Abstract
Background
Efavirenz has been a mainstay of antiretroviral therapy (ART) for over 15 years in the US. Its association with neuropsychiatric side effects may influence clinical prescribing and management.
Methods
We included HIV-infected adults enrolled in care at 7 sites across the US, who initiated combination ART between 1999 and 2015. We examined the proportion initiating and continuing on efavirenz, overall and by mental health status. Log binomial and Cox models were used to estimate associations between mental health, clinical, and sociodemographic characteristics and initiating or switching from efavirenz as first line ART.
Results
Of the 8,230 participants included, 3,710 (45%) initiated efavirenz. In multivariable analyses, prior mono or dual ART, ART initiation after 2006, being female, intravenous drug use, antidepressant prescription, previous mental health diagnosis and baseline CD4 >350 were inversely associated with initiating efavirenz. Participants initiating efavirenz had a faster time to a regimen switch, compared to those initiating an efavirenz-free regimen (p-value <0.01). Among efavirenz initiators, starting efavirenz in more recent time-periods and a previous mental health diagnosis were associated with faster time to switching from efavirenz. Despite this, 40–50% of participants with a previous mental health diagnosis initiated and continued on efavirenz for much of the follow-up period.
Conclusions
Multiple clinical factors, including mental health diagnoses, appeared to influence efavirenz use. While mental health diagnosis status and more recent treatment starts were associated with shorter duration of efavirenz therapy, a previous mental health diagnosis did not preclude efavirenz initiation or continuation in many participants.
Introduction
For over 15 years, efavirenz has been a central component of antiretroviral therapy (ART) in the United States (US)(1, 2) and continues to be recommended by the World Health Organization (WHO) globally.(3, 4) Until 2014, a 600 mg dose of efavirenz (hereafter ‘efavirenz’) was a preferred first line antiretroviral (ARV) agent in the US due to its durable viral suppression, generic formulation, and availability as a fixed-dose combination pill.(1) Efavirenz continues to be widely taken by HIV-infected adults in the US, and remains a preferred first line agent for millions of HIV-infected persons in low and middle-income countries.(5–9)
Despite efavirenz’s desirable treatment qualities, it has been associated with important neuropsychiatric side effects including mood changes, sleep disturbances, and depression.(5, 10, 11) Evidence about severe side effects, such as suicidal thoughts or suicide, remains conflicting (11–16). As a result, clinicians have faced challenging questions about whether to prescribe efavirenz to patients with mental health diagnoses or to switch patients who develop mental health diagnoses from efavirenz(10) For patients already on efavirenz, clinicians face difficult choices about whether to continue efavirenz or to switch patients to newer agents, regardless of clinically evident neuropsychiatric side effects. Given these concerns, a clear understanding of factors associated with initiating and switching from efavirenz in needed.
The goal of our analysis was to examine trends in efavirenz initiation in a large cohort of HIV-infected adults, during the time-period when efavirenz was recommended and widely used for first-line combination ART in the US. We examine temporal trends in efavirenz use, as well as whether clinical, sociodemographic, and mental health factors influenced efavirenz prescription or continuation during an evolving ART treatment era from 1999–2015.
Methods
Data for the present analysis come from the Centers for AIDS Research (CFAR) Network of Integrated Clinical Systems (CNICS) cohort. The CNICS cohort includes over 32,000 HIV-infected adults in routine HIV clinical care at 8 academic medical centers across the US.(17) Many patients receiving care at the sites participating in CNICs access ART at no charge or low cost through private or federal insurance programs, or through state administered federal assistance programs, such as the Ryan White Program. Since 1997, CNICS collects detailed information on demographic characteristics, medication prescriptions, HIV/AIDS clinical events, ART prescription, co-morbid conditions, CD4 count, viral load and vital status on patients who consent to participate. In 2005 participants in CNICS also began completing self-administered questionnaires, called Patient-Reported Outcomes (PROs), on touch-screen tablets or personal computers every 4–6 months as part of routine clinical visits. Patients receiving care at each CNICS site provide written informed consent to participate in CNICS and participation is near universal.(18) Ethical approval for the use of routinely collected clinical data was provided by the institutional review board at each CNICS site.
Study Population
Efavirenz was recommended as part of first-line therapy by the Department of Health and Human Services in the US from 1999 to 2014.(2) To cover this period and the year that guidelines changed in the US, we included participants in CNICS who initiated combination ART between 1999 and 2015 (n=9,796) at 7 of 8 sites where PROs were collected.(1) A combination ART (hereafter ART) regimen was defined as treatment with at least 3 antiretroviral drugs, including at least 1 non-nucleoside reverse transcriptase inhibitor, protease inhibitor, or integrase inhibitor for > 21 days.(19) Participants with incomplete data on ART use (n=517), a missing (n=623) or undetectable viral load (defined as < 75 copies/mL) at ART initiation (n=426, possibly indicating possible previous ART treatment) were excluded for a final study population of 8,230. We followed participants from ART initiation until the first of the following dates: a switch from their initial regimen (defined as switching to an efavirenz-containing or efavirenz-free regimen, depending on initial ART regimen), discontinuing ART entirely, death, loss to care (>12 months with no attended HIV appointments), or administrative censoring (October 2014-September 2015, depending on site).
