Abstract
Objective
Preterm delivery for preeclampsia or placental insufficiency (PREPI) is a clinical criterion for antiphospholipid syndrome (APS), but no prior prospective studies use the international classification criteria for APS. Our objective was to determine the proportion of women with PREPI who test positive for aPL using international criteria for antiphospholipid antibody (aPL) assays.
Methods
Prospective, case-control study of 148 women delivered <36 weeks due to PREPI compared to 148 controls. PREPI cases delivered <36 weeks were compared to matched controls. Cases and controls were tested for aPL. Demographic variables were compared with chi-squared and Wilcoxon-rank-sum statistics. Rates of +aPL were compared using adjusted odds ratios (aOR) for maternal BMI and Caucasian race. Positive aPL (+aPL) was defined as lupus anticoagulant (LAC), anti-cardiolipin (aCL) IgG (GPL) or IgM (MPL) ≥40, or anti-β2-glycoprotein I (aβ2GPI) IgG (SGU) or IgM (SMU) ≥40.
Results
Controls were more likely to be Caucasian (87% versus 70%, p=0.006) and had lower BMIs (BMI 26 versus 33, p<0.001). Positive aPL were found more commonly in cases than controls (11.5 versus 1.4%, aOR 8.9 [95% CI 1.9–41.4]). In +aPL cases, 76% had +LAC, 41% had +aCL, and 24% had +aβ2GPI.
Conclusion
Women requiring early delivery for PREPI are more likely to have aPL (and thus APS) than controls. This is the first prospective study using both obstetric definitions and laboratory criteria in accordance with APS international criteria.
Introduction
Antiphospholipid syndrome (APS) is a disorder of concern to obstetricians, rheumatologists, and hematologists. Modified Sapporo classification criteria for APS include specified levels of at least one antiphospholipid antibody (aPL) and at least one clinical criterion(1). Clinical criteria include vascular thrombosis and/or pregnancy morbidity. Pregnancy morbidity includes one or more unexplained fetal deaths, one or more premature births due to preeclampsia or placental insufficiency, and/or three or more unexplained, consecutive early pregnancy losses(1). Preeclampsia and placental insufficiency (PREPI) occurring prior to term are typically associated with abnormal placental function. In severe cases, the treatment of preeclampsia and placental insufficiency is delivery, which in preterm cases may result in neonatal morbidity.
The pathogenesis of preeclampsia and placental insufficiency is multifactorial, but some proportion of cases are caused by maternal aPL. Both case-control and cohort studies report associations between aPL and PREPI(2–33). However, previous studies used widely variable definitions of preeclampsia and/or placental insufficiency, and many included women who developed PREPI at term. These studies also used various thresholds to define positive levels of aPL. Many included women with only low positive titers, which would not meet modified Sapporo criteria for APS. Also, few studies repeated testing to confirm aPL, as recommended in order to make the diagnosis of APS. Finally, small numbers of patients in most previous studies make it difficult to determine the true frequency of aPL in the setting of preterm PREPI. Knowing the prevalence of aPL in women with PREPI would help practitioners understand the true utility of aPL testing in women with preterm PREPI.
The Obstetric task forces at the 14th and 15th International Congress on Antiphospholipid Antibodies noted that high quality studies defining the association of aPL with pregnancy morbidity are lacking (34). Thus, our objective was to prospectively measure the association of aPL with APS obstetric criterion of preterm delivery due to preeclampsia or placental insufficiency.
Materials and Methods
Study Design and Participants
We conducted a case-control study comparing frequency of aPL presence in women with and without delivery prior to 36 weeks for preeclampsia or placental insufficiency. Cases were women who were delivered prior to 36 0/7 weeks’ gestation due to either preeclampsia with severe features or placental insufficiency. All women delivering at University of Utah, Salt Lake City, Utah or Intermountain Medical Center, Murray, Utah from February 2014 until May 2017 were eligible for participation. No subjects were excluded on the basis of chronic medical condition or use of any specific medication during pregnancy, including use of aspirin or heparin. Women with multiple gestations were excluded. Women with intellectual disability and incarcerated women were excluded. Gestational age was determined using standard recommendations per the American Congress of Obstetricians and Gynecologists (ACOG) (35). Last menstrual period (LMP) was used to establish the estimated due date (EDD) and was considered consistent with ultrasound dating if the dates were within 5 days prior to 9 0/7 weeks, within 7 days from 9 0/7 – 15 6/7 weeks, and within 10 days if from 16 0/7 weeks-21 6/7 weeks. If ultrasound assessment of EDD was not consistent with LMP, the EDD was then based on the discrepant ultrasound.
The study was approved by Institutional Review Boards at the University of Utah and Intermountain Healthcare and written informed consent was obtained from all participants. Consent documents were available in English and Spanish - thus, women who did not speak these languages were also excluded.
