Abstract
Respiratory assist devices, that utilize ~2 m2 of hollow fiber membranes (HFMs) to achieve desired gas transfer rates, have been limited in their adoption due to such blood biocompatibility limitations. This study reports two techniques for the functionalization and subsequent conjugation of zwitterionic sulfobetaine (SB) block copolymers to polymethylpentene (PMP) HFM surfaces with the intention of reducing thrombus formation in respiratory assist devices. Amine or hydroxyl functionalization of PMP HFMs (PMP-A or PMP-H) was accomplished using plasma-enhanced chemical vapor deposition (PECVD). The generated functional groups were conjugated to low molecular weight SB block copolymers with N-hydroxysuccinimide ester or siloxane groups (SBNHS or SBNHSi) that were synthesized using reversible addition fragmentation chain transfer polymerization. The modified HFMs (PMP-A-SBNHS or PMP-H-SBNHSi) showed 80–95% reduction in platelet deposition from whole ovine blood, stability under the fluid shear of anticipated operating conditions, and uninhibited gas exchange performance relative to non-modified HFMs (PMP-C). Additionally, the functionalization and SBNHSi conjugation technique was shown to reduce platelet deposition on polycarbonate and poly(vinyl chloride), two other materials commonly found in extracorporeal circuits. The observed thromboresistance and stability of the SB modified surfaces, without degradation of HFM gas transfer performance, indicate that this approach is promising for longer term pre-clinical testing in respiratory assist devices and may ultimately allow for the reduction of anticoagulation levels in patients being supported for extended periods.
Keywords: respiratory assist devices, reduction of thrombus formation, surface functionalization and conjugation, zwitterionic sulfobetaine block copolymers, gas transfer performance
Introduction
Balancing required blood anticoagulation while maintaining adequate levels of hemostatic activity is a major challenge that presents with a wide variety of blood contacting medical devices. This issue is particularly challenging in extracorporeal circuits such as respiratory assist devices, which expose patient blood to large surface areas (~2 m2) of synthetic material1–5. Blood contact with these artificial surfaces results in activation of the coagulation cascade, and platelet deposition onto the foreign surfaces6,7, leading to reduced device performance and emboli formation8–10. Additionally, prolonged support may deplete the patient of critical coagulation factors and reduce platelet numbers, causing systemic hemostatic issues11,12. Physicians manage these thrombotic challenges with use of anticoagulants, most commonly systemic administration of heparin. However excess anticoagulation, particularly when administered systemically, may cause bleeding problems13,14. In fact bleeding issues are the most common complication in extracorporeal membrane oxygenation (ECMO), occurring in between 10–30% of cases, and the thrombotic complications that anticoagulation is meant to blunt, commonly lead to reduced device lifespan9,14–16. The risks associated with limited device blood biocompatibility and the pharmacologic interventions utilized to address this limitation clearly restrict the broader adoption of life saving extracorporeal therapies.
A great deal of effort has been directed at improving the hemocompatibility of extracorporeal circuits, by better pharmacologic management, more efficient circuit design and improved surfaces. These advances have led to broader use of dialysis and increasing use of ECMO and respiratory dialysis, particularly for low flow CO2 removal1,10,17–19. ECMO and respiratory dialysis devices utilize gas permeable hollow fiber membranes (HFMs) to add oxygen and remove carbon dioxide from blood independent of the lung. Innovations in HFMs, such as the introduction of polymethylpentene (PMP) HFMs in oxygenators, have facilitated extended patient support periods by preventing plasma leakage into the fiber lumen, which was common in older generation, porous wall polypropylene fibers20. PMP fibers greatly extended the circuit life of the oxygenator by resisting plasma leakage, but thrombotic deposition on fiber surfaces remained as a problem. Local anticoagulation using citrate is a potential solution, but it requires a more complex loop, restricted flow rates and its use is not widespread21,22.
Surface modification of synthetic fiber materials provides a potential resolution to the coagulation dilemma. Direct coating of the fiber may allow for local inhibition of thrombotic deposition, reducing the need for systemic anticoagulation and the resulting complications for the patient. Heparin coating of blood-contacting surfaces in extracorporeal perfusion circuits is common, but it is debatable as to the how much this surface modification has resulted in clinical benefit23. Surface conjugation of polymers bearing zwitterionic groups (e.g. phosphorylcholine, sulfobetaine (SB), carboxybetaine) have been described and examined as anti-fouling, non-thrombogenic and anti-bacterial materials24–27, but many of these approaches are limited by cost and stability28–30. Given the potential for zwitterionic surface modification to provide improved hemocompatibility in many instances, development of new surface modification strategies using this basic concept are warranted where durability and cost efficiency are design features.
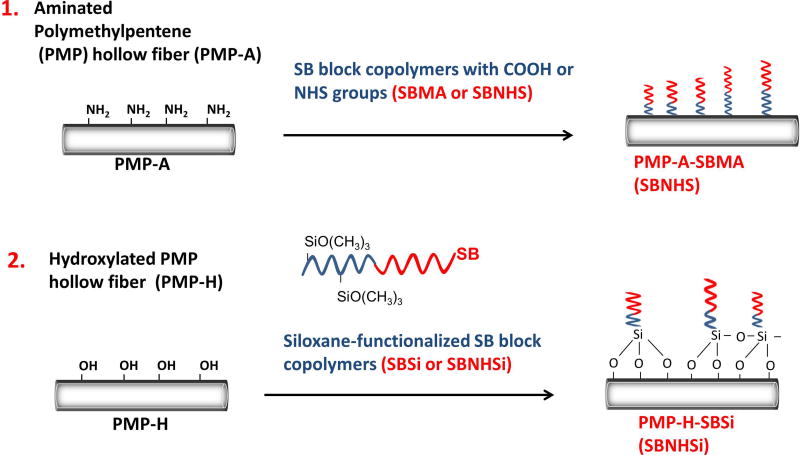
In this manuscript, a new SB block copolymer with N-hydroxysuccinimide ester groups (SBNHS) was synthesized to simplify conjugation to functionalized PMP HFMs. Two coating strategies, which pair PMP surface functionalization and zwitterionic molecule conjugation, were developed and evaluated. The first strategy employs plasma-enhanced chemical vapor deposition (PECVD) to generate amine functional groups on the PMP fiber surface that are subsequently conjugated to functional SB block copolymers, building upon our previous work with PP fibers31. A second strategy utilized surface hydroxylation32 to conjugate an SB block copolymer with siloxane groups (Figure 1). The zwitterion-modified PMP surfaces were evaluated for acute thrombotic deposition, surface modification stability under anticipated shear flow conditions, and for the impact of the surface modification on gas exchange performance. These coatings address the shortcomings of currently available alternatives as they are stable under high fluid shear stress, do not inhibit membrane transport, and employ economically attractive SB moieties as opposed to phosphorylcholine. This study evaluates the coatings for use specifically in respiratory assist devices, but the coatings are shown to be effective on several clinically relevant materials.
Figure 1.
Approaches for pre-functionalization of PMP hollow fibers & post-conjugation of SB molecules.
Materials and Methods
Materials
Polymethylpentene hollow fibers (Oxyplus™; OD: 380 µm, ID: 200 µm), of the same type used in commercial oxygenators, were obtained from Membrana GmbH (Wuppertal, Germany). N-(3-sulfopropyl)-N-(methacryloxyethyl)-N,N-dimethylammonium betaine (SMDAB) monomer, acrylic acid N-hydroxysuccinimide ester (AANHS), 4-cyano-4-(phenylcarbonothioylthio) pentanoic acid (CPPA as a chain transfer agent), 4,4′-azobis(4-cyanopentanoic acid) (V-501) (ACPA, initiator), 3-aminopropyl-trimethoxysilane (APSi), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), N-hydroxysuccinimide (NHS), 2,2,2-trifluoroethanol (TFE), and buffer reagents were purchased from Sigma-Aldrich. Methacrylic acid (MA, Sigma-Aldrich) was distilled before its use. [Sulfo-succinimidyl-4-O-(4,4´-dimethoxytrityl)-butyrate] (Sulfo-SDTB) was purchased from prochemonline.
