Supplemental Digital Content is Available in the Text.
Keywords: Opioids, Chronic pain, Cognition, Inflammation, Attention, Self-efficacy
Abstract
Introduction:
Cognitive performance and inflammation are altered in people with chronic low back pain (CLBP). Yet, the magnitude of these changes has been unclear because of the potential influence of opioid analgesics.
Objectives:
This cross-sectional pilot study aimed to explore whether patients with CLBP receiving long-term opioid analgesics differed from patients not taking opioids on measures of cognitive performance and plasma cytokine concentrations.
Methods:
Patients with CLBP who were either taking (N = 18) or not taking (N = 22) opioids daily for 3 or more months were recruited from a tertiary care private hospital and compared with healthy adults (N = 20). All groups were administered validated questionnaires to assess depression, anxiety, and stress; a cognitive test of memory, attention, and executive function; and a peripheral blood draw to measure proinflammatory (IL-1β, IL-2, IL-8, IL-12p70, TNF-α, and IFN-γ), anti-inflammatory (IL-4, IL-10, and IL-13), and pleiotropic (IL-6) cytokine concentrations. Patients also completed pain-specific questionnaires.
Results:
Patients receiving opioid analgesics performed significantly (P < 0.05) worse in attention and had significantly (P < 0.05) lower pain self-efficacy beliefs than those patients not taking opioids. Patient groups did not differ in mean pain severity or pain interference scores, tests of memory and executive function, and mean plasma cytokine concentrations, despite long-term opioid analgesics.
Conclusion:
Patients receiving long-term opioid analgesics for CLBP have minor differences when compared with patients not taking opioids. This has important clinical implications when considering long-term treatment for patients with CLBP.
1. Introduction
Chronic low back pain (CLBP) is a complex, multifactorial condition that is difficult to treat. Now the leading cause of disability,26 CLBP places a huge economic burden on the health care system.22 The multidisciplinary management of CLBP is the preferred treatment approach yet, the prescription of opioid analgesics continues to increase in high-income countries while the long-term use of opioids remains controversial.1
People with CLBP experience greater cognitive difficulties in memory,38 attention,37 and executive function7 compared with people who do not have pain. In addition, people with CLBP may experience coexisting disorders such as depression49 and/or anxiety,3 respond with catastrophizing thoughts and feelings or experience low self-efficacy beliefs.19 Accounting for cognitive decline, coexisting disorders, and contributing psychosocial factors experienced by people with CLBP is key in understanding the overall pain experience. Therefore, this cross-sectional pilot study has included tests and questionnaires that measure cognitive performance, symptoms of depression, anxiety and stress, and pain catastrophizing and pain self-efficacy beliefs.
Inflammation contributes to the biological mechanisms underpinning chronic pain.4 Once activated, immune cells secrete proteins, such as cytokines, that drive the process of inflammation. Studies have found an imbalance between proinflammatory and anti-inflammatory cytokines in the periphery of people with CLBP.5,8 However, to date, there has been considerable between-study variability in plasma sampling times, sample handling procedures, the type of cytokines measured, and/or assay methodologies used.2,27,41,53 Hence, our pilot study uses, for the first time in human plasma samples, multiplexed technology to quantify multiple proinflammatory (IL-1β, IL-2, IL-8, IL-12p70, TNF-α, and IFN-γ), anti-inflammatory (IL-4, IL-10, and IL-13), and pleiotropic (IL-6) cytokines to provide recommendations for future research.
It is now established that cognitive processing and inflammation are interconnected in the neurobiology of chronic pain.32 Opioids have been found to independently impair cognitive performance46,48 and alter cytokine concentrations55 in people with CLBP. Yet, previous studies have not controlled for opioids or collectively measured cognitive performance and cytokine concentrations in patients with CLBP, despite the strong biological relationship. Thus, previous studies have not adequately attributed cognitive decline or altered cytokine concentrations in people with chronic pain to the opioids, other medications, or the pain itself. Our study controls for the use of opioids by replicating the design of Schiltenwolf et al.,46 which uses 2 patient groups and healthy controls (HCs), to better delineate the differences between patients who do and do not take long-term opioid analgesics. As this is the first time cognitive performance and cytokine concentrations have been measured collectively, we designed a pilot study to explore the differences between patients with CLBP who were and were not taking long-term opioid analgesics. We hypothesized that patients with CLBP who were established on long-term opioid analgesics would perform worse on cognitive tasks and experience further alterations in inflammatory plasma cytokine profiles than those patients who were not using opioid analgesics.
2. Methods
2.1. Participants
Forty consecutive patients were recruited by invitation from their Pain Specialist at a private tertiary hospital in Brisbane, Australia. Patients were included if they: (1) had been diagnosed with CLBP by a Pain Specialist (B.M. or J.O.C.), which was not a result of stroke, postherpetic neuralgia, diabetic neuropathy, polymyalgia, or cancer, (2) were not currently taking nonsteroidal anti-inflammatory drugs (NSAIDs) or had refrained from taking NSAIDs 7 days before the blood draw, (3) were English language literate, (4) were 18 years and older, and (5) were able to provide written informed consent. Participants were excluded if they had a major psychiatric disorder such as schizophrenia and/or psychosis.
No participant declined the invitation to participate or received an incentive to participate. Patients were categorised into those who had been established on long-term opioids, defined as consecutive opioid prescriptions for 3 or more months9 (OP; N = 18), and those who were opioid-naive, defined as having no daily opioid dosing for 3 or more months (NO; N = 22). Previous studies have defined opioid-naive patients as those who have abstained from repeat opioid dosing for a 2- to 3-week period.30,46 Therefore, our conservative limit of 3 or more months ensures that patients in the nonopioid group well represent an opioid-naive cohort. The patient subgroups were assigned based on the medication listed in the patient's medical record. After this information was obtained, the patient's opioid status (ie, if or when the patient commenced or ceased opioids) was confirmed by the patient and their treating Pain Specialist (B.M. or J.O.C.). An additional group of adults without chronic pain, who were not taking any regular medications that may affect cognition or inflammation, were recruited by word of mouth and classified as HCs (N = 20). The categorisation of participants, OP, NO, and HC, replicates the subgroups used by Schiltenwolf et al.46 No power calculations were performed to determine the sample size, as this is a pilot study; however, the number of participants per group was based on those included in previous studies.29,47,51
2.2. Procedure
This study was approved by the Human Research Ethics Committee at Greenslopes Private Hospital (Protocol 15/09) and the Institutional Human Research Ethics Committee at The University of Queensland (Number: 2015000615). After a patient was invited to participate by their Pain Specialist and/or an HC was identified, the objectives and the tasks involved in the study were explained verbally by the researcher and supplemented with an information sheet. Written informed consent was then obtained from all participants before any study procedure was commenced. The researcher then interviewed or scheduled a suitable time to interview the participant to complete the questionnaires, administer the neuropsychological assessment, obtain the blood sample, and process the blood sample to produce plasma that was frozen for its later analysis. All data collected were deidentified and stored in a secure manner as per the ethical guidelines and good clinical practice.