Case and Control Definitions
Cases were women delivered at or before 36 weeks’ gestation due to preeclampsia with severe features ore placental insufficiency (PREPI). We chose delivery at 36 weeks’ or earlier as our gestational age criterion for case definition because women without severe features would not be delivered until 37 weeks, thus clearly delineating severe disease. Our goal was to be inclusive of severe presentations of PREPI. However, the international classification criteria for APS use PREPI prior to 34 weeks’ gestation, Thus, we repeated the analysis of aPL presence using only cases who delivered prior to 34 weeks and their matched controls. We believe both are valuable as 34 weeks is in accordance with international classification criteria, yet 36 weeks is more inclusive of clinical practice and the true spectrum of disease. Preeclampsia with severe features was defined per ACOG criteria (36). Women were diagnosed with preeclampsia with severe features if they had new onset elevated blood pressure (with systolic blood pressure greater than 140 mmHg and/or diastolic blood pressure greater than 90 mmHg) or acute worsening of chronic blood pressure, with or without proteinuria, and with presence of one or more severe feature. Severe features included systolic blood pressure greater than 160 mmHg and/or diastolic blood pressure greater than 110 mmHg, platelet count <100,000/microliter, liver enzymes twice normal concentration or symptoms of liver failure, right upper quadrant or epigastric pain, serum creatinine >1.1 mg/dL, pulmonary edema, and/or new onset cerebral or visual disturbances.
Placental insufficiency was defined according to the international consensus for the diagnosis of APS(1). Placental insufficiency was defined as delivery due to fetal growth restriction (FGR) with estimated fetal weight less than the 10th percentile or abdominal circumference less than the 5th percentile for gestational age, oligohydramnios with amniotic fluid index < 5 cm or deepest vertical pocket < 2 cm, abnormal Doppler velocimetry suggestive of fetal hypoxemia (absent or reversed end diastolic flow), abnormal non-stress test, positive contraction stress test, or biophysical profile score <6.
Controls were women with ongoing pregnancies without PREPI at time of enrollment. Pregnant controls were matched to cases based on 1) gestational age within one week at time of first sample draw, 2) maternal age within 5 years, and 3) parity. Research staff surveyed labor and delivery daily logs and approached women meeting case criteria. Controls were recruited during their scheduled obstetric clinic visits at participating institutions. Women were eligible for participation if they received medical care at one of the participating institutions and had a singleton pregnancy.
Patients were not involved in the design of the study or the analysis/interpretation of the findings. Core outcome sets are not available for the outcome of antiphospholipid antibodies.
Laboratory Definitions and Assay Characteristics
The primary exposure evaluated was presence or absence of aPL. Presence of aPL was defined as any single positive antiphospholipid test, including LAC, aCL IgG (GPL) or IgM (MPL) >40, and aB2GPI IgG (SGU) or IgM (SMU) >40. Although a single positive test is sufficient to meet criteria for antiphospholipid antibody presence, an individual may test positive for multiple aPL in combination. Thus, our secondary exposure evaluated was each individual antiphospholipid antibody test. Every participant with positive antiphospholipid antibody testing was contacted for repeat testing at 12 weeks or greater after initial positive test.
At time of consent, routine venipuncture was performed to obtain samples of peripheral blood for serum and plasma. Laboratory testing was performed at ARUP Laboratories (a National Reference Laboratory) by an experienced technician who was blinded to the samples’ status as cases or controls. LAC testing was performed according to the updated guidelines of the International Society on Thrombosis and Hemostasis (ISTH) (37) on a STA-R automated coagulation analyzer. Screening on platelet-poor citrated plasma used two different clotting assay principles: a sensitive activated partial thromboplastin time (aPTT) and dilute Russell’s viper venom time (dRVVT), each performed according to the manufacturer’s recommendations. If one or both screening tests was prolonged, a mixing study was performed using a 1:1 mix of patient sample and pooled normal plasma (PNP), followed by repeat testing of the aPPT and/or the dRVVT clotting time without incubation. Results from mixing studies that failed to correct to within the local cutoff values, and thus suggested an inhibitory effect, underwent reflexive confirmation (neutralization) testing to demonstrate antiphospholipid antibody dependency by increasing the concentration and/or composition of aPL (aPL). Hexagonal phase assay with increased PL was used when mixing studies for aPPT or dRVVT showed an inhibitory effect, but the confirmatory steps were negative. PTT heparin neutralization is performed to account for potential heparin effect. LAC was considered present when one or more of the clotting assays was positive (i.e. prolonged aPTT or dRVVT in mixing studies with positive confirmatory testing or positive hexagonal phase in the case of negative confirmatory testing).
Analysis and Statistics
Participant medical history, obstetric characteristics, and delivery information were obtained using a combination of interview and chart abstraction. Diagnosis of small for gestational age (SGA) was made using recorded birthweight and standardized birth tables from Oken et al. in which data from the 1999 and 2000 National Center for Health Statistics Natality Data Sets were used to generate a nearly continuous reference measure for gestational age birth weight (38). SGA was defined as birth weight less than the 10th percentile for gestational age. All data were de-identified and stored in a secure REDCap database(39).
