Abstract
Social play is a highly rewarding and motivated behavior predominately displayed by juveniles and expressed by nearly all mammalian species. Prior work suggested that the vasopressin (AVP) and oxytocin (OT) systems can regulate the expression of social play in sex-specific ways. Here we investigated whether there are sex differences in the recruitment of vasopressinergic and oxytocinergic brain regions following social play exposure in juvenile rats. Single-housed rats were allowed to play, in their home cage, with an age- and sex-matched unfamiliar conspecific for 10 min, or received similar handling but no partner. Double-labeled fluorescent immunohistochemistry for Fos and either AVP or OT was completed in adjacent series of tissue to determine recruitment of AVP- and OT-immunoreactive neurons in response to social play. Exposure to social play did not increase recruitment of AVP or OT neurons in the supraoptic (SO) or paraventricular (PVH) hypothalamic nuclei of either sex compared to the no-play control condition. Interestingly, there was a robust sex difference in SO recruitment, irrespective of social play condition, with males exhibiting twice the recruitment of SO-AVP and SO-OT neurons compared to females. Lastly, exposure to social play increased recruitment of the posterior bed nuclei of the stria terminalis (pBST) and the posterodorsal medial amygdalar nucleus (MEApd) compared to the no-play control condition, and this effect was most pronounced in females. Our findings revealed sex differences in the recruitment of brain regions (i) independent of play condition (i.e., SO) possibly representing a sex difference in the baseline levels of AVP and OT signaling required for typical functioning and (ii) specific to play condition (i.e., pBST, MEApd). In sum, this study provides further evidence that the neural substrates underlying social play behavior are sex-specific.
Keywords: vasopressin, oxytocin, medial amygdala, bed nucleus of the stria terminalis, sex differences
1 INTRODUCTION
Social play behavior is predominately displayed by juveniles of both sexes across nearly all mammalian species (1, 2). This evolutionary conservation is likely due to the importance of social play in the development of social, emotional, and cognitive skills that are essential for appropriate social interactions in adulthood (3–6). Moreover, social play is a highly rewarding behavior (7–9) and can be modulated by motivational state (10, 11). Understanding the neurobiological underpinnings of social play may have important implications for neurodevelopmental disorders like autism spectrum disorders (ASD), where the motivation to engage in social play is impaired (12, 13).
The neuropeptides vasopressin (AVP) and oxytocin (OT) have been implicated in the regulation of many social behaviors (14–16), including social play (17–22). These neuropeptides are evolutionally conserved across the animal kingdom (15, 16, 23), and have been implicated in neurodevelopmental disorders like ASD (24, 25). Notably, clinical trials have administered these neuropeptides to human subjects to test their ability to improve social functioning (clinicaltrials.gov).
We recently demonstrated that the regulation of social play by AVP and OT is sex-specific in juvenile rats. Specifically, central blockade of the AVP 1a receptor (V1aR) decreased social play behavior in males and increased social play behavior in females (18), while local V1aR blockade in the lateral septum (LS) produced the opposite pattern of results (18, 19). Although central or intra-LS blockade of the OT receptor (OTR) did not alter social play expression in either sex (18, 19), local infusion of OT into the LS decreased social play in juvenile females and did not alter social play in males (19). However, it is unclear whether the sex-specific regulation of social play by the AVP and OT systems corresponds with sex differences in the activation of vasopressinergic and oxytocinergic brain regions during social play.
In the current study, we determined whether there are sex differences in the recruitment of vasopressinergic and oxytocinergic brain regions in response to social play exposure in juvenile rats. We analyzed recruitment of the supraoptic (SO) and paraventricular (PVH) hypothalamic nuclei, which are the largest sources of AVP and OT (26). We also analyzed recruitment of regions of extrahypothalamic AVP synthesis, specifically the posterior region of the bed nuclei of the stria terminalis (pBST) and the posterodorsal part of the medial amygdalar nucleus (MEApd) (27–29). Recruitment was measured via fluorescent double-labeled immunohistochemistry for AVP or OT combined with Fos, a commonly used indirect measure of neuronal activation (30, 31). We hypothesized that Fos induction within AVP and OT neurons would be increased in rats that were exposed to social play compared to those that were not exposed to social play, and that sex differences in Fos induction patterns might be observed.
2 MATERIAL AND METHODS
2.1 Animals
Experimentally naïve male and female Wistar rats (28 days old at arrival; Charles River Laboratories, Kingston, NY) were housed in single sex groups of two to four in standard rat cages (48 × 27 × 20 cm) and maintained under standard laboratory conditions (12 hr light/dark cycle, lights off at 14:00 h, 22 C, 50% humidity, food and water ad libitum). All housing and testing was in accordance with the National Institute of Health Guidelines for Care and Use of Laboratory Animals and the Boston College Institutional Animal Care and Use Committee.
2.2 Behavioral procedure and analysis
Following arrival, rats were acclimated to the housing room for at least 24 hrs prior to the start of daily handling. At 31–32 days of age, experimental rats were individually housed in clean cages approximately 4 h prior to the onset of the dark phase and were maintained individually housed for the remainder of the experiment (stimulus rats remained group housed). That same day, during the first hour of the dark phase, experimental rats received the first of two habituation sessions (that occurred on consecutive days) to the social play procedure: home cages were removed from the cage rack and placed on the floor of the housing room, wire cage lids were removed and replaced with a Plexiglas lid, and a tripod and video camera were set up above each cage. After ten minutes, wire lids were replaced and cages returned to the cage rack. During habituation sessions, no social play partners were present. The day after the last habituation session, subjects underwent the social play test (at 33–34 days old). The 10 min social play test procedure was identical to the habitation sessions described above, except that the Play groups (males n=8, females n=8) were exposed to an age- and sex-matched unfamiliar stimulus rat during the test while the No-Play groups (males n=8, females n=8) were not given access to a conspecific. Stimulus rats were striped with a permanent marker 30–60 min prior to testing in order to distinguish them from the experimental rats during later video analysis. After the test, rats were left undisturbed in the housing room until sacrifice 80 min later (see 2.3 Histological procedure). Food and water were not available during the 10 min habituation or testing sessions, but were immediately returned when each session was complete. All habituation and testing took place during the first hour of the dark phase, and testing order was counterbalanced across sex and play conditions. All sessions were videotaped for future behavioral analysis.