Synthesis of SB block copolymers with functional groups
SB block copolymers with functional groups (COOH or NHS) were synthesized using a standard reversible addition fragmentation chain transfer (RAFT) polymerization technique previously described31. Briefly, after being dissolved in TFE solvent, SB prepolymer was synthesized from SMDAB monomer, CPPA chain transfer agent (CTA) and V-501 (ACPA) initiator at a determined molar ratio (SMDAB: CTA: ACPA= 10:1:0.2) to yield SB-CTA. Argon gas was bubbled into the solution for 30 min to remove oxygen, then the solution was sealed under argon for the duration of the synthesis. After stirring for 15 h at 70°C, the synthesized product was precipitated into anhydrous methanol. The obtained pink gel-like product (SB-CTA prepolymer) was further rinsed with excess methanol and dried in a vacuum oven for 24 h. Next, macro SB-CTA and MA or AANHS monomer was dissolved in TFE at a predetermined molar ratio (SB-CTA: MA=1:10 for SBMA, SB-CTA: AANHS=1:10 for SBNHS). After adding ACPA initiator (SB-CTA:ACPA= 1:0.2) and bubbling argon gas into the solution for 30 min, the reaction mixture was stirred for 15 h at 70°C. Following precipitation in anhydrous methanol, centrifugation, and rinsing to remove unreacted reagents, the synthesized SBMA or SBNHS block copolymers were obtained after vacuum drying. The siloxane functionalized SB block copolymers (SBSi or SBNHSi) were also prepared from the block copolymers after reaction with APSi via a standard EDC/NHS conjugation technique31,33. Multiple feeding ratios of the SMDAB and MA or AANHS monomers (e.g. 20:20, 10:10, 10:5) were also explored (defined as SBMA(20/20), SBMA(10/10) SBNHS(10/10) and SBNHS(10/5)). The chemical structure and the polymerization degree were confirmed using proton nuclear magnetic resonance (1H NMR, BrukerBiospin Co., Billerica, MA).
PMP fiber functionalization and characterization
Hollow fiber functionalization
Unmodified polymethylpentene (PMP) hollow fiber membranes were either amine or hydroxyl functionalized using plasma enhanced chemical vapor deposition (PECVD) with a PVA TePla Ion 40 system. Amine functionalization was completed according to a technique established by Arazawa et al34. Briefly, hollow fiber membrane samples, in a mat format, were cut to size (102 cm2), washed with 0.5% Tween-20 in 100 mM phosphate buffer pH 8.5, then rinsed with deionized water. Upon adding samples, the chamber was evacuated to 50 mTorr. Allylamine was introduced to the chamber at a rate of 180 mL/min and 300 W of power at 150 Hz and a 20% duty cycle was introduced for times ranging from 1 (A1) to 5 min (A2). Samples were removed and immediately rinsed three times with 0.5% Tween-20 in 100 mM phosphate buffer pH 8.5 then thoroughly rinsed with deionized water. Hydroxyl groups were generated using oxygen plasma32. PMP hollow fiber samples were prepared in a similar manner to amine functionalization, but after establishing 50 mTorr in the chamber, oxygen was added to the chamber at 500 mL/min then powers ranging from 100–600 W at 150 Hz and 20% duty cycled was applied for 30–300 sec. Additional materials, including polycarbonate sheet (Makrolon®, Bayer CropScience Ltd.) and a polyvinylchloride tubing (Fisherbrand Clear PVC Tubing, Fisher Scientific Inc.), were functionalized in the same manner.
Quantification of surface amine groups using sulfo-SDTB amine assay
Surface amine density was varied by adjusting plasma exposure time. Increased time exposure raises amine density, but may adversely inhibit gas exchange performance. 4 mL of 2.4 mM Sulfo-SDTB was added to a 16 cm2 fiber sample and incubated at room temperature for 2 hr. Fibers were then washed with 0.5% Tween-20 in deionized water to remove unbound reagent. 35% perchloric acid was added to cleave immobilized sulfo-SDTB and induce color change. Harvested solutions were read using a spectrometer (Thermo Genysis UV-Vis) at 498nm and amine concentration was calculated using a standard curve.
SB block copolymer conjugation
To prepare an SB block copolymer conjugated surface (PMP-A-SBMA or PMP-A-SBNHS), the amine functionalized PMP fiber (PMP-A) mats were immersed in a container with 0.1 wt% of aqueous SBNHS (NHS functionalized) solution or SBMA (carboxyl functionalized) with EDC/NHS31 and placed on a rocker at RT for 15 hr. For PMP-H-SBSi or PMP-H-SBNHSi, the hydroxyl functionalized PMP fiber (PMP-H) mats were immersed SBSi and SBNHSi solutions that were reacted with APSi in situ condition. The surface compositions were analyzed by X-ray photoelectron spectroscopy (XPS) using a Surface Instruments S-probe spectrometer with a takeoff angle 55°, performed at NESAC-BIO, University of Washington. Additional materials, including polycarbonate sheet (Makrolon®, Bayer CropScience Ltd.) and a polyvinylchloride tubing (Fisherbrand Clear PVC Tubing, Fisher Scientific Inc.), were modified and analyzed in the same manner.
In vitro blood contact test and platelet deposition characterization
Surface platelet deposition was analyzed after prolonged material contact with fresh whole ovine blood as described in previous studies31,35,36. In short, whole ovine blood was collected by jugular venipuncture with an 18½ gauge needle feeding directly into a syringe with a citrate solution (10mM/mL). The first 3 mL of blood were discarded to ensure sample uniformity. National Institutes of Health guidelines for the care and use of laboratory animals were observed for all donor sheep, and all procedures were approved by the Institutional Animal Care and Use Committee (IACUC). PMP fiber swatches were cut from fiber mats into 5 cm long segments for analysis. The fiber ends were sealed using a hot glue gun to prevent blood from reaching the inner lumen. Samples were sterilized by ethanol emersion then washed with DPBS. The fibers were then placed into a vacutainer tube (BD Vacutainer, no additives) filled with 5 mL of the fresh, citrated ovine blood and rocked for 3 h at 37°C using a hematology mixer (Fisher Scientific, Pittsburgh, PA).
After removal from the tubes, samples were rinsed with DPBS at least 10 times, according to the previously described protocol, in preparation for scanning electron microscopy (SEM, JEOL USA model JSM-6330F) analysis31. In addition to direct visualization of the surface, a lactate dehydrogenase assay (LDH, Cytotoxicity Detection Kit, Clontech Laboratories, Inc.) was used to quantify platelet deposition31.
The results are presented as mean ± standard deviation (SD). Data were analyzed by one-way ANOVA followed by a post hoc Neuman-Keuls testing. Significant differences were considered to exist at p <0.05.
SB coated fiber stability testing
PMP hollow fibers (102 cm2) were functionalized and coated with SBMA, SBSi, SBNHS, or SBNHSi as detailed above. Fiber mats were potted directly into polycarbonate mini-modules using 5 min epoxy (Devcon, Danvers, MA). The mini-modules were scaled from full sized oxygenator devices manufactured by Medtronic, Terumo and Capiox and simulate the surface area to volume ratio for gas exchange31,34 The estimated blood wall shear rate experienced in these modules was estimated to be comparable to commercially available devices. Mini-modules with open ports were sterilized using a standard ethylene oxide (EtO) sterilization cycle. Peristaltic pumps and L/S 16 Masterflex tubing were used to circulate 200 mL PBS with sodium azide (to prevent pathogen growth) through the modules at 45 mL/min for 2 wk at 37°C. The PBS solution was replaced at one week. After recirculation was complete, samples were removed from modules and unrolled. Randomly selected 1 by 1 cm2 fiber mats were cut and sent for XPS analysis and LDH platelet deposition testing.