2.3. Measures
Participants were asked to complete the following questionnaires:
2.3.1. Demographics and medical history
This questionnaire collected basic information about the participant including age, sex, residential distance from the central business district, place of birth, ethnic background, employment status, and a brief medical history.
2.3.2. Pain-related questionnaires
The two patient groups were asked to report on their use of health care services for their pain over the past 3 months including the number of visits to: their general practitioner, medical specialists, health professionals, the emergency department, hospital admissions, and number of diagnostic tests. As both patient groups were taking an extensive array of medications, only pain-related drug classes, commonly used by patients with chronic pain,43 were recorded and analyzed. These latter medications included paracetamol, NSAIDs, antidepressants, anticonvulsants, and benzodiazepines. Patients self-reported the number of years since CLBP diagnosis from their Pain Specialist (B.M. or J.O.C.).
For patients receiving opioids, their daily dosage was determined by converting all opioids to a standardised oral morphine equivalent daily dose (oMEDD) using the iOS application developed by the Australian and New Zealand College of Anaesthetists Faculty of Pain Medicine guidelines.21 Given the complex pharmacokinetics of methadone, the guidelines from the Washington State Agency Medical Directors' Group13 were used to calculate the oMEDD for patients receiving methadone.
Patients were administered the Brief Pain Inventory11 to measure additional areas of pain, the severity of pain, and the impact of pain on daily functions; the Depression Anxiety Stress Scales 21 (DASS-21)33 to measure psychological symptoms of depression, anxiety, and stress; the Pain Self-Efficacy Questionnaire (PSEQ)36 to measure one's beliefs in their ability to accomplish a range of activities, despite their pain; and the Pain Catastrophizing Scale50 to measure one's catastrophic thinking related to the pain experience. Each questionnaire has been validated, and further information is summarized in (Supplemental Table 1, http://links.lww.com/PR9/A21).
2.3.3. Cognitive performance tests
A battery of cognitive tests was administered to all participants to measure premorbid ability, memory, attention, and executive function. These tests included: the Wechsler Test of Adult Reading,24 the California Verbal Learning Test,15 the Everyday Memory Questionnaire–Revised,45 the Brief Assessment of Prospective Memory,34 the California Older Adult Stroop Test (COAST),39 the Letter-Number Sequencing (LNS), and Matrix Reasoning.24 Further information on each test is summarized in (Supplemental Table 1, http://links.lww.com/PR9/A21).
2.3.4. Plasma cytokine concentrations
One 10-mL peripheral blood sample was obtained using standard phlebotomy techniques for each participant and transferred into two 5 mL tubes containing the anticoagulant ethylenediaminetetraacetic acid. The samples were centrifuged immediately (2000g for 10 minutes at 4°C), separated, and stored at −80°C for later analysis. Three K15049D-1 V-PLEX Proinflammatory Panel 1 (human) Kits were used for the quantification of the plasma concentrations of IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, TNF-α, and IFN-γ. The assay was performed in duplicate according to the manufacturer's instructions,16 using a 1:1 dilution factor. Each 96-well plate contained plasma samples from each group of study participants. A Meso Scale Discovery (MSD) Sector Imager 2400 was used for the cytokine assays. The standard curve produced by MSD Sector Imager software with inverse prediction was used to determine the plasma concentrations for each cytokine of interest.
2.4. Statistical analyses
The data were analyzed using the Statistical Package for the Social Sciences (SPSS) version 25 for Windows.12 Range, mean, and SD were reported for all continuous variables. To reduce the errors associated with multiple comparisons, only essential parameters were chosen for independent statistical analyses. One-way analysis of variance was used to determine differences between groups for continuous variables. Significant differences were further evaluated using Gabriel post-hoc t tests for multiple comparisons, which are recommended when there are an unequal number of participants across groups.20 Pairwise independent t tests were used for comparisons between patient groups for the pain variables, and χ2 tests were performed for the categorical variables. The significance level was set at P ≤ 0.05 for each statistical test.
3. Results
3.1. Demographics and medical history
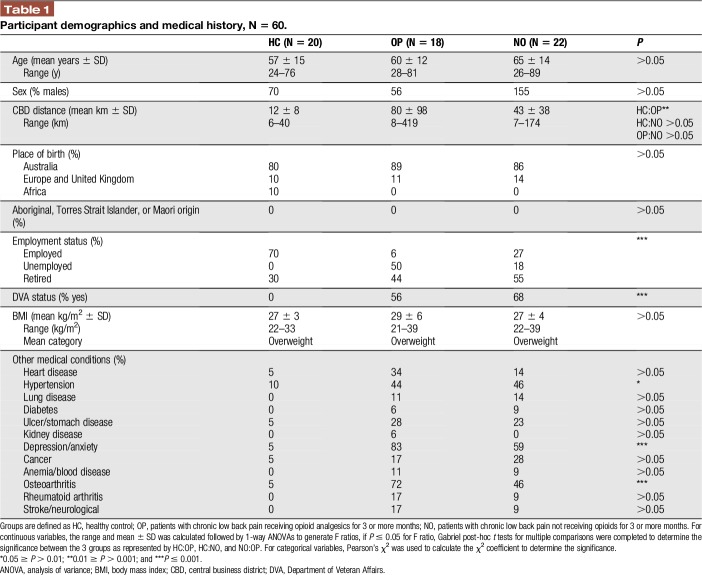
Study participants had comparable demographics with no significant differences between groups for age, sex, place of birth, ethnicity, and body mass index as summarized in Table 1 (all P's > 0.05). Patients receiving opioids lived farther away from the city compared with participants in the HC group, and this difference was significant (P ≤ 0.05). Patients were significantly more likely to be unemployed, retired, and/or supported by the Department of Veteran Affairs than participants in the HC group (P ≤ 0.001). Hypertension, depression/anxiety, and osteoarthritis were self-reported significantly more by patients than the HC group (all P's ≤ 0.05).
Table 1.
Participant demographics and medical history, N = 60.