Behavior during the test was analyzed using JWatcher software (jwatcher.ucla.edu), by a trained observer unaware of the sex of each subject. Videos for subjects in the Play groups were analyzed for the amount of time spent engaged in social play behavior (playful interactions with the intruder), social investigation (sniffing the anogenital region of intruder), allogrooming (grooming head and neck of intruder), and non-social behavior (e.g., cage exploration, self-grooming). The frequency of main stereotypical play behaviors, specifically, nape attacks (resident attacks or makes nose contact with nape of intruder), pins (resident holds intruder in supine position) and supine positions (resident is pinned on its back by intruder) was also analyzed (individually as well as total), according to (18). Nape attacks and pins are considered measures of social play motivation, while supine positions represent play receptivity (5, 10, 11) and are less frequently expressed by experimental rats in our testing paradigm (18, 19, 32). Videos for subjects in the No-Play groups were watched to confirm the presence of typical behaviors (such as cage exploration and self-grooming) and the absence of abnormal behaviors.
2.3 Histological procedure
Rats were deeply anesthetized with isoflurane, then transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M borate buffer (pH 9.5). Brains were extracted and post-fixed overnight in a solution of 12% sucrose dissolved in the perfusion liquid, then rapidly frozen in hexanes cooled in dry ice, and stored at −45°C. Brains were sliced into 30μm coronal sections using a sliding microtome (Leica SM2000R) and collected into four series. Three series were put into cryoprotectant solution (0.05 M sodium phosphate buffer, 30% ethylene glycol, 20% glycerol) and stored at −20°C until later immunohistochemical processing. One series was collected into tris-buffered saline solution (TBS; 50 mM, pH 7.6), mounted onto gelatin-coated slides, and stained with thionin (as described in: 33) for identification of cytoarchitectonic borders of brain regions as defined in Swanson’s rat brain atlas (34).
Separate series of brain sections were processed using double-label fluorescent immunohistochemistry for Fos and either AVP or OT. AVP and OT were visualized using highly specific monoclonal antibodies raised against mammalian AVP and OT-associated neurophysins and exhibit no cross-reactivity (35). Tissue was processed sequentially, first for Fos and then for AVP or OT. The histological procedures described below were completed in semi-darkness and out of direct light to preserve fluorescence.
For each series, tissue was removed from cryoprotectant storage solution and thoroughly rinsed in TBS. To process for Fos, tissue was incubated for 24 h at 4°C in a blocking solution [TBS with 0.3% Triton X-100 and 2% normal donkey serum (017-000-121, Jackson ImmunoResearch, West Grove, PA)] with the primary antibody anti-cFos raised in rabbit (1:7.5K; ABE457, Millipore, Billerica, MA). After rinses in TBS, tissue was incubated for one hour in the blocking solution containing the secondary antibody Alexa 594 anti-rabbit raised in donkey (1:500; 711-585-152, Jackson ImmunoResearch, West Grove, PA). Following rinses in TBS, tissue was then processed for AVP or OT by incubation for 24 h at 4°C in the blocking solution containing either anti-AVP-associated neurophysin raised in mouse (1:10K; PS41, Dr. Harold Gainer, NIH, Bethesda, MD) or anti-OT-associated neurophysin raised mouse (1:10K; PS38, Dr. Harold Gainer, NIH, Bethesda, MD). Tissue was then rinsed in TBS and incubated for 1 h in the blocking solution containing the secondary antibody Alexa 488 anti-mouse raised in donkey (1:500; 715-545-150, Jackson ImmunoResearch, West Grove, PA). Following rinses in TBS, tissue was mounted onto gelatin-coated slides, air-dried, coverslipped with Vectashield HardSet Mounting Medium with DAPI (4′,6-diamidino-2-phenylindole; H-1500; Vector Labs, Burlingame, CA, USA), and stored at 4 °C.
2.4 Image acquisition and analysis
For the SO and PVH, images of the processed tissue sections were acquired with a 20X objective on a Zeiss AxioImager fluorescence microscope with ApoTome attachment (Carl Zeiss Microscopy GmbH, Jena, Germany), Hamamatsu Orca-R2 camera (Bridgewater, NJ), and Zen software (Carl Zeiss Microscopy GmbH, Jena, Germany). For the SO, three bilateral sets of images were taken at the mid-rostral, middle, and mid-caudal points of the region (corresponding to atlas levels 21, 23, and 25; (34); Figure 1A). For the PVH, bilateral images were taken across three adjacent sections of the most AVP- and OT-dense area (corresponding a region spanning from caudal atlas level 25 thru rostral atlas level 27 (34); Figure 1B). For both the SO and PVH, single or tiled images were taken as needed to capture the entirety of the AVP or OT population at each sampling location, and counts were done within the neuroanatomical borders of each brain region (Figure 1A, 1B) based on neuropeptide distribution, reference to the adjacent Nissl-stained series, and the Swanson rat brain atlas (34).
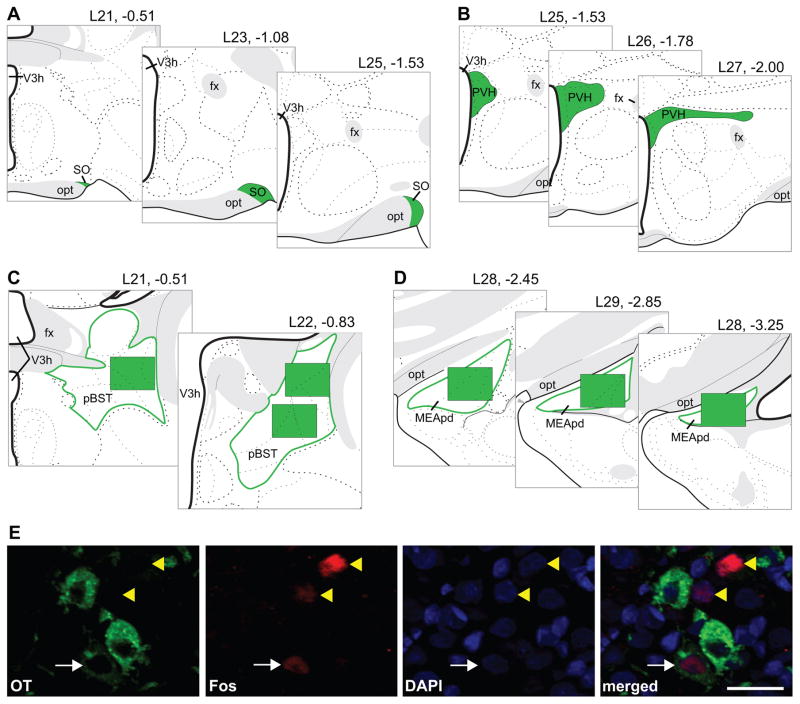
Figure 1.
Representation of sampling locations shown on modified rat brain atlas templates (34) for the SO (A), PVH (B), pBST (C), and MEApd (D); headings refer to atlas level and distance in mm from bregma; green-filled areas refer to the analyzed locations. Example of analysis procedure (E) showing OT cytoplasmic labeling in green, Fos nuclear staining in red, and DAPI nuclear counterstain in blue. The number of double-labeled (white arrow) and single-labeled Fos-immunoreactive nuclei (yellow arrowheads) were counted. Scale bar = 25 μm, opt = optic tract, fx = fornix.