Data analysis was performed using Stata v13.1 (StataCorp LP, College Station, TX). Univariate analysis used student’s t-test, Wilcoxon-rank sum test (if variable was not normally distributed), Chi squared test, or Fisher’s exact test as appropriate. Normality was assessed using the Shapiro-Wilk test. Odds ratios were calculated for presence of positive aPL test. Odds ratios were adjusted using multivariable logistic regression with a model including the potential confounders maternal BMI and Caucasian race. Cases underwent a secondary nested case-control analysis comparing demographic and obstetric factors between +aPL cases and -aPL cases. We predicted we would detect aPL in 7.9% of cases and 0.5% of controls based on previous literature(8, 10, 14, 18). Thus, we estimated needing 140 participants per group to achieve 80% power to detect a difference with a two-sided alpha of 0.05.
Results
Out of 1590 women delivering prior to 36 weeks, 295 were delivered because of preeclampsia and/or placental insufficiency, and 148 of these consented to participate in this study (Figure 1). 148 women with PREPI indicated delivery prior to 36 weeks were compared to 148 matched controls. Demographics for cases and controls are shown in Table 1. Cases were less likely to be Caucasian, more likely to be obese, and more likely to have had a previous pregnancy loss. Overall, 137 had a BMI at the time of delivery of greater than or equal to 30 (46.3%). Concomitant use of heparin or low molecular weight heparin (LMWH) was more frequent in cases than controls (18.2 versus 0%, p<0.001). There was only one case of therapeutic LMWH use; the remainder was prophylactic dosing, typically 40 units of LMWH subcutaneous daily. Additionally, cases were delivered at an earlier gestational age (32.9 versus 39.4 weeks, p<0.001) and were more likely to deliver an SGA neonate (44.9% versus 9.7%, p<0.001). Despite being matched for overall parity, pregnancy histories differed between groups. Cases were more likely to have experienced any pregnancy loss (31.3 versus 16.4%, p=0.003) and early pregnancy loss (26.5 versus 12.3%, p=0.002). Other characteristics were similar and shown in Table 1.
Figure 1.
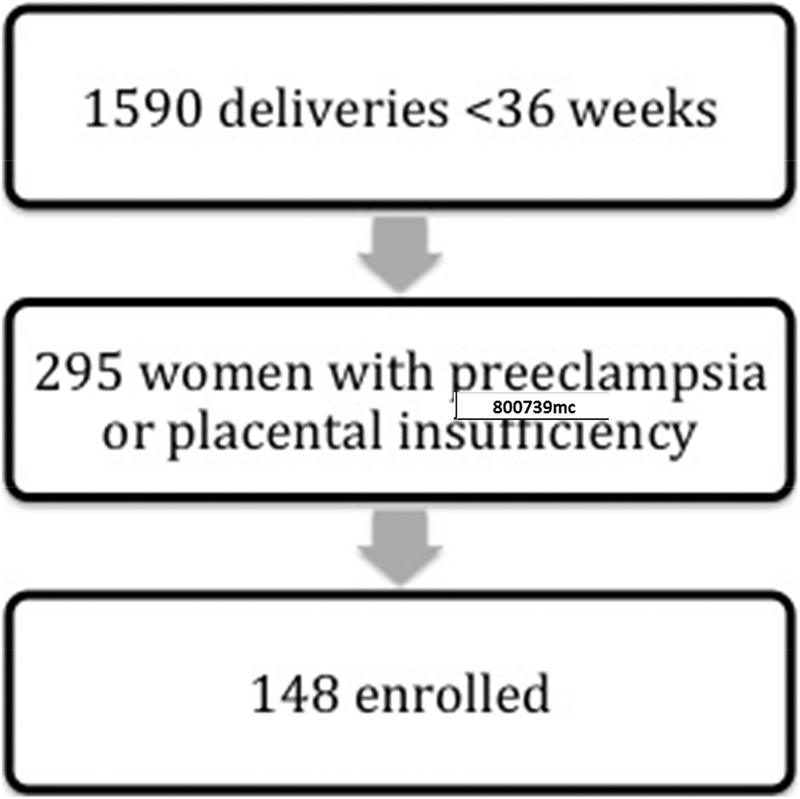
Screening, eligibility, and enrollment of cases with preeclampsia and/or placental insufficiency and delivery prior to 36 weeks.
Table 1.
Demographic and obstetric factors of PREPI cases and matched controls.
| Variable | Controls (N=148) | PREPI (N=148) | p-value |
|---|---|---|---|
| Maternal age (years) | 29 (26–33) | 29 (25–34) | 0.711 |
| Race | 0.006 | ||
| Non-Hispanic White | 126 (86.9) | 100 (69.9) | |
| Non-Hispanic Black | 0 (0) | 2 (1.4) | |
| Hispanic | 10 (6.9) | 20 (14.0) | |
| Asian/Pacific-Islander | 6 (4.1) | 10 (7.0) | |
| Other/Unknown | 3 (2.1) | 11 (7.7) | |
| Tobacco Use | 6 (4.1) | 8 (5.4) | 0.310 |
| Alcohol Use | 10 (6.7) | 8 (5.4) | 0.329 |
| BMI | 26.3 (23.6–30.0) | 32.5 (26.7–38.1) | <0.001 |
| Obese (BMI > 30 m/kg2) | 39 (26.4) | 98 (67.1) | <0.001 |
| Heparin or LMWH use | 0 (0) | 27 (18.2) | <0.001 |
| Aspirin use | 2 (1.4) | 6 (4.1) | 0.282 |
| Parous | 58 (43.9) | 55 (47.0) | 0.627 |
| Previous pregnancy loss | 24 (16.4) | 46 (31.3) | 0.003 |
| Total pregnancy losses | 0(0–0) | 0 (0–1) | 0.003 |
| Previous stillbirth | 1 (0.7) | 4 (2.7) | 0.371 |
| Previous previable delivery | 0 (0) | 2 (1.4) | 0.498 |
| Previous fetal loss | 2 (1.4) | 7 (4.8) | 0.173 |
| Previous early pregnancy loss | 18 (12.3) | 39 (26.5) | 0.002 |
| Number of previous early pregnancy losses | 0(0–0) | 0 (0–1) | 0.002 |
| Delivery Gestational Age (weeks) | 39.4 (38–7–40.3) | 32.9 (30.1–34.4) | <0.001 |
| SGA | 14 (9.7) | 66 (44.9) | <0.001 |
Data are median and IQR or n(%). LMWH = low molecular weight heparin.
