Abstract
2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate was designed to replace perfluorooctanoic acid (PFOA), which has been mostly phased out of U.S. production due to environmental persistence, detectable human and wildlife serum concentrations, and reports of systemic toxicity. In rodent models, PFOA exposure suppresses T cell-dependent antibody responses (TDAR) and vaccine responses in exposed humans. To determine replacement compound effects on TDAR and related parameters, male and female C57BL/6 mice were gavaged with 0, 1, 10, or 100 mg/kg/day for 28 days. Mice immunized with antigen on day 24 were evaluated for TDAR and splenic lymphocyte subpopulations five days later. Serum and urine were collected for test compound concentrations and liver peroxisome proliferation was measured. Relative liver weight at 10 and 100 mg/kg and peroxisome proliferation at 100 mg/kg were increased in both sexes. TDAR was suppressed in females at 100 mg/kg. T lymphocyte numbers were increased in males at 100 mg/kg; B lymphocyte numbers were unchanged in both sexes. Females had less serum accumulation and higher clearance than males, and males had higher urine concentrations than females at all times and doses. While this PFOA-replacement compound appears less potent at suppressing TDAR relative to PFOA, it produces detectable changes in parameters affected by PFOA; further studies are necessary to determine its full immunomodulatory profile and potential synergism with other per- and polyfluoroalkyl substances of environmental concern.
Keywords: Per- and polyfluoroalkyl substances (PFASs), TDAR, immunotoxicity, perfluorooctanoic acid (PFOA)
Per- and polyfluoroalkyl substances (PFASs) are anthropogenic organic compounds composed of strong carbon–fluorine bonds that make them extremely useful as polymerization aids and surfactants for the processing of myriad consumer and industrial products. However, the characteristics that make PFASs beneficial in industrial processes often make them problematic from an environmental health standpoint: perfluoroalkyl acids (PFAAs), a class of PFASs, are extremely persistent in the environment, and some bioaccumulate in wildlife and humans. As well as being multisystem toxicants, they have also been associated with immunotoxicity and similar effects on the immune system have been observed in both exposed experimental animal models and humans (Keil, 2015). Among the most well-characterized PFASs are perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS), which have been reported to potently suppress T cell-dependent antibody responses (TDAR) in experimental rodent models (DeWitt et al., 2008; DeWitt et al., 2009, 2016; Loveless et al., 2008; Peden-Adams et al., 2008; Yang et al., 2002) and responses to vaccinations in exposed humans (Grandjean et al., 2012; Granum et al., 2013; Looker et al., 2014). Largely due to environmental persistence and growing reports of toxicity, PFOS was phased out of production by its major manufacturer in 2002 and PFOA was phased out of production by its major U.S. manufacturers in 2015. However, their usefulness in industrial processes has prompted research into alternative PFASs that will provide optimal industrial usefulness but with reduced toxicity.
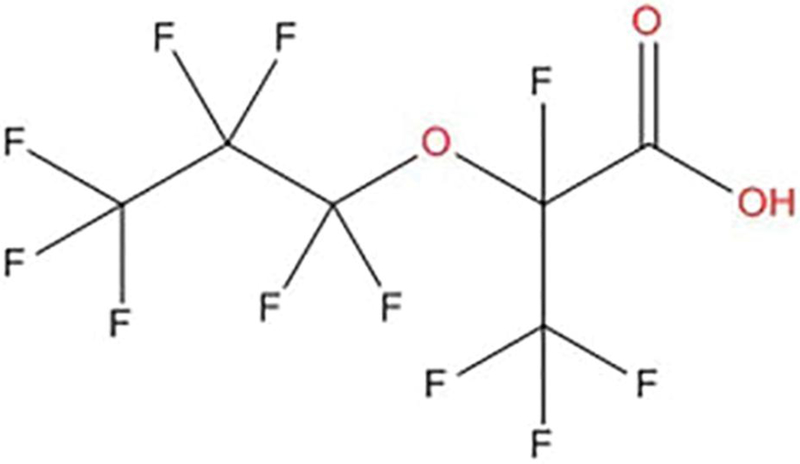
The search for PFOA alternatives has focused on shorter-chain PFAAs and perfluoroether substances based on the notion that they are less persistent and toxic (Stahl et al., 2009; Wang et al., 2015). However, evidence to date suggests that perfluoroether substituted chemicals may be just as persistent as PFAAs under environmentally relevant conditions (Wang et al., 2015). One such perfluoroether replacement is a 6-carbon compound known as 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate (C6HF11O3), with the trade name “GenX” (Figure 1). It was introduced to fluoropolymer manufacturing processes in 2009 by a major U.S. manufacturer (DuPont, 2010). Data from publicly available documents associated with a National Pollutant Discharge Elimination System (NPDES) permit at a manufacturing facility indicate a “favorable toxicological profile” (WV DEP, 2012). Unpublished industry studies summarized in the NPDES permit documentation list a no observed adverse effect level (NOAEL) of 10 mg/kg based on the evidence of regenerative anemia in male rats exposed to 100 mg/kg and female rats exposed to 1000 mg/kg for 90 days; the exposure route and strain of rat was not specified. At the NOAEL concentration, male rats also had a reduced red blood cell count, hematocrit, and hemoglobin. A study by Rae et al. (2015) on the chronic toxicity and carcinogenicity of the test compound in Sprague–Dawley rats reported a NOAEL of 0.1 mg/kg and 1 mg/kg for males and females, respectively, based on liver and kidney effects. Therefore, our goal in undertaking this initial study was to determine serum and urine concentrations of the test compound as well as its potential to affect the TDAR with or without concomitant effects on splenic lymphocyte subpopulations in mice as a way to compare its immunomodulatory potential to that of PFOA. The current study was based on the U.S. Environmental Protection Agency’s (EPA) Immunotoxicity Harmonized Test Guideline (OPPTS 870.7800) to assess the potential immunotoxicity of the test compound.
FIG. 1.
Chemical structure of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate.
MATERIALS AND METHODS
Experimental design
A total of 96 C57BL/6 mice in 2 replicates of 48 animals each, spaced about 8 weeks apart, were used for this experiment and were obtained from Charles River Laboratories at 7 weeks of age. The second replicate was performed 5 months after the first replicate to verify the findings of the first replicate. Upon arrival to AAALAC accredited animal facilities at East Carolina University (ECU), mice were housed in groups of 3 and separated by sex. Distribution of animals from shipping containers for each replicate was done semi-randomly with the first animal being added to the first cage, the second to the second cage, the third to the third cage, and so forth, to avoid biasing certain doses toward more or less active animals. After distribution into cages, animals were weighed and if necessary, redistributed to different cages to equalize body weights among cages so that pre-dosing body weights did not differ statistically by cage within sex (P < .05). Food and water were provided ad libitum, and animals were kept on a 12-h light-dark cycle, 20ºC –24ºC, and a relative humidity of 60%–65%. Bedding was changed twice weekly and the health of mice was monitored daily by both researchers and animal care staff. Mice were given 5 days to acclimate to their new housing arrangements before dosing began and the ECU Institutional Animal Care and Use Committee approved all procedures in advance.