For the pBST and MEApd, images of the processed tissue sections were acquired with a 20X objective on a Keyence BZ-X700E/BZ-X710 fluorescence microscope and associated BZ-H3AE software (Keyence Corporation of America, Elmwood Park, New Jersey). For the pBST, three bilateral sets of images were taken: one at atlas level 21, and two (one dorsal, one ventral) at atlas level 22 ((34); Figure 1C). For the MEApd, three bilateral sets of images (taken at atlas levels 28–30 (34)) sampled the rostocaudal extent of the region (Figure 1D). Counts for the pBST and MEApd were done for the entirety of each image obtained, and the image field-of-view was 724.69 μm × 543.52 μm (Figure 1C, 1D).
Analysis was conducted from the triple-merged images (composite images showing each fluorophore/stain used; Figure 1E, far-right image); however, single-label images (Figure 1E, first three images) were consulted as needed to confirm the cell and stain type. A neuron was counted as AVP- or OT-immunoreactive (-ir) only if both the cell body and the nucleus were visible. A neuron was counted as Fos-ir when the Fos labeling was contained within the nucleus. The nuclear counterstain, DAPI, was used in all images to identify nuclei. All analysis was conducted by an experimenter unaware of the experimental condition or sex of the rats corresponding to the images.
For the SO and PVH, the number of double-labeled neurons (AVP-ir + Fos-ir, OT-ir + Fos-ir) and the total number of Fos-ir neurons were quantified. The percent of the total Fos induction that occurred within AVP-ir and OT-ir neurons was then calculated separately for each series [(number of double-labeled neurons in one series)/(total number of Fos-ir neurons in the same series)*100]. Due to the high density of AVP- and OT-synthesizing neurons in the SO and PVH, the total number of AVP-ir or OT-ir neurons was not counted. AVP-ir neurons in the pBST and MEApd could not be consistently identified; thus, only total Fos induction in these brain regions was quantified. The number of double-labeled neurons (for SO and PVH) and total number of Fos-ir neurons (for all brain regions analyzed) were averaged by hemisphere at each sampling location. Although multiple sampling locations were analyzed across each brain region, no significant regional differences were found in the pattern of Fos induction (i.e., in repeated measures ANOVAs there were no significant within-subjects interactions with sampling position). Therefore, counts were summed across samples for each brain region.
2.5 Statistical analysis
For the Play groups, independent sample t-tests were used to analyze the effect of sex on social behaviors during the test. Two-way analysis of variances (ANOVAs) were used to analyze the effects of play condition and sex on Fos induction within AVP or OT neurons in the SO and PVH, and total Fos induction in the pBST and MEApd. Mixed design (tissue series × play condition × sex) ANOVAs were used to analyze total Fos induction within the SO and PVH. If a main effect of play condition was reported, then post hoc independent samples t-tests were completed and Cohen’s d effect size measures computed to determine if the effect was similar in males and females. Pearson correlations (using all subjects in the play condition) were completed to explore whether the number of double-labeled neurons in SO or total Fos induction in the pBST and MEApd (neuronal populations that had robust and/or differential Fos induction) was related to the time spent engaged in social play behavior (as an indicator of overall playfulness). All data were assessed for normality at the group level via Shapiro-Wilk; data analyzed with t-Tests were assessed with Levene’s test for equality of variances and adjusted t/df values were used as directed by this test. Due to tissue damage, Fos data for the following were not collected: SO (AVP: 2 females play; OT: 2 females play, 1 female no-play), PVH (AVP: 1 female play, 1 male no-play; OT: 1 female play), pBST (1 male play, 1 male no-play, 1 female play), MEApd (2 males no-play condition, 1 female no-play condition). Data were analyzed using IBM SPSS Statistics 24, and statistical significance was set at p < 0.05.
3 RESULTS
3.1 Effect of Sex on Social Play Behavior
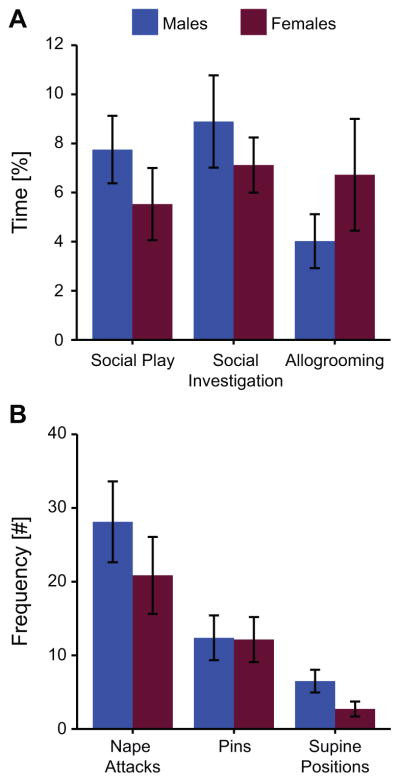
Males and females in the Play groups exhibited similar behavior during the test (Figure 2). Specifically, there were no significant sex differences in the duration of social play behavior (t(14) = 1.10, p = 0.30), social investigation (t(14) = 0.81, p = 0.43), or allogrooming (t(14) = 1.07, p = 0.30). There were also no significant sex differences in social play motivation as measured by the number of nape attacks (t(14) = 0.95, p = 0.40) and pins (t(14) = 0.05, p = 0.96), but there was a trend for females exhibiting reduced play receptivity compared to males as measured by the number of supine positions (t(14) = 1.99, p = 0.07). However, the total number of all three play elements combined was similar between the sexes (males: 47 ± 9, females 36 ± 9; t(14) = 0.87, p = 0.40).
Figure 2.
The percent of time juvenile male and female rats spent engaged in social behaviors (A) and the frequency rats expressed stereotypical social play elements (B) during the 10 min test in which each rat is exposed in its home cage to an age- and sex-matched unfamiliar stimulus rat. Data presented as mean ± SEM.