Table 2 demonstrates aPL results in cases and controls. Overall, there were more women with +aPL in the PREPI case group (11.5% vs 1.4%, aOR 8.9, 95% CI 1.941.4). Individual aPL that were more common in the PREPI group included LAC (11.5 versus 1.4%, aOR 7.0, 95% CI 1.5–33.8), and aCL (8.8 versus 1.4%, p=0.004). LAC was the most common aPL detected, and was the only positive lab test detected in the control group. When the cutoff for a positive immunoassay titer was lowered to 20 U/mL, +aPL remained more common in the PREPI group (14.2 versus 3.4%, aOR 4.3, 95% CI 1.5–12.5), and aβ2GPI was more common individually (Table 2). There was no difference in frequency of +aPL based on type of case (preeclampsia versus placental insufficiency), with 10 (11.8%) severe preeclampsia cases and 7 (11.3%) placental insufficiency cases testing positive for aPL (p=0.929). There were five cases with both severe preeclampsia and placental insufficiency and +aPL. Of the 19 women with +aPL, 10 returned for repeat testing. Of those, 8 (80%) remained positive. One participant had completely negative testing on repeat, and one who originally was positive for LAC retested negative for LAC but had an aCL IgM elevated at 26.8 U/mL. Details of each participant with +aPL are shown in Table 3, including repeat testing.
Table 2.
Comparison of aPL between PREPI cases and matched controls.
| aPL | Control (N=148) | PREPI Cases (N=148) | p-value | OR | aOR* |
|---|---|---|---|---|---|
| LAC+ or +aPL (IgG or IgM)>40 | 2 (1.4) | 17 (11.5) | <0.001 | 9.5 (2.2–85.6) | 8.9 (1.9–41.4) |
| LAC+ | 2 (1.4) | 13 (8.8) | 0.006 | 7.0 (1.5–64.9) | 7.0 (1.5–33.8) |
| +aPL IgG >40 | 0 (0) | 2 (1.4) | 0.498 | -- | -- |
| +aCL IgG >40 | 0 (0) | 2 (1.4) | 0.498 | -- | -- |
| + aβ2-g-I IgG >40 | 0 (0) | 1 (0.7) | >0.999 | -- | -- |
| +aPL IgM >40 | 0 (0) | 6 (4.1) | 0.030 | -- | -- |
| +aCL IgM >40 | 0 (0) | 6 (4.1) | 0.030 | -- | -- |
| + aβ2-g-I IgM >40 | 0 (0) | 3 (2.0) | 0.247 | -- | -- |
| +aPL IgG >20 | 0 (0) | 9 (6.1) | 0.003 | -- | -- |
| +aCL IgG >20 | 0 (0) | 8 (5.4) | 0.007 | -- | -- |
| + aβ2-g-I IgG >20 | 0 (0) | 2 (1.4) | 0.498 | -- | -- |
| +aPL IgM >20 | 3 (2.0) | 8 (5.4) | 0.218 | 2.8 (0.6–16.4) | 2.9 (0.7–12.2) |
| +aCL IgM >20 | 3 (2.0) | 7 (4.7) | 0.335 | 2.4 (0.5–14.6) | 2.9 (0.7–12.2) |
| + aβ2-g-I IgM >20 | 0 (0) | 5 (3.4) | 0.060 | -- | -- |
logistic regression model includes maternal BMI and Caucasian race.
Table 3.