Within each replicate, mice were randomly assigned by cage to 4 different dose groups with 6 males and 6 females per group and 2 cages/sex/group housing 3 animals each. Doses were selected based on the reported NOAEL from the rat data extracted from the NPDES permit, with the highest dose reduced by an order of magnitude due to uncertainty about the sensitivity of mice relative to rats and lower doses reduced by a factor of 10 from the highest dose. Doses, therefore, were 0, 1, 10, or 100 mg/kg per day. The test compound (CAS# 13252–13-6) was acquired from Synquest Laboratories and dosing solution was prepared fresh at the beginning of each week with sterile water and 0.5% Tween-20 vehicle to ensure emulsification into the dosing water. Concentrations of dosing solutions were 0, 0.1, 1, and 10 mg/ml, which when given at 0.1 ml/10 g of body weight resulted in the appropriate mg/kg dose per day. Each mouse was dosed daily via oral gavage for 28 days. Body weights and deviations from normal appearance suggestive of systemic toxicity (i.e., piloerection, hunched posture, failure to move appropriately) were recorded daily before dosing for that day.
Organ endpoints
One day after the final gavage exposure, mice were humanely euthanized via IACUC-approved methods and necropsied. Spleen, liver, and thymus were harvested and weighed immediately. Livers were stored at −80ºC for later analysis of peroxisome proliferation, spleens were stored on ice in 3 ml of complete medium (RPMI, 10% fetal calf serum, 50 IU penicillin, and 50 µg streptomycin), and thymuses were discarded after weights were recorded. Livers were homogenized and the activity of the acyl-CoA oxidase enzyme, as a measure of hepatic peroxisomal fatty acid oxidation, was assayed fluorimetrically with palmitoyl-CoA as a substrate as per Poosch and Yamazaki (1986).
Immunophenotyping
Only one experimental replicate (48 total animals) was used to assess immunophenotype. Spleens from mice that had been immunized with sheep red blood cells (SRBCs) were aseptically processed into single-cell suspensions by gentle grinding and passage through a 70 μm nylon filter, followed by the addition of 7 ml of complete medium. An aliquot of each spleen suspension was counted on a Nexcelom Bioscience Cellometer Auto 2000 cell counter (Nexcelom Bioscience LLC) to determine the number of live cells. The total number of live cells per spleen (cellularity), adjusted by the weight of each organ, was determined for each animal. Suspensions were adjusted to 2 × 107 cells/ ml. Optimal concentrations of flow antibodies, reagents, and isotype controls to estimate non-specific binding were determined in previous experiments (DeWitt et al., 2016). All experimental replicates also included unstained cells as negative controls and single color controls as positive controls to determine color compensation. Monoclonal antibodies (eBioscience, Inc.) coupled to fluorochromes specific for the following markers were used: anti-mouse APC anti-mouse CD3e, FITC anti-mouse CD4, PE anti-mouse CD8a, and FITC anti-mouse CD45RB. Flow cytometric analysis was performed using an Accuri C6 flow cytometer (BD Biosciences) and 10 000 events were collected from each sample. Dead cells and debris were excluded from analysis by using forward scatter and 90° light scatter to establish a gate around the viable lymphocyte population. Non-stained cells, isotype controls, and fluorescence minus one (FMO) controls were used to distinguish the negative populations from positive populations for B cells and T cells. Cells were gated based on CD3 expression for the subsequent analysis of CD4/CD8 T-subpopulations, but not B cell subpopulations. The total number of each cell type was determined from spleen cellularity.
Measurement of TDAR
On the 24th day after the initial dose, all mice in both replicates and in all dose groups were immunized with SRBCs via tail vein injections. SRBCs were adjusted to 4 × 107 cells in 0.2 ml of sterile saline. Sera for measurement of SRBC-specific IgM antibodies were collected and IgM antibody titers were determined as described by DeWitt et al. (2016). Briefly, flat bottom 96-well Immunolon-2 ELISA microtiter plates (Dynatech Labs) were coated with 125 μl of 2 μg/ml of SRBC membrane [1.46 mg/ml stock solution diluted in phosphate-buffered saline (PBS); prepared according to Temple et al. (1995) and then incubated at 4 °C for at least 16 h. Each plate included 20 wells coated with pooled serum collected from healthy mice 5 days after primary immunization with SRBC, as assay positive controls, and 16 wells contained 100 μl PBS as blanks. After washing, blocking of non-specific binding, and addition of serum samples (serially diluted 1:2, starting at 1:8), secondary antibody (goat anti-mouse IgM horseradish peroxidase; Accurate Chemical and Scientific Corp.) was added to the wells. Following 3 washes and addition of substrate [10 mg 2,2′-azino-di-(3 ethylbenz-thiazoline sulfonic acid, ABTS, Sigma) added to 50 ml phosphate-citrate buffer with one tablet of urea hydroxide peroxide (Sigma) in 100 ml distilled water, 0.05 M final solution], plates were incubated for 45 min at room temperature and then the absorbance in each well evaluated at 410 nm on a BioTek Synergy HT plate reader (BioTek Instruments, Inc.). IgM anti-body titers were processed using SOFTmax Pro software (Molecular Devices, LLC) to determine the log2 serum titers for an optical density of 0.5 U from the log–log curve of optical density versus dilution, as described by Temple et al. (1995).
Sera and urine concentrations
Only one experimental replicate (48 total animals) was used to determine serum and urine concentrations. Blood was collected 1, 5, 14, and 28 days after the initial dose. Prior to the terminal bleed 1 day after exposure ended, blood was collected via the submandibular vascular bundle; blood at terminus was collected via neck vein transection from anesthetized animals. Blood was allowed to clot at room temperature for 30 minutes, serum was separated from the clot by centrifugation at 4ºC, and then frozen at −80ºC for later analyses. Urine was collected non-invasively by placing each of the 3 animals/cage into a clean cage free of bedding and collecting deposited urine. This pooled urine was collected 1, 2, 3, and 14 days after exposure began. Sera and urine samples from each time point were evaluated using the methods developed for PFOA analysis with minor modifications (Reiner et al., 2009). In brief, serum and urine samples were denatured with a solution of 0.1 M formic acid followed by an acetonitrile protein crash. Constructed standard curves were created using blank CD1 mouse serum (Pel Freez Biologicals) or control animal urine depending on the matrix. Analyte in dosed and control serum/urine were quantitated using the stable isotope dilution method with 13C2-perfluorohexanoic acid purchased from Wellington Labs as an internal standard. At the time of this analysis, no stable isotope labeled test compound was available. Calibration curves, process blanks, matrix blanks, and replicate samples were run with every assay to maintain quality control.