3.2 Effects of Play condition and Sex on Fos induction in the SO
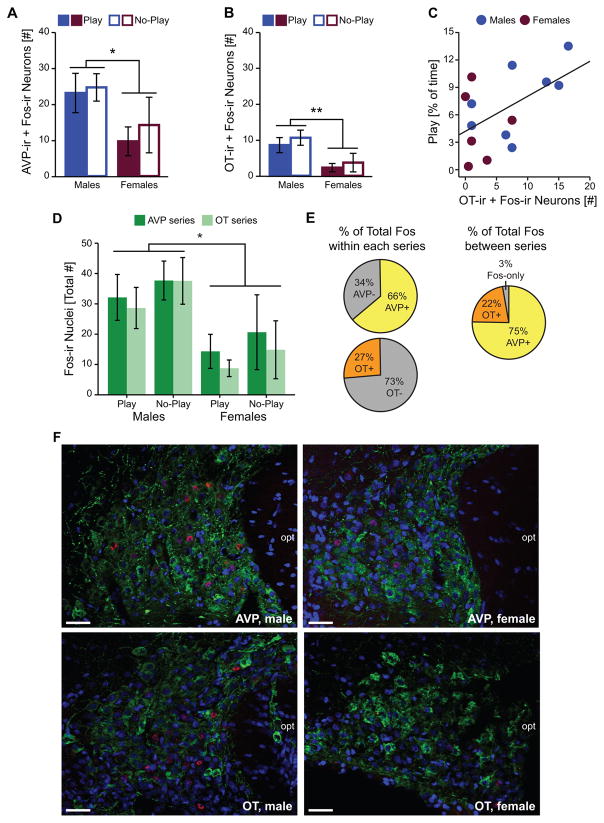
In the SO, there was no effect of play condition on Fos induction within AVP-ir neurons or OT-ir neurons. However, Fos induction was significantly greater in males compared to females within AVP-ir neurons (Figure 3A, F) and OT-ir neurons (Figure 3B, F; see Table 1 for complete statistics). Fos induction within OT-ir, but not AVP-ir, neurons significantly correlated with the percent of time subjects were engaged in social play (OT: r(14) = 0.54, p < 0.05; AVP: r(14) = 0.35, p = 0.22; Figure 3C). Although this correlation effect appeared to be driven by the males, it did not reach significance for either sex when examined separately (males: r(8) = 0.62, p = 0.10; females: r(6) = −0.10, p = 0.85).
Figure 3.
Effects of play condition and sex on Fos induction within the SO. Fos induction within AVP-ir (A) and OT-ir (B) neurons. (C) Correlation between the time rats spent engaged in social play and Fos induction within OT-ir neurons. (D) Total SO Fos induction in AVP and OT tissue series. (E) Percent of total Fos induction that occurred within SO AVP-ir and SO OT-ir neurons within each series (left), and estimated between series (right). (F) Representative photomicrographs illustrate Fos induction (red), AVP-ir or OT-ir neurons (green), and the nuclear counterstain DAPI (blue). Bar graphs display mean ± SEM; *p < 0.05, **p < 0.01; opt = optic tract; scale bars = 50 μm.
Table 1.
ANOVA statistics for measures of Fos-ir. Significant effects are bolded.
| Play Condition | Sex | Play Condition X Sex | Effects or Interactions with Tissue Series | |
|---|---|---|---|---|
| SO | ||||
| AVP-ir + Fos-ir | F(1,30) = 0.28, p = 0.60 | F(1,30) = 4.34, p = 0.047 | F(1,30) = 0.067, p = 0.78 | n/a |
| OT-ir + Fos-ir | F(1,29) = 0.66, p = 0.43 | F(1,29) = 9.54, p = 0.005 | F1,29) < 0.10, p = 0.88 | n/a |
| Total Fos-ir | F(1,25) = 0.75, p = 0.39 | F(1,25) = 6.22, p = 0.020 | F(1,25) < 0.10, p = 0.95 | F(1,25) < 4.05, p > 0.055, all |
| % of Total Fos-ir in AVP-ir | F(1,30) < 0.10, p = 0.95 | F(1,30) = 0.24, p = 0.63 | F(1,30) = 0.22, p = 0.64 | n/a |
| % of Total Fos-ir in OT-ir | F(1,29) = 0.47, p = 0.50 | F(1,29) = 0.72, p = 0.40 | F(1,30) = 0.81, p = 0.38 | n/a |
|
| ||||
| PVH | ||||
| Total Fos-ir | F(1,26) = 2.45, p = 0.13 | F(1,26) = 0.62, p = 0.44 | F(1,26) = 0.46, p = 0.51 | F(1,26) < 1.25, p > 0.28, all |
|
| ||||
| pBST | ||||
| Total Fos-ir | F(1,29) = 11.3, p = 0.002 | F(1,29) = 3.48, p = 0.074 | F(1,29) = 3.84, p = 0.061 | n/a |
|
| ||||
| MEApd | ||||
| Total Fos-ir | F(1,29) = 11.2, p = 0.003 | F(1,29) < 0.10, p = 0.86 | F(1,29) = 3.20, p = 0.086 | n/a |
The patterns of SO-AVP and SO-OT activation reflected the pattern of total Fos induction within the SO. Specifically, the total number of Fos-ir nuclei in the SO was greater in males than in females (Figure 3D, F), but was not affected by play condition and did not differ between the two series of tissue (see Table 1 for complete statistics). This resulted in the percent of the total SO Fos induction that occurred within AVP-ir or OT-ir neurons being similar across sexes and play conditions (AVP: 66.6% ± 2.7, OT: 27.3% ± 3.2; see Table 1 for statistics and Section 2.4 for calculation). Since total Fos induction was similar in the two series of tissue and AVP and OT rarely colocalize in the SO (36), we can further estimate the percent of Fos-ir only nuclei (those that did not occur within AVP-ir and OT-ir cell bodies) by recalculating the percent of total SO Fos induction that occurred within AVP-ir or OT-ir neurons from the average amount of total Fos induction between the two series (AVP: 75.4% ± 4.1, OT: 22.1% ± 1.9). Estimates from both the within series (Figure 3E, left) and between series (Figure 3E, right) calculations suggest that Fos induction within AVP-ir and OT-ir neurons accounted for > 90% of the total Fos induction observed in the SO.
3.3 Effects of Play Condition and Sex on Fos induction in the PVH
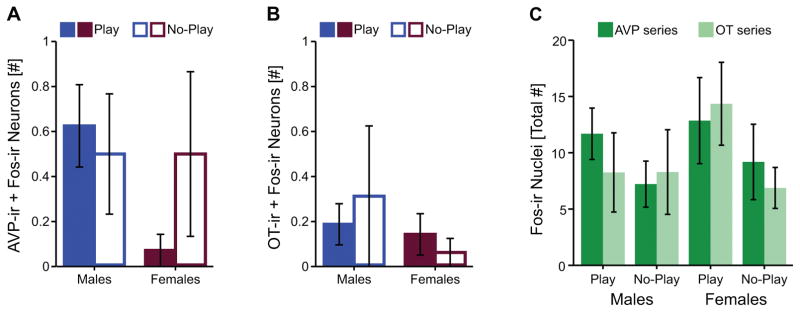
In the PVH, there was negligible Fos induction within AVP or OT neurons (Figure 4A, B). Some Fos induction was observed in non-AVP-ir or non-OT-ir neurons in the PVH, but total PVH Fos-ir was similar across groups and between the two series of tissue (see Table 1 for statistics; Figure 4C). Therefore, no further analyses were justified.