Among people who initiated efavirenz, we identified a sub-population who had a PRO at least 1 week after and within 9 months of ART initiation and who were still on efavirenz at the time of the PRO measurement (n=523). In this sub-population, we evaluated associations between baseline and time-updated characteristics (measured on the PRO) and switching from an initial efavirenz-containing regimen.
Measures
CNICS systematically collects information on demographics, prior mono or dual ARV use, antidepressant medication prescription, HIV acquisition risk group (male-to-male sexual contact [MSM], intravenous drug use [IDU], heterosexual contact, or other), previous chart-documented mental health diagnoses or chart-documented medical diagnoses (such as hypertension or diabetes), CD4 count and viral load lab values. We defined a pervious mental health diagnosis as any clinician-documented diagnosis of depression, bipolar disorder, post-traumatic stress disorder or psychosis in a participant’s medical chart, since many of these conditions are associated with efavirenz use and may influence efavirenz prescription.(20, 21)
Among the sub-population of who participants who initiated efavirenz after PRO collection began, CNICS PRO’s also capture validated measures of depressive symptoms (Patient Health Questionnaire-9, defined as a score ≥10),(22) panic disorder (Patient Health Questionnaire-5, defined as no panic symptoms, some panic symptoms or panic disorder),(23) high risk alcohol use (Alcohol Use Disorders Identification Test (AUDIT-C), defined as an AUDIT-C score ≥ 4 for males and ≥ 3 for females),(24), current, past or no illicit drug use, including cocaine/crack, heroin/opiates, crystal/amphetamine use, but excluding marijuana (The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST), defined as past, current or no drug use)(25, 26) and ART adherence (AIDS Clinical Trials Unit-4 Visual Analog Scale, defined as no missed doses in the past week (27, 28)).
Statistical Analysis
The goal of the present analysis was to describe trends in initial efavirenz prescription and continuation over time in the US and to identify associations between sociodemographic, clinical, and mental health characteristic and initiating or switching from efavirenz as first line ART. To investigate trends in efavirenz prescription, we examined the proportion of new ART users initiating efavirenz-containing ART, as well as the net proportion (defined as the cumulative proportion of persons who initiated efavirenz after subtracting out censored participants) of individuals on efavirenz during the follow-up period. Since a provider’s decision to prescribe efavirenz may be influenced by a patient’s mental health status, we also stratified the proportion of participants initiating efavirenz and the net proportion of participants on efavirenz each year by previous mental health diagnosis status at ART initiation.
Among all participants, we used log binomial models to estimate prevalence ratios (PR) for associations between baseline (collected at ART initiation) demographic, mental health, and clinical factors and initiating efavirenz-containing ART. We used the compliment of unadjusted Kaplan-Meier survival curves to estimate time from ART initiation to initial regimen switch among all participants, stratified by initial ART regimen (efavirenz-containing versus efavirenz-free).
Among participants initiating efavirenz-containing ART, we used Cox proportional hazards models to examine associations between baseline characteristics and time to switching from an efavirenz-containing regimen. In a secondary analysis including participants who initiated efavirenz after PRO collection began, we included both baseline and time-updated predictors for switching from an efavirenz-containing regimen. The values for all time-updated characteristics were carried forward until the next PRO measurement. Median depression score values over time, measured using the PHQ-9 and modeled using restricted splines, were compared between participants who continued on efavirenz and those who switched from efavirenz. Patient characteristics between participants who initiated efavirenz in the primary study population (n=3,170) and the subset who initiated efavirenz after PRO collection began (n=523) were compared using chi-squared tests.
Due to model convergence issues, the “other” categories for HIV acquisition risk group and race/ethnicity was collapsed with the largest category for each variable in statistical analyses. Given the strong time-trends in efavirenz prescription, year of ART initiation was included as a covariate in all analyses. For all analyses, characteristics with a Wald p-value of ≤ 0.10 in bivariable analyses were included in the multivariable model. Statistical analyses were conducted using Stata version 13 (StataCorp, College Station, Texas).
Results
In total, 8,230 CNICS participants with a detectable viral load initiated ART between 1999 and 2015. Of those, 3,710 (45%) initiated an efavirenz-containing regimen. Of the 3,710 who started EFV-containing ART, 685 (18%) switched from their initial efavirenz regimen and 3,025 (82%) continued on efavirenz until a censoring event or the end of follow-up. Over the follow-up period, 31% overall were lost to care, 4% died, 12% switched from their initial ART regimen, 22% discontinued ART entirely, and 31% were administratively censored. Participants who were lost to care were similar to those who were not, with the exceptions of being more likely to have initiated ART later (during 2012–2015) and less likely to have contracted HIV through male-to-male sexual contact (Supplemental Table 1). Participants were followed for a median of 24 months and contributed a total of 293,168 person-months of follow-up.
Our study population was predominately male (83%), white, non-Hispanic (44%) and self-reported contracting HIV through male-to-male sexual contact (57%). Nearly 16% of participants had an antidepressant prescription at ART initiation and 25% had a prior clinician-documented mental health diagnosis. The majority of the study population (68%) had a CD4 count ≤ 350 cells/mm3 at ART initiation (Table 1).
Table 1.