Statistical analysis
Data are presented as mean ± standard deviation unless otherwise indicated and all statistical analyses were performed with the Statistical Analysis System (SAS Institute). Data sets with experimental readouts from both sets of 48 animals were evaluated for homogeneity and combined as no heterogeneity was detected within sex and within dose between replicates. Therefore, body and organ weights and the TDAR reflect N = 12 animals/sex/dose. Immunophenotype, serum, and urine concentrations were determined from only one experimental replicate (N = 6 animals/sex/dose). However, due to low blood collection volumes from individual animals at individual time points, serum concentrations reflect N = 1–6; concentrations based only on N = 1 are reflected in figure legends. Urine samples reflect pooled samples of 3 animals/cage and 2 pooled samples/dose as animals were housed 3/cage with 2 cages/dose. Urine samples, therefore, reflect N = 1 or 2 based on the volume of urine non-invasively excreted by animals on a particular collection day. Body weights over the course of the study were evaluated by 2-way analysis of variance (ANOVA) with a repeated factor; all other data were evaluated between sex and across dose by a 2-way ANOVA and separately within sex and across dose by one-way ANOVA with appropriate post-hoc t-tests when the overall ANOVA revealed an F-statistic with a P value < .05.
RESULTS
Body and Organ Endpoints
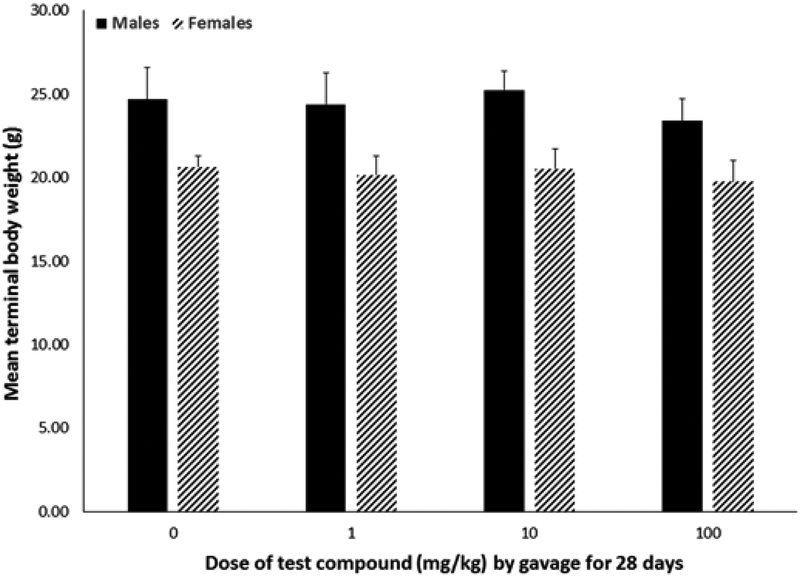
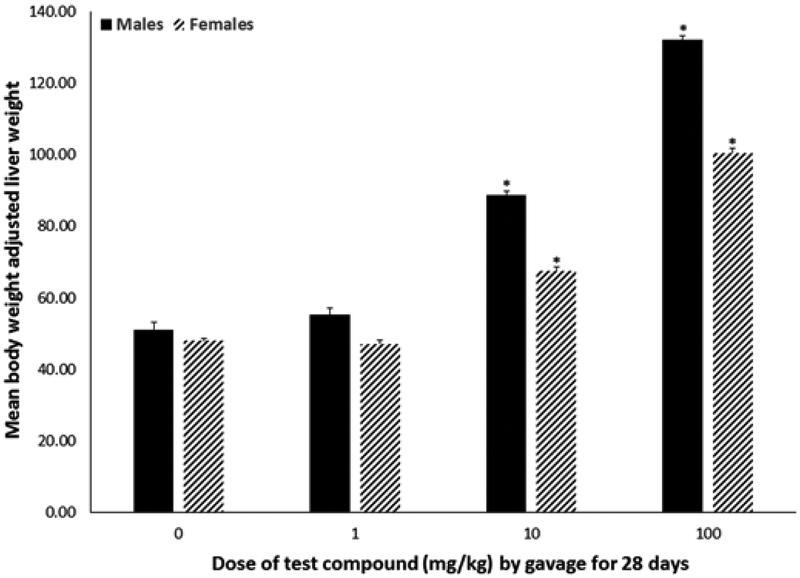
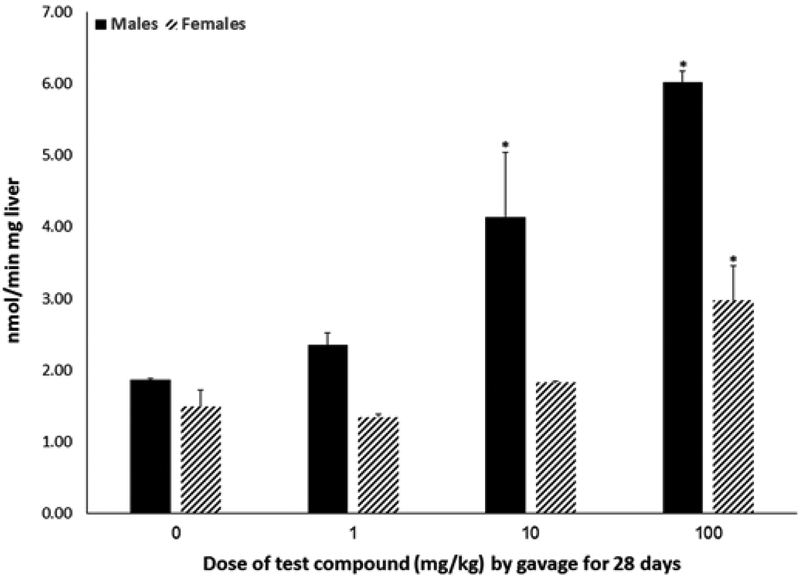
Body weight did not differ by dose within sex over the course of exposure or at experimental terminus (Figure 2) nor did animals show outward signs of systemic toxicity. Absolute (data not shown) and relative (Figure 3) liver weights were increased by 40%–160% (P < .0001) in both males and females exposed to 10 or 100 mg/kg, with males having a greater percent increase relative to the 0 mg/kg group compared with females. Absolute and relative thymus weights did not differ statistically by dose within either sex (data not shown). Absolute and relative spleen weights from females exposed to 100 mg/kg were 16.5% and 11.3% lower (P < .002) compared with weights from the 0 mg/kg group (Table 1); male spleen weights did not differ statistically. No statistical differences in splenic lymphocyte cellularity were noted across doses for either sex. Peroxisomal fatty acid oxidation was increased in both males and females (Figure 4). Hepatic acyl-CoA oxidase activity was increased 122% and 222% in livers of male animals exposed to 10 or 100 mg/kg, respectively (P < .03). In female animals, only exposure to 100 mg/kg statistically increased acyl-CoA oxidase activity (by 100%; P < .02).
FIG. 2.
Body weights (mean ± SD) of male or female C57Bl/6 mice given 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate via gavage for 28 days. No statistically significant differences in mean body weights collected at study terminus (1 day after exposure ended) were associated with test compound exposure.