Figure 4.
Fos induction within the PVH. Fos induction within AVP-ir (A) and OT-ir (B) neurons, and total PVH Fos induction in AVP and OT tissue series (C). Bar graphs display mean ± SEM.
3.4 Effects of Play Condition and Sex on Fos induction in the pBST and MEApd
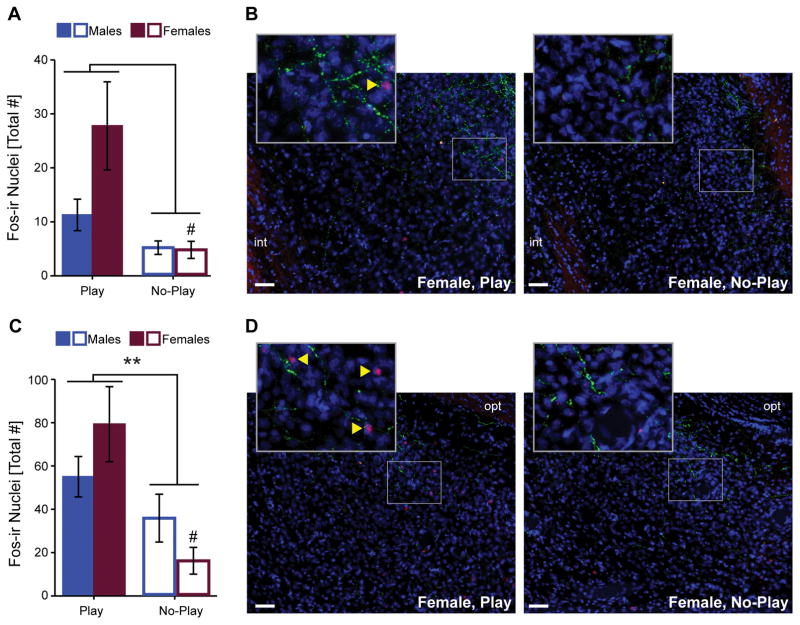
AVP-ir neurons in the pBST and MEApd could not be consistently identified (see also sections 2.4 and 4.3); thus, only total Fos induction in these brain regions was quantified. In the pBST, Fos induction was significantly greater in subjects in the Play compared to the No Play groups (see Table 1 for statistics; Figure 5A, B). Females had greater Fos induction than males which was reflected by the main effect of sex as well as the play condition by sex interaction approaching significance (see Table 1 for statistics). Post hoc tests to assess whether the effect of play condition on pBST Fos induction was similar between the sexes showed that there was a significant difference between the Play and No Play groups in females (t(6.5) = 2.76, p <0.05, d = 1.47), and this comparison approached significance in males (t(12) = 1.91, p = 0.08, d = 1.02). Fos induction in the pBST did not correlate with the percent of time subjects were engaged in social play (r(14)= 0.01, p = 0.99).
Figure 5.
Fos induction in pBST (A) and MEApd (C). Representative photomicrographs of pBST (B) and MEApd (D) illustrate Fos induction (red), AVP-ir fibers (green), and the nuclear counterstain DAPI (blue). Bar graphs displays mean ± SEM; yellow arrowheads on insets indicate Fos-ir nuclei; **p < 0.01; # p<0.05 between Play and No-Play groups of the same sex; int = internal capsule, opt = optic tract; scale bars = 50 μm.
In the MEApd, Fos induction was significantly greater in subjects in the Play compared to the No Play groups (see Table 1 for statistics; Figure 5C, D). There was no effect of sex on MEApd Fos induction, but a sex by play condition interaction approached significance (see Table 1 for statistics). Post hoc tests to assess whether the effect of play condition on MEApd Fos induction was similar between the sexes showed a significant difference between the Play and No Play groups in females (t(8.7) = 3.42, p <0.01, d = 1.73), but not in males (t(12)=1.33, p = 0.21, d = 0.72). Fos induction in the MEApd did not correlate with the percent of time subjects were engaged in social play (r(16)= −0.19, p = 0.49).
4 DISCUSSION
In the current study, we examined recruitment of vasopressinergic and oxytocinergic brain regions, and observed region- and sex-specific patterns of Fos induction in response to social play exposure in juvenile rats. Contrary to our hypothesis, exposure to social play did not increase Fos induction of AVP- or OT-synthesizing neurons in the SO or PVH of either sex compared to the no-play control condition. However, a positive correlation was found between recruitment of SO-OT neurons and the percentage of time spent engaged in social play. Interestingly, we observed a robust sex difference in Fos induction in the SO, irrespective of social play condition, with males exhibiting twice the recruitment of SO-AVP and SO-OT neurons compared to females. In contrast, there was very little recruitment of PVH-AVP or PVH-OT neurons across all conditions. Although AVP neurons could not be quantified in the pBST or MEApd, these two regions showed increased Fos induction in the social play compared to the no-play control condition, and this effect was most pronounced in females. Despite sex differences in neural recruitment patterns, males and females displayed similar levels of social play behavior which was consistent with prior reports (18, 19, 32). Together, the sex-specific recruitment of the SO (irrespective of play condition), and pBST and MEApd (specific to the social play condition) strongly suggest that the functional neural systems that support the expression of social play behavior are different in males and females.
4.1 Sex differences in baseline activation of the SO
Irrespective of play condition, juvenile male rats exhibited twofold greater recruitment of SO-AVP and SO-OT neurons compared to females. This sex difference in recruitment could be reflective of sex differences in the number of AVP or OT neurons in the SO (which was not assessed in the current study, see section 2.4). Indeed, the area of the SO expressing AVP neurons has been reported to be larger in juvenile and adult male compared to female rats (37, 38). However, this difference in area appears to be due to differences in neuronal size as opposed to neuronal number (37). Moreover, there is no sex difference in SO-OT expression in juvenile or adults rats (39, 40). Therefore, our results likely suggest a baseline difference in the activation of vasopressinergic and oxytocinergic neurons in the SO, and the potential for higher basal AVP and OT signaling in juvenile male compared to female rats.
In both sexes and across play conditions, activation of AVP and OT neurons represented nearly all of the observed SO Fos induction, consistent with the composition of the SO (36, 41). We estimated that < 10% of the total Fos induction was outside of AVP-ir and OT-ir neurons; this non-AVP and non-OT Fos induction is more likely to represent activation of excitatory neuronal or astrocytic populations (41), rather than inhibitory neuronal populations (42, 43). This additional Fos induction could also be the result of sampling error (e.g., due to the nature of 2D imaging, the counts for AVP and OT being completed in separate series of tissue, or weakly labeled AVP/OT neurons that were not classified as AVP- or OT-positive).