Gas exchange testing of SB coated hollow fibers
Scaled polycarbonate gas exchange mini-modules were constructed from uncoated and SBNHS and SBNHSi modified fibers as described above. 5 min epoxy potting was used to separate the gas and fluid pathways. A gas exchange recirculation loop (see Figure S1) was assembled to measure CO2 removal from freshly drawn bovine blood. Bovine blood was freshly harvested from a local slaughterhouse and anticoagulated with heparin. Gentamycin (0.1g/mL) was added and blood was filtered (Haemonetics SQ40s 40 µm transfusion filter) to remove particulates. PBS was added to adjust hemoglobin to 12 ± 1 g/dL and glucose was adjusted to 10 mmol/dL ± 5 mmol/dL according to ISO 7199:2009. Bovine blood, obtained from a local slaughterhouse, was used for this testing due to the convenience in obtaining large volumes of fresh blood. The fluid side gas exchange loop contained (in series) a 1L compliant blood reservoir, peristaltic pump, Sorin Lilliput 2 D902 pediatric de-oxygenator, heat exchanger, and a mini-module before returning to the fluid reservoir. The de-oxygenator sweep gas was composed of carbon dioxide, oxygen, and nitrogen. These ratios were adjusted to set inlet blood CO2 partial pressure at 50 mmHg ± 5 mmHg as measured by a RapidPoint 305 blood gas analyzer (Siemans, Deerfield, IL). The oxygen sweep gas originates in a pressurized oxygen tank regulated by mass flow controller before passing through the mini-module. Sweep gas then passed through a condenser, vacuum pump, and CO2 analyzer. Triplicates of unmodified control fibers, PMP-SBNHS, and SBNHSi were each tested in triplicate at a blood flow rate of 45 mL/min. The sweep gas flow rate was modulated to maintain a 3000 ± 100 ppm CO2 concentration at the gas outlet. A one way ANOVA analysis was completed using SPSS 23 (IBM Analytics, Armonk, NY) with a p value<=0.05
Results
Synthesis of SB block copolymers with functional groups
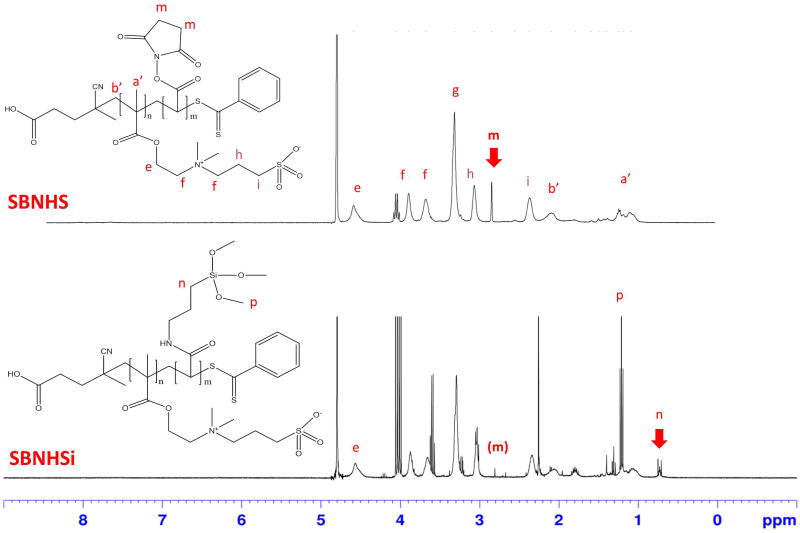
Chemical structures were confirmed by 1H NMR. For the newly synthesized SBNHS in D2O, peaks were: δ (ppm) = 0.90–1.30 (α-CH3), 1.70–2.20 (CH2C), 2.25–2.35 (CH2CH2SO3), 2.80–2.85 (CH2-CH2), 2.90–3.00 (CH2CH2SO3), 3.20–3.40 (N(CH3)2), 3.50–3.85 (CH2N(CH3)2CH2) and 4.35–4.55 (OCH2). The peak area integration ratio of 2.80–2.85 (CH2-CH2, m) and 4.35–4.55 (OCH2, e) was used to define the polymer composition. A SBNHS block copolymer, which was synthesized with a 2:1 monomer feed ratio of SBDMB and AANHS, resulted in a peak area integration ratio (e/m) of 2.7. After APSi conjugation (SBNHSi), the peak from the NHS groups, δ (ppm)=2.80–2.85 (CH2-CH2, m) was decreased and new peaks were measured at 0.65–0.75 (CH2-Si, n) and 1.20–1.30 (OCH3, p), indicating the presence of conjugated APSi (Figure 2). The molecular weights of the zwitterionic SB copolymers, with varied monomer feed ratios, were estimated from NMR spectra using the peak integration ratio of the NHS groups and CTA(CH2-COOH). The estimated molecular weights were 3588 ± 285 for SBMA(10/10), 3755 ± 570 for SBNHS (10/10), and 3050 ± 350 for SBNHS (10/5).
Figure 2.
1H NMR spectra of functional zwitterionic block copolymers (SBNHS or SBNHSi). SBMA has COOH instead of the N-Hydroxysuccinimide (NHS) groups in the SBNHS structure. SBSi structure is equivalent to SBNHSi.
HFM amine functionalization
PMP fibers aminated with PECVD showed that the plasma treatment time period (A1: 1 min, A2: 5 min) could be adjusted to vary HFM amine density, as measured by the sulfo-SDTB amine assay and XPS analysis (Table 1). A2 showed a 3% increase in N percentage compared to A1, indicating enhanced amination. All amine functionalized HFM conjugations were completed using 5 min time, hereby referred to as PMP-A.
Table 1.
Atomic percentages for plasma treated hollow fiber surfaces as determined by X-ray photoelectron spectroscopy.
| C | O | N | Si | S | |
|---|---|---|---|---|---|
| PMP-C | 95.1 (±1.3) | 4.9 (±1.3) | - | - | - |
| PMP-A1 | 73.0 (±1.1) | 18.2 (±0.5) | 5.2 (±0.5) | 3.4 (±0.1) | 0.2 (±0.0) |
| PMP-A2 | 68.9 (±0.6) | 18.7 (±0.2) | 8.2 (±0.3) | 3.6 (±3.4) | 0.3 (±0.0) |
| PMP-H (30s) | 87.8 (±0.6) | 9.9 (±0.8) | - | - | - |
| PMP-H (60s) | 88.3 (±2.4) | 12.0 (±0.4) | 0.3 (±0.4) | 0.3 (±0.4) | - |
| PMP-H (120s) | 88.9 (±0.4) | 10.9 (±0.3) | - | 0.5 (±0.5) | - |
PMP-C: unmodifed and cleaned HFM. PMP-A1 = 1 min, PMP-A2 = 5 min of plasma treatment with allyl amine. For PMP-A1 amine density was 0.09 nM/cm2 and for PMP-A2 0.34 nM/cm2 as measured by sulfo-SDTB assay. PMP-H: O2 plasma treated by PECVD (100W) for different exposure times (n=3, mean ± st. dev.).
