Abstract
Background
Although depression and anxiety have been associated with shorter telomeres in cross-sectional studies, the data regarding the prospective relations of depression and anxiety to accelerated telomere length shortening are limited and findings are mixed. We prospectively examined relations of baseline depression and phobic anxiety to subsequent 11-year change in relative leukocyte telomere lengths (LTLs).
Methods
We selected 1,250 women from a sub-cohort of the Nurses’ Health Study who provided blood specimens at both blood collections (1989–1990 and 2000–2001). Depression was defined by self-reported regular antidepressant use or presence of severe depressive symptoms; anxiety symptoms were assessed using the Crown-Crisp Experiential Index. Using quantitative real-time polymerase chain reaction assay, LTLs were measured as the copy number ratio of telomere repeat to a single control gene. Changes in LTLs were defined in 3 ways: absolute change, symmetrized percent change, and decile shift.
Results
Overall, there were no statistically significant associations of depression or phobic anxiety to subsequent 11-year LTL shortening, despite a point estimates in the direction of greater telomere shortening among participants with vs. without depression, across all three metrics of telomere change. The strongest predictor of LTL change was baseline telomere length, and regression-to-the-mean was observed.
Conclusion
Baseline depression and phobic anxiety were not significantly associated with 11-year attrition in LTLs among 1,250 mid-life and older women. However, a suggestion of depression and greater subsequent LTL attrition, while not statistically significant, may warrant further inquiry, particularly in prospective studies with larger sample sizes and broader windows of the lifespan.
Keywords: prospective, women, telomere length, depression, phobic anxiety, telomere change
INTRODUCTION
Depression and anxiety are major mental disorders in the U.S. and worldwide (Global Burden of Disease Study 2015). Both conditions are highly prevalent and are leading causes of disease burden and disability (Kessler et al. 2009; Whiteford et al. 2013). Depression and anxiety have also been associated with higher risk of major somatic conditions and adverse health outcomes (Batelaan et al. 2016; Gan et al. 2014; Mezuk et al. 2008; Luppino et al. 2011; Gulpers et al. 2016; Cuijpers et al. 2013; Chodosh et al. 2007). Although the underlying biological mechanisms involved in depression and anxiety are unknown, it has been hypothesized that immune dysregulation or dysfunction, oxidative stress and/or inflammation may be involved (Raison, Capuron, and Miller 2006; Dantzer et al. 2008). Further, it has been suggested that such systemic dysfunction may lead to cellular damage, including to the telomeres of cells (Dowlati et al. 2010; Wolkowitz et al. 2010). Telomeres are repetitive nucleotide sequences at the ends of eukaryotic chromosomes and serve to protect DNA from damage; they naturally shorten over time with mitosis, and cells may undergo senescence or apoptosis after telomeres reach critically short lengths (Bojesen 2013). However, under conditions of heightened oxidative, inflammatory or other cellular stress, telomere shortening may be accelerated. Therefore, telomere shortening may serve as an indicator of biological or cellular aging, reflecting damage accumulated over time. Telomere length has associated with mortality and age-related morbidity, including cancer and cardiovascular disease (Zhu, Belcher, and van der Harst 2011; Willeit et al. 2010; Fitzpatrick et al. 2007), and could potentially mediate the pathophysiology between depression or anxiety and subsequent somatic conditions (Gotlib et al. 2015).
Nevertheless, the nature and direction of the relationships of depression and anxiety to leukocyte telomere length are not entirely clear. It seems possible that depression and anxiety may precede telomere attrition (Shalev et al. 2014; Hoen et al. 2013) or vice versa (Ramin et al. 2015; Wium-Andersen et al. 2017); it is also plausible that depression and telomere shortening share common causes such as lifestyle factors or genetic predisposition. Several cross-sectional studies and a few prospective studies have examined these associations with inconsistent findings (Darrow et al. 2016; Lin, Huang, and Hung 2016; Schutte and Malouff 2015). These inconsistencies may be explained by differences in study design (cross-sectional vs. longitudinal) (Schutte and Malouff 2015), sample composition (clinical sample vs. general population), age range (younger, mid-life, or older), measurement of psychiatric variables (self-report vs. diagnostic interview, symptoms vs. diagnoses), psychiatric illness status (past/remitted vs. current case), type of telomere length assay (Southern blot, fluorescence in situ hybridization (FISH), or quantitative PCR (qPCR))(Lin, Huang, and Hung 2016), and the level of detail in adjustment for confounders. Most positive associations come from cross-sectional studies, nearly half of which featured small sample sizes (N<100).
Prospective studies of associations between depression and/or anxiety and subsequent telomere length shortening featured variable follow-up periods, ranging from 2 to 12 years, and findings have been inconsistent (Verhoeven et al. 2016; Shalev et al. 2014; Hoen et al. 2013; Hoen et al. 2011; Rius-Ottenheim et al. 2012). Specifically, significant associations were seen among young male adults and older men with heart disease in which significance disappeared after further adjustment for covariates. Potential sources of the discrepant findings across prospective studies include variations in sample size, age, sex, health characteristics (healthy population/patients with heart disease) and follow-up durations. In addition, several studies examined the impact of changes of depressive symptoms on changes of telomere lengths across two time points (Rius-Ottenheim et al. 2012; Shalev et al. 2014; Verhoeven et al. 2016); however, changes in depression and telomere length were assessed concurrently (i.e., overlapping) rather than sequentially. Because these designs did not feature a baseline period of change in depression, followed by a subsequent period of change in telomeres, the nature of the temporal association was unclear. In addition, it is not known whether depression and anxiety have similar relations to telomere shortening. For example, Verhoeven et al. found people with depression and people with anxiety both had consistently shorter telomere lengths at follow-up (2016), while Hoen et a. identified decreasing LTLs only among people with the presence of anxiety but not depression (2013). Overall it remains unclear whether depression and anxiety leads to accelerated shortening of telomere length over time.