FIG. 3.
Relative liver weights (absolute liver weight adjusted for body weight; mean ± SD) of male or female C57Bl/6 mice given 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate via gavage for 28 days. Livers were collected 1 day after exposure ended. *A statistically significant increase in relative liver weight relative to the 0 mg/kg group of the matching sex (P < .05).
TABLE 1.
Splenic lymphocyte subpopulations in adult C57BL/6 male and female mice orally exposed to 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate for 28 days
| Test compound (mg/kg) |
Relative spleen wta |
B cells (cells × 107) |
CD4+(cells × 107) |
CD8+(cells × 107) |
CD4+/CD8+(cells × 106) |
CD4−/CD8−(cells × 106) |
|---|---|---|---|---|---|---|
| Males | ||||||
| 0 | 3.81 ± 0.40 | 2.76 ± 1.07 | 2.36 ± 0.89 | 1.41 ± 0.51 | 0.60 ± 0.23 | 2.67 ± 1.39 |
| 1 | 3.59 ± 0.61 | 3.69 ± 0.78 | 3.27 ± 0.80 | 2.03 ± 0.37 | 0.73 ± 0.16 | 4.96 ± 1.25 |
| 10 | 3.78 ± 0.47 | 2.94 ± 1.00 | 2.55 ± 0.85 | 1.53 ± 0.48 | 0.59 ± 0.13 | 3.46 ± 0.96 |
| 100 | 4.07 ± 0.99 | 3.88 ± 1.22 | 3.62 ± 1.08 | 2.15 ± 0.61* | 1.02 ± 0.27* | 5.32 ± 2.27* |
| Females | ||||||
| 0 | 5.34 ± 0.39 | 3.25 ± 1.11 | 2.54 ± 0.83 | 1.55 ± 0.45 | 0.57 ± 0.17 | 6.60 ± 2.34 |
| 1 | 5.15 ± 0.56 | 4.26 ± 0.77 | 3.39 ± 0.78 | 1.96 ± 0.19 | 0.85 ± 0.25 | 7.62 ± 2.09 |
| 10 | 5.01 ± 0.77 | 3.72 ± 0.70 | 2.67 ± 0.33 | 1.70 ± 0.40 | 0.74 ± 0.36 | 7.00 ± 4.06 |
| 100 | 4.65 ± 0.56* | 3.33 ± 1.11 | 2.84 ± 0.97 | 1.62 ± 0.47 | 0.77 ± 0.44 | 5.93 ± 2.11 |
A Data are presented as the mean cell number ± standard deviation based on a total of 10 000 events collected. N = 6 animals/sex/dose.
P < .05 from the 0 mg/kg group of the same sex.
FIG. 4.
Hepatic peroxisome proliferation (mean ± SD) of male or female C57Bl/6 mice given 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate via gavage for 28 days. Acyl-CoA oxidase activity was measured in archived livers that had been collected from animals 1 day after exposure ended. *A statistically significant increase in acyl-CoA oxidase activity relative to the 0 mg/kg group of the matching sex (P < .05).
Immunophenotyping
In both males and females, splenic B-cell subpopulations were not altered in any of the dose groups (Table 1). Splenic T-cell subpopulations in males, however, were statistically altered (P < .05) by exposure to the test compound. The number of CD8+, CD4+/CD8+, and CD4−/CD8− T cells was increased, on an average, by 74% following exposure to 100 mg/kg (Table 1).
T-Cell-Dependent Antibody Responses
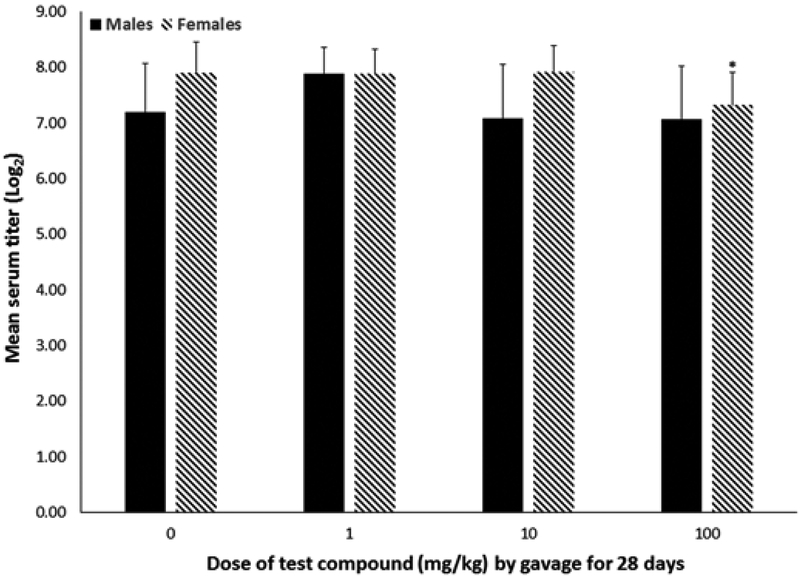
SRBC-specific IgM antibody responses were suppressed by 7.3% (P < .009) in females exposed to 100 mg/kg, whereas males did not experience any statistically significant differences in IgM antibody production (Figure 5).
FIG. 5.
Antigen-specific IgM antibody production (mean ± SD) of male or female C57Bl/6 mice given 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate via gavage for 28 days. IgM serum titers were measured in archived sera that had been collected from animals 1 day after exposure ended. *A statistically significant reduction in IgM serum titer relative to the 0 mg/kg group of the matching sex (P < .05).