3.2. Pain-related questionnaires
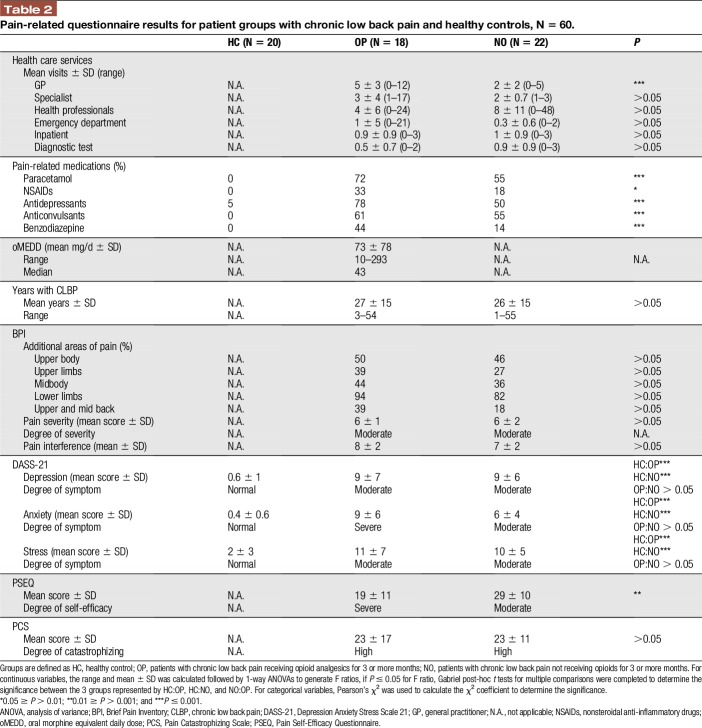
No significant differences were found between the patient groups on the Brief Pain Inventory, DASS-21, or Pain Catastrophizing Scale measures (all P's > 0.05, Table 2). This included, no difference in the: mean number of years with CLBP, number of additional areas with pain, mean pain severity scores, mean score of pain interference on daily functions, mean scores for symptoms of depression, anxiety, and stress, and mean score for pain catastrophizing thoughts and feelings. Compared with the HC group, patients reported significantly more symptoms of depression, anxiety, and stress (all P's ≤ 0.001, Table 2).
Table 2.
Pain-related questionnaire results for patient groups with chronic low back pain and healthy controls, N = 60.
Patients receiving opioids visited their general practitioners significantly more for their pain, were taking more pain-related medications, and had a significantly lower mean PSEQ score, than those patients not taking opioids (all P's ≤ 0.05). Figure 1 illustrates the distribution in PSEQ scores. Scores for patients receiving opioids were skewed to the right (ie, concentrated in the left of the figure), where low scores indicate poor self-efficacy beliefs (Fig. 1). Opioid doses ranged from 10 to 293 mg/d, with the majority of patients (N = 12) in the OP group being prescribed doses less than 50 mg/d (Fig. 2).
Figure 1.
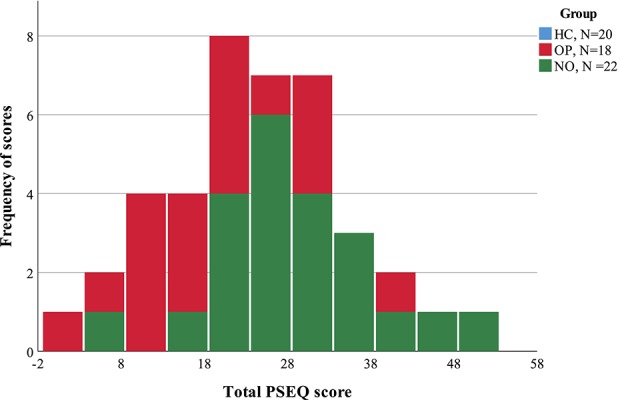
This histogram illustrates the scores for the Pain Self-Efficacy Questionnaire (PSEQ) in patients with chronic low back pain. Patients receiving opioid analgesics (OP, N = 18, red bars) had a mean PSEQ score that was significantly (P ≤ 0.05) lower than patients who were not taking opioid (NO, N = 22, green bars). HC, healthy control.
Figure 2.
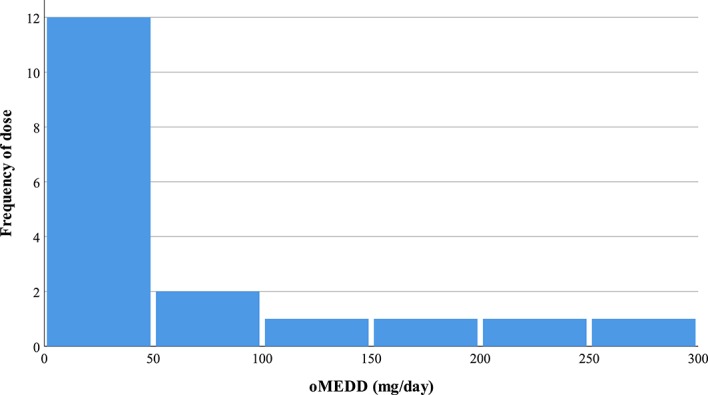
This histogram illustrates the various dosages of opioid analgesics for patients with chronic low back pain participating in the study (N = 18). The opioid dose for each eligible patient was converted to an oral morphine equivalent daily dose (oMEDD). Most patients were receiving moderate doses between 10 mg/d and 49 mg/d, whereas few patients were on high doses of opioids, ≥100 mg/d.
3.3. Cognitive performance tests
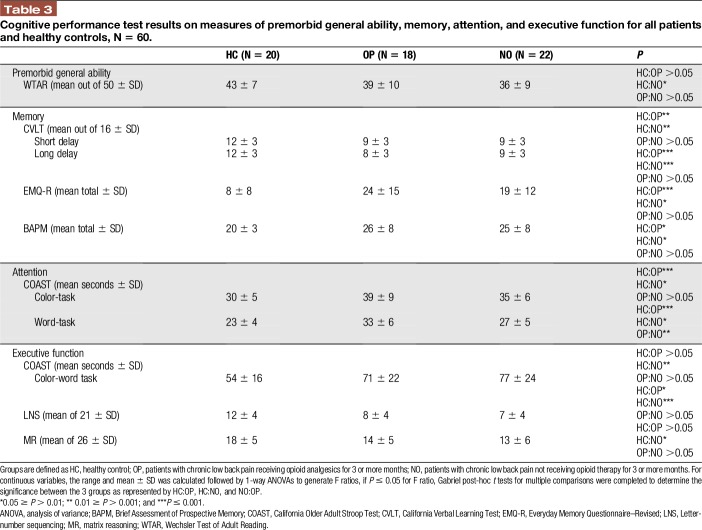
The mean scores on cognitive tests are presented in Table 3, with the key findings described below.
Table 3.
Cognitive performance test results on measures of premorbid general ability, memory, attention, and executive function for all patients and healthy controls, N = 60.