To address the above limitations and gaps in the knowledge base, we aimed to investigate the prospective relationship between baseline depression and phobic anxiety, typically a chronic form of anxiety, and subsequent 11-year changes in telomere length in a well-characterized sample of mid-life and older U.S. women (N=1,250), which particularly addressed potential age- and gender-specific associations in the literature. We hypothesized that women with depression or phobic anxiety at baseline would have acceleration in telomere attrition after 11 years compared to those without these conditions.
METHODS AND MATERIALS
Study population
The Nurses’ Health Study (NHS) is a prospective cohort that began in 1976 when 121,700 female nurses living in 11 U.S. states, aged 30–55 years, returned a mailed questionnaire regarding lifestyle and medical history. Participants have received questionnaires biennially since then, with >90% follow-up in each 2-year cycle. Between 1989 and 1990 (T1), blood samples, along with information from a supplementary questionnaire, were collected from 32,826 NHS participants. Details of the blood collection have been described previously (Hankinson et al. 1995). Among this blood sub-cohort, a subset of 18,717 women also provided a second blood sample in 2000–2001 (T2). For the current study, we generated a random sample of 1,700 adults who provided blood samples at both T1 and T2, had information on phobic anxiety on the 1988 questionnaire and depressive symptoms on the 1992 questionnaire, were free of major chronic diseases including cancer and cardiovascular disease, and had not been selected as cases or controls in prior NHS telomere studies. Following exclusions described in detail below, the final sample for analysis included 1,250 women.
Assessment of depression and phobic anxiety
Antidepressant use was ascertained from the supplementary questionnaire that accompanied the first blood collection in 1989–1990. Information on depressive symptoms were first assessed with the 1992 cohort questionnaire using the Mental Health Inventory-5 (MHI-5) subscale of the 36-item Short-Form Health Status Survey (Ware and Sherbourne 1992); the MHI-5 has been validated for detecting clinical depression and severe depressive symptoms (Berwick et al. 1991; Ware and Sherbourne 1992). Baseline depression was defined by self-reported antidepressant use or the presence of severe depressive symptoms (MHI-5 ≤ 52) (Yamazaki, Fukuhara, and Green 2005). In a secondary analysis, depression was alternatively defined by presence of both antidepressant use and severe symptoms; such a Boolean AND definition tends to maximize case specificity, and potentially reduce bias in estimates, but to have substantially lower case detection.
Phobic anxiety was assessed using the phobic subscale of the Crown-Crisp Experiential Index (CCI) (Crown and Crisp 1966) on the 1988 cohort questionnaire. The CCI contained 8 items assessing fear and desire for avoidance; each item has 2–3 levels of possible response (between 0 and 2 points), leading a sum score for all items ranging from 0 to 16 points. Higher scores indicate greater levels of phobic anxiety. The CCI has been validated in psychiatric outpatient clinics and has been shown to have good discrimination (Crown and Crisp 1966; Burgess, Mazzocco, and Campbell 1987). CCI scores are not normally distributed (highly right-skewed), and in keeping with other work (Haines et al. 2001; McGrath et al. 2004; Okereke et al. 2012), CCI scores were categorized into five categories according to total score: 0–1 (reference group), 2, 3, 4–5, and 6+ points.
Assessment of covariates
Information on covariates was obtained by either the supplementary questionnaire that accompanied the T1 blood collection or the most proximal biennial NHS questionnaire (i.e., 1988–1990 questionnaire cycle). Potential confounders were selected a priori based on prior literature (involving NHS or other cohorts), in which they were significantly associated with telomere lengths: age (in years); paternal age at participant’s birth (in years)(Kimura et al. 2008; Prescott et al. 2012); cigarette smoking (pack-years; never, >0–20, >20–40, >40, missing)(McGrath et al. 2007; Valdes et al. 2005); body mass index (BMI, in kg/m2)(Muezzinler, Zaineddin, and Brenner 2014); and physical activity (total metabolic equivalent hours of activity per week; MET-hours/week)(Du et al. 2012; Latifovic et al. 2016).
Assessment of relative leukocyte telomere length
Leukocyte telomere lengths in genomic DNA were measured using a modified, high-throughput version of the quantitative real-time polymerase chain reaction-based telomere assay (qPCR) (Crown and Crisp 1966; Wang et al. 2008) that was run on the Applied Biosystems 7900HT Sequence Detection System (Foster City, CA, USA). Specimens collected at T1 and T2 for each participant were placed side-by-side on the same plate to minimize influences of random measurement error on the paired samples. Experienced laboratory personnel were blinded to participants’ characteristics, and all assays were processed in triplicate by the same technician and under identical conditions.
The amplification of genomic DNA from peripheral blood leukocytes was done for both telomere repeat copy number (T) and a single control gene (36B4) copy number (S). The average relative telomere length was calculated, and the ratio of telomere repeat copy number to a single gene copy number (T/S ratio) was proportional to the average telomere length (Cawthon 2002). Relative leukocyte telomere lengths (LTLs) were reported as the exponentiated relative T/S ratio corrected for a reference sample (Nan et al. 2011); this measure correlates highly (r=0.68–0.85; p<0.001) with Southern blot measurement of absolute telomere lengths (Aviv et al. 2011; Cawthon 2002). The coefficient of variations (CVs) for the telomere and the single-gene assays in this study ranged from 0.10–1.98% and 0.04–1.57%, respectively. The CVs for the relative telomere lengths of quality control samples were 11.3–19.2%.