Serum and Urine Concentrations
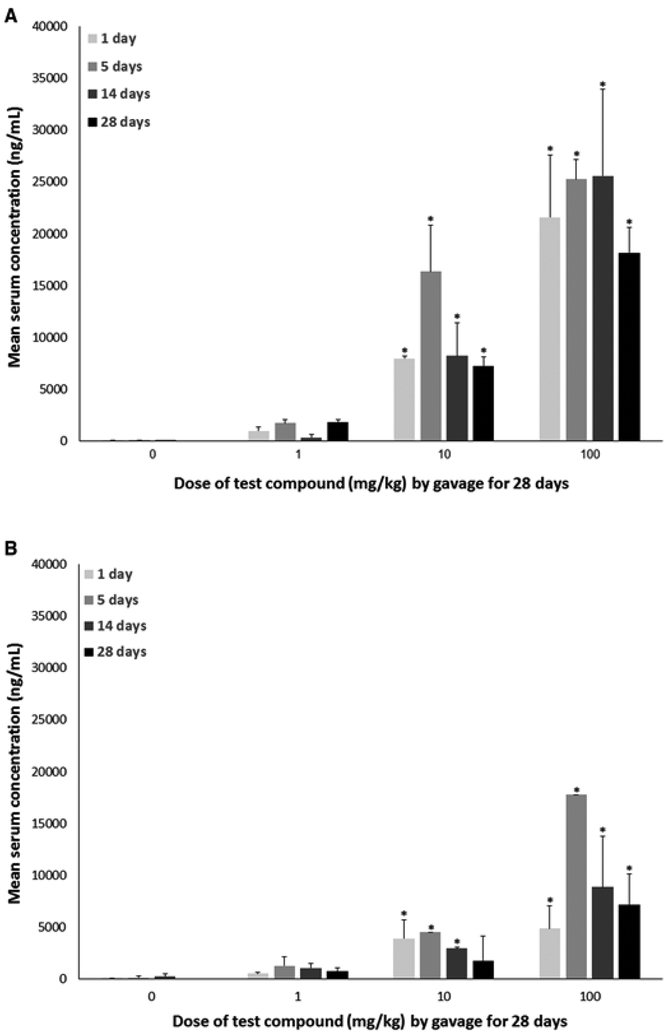
Relative to the 0 mg/kg group, serum concentrations of the test compound were statistically elevated at all time points evaluated (after 1, 5, 14, and 28 days of exposure) in animals exposed to 10 or 100 mg/kg; serum concentrations in animals exposed to 1 mg/kg did not differ statistically from the 0 mg/kg group at any measured time point (P < .05; Figure 6). In both males and females exposed to 10 mg/kg, serum concentrations dropped by about 50% between 5 and 14 days of exposure. In females, serum concentrations dropped again by about 40% following 14 days of exposure to 1 day after exposure ended. In contrast, serum concentrations of males exposed to 10 mg/kg dropped only by about 10% after 14 days of exposure to 1 day after exposure ended. In animals exposed to 100 mg/kg, maximal concentrations were reached after 5 days of exposure. In males, this was maintained until after 14 days of exposure whereas in females, serum concentrations, similar to the 10 mg/kg group, dropped by about 50% between 5 and 14 days of exposure and by about 20% after 14 days of exposure to 1 day after exposure ended. In males, serum concentrations dropped by about 30% from after 14 days of exposure to 1 day after exposure ended.
FIG. 6.
Serum concentrations (mean ± SD) of male or female C57Bl/6 mice given 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate via gavage for 28 days. Sera samples were collected after 1, 5, 14, and 28 days of exposure. A, Males. B, Females. *A statistically significant increase in serum concentrations relative to the 0 mg/kg group at the same time point (P < .05). N = 1–6/dose. Samples where N = 1 occurred only in females on day 5 for the 10 and 100 mg/kg dose groups.
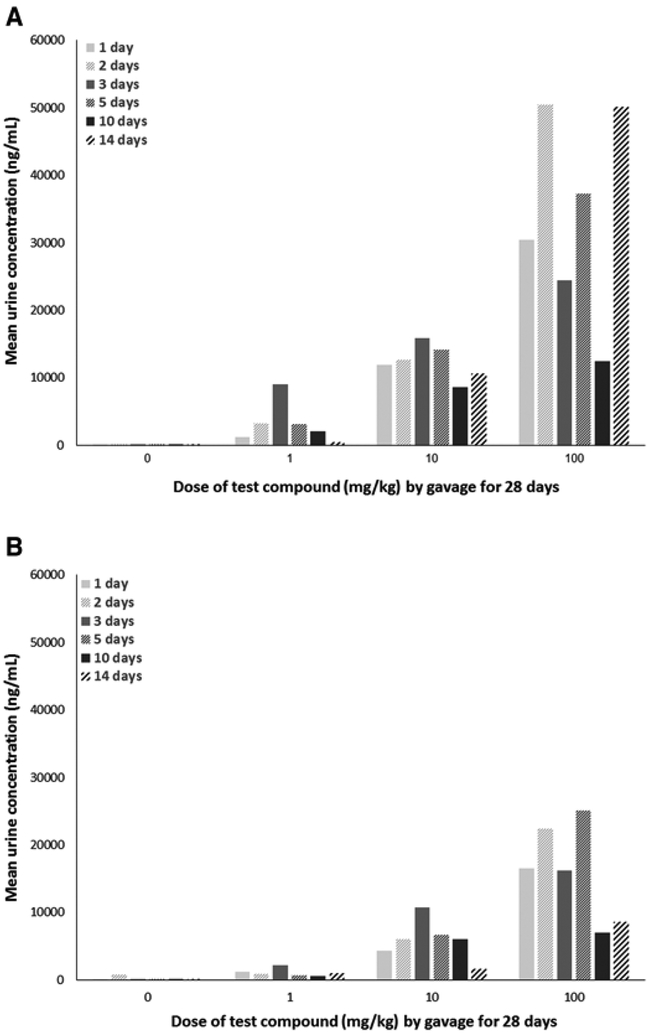
As full urine samples were not available on each day for all groups due to lack of urine excretion, it is challenging to meaningfully interpret urinary excretion trends. What is abundantly clear from the urine data, however, is that males excreted a much higher concentration of the test compound across all concentrations and time points collected relative to females (Figure 7).
FIG. 7.
Urine concentrations (mean) of male or female C57Bl/6 mice given 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate via gavage for 28 days. Urine samples were collected after 1, 2, 3, 5, 10, and 14 days of exposure. A, Males. B, Females. Samples are pooled samples of 3 animals/sex/cage and 2 pooled samples/dose; sample sizes are, therefore, N = 1 or 2/dose depending on the quantity of urine non-invasively excreted on a particular sample collection day.
DISCUSSION
2,3,3,3-Tetrafluoro-2-(heptafluoropropoxy)-propanoate is a compound that was designed to replace PFOA in various manufacturing processes and is marketed as a processing aid with a toxicological profile more favorable than PFOA due to its rapid bioelimination (DuPont, 2010). It was introduced to fluoropolymer-manufacturing processes in 2009 and, to our knowledge, only one peer-reviewed report about its toxicological effects exists (Rae et al., 2015). In our study, we chose the C57Bl/6 mouse model as it is a rodent model that is sensitive to the immunomodulatory effects of PFOA exposure (DeWitt et al., 2009). We also chose to evaluate the test compound following a standard immunotoxicity testing protocol, employing a 28-day exposure duration and evaluating antigen-specific antibody responses (i.e., TDAR) and enumeration of splenic lymphocytes. TDAR also was chosen as suppression of the TDAR has been reported in various rodent species after exposure to ammonium perfluorooctanoate (APFO, the ammonium salt of PFOA), PFOA, or PFOS (DeWitt et al., 2008; DeWitt et al., 2009, 2016; Loveless et al., 2008; Peden-Adams et al., 2008; Yang et al., 2002) and in humans as reduced responses to vaccine administration (Grandjean et al., 2012; Granum et al., 2013; Looker et al., 2014). In addition, we evaluated peroxisome proliferation, which is a biomarker of PFOA exposure, general markers of toxicity, and measured serum and urine concentrations over the course of the study. Therefore, our major goal was to compare the results of this study to similar studies of PFOA to attempt to discern if the test compound produced immunotoxicological effects and associated effects similarly to or differently from PFOA.