Exposure to social play did not increase Fos induction within any of the AVP or OT populations investigated in the current study. When considered in isolation, one possible interpretation of our data is that these neuropeptides are unimportant for the expression of social play behavior. However, prior studies in juvenile rats have shown this is not the case (18–20). Instead of direct real-time control of social play behavior, it could be that a specific baseline level of AVP and OT signaling is required for normal expression of social play behavior, and that alternations to these baseline levels via pharmacological manipulations (18, 19) or a congenital absence of AVP signaling (20) results in alternations in social play. If this were the case, then the robust sex difference in baseline activation of SO-AVP and SO-OT neurons could represent a new example of the dual-function hypothesis (44), suggesting that these sex differences in the brain exist in order to prevent sex differences in levels of social play behavior. This interpretation is consistent with earlier data in juvenile rats showing the emergence of sex differences in social play behavior when AVP signaling is disrupted (18, 19), and OT signaling is increased (19).
Although we did not observe differences in the recruitment of AVP- and OT-synthesizing neurons in subjects exposed to social play compared to no-play controls in the present study, not all sources of AVP and OT were investigated. For example, AVP- and OT-synthesizing neurons are found in scattered islands of accessory magnocellular groups (26) and few studies have explored the function of these neurons (45). There might also be species differences in the activation of these neuronal populations in response to social play, as a social play-induced increase in SO-AVP Fos induction was observed in juvenile male golden hamsters (21). Lastly, immediate early gene induction mapping comes with limitations, and in the current study only one marker (Fos) was used to assess neuronal recruitment (30, 31). Thus, mapping the induction of other immediate early genes or performing electrophysiological recordings could reveal social play-specific recruitment of AVP or OT neuronal populations.
4.2 A potential role for SO-OT neurons in social play expression
SO-OT activation was positively correlated with the percentage of time rats spent engaged in social play behavior. We postulate that individual differences in the level of social play expression could account for this correlation, in the absence of a main effect of social play exposure on SO-OT recruitment. Correlations derived from a small sample tend to be underpowered and should be interpreted with caution. Indeed, post hoc power analyses using G*Power (46) showed the statistical power of this correlation to be 0.55 (the sex-specific correlations had even lower power, 0.40 for males and 0.05 for females). Therefore, this finding warrants future investigations to more directly assess a potential role for SO-OT neurons in social play expression. Interestingly, recent evidence has implicated SO-OT neurons in other social behaviors. For example, SO-OT neurons are recruited during conditioned mate guarding in female rats (47) and pup exposure in female mice (48), and may facilitate social recognition in male mice and rats (49). SO-OT recruitment could be a consequence of social play expression, since tactile stimulation increased Fos induction in SO-OT neurons of adult male rats (50) and central or LS-specific blockade of OT signaling did not alter social play expression in juvenile male or females rats (18, 19). Conversely, SO-OT could regulate social play expression by activation of specific pathways and/or target regions. In support, local brain region-specific manipulations of OT signaling altered social play expression in juvenile female rats (19). To better understand the possible role of SO-OT neurons in social play behavior, future work could investigate the efferent targets of SO-OT neurons that are recruited during social play, and whether inhibition or stimulation of SO-OT neurons alters social play expression.
4.3 Social play recruits the pBST and MEApd in sex-specific ways
We observed increased Fos induction in the social play compared to the no-play control condition in the pBST and MEApd. Importantly, the effect sizes of these comparisons were larger in females compared to males, especially in the MEApd. Thus, the pBST and MEApd are potentially important regions in regards to the sex-specific regulation of social play, and may serve as additional examples of the dual-function hypothesis (44). That is, greater play-specific recruitment of the pBST and MEApd in females compared to males may be important for preventing sex differences in the levels of social play behavior. Our pBST results are in agreement with increased BST Fos induction (in a region rostral to that analyzed in the current study) in juvenile male rats (51) and increased pBST Fos induction in juvenile male golden hamsters (21) in response to social play. Further, while social play exposure increased MEApd Fos induction in juvenile male golden hamsters (21), prior studies in juvenile male rats did not observe increased Fos induction when the entire amygdala (52) or entire MEA (51) were assessed. Together, this highlights the importance of neuroanatomical specificity and the inclusion of both sexes in studies investigating the neural substrates underlying social play behavior.
Whether the Fos induction observed in the pBST or MEApd reflected activation of AVP neurons remains unknown as we were unable to confidently identify AVP-ir neurons in these regions. This is most likely due to a combination of factors including the lower density, smaller size, and lighter staining of AVP-ir neurons in the pBST and MEApd compared to those in the SO and PVH (53, 54), intermingled placement of extrahypothalamic AVP-ir neurons among AVP-ir fibers (53, 54), as well as the low number of extrahypothalamic AVP-ir neurons in juveniles compared to adults (53). Methodological limitations may have also contributed to our difficulty in identifying extrahypothalamic AVP-ir neurons. In situ hybridization techniques tend to report higher numbers of AVP-positive neurons in juvenile rats compared to immunohistochemical methods (38, 53).
It is important to note that extrahypothalamic sources of AVP likely contribute to the sex-specific regulation of social play behavior. Specifically, pBST-AVP mRNA expression negatively correlated with social play expression in juvenile male, but not female, rats (17). This seems in line with the observation that social play behavior increased in juvenile male, but not female, rats upon V1aR antagonist administration in the LS (18, 19), a brain region that receives AVP fiber projections from the pBST (55). Our finding of sex differences in the recruitment of the pBST and MEApd during social play provides a means by which differential activation of extrahypothalamic-AVP to LS pathways (55, 56) could support the sex-specific regulation of social play. Obtaining direct evidence for this hypothesized functional pathway is an important avenue for future research.
4.4 Weak recruitment of the PVH across conditions
There was negligible Fos induction of AVP and OT neurons in the PVH across sexes and experimental conditions, suggesting that, despite prior evidence that PVH-AVP expression positivity correlates with social play in juvenile male rats (17), there is little activation of these populations during social play. Further, although Fos induction was reported in AVP-ir neurons following social play exposure in juvenile male golden hamsters, the level of Fos induction did not differ from the control condition (21). We observed some Fos induction in cells not labeled for either AVP or OT; this weak PVH recruitment was similar between the sexes and there was no effect of social play exposure of total Fos induction, consistent with prior reports in juvenile male rats (52). The PVH is a highly heterogeneous brain region, containing multiple distinct populations (57) and determining the phenotypes of PVH cells recruited during this behavioral paradigm should be investigated in future research.