The mean duration between T1 and T2 among the study participants was 11.1 years (st.d. 0.5 years; range of 9.6–12.5 years). We calculated 11-year changes in LTLs using three metrics. First, we computed absolute change (AC), defined as absolute difference of LTLs between T2 and T1: LTL(T2)-LTL(T1). Second, we computed symmetrized percent change (SPC)(Berry and Ayers 2002), defined as [LTL(T2)-LTL(T1)]/[LTL(T2)+LTL(T1)]×100; the advantage of SPC is that it is less sensitive to outliers and also takes into account baseline telomere length. Lastly, we calculated the decile shift (DS) (Benetos et al. 2013), defined as the difference of decile ranking in LTLs between T2 and T1. We created the following DS categories: “stable” was defined as either maintenance of identical decile rank or a decile shift within ±1 decile; “shifting upward” and “shifting downward” were defined as >1 decile increase and >1 decile decrease, respectively, comparing T2 and T1 (Benetos et al. 2013). Because rankings are used, instead of actual LTL values, DS is also less sensitive to outliers. The main goal was to examine the robustness and consistency of the association between depression and anxiety and changes in telomere length between three different metrics of change measures. Among 1700 participants, we excluded: 17 with samples at T1 with inadequate specimen volume, 22 with samples that failed the telomere assay runs at both time points, and an additional 229 with samples at T1 and 211 with samples at T2 that had within-triplicate CVs for the T/S ratio >20%; thus, 1432 and 1467 participants remained with available LTLs at T1 and T2, respectively. A total of 1,250 women with LTLs available at both time points were included in the final analysis.
Statistical analysis
For the outcomes of AC and SPC, we used linear regression models to investigate the relations of baseline depression and phobic anxiety to 11-year changes in LTLs. Adjusted least squares mean 11-year changes in LTLs (and their corresponding 95% confidence intervals; 95% CIs) were calculated across depression and phobic anxiety categories using generalized linear models. For the DS outcome, multinomial logistic regressions were used. Model 1 adjusted for age and LTLs at baseline (i.e., T1). Model 2 additionally adjusted for potential confounders described above in “Assessment of covariates”. Statistical analyses were conducted using SAS v. 9.4 (SAS Institute Inc., Cary, NC). All p-values were two-sided, and a nominal α level of 0.05 was used for statistical significance.
RESULTS
The age range of the participants was 48–69 years at baseline, with a mean of 56.9 years (st.d. 5.5 years). There was a modest but consistent inverse correlation between LTLs and age at both T1 and T2 (Figure 1): a one-year increase in age was correlated with a decrease in LTLs by 0.003 at both T1 and T2. The Pearson correlation coefficient was 0.72 (p < 0.0001) between LTLs at T1 and T2 11 years apart (Figure S1); the coefficient remained positive and statistically significant after adjusting for covariates (β=0.70, p < 0.0001). The majority of participants (61%) had stable decile rankings in LTLs over the 11-year follow-up; the remaining 20% and 19% had upward and downward decile ranking shifts, respectively (Figure S2).
Figure 1.
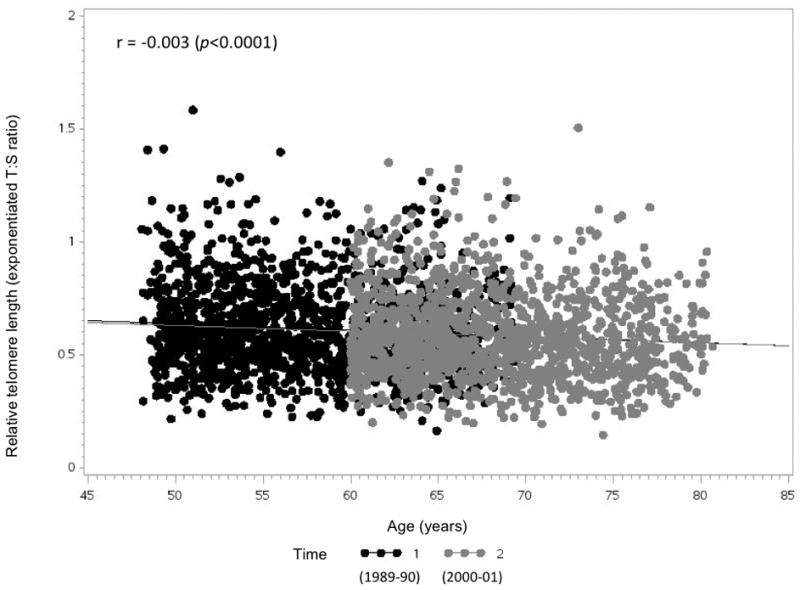
Relationship between relative leukocyte telomere length and age by time of blood draw (N=1,250)
The mean ages and age-standardized characteristics of the study participants at T1 are shown in Table 1, by DS of LTLs between T1 and T2. Compared to women whose rankings in LTLs were stable between T2 and T1, those with upward DS in LTL rank had a slightly higher prevalence of antidepressant use at baseline. Women with downward DS in LTL rank had higher prevalence of severe depressive symptoms at baseline (defined by MHI-5 ≤ 52).
Table 1.