Clinical, demographic, and mental health characteristics at ART initiation of 8,230 new ART users in CNICS: 1999–2015
| Baseline Characteristic | N(%) |
|---|---|
| Initial ART regimen | |
| EFV-free ART | 4,520 (54.9) |
| EFV-containing ART | 3,710 (45.1) |
| Prior mono or dual ARV use | |
| No | 7,837 (95.2) |
| Yes | 393 (4.8) |
| Year initiated cART | |
| 1999–2006 | 3,310 (40.2) |
| 2007–2011 | 2,532 (30.8) |
| 2012–2015 | 2,388 (29.0) |
| Age, median (IQR) | 38 (30, 45) |
| Gender | |
| Male | 6,791 (82.5) |
| Female | 1,439 (17.5) |
| Race/ethnicity | |
| White, non-Hispanic | 3,599 (44.2) |
| Black, non-Hispanic | 2,961 (36.3) |
| Hispanic | 1,148 (14.1) |
| Other | 452 (5.5) |
| HIV risk group | |
| MSM | 4,616 (57.0) |
| IDU | 1,295 (16.0) |
| Heterosexual | 2,066 (25.5) |
| Other | 129 (1.6) |
| Antidepressant use | |
| Not on antidepressants | 6,958 (84.5) |
| On antidepressants | 1,272 (15.5) |
| Previous mental health diagnosis2 | |
| No | 6,146 (74.7) |
| Yes | 2,084 (25.3) |
| Previous medical diagnosis3 | |
| No | 7,214 (87.7) |
| Yes | 1,016 (12.4) |
| CD4 count, cells/mm3 | |
| ≤ 350 | 5,532 (68.0) |
| > 350 | 2,599 (32.0) |
Missing data: race/ethnicity n=70 (0.9%), HIV risk group n=124 (1.5%), CD4 count n=99 (1.2%).
Defined as switching to an efavirenz-containing or efavirenz-free regimen, depending on initial ART regimen.
Defined as a clinician-documented diagnosis of depression, bipolar disorder, post-traumatic stress disorder or psychosis in a participant’s medical chart.
Defined as clinician-documented diagnosis of hypertension or diabetes.
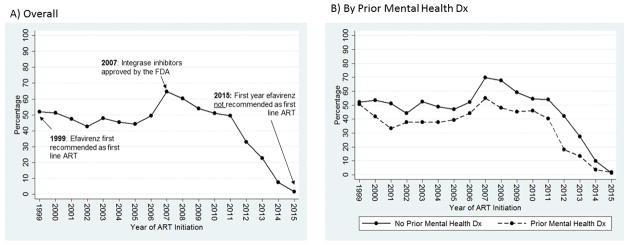
Efavirenz prescription for first-line ART was common during the study period. Between 1999 and 2006, 40% – 50% of participants initiating ART started on an efavirenz-containing regimen (Figure 1a). Efavirenz prescription peaked in 2007 at 65% of all new ART users and declined rapidly thereafter. By 2014, the last year the Department of Health and Human Services recommended efavirenz as a part of first-line therapy, just 7% of those initiating ART in our study population started on efavirenz.
Figure 1.
Proportion of 8,230 HIV-infected Adults Initiating Efavirenz-containing ART as First Line Therapy from 1999 to 2015 in CNICS: A) Overall and B) by Prior Mental Health Diagnosis (Dx).
Throughout the study period, persons with a previous mental health diagnosis at ART initiation were approximately 10–15% less likely to initiate efavirenz-containing ART, compared to other participants (Figure 1b and 1c). However, the net proportion of participants who continued on efavirenz-containing ART remained high over the follow-up period, regardless of previous mental health diagnoses status (Supplemental Figure 1). Among participants with no previous mental health diagnoses, roughly 50% or more of participants were on efavirenz-containing ART through 2014, when the overall net proportion of participants on efavirenz began declining due to the fact that some patients reached the end of their follow-up time (e.g. they were administratively censored). By the end of follow-up in 2015, approximately 20% of participants with no previous mental health diagnoses remained on efavirenz-containing ART (Supplemental Figure 1a). Among those with a previous mental health diagnosis, the proportion of participants on an efavirenz-containing regimen was lower, but remained between 40% and 50% for much of the follow-up period. In 2015, approximately 16% of participants with previous mental health diagnoses remained on efavirenz (Supplemental Figure 1b).
In multivariable analyses, baseline factors inversely associated with initiating efavirenz as first line ART included: prior mono or dual ARV (PR 0.78, 95% CI 0.68, 0.89), initiating ART from 2007–2015 (PR 0.95, 95% CI 0.90, 0.99), contracting HIV through IDU (PR 0.85, 95% CI 0.79, 0.92), being on antidepressants (PR 0.91, 95% CI 0.84, 0.98), having a previous mental health diagnosis (PR 0.80, 95% CI 0.75, 0.86), being female (PR 0.80, 95% CI 0.74, 0.87), and having a CD4 count >350 (PR 0.94, 95% CI 0.89, 0.99) (Table 2). The effect of gender on efavirenz initiation was primarily driven by the fact that women of childbearing age (ages 15–44) were less likely to initiate efavirenz (PR 0.71, 95% CI 0.64, 0.78), compared to older women (ages ≥ 45; PR 0.92, 95% CI 0.81, 1.03). Similarly, the association between CD4 count >350 and efavirenz initiation was most prominent for participants initiating ART between 2007 and 2015 (PR 0.89, 95% CI 0.97, 1.17), compared to between 1995 and 2006 (PR 1.06, 95% CI 0.97, 1.17).