3.3.1. Premorbid general ability
No significant differences in premorbid general ability were found between the patient groups or between HC and OP. However, premorbid ability was significantly lower for patients in the NO group relative to the HC group, where P ≤ 0.05.
3.3.2. Memory
Memory performance was significantly reduced in patients compared with the HC group. Patients performed significantly worse in their ability to consolidate and retain material over time (as evident in California Verbal Learning Test scores) and self-reported significantly more memory retrieval (Everyday Memory Questionnaire–Revised; P ≤ 0.05) and prospective memory problems (on the Brief Assessment of Prospective Memory; P ≤ 0.05) than the HC group. No significant differences were found between the 2 patient groups in memory performance.
3.3.3. Attention and executive function
Patients with CLBP performed significantly worse in areas of attention (COAST) and executive working memory (LNS) than the HC participants. Patients took significantly longer time to complete the COAST-color and COAST-word tasks, and performed significantly worse on the LNS task compared with the HC group (all P's ≤ 0.05). Patients receiving opioids had a further reduction in attention (COAST-word task) than those patients not taking opioids, and this was a significant finding (0.01 ≥ P > 0.001). No other significant differences in mean completion times and scores were found between patient groups.
NO patients took significantly longer time to perform the COAST-color-word task (P ≤ 0.05) and scored significantly worse on the Matrix Reasoning task (P ≤ 0.05) than HC participants, whereas this difference was not found between OP and HC. This finding between NO and HC may be attributed to the reduced premorbid general ability of NO patients as described in section 3.3.1.
3.4. Plasma cytokine concentrations
No significant differences were found in the mean plasma concentrations of proinflammatory (IL-1β, IL-2, IL-8, IL-12p70, TNF-α, and IFN-γ), anti-inflammatory (IL-4, IL-10, and IL-13), and pleiotropic (IL-6) cytokines between the 3 groups. Figure 3 illustrates the variation in concentrations that exist between the 3 groups and between the different individual cytokine types. Unfortunately, some concentrations for IL-1β, IL-2, IL-12p70, and IL-4 could not be analyzed from the plasma samples, as concentrations fell below the lower limit of quantification. The data for each cytokine and patient group are summarized in (Supplemental Table 2, http://links.lww.com/PR9/A21).
Figure 3.
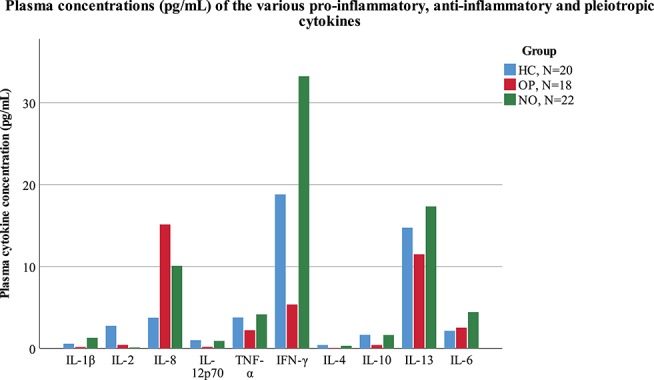
This graph illustrates the plasma concentrations (pg/mL) for various proinflammatory (IL-1β, IL-2, IL-8, IL-12p70, TNF-α, and IFN-γ), anti-inflammatory (IL-4, IL-10, and IL-13), and pleiotropic (IL-6) cytokines in patients with chronic low back pain who were receiving opioid analgesics (OP, N = 18, red bars), not receiving opioids (NO, N = 22, green bars), and healthy controls (HCs, N = 20, blue bars). No significant differences were found between the 3 groups (P ≤ 0.05).
4. Discussion
This cross-sectional pilot study aimed to explore the differences between patients with CLBP who were and were not taking opioid analgesics on measures of cognitive performance and plasma cytokine concentrations. The hypothesis that patients with CLBP on long-term opioid analgesics would have poorer cognitive performance and further alterations in plasma cytokine concentrations than those patients not using opioids was partially supported. We found that patients on long-term opioids performed significantly worse on attention (COAST-word task) and had significantly lower self-efficacy beliefs (PSEQ) over patients not receiving opioids. However, no further differences were found between the patient groups in pain severity, pain interference, tests of memory and executive function, and mean plasma cytokine concentrations, despite long-term opioid analgesics.
It is well understood that both pain and opioid analgesics affect the central nervous system.23 Yet, there is contradictory evidence concerning the effects of opioid analgesics on cognitive performance. Studies examining long-term opioid use in patients with chronic pain have found such treatment to improve cognitive function,25,51 whereas others have found that it is impaired46,48 or is unaffected by the use of opioids.31 However, most previous studies have not included a pain-free comparison group and thus were unable to control for potentially confounding variables such as age and sex. Our study incorporated a HC group and found no differences between the patient groups on most tests of cognitive performance, except for attention, which highlights the strong contribution of the pain itself in reducing cognitive performance in people with CLBP. This is consistent with previous studies31,38,54 and may be explained by the continuous nociceptive inputs associated with chronic pain, which may be competing with other sensory inputs.18
Pain self-efficacy assesses one's confidence in participating in activities such as household chores, work, socialising, and coping with life despite pain. Our finding that patients receiving opioids had lower self-efficacy beliefs than those patients not taking opioids replicates the work by Morasco et al.35 This finding is clinically important, as lower pain self-efficacy scores in patients prescribed opioids have been independently associated with suicide ideation-to-action10 and the development of depression.49 However, a prospective study design would be required to identify the type of relationship between long-term opioid use and pain self-efficacy beliefs.
The interaction between attention and self-efficacy beliefs in patients with CLBP has not yet been thoroughly investigated. Our findings indicate that further research is required to determine whether self-efficacy beliefs impact attentional processing and, where they do, what treatment strategies can be implemented to enhance and maintain self-efficacy.
Our findings replicate the work of others28,44 by highlighting the significant impact of CLBP on psychosocial variables. Both patient groups had significantly more symptoms of psychological distress (ie, depression, anxiety, and stress) and catastrophizing than HCs. Roth et al.44 found depressive symptoms and catastrophizing to be correlated with complaints of cognitive impairment in patients with chronic pain, whereas Kurita et al.28 found anxiety and depression to be associated with poor cognitive performance. Therefore, it is possible that the high prevalence of psychological distress and catastrophizing found in our patients may be contributing to the performance decline observed. However, an alternative study design would be required to determine the mechanism of this relationship.