Age-standardized characteristics of study population at initial blood draw, by decile shift in ranking of relative leukocyte telomere lengths (N=1,250)1,2
| Shift upward (>1 decile increase) (N=250) | Stable (within ±1 decile shift) (N=763) | Shift downward (>1 decile decrease) (N=237) | |
|---|---|---|---|
| Age, years3 | 57.1(5.5) | 56.8(5.6) | 56.8(5.2) |
| Crown-Crisp Phobic Index, % | |||
| 0–1 | 30.9 | 33.3 | 39.9 |
| 2 | 20.1 | 19.9 | 16.0 |
| 3 | 17.1 | 16.1 | 13.6 |
| 4–5 | 22.9 | 19.2 | 20.7 |
| 6+ | 8.9 | 11.5 | 9.8 |
| Antidepressant use, % | 4.4 | 2.3 | 2.5 |
| Mental Health Index-5 ≤ 52, % | 5.7 | 4.8 | 8.0 |
| Baseline relative leukocyte telomere length | 0.5(0.1) | 0.6(0.2) | 0.7(0.1) |
| Paternal age at birth, years | 29.0(8.9) | 30.2(9.3) | 29.8(8.7) |
| Body mass index (continuous) | 24.8(3.9) | 25.2(4.5) | 25.0(3.6) |
| Body mass index (category) | |||
| Normal | 60.7 | 55.1 | 58.0 |
| Overweight | 28.5 | 30.7 | 29.2 |
| Obese | 10.7 | 14.2 | 12.8 |
| Physical activity (Mets/week) | 15.7(15.7) | 17.5(28.7) | 16.2(16.4) |
| Alcohol (grams/day) | 5.6(8.1) | 4.4(7.4) | 5.1(6.8) |
| Smoking in categories, % | |||
| Never smokers | 45.5 | 48.9 | 49.8 |
| >0–20 pack-years | 29.4 | 30.2 | 30.5 |
| >20–40 pack-years | 17.7 | 13.9 | 12.7 |
| >40 pack-years | 6.4 | 6.1 | 5.1 |
| Hypertension, % | 24.7 | 24.8 | 22.9 |
| Hypercholesterolemia, % | 43.6 | 39.8 | 41.5 |
| Type 2 diabetes, % | 2.0 | 2.8 | 2.1 |
Relative telomere length was estimated by the exponentiated T/S ratio
Values are means (SD) or percentages and are standardized to the age distribution of the study population
Value is not age-adjusted.
Although age-at-blood draw and paternal age at participant’s birth, respectively, had statistically significant inverse (F1,1249=10.38, p=0.001) and positive (F1,1249=11.50, p<0.0001) correlations with LTLs at baseline, they were not significantly associated with 11-year absolute change in LTLs (all p>0.05). None of the other covariates had statistically significant associations with DS over 11 years in the age-adjusted models (all p>0.05). The only factor that showed a significant association with DS of LTLs was baseline LTLs (F1,1249=300.10, p < 0.0001). A significant inverse relation also existed between baseline LTL and 11-year LTL change in absolute values (β=−0.29, p <0.0001 after adjusting for covariates). Specifically, participants with longer baseline LTLs were more likely to be observed to have subsequent TL shortening, while apparent telomere lengthening was more likely to be observed among those with shorter baseline LTLs (i.e., “regression-to-the-mean” phenomenon) (Figure S3).
Overall, there was no statistically significant association between baseline depression status and subsequent accelerated telomere shortening over 11 years. Examining point estimates, however, participants with depression at baseline had respective non-significant 1.6-fold (F1,1249=1.72, p=0.19)(Table 2) and 1.8-fold (F1,1249=2.51, p=0.11)(Table 3) decreases in in AC and SPC over 11 years, relative to those without depression, and 1.5-fold (95% CI, 0.87–2.54) of relative likelihood of having a downward shift in DS over 11 years (Table 4). When depression was alternatively defined by both antidepressant use and the presence of severe depressive symptoms, those with depression had greater declines telomere length on both the AC (1.8-fold) and SPC (2.6-fold) metrics; however, the number of depression cases was reduced substantially (N=12), as expected, by this Boolean AND definition.
Table 2.
Least-squares mean (95% CI) 11-year absolute change in relative leukocyte telomere length, by categories of baseline mental health outcomes1
| Exposure | Category | N | Age, baseline LTL-adjusted2 | F | p | Multivariable-adjusted3 | F | p |
|---|---|---|---|---|---|---|---|---|
| Depression4 | No | 1098 | −0.033 (−0.040, −0.025) | F1,1249=1.57 | 0.21 | −0.032 (−0.040, −0.025) | F1,1249=1.72 | 0.19 |
| Yes | 86 | −0.049 (−0.074, −0.024) | −0.050 (−0.075, −0.025) | |||||
| Phobic anxiety symptom score5 | 0–1 | 440 | −0.036 (−0.047, −0.024) | F4,1249=0.15 | 0.96 | −0.036 (−0.047, −0.024) | F4,1249=0.19 | 0.94 |
| 2 | 240 | −0.030 (−0.045, −0.015) | −0.029 (−0.044, −0.014) | |||||
| 3 | 185 | −0.038 (−0.055, −0.021) | −0.039 (−0.056, −0.021) | |||||
| 4–5 | 248 | −0.034 (−0.049, −0.019) | −0.034 (−0.049, −0.019) | |||||
| 6+ | 137 | −0.036 (−0.056, −0.016) | −0.037 (−0.057, −0.016) |
11-year absolute change in relative leukocyte telomere length was defined by: exponentiated T/S ratio at T2 − exponentiated T/S ratio at T1
Model adjusted for age at first blood draw (continuous) and baseline LTL (continuous)
Model adjusted for age at first blood draw (continuous), baseline LTL (continuous), paternal age at birth (continuous), pack-years of smoking (never, >0–20, >20–40, >40, missing), body mass index (continuous), and physical activity (continuous)
Depression was defined by antidepressant use at initial blood draw or MHI-5 ≤ 52 on the 1992 questionnaire; 66 participants who did not answer question on antidepressant use at initial blood draw were not included
Phobic anxiety score was measured by Crown-Crisp Phobic Index on the 1988 questionnaire
Table 3.