No statistically significant effects on body weights were observed nor were any overt signs of systemic toxicity noted in our study at doses of the test compound up to 100 mg/kg given by gavage for 28 days (Figure 2). In contrast, when female C57Bl/6 mice were given 30 mg/kg of PFOA in drinking water for 15 days, body weight was statistically reduced starting at 8 days of exposure through the end of the study when compared with body weights of control animals (DeWitt et al., 2008). In the same study, mice given 15 mg/kg of PFOA had reduced body weight relative to the control animals by the end of the study (DeWitt et al., 2008). Loveless et al. (2008) reported body weight loss in male Crl:CD-1(ICR)BR mice given 10 or 30 mg/kg APFO via gavage for 29 days as did Yang et al. (2001) in male C57Bl/6 mice given 40 mg/kg of PFOA in the diet for 10 days. When strictly compared on a dose basis, it appears as if a much higher dose of the test compound (100 mg/kg versus 10–40 mg/kg) was not sufficient to reduce body weights of male or female C57Bl/6 mice, even when given for a longer exposure period (28 days versus up to 29 days). However, in a chronic toxicity study of male and female Sprague–Dawley rats gavaged with 0, 0.1, 1, or 50 mg/kg (males) or 0, 1, 50, or 500 mg/kg (females) of the test compound once daily for 2 years, Rae et al. (2015) reported reductions in body weight, body weight gain, and food efficiency in females given 500 mg/kg. In the same study (Rae et al., 2015), ∼9% of females exposed to 500 mg/kg died prior to the end of the study and had test compound-related papillary necrosis and kidney inflammation. Overall survivorship for either sex, however, was not reported as being associated with the test compound although female animals were euthanized 3 weeks prior to the study terminus due to low survivorship in all groups, including the control.
In our study, exposure to the test compound increased both absolute and relative liver weights. Both males and females exposed to 10 or 100 mg/kg exhibited liver weight increases compared to liver weights of animals exposed to 0 mg/kg (Figure 3). However, exposure to 1 mg/kg did not result in increased liver weights. In the chronic toxicity study of Sprague–Dawley rats, only female animals had increases in liver weights (Rae et al., 2015). In that study, female rats gavaged with 50 mg/kg had livers 16% heavier than controls and those gavaged with 500 mg/kg had livers 69% heavier than controls. Similar doses of PFOA do result in increased liver weights in mice; exposure to either 0.94 or 1.88 mg/kg of PFOA via drinking water for 15 days increased absolute and relative liver weights in female C57Bl/6 mice when evaluated at the end of the study (DeWitt et al., 2008). A higher dose of PFOA, 40 mg/kg, was reported to increase liver weights in male C57Bl/6 mice after only 2 days of exposure (Yang et al., 2000b) and liver weight increases were reported in male Crl:CD-1(ICR)BR mice given APFO for 29 days (Loveless et al., 2008). Together, these data indicate that mice appear to be more sensitive to test compound-associated liver weight increases relative to rats and that PFOA produces more robust increases in liver weight compared to the test compound.
Hepatic acyl-CoA oxidase activity (Figure 4), a marker of hepatic peroxisome proliferation, was increased in both males and females exposed to 100 mg/kg and in males exposed to 10 mg/kg. An earlier study on the potential immunotoxicity of PFOA reported increases in hepatic acyl-Co-A at a low dose of about 2 mg/kg after 10 days of dietary exposure in male C57Bl/6 mice (Yang et al., 2001). It appears as if the test compound is not as potent in inducing hepatic peroxisome proliferation as PFOA.
Lymphoid organs also appeared to be less sensitive to test compound exposure than to PFOA exposure. The high dose (100 mg/kg) was associated with a reduction in absolute and relative spleen weights in female animals whereas PFOA and APFO have been reported to reduce both spleen and thymus weights at lower doses (10–40 mg/kg) and after shorter or similar exposure durations (DeWitt et al., 2008; Loveless et al., 2008; Yang et al., 2001, 2000b). Rae et al. (2015) reported no changes in weights of lymphoid organs in male and female Sprague–Dawley rats exposed to the test compound for 2 years. It is probable, given the weight loss associated with these relatively higher doses of PFOA that lymphoid organ atrophy occurred as a result of systemic toxicity rather than immune system-specific effects of PFOA exposure.
The effect of PFOA exposure on antigen-specific antibody production has been documented in several strains of mice and is supported by epidemiological studies that associated serum PFOA concentrations with reduced responses to vaccines. These data are particularly robust, as there is agreement among the studies, although interpretation of the results varies across studies. The TDAR is a measure of immune function that is highly predictive of the ability of an agent to induce immunotoxicity in exposed humans if it is altered in experimental animal models (Luster et al., 1992). The lowest observed adverse effect level (LOAEL) reported for female C57Bl/6 mice given PFOA via drinking water for 15 days was 3.75 mg/kg, with a benchmark dose of 3.06 mg/kg (DeWitt et al., 2008). Loveless et al. (2008) revealed a LOAEL of 10 mg/kg in male Crl:CD-1(ICR)BR mice given APFO via gavage for 29 days and Yang et al. (2002) reported suppression of the TDAR in male C57Bl/6 mice fed 40 mg/kg of PFOA for 10 days. Loveless et al. (2008) suggested that this suppression of the TDAR was a result of systemic toxicity rather than immune system-specific effects of PFOA or APFO exposure. Doses of APFO or PFOA ≥ 10 mg/kg have been associated with statistically significant reductions in body weight, a sign of systemic toxicity. Loveless et al. (2008) reported a negative correlation between serum corticosterone, a measure of systemic toxicity and stress, and the TDAR in APFO-exposed mice. As no reduction in body weight or the TDAR was correlated with increases in serum corticosterone at APFO doses of 0.3 or 1 mg/kg, they concluded that APFO was not immunotoxic at their tested doses. In a follow-up study, DeWitt et al. (2009) exposed adrenalectomized (adx) C57Bl/6 female mice to PFOA via drinking water for 10 days and reported suppression of the TDAR at a dose of 7.5 mg/kg, which was not associated with body weight loss or elevated corticosterone production in the adx animals. While systemic toxicity and stress cannot be discounted as factors contributing to the immune effects of PFOA in mice given relatively high doses (≥ 10 mg/kg), it does not appear to be a factor in the immune effects of PFOA given at lower doses. In terms of the effects of the test compound on the TDAR, suppression of this response was observed only in female animals exposed to 100 mg/kg (Figure 5). This suppression was relatively mild; IgM production was 7.3% lower in treated animals relative to control animals. This suppression also was not associated with a reduction in body weight or overt signs of systemic toxicity. The TDAR was not suppressed in male animals even though the serum concentration of the test article was much higher. Variability of this response in male animals at 100 mg/kg was higher relative to female animals (standard deviations of 0.95 vs 0.59, respectively), which is a possible explanation for lack of detectable effects in the TDAR for male animals. Another possibility is that male C57BL/6 mice are less sensitive to this PFAS-mediated effect than are female C57BL/6 mice; further experimentation would be necessary, however, to verify this explanation. Relative to PFOA, the test compound appears to be less potent at affecting the TDAR.