Table 2.
Baseline correlates of initiating EFV-containing ART, among 8,230 new ART users in CNICS.
| Baseline Characteristics | Bivariable | Multivariable1 | ||
|---|---|---|---|---|
| PR (95% CI) | p-value | PR (95% CI) | p-value | |
|
|
|
|||
| Prior mono or dual ARV use | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.81 (0.71, 0.93) | <0.01 | 0.78 (0.68, 0.89) | <0.01 |
| Year initiated ART | ||||
| 1995–2006 | 1.00 | 1.00 | ||
| 2007–2015 | 0.91 (0.87, 0.96) | <0.01 | 0.95 (0.90, 0.99) | 0.03 |
| Age, per year | 1.00 (1.00, 1.00) | 0.95 | -- | -- |
| Gender | ||||
| Male | 1.00 | 1.00 | ||
| Female | 0.77 (0.72, 0.83) | <0.01 | 0.80 (0.74, 0.87) | <0.01 |
| Race/ethnicity | ||||
| White, non-Hispanic or other | 1.00 | -- | -- | |
| Black, non-Hispanic | 1.00 (0.94, 1.05) | 0.88 | -- | -- |
| Hispanic | 1.04 (0.97, 1.12) | 0.26 | -- | -- |
| HIV risk group | ||||
| MSM or Other | 1.00 | 1.00 | ||
| IDU | 0.80 (0.75, 0.87) | <0.01 | 0.85 (0.79, 0.92) | <0.01 |
| Heterosexual | 0.88 (0.83, 0.93) | <0.01 | 0.96 (0.89, 1.03) | 0.24 |
| Antidepressant use | ||||
| No on antidepressants | 1.00 | 1.00 | ||
| On antidepressants | 0.82 (0.76, 0.88) | <0.01 | 0.91 (0.84, 0.98) | 0.02 |
| Previous mental health diagnosis | ||||
| No | 1.00 | 1.00 | ||
| Yes | 0.76 (0.71, 0.81) | <0.01 | 0.80 (0.75, 0.86) | <0.01 |
| Previous medical diagnosis | ||||
| No | 1.00 | -- | -- | |
| Yes | 0.94 (0.87, 1.02) | 0.13 | -- | -- |
| CD4 count, cells/mm3 | ||||
| ≤ 350 | 1.00 | 1.00 | ||
| >350 | 0.92 (0.87, 0.97) | <0.01 | 0.94 (0.89, 0.99) | 0.03 |
|
|
|
|||
Model adjusted for all variables in the table and additionally adjusted for site.
Among all study participants (n=8,230), initiating efavirenz was associated with a faster time to switching from one’s initial ART regimen (log-rank p-value <0.01; Figure 2). For example, by 96 months after ART initiation, persons who initiated an efavirenz-containing regimen had a 34% (95% CI 31%, 37%) probability of switching, compared to 17% (95% CI 14% to 20%) among those who initiated an efavirenz-free regimen.
Figure 2.

Time from ART initiation to initial regimen switch among 8,230 new ART users, stratified by initial ART regimen. ART regimen switch is defined as switching to an efavirenz-containing or efavirenz-free regimen, depending on initial ART regimen (log-rang p-value <0.01.).
Among all participants who initiated efavirenz (n=3170), baseline factors associated with switching from efavirenz in multivariable analyses included initiating efavirenz from 2007–2011 (HR 1.70, 95% CI 1.40, 2.05) or 2012–2015 (HR 3.68, 95% CI 2.93, 4.62), and having a previous mental health diagnosis (HR 1.33, 95% CI 1.11, 1.59; Table 3).
Table 3.
Baseline predictors of switching from efavirenz-containing ART, among 3,710 persons new efavirenz users in CNICS.
| Baseline Characteristics | Bivariable | Multivariable1 | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Prior mono or dual ARV use | ||||
| No | 1.00 | -- | -- | |
| Yes | 1.07 (0.73, 1.61) | 0.73 | -- | -- |
| Year initiated ART | ||||
| 1999–2006 | 1.00 | 1.00 | ||
| 2007–2011 | 1.64 (1.36, 1.98) | <0.01 | 1.70 (1.40, 2.05) | <0.01 |
| 2012–2015 | 3.24 (2.59, 4.05) | <0.01 | 3.68 (2.93, 4.62) | <0.01 |
| Age, per year | 0.99 (0.99, 1.00) | 0.14 | -- | -- |
| Gender | ||||
| Male | 1.00 | -- | -- | |
| Female | 1.16 (0.94, 1.44) | 0.16 | -- | -- |
| Race/ethnicity | ||||
| White, non-Hispanic or Other | 1.00 | 1.00 | ||
| Black, non-Hispanic | 1.15 (0.98, 1.36) | 0.09 | 1.08 (0.90, 1.29) | 0.40 |
| Hispanic | 0.97 (0.78, 1.22) | 0.82 | 1.00 (0.79, 1.26) | 0.99 |
| HIV risk group | ||||
| MSM or Other | 1.00 | -- | -- | |
| IDU | 1.03 (0.80, 1.31) | 0.83 | -- | -- |
| Heterosexual | 1.03 (0.87, 1.23) | 0.71 | -- | -- |
| Antidepressant use | ||||
| No on antidepressants | 1.00 | -- | -- | |
| On antidepressants | 1.09 (0.88, 1.34) | 0.45 | -- | -- |
| Previous mental health diagnosis | ||||
| No | 1.00 | 1.00 | ||
| Yes | 1.33 (1.12, 1.59) | <0.01 | 1.33 (1.11, 1.59) | <0.01 |
| Previous medical diagnosis | ||||
| No | 1.00 | -- | -- | |
| Yes | 1.08 (0.86, 1.34) | 0.51 | -- | -- |
| CD4 count, cells/mm3 | ||||
| ≤ 350 | 1.00 | -- | -- | |
| >350 | 1.07 (0.91, 1.27) | 0.41 | -- | -- |
Model adjusted for all variables in the table and site.