Patients did not differ in their pain severity or pain interference scores, despite daily opioid analgesics. This finding illustrates that both groups were equally treated for their pain. Alternatively, this may be attributed to the development of opioid tolerance. The long-term use of opioids can lead to the loss of analgesic potency and effectiveness, depending on the specific opioid prescribed.6 However, a different study design would be necessary to explore the efficacy of opioids in this cohort of patients with CLBP. Therefore, both patient groups were receiving treatments with the same outcome on pain intensity and function.
It has been proposed that altered plasma concentrations of proinflammatory and anti-inflammatory cytokines may indicate a shift in biological equilibrium and contribute to the disease pathology of chronic pain.16 In patients with chronic pain, the balance appears to be shifted towards a proinflammatory state with a concurrent reduction in anti-inflammatory cytokines.5 However, failure to confirm this finding is not uncommon.52 Cytokines have a broad range of roles in many organs and body systems,42 especially in patients diagnosed with depression and osteoarthritis.14,40 In our present work, we found no differences in the mean plasma concentrations of various cytokines between the 3 groups, and thus, this did not support our hypothesis. However, the patients in our study reported significantly more comorbidities including hypertension, depression, anxiety, and osteoarthritis as well as taking a broad array of other medications. Hence, the considerable interindividual variability in the circulating plasma cytokine concentrations may reflect the disease complexity of patients with CLBP.
Our study is not without its limitations. Data were absent for some cytokine concentrations, as results fell below the lower limit of quantification despite using prevalidated multiplexed kits with high specificity and sensitivity.16 We determined opioid status from medical records, with confirmation from patients and prescribers, and did not measure opioid intake using screening tools, such as urine drug tests, to verify the opioid status of each participant. This means we could not account for opioid receipt from nonmedical sources nor confirm adherence to medications. Patients were receiving heterogeneous opioid doses, and hence, a stratified analysis based on the range of opioid dosages (ie, 10–50 mg/d, 51–100 mg/d, and 101–300 mg/d of oMEDD) would be preferred. However, this was not possible because of the limited number of included patients (N = 18). Yet, sample size was not a direct limitation, as it was based on those included in previous studies29,47,51 and acceptable for this preliminary study design. Nevertheless, the patients in our study represent a doctor-diagnosed cohort rather than self-reported pain as used in other studies.17 One trained researcher (G.C.R.) administered all study measures and processed all blood samples, which reduced variation that may otherwise arise from such procedures. Our preliminary results can be used to design a larger prospective study that is adequately powered. Overall, this study was unique in its multidimensional assessment of the pain experience, cognitive performance, and plasma cytokine concentrations, which raises important clinical implications when considering long-term opioid analgesics for patients with CLBP.
To the best of our knowledge, this is the first pilot study that has controlled for the use of long-term opioids while measuring cognitive performance, psychosocial variables, and plasma cytokine concentration in patients with CLBP. We found that patients receiving long-term opioid analgesics for CLBP have minor differences when compared with patients not taking opioids. The frequency of psychological distress and poor cognitive performance found in our patients supports a multidisciplinary biopsychosocial approach to pain management.
Disclosures
The authors have no conflict of interest to declare.
The University of Queensland provided the finances to purchase the laboratory equipment and run the cytokine assays.
This abstract was presented as a poster at the IASP 16th World Congress on Pain in 2016.
Acknowledgements
The authors thank Dr Chin Lin Wong from the Centre for Integrated Preclinical Drug Development (CIPDD), School of Biomedical Sciences, Faculty of Medicine, at The University of Queensland (UQ) for her invaluable assistance in performing the multiplexed plasma cytokine assays. The authors thank Gallipoli Medical Research Foundation and the CIPDD for access to laboratory facilities for the separation and storage of plasma samples. The authors thank the staff at Queensland Medical Laboratories (St Lucia, Queensland, Australia) who collected the peripheral blood samples for the HC group. The authors would also like to thank the participants who volunteered their time for this study.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PR9/A21.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painrpts.com).
References
- [1].Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Intern Med 2016;176:958–68. [DOI] [PubMed] [Google Scholar]
- [2].Alexander GM, Peterlin BL, Perreault MJ, Grothusen JR, Schwartzman RJ. Changes in plasma cytokines and their soluble receptors in complex regional pain syndrome. J Pain 2012;13:10–20. [DOI] [PubMed] [Google Scholar]
- [3].Asmundson GJ, Katz J. Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety 2009;26:888–901. [DOI] [PubMed] [Google Scholar]
- [4].Austin PJ, Moalem-Taylor G. The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol 2010;229:26–50. [DOI] [PubMed] [Google Scholar]
- [5].Backonja MM, Coe CL, Muller DA, Schell K. Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. J Neuroimmunol 2008;195:157–63. [DOI] [PubMed] [Google Scholar]
- [6].Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R. Opioid complications and side effects. Pain Physician 2008;11:S105–20. [PubMed] [Google Scholar]
- [7].Berryman C, Stanton TR, Bowering KJ, Tabor A, McFarlane A, Moseley GL. Do people with chronic pain have impaired executive function? A meta-analytic review. Clin Psychol Rev 2014;34:563–79. [DOI] [PubMed] [Google Scholar]