4.5 Conclusion
We identified neural substrates whose Fos induction patterns were associated with the expression of social play behavior by juvenile rats that were exposed in their home cage to a novel conspecific: the pBST, the MEApd, and possibly SO-OT neurons. Furthermore, we observed sex differences in Fos induction patterns for the SO, pBST, and MEApd. Because males and females show similar levels of social play behavior, our findings may indicate that these sex differences in the brain act to prevent sex differences in the expression of social play. Specifically, we propose that the robust sex difference in the activation of the SO represents a sex difference in the baseline levels of AVP and OT signaling required for typical functioning, and that females require greater increases from baseline pBST and MEApd activation in order to show levels of social play behavior similar to that of males.
Acknowledgments
We thank Remco Bredewold for instruction in social play testing and video analysis, Suhana Posani for technical assistance, current members of the Veenema lab for their critical reading of the manuscript, and the 2017 World Congress on Neurohypophysial Hormones for the Glenn I. Hatton Memorial Travel Award given to CJR. This research was supported by NIMH Grant R01MH102456 to AHV.
Footnotes
AUTHORS’ CONTRIBUTIONS
CJR and AHV designed the experiment; CJR and CKG performed the behavioral and histological procedures; CKG analyzed the behavioral videos, and CJR performed the imaging and analysis; CJR wrote the manuscript, with input from CKG and AHV.
CONFLICTS OF INTEREST
The authors of the manuscript have no conflicts of interest to declare.
References
- 1.Pellis SM, Iwaniuk AN. Comparative analyses of the role of postnatal development on the expression of play fighting. Dev Psychobiol. 2000;36(2):136–47. [PubMed] [Google Scholar]
- 2.Bekoff M, Byers JA. Animal Play: Evolutionary, Comparative, and Ecological Perspectives. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- 3.Siviy SM. A brain motivated to play: insights into the neurobiology of playfulness. Behavior. 2016;153(6–7):819–44. doi: 10.1163/1568539X-00003349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Berg CL, Hol T, Van Ree JM, Spruijt BM, Everts H, Koolhaas JM. Play is indispensable for an adequate development of coping with social challenges in the rat. Developmental Psychobiology. 1999;34(2):129–38. [PubMed] [Google Scholar]
- 5.Vanderschuren LJMJ, Niesink RJM, VanRee JM. The neurobiology of social play behavior in rats. Neurosci Biobehav R. 1997;21(3):309–26. doi: 10.1016/s0149-7634(96)00020-6. [DOI] [PubMed] [Google Scholar]
- 6.Spinka M, Newberry RC, Bekoff M. Mammalian play: Training for the unexpected. Q Rev Biol. 2001;76(2):141–68. doi: 10.1086/393866. [DOI] [PubMed] [Google Scholar]
- 7.Calcagnetti DJ, Schechter MD. Place conditioning reveals the rewarding aspect of social-interaction in juvenile rats. Physiology & Behavior. 1992;51(4):667–72. doi: 10.1016/0031-9384(92)90101-7. [DOI] [PubMed] [Google Scholar]
- 8.Humphreys AP, Einon DF. Play as a reinforcer for maze-learning in juvenile rats. Anim Behav. 1981 Feb;29:259–70. [Google Scholar]
- 9.Achterberg EJM, van Kerkhof LWM, Servadio M, van Swieten MMH, Houwing DJ, Aalderink M, Driel NV, Trezza V, Vanderschuren LJMJ. Contrasting roles of dopamine and noradrenaline in the motivational properties of social play behavior in rats. Neuropsychopharmacology. 2016;41(3):858–68. doi: 10.1038/npp.2015.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panksepp J, Beatty WW. Social deprivation and play in rats. Behav Neural Biol. 1980;30(2):197–206. doi: 10.1016/s0163-1047(80)91077-8. [DOI] [PubMed] [Google Scholar]
- 11.Ikemoto S, Panksepp J. The effects of early social-isolation on the motivation for social play in juvenile rats. Developmental Psychobiology. 1992;25(4):261–74. doi: 10.1002/dev.420250404. [DOI] [PubMed] [Google Scholar]
- 12.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16(4):231–9. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan R. Social play and autistic spectrum disorders - A perspective on theory, implications and educational approaches. Autism. 2003;7(4):347–60. doi: 10.1177/1362361303007004002. [DOI] [PubMed] [Google Scholar]
- 14.Dumais KM, Veenema AH. Vasopressin and oxytocin receptor systems in the brain: Sex differences and sex-specific regulation of social behavior. Front Neuroendocrin. 2016:401–23. doi: 10.1016/j.yfrne.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900–4. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 16.Goodson JL, Thompson RR. Nonapeptide mechanisms of social cognition, behavior and species-specific social systems. Current Opinion in Neurobiology. 2010;20(6):784–94. doi: 10.1016/j.conb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Paul MJ, Terranova JI, Probst CK, Murray EK, Ismail NI, de Vries GJ. Sexually dimorphic role for vasopressin in the development of social play. Frontiers in Behavioral Neuroscience. 2014:8. doi: 10.3389/fnbeh.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veenema AH, Bredewold R, de Vries GJ. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology. 2013;38(11):2554–61. doi: 10.1016/j.psyneuen.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bredewold R, Smith CJW, Dumais KM, Veenema AH. Sex-specific modulation of juvenile social play behavior by vasopressin and oxytocin depends on social context. Frontiers in Behavioral Neuroscience. 2014:8. doi: 10.3389/fnbeh.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paul MJ, Peters NV, Holder MK, Kim AM, Whylings J, Terranova JI, de Vries GJ. Atypical social development in vasopressin-deficient brattleboro rats. Eneuro. 2016;3(2) doi: 10.1523/ENEURO.0150-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng SY, Taravosh-Lahn K, Delville Y. Neural circuitry of play fighting in golden hamsters. Neuroscience. 2008;156(2):247–56. doi: 10.1016/j.neuroscience.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 22.Cheng SY, Delville Y. Vasopressin facilitates play fighting in juvenile golden hamsters. Physiol Behav. 2009;98(1–2):242–6. doi: 10.1016/j.physbeh.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 23.de Vries GJ, Panzica GC. Sexual differentiation of central vasopressin and vasotocin systems in vertebrates: Different mechanisms, similar endpoints. Neuroscience. 2006;138(3):947–55. doi: 10.1016/j.neuroscience.2005.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 25.Zhang R, Zhang HF, Han JS, Han SP. Genes related to oxytocin and arginine-vasopressin pathways: Associations with autism spectrum disorders. Neuroscience bulletin. 2017;33(2):238–46. doi: 10.1007/s12264-017-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhodes CH, Morrell JI, Pfaff DW. Immunohistochemical analysis of magnocellular elements in rat hypothalamus - distribution and numbers of cells containing neurophysin, oxytocin, and vasopressin. Journal of Comparative Neurology. 1981;198(1):45–64. doi: 10.1002/cne.901980106. [DOI] [PubMed] [Google Scholar]