Of the 523 persons who initiated efavirenz after PRO collection began, 140 (27%) switched from their initial efavirenz regimen. Over one fifth (22%) of switches happened within 7 days of a PRO measurement, providing a reasonable measure of the psychiatric symptoms present at the time of the switch, and 25% within 30 days. In multivariable analyses, time-updated depressive symptoms (HR 1.62, 95% CI 1.01, 2.57) and ART non-adherence (HR 1.62, 95% CI 1.01, 2.61) were associated with faster time to switching from an efavirenz-containing regimen (Supplemental Table 2). Median PHQ-9 scores for depressive severity were higher among participants who eventually switched from efavirenz, compared to those who continued on efavirenz during the study period, but remained below the level indicating depressive symptoms (Supplemental Figure 2). Participants who initiated efavirenz after PRO collection began, compared to efavirenz initiators in the entire study population, were more likely to switch from their initial regimen (27% versus 19%), but less likely to discontinue ART entirely (6% versus 20%, combined p-value <0.01), and were more likely to be male (92% versus 86%, p-value <0.01), MSM (75% versus 61%, p-value <0.01), and to have a CD4 count at ART initiation >350 cells/mm3 (42% versus 30%, p-value <0.01; Supplemental Table 3).
Discussion
Our analysis examines efavirenz use among a large cohort of HIV-infected adults during the 15 years it was recommended as first-line ART in the US. After its introduction in 1999, efavirenz use expanded rapidly in our cohort reaching its peak in 2007 when 60% of treatment naïve participants were initiated on efavirenz-containing ART. Between 1999 and 2015, patients with a previous mental health diagnosis were less likely to be prescribed efavirenz as initial therapy and more likely to switch from an initial efavirenz-containing regimen. Despite this, efavirenz initiation was relatively common among people with previous mental health diagnoses. Through 2011, over 30% of patients with a previous mental health diagnosis initiated efavirenz-containing ART each year and 40–50% of this population continued on the drug. Over the study period, participants initiating efavirenz were more likely to switch from their initial regimen, compared with participants initiating an efavirenz-free regimen.
Efavirenz has been associated with a range of adverse mental health outcomes, including mood changes, insomnia, depression and suicidal thoughts.(5, 10) Our results demonstrate that patients with mental health concerns were less likely to be prescribed efavirenz as initial ART. In multivariable analyses those with an antidepressant prescription or a previous mental health diagnosis at ART initiation, as well as women, IDUs, those previously on mono or dual ARVs, those with a CD4 count >350, and those starting ART after 2006 were all less likely to initiate efavirenz-containing ART. IDUs may have been less likely to be prescribed efavirenz due to concerns about adherence and efavirenz low genetic barrier to resistance. (29) HIV-infected women have been reported to have a higher prevalence of depression, compared to HIV-infected men (30), which may influence efavirenz prescription. However, concerns about teratogenicity with efavirenz use (31–33) also likely led to fewer women of childbearing age initiating efavirenz. Participants with a CD4 count >350 were less likely to initiate efavirenz after 2007, when integrase inhibitors became available, possibly reflecting a clinical preference for prescribing newer or alternative agents.(34, 35)
Among participants who initiated efavirenz, mental health status, ART initiation after 2006, depressive symptoms and poor ART adherence were associated with switching off the drug. By 2006, there was a growing body of evidence reporting associations between efavirenz and mental health side effects.(36, 37) In addition, integrase inhibitors, which have superior viral suppression and fewer side effects than efavirenz, became available in 2007 and were quickly adopted by clinicians as the preferred choice for first-line therapy.(38, 39) Concerns over efavirenz’s influence on mental health side effects, coupled with the availability of alternative agents, including some single tablet regimens, may have influenced patient and provider decision-making related to ART prescriptions. Indeed, our results demonstrate a shorter time to switching from efavirenz in those who initiated ART after 2006 and those who had a previous mental health diagnosis at ART initiation. Similarly, among those who initiated efavirenz in the PRO era, patients who reported depressive symptoms and who missed ART doses over time, possibly due to side effects, were more likely to switch from efavirenz. Over time, depressive symptom scores were higher among participants who switched from efavirenz, compared to those who continued on efavirenz throughout the study period, but remained below the threshold indicated depressive symptoms.