OH functionalized fiber SEMs
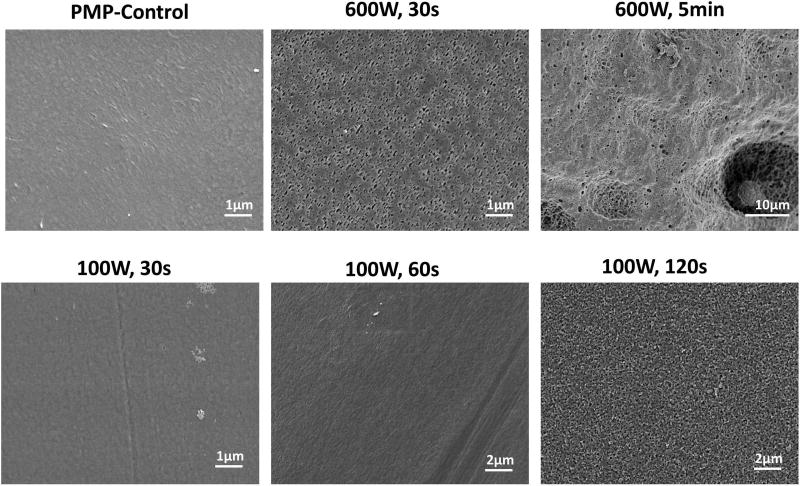
A new functionalization approach using hydroxyl groups (-OH) was developed. Unlike surface amine groups, hydroxyl groups are not anticipated to negatively impact biocompatibility and may increase the conjugation efficiency and loading of SB block copolymer. For hydroxylated PMP fibers (PMP-H) prepared by oxygen plasma treatment using PECVD, excessive power settings may damage the PMP fiber surface, so SEM images were taken for visual inspection (Figure 3). Damage to the fiber surface was observed in the 600 W, 30 sec and 5 min samples. Reduction of power to 100 W allowed for a plasma exposure time of 60 sec without visual signs of surface damage. X-ray photoelectron spectroscopy (XPS) (Table 1) was used to confirm surface hydroxylation. The oxygen percentage peaked at 12% ± 0.4% in the 100 W, 60 sec sample. All hydroxylated PMP HFM conjugations were completed using 100W, 60 sec sample, hereby referred to as PMP-H.
Figure 3.
Surface morphology change in PMP HFMs after exposure to O2 plasma (PECVD) at indicated power levels and exposure times.
SB block copolymer conjugation and platelet deposition on HFMs after ovine blood contact
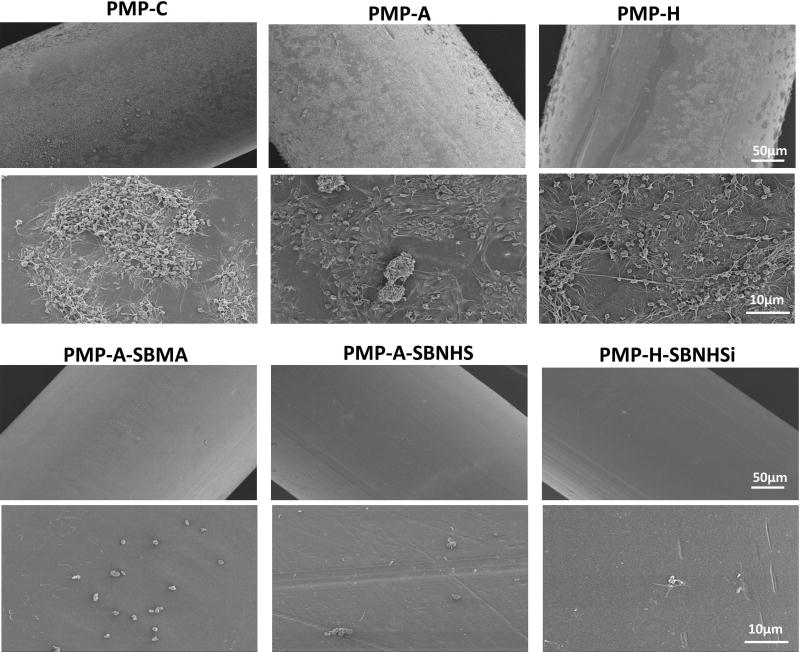
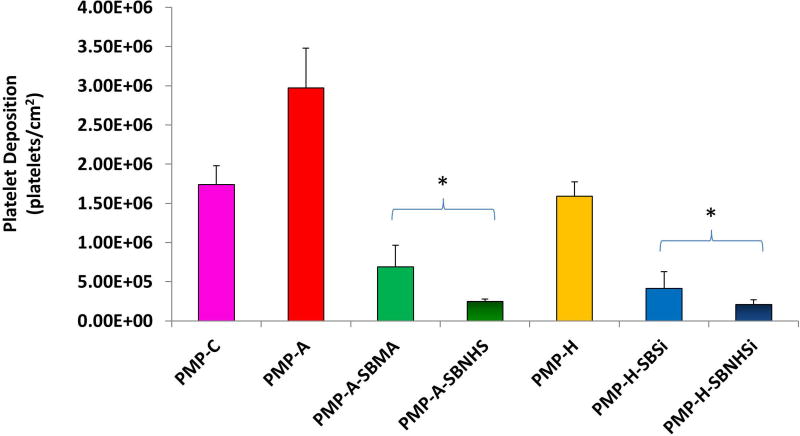
SB block copolymers SBMA, SBNHS, SBSi, and SBNHSi were directly conjugated to functionalized PMP fibers (PMP-A: 5 min or PMP-H: 100 W, 60 s). The loading capacity of SBMA onto the fibers can be measured by observing changes in the surface sulfur (S) content. XPS analysis results in Table 2 show S content ranging from 1.0± 0.1% to 1.2± 0.1%, compared to 0.3 for PMP-A and undetectable levels for PMP-C and PMP-H. Additional XPS analysis (Table S1) indicated increased S content for the lower molecular weight SBMA(10/10) and SBNHS(10/10) samples relative to that observed for the higher molecular weight SBMA(20/20). Platelet deposition on the controls, pre-functionalized, and modified PMP hollow fibers after contact with fresh ovine blood for 3 hr is shown in Figure 4 and Figure S2. The control (PMP-C) and pre-functionalized surfaces (PMP-A and PMP-H) showed large quantities of deposited platelets with aggregates spread uniformly over the entire fiber surface. The average amount of deposition on PMP-A samples showed a higher deposition than the PMP-C as well as PMP-H based on the SEM observation and LDH assay results (Figure 5 and Figure S3). Platelet deposition and the aggregates were dramatically reduced on all of the SB block copolymer conjugated surfaces compared to control and pre-functionalized surfaces (p<0.05).
Table 2.
Atomic percentages for SB block copolymers modified hollow fiber surfaces as determined by X-ray photoelectron spectroscopy.
| C | O | N | Si | S | |
|---|---|---|---|---|---|
| PMP-A-SBMA | 79.6 (±1.4) | 11.1 (±0.8) | 5.6 (±0.5) | 0.3 (±0.1) | 1.0 (±0.1) |
| PMP-H-SBSi | 85.6 (±0.7) | 9.8 (±2.1) | 1.1 (±0.4) | 0.6 (±0.1) | 1.0 (±0.1) |
| PMP-A-SBNHS | 69.3 (±0.5) | 18.3 (±0.2) | 7.5 (±0.2) | 0.9 (±0.0) | 1.1 (±0.1) |
| PMP-H-SBNHSi | 79.8 (±1.2) | 15.3 (±0.6) | 1.9 (±0.1) | 1.0 (±0.1) | 1.2 (±0.1) |
PMP-C: unmodifed and cleaned HFM. PMP-A = 5 min of plasma treatment with allyl amine (0.34 nM/cm2 as measured by sulfo-SDTB assay). PMP-H: O2 plasma treated by PECVD (100W) for 60 s exposure times (n=3, mean ± st. dev.).
