] [PubMed] [Google Scholar]
- [15].Liu Y, Logan JE, Paulozzi LJ, Zhang K, Jones CM. Potential misuse and inappropriate prescription practices involving opioid analgesics. Am J Manag Care 2013;19:648–65. [PubMed] [Google Scholar]
- [16].McCauley JL, Hyer JM, Ramakrishnan VR, Leite R, Melvin CL, Fillingim RB, Frick C, Brady KT. Dental opioid prescribing and multiple opioid prescriptions among dental patients: administrative data from the South Carolina prescription drug monitoring program. J Am Dent Assoc 2016;147:537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McCauley JL, Leite RS, Melvin CL, Fillingim RB, Brady KT. Dental opioid prescribing practices and risk mitigation strategy implementation: identification of potential targets for provider-level intervention. Subst Abus 2016;37:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mundkur ML, Rough K, Huybrechts KF, Levin R, Gagne JJ, Desai RJ, Patorno E, Choudhry NK, Bateman BT. Patterns of opioid initiation at first visits for pain in United States primary care settings. Pharmacoepidemiol Drug Saf 2017. 10.1002/pds.4322. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Olfson M, Wang S, Iza M, Crystal S, Blanco C. National trends in the office-based prescription of schedule II opioids. J Clin Psychiatry 2013;74:932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ontario Ministry of Health and Long-term Care. The Narcotics safety and awareness Act. Toronto, ON, Canada: Ontario Lao, 2010. [Google Scholar]
- [21].Rasubala L, Pernapati L, Velasquez X, Burk J, Ren YF. Impact of a mandatory prescription drug monitoring program on prescription of opioid analgesics by dentists. PLoS One 2015;10:e0135957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Prescription of long-acting opioids and mortality in patients with chronic noncancer pain. JAMA 2016;315:2415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Mil Med 2016;181:397–9. [DOI] [PubMed] [Google Scholar]
- [24].Schoenfeld AJ, Jiang W, Chaudhary MA, Scully RE, Koehlmoos T, Haider AH. Sustained prescription opioid use among previously opioid-naive patients insured through TRICARE (2006-2014). JAMA Surg 2017;152:1175–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006-2015. MMWR Morb Mortal Wkly Rep 2017;66:265–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shah A, Hayes CJ, Martin BC. Factors influencing long-term opioid use among opioid naive patients: an examination of initial prescription characteristics and pain etiologies. J Pain 2017;18:1374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Statistics Canada. Ontario [province] and Canada [country] (table). Census profile. 2016 census. Statistics Canada catalogue no. 98-316-X2016001. Ottawa: 2017. [Google Scholar]
- [28].Tepper SJ. Opioids should not be used in migraine. Headache 2012;52(suppl 1):30–4. [DOI] [PubMed] [Google Scholar]
- [29].U.S. Department of Health and Human Services. National pain strategy: a comprehensive population health-level strategy for pain. Washington, D.C.:U.S: Department of Health and Human Services, 2016. [Google Scholar]
- [30].White AG, Birnbaum HG, Schiller M, Tang J, Katz NP. Analytic models to identify patients at risk for prescription opioid abuse. Am J Manag Care 2009;15:897–906. [PubMed] [Google Scholar]
- [31].World Health Organization. Clinical guidelines for withdrawal management and treatment of drug dependence in closed settings. Geneva: 2009. [PubMed] [Google Scholar]