While mental health factors led to switching from efavirenz in some patients, our results also demonstrate that 40–50% of participants with a previous mental health diagnosis at ART initiation successfully remained on efavirenz for much of the follow-up period. These disparate results suggest that while some patients initiate efavirenz and develop mental health side effects necessitating a regimen change, other patients, including some with a history of mental health diagnoses, do well on the drug. Provider practices around when to switch a patient’s ART regimen also vary and may be strongly influenced by the availability of alternative ART agents.(34, 40) Indeed, our results suggest that the presence of psychiatric symptoms on PRO measures did not drive decisions about switching from efavirenz – just 22% of switches occurred within 7 days of a PRO measure and 25% occurred within 30 days.
Our analysis had several limitations. First, while our analysis makes use of one of the largest clinical cohorts of HIV-infected adults in the US, white males made up the majority of our cohort. We had limited data on patients who completed PRO measures (including time-updated measures of depressive symptoms, panic disorder, and antidepressant use) over time. Further, the subset of participants with PRO measures differed in several important ways from the larger cohort. Second, despite examining a range of clinical, sociodemographic, and mental health characteristics, we did not have data on the cost of individual drugs, drug side effects not related to mental health, or a patient’s provider, all of which may have influenced efavirenz prescription and switching patterns. Similarly, data on reasons for regimen switch, discontinuation, or provider attitudes towards efavirenz use are not available in our data.
Conclusions
In our cohort of HIV-infected adults initiating ART between 1999 and 2015, a variety of factors, including mental health status and antidepressant use, influenced the likelihood of being prescribed or switching from efavirenz as first line ART. Our results corroborate previous evidence showing that clinicians are often hesitant to prescribe efavirenz to persons with mental health diagnoses, and may switch participants more quickly with mental health diagnoses from efavirenz, due to its association with neuropsychiatric side effects.(5, 10) However, our results also demonstrate that a sizeable proportion of participants, including those with previous mental health diagnoses, initiated efavirenz and successfully continued on the drug during the study period. Taken together, these results suggest that for patients on efavirenz who are virally suppressed and monitored for ongoing neuropsychiatric side effects, the benefits of remaining on a stable regimen may outweigh possible risks of developing neuropsychiatric side effects or new toxicities associated with switching to a different regimen. For example, some newer agents, such as dolutegravir, have also been associated with neuro-psychiatric side-effects.(41–44) Additional data is needed on mental health indicators over time in patients currently on efavirenz, as well as ongoing monitoring of neuropsychiatric side effects for patients on efavirenz, or other agents, in developing world, in order to ensure access to safe, tolerable ART regimens for all people living with HIV.
Supplementary Material
The net proportion of participants on efavirenz-containing ART as first line therapy in CNICS between 1999 and 2015, stratified by a participant’s previous mental health diagnoses status at ART initiation. The net proportion of participants on efavirenz as first line therapy is calculated as the cumulative proportion of participants on efavirenz, minus the number of participants who have been censored, on each day.
Median Patient Health Questionnaire-9 (PHQ-9) values for depressive symptom severity over time, among 523 participants who initiated efavirenz (EFV) and either continued on efavirenz or eventually switched from efavirenz during the follow-up period. Median values across time are fit using a cubic spline. A PHQ-9 value of ≥ 10 indicates depressive symptoms; < 10 indicates no depressive symptoms.
Acknowledgments
This work was supported by the National Institutes of Health [grant numbers K99MH112413, R01MH100970, R24AI067039, U01 AI069918]. The Center for AIDS Research sites involved in CNICS include: University of Alabama at Birmingham (P30 AI027767), University of Washington (P30 AI027757), University of California San Diego (P30 AI036214), University of California San Francisco (P30 AI027763), Case Western Reserve University (P30 AI036219), Johns Hopkins University (P30 AI094189, U01 DA036935), Fenway Health/Harvard (P30 AI060354), and University of North Carolina Chapel Hill (P30 AI50410).
Footnotes
Presentation
A version of this work was presented at the Conference on Retroviruses and Opportunistic Infections (CROI) in Seattle, Washington, February 13-16, 2017.
Conflicts of Interest
KM has received research support from grants awarded to UNC from Merck, AbbVie, and Gilead. All other authors have no conflicts of interest to report.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2015 [updated April 8, 2015. Available from: Available at https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL003400.pdf.