Description of participants with +aPL.
| Study cohort | Specific aPL | Repeat Labs | Gestational Age (weeks) | Birthweight (grams) | Maternal age (years) | Obese | Comorbidities |
|---|---|---|---|---|---|---|---|
| PI | aCL IgM, aβ2-g-I IgM | aCL IgM, aβ2-g-I IgM | 35 | 1800 (SGA) | 32 | Yes | Hypothyroid |
| Both PE/PI | LAC | -- | 25.1 | 500 (SGA) | 25 | Yes | |
| Both PE/PI | LAC | -- | 36 | 2039 (SGA) | 29 | Yes | Renal insufficiency |
| PI | LAC | -- | 35.5 | 1730 (SGA) | 37 | Yes | Chronic HTN |
| Both PE/PI | LAC | Negative | 34.2 | 1790 (SGA) | 27 | Yes | |
| PE | LAC | LAC | 35 | 2560 | 36 | Yes | Hypothyroid, chronic HTN |
| PE | aCL IgM | aCL IgM, aβ2-g-IIgM | 32.5 | 1580 | 23 | No | Seizure disorder |
| Both PE/PI | LAC, aβ2-g-I IgG | LAC, aCL IgG, aβ2-g-I IgG | 26.9 | 410 (SGA) | 25 | No | Hypothyroid, ITP |
| PE | LAC | -- | 35 | 2540 | 21 | Yes | Chronic HTN |
| PE | LAC | LAC | 20.4 | 130 | 24 | Yes | SLE, chronic HTN |
| PE |
aCL IgM | aCL IgM 29 | 35.1 | 1880 (SGA) | 41 | Yes | Chronic HTN |
| Control | LAC | -- | 38.6 | 3420 | 33 | No | |
| PE | LAC | aCL IgM 26.8, LAC neg | 32.7 | 1420 (SGA) | 41 | No | |
| PE | LAC | LAC | 31.1 | 1710 | 34 | No | VTE, Takayasu’s arteritis, Chronic HTN |
| PE | aCL IgM | -- | 32 | 1490 | 31 | Yes | GDM |
| PE | LAC | -- | 29.1 | 1210 | 28 | Yes | Chronic HTN |
| Control | LAC | -- | 38.7 | 3540 | 30 | No | Multiple sclerosis, Psoriatic arthritis |
| Both PE/PI | LAC, aCL IgM, aCL IgG | LAC, aCL IgM, aCL IgG | 23.9 | 370 (SGA) | 21 | Yes | |
| PE | LAC | -- | 31.7 | 1180 (SGA) | 28 | Yes |
PE=preeclampsia; PI=placental insufficiency; LAC=lupus anticoagulant, aCL=anti-cardiolipin antibody, aβ2-g-I= anti-β2-glycoprotein-I antibody
We performed an analysis of frequency of positive LAC based on use of heparin or LMWH. Positive LAC was more common in women using heparin or LMWH than in those not using heparin (18.5% vs 3.7%, p=0.001). At time of confirmatory testing, no women were using heparin or LMWH. Of the five women using heparin or LMWH who originally tested positive for LAC, four had confirmatory positive LAC testing and one did not complete testing.
Limiting analysis to deliveries prior to 34 weeks excluded 50 cases and their controls (n=98 PREPI cases prior to 34 weeks). Positive aPL were noted in 10.2% of cases less than 34 weeks and 1.0% of their controls, with adjusted OR of 13.0 (95% CI 1.5–110.3).
When demographic and obstetric factors were compared between cases with -aPL and cases with +aPL, the only difference was an increased frequency of history of stillbirth in cases with +aPL (17.7% versus 0.8%, p=0.005) (data not shown).
Discussion
Main Findings
We found that 11.5% or one out of every nine women with preeclampsia or placental insufficiency requiring preterm delivery had presence of aPL. This was considerably more than the 1.4% of women with uncomplicated pregnancies. We were also able to demonstrate repeatedly positive tests in 80% of those who underwent repeat testing.
Interpretation
Two meta-analyses summarised studies assessing the association of aCL and preeclampsia or placental insufficiency. The first included 12 studies and found a pooled odds ratio (OR) for aCL with preeclampsia of 2.86 (95% CI 1.37–5.98). The OR for aPL with severe preeclampsia was 11.15 (95% CI 2.66–46.75). Only 6 of the 12 studies defined criteria for severe preeclampsia(6). A second meta-analysis by Abou-Nassar evaluated the relationships between lupus anticoagulant (LAC), aCL, or aβ2GPI and preeclampsia, late fetal loss, FGR, and placental abruption(7). Overall, the investigators found an association between LAC and preeclampsia (OR 2.34, 95% CI 1.18–4.64) in case-control studies, but not in cohort studies. FGR was also associated with LAC (OR 4.65, 95% CI 1.29–16.71) in case-control studies and with aβ2GPI in cohort studies (OR 20.03, 95% CI 4.59–87.43). aCL IgG was associated with preeclampsia in case-control studies (OR 1.52, 95% CI 1.05–2.2), and aβ2GPI was associated with preeclampsia in cohort studies (OR 19.14, 95% CI 6.34–57.77). Although these associations suggest a link between preeclampsia, placenta insufficiency, and aPL, study inclusion criteria and outcomes are heterogeneous. Many studies used low thresholds for aCL and aβ2GPI (>5 GPL/MPL or SGU/MPU). Of the 28 studies included, only 13 tested for LAC and 6 tested for aβ2GPI. Very few studies included repeat testing. Severe disease and premature delivery were not part of the outcome measures.
These epidemiologic findings are consistent with findings in animal studies. Murine studies of the effects of aPL have found complement mediated fetal injury in antiphospholipid syndrome(40–44) and increased expression of tissue factor(45). Placentas from human pregnancies affected by antiphospholipid syndrome also show increased complement deposition(40, 46). Thus, complement activation is a plausible pathway through which aPL lead to adverse pregnancy outcomes.