Least-squares mean (95% CI) symmetrized percent change in relative leukocyte telomere length, by categories of baseline mental health outcomes1
| Exposure | Category | N | Age-adjusted2 | F | p | Multivariable-adjusted3 | F | p |
|---|---|---|---|---|---|---|---|---|
| Depression4 | No | 1098 | −2.54 (−3.18, −1.89) | F1,1249=2.16 | 0.14 | −2.53 (−3.17, −1.88) | F1,1249=2.51 | 0.11 |
| Yes | 86 | −4.34 (−6.66, −2.03) | −4.48 (−4.80, −2.15) | |||||
| Phobic anxiety symptom score5 | 0–1 | 440 | −2.89 (−3.91, −1.87) | F4,1249 =0.14 | 0.97 | −2.94 (−3.96, −1.91) | F4,1249 =0.21 | 0.93 |
| 2 | 240 | −2.29 (−3.67, −0.92) | −2.19 (−3.58, −0.80) | |||||
| 3 | 185 | −2.79 (−4.36, −1.22) | −2.85 (−4.43, −1.27) | |||||
| 4–5 | 248 | −2.85 (−4.21, −1.49) | −2.80 (−4.16, −1.44) | |||||
| 6+ | 137 | −2.98 (−4.81, −1.16) | −3.04 (−4.88, −1.20) |
11-year change in relative leukocyte telomere length was calculated by symmetrized percent change, defined by (exponentiated T/S ratio at T2 − exponentiated T/S ratio at T1)/(exponentiated T/S ratio at T2 + exponentiated T/S ratio at T1) * 100
Model adjusted for age at first blood draw (continuous)
Model adjusted for age at first blood draw (continuous), paternal age at birth (continuous), pack-years of smoking (never, >0–20, >20–40, >40, missing), body mass index (continuous), and physical activity (continuous)
Depression was defined by antidepressant use at initial blood draw or MHI-5 ≤ 52 on the 1992 questionnaire
Phobic anxiety score was measured by Crown-Crisp Phobic Index on the 1988 questionnaire
Table 4.
Odds ratio (95% CI) of 11-year decile shift in relative leukocyte telomere length, by categories of baseline mental health outcomes1
| Exposure | Category | 11-year decile shift in LTL
|
||||||
|---|---|---|---|---|---|---|---|---|
| N | Age, baseline LTL-adjusted model2 | Multivariable-adjusted model3 | ||||||
|
| ||||||||
| Stable | Shift upward | Shift downward | Shift upward | Shift downward | Shift upward | Shift downward | ||
| Depression4 | No | 674 | 221 | 203 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Yes | 48 | 16 | 22 | 1.16 (0.64, 2.13) | 1.48 (0.87, 2.52) | 1.11 (0.60, 2.04) | 1.48 (0.87, 2.54) | |
| Phobic anxiety symptom score5 | 0–1 | 263 | 84 | 93 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| 2 | 153 | 47 | 40 | 0.92 (0.60, 1.39) | 0.74 (0.48, 1.13) | 0.85 (0.55, 1.30) | 0.70 (0.46, 1.08) | |
| 3 | 113 | 39 | 33 | 1.01 (0.64, 1.58) | 0.84 (0.53, 1.32) | 0.98 (0.62, 1.55) | 0.84 (0.53, 1.33) | |
| 4–5 | 147 | 55 | 46 | 1.24 (0.83, 1.87) | 0.85 (0.57, 1.29) | 1.20 (0.80, 1.81) | 0.84 (0.56, 1.28) | |
| 6+ | 87 | 25 | 25 | 0.91 (0.54, 1.53) | 0.81 (0.49, 1.35) | 0.87 (0.51, 1.47) | 0.81 (0.48, 1.35) | |
Decile shift was defined as the difference of decile ranking in relative telomere lengths estimated by exponentiated T/S ratio between second and initial blood draw: referent group being stable in decile shift over 10 years of LTL (within ±1 decile shift); shifting upward was defined as >1 decile increase; shifting downward was defined as >1 decile decrease. Multinomial logistic regression was used to examine the associations
Model adjusted for age at first blood draw (continuous) and baseline LTL (continuous)
Model adjusted for age at first blood draw (continuous), baseline LTL (continuous), paternal age at birth (continuous), pack-years of smoking (never, >0–20, >20–40, >40, missing), body mass index (continuous), and physical activity (continuous)
Depression was defined by antidepressant use at initial blood draw or MHI-5 ≤ 52 on the 1992 questionnaire. 66 participants who did not answer question on antidepressant use at initial blood draw were not included.
Phobic anxiety score was measured by Crown-Crisp Phobic Index on the 1988 questionnaire.
We did not observe significant associations between baseline phobic anxiety and higher LTL attrition rate between two time points. The age-adjusted and multivariable-adjusted analyses yielded similar results. In the multivariable-adjusted models, compared to participants with minimal phobic anxiety symptoms (CCI ≤ 1), those with CCI scores of 2, 3, 4–5, 6+ had similar levels of LTL shortening for both the AC (F4,1249=0.19, p=0.94; Table 2) or SPC (F4,1249=0.21, p=0.93; Table 3) outcome, and they did not have a significantly greater odds of having downward DS in telomere lengths (Table 4).