Of particular interest regarding the effects of PFOA on the TDAR is that IgM antibody suppression occurs at doses that do not produce notable decreases in splenic lymphocyte numbers overall or on the percentage of B and T cell subtypes. Higher doses of PFOA and APFO have been associated with reduced splenic and thymic lymphocyte numbers (Loveless et al., 2008; Yang et al., 2001,, 2000b) as well as reductions in T and B cell subtypes Yang et al. (2001). However, lower doses of PFOA have not been reported to consistently alter lymphocyte numbers in lymphoid organs or T and B cell subtypes (DeWitt et al., 2016). In this study, exposure to the test compound was not associated with decreases in splenic lymphocyte numbers or T cell subpopulations when compared to control values (Table 1). In male animals, however, the number of CD8+, CD4+/CD8+, and CD4−/CD8− T cells was increased, on average, by 74% following exposure to 100 mg/kg. The increase in these subpopulations was not accompanied by a statistically significant change in spleen weight or cellularity in this dose group. At this dose, spleen cellularity adjusted by terminal body weight also was statistically significantly larger relative to the 0 mg/kg group (data not show), but neither spleen cellularity nor terminal body weight alone were statistically significant. In the absence of an accompanying effect on the TDAR in male animals and further analyses, it is difficult to determine what these increases in T cell subpopulations mean for the health of the exposed organism. However, taken together, these data suggest that the test compound may affect the TDAR via different pathways than does PFOA. Several studies have attempted to determine the mechanism(s) by which PFOA impacts the TDAR and to date, no consensus has been demonstrated by the data. Some studies (DeWitt et al., 2016; Yang et al., 2002a) have attempted to link peroxisome proliferation via activation of the peroxisome proliferator activated receptor alpha (PPARα) with PFOA-induced suppression of T and B cell subpopulations or the TDAR, but even though PPARα-null animals have somewhat attenuated responses to PFOA, they still exhibit suppression of the TDAR. Other studies have included in vitro and ex vivo assays to evaluate cytokine production following PFOA exposure, but no studies have specifically associated changes in cytokine production with suppression of the TDAR associated with PFOA exposure. Additional studies with PFOA, the test compound, and additional PFASs are warranted to determine the mechanism(s) associated with suppression of the TDAR and whether or not the mechanism is associated with chain length, functional group, per- or poly-substitution, or other factors.
A recently published study highlighted the absorption, distribution, metabolism, excretion, and kinetics of the test compound in rats, mice, and cynomologus monkeys following a single dose (Gannon et al., 2015). In one experiment presented in this study, Sprague–Dawley rats of each sex and Crl:CD1 (ICR) mice of each sex were given a single oral dose of 10 or 30 mg/kg of the ammonium form of the test compound (C6F11O3−NH4+) and blood samples were collected at various times within the first 24 h following administration of the test compound and then once daily for 7 days; urine samples also were collected during this time. Biphasic kinetics were observed in both species, with mice having slower plasma elimination than rats and female animals having more rapid plasma elimination, overall, compared with male animals (Gannon et al., 2015). Urine data indicated rapid and complete elimination without metabolism (Yang et al., 2002a). In another experiment presented in the Gannon et al. (2015) study, male and female Sprague–Dawley rats and cynomologus monkeys were give a single intravenous dose of 10 mg/kg (rats and monkeys) or 50 mg/kg (rats only). Biphasic kinetics also were observed in both species, with plasma elimination being similar to the orally administered dose (Gannon et al., 2015). Gannon et al. (2015) reported that the test compound was cleared from plasma more quickly in female mice than in male mice, that urinary excretion was rapid and complete, and that it was more rapidly eliminated compared with PFOA. Our data support this general trend although our study was not conducted according to pharmacokinetic study guidelines. In our study, male animals had much higher serum and urine concentrations as the study progressed relative to female animals, suggesting slower plasma and, therefore, urinary clearance (Figs. 6 and 7). Relative to PFOA, and in support of the Gannon et al. (2015) study, it appears as if the test compound has a shorter serum half-life and more rapid excretion in mice. It appears as if the test compound is accumulated and excreted at a different rate in male and female mice; pharmacokinetic studies and serum measures of PFOA suggest that male and female mice accumulate and excrete PFOA fairly similarly. Lau et al. (2006)reported relatively similar serum concentrations in male and female CD1 mice given 20 mg/kg of PFOA for 17 days. Serum PFOA concentration in male mice was ∼200 µg/ ml and was ∼175 µg/ ml in female mice, a difference of about 12% (Lau et al., 2006). In our study, serum concentration in the 10 mg/kg group after 14 days of exposure (the closest comparator in dose and time) was about 65% greater in males than in females. Certainly this difference between PFOA and the test compound could be strain dependent or concentration dependent. However, these data, combined with the results of the Gannon et al. (2015) study, indicate that unlike PFOA, male and female mice accumulate and excrete the test compound at very different rates. In previous studies with experimental rodent models, rats were the only species with significant sex-related differences in urinary excretion of PFOA. This difference is thought to be testosterone dependent at the level of the renal tubular cells as castrated male rats excrete PFOA more rapidly and when treated with testosterone, excrete PFOA more slowly (Kudo and Kawashima, 2003). The results of our study and the Gannon et al. (2015) study suggest that the test compound also may induce sex-specific effects. Therefore, future studies with mice should evaluate toxicological endpoints in both sexes.
CONCLUSIONS/SUMMARY
Our study is the first to report on the potential immunotoxicity of oral 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate exposure in C57Bl/6 mice. Unlike PFOA, the test compound did not potently suppress the TDAR, even at doses that would induce high mortality in mice given PFOA. Effects on immune parameters, liver weights, and peroxisome proliferation, while less robust than PFOA, were still evident. However, while this study evaluated only a few endpoints, the data suggest that the test compound may differ from PFOA in its mechanism of action on the immune system and may have sex-specific effects related to accumulation and excretion in mice. Additionally, given the absence of data about levels of the test compound in environmental media, wildlife, and humans, the doses used in this study may not reflect actual exposures and may be over- or underestimating exposure concentrations to potentially exposed populations. Further, the extent of occupational exposure to the test compound and potential sera concentrations in occupational populations cannot yet be estimated or compared to PFOA as the full occupational use of the test compound is not known. While the shorter chain length and perfluoroether makeup of the test compound may be associated with fewer immunotoxicological effects than PFOA, additional data, especially related to sex differences, are warranted to fully understand its toxicological profile and, therefore, suitability as a less toxic alternative to PFOA.
Acknowledgments
FUNDING
This study was supported, in part, by the Summer Biomedical Research Program (Blake Rushing) at East Carolina University.