Strengths and Limitations
Our study has numerous strengths. We have a clearly defined phenotype of PREPI that results in need for preterm delivery - a group of women with severe disease. Focusing on these pregnancies allows for a more precise measure of association with aPL. We also measured all relevant aPL, as defined by the modified Sapporo criteria. Many prior studies excluded LAC or aβ2GPI, which precludes true assessment of aPL prevalence in this population. We also made a rigorous attempt to obtain repeat testing for women who tested positive for aPL, although we were only successful in achieving repeat testing in approximately half of women with initial presence of aPL. Relevant levels of aPL persisted in 80% of women undergoing repeat testing.
There is some concern about whether use of heparin or LMWH can result in false positive LAC. Although we did find a higher rate of positive LAC in women using heparin or LMWH, the majority (81.5%) of women using heparin or LMWH had a negative LAC result. Additionally, four out of five women in our study who were using heparin or LMWH at time of initial positive LAC ultimately had positive LAC confirmed when no longer using heparin or LMWH. We suspect the initial higher rate of positive LAC in women using heparin or LMWH simply reflects appropriate medical care in the setting of increased thrombosis risk.
One of our limitations was our modest success at obtaining repeat testing. Although we made concerted efforts to obtain repeat testing, ultimately only 52.6% of aPL positive women were willing to complete this. We believe it is clinically relevant for researchers and clinicians to pursue repeat, confirmatory testing, but we acknowledge the obstacles to successfully completing it. Additionally, we used a more liberal definition of preterm than the modified Sapporo criteria (36 as opposed to 34 weeks). We believe this is appropriate because obstetric management of PREPI at these gestational ages is similar. Inasmuch as 7 of our cases were >34 weeks (though all less than 36 weeks), one might argue for reconsidering the <34 week threshold for considering the diagnosis of APS (1).
The two cases of PREPI with transiently positive levels of aPL illustrate the need for repeat testing. It is unclear if these women truly have APS or whether treatment will improve obstetric outcomes in subsequent pregnancies. More data are needed regarding the prognosis for women with transiently positive levels of aPL.
Conclusion
Our findings confirm the association between preterm PREPI and aPL. In our population, the prevalence of aPL in preterm PREPI was 11.5%, or 1 in 9. This is common enough to support routine testing for aPL when delivering a patient < 36 weeks gestation due to PREPI. However, the utility of an APS diagnosis, or any diagnosis, hinges on our ability to use that information to improve future health outcomes, and well-designed studies to show that treatment, e.g., with a heparin agent and/or low dose aspirin improve pregnancy outcomes in women with APS diagnosed on the basis of PREPI are lacking. Our findings emphasize the importance of working to develop therapy that can be targeted to this population in order to improve both maternal and neonatal health outcomes.
Acknowledgements
We wish to express our appreciation for the women who participated in this study.
Funding: Karen J. Gibbins received support from the University of Utah WRHR program 1K12HD085816 NICHD. REDCap use is supported by 8UL1TR000105 NCATS/NIH
Footnotes
Disclosure of Interests: None of the authors have any conflicts of interest.
Contribution of Authorship: Karen J. Gibbins, MD, MSCI (KJG), Anne E. Tebo, PhD (AET), and D. Ware Branch, MD (DWB) made substantial contributions to the conception and design of the study. Samantha K. Nielsen (SKN) participated in the design of the study and the acquisition of the data. KJG primarily performed the analysis, but KJG, AET, SKN, and DWB all participated in the interpretation of the data. All authors were involved drafting or revising the manuscript, and all authors give final approval of this version for publication. We agree that we are all accountable for all aspects of the work.
Details of Ethics Approval: This study was approved by University of Utah IRB on 11/21/2013, IRB #00068989. It was approved by the Intermountain Healthcare Research IRB on 9/11/2014, IRB#1024966.