DISCUSSION
In this prospective study involving 1,250 mid-life and older U.S. women, we did not observe statistically significant associations of depression or anxiety with change in telomere lengths over 11 years. When considering all three metrics of telomere length change, point estimates were in the direction of more accelerated telomere shortening among persons with vs. without depression at baseline; however, these associations were not statistically significant. The largest determinant of observed changes in telomere length was the baseline telomere length, and regression-to-the-mean was present.
Regarding the association between depression and telomere length in the literature, individual studies have shown mixed results; however, an overall significant association between depressive disorders and shorter telomere length has been observed in meta-analyses (Schutte and Malouff 2015; Darrow et al. 2016; Lin, Huang, and Hung 2016). Nevertheless, most studies were conducted cross-sectionally, and there have been few prospective studies that could address temporality. Shalev et al (Shalev et al. 2014) found that the persistence of internalizing disorders (including major depressive disorder (MDD), generalized anxiety disorder (GAD), and post-traumatic stress disorder) between adolescence and mid-life (ages 11 and 38 years) significantly predicted shorter LTLs at 38 years old. In addition, people diagnosed with incident MDD had significantly greater LTL erosion between two time points (ages 26 and 38 years), but this association was only seen among men. In a paper by Verhoeven and colleagues (Verhoeven et al. 2016), 2,292 middle-aged male and female patients with depressive or anxiety disorders, either remitted or current, consistently had shorter telomere length compared to 644 controls without any history of depression or anxiety – both at the baseline and 6-year time points. However, changes in disease course of depression or anxiety over 6 years were not associated with greater LTL attrition during the same 6-year period; the authors interpreted this as evidence of a between-person rather than within-person association of depression with telomere length. Similarly, Rius-Ottenheim et al (Rius-Ottenheim et al. 2012) did not observe significant associations between changes in depressive symptoms and changes in telomere lengths between baseline and 7 years later among 75 older males. Hoen et al (Hoen et al. 2013) also found no association between depression and shorter telomeres at 2-year follow-up among 974 mid-life men and women. Interestingly, in a study (Hoen et al. 2011) of 608 predominantly older male patients with coronary heart disease, depression appeared associated with a lower likelihood of 5-year subsequent telomere shortening; however, this association was attenuated and no longer statistically significant after adjusting for anxiety and baseline telomere length. Although these investigations differed by study design, sample size, follow-up durations, and participants’ ages, sex and health characteristics (e.g., healthy population vs. patients with heart disease), the results are generally consistent with our current finding of a lack of statistically significant associations between depression and LTL shortening over time. Nevertheless, a pattern suggestive of greater decreases in telomere lengths with depression at baseline was consistently noted on all three LTL change metrics. This finding may warrant further investigation, especially in studies with somewhat larger sample sizes that can overcome intrinsic limitations of statistical power due to random measurement error in the qPCR telomere measure.
Anxiety has also been studied with regard to telomere length, although its relation to telomere length has been examined more frequently in conjunction with depression (Verhoeven et al. 2016; Hoen et al. 2013; Shalev et al. 2014). In work by Hoen et al (Hoen et al. 2013), the presence of anxiety disorders was significantly associated with shorter telomeres at 2-year follow-up among mid-life men and women, but the association was no longer significant after adjustment for baseline telomere length. In the paper by Shalev et al (Shalev et al. 2014), participants who experienced GAD between ages 26 and 38 years showed significantly accelerated LTL erosion during this same 12-year time frame compared to those without GAD; however, as was the case for MDD and LTL erosion, this association was only observed among men. Although our prior finding in the NHS cohort identified a significant cross-sectional association of phobic anxiety with shorter telomere lengths, we did not observe a prospective association between phobic anxiety and telomere length shortening over 11 years in the current study. It is notable, however, that our data cannot inform the issue of whether relations of anxiety to accelerated telomere shortening may exist among younger people.
The strengths of the study include: a well-characterized sample of generally healthy, community-dwelling participants; prospective study design; high-quality information on relevant health, lifestyle, and demographic factors. Potential limitations should also be noted. First, the study examined the presence of depression and phobic anxiety with respect to changes in telomere length between ages 57 and 68 years. This may not be the most sensitive time window for addressing these associations, as the rate of telomere attrition is most rapid in earlier life periods (Weng 2008). Second, compared to prior studies which measured LTLs using the Southern blot method (Aviv et al. 2009), more participants showed gain in telomere length in our study, in which LTLs were measured by qPCR; this suggests importance of technical differences in the telomere assay methods. There are two key sources of random measurement error in the qPCR-based T/S ratio calculation at each time point (i.e., from both the telomere and single control gene assays). As even greater random measurement error is involved in computing changes in T/S ratios between two time points, associations may be biased towards the null (Aviv et al. 2011). Third, despite a comparatively large sample size, adequate power was an issue for some analyses: for example, in this generally healthy sample of 1,250 women, there were fewer than 100 participants who regularly used antidepressants or had severe depressive symptoms at baseline. Furthermore, the adverse impact of random error on statistical power may be even more pronounced in this study, where the available sample size was lower than those in prior analyses of anxiety and telomere lengths in this cohort (Okereke et al. 2012; Ramin et al. 2015). Fourth, misclassification of depression was possible; as such misclassification is more likely to be non-differential, it could bias results towards the null. Fifth, residual confounding is possible, as in all observational designs. However, we performed multivariable-adjusted analyses that controlled for carefully measured potential confounders, and results were similar when compared to age-adjusted estimates; therefore, residual confounding may be less of a concern. Lastly, the study was conducted among mid-life and older women who were predominantly non-Latino white (prevalence of minority race/ethnicity is 5% in the NHS). Homogeneity of study participants may strengthen internal validity, but the findings may not be generalizable to males or to other racial, ethnic and/or age groups (Diez Roux et al. 2009; Shalev et al. 2014; Phillips et al. 2013).