- [8].Block C, Cianfrini L. Neuropsychological and neuroanatomical sequelae of chronic non-malignant pain and opioid analgesia. NeuroRehabilitation 2013;33:343–66. [DOI] [PubMed] [Google Scholar]
- [9].Boudreau D, Von Korff M, Rutter CM, Saunders K, Ray GT, Sullivan MD, Campbell CI, Merrill JO, Silverberg MJ, Banta-Green C, Weisner C. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf 2009;18:1166–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Campbell G, Bruno R, Darke S, Shand F, Hall W, Farrell M, Degenhardt L. Prevalence and correlates of suicidal thoughts and suicide attempts in people prescribed pharmaceutical opioids for chronic pain. Clin J Pain 2016;32:292–301. [DOI] [PubMed] [Google Scholar]
- [11].Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129–38. [PubMed] [Google Scholar]
- [12].Corp IBM. SPSS statistics for Windows. Armonk: IBM Corp, 2013. [Google Scholar]
- [13].Cramb S. Total opioid dose calculator. Washington: Agency Medical Directors' Group, 2015. [Google Scholar]
- [14].Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008;9:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test (CVLT): adult version: manual. P Corporation, San Antonio, TX, 1987. [Google Scholar]
- [16].Diagnostics MSD. MSD instrument manual: sector imager models 2400 & 6000. Vol. 3 Maryland: Meso Scale Diagnostics, LLC, 2013. [Google Scholar]
- [17].Dominick CH, Blyth FM, Nicholas MK. Unpacking the burden: understanding the relationships between chronic pain and comorbidity in the general population. PAIN 2012;153:293–304. [DOI] [PubMed] [Google Scholar]
- [18].Eccleston C, Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol Bull 1999;125:356–66. [DOI] [PubMed] [Google Scholar]
- [19].Ferrari S, Chiarotto A, Pellizzer M, Vanti C, Monticone M. Pain self-efficacy and fear of movement are similarly associated with pain intensity and disability in Italian patients with chronic low back pain. Pain Pract 2015;16:1040–47. [DOI] [PubMed] [Google Scholar]
- [20].Field AP. Discovering statistics using SPSS: and sex and drugs and rock “n” roll. London: Sage, 2013. [Google Scholar]
- [21].FPMANZCA. Opioid dose equivalence. Calculation of oral morphine equivalent daily dose (oMEDD). Faculty of Pain Medicine Australian and New Zealand College of Anaesthetists, 2014. Available at: http://www.opioidcalculator.com.au/. Accessed May 2017. [Google Scholar]
- [22].Gore M, Sadosky A, Stacey BR, Tai KS, Leslie D. The burden of chronic low back pain: clinical comorbidities, treatment patterns, and health care costs in usual care settings. Spine 2012;37:E668–77. [DOI] [PubMed] [Google Scholar]
- [23].Hojsted J, Kurita GP, Kendall S, Lundorff L, de Mattos Pimenta CA, Sjogren P. Non-analgesic effects of opioids: the cognitive effects of opioids in chronic pain of malignant and non-malignant origin. Curr Pharm Des 2012;18:6116–22. [DOI] [PubMed] [Google Scholar]
- [24].Holdnack JA. Wechsler test of adult reading. San Antonio: Psychological Corporation, 2001. [Google Scholar]
- [25].Jamison RN, Schein JR, Vallow S, Ascher S, Virsanger GJ, Katz NP. Neuropsychological effects of longterm opioid use in chronic pain patients. J Pain Symptom Manage 2003;26:913–21. [DOI] [PubMed] [Google Scholar]
- [26].Kassebaum NJ, Arora M, Barber RM, Bhutta ZA, Carter A, Casey DC, Charlson FJ, Coates MM, Coggeshall M, Cornaby L, Dandona L, Dicker DJ, Erskine HE, Ferrari AJ, Fitzmaurice C, Foreman K, Forouzanfar MH, Fullman N, Gething PW, Goldberg EM, Graetz N, Haagsma JA, Johnson C, Kemmer L, Khalil IA, Kinfu Y, Kutz MJ, Kyu HH, Leung J, Liang XF, Lim SS, Lim SS, Lozano R, Mensah GA, Mikesell J, Mokdad AH, Mooney MD, Naghavi M, Nguyen G, Nsoesie E, Pigott DM, Pinho C, Rankin Z, Reinig N, Salomon JA, Sandar L, Smith A, Sorensen RJD, Stanaway J, Steiner C, Teeple S, Thomas BA, Troeger C, VanderZanden A, Wagner JA, Wanga V, Whiteford HA, Zhou M, Zoeckler L, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abraham B, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NME, Achoki T, Ackerman IN, Adebiyi AO, Adedeji IA, Adsuar JC, Afanvi KA, Afshin A, Agardh EE, Agarwal A, Kumar S, Ahmed MB, Kiadaliri AA, Ahmadieh H, Akseer N, Al-Aly Z, Alam K, Alam NKM, Aldhahri SF, Alegretti MA, Aleman AV, Alemu ZA, Alexander LT, Raghib A, Alkerwi A, Alla F, Allebeck P, Alsharif U, Altirkawi KA, Martin EA, Alvis-Guzman N, Amare AT, Amberbir A, Amegah AK, Amini H, Ammar W, Amrock SM, Anderson GM, Anderson BO, Antonio CAT, Anwari P, Arnlov J, Arsenijevic VSA, Artaman A, Asayesh H, Asghar RJ, Avokpaho EFGA, Awasthi A, Quintanilla BPA, Azzopardi P, Bacha U, Badawi A, Balakrishnan K, Banerjee A, Barac A, Barker-Collo SL, Barnighausen T, Barregard L, Barrero LH, Basu S, Bayou TA, Beardsley J, Bedi N, Beghi E, Bell B, Bell ML, Benjet C, Bennett DA, Bensenor IM, Berhane A, Bernabe E, Betsu BD, Beyene AS, Bhala N, Bhansali A, Bhatt S, Biadgilign S, Bienhofff K, Bikbov B, Bin Abdulhak AA, Bisanzio D, Bjertness E, Blore JD, Borschmann R, Boufous S, Bourne RRA, Brainin M, Brazinova A, Breitborde NJK, Brugha TS, Buchbinder R, Buckle GC, Butt ZA, Calabria B, Campos-Nonato IR, Campuzano JC, Carabin H, Carapetis JR, Cardenas R, Carrero JJ, Castaneda-Orjuela CA, Rivas JC, Catala-Lopez F, Cavalleri F, Chang JC, Chiang PPC, Chibalabala M, Chibueze CE, Chisumpa VH, Choi JYJ, Choudhury L, Christensen H, Ciobanu LG, Colistro V, Colomar M, Colquhoun SM, Cortinovis M, Crump JA, Damasceno A, Dandona R, Dargan PI, Das Neves J, Davey G, Davis AC, De Leo D, Degenhardt L, Del Gobbo LC, Derrett S, Des Jarlais DC, Deveber GA, Dharmaratne SD, Dhillon PK, Ding EL, Doyle KE, Driscoll TR, Duan L, Dubey M, Duncan BB, Ebrahimi H, Ellenbogen RG, Elyazar I, Endries AY, Ermakov SP, Eshrati B, Esteghamati A, Estep K, Fahimi S, Farid TA, Farinha CSES, Faro A, Farvid MS, Farzadfar F, Feigin VL, Fereshtehnejad SM, Fernandes JG, Fernandes JC, Fischer F, Fitchett JRA, Foigt N, Fowkes FGR, Franklin RC, Friedman J, Frostad J, Furst T, Futran ND, Gabbe B, Gankpe FG, Garcia-Basteiro AL, Gebrehiwot TT, Gebremedhin AT, Geleijnse JM, Gibney KB, Gillum RF, Ginawi IAM, Giref AZ, Giroud M, Gishu MD, Godwin WW, Gomez-Dantes H, Gona P, Goodridge A, Gopalani SV, Gotay CC, Goto A, Gouda HN, Guo Y, Gupta R, Gupta R, Gupta V, Gutierrez RA, Hafezi-Nejad N, Haile D, Hailu AD, Hailu GB, Halasa YA, Ribhi R, Hamadeh RR, Hamidi