- 27.de Vries GJ, Buijs RM, Swaab DF. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat-brain - presence of a sex difference in the lateral septum. Brain Research. 1981;218(1–2):67–78. doi: 10.1016/0006-8993(81)90989-6. [DOI] [PubMed] [Google Scholar]
- 28.van Leeuwen FW, Caffe AR, de Vries GJ. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: sex differences and the influence of androgens. Brain Res. 1985;325(1–2):391–4. doi: 10.1016/0006-8993(85)90348-8. [DOI] [PubMed] [Google Scholar]
- 29.Caffe AR, van Leeuwen FW. Vasopressin-immunoreactive cells in the dorsomedial hypothalamic region, medial amygdaloid nucleus and locus coeruleus of the rat. Cell and tissue research. 1983;233(1):23–33. doi: 10.1007/BF00222229. [DOI] [PubMed] [Google Scholar]
- 30.Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991:14421–51. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhuri A. Neural activity mapping with inducible transcription factors. Neuroreport. 1997;8(16):v–ix. [PubMed] [Google Scholar]
- 32.Bredewold R, Schiavo JK, van der Hart M, Verreij M, Veenema AH. Dynamic Changes in Extracellular Release of Gaba and Glutamate in the Lateral Septum during Social Play Behavior in Juvenile Rats: Implications for Sex-Specific Regulation of Social Play Behavior. Neuroscience. 2015:307117–27. doi: 10.1016/j.neuroscience.2015.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmons DM, Swanson LW. The Nissl stain. Neurosci Protocols. 1993 050-12-01-07. [Google Scholar]
- 34.Swanson LW. Brain Maps: Structure of the Rat Brain. A Laboratory Guide with Printed and Electronic Templates for Data, Models and Schematics. Elsevier; Amsterdam: 2004. [Google Scholar]
- 35.Ben-Barak Y, Russell JT, Whitnall MH, Ozato K, Gainer H. Neurophysin in the hypothalamo-neurohypophysial system. I. Production and characterization of monoclonal antibodies. J Neurosci. 1985;5(1):81–97. doi: 10.1523/JNEUROSCI.05-01-00081.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gainer H. Cell-type specific expression of oxytocin and vasopressin genes: an experimental odyssey. J Neuroendocrinol. 2012;24(4):528–38. doi: 10.1111/j.1365-2826.2011.02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Madeira MD, Sousa N, Cadeteleite A, Lieberman AR, Paulabarbosa MM. The supraoptic nucleus of the adult-rat hypothalamus displays marked sexual dimorphism which Is dependent on body-weight. Neuroscience. 1993;52(3):497–513. doi: 10.1016/0306-4522(93)90402-2. [DOI] [PubMed] [Google Scholar]
- 38.Taylor PV, Veenema AH, Paul MJ, Bredewold R, Isaacs S, de Vries GJ. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol Sex Differ. 2012:3. doi: 10.1186/2042-6410-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minhas S, Liu C, Galdamez J, So VM, Romeo RD. Stress-induced oxytocin release and oxytocin cell number and size in prepubertal and adult male and female rats. General and comparative endocrinology. 2016:234103–9. doi: 10.1016/j.ygcen.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav. 2013;64(4):693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Ponzio TA, Ni Y, Montana V, Parpura V, Hatton GI. Vesicular glutamate transporter expression in supraoptic neurones suggests a glutamatergic phenotype. J Neuroendocrinol. 2006;18(4):253–65. doi: 10.1111/j.1365-2826.2006.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roland BL, Sawchenko PE. Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J Comp Neurol. 1993;332(1):123–43. doi: 10.1002/cne.903320109. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Ennis M, Szabo G, Armstrong WE. Characteristics of GABAergic and cholinergic neurons in perinuclear zone of mouse supraoptic nucleus. J Neurophysiol. 2015;113(3):754–67. doi: 10.1152/jn.00561.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145(3):1063–8. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- 45.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73(3):553–66. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 46.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior research methods. 2009;41(4):1149–60. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 47.Holley A, Bellevue S, Vosberg D, Wenzel K, Roorda S, Jr, Pfaus JG. The role of oxytocin and vasopressin in conditioned mate guarding behavior in the female rat. Physiol Behav. 2015:1447–14. doi: 10.1016/j.physbeh.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 48.Okabe S, Tsuneoka Y, Takahashi A, Ooyama R, Watarai A, Maeda S, Honda Y, Nagasawa M, Mogi K, Nishimori K, Kuroda M, Koide T, Kikusui T. Pup exposure facilitates retrieving behavior via the oxytocin neural system in female mice. Psychoneuroendocrinology. 2017:7920–30. doi: 10.1016/j.psyneuen.2017.01.036. [DOI] [PubMed] [Google Scholar]
- 49.Takayanagi Y, Yoshida M, Takashima A, Takanami K, Yoshida S, Nishimori K, Nishijima I, Sakamoto H, Yamagata T, Onaka T. Activation of supraoptic oxytocin neurons by secretin facilitates social recognition. Biol Psychiatry. 2017;81(3):243–51. doi: 10.1016/j.biopsych.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 50.Okabe S, Yoshida M, Takayanagi Y, Onaka T. Activation of hypothalamic oxytocin neurons following tactile stimuli in rats. Neurosci Lett. 2015:60022–7. doi: 10.1016/j.neulet.2015.05.055. [DOI] [PubMed] [Google Scholar]
- 51.van Kerkhof LW, Trezza V, Mulder T, Gao P, Voorn P, Vanderschuren LJ. Cellular activation in limbic brain systems during social play behaviour in rats. Brain Struct Funct. 2014;219(4):1181–211. doi: 10.1007/s00429-013-0558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon NS, Kollack-Walker S, Akil H, Panksepp J. Expression of c-fos gene activation during rough and tumble play in juvenile rats. Brain Res Bull. 2002;57(5):651–9. doi: 10.1016/s0361-9230(01)00762-6. [DOI] [PubMed] [Google Scholar]
- 53.DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH. Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J Comp Neurol. 2017;525(11):2549–70. doi: 10.1002/cne.24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rood BD, De Vries GJ. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J Comp Neurol. 2011;519(12):2434–74. doi: 10.1002/cne.22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Vries GJ, Buijs RM. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 1983;273(2):307–17. doi: 10.1016/0006-8993(83)90855-7. [DOI] [PubMed] [Google Scholar]
- 56.Caffe AR, van Leeuwen FW, Luiten PG. Vasopressin cells in the medial amygdala of the rat project to the lateral septum and ventral hippocampus. J Comp Neurol. 1987;261(2):237–52. doi: 10.1002/cne.902610206. [DOI] [PubMed] [Google Scholar]
- 57.Simmons DM, Swanson LW. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. J Comp Neurol. 2009;516(5):423–41. doi: 10.1002/cne.22126. [DOI] [PubMed] [Google Scholar]