References
- 1.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 2.Yamada H, Atsumi T, Kobashi G, Ota C, Kato EH, Tsuruga N, et al. Antiphospholipid antibodies increase the risk of pregnancy-induced hypertension and adverse pregnancy outcomes. J Reprod Immunol. 2009;79(2):188–95. [DOI] [PubMed] [Google Scholar]
- 3.Yamada H, Atsumi T, Amengual O, Koike T, Furuta I, Ohta K, et al. Anti-beta2 glycoprotein-I antibody increases the risk of pregnancy-induced hypertension: a case-controlled study. J Reprod Immunol. 2010;84(1):95–9. [DOI] [PubMed] [Google Scholar]
- 4.Chauleur C, Galanaud JP, Alonso S, Cochery-Nouvellon E, Balducchi JP, Mares P, et al. Observational study of pregnant women with a previous spontaneous abortion before the 10th gestation week with and without antiphospholipid antibodies. J Thromb Haemost. 2010;8(4):699–706. [DOI] [PubMed] [Google Scholar]
- 5.Polzin WJ, Kopelman JN, Robinson RD, Read JA, Brady K. The association of antiphospholipid antibodies with pregnancies complicated by fetal growth restriction. Obstet Gynecol. 1991;78(6):1108–11. [PubMed] [Google Scholar]
- 6.do Prado AD, Piovesan DM, Staub HL, Horta BL. Association of anticardiolipin antibodies with preeclampsia: a systematic review and meta-analysis. Obstet Gynecol. 2010;116(6):1433–43. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Nassar K, Carrier M, Ramsay T, Rodger MA. The association between antiphospholipid antibodies and placenta mediated complications: a systematic review and meta-analysis. Thromb Res. 2011;128(1):77–85. [DOI] [PubMed] [Google Scholar]
- 8.Branch DW, Andres R, Digre KB, Rote NS, Scott JR. The association of antiphospholipid antibodies with severe preeclampsia. Obstet Gynecol. 1989;73(4):541–5. [PubMed] [Google Scholar]
- 9.Yasuda M, Takakuwa K, Tokunaga A, Tanaka K. Prospective studies of the association between anticardiolipin antibody and outcome of pregnancy. Obstet Gynecol. 1995;86(4):555–9. [DOI] [PubMed] [Google Scholar]
- 10.Lee R, Brown MA, Branch DW, Ward K, Silver RM. Anticardiolipin and anti-β2-glycoprotein-I antibodies in preeclampsia. Obstetrics & Gynecology. 2003;102(2):294–300. [DOI] [PubMed] [Google Scholar]
- 11.Dreyfus M, Hedelin G, Kutnahorsky R, Lehmann M, Viville B, Langer B, et al. Antiphospholipid antibodies and preeclampsia: a case-control study. Obstet Gynecol. 2001;97(1):29–34. [DOI] [PubMed] [Google Scholar]
- 12.Allen JY, Tapia-Santiago C, Kutteh WH. Antiphospholipid antibodies in patients with preeclampsia. Am J Reprod Immunol. 1996;36(2):81–5. [DOI] [PubMed] [Google Scholar]
- 13.Facchinetti F, Marozio L, Frusca T, Grandone E, Venturini P, Tiscia GL, et al. Maternal thrombophilia and the risk of recurrence of preeclampsia. Am J Obstet Gynecol. 2009;200(1):46 e1–5. [DOI] [PubMed] [Google Scholar]
- 14.Mello G, Parretti E, Marozio L, Pizzi C, Lojacono A, Frusca T, et al. Thrombophilia is significantly associated with severe preeclampsia: results of a large-scale, case-controlled study. Hypertension. 2005;46:1270–4. [DOI] [PubMed] [Google Scholar]
- 15.Faden D, Tincani A, Tanzi P, Spatola L, Lojacono A, Tarantini M, et al. Antibeta 2 glycoprotein I antibodies in a general obstetric population: preliminary results on the prevalence and correlation with pregnancy outcome. Anti-beta 2 glycoprotein I antibodies are associated with some obstetrical complications, mainly preeclampsia-eclampsia. Obstet Gynecol. 1997;73:37–42. [DOI] [PubMed] [Google Scholar]
- 16.Bowen RS, Moodley J, Dutton MF, Fickl H. Antibodies to oxidised low-density lipoproteins and cardiolipin in pre-eclampsia and eclampsia. J Obstet Gynaecol. 2002;22(2):123–6. [DOI] [PubMed] [Google Scholar]
- 17.Harris EN, Spinnato JA. Should anticardiolipin tests be performed in otherwise healthy pregnant women? Am J Obstet Gynecol. 1991;165(5 Pt 1):1272–7. [DOI] [PubMed] [Google Scholar]
- 18.Kupferminc MJ, Fait G, Many A, Gordon D, Eldor A, Lessing JB. Severe preeclampsia and high frequency of genetic thrombophilic mutations. Obstet Gynecol. 2000;96(1):45–9. [DOI] [PubMed] [Google Scholar]
- 19.Pattison NS, Chamley LW, McKay EJ, Liggins GC, Butler WS. Antiphospholipid antibodies in pregnancy: prevalence and clinical associations. Br J Obstet Gynaecol. 1993;100(10):909–13. [DOI] [PubMed] [Google Scholar]
- 20.Alfirevic Z, Mousa HA, Martlew V, Briscoe L, Perez-Casal M, Toh CH. Postnatal screening for thrombophilia in women with severe pregnancy complications. Obstet Gynecol. 2001;97(5):753–9. [DOI] [PubMed] [Google Scholar]
- 21.Kaleli B, Kaleli I, Aktan E, Turan C, Aksit F. Antiphospholipid antibodies in eclamptic women. Gynecol Obstet Invest. 1998;45(2):81–4. [DOI] [PubMed] [Google Scholar]