In summary, we did not observe that either depression or anxiety was significantly associated with subsequent change in telomere lengths over 11 years among middle-aged and older women. However, when considering all three metrics of telomere length change, the point estimates suggested a pattern of more accelerated telomere shortening over follow-up among persons with vs. without depression, and this finding may warrant further inquiry. Future prospective studies would be enhanced by addressing broader windows of the lifespan, including larger sample sizes in order to increase power, and using psychological measures that maximize contrasts in characterizing persons with depression or anxiety vs. those without these conditions.
Supplementary Material
Acknowledgments
The authors thank the participants and staff of the Nurses’ Health Study for their valuable contributions.
This work was supported by funding from the National Institutes of Health (UM1 CA186107, R01 CA49449, and R01 MH096776). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The contents hereof are solely the responsibility of the authors.
Footnotes
FINANCIAL DISCLOSURES
None
References
- Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323–9. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;39:e134. doi: 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batelaan NM, Seldenrijk A, Bot M, van Balkom AJ, Penninx BW. Anxiety and new onset of cardiovascular disease: critical review and meta-analysis. Br J Psychiatry. 2016;208:223–31. doi: 10.1192/bjp.bp.114.156554. [DOI] [PubMed] [Google Scholar]
- Benetos A, Kark JD, Susser E, Kimura M, Sinnreich R, Chen W, Steenstrup T, Christensen K, Herbig U, von Bornemann Hjelmborg J, Srinivasan SR, Berenson GS, Labat C, Aviv A. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12:615–21. doi: 10.1111/acel.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DA, Ayers GD. Symmetrized Percent Change for Treatment Comparisons. The American Statistician. 2002;60:27–31. [Google Scholar]
- Berwick DM, Murphy JM, Goldman PA, Ware JE, Jr, Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Med Care. 1991;29:169–76. doi: 10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Bojesen SE. Telomeres and human health. J Intern Med. 2013;274:399–413. doi: 10.1111/joim.12083. [DOI] [PubMed] [Google Scholar]
- Burgess PM, Mazzocco L, Campbell IM. Discriminant validity of the Crown-Crisp Experiential Index. Br J Med Psychol. 1987;60(Pt 1):61–9. [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodosh J, Kado DM, Seeman TE, Karlamangla AS. Depressive symptoms as a predictor of cognitive decline: MacArthur Studies of Successful Aging. Am J Geriatr Psychiatry. 2007;15:406–15. doi: 10.1097/01.JGP.0b013e31802c0c63. [DOI] [PubMed] [Google Scholar]
- Crown S, Crisp AH. A short clinical diagnostic self-rating scale for psychoneurotic patients. The Middlesex Hospital Questionnaire (M.H.Q.) Br J Psychiatry. 1966;112:917–23. doi: 10.1192/bjp.112.490.917. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Differential mortality rates in major and subthreshold depression: meta-analysis of studies that measured both. Br J Psychiatry. 2013;202:22–7. doi: 10.1192/bjp.bp.112.112169. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow SM, Verhoeven JE, Revesz D, Lindqvist D, Penninx BW, Delucchi KL, Wolkowitz OM, Mathews CA. The Association Between Psychiatric Disorders and Telomere Length: A Meta-Analysis Involving 14,827 Persons. Psychosom Med. 2016;78:776–87. doi: 10.1097/PSY.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, Seeman T. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell. 2009;8:251–7. doi: 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Du M, Prescott J, Kraft P, Han J, Giovannucci E, Hankinson SE, De Vivo I. Physical activity, sedentary behavior, and leukocyte telomere length in women. Am J Epidemiol. 2012;175:414–22. doi: 10.1093/aje/kwr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Walston J, Kimura M, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Gan Y, Gong Y, Tong X, Sun H, Cong Y, Dong X, Wang Y, Xu X, Yin X, Deng J, Li L, Cao S, Lu Z. Depression and the risk of coronary heart disease: a meta-analysis of prospective cohort studies. BMC Psychiatry. 2014;14:371. doi: 10.1186/s12888-014-0371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Study, Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joormann J, Lin J, Wolkowitz OM. Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry. 2015;20:615–20. doi: 10.1038/mp.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulpers B, Ramakers I, Hamel R, Kohler S, Oude Voshaar R, Verhey F. Anxiety as a Predictor for Cognitive Decline and Dementia: A Systematic Review and Meta-Analysis. Am J Geriatr Psychiatry. 2016;24:823–42. doi: 10.1016/j.jagp.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Haines A, Cooper J, Meade TW. Psychological characteristics and fatal ischaemic heart disease. Heart. 2001;85:385–9. doi: 10.1136/heart.85.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2–3-year period. Cancer Epidemiol Biomarkers Prev. 1995;4:649–54. [PubMed] [Google Scholar]
- Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel E, Lin J, Blackburn E, Whooley MA. Depression and leukocyte telomere length in patients with coronary heart disease: data from the Heart and Soul Study. Psychosom Med. 2011;73:541–7. doi: 10.1097/PSY.0b013e31821b1f6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen PW, Rosmalen JG, Schoevers RA, Huzen J, van der Harst P, de Jonge P. Association between anxiety but not depressive disorders and leukocyte telomere length after 2 years of follow-up in a population-based sample. Psychol Med. 2013;43:689–97. doi: 10.1017/S0033291712001766. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Aguilar-Gaxiola S, Alonso J, Chatterji S, Lee S, Ormel J, Ustun TB, Wang PS. The global burden of mental disorders: an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18:23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, Cupples A, Hunkin JL, Gardner JP, Lu X, Cao X, Sastrasinh M, Province MA, Hunt SC, Christensen K, Levy D, Spector TD, Aviv A. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4:e37. doi: 10.1371/journal.pgen.0040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latifovic L, Peacock SD, Massey TE, King WD. The Influence of Alcohol Consumption, Cigarette Smoking, and Physical Activity on Leukocyte Telomere Length. Cancer Epidemiol Biomarkers Prev. 2016;25:374–80. doi: 10.1158/1055-9965.EPI-14-1364. [DOI] [PubMed] [Google Scholar]
- Lin PY, Huang YC, Hung CF. Shortened telomere length in patients with depression: A meta-analytic study. J Psychiatr Res. 2016;76:84–93. doi: 10.1016/j.jpsychires.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Luppino FS, van Reedt Dortland AK, Wardenaar KJ, Bouvy PF, Giltay EJ, Zitman FG, Penninx BW. Symptom dimensions of depression and anxiety and the metabolic syndrome. Psychosom Med. 2011;73:257–64. doi: 10.1097/PSY.0b013e31820a59c0. [DOI] [PubMed] [Google Scholar]
- McGrath M, Kawachi I, Ascherio A, Colditz GA, Hunter DJ, De Vivo I. Association between catechol-O-methyltransferase and phobic anxiety. Am J Psychiatry. 2004;161:1703–5. doi: 10.1176/appi.ajp.161.9.1703. [DOI] [PubMed] [Google Scholar]
- McGrath M, Wong JY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16:815–9. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–90. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muezzinler A, Zaineddin AK, Brenner H. Body mass index and leukocyte telomere length in adults: a systematic review and meta-analysis. Obes Rev. 2014;15:192–201. doi: 10.1111/obr.12126. [DOI] [PubMed] [Google Scholar]
- Nan H, Du M, De Vivo I, Manson JE, Liu S, McTiernan A, Curb JD, Lessin LS, Bonner MR, Guo Q, Qureshi AA, Hunter DJ, Han J. Shorter telomeres associate with a reduced risk of melanoma development. Cancer Res. 2011;71:6758–63. doi: 10.1158/0008-5472.CAN-11-1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okereke OI, Prescott J, Wong JY, Han J, Rexrode KM, De Vivo I. High phobic anxiety is related to lower leukocyte telomere length in women. PLoS One. 2012;7:e40516. doi: 10.1371/journal.pone.0040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Robertson T, Carroll D, Der G, Shiels PG, McGlynn L, Benzeval M. Do symptoms of depression predict telomere length? Evidence from the west of Scotland twenty-07 study. Psychosom Med. 2013;75:288–96. doi: 10.1097/PSY.0b013e318289e6b5. [DOI] [PubMed] [Google Scholar]
- Prescott J, Du M, Wong JY, Han J, De Vivo I. Paternal age at birth is associated with offspring leukocyte telomere length in the nurses’ health study. Hum Reprod. 2012;27:3622–31. doi: 10.1093/humrep/des314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramin C, Wang W, Prescott J, Rosner B, Simon NM, De Vivo I, Okereke OI. A prospective study of leukocyte telomere length and risk of phobic anxiety among women. Psychiatry Res. 2015;230:545–52. doi: 10.1016/j.psychres.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rius-Ottenheim N, Houben JM, Kromhout D, Kafatos A, van der Mast RC, Zitman FG, Geleijnse JM, Hageman GJ, Giltay EJ. Telomere length and mental well-being in elderly men from the Netherlands and Greece. Behav Genet. 2012;42:278–86. doi: 10.1007/s10519-011-9498-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM. The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety. 2015;32:229–38. doi: 10.1002/da.22351. [DOI] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, Harrington HL, Houts RM, Israel S, Poulton R, Robertson SP, Sugden K, Williams B, Caspi A. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry. 2014;19:1163–70. doi: 10.1038/mp.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–4. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, van Oppen P, Revesz D, Wolkowitz OM, Penninx BW. Depressive and Anxiety Disorders Showing Robust, but Non-Dynamic, 6-Year Longitudinal Association With Short Leukocyte Telomere Length. Am J Psychiatry. 2016;173:617–24. doi: 10.1176/appi.ajp.2015.15070887. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen H, Gao X, McGrath M, Deer D, De Vivo I, Schwarzschild MA, Ascherio A. Telomere length and risk of Parkinson’s disease. Mov Disord. 2008;23:302–5. doi: 10.1002/mds.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- Weng NP. Telomere and adaptive immunity. Mech Ageing Dev. 2008;129:60–6. doi: 10.1016/j.mad.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–86. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstatter A, Kronenberg F, Kiechl S. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304:69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen MK, Orsted DD, Rode L, Bojesen SE, Nordestgaard BG. Telomere length and depression: prospective cohort study and Mendelian randomisation study in 67 306 individuals. Br J Psychiatry. 2017;210:31–38. doi: 10.1192/bjp.bp.115.178798. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27:327–38. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Fukuhara S, Green J. Usefulness of five-item and three-item Mental Health Inventories to screen for depressive symptoms in the general population of Japan. Health Qual Life Outcomes. 2005;3:48. doi: 10.1186/1477-7525-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Belcher M, van der Harst P. Healthy aging and disease: role for telomere biology? Clin Sci (Lond) 2011;120:427–40. doi: 10.1042/CS20100385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