Figure 4.
1. Scanning electron micrographs of PMP control and the modified surfaces after contact with citrated ovine whole blood for 3 h at 37°C.
2. Scanning electron micrographs of PMP control and the modified surfaces after contact with citrated ovine whole blood for 3 h at 37°C.
Figure 5.
Platelet deposition on a fiber mat unit (1 cm2), including binding fibers, after contact with fresh ovine blood for 3 h at 37°C as determined by a lactate dehydrogenase (LDH) assay (*P<0.05 vs. PMP-C, PMP-A2 and PMP-H controls, n=3).
SB coating stability testing
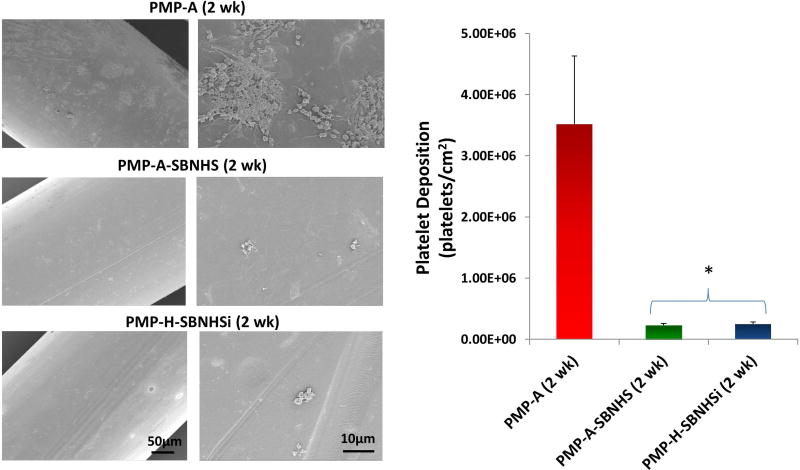
The surface compositions of coated fibers were also measured after exposure to elevated fluid shear levels for 2 wk in PBS at 37°C (Table 3). Both PMP-A-SBMA (2 wk) and PMP-H-SBSi (2 wk) decreased over 50% compared to pre-flow exposure (Table 2). SBNHS and SBNHSi coated surfaces better preserved surface S, remaining above 0.9 ± 0.1% and 1.0 ± 0.1% respectively. SEM images and LDH assay results (Figure 6) also provide evidence of coating integrity, exhibiting significantly reduced platelet deposition in both samples compared to PMP aminated controls (p<0.05).
Table 3.
Atomic percentages for the 2wk rinsed HFM surfaces as determined by X-ray photoelectron spectroscopy.
| C | O | N | Si | S | |
|---|---|---|---|---|---|
| PMP-A (2 wk) | 78.0 (±0.8) | 14.2 (±0.4) | 6.0 (±0.5) | 0.5 (±0.1) | 0.2 (±0.0) |
| PMP-A-SBMA (2 wk) | 75.5 (±0.6) | 19.7 (±0.5) | 2.9 (±0.2) | 0.4 (±0.1) | 0.4 (±0.1) |
| PMP-H-SBSi (2 wk) | 76.4 (±0.4) | 19.3 (±1.3) | 2.2 (±0.2) | 1.8 (±0.5) | 0.3 (±0.1) |
| PMP-A-SBNHS (2 wk) | 71.8 (±1.1) | 17.7 (±0.7) | 6.2 (±0.1) | 0.7 (±0.1) | 0.9 (±0.1) |
| PMP-H-SBNHSi (2 wk) | 81.4 (±0.2) | 14.3 (±0.2) | 2.0 (±0.3) | 0.8 (±0.1) | 1.0 (±0.1) |
All rinsed samples (2 wk) were EtO treated then continuously rinsed with PBS (with sodium azide 0.02%) at 37°C for 2 wk (n=3, mean ± st. dev.).
Figure 6.
Platelet deposition on the stability tested PMP hollow fibers (PBS rinsed for 2 wk) after contact with fresh ovine blood for 3 h at 37°C as determined by a lactate dehydrogenase (LDH) assay (*P<0.05 vs. the controls).
Gas exchange testing
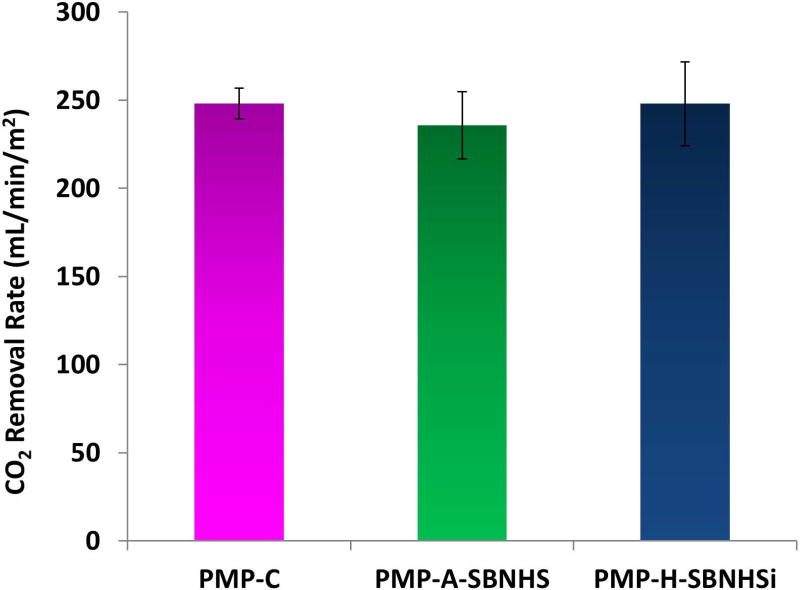
Gas exchange performance of hollow fibers was evaluated by examining carbon dioxide removal in a scaled experiment which simulates a clinical gas exchange setup. CO2 removal rates were normalized using inlet CO2 partial pressure to account for variations over the test span, then divided by fiber surface area. Only PMP-A-SBNHS and PMP-H-SBNHSi modifications were evaluated due to their superior stability characteristics. The unmodified control fiber removal rate of 248 ± 8 (mL/min/m2) is comparable to previous testing using scaled modules31. Aminated SBNHS coating samples (PMP-A-SBNHS) exhibited a 6% reduction in mean CO2 removal rate (236 ± 19), while hydroxyl activated SBNHSi fibers (PMP-H-SBNHSi) (248 ± 23) did not vary substantially from the mean control fiber CO2 removal rate. The statistical analysis determined a p-value of 0.08 (Figure 7).
Figure 7.
CO2 removal rate by HFMs in bovine blood using a scaled down gas exchange module (n=3).
Polycarbonate and PVC surface modification
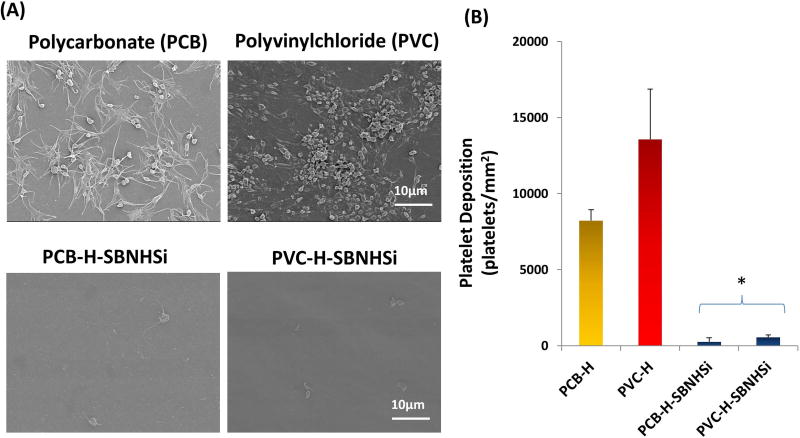
Both unmodified polycarbonate and polyvinylchloride (PVC) control surfaces showed widespread platelet deposition with a spread morphology and platelet aggregates after 3 h contact with fresh ovine blood contact, although polycarbonate visually exhibited less platelet deposition and aggregation than PVC, as shown in Figure 8(A). After the surface modification using SBNHSi, the amount of platelet deposition was reduced on both surfaces by up to 95% as shown in the LDH assay results (Figure 8(B)).