- 22.Mello G, Parretti E, Martini E, Mecacci F, La Torre P, Cioni R, et al. Usefulness of screening for congenital or acquired hemostatic abnormalities in women with previous complicated pregnancies. Haemostasis. 1999;29(4):197–203. [DOI] [PubMed] [Google Scholar]
- 23.Milliez J, Lelong F, Bayani N, Jannet D, el Medjadji M, Latrous H, et al. The prevalence of autoantibodies during third-trimester pregnancy complicated by hypertension or idiopathic fetal growth retardation. Am J Obstet Gynecol. 1991;165(1):51–6. [DOI] [PubMed] [Google Scholar]
- 24.Moodley J, Bhoola V, Duursma J, Pudifin D, Byrne S, Kenoyer DG. The association of antiphospholipid antibodies with severe early-onset pre-eclampsia. S Afr Med J. 1995;85(2):105–7. [PubMed] [Google Scholar]
- 25.Rix P, Stentoft J, Aunsholt NA, Dueholm M, Tilma KA, Hoier-Madsen M. Lupus anticoagulant and anticardiolipin antibodies in an obstetric population. Acta Obstet Gynecol Scand. 1992;71(8):605–9. [DOI] [PubMed] [Google Scholar]
- 26.Wharfe G, Fletcher H, Reid M. Lupus anticoagulant in Jamaican primiparae and the clinical significance in asymptomatic patients. West Indian Med J. 1999;48(3):126–8. [PubMed] [Google Scholar]
- 27.Katano K, Aoki A, Sasa H, Ogasawara M, Matsuura E, Yagami Y. beta 2-Glycoprotein I-dependent anticardiolipin antibodies as a predictor of adverse pregnancy outcomes in healthy pregnant women. Hum Reprod. 1996;11(3):509–12. [DOI] [PubMed] [Google Scholar]
- 28.Lynch AM, Rutledge JH, Stephens JK, Murphy JR, Marlar RA, Davila GH, et al. Longitudinal measurement of anticardiolipin antibodies during normal pregnancy: a prospective study. Lupus. 1995;4:365–9. [DOI] [PubMed] [Google Scholar]
- 29.Stuart RA, Kornman LH, McHugh NJ. A prospective study of pregnancy outcome in women screened at a routine antenatal clinic for anticardiolipin antibodies. Br J Obstet Gynaecol. 1993;100(6):599–600. [DOI] [PubMed] [Google Scholar]
- 30.Uncu G, Ozan H, Kucukerdogan I, Cengiz C. Anticardiolipin antibodies in pregnancy induced hypertension. Obstet Gynecol. 1996;70:97–100. [DOI] [PubMed] [Google Scholar]
- 31.Valdes-Macho E, Cabiedes J, Villa AR, Cabral AR, Alarcon-Segovia D. Anticardiolipin and anti-beta2-glycoprotein-I antibodies in hypertensive disorders of pregnancy. Arch Med Res. 2002;33:460–5. [DOI] [PubMed] [Google Scholar]
- 32.van Pampus MG, Dekker GA, Wolf H, Huijgens PC, Koopman MMW, von Blomberg ME, et al. High prevalence of hemostatic abnormalities in women with a history of severe preeclampsia. Am J Obstet Gynecol. 1999;180:1146–50. [DOI] [PubMed] [Google Scholar]
- 33.De Carolis S, Caruso A, Ferrazzani S, Carducci B, De Santis L, Mancuso S. Poor pregnancy outcome and anticardiolipin antibodies. Fetal Diagn Ther. 1994;9(5):296–9. [DOI] [PubMed] [Google Scholar]
- 34.de Jesus GR, Agmon-Levin N, Andrade CA, Andreoli L, Chighizola CB, Porter TF, et al. 14th International Congress on Antiphospholipid Antibodies Task Force report on obstetric antiphospholipid syndrome. Autoimmun Rev. 2014;13(8):795–813. [DOI] [PubMed] [Google Scholar]
- 35.Gynecologists TACoOa, Publications SfM-FM, Medicine AIoUi. Committee Opinion: Methods for estimating the due date. 2017.
- 36.Roberts JM, August PA, Bakris G, Barton JR, Bernstein IM, Druzin M, et al. Hypertension in Pregnancy: Executive Summary. Obstet Gynecol. 2013;122(5):1122–31. [Google Scholar]
- 37.Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost. 2009;7(10):1737–40. [DOI] [PubMed] [Google Scholar]
- 38.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen D, Buurma A, Goemaere NN, Girardi G, le Cessie S, Scherjon S, et al. Classical complement activation as a footprint for murine and human antiphospholipid antibody-induced fetal loss. J Pathol. 2011;225(4):502–11. [DOI] [PubMed] [Google Scholar]
- 41.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. Journal of Clinical Investigation. 2003;112(11):1644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195(2):211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pierangeli SS, Girardi G, Vega-Ostertag M, Liu X, Espinola RG, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum. 2005;52(7):2120–4. [DOI] [PubMed] [Google Scholar]
- 44.Salmon JE, Girardi G, Holers VM. Activation of complement mediates antiphospholipid antibody-induced pregnancy loss. Lupus. 2003;12(7):535–8. [DOI] [PubMed] [Google Scholar]
- 45.Girardi G, Mackman N. Tissue factor in antiphospholipid antibody-induced pregnancy loss: a pro-inflammatory molecule. Lupus. 2008;17(10):931–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shamonki JM, Salmon JE, Hyjek E, Baergen RN. Excessive compement activation is associated with placental injury in patients with antiphospholipid antibodies. Am J Obstet Gynecol. 2007;196(2). [DOI] [PMC free article] [PubMed] [Google Scholar]