- 2.Panel on Clinical Practices for Treatment of HIV Infection. Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. Department of Health and Human Services and the Henry J. Kaiser Family Foundation; 1999. [updated May 5, 1999. Available from: https://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL05051999011.pdf. [Google Scholar]
- 3.Raffi F, Pozniak AL, Wainberg MA. Has the time come to abandon efavirenz for first-line antiretroviral therapy? The Journal of antimicrobial chemotherapy. 2014;69(7):1742–7. doi: 10.1093/jac/dku058. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Recommendations for a public health approach. 2010. Antiretroviral therapy for HIV infection in adults and adolescents. Contract No.: Report. [PubMed] [Google Scholar]
- 5.Kryst J, Kawalec P, Pilc A. Efavirenz-Based Regimens in Antiretroviral-Naive HIV-Infected Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS One. 2015;10(5):e0124279. doi: 10.1371/journal.pone.0124279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coovadia A, Abrams EJ, Strehlau R, Shiau S, Pinillos F, Martens L, et al. Efavirenz-Based Antiretroviral Therapy Among Nevirapine-Exposed HIV-Infected Children in South Africa: A Randomized Clinical Trial. Jama. 2015;314(17):1808–17. doi: 10.1001/jama.2015.13631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwuji CC, Orne-Gliemann J, Larmarange J, Balestre E, Thiebaut R, Tanser F, et al. Universal test and treat and the HIV epidemic in rural South Africa: a phase 4, open-label, community cluster randomised trial. The lancet HIV. 2017 doi: 10.1016/S2352-3018(17)30205-9. [DOI] [PubMed] [Google Scholar]
- 8.Masimirembwa C, Dandara C, Leutscher PD. Rolling out Efavirenz for HIV Precision Medicine in Africa: Are We Ready for Pharmacovigilance and Tackling Neuropsychiatric Adverse Effects? Omics : a journal of integrative biology. 2016;20(10):575–80. doi: 10.1089/omi.2016.0120. [DOI] [PubMed] [Google Scholar]
- 9.Sonderup MW, Maughan D, Gogela N, Setshedi M, Wainwright H, Meintjes G, et al. Identification of a novel and severe pattern of efavirenz drug-induced liver injury in South Africa. Aids. 2016;30(9):1483–5. doi: 10.1097/QAD.0000000000001084. [DOI] [PubMed] [Google Scholar]
- 10.Kenedi CA, Goforth HW. A systematic review of the psychiatric side-effects of efavirenz. AIDS Behav. 2011;15(8):1803–18. doi: 10.1007/s10461-011-9939-5. [DOI] [PubMed] [Google Scholar]
- 11.Squibb B-M, editor. Efavirenz [Package insert] Princeton, NJ: 2013. [Google Scholar]
- 12.Mollan KR, Smurzynski M, Eron JJ, Daar ES, Campbell TB, Sax PE, et al. Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med. 2014;161(1):1–10. doi: 10.7326/M14-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arenas-Pinto Alejandro, Grund Birgit, Sharma Shweta, Martinez Esteban, Cummins Nathan, Fox Julie, et al. AIDS. Durban, South Africa: 2016. Increased risk of suicidal behaviour with use of efavirenz: Results from the START Trial. [Google Scholar]
- 14.Napoli AA, Wood JJ, Coumbis JJ, Soitkar AM, Seekins DW, Tilson HH. No evident association between efavirenz use and suicidality was identified from a disproportionality analysis using the FAERS database. J Int AIDS Soc. 2014;17:19214. doi: 10.7448/IAS.17.1.19214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nkhoma ET, Coumbis J, Farr AM, Johnston SS, Chu BC, Rosenblatt LC, et al. No Evidence of an Association Between Efavirenz Exposure and Suicidality Among HIV Patients Initiating Antiretroviral Therapy in a Retrospective Cohort Study of Real World Data. Medicine. 2016;95(3):e2480. doi: 10.1097/MD.0000000000002480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith C, Ryom L, Monforte A, Reiss P, Mocroft A, El-Sadr W, et al. Lack of association between use of efavirenz and death from suicide: evidence from the D:A:D study. J Int AIDS Soc. 2014;17(4 Suppl 3):19512. doi: 10.7448/IAS.17.4.19512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol. 2008;37(5):948–55. doi: 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saag Michael. Participation rate at CNICS sites. Personal email communication. 2016.
- 19.Department of Health and Human Services, Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2016 Available from: http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- 20.Akiskal HS, Benazzi F. Psychopathologic correlates of suicidal ideation in major depressive outpatients: is it all due to unrecognized (bipolar) depressive mixed states? Psychopathology. 2005;38(5):273–80. doi: 10.1159/000088445. [DOI] [PubMed] [Google Scholar]
- 21.Ashrafioun L, Pigeon WR, Conner KR, Leong SH, Oslin DW. Prevalence and correlates of suicidal ideation and suicide attempts among veterans in primary care referred for a mental health evaluation. J Affect Disord. 2016;189:344–50. doi: 10.1016/j.jad.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737–44. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 24.Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. Guidelines for Use in Primary Care. Geneva, Switzerland: World Health Organization, Dependence DoMHaS; 2001. AUDIT, The Alcohol Use Disorders Identification Test. [Google Scholar]