Figure 8.
Platelet deposition on other commonly used blood-contacting materials. (A) Scanning electron micrographs of blood-contacted polycarbonate (PCB), polyvinylchloride (PVC) and the SBNHSi modified surfaces. (B) Platelet deposition as determined by a lactate dehydrogenase (LDH) assay after contact with citrated ovine whole blood for 2 h at 37°C (*P<0.01 vs. PCB-H and PVC-H controls).
Discussion
This manuscript describes the development of zwitterionic surface modification approaches for use in extracorporeal blood-contacting medical devices such as artificial lungs. Two surface functionalization techniques, designed for amine or hydroxyl functionalized HFM surfaces respectively, were evaluated with the objective of designing a robust and simple process compatible with coating an entire extracorporeal circuit. The modified HFMs were shown to significantly reduce acute platelet deposition from whole blood, even after 2 wk exposure to continuous fluid flow. Additionally, gas exchange studies performed with the modified HFMs showed no significant reduction in gas transport, a critical feature for the application of this methodology in respiratory assist devices.
Amine functionalization of porous polypropylene hollow fibers has been studied by our group in the past31,36. With this approach, allylamine monomer was deposited on the fiber surface using PECVD, generating stable amine groups, but also decreasing fiber permeance37. Excess allylamine deposition may lead to decreased gas exchange performance, particularly if paired with a high molecular weight coating. Additionally, unreacted amines may result in increased platelet deposition. Variations of hydroxyl functionalization have been implemented in a variety of prototype extracorporeal devices38,39. With PMP fibers, excessive oxygen plasma runs the risk of damaging the thin outer fiber film which prevents plasma leakage40. This damage can be seen in SEMs of HFMs treated with elevated power and time settings (Figure 3: 600 W / 30 sec, 600 W / 5 min & 100 W / 120 sec), but was not present at the lower power settings (100 W / 60 sec) which were used for conjugation. The selected instrument settings did not present visible fiber damage and also exhibited the highest surface oxygen content (12.0 ± 0.4 %, Table 1), indicating these parameters balance avoidance of fiber damage and degree of functionalization.
For decades, various polymers bearing zwitterionic groups have been developed and studied as anti-fouling, non-thrombogenic and anti-inflammatory materials24–27. Studies by Cook41–43, Li38, and Gong44 have incorporated zwitterionic coatings into prototype artificial lung devices with success on the bench. Phosphorylcholine (PC) polymer coatings have also been incorporated into CE-approved oxygenators (ALONE ECMO, EUROSETS, Italy), but there are still many potential improvements that may benefit clinical efficacy and application in the challenging extended perfusion periods associated with ECMO support. Additionally, PC coatings are relatively expensive and difficult to handle compared to other zwitterionic polymers28,29. These limitations of PC coatings challenge widespread integration into extracorporeal devices, leading to exploration of alternative zwitterionic polymers. SB polymers in particular have shown promise, with comparable improvements in hemocompatibility and platelet deposition to PC counterparts at a lower price with higher stability26,28,31,45.
Our previous work developed functional zwitterionic PC macromolecules and low molecular weight (LMW <10,000) sulfobetaine (SB) block copolymer with carboxyl groups (SBMA-COOH) for polypropylene HFM surface modification31. These compounds are water soluble and can be conjugated onto an aminated polypropylene HFM to reduce thrombotic deposition without significantly inhibiting gas performance. Although the previous experimental coatings using the LMW SB block copolymer were promising, they have not been evaluated using PMP HFMs which are increasingly used in commercial oxygenators and are critical for the extended blood contact in chronic respiratory assist devices. The modifications have also not been tested for extended exposure to fluid flow rates commonly seen in clinical practice or on other material surfaces included in a typical extracorporeal loop (e.g. cannulae, tubing and device housing material).
Given that the application of extracorporeal oxygenators is necessarily associated with continuous exposure to flowing blood and wall shear stresses, the surface modification stability is of concern, and the instability of common device coatings such as heparin and PC have been documented45–47. Many techniques have been developed to improve stability and these show short term improvements in thromboresistance48–50, but few studies have examined efficacy after long term exposure to anticipated fluid shear conditions. In this study, a 2-week perfusion with buffered saline was completed to simulate the extended operating conditions becoming more prevalent in ECMO and more recent CO2 removal devices. Additionally, prior to the recirculation studies, the modules employed were EtO sterilized to simulate commercial devices. Results in Table 3 indicate previously developed coatings (SBMA and SBSi), which did not incorporate multiple active NHS groups, exhibited a reduction in surface sulfur groups, suggesting leaching of the conjugate from the HFM or degradation of the zwitterionic compounds. However, SBNHS and SBNHSi coatings did not experience the same degree of reduction, indicating greater stability under the anticipated operating shear and temperature. This stability, which has not been exhibited by other coatings, would be critical to achieving prolonged reductions in systemic anticoagulation. However, it should be noted that extended perfusion with patient blood would be a more rigorous situation, given the array of protein (especially enzyme) and cellular components that could act to foul or degrade the surface activity. The resistance of the SB modified surfaces to resist such interactions would potentially act to retard this effect.
Adding any coating to HFMs raises concerns of decreased gas exchange due to increased gas transfer resistance. Higher molecular weight coatings in particular run this risk. Thus, we synthesized low molecular weight functional block copolymers (SBMA or SBNHS) for the surface conjugation using a RAFT polymerization technique. The employment of RAFT polymerization allows for generation of well-controlled, low molecular weight block copolymers with versatile functional groups51. A synthesis strategy using SB block copolymer with NHS groups is particularly flexible as it could be used in one approach to provide direct SB conjugation to amines on the target surface with a high reactivity, or in an alternate approach, to generate a polymer such as SBNHSi to react with surface hydroxyl groups. Gas exchange testing showed no statistical difference in CO2 removal rates between the SB block copolymers modified and control HFMs. Comparable gas exchange evaluation on coated PMP fibers was completed by Huang38 on PC coated PMP fibers. This testing showed a 15% reduction in mean CO2 removal rate. Additionally, no blood plasma leakage was observed, indicating that the outer layer of the PMP fibers remained intact after surface functionalization and modification, supporting the SEM images (Figure 3), which did not show surface damage at the selected plasma instrument settings. Any breach in this outer layer would result in fluid leakage into the gas side of the fibers and a significant decrease in gas exchange efficiency over time. While it is also possible that carbonyl groups are generated after the oxygen plasma process38, their presence on the functionalized surface before conjugation would not be expected to decrease gas exchange efficiency. In addition to the XPS data supporting conjugation, a decrease in surface hydrophobicity was observed after coating (qualitative observation of water contact angle). This change did not appear to have any influence on the CO2 removal rate. However, platelet deposition was notably reduced on the SB block copolymer modified surfaces. The reduction of platelet deposition associated with SB block copolymer conjugation are likely related to changes of the surface properties and the reduced protein (e.g. fibrinogen) adsorption, as confirmed in our previous study52. The antifouling mechanisms associated with zwitterion-modified surfaces have also been extensively considered in terms of surface property changes (interactions with water and salts) in previous publications53–55 while the antifouling effectiveness on the SB modified surface could be variable based upon salt concentration, buffer composition, solution pH level and temperature56,57.”