S, Hammami M, Handal AJ, Hankey GJ, Harb HL, Harikrishnan S, Haro JM, Hassanvand MS, Hassen TA, Havmoeller R, Hay RJ, Hedayati MT, Heredia-Pi IB, Heydarpour P, Hoek HW, Hoffman DJ, Horino M, Horita N, Hosgood HD, Hoy DG, Hsairi M, Huang H, Huang JJ, Iburg KM, Idrisov BT, Innos K, Inoue M, Jacobsen KH, Jauregui A, Jayatilleke AU, Jeemon P, Jha V, Jiang GH, Jiang Y, Jibat T, Jimenez-Corona A, Jin Y, Jonas JB, Kabir Z, Kajungu DK, Kalkonde Y, Kamal R, Kan HD, Kandel A, Karch A, Karema CK, Karimkhani C, Kasaeian A, Katibeh M, Kaul A, Kawakami N, Kazi DS, Keiyoro PN, Kemp AH, Kengne AP, Keren A, Kesavachandran CN, Khader YS, Khan AR, Khan EA, Khang YH, Khoja TAM, Khubchandani J, Kieling C, Kim CI, Kim D, Kim YJ, Kissoon N, Kivipelto M, Knibbs LD, Knudsen AK, Kokubo Y, Kolte D, Kopec JA, Koul PA, Koyanagi A, Defo BK, Kuchenbecker RS, Bicer BK, Kuipers EJ, Kumar GA, Kwan GF, Lalloo R, Lallukka T, Larsson A, Latif AA, Lavados PM, Lawrynowicz AEB, Leasher JL, Leigh J, Leung R, Li YC, Li YM, Lipshultz SE, Liu PY, Liu Y, Lloyd BK, Logroscino G, Looker KJ, Lotufo PA, Lucas RM, Lunevicius R, Lyons RA, El Razek HMA, Mandavi M, Majdan M, Majeed A, Malekzadeh R, Malta DC, Marcenes W, Martinez-Raga J, Masiye F, Mason-Jones AJ, Matzopoulos R, Mayosi BM, McGrath JJ, Mckee M, Meaney PA, Mehari A, Melaku YA, Memiah P, Memish ZA, Mendoza W, Meretoja A, Meretoja TJ, Mesfin YM, Mhimbira FA, Miller TR, Mills EJ, Mirarefin M, Mirrakhimov EM, Mitchell PB, Mock CN, Mohammad KA, Mohammadi A, Mohammed S, Monasta L, Hernandez JCM, Montico M, Moradi-Lakeh M, Mori R, Mueller UO, Mumford JE, Murdoch ME, Murthy GVS, Nachega JB, Naheed A, Naldi L, Nangia V, Newton JN, Ng M, Ngalesoni FN, Le Nguyen Q, Nisar MI, Pete PMN, Nolla JM, Norheim OF, Norman RE, Norrving B, Obermeyer CM, Ogbo FA, Oh IH, Oladimeji O, Olivares PR, Olusanya BO, Olusanya JO, Oren E, Ortiz A, Ota E, Oyekale AS, Pa M, Park EK, Parsaeian M, Patten SB, Patton GC, Pedro JM, Pereira DM, Perico N, Pesudovs K, Petzold M, Phillips MR, Piel FB, Pillay JD, Pishgar F, Plass D, Polinder S, Popova S, Poulton RG, Pourmalek F, Prasad NM, Qorbani M, Rabiee RHS, Radfar A, Rafay A, Rahimi K, Rahimi-Movaghar V, Rahman M, Rahman MHU, Rahman SU, Rai D, Rai RK, Rajsic S, Raju M, Ram U, Ranganathan K, Refaat AH, Reitsma MB, Remuzzi G, Resnikoff S, Reynolds A, Ribeiro AL, Ricci S, Roba HS, Rojas-Rueda D, Ronfani L, Roshandel G, Roth GA, Roy A, Sackey BB, Sagar R, Sanabria JR, Sanchez-Nino MD, Santos IS, Santos JV, Sarmiento-Suarez R, Sartorius B, Satpathy M, Savic M, Sawhney M, Schmidt MI, Schneider IJC, Schutte AE, Schwebel DC, Seedat S, Sepanlou SG, Servan-Mori EE, Shahraz S, Shaikh MA, Sharma R, She J, Sheikhbahaei S, Shen J, Sheth KN, Shibuya K, Shigematsu M, Shin MJ, Shin R, Sigfusdottir ID, Silva DAS, Silverberg JI, Simard EP, Singh A, Singh JA, Singh PK, Skirbekk V, Skogen JC, Soljak M, Soreide K, Sorensen RJD, Sreeramareddy CT, Stathopoulou V, Steel N, Stein DJ, Stein MB, Steiner TJ, Stovner LJ, Stranges S, Stroumpoulis K, Sunguya BF, Sur PJ, Swaminathan S, Sykes BL, Szoeke CEI, Tabares-Seisdedos R, Landon N, Tanne D, Tavakkoli M, Taye B, Taylor HR, Ao BJT, Tegegne TK, Tekle DY, Terkawi AS, Tessema GA, Thakur JS, Thomson AJ, Thorne-Lyman AL, Thrift AG, Thurston GD, Tobe-Gai R, Tonelli M, Topor-Madry R, Topouzis F, Tran BX, Dimbuene ZT, Tsilimbaris M, Tura AK, Tuzcu EM, Tyrovolas S, Ukwaja KN, Undurraga EA, Uneke CJ, Uthman OA, van Gool CH, van Os J, Vasankari T, Vasconcelos AMN, Venketasubramanian N, Violante FS, Vlassov VV, Vollset SE, Wagner GR, Wallin MT, Wang LH, Weichenthal S, Weiderpass E, Weintraub RG, Werdecker A, WestermaM R, Wijeratne T, Wilkinson JD, Williams HC, Wiysonge CS, Woldeyohannes SM, Wolfe CDA, Won S, Xu G, Yadav AK, Yakob B, Yan LL, Yan Y, Yaseri M, Ye P, Yip P, Yonemoto N, Yoon SJ, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zeeb H, Zodpey S, Zonies D, Zuhlke LJ, Zeeb H, Zodpey S, Zonies D, Zuhlke LJ, Vos T, Lopez AD, Murray CJL, Hale GD. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1603–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kraychete DC, Sakata RK, Issy AM, Bacellar O, Santos-Jesus R, Carvalho EM. Serum cytokine levels in patients with chronic low back pain due to herniated disc: analytical cross-sectional study. Sao Paulo Med J 2010;128:259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kurita GP, de Mattos Pimenta CA, Braga PE, Frich L, Jorgensen MM, Nielsen PR, Hojsted J, Sjogren P. Cognitive function in patients with chronic pain treated with opioids: characteristics and associated factors. Acta Anaesthesiol Scand 2012;56:1257–66. [DOI] [PubMed] [Google Scholar]
- [29].Kurita GP, Malver LP, Andresen T, Polianskis R, Drewes AM, Christrup L, Hojsted J, Sjogren P. Does mutual compensation of the cognitive effects induced by pain and opioids exist? an experimental study. Psychopharmacol (Berl) 2015;232:1373–81. [DOI] [PubMed] [Google Scholar]
- [30].Lail S, Sequeira K, Lieu J, Dhalla IA. Prescription of opioids for opioid-naive medical inpatients. Can J Hosp Pharm 2014;67:337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Landrø NI, Fors EA, Våpenstad LL, Holthe O, Stiles TC, Borchgrevink PC. The extent of neurocognitive dysfunction in a multidisciplinary pain centre population. Is there a relation between reported and tested neuropsychological functioning? PAIN 2013;154:972–7. [DOI] [PubMed] [Google Scholar]
- [32].Linnman C, Becerra L, Borsook D. Inflaming the brain: CRPS a model disease to understand neuroimmune interactions in chronic pain. J Neuroimmune Pharmacol 2013;8:547–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther 1995;33:335–43. [DOI] [PubMed] [Google Scholar]
- [34].Man DW, Fleming J, Hohaus L, Shum D. Development of the Brief Assessment of Prospective Memory (BAPM) for use with traumatic brain injury populations. Neuropsychol Rehabil 2011;21:884–98. [DOI] [PubMed] [Google Scholar]
- [35].Morasco BJ, Yarborough BJ, Smith NX, Dobscha SK, Deyo RA, Perrin NA, Green CA. Higher prescription opioid dose is associated with worse patient-reported pain outcomes and more health care utilization. J Pain 2017;18:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nicholas MK. Self-efficacy and chronic pain. Annual Conference of the British Psychological Society, St Andrews, Scotland, 1989.