- 25.WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97(9):1183–94. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 26.Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction. 2008;103(6):1039–47. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 27.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12(3):255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 28.Amico KR, Fisher WA, Cornman DH, Shuper PA, Redding CG, Konkle-Parker DJ, et al. Visual analog scale of ART adherence: association with 3-day self-report and adherence barriers. J Acquir Immune Defic Syndr. 2006;42(4):455–9. doi: 10.1097/01.qai.0000225020.73760.c2. [DOI] [PubMed] [Google Scholar]
- 29.Vrouenraets SM, Wit FW, van Tongeren J, Lange JM. Efavirenz: a review. Expert opinion on pharmacotherapy. 2007;8(6):851–71. doi: 10.1517/14656566.8.6.851. [DOI] [PubMed] [Google Scholar]
- 30.Bengtson AM, Pence BW, Crane HM, Christopoulos K, Fredericksen RJ, Gaynes BN, et al. Disparities in Depressive Symptoms and Antidepressant Treatment by Gender and Race/Ethnicity among People Living with HIV in the United States. PLoS One. 2016;11(8):e0160738. doi: 10.1371/journal.pone.0160738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chersich MF, Urban MF, Venter FW, Wessels T, Krause A, Gray GE, et al. Efavirenz use during pregnancy and for women of child-bearing potential. AIDS research and therapy. 2006;3:11. doi: 10.1186/1742-6405-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mirochnick M, Best BM, Clarke DF. Antiretroviral pharmacology: special issues regarding pregnant women and neonates. Clin Perinatol. 2010;37(4):907–27. xi. doi: 10.1016/j.clp.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 33.De Santis M, Carducci B, De Santis L, Cavaliere AF, Straface G. Periconceptional exposure to efavirenz and neural tube defects. Archives of Internal Medicine. 2002;162(3):355. doi: 10.1001/archinte.162.3.355. [DOI] [PubMed] [Google Scholar]
- 34.Eaton EF, Tamhane AR, Burkholder GA, Willig JH, Saag MS, Mugavero MJ. Unanticipated Effects of New Drug Availability on Antiretroviral Durability: Implications for Comparative Effectiveness Research. Open forum infectious diseases. 2016;3(2):ofw109. doi: 10.1093/ofid/ofw109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.FDA notifications. Accelerated approval for raltegravir tablets. AIDS alert. 2007;22(12):143–4. [PubMed] [Google Scholar]
- 36.Juethner SN, Seyfried W, Aberg JA. Tolerance of efavirenz-induced central nervous system side effects in HIV-infected individuals with a history of substance abuse. HIV clinical trials. 2003;4(3):145–9. doi: 10.1310/P7MJ-K2WD-T1FY-AU8L. [DOI] [PubMed] [Google Scholar]
- 37.Fumaz CR, Munoz-Moreno JA, Molto J, Negredo E, Ferrer MJ, Sirera G, et al. Long-term neuropsychiatric disorders on efavirenz-based approaches: quality of life, psychologic issues, and adherence. J Acquir Immune Defic Syndr. 2005;38(5):560–5. doi: 10.1097/01.qai.0000147523.41993.47. [DOI] [PubMed] [Google Scholar]
- 38.Steigbigel RT, Cooper DA, Kumar PN, Eron JE, Schechter M, Markowitz M, et al. Raltegravir with optimized background therapy for resistant HIV-1 infection. N Engl J Med. 2008;359(4):339–54. doi: 10.1056/NEJMoa0708975. [DOI] [PubMed] [Google Scholar]
- 39.D’Abbraccio M, Busto A, De Marco M, Figoni M, Maddaloni A, Abrescia N. Efficacy and Tolerability of Integrase Inhibitors in Antiretroviral-Naive Patients. AIDS reviews. 2015;17(3):171–85. [PubMed] [Google Scholar]
- 40.Cotte L, Ferry T, Pugliese P, Valantin MA, Allavena C, Cabie A, et al. Effectiveness and tolerance of single tablet versus once daily multiple tablet regimens as first-line antiretroviral therapy - Results from a large french multicenter cohort study. PLoS One. 2017;12(2):e0170661. doi: 10.1371/journal.pone.0170661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fettiplace A, Stainsby C, Winston A, Givens N, Puccini S, Vannappagari V, et al. Psychiatric Symptoms in Patients Receiving Dolutegravir. J Acquir Immune Defic Syndr. 2017;74(4):423–31. doi: 10.1097/QAI.0000000000001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kheloufi F, Allemand J, Mokhtari S, Default A. Psychiatric disorders after starting dolutegravir: report of four cases. Aids. 2015;29(13):1723–5. doi: 10.1097/QAD.0000000000000789. [DOI] [PubMed] [Google Scholar]
- 43.Parant F, Miailhes P, Brunel F, Gagnieu MC. Dolutegravir-Related Neurological Adverse Events: A case Report of Successful Management with Therapeutic Drug Monitoring. Current drug safety. 2018 doi: 10.2174/1574886313666180116124046. [DOI] [PubMed] [Google Scholar]
- 44.Scheper H, van Holten N, Hovens J, de Boer M. Severe depression as a neuropsychiatric side effect induced by dolutegravir. HIV Med. 2017 doi: 10.1111/hiv.12538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The net proportion of participants on efavirenz-containing ART as first line therapy in CNICS between 1999 and 2015, stratified by a participant’s previous mental health diagnoses status at ART initiation. The net proportion of participants on efavirenz as first line therapy is calculated as the cumulative proportion of participants on efavirenz, minus the number of participants who have been censored, on each day.
Median Patient Health Questionnaire-9 (PHQ-9) values for depressive symptom severity over time, among 523 participants who initiated efavirenz (EFV) and either continued on efavirenz or eventually switched from efavirenz during the follow-up period. Median values across time are fit using a cubic spline. A PHQ-9 value of ≥ 10 indicates depressive symptoms; < 10 indicates no depressive symptoms.