The majority of studies developing thromboresistant coatings have focused on a single material and application31,38,44. However, most extracorporeal circuits are composed of a variety of materials and surfaces which may contribute to thrombus formation and subsequent embolization. To evaluate the versatility of the reported surface modification approach, a peripheral evaluation of acute thromboresistance was completed for two materials commonly found in non-gas transferring surfaces included in extracorporeal devices. Polycarbonate, which is commonly used for injection molded housings, and PVC, which is a widely used tubing material, were functionalized and modified under the same parameters as the PMP HFMs. With the effectiveness of the modification strategy shown on these surfaces, the data suggest that it may be possible to uniformly modify all blood contacting surfaces in an extracorporeal circuit, to further improve the blood biocompatibility and potentially allow for the reduction in systemic anticoagulation.
While this report has established a technique capable of reducing acute platelet deposition on a variety of materials commonly used in respiratory assist devices, without significantly reducing device performance, there are several limitations that should be noted. First, the surface modification strategy has only been evaluated in vitro and for limited contact periods with anticoagulated animal blood. In vivo testing would be the next step to more fully verify device gas transfer performance and thromboresistance. The notion that systemic anticoagulation might be reducible in clinical practice with such surface modifications would similarly need to first be evaluated in a large animal model. As noted previously, stability testing established modification stability at shear rates comparable to that experienced in full-sized devices, however it is unknown how the coating would perform after exposure to the proteases and high protein concentrations present in blood, not to mention with extended contact with leukocytes and platelets. The LDH assay, used to measure platelet deposition, could also measure any adhered red blood cells58, potentially interfering with the platelet adhesion results. However, the SEM images (Figures 4, 6, & 8) consistently showed little to no red blood cell deposition and this was also not observed macroscopically on the fibers, indicating that such interference with the LDH assay was minimal. While a range of functionalization and conjugation parameters were evaluated, a complete optimization was not completed and further improvements in performance and stability of the reported modifications may be possible. Finally, all testing was completed on scaled-down devices or HFM swatches. It is believed the process would scale to full sized devices, but a strategy for surface modification would need to be developed. For instance, determining whether the fibers would be modified independently of the device housing or whether an assembled oxygenator could be modified.
Conclusions
This study reports two techniques for the functionalization and subsequent conjugation of SB block copolymers to PMP HFMs with the intention of reducing thrombus formation in respiratory assist devices. Amine or hydroxyl functionalization of HFMs was accomplished using PECVD and the generated functional groups were then conjugated to newly synthesized SBNHS or SBNHSi block copolymers. The modified HFMs showed markedly reduced platelet deposition from whole ovine blood, stability under the fluid shear of anticipated operating conditions and uninhibited gas exchange performance. Additionally, the functionalization and conjugation techniques were shown to reduce platelet deposition on other materials which are common in extracorporeal circuits such as polycarbonate and PVC tubing. This flexible surface modification approach may thus allow for coating of an entire blood contacting extracorporeal circuit using recirculated reagents at room temperature. Improvements in the thromboresistance of the blood-contacting surfaces in large extracorporeal devices intended for chronic support are essential to allow for the reduction of anticoagulation levels in the supported patients. Without such advancements, the implementation of these devices will be hampered since the technology will continue to be associated with limited patient mobility, a high-level of patient oversight and management, and substantial patient morbidity.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (HL117637 and T32HL076124). The authors would like to thank the Center for Biological Imaging (CBI) of the University of Pittsburgh for their kind assistance in collecting the SEM images. The surface analysis experiments done at NESAC/BIO and were supported by NIH, NIBIB grant EB-002027. The authors also would like to thank Dr. Venkat Shankarraman, Joseph Hanke, Teri Horgan and others who obtained the fresh ovine blood used in this study.
Footnotes
In vitro gas exchange analysis setup using a scaled down gas exchange module. Not shown are the CO2, N2 and O2 gas lines, the oxygen gas flow meter, condenser, and CO2 analyzer (Figure S1), atomic percentages for hollow fiber surfaces as determined by X-ray photoelectron spectroscopy (Table S1), scanning electron micrographs of PMP control and the modified surfaces after contact with citrated ovine whole blood for 3 h at 37°C (Figure S2) and platelet deposition on a fiber mat unit (1 cm2), including binding fibers, after contact with fresh ovine blood for 3 h at 37°C as determined by a lactate dehydrogenase (LDH) assay (*P<0.05 vs. PMP-C, PMP-A2 and PMP-H controls, n=3) (Figure S3). This material is available free for charge via the Internet at hppt://pubs.acs.org.
The authors declare not competing financial interest.
References
- 1.Nolan H, Wang D, Zwischenberger JB. Artificial lung basics. Organogenesis. 2011;7:23–7. doi: 10.4161/org.7.1.14025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Federspiel WJ, Henchir KA. Lung, artificial: basic principles and current applications. Encycl Biomater Biomed Eng. 2004;9:910. [Google Scholar]
- 3.Esper SA, Levy JH, Waters JH, Welsby IJ. Extracorporeal Membrane Oxygenation in the Adult: A Review of Anticoagulation Monitoring and Transfusion. Anesth Analg. 2014;118:731–43. doi: 10.1213/ANE.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 4.Galbusera M, Remuzzi G, Boccardo P. Treatment of Bleeding in Dialysis Patients. Semin Dial. 2009;22:279–86. doi: 10.1111/j.1525-139X.2008.00556.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaw D, Malhotra D. HEMATOLOGY: ISSUES IN THE DIALYSIS PATIENT: Platelet Dysfunction and End-Stage Renal Disease. Semin Dial. 2006;19:317–22. doi: 10.1111/j.1525-139X.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- 6.Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI. Medical device-induced thrombosis: what causes it and how can we prevent it? J Thromb Haemost. 2015;13:S72–81. doi: 10.1111/jth.12961. [DOI] [PubMed] [Google Scholar]
- 7.Wendel HP, Ziemer G. Coating-techniques to improve the hemocompatibility of artificial devices used for extracorporeal circulation. Eur J Cardiothorac Surg. 1999;16:342–50. doi: 10.1016/s1010-7940(99)00210-9. [DOI] [PubMed] [Google Scholar]
- 8.Gorbet MB, Sefton MV. Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials. 2004;25:5681–703. doi: 10.1016/j.biomaterials.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Annich GM. Extracorporeal life support: the precarious balance of hemostasis. J Thromb Haemost. 2015;13:S336–42. doi: 10.1111/jth.12963. [DOI] [PubMed] [Google Scholar]