- [37].Oosterman J, Derksen L, Van Wijck A, Kessels R, Veldhuijzen D. Executive and attentional functions in chronic pain: does performance decrease with increasing task load? Pain Res Manag 2012;3:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Oosterman JM, Derksen LC, van Wijck AJ, Veldhuijzen DS, Kessels RP. Memory functions in chronic pain: examining contributions of attention and age to test performance. Clin J Pain 2011;27:70–5. [DOI] [PubMed] [Google Scholar]
- [39].Pachana NA, Marcopulos B, Yoash-Gantz R, Thompson LW. California Older Adult Stroop Test (COAST). Gerontologist 1995;35:219. [Google Scholar]
- [40].Penninx BWJH, Abbas H, Ambrosius W, Nicklas BJ, Davis C, Messier SP, Pahor M. Inflammatory markers and physical function among older adults with knee osteoarthritis. J Rheumatol 2004;31:2027–31. [PubMed] [Google Scholar]
- [41].Pernambuco AP, Schetino LP, Alvim CC, Murad CM, Viana RS, Carvalho LS, Reis DA. Increased levels of IL-17A in patients with fibromyalgia. Clin Exp Rheumatol 2013;31:S60–3. [PubMed] [Google Scholar]
- [42].Rodriguez-Pinto I, Agmon-Levin N, Howard A, Shoenfeld Y. Fibromyalgia and cytokines. Immunol Lett 2014;161:200–3. [DOI] [PubMed] [Google Scholar]
- [43].Rosser BA, McCracken LM, Velleman SC, Boichat C, Eccleston C. Concerns about medication and medication adherence in patients with chronic pain recruited from general practice. PAIN 2011;152:1201–05. [DOI] [PubMed] [Google Scholar]
- [44].Roth RS, Geisser ME, Theisen-Goodvich M, Dixon PJ. Cognitive complaints are associated with depression, fatigue, female sex, and pain catastrophizing in patients with chronic pain. Arch Phys Med Rehabil 2005;86:1147–54. [DOI] [PubMed] [Google Scholar]
- [45].Royle J, Lincoln NB. The Everyday Memory Questionnaire-revised: development of a 13-item scale. Disabil Rehabil 2008;30:114–21. [DOI] [PubMed] [Google Scholar]
- [46].Schiltenwolf M, Akbar M, Hug A, Pfuller U, Gantz S, Neubauer E, Flor H, Wang H. Evidence of specific cognitive deficits in patients with chronic low back pain under long-term substitution treatment of opioids. Pain Physician 2014;17:9–20. [PubMed] [Google Scholar]
- [47].Schmidt-Wilcke T, Leinisch E, Ganssbauer S, Draganski B, Bogdahn U, Altmeppen J, May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. PAIN 2006;125:89–97. [DOI] [PubMed] [Google Scholar]
- [48].Sjogren P, Thomsen AB, Olsen AK. Impaired neuropsychological performance in chronic nonmalignant pain patients receiving long-term oral opioid therapy. J Pain Symptom Manage 2000;19:100–8. [DOI] [PubMed] [Google Scholar]
- [49].Smith K, Mattick RP, Bruno R, Nielsen S, Cohen M, Campbell G, Larance B, Farrell M, Degenhardt L. Factors associated with the development of depression in chronic non-cancer pain patients following the onset of opioid treatment for pain. J Affect Disord 2015;184:72–80. [DOI] [PubMed] [Google Scholar]
- [50].Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess 1995;7:524–32. [Google Scholar]
- [51].Tassain V, Attal N, Fletcher D, Brasseur L, Degieux P, Chauvin M, Bouhassira D. Long term effects of oral sustained release morphine on neuropsychological performance in patients with chronic non-cancer pain. PAIN 2003;104:389–400. [DOI] [PubMed] [Google Scholar]
- [52].van de Beek WJT, Remarque EJ, Westemdorp RGJ, van Hilten JJ. Innate cytokine profile in patients with complex regional pain syndrome is normal. PAIN 2001;91:259–61. [DOI] [PubMed] [Google Scholar]
- [53].Wang H, Schiltenwolf M, Buchner M. The role of TNF-a in patients with chronic low back pain—a prospective comparative longitudinal study. Clin J Pain 2008;24:273–8. [DOI] [PubMed] [Google Scholar]
- [54].Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med 2006;7:60–70. [DOI] [PubMed] [Google Scholar]
- [55].Zin C, Nissen LM, O'Callaghan JP, Moore BJ, Smith MS. Preliminary study of the plasma and cerebrospinal fluid concentrations of IL-6 and IL-10 in patients with chronic pain recieveing intrathecal opioid infusions by chronically implanted pump for pain management. Pain Med 2010;11:550–61. [DOI] [PubMed] [Google Scholar]