Footnotes
DISCLAIMER
This report has been reviewed by the Environmental Protection Agency’s Office of Research and Development, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. The authors have no conflicts of interest to declare.
REFERENCES
- DeWitt JC, Copeland CB, Luebke RW (2009). Suppression of humoral immunity by perfluorooctanoic acid is independent of elevated serum corticosterone concentration in mice. Toxicol. Sci 109, 106–112. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Copeland CB, Strynar MJ, Luebke RW (2008). Perfluorooctanoic acid-induced immunomodulation in adult C57BL/6J or C57BL/6N female mice. Environ. Health Perspect 116, 644–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWitt JC, Shnyra A, Badr MZ, Loveless SE, Hoban D, Frame SR, Cunard R, Anderson SE, Meade BJ, Peden-Adams MM, et al. (2009). Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit. Rev. Toxicol 39, 76–94. [DOI] [PubMed] [Google Scholar]
- DeWitt JC, Williams WC, Creech NJ, Luebke RW (2016). Suppression of antigen-specific antibody responses in mice exposed to perfluorooctanic acid: Role of PPARα and T- and B-cell targeting. J. Immunotoxicol 13, 38–45. [DOI] [PubMed] [Google Scholar]
- DuPont. (2010). DuPontTM GenX processing aid for making fluoropolymer resins. Setting a new industry standard for sustainable replacement technology. Available at: https://www.chemours.com/Industrial_Bakery_Solutions/en_GB/assets/downloads/Chemours_GenX_Brochure_Final_07July2010.pdf. Accessed October 10, 2016.
- Gannon SA, Fasano WJ, Mawn MP, Nabb DL, Buck RC, Buxton LW, Jepson GW, Frame SR (2015). Absorption, distribution, metabolism, excretion, and kinetics of 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy) propanoic acid ammonium salt following a single dose in rat, mouse, and cynomologus monkey. Toxicology 340, 1–9. [DOI] [PubMed] [Google Scholar]
- Grandjean P, Anderson EW, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, Heilmann C (2012). Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307, 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granum B, Haug LS, Namork E, Stølevik SB, Thomsen C, Aaberge IS, van Loveren H, Løvik M, Nygaard UC (2013). Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J. Immunotoxicol 10, 373–379. [DOI] [PubMed] [Google Scholar]
- Keil DK (2015). Immunotoxicity of perfluoroalkylated compounds. In Toxicological Effects of Perfluoroalkyl and Polyfluoroalyl Substances (DeWitt JC, Ed.), Molecular and Integrative Toxicology, pp. 239–248. Humana Press, Springer International Publishing, Switzerland. [Google Scholar]
- Kudo N, Kawashima Y (2003). Toxicity and toxicokinetics of perfluorooctanoic acid in humans and animals. J. Toxicol. Sci 28, 49–57. [DOI] [PubMed] [Google Scholar]
- Lau C, Thibodeaux JR, Hanson RG, Narotsky MG, Rogers JM, Linstrom AB, Strynar MJ (2006). Effects of perfluorooctanoic acid exposure during pregnancy in the mouse. Toxicol. Sci 90, 510–518. [DOI] [PubMed] [Google Scholar]
- Looker C, Luster MI, Calafat AM, Johnson VJ, Burleson GR, Burleson FG, Fletcher T (2014). Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol. Sci 138, 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveless SE, Hoban D, Sykes G, Frame SR, Everds NE (2008). Evaluation of the immune system in rats and mice administered linear ammonium perfluorooctanoate. Toxicol. Sci 105, 86–96. [DOI] [PubMed] [Google Scholar]
- Luster MI, Portier C, Pait DG, White KL Jr, Gennings C, Munson AE, Rosenthal GJ (1992). Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol 18, 200–210. [DOI] [PubMed] [Google Scholar]
- Peden-Adams MM, Keller JM, EuDaly JG, Berger J, Gilkeson GS, Keil DE (2008). Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol. Sci 104, 144–154. [DOI] [PubMed] [Google Scholar]
- Poosch MS, Yamazaki RK (1986). Determination of peroxisomal fatty acid acyl-CoA oxidase activity using a lauroyl-CoA-based fluorometric assay. Biochim. Biophys. Acta 884, 585–593. [DOI] [PubMed] [Google Scholar]
- Rae JMC, Craig L, Slone TW, Frame SR, Buxton LW, Kennedy GL (2015). Evaluation of chronic toxicity and carcinogenicity of ammonium 2,3,3,3-tetrafluoro-2-(heptafluoropropoxy)-propanoate in Sprague–Dawley rats. Toxicol. Rep 2, 939–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner JL, Nakayama SF, Delinsky AD, Stanko JP, Fenton SE, Lindstrom AB, Strynar MJ (2009). Analysis of PFOA in dosed CD1 mice: Part 1. Methods development for the analysis of tissues and fluids from pregnant and lactating mice and their pups. Reprod. Toxicol 27, 360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl T, Heyn J, Thiele H, Hüther J, Failing K, Georgii S, Brunn H (2009). Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plants. Arch. Environ. Contam. Toxicol 57, 89–298. [DOI] [PubMed] [Google Scholar]
- Temple L, Butterworth TT, Kawabata AE, Munson AE, White KL Jr. (1995). ELISA to measure SRBC specific serum IgM: Method and data evaluation In Methods in Immunology (Burleson GR, Dean JH, Munson AE, Eds.), Vol. I, pp. 137–157. Wiley-Liss, Inc., New York, USA. [Google Scholar]
- Wang Z, Cousins IT, Scheringer M, Hungerbuehler K (2015). Hazard assessment of fluorinated alternatives to long-chain perfluoroalkyl acids (PFAAs) and their precursors: Status quo, ongoing challenges and possible solutions. Envrion. Int 75, 172–179. [DOI] [PubMed] [Google Scholar]
- West Virginia Department of Environmental Protection (WV DEP). (2012). Memo regarding WV/NPDES Permit No. WV0001279.
- Yang Q, Abedi-Valugerdi M, Xie Y, Zhao X, Moller G, Nelson BD, DePierre JW (2002). Potent suppression of the adaptive immune response in mice following dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid. Int. Immunopharmacol 2, 389–397. [DOI] [PubMed] [Google Scholar]
- Yang Q, Xie Y, Alexson SHE, Nelson BD, DePierre JW (2002a). Involvement of the peroxisome proliferator-activated receptor alpha in the immunomodulation caused by peroxisome proliferators in mice. Biochem. Pharmacol 63, 1893–1900. [DOI] [PubMed] [Google Scholar]
- Yang Q, Xie Y, DePierre JW (2000b). Effects of peroxisome proliferators on the thymus and spleen of mice. Clin. Exp. Immunol 122, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Xie Y, Eriksson AM, Nelson BD, DePierre JW (2001). Further evidence for the involvement of inhibition of cell proliferation and development in thymic and splenic atrophy induced by the peroxisome proliferator perfluorooctanoic acid in mice. Biochem. Pharmacol 62, 1133–1140. [DOI] [PubMed] [Google Scholar]