- 10.Strueber M. Artificial Lungs. Thorac Surg Clin. 2015;25:107–13. doi: 10.1016/j.thorsurg.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 11.McManus ML, Kevy SV, Bower LK, Hickey PR. Coagulation factor deficiencies during initiation of extracorporeal membrane oxygenation. J Pediatr. 1995;126:900–4. doi: 10.1016/s0022-3476(95)70205-9. [DOI] [PubMed] [Google Scholar]
- 12.Despotis GJ, Avidan MS, Hogue CW., Jr Mechanisms and attenuation of hemostatic activation during extracorporeal circulation. Ann Thorac Surg. 2001;72:S1821–31. doi: 10.1016/s0003-4975(01)03211-8. [DOI] [PubMed] [Google Scholar]
- 13.Robba C, Ortu A, Bilotta F, Lombardo A, Sekhon MS, Gallo F, Matta BF. Extracorporeal membrane oxygenation for adult respiratory distress syndrome in trauma patients: A case series and systematic literature review. J Trauma Acute Care Surg. 2017;82:165–73. doi: 10.1097/TA.0000000000001276. [DOI] [PubMed] [Google Scholar]
- 14.Makdisi G, Wang I. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7:E166–76. doi: 10.3978/j.issn.2072-1439.2015.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews J, Winkler AM. Challenges with Navigating the Precarious Hemostatic Balance during Extracorporeal Life Support: Implications for Coagulation and Transfusion Management. Transfus Med Rev. 2016;30:223–9. doi: 10.1016/j.tmrv.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, Patroniti N, Antonelli M, Pesenti A, Pappalardo F, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013;15:172. [PubMed] [Google Scholar]
- 17.Pesenti A, Patroniti N, Fumagalli R. Carbon dioxide dialysis will save the lung: Crit Care Med. 2010;38:S549–54. doi: 10.1097/CCM.0b013e3181f1fe0c. [DOI] [PubMed] [Google Scholar]
- 18.Lund LW, Federspiel WJ. Removing extra CO2 in COPD patients. Curr Respir Care Rep. 2013;2:131–8. doi: 10.1007/s13665-013-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crotti S, Lissoni A, Tubiolo D, Azzari S, Tarsia P, Caspani L, Gattinoni L. Artificial lung as an alternative to mechanical ventilation in COPD exacerbation. Eur Respir J. 2012;39:212–5. doi: 10.1183/09031936.00021111. [DOI] [PubMed] [Google Scholar]
- 20.Montoya JP, Shanley CJ, Merz SI, Bartlett RH. Plasma Leakage through Microporous Membranes: Role of Phospholipids. ET J. 1992;38 doi: 10.1097/00002480-199207000-00064. [DOI] [PubMed] [Google Scholar]
- 21.Wu M-Y, Hsu Y-H, Bai C-H, Lin Y-F, Wu C-H, Tam K-W. Regional Citrate Versus Heparin Anticoagulation for Continuous Renal Replacement Therapy: A Meta-Analysis of Randomized Controlled Trials. Am J Kidney Dis. 2012;59:810–8. doi: 10.1053/j.ajkd.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 22.Liu C, Mao Z, Kang H, Hu J, Zhou F. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: a meta-analysis with trial sequential analysis of randomized controlled trials. [cited 2017 Jan 9];Crit Care [Internet] 2016 20 doi: 10.1186/s13054-016-1299-0. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4866420/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvetti S, Koster A, Pappalardo F. Do We Need Heparin Coating for Extracorporeal Membrane Oxygenation? New Concepts and Controversial Positions About Coating Surfaces of Extracorporeal Circuits. Artif Organs. 2015;39:176–9. doi: 10.1111/aor.12335. [DOI] [PubMed] [Google Scholar]
- 24.Ye SH, Watanabe J, Iwasaki Y, Ishihara K. Antifouling blood purification membrane composed of cellulose acetate and phospholipid polymer. Biomaterials. 2003;24:4143–52. doi: 10.1016/s0142-9612(03)00296-5. [DOI] [PubMed] [Google Scholar]
- 25.Sin M-C, Chen S-H, Chang Y. Hemocompatibility of zwitterionic interfaces and membranes. Polym J. 2014;46:436–43. [Google Scholar]
- 26.Schlenoff JB. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir. 2014;30:9625–36. doi: 10.1021/la500057j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He M, Gao K, Zhou L, Jiao Z, Wu M, Cao J, You X, Cai Z, Su Y, Jiang Z. Zwitterionic materials for antifouling membrane surface construction. Acta Biomater. 2016;40:142–52. doi: 10.1016/j.actbio.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Chen S, Chang Y, Jiang S. Surface Grafted Sulfobetaine Polymers via Atom Transfer Radical Polymerization as Superlow Fouling Coatings. J Phys Chem B. 2006;110:10799–804. doi: 10.1021/jp057266i. [DOI] [PubMed] [Google Scholar]
- 29.Ueda T, Oshida H, Kurita K, Ishihara K, Nakabayashi N. Perparation of 2-Methacryloyloxyethyl Phosphorylcholine Copolymers with Alkyl Methacrylates and Their Blood Compatibility. Polym J. 1992;24:1259–69. [Google Scholar]
- 30.Wang D, Williams CG, Li Q, Sharma B, Elisseeff JH. Synthesis and characterization of a novel degradable phosphate-containing hydrogel. Biomaterials. 2003;24:3969–80. doi: 10.1016/s0142-9612(03)00280-1. [DOI] [PubMed] [Google Scholar]
- 31.Ye S-H, Arazawa DT, Zhu Y, Shankarraman V, Malkin AD, Kimmel JD, Gamble LJ, Ishihara K, Federspiel WJ, Wagner WR. Hollow Fiber Membrane Modification with Functional Zwitterionic Macromolecules for Improved Thromboresistance in Artificial Lungs. Langmuir. 2015;31:2463–71. doi: 10.1021/la504907m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixit CK, Vashist SK, MacCraith BD, O’Kennedy R. Multisubstrate-compatible ELISA procedures for rapid and high-sensitivity immunoassays. Nat Protoc. 2011;6:439–45. doi: 10.1038/nprot.2011.304. [DOI] [PubMed] [Google Scholar]
- 33.Fischer ME. Amine Coupling Through EDC/NHS: A Practical Approach. In: Mol NJ, Fischer MJE, editors. Surf Plasmon Reson [Internet] Humana Press; 2010. [cited 2017 Jan 10]. Available from: [DOI] [Google Scholar]
- 34.Arazawa DT, Kimmel JD, Finn MC, Federspiel WJ. Acidic sweep gas with carbonic anhydrase coated hollow fiber membranes synergistically accelerates CO2 removal from blood. Acta Biomater. 2015;25:143–9. doi: 10.1016/j.actbio.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arazawa DT, Oh H-I, Ye S-H, Johnson CA, Jr, Woolley JR, Wagner WR, Federspiel WJ. Immobilized carbonic anhydrase on hollow fiber membranes accelerates CO2 removal from blood. J Membr Sci. 2012;403–404:25–31. doi: 10.1016/j.memsci.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimmel JD, Arazawa DT, Ye S-H, Shankarraman V, Wagner WR, Federspiel WJ. Carbonic anhydrase immobilized on hollow fiber membranes using glutaraldehyde activated chitosan for artificial lung applications. J Mater Sci Mater Med. 2013;24:2611–21. doi: 10.1007/s10856-013-5006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calderon JG, Harsch A, Gross GW, Timmons RB. Stability of plasma-polymerized allylamine films with sterilization by autoclaving. J Biomed Mater Res. 1998;42:597–603. doi: 10.1002/(sici)1097-4636(19981215)42:4<597::aid-jbm16>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 38.Huang X, Wang W, Zheng Z, Fan W, Mao C, Shi J, Li L. Surface monofunctionalized polymethyl pentene hollow fiber membranes by plasma treatment and hemocompatibility modification for membrane oxygenators. Appl Surf Sci. 2016;362:355–63. [Google Scholar]
- 39.Didar TF, Cartwright MJ, Rottman M, Graveline AR, Gamini N, Watters AL, Leslie DC, Mammoto T, Rodas MJ, Kang JH, Waterhouse A, Seiler BT, Lombardo P, Qendro EI, Super M, Ingber DE. Improved treatment of systemic blood infections using antibiotics with extracorporeal opsonin hemoadsorption. Biomaterials. 2015;67:382–92. doi: 10.1016/j.biomaterials.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 40.Toomasian JM, Schreiner RJ, Meyer DE, Schmidt ME, Hagan SE, Griffith GW, Bartlett RH, Cook KE. A Polymethylpentene Fiber Gas Exchanger for Long-Term Extracorporeal Life Support: ASAIO J. 2005;51:390–7. doi: 10.1097/01.mat.0000169111.66328.a8. [DOI] [PubMed] [Google Scholar]
- 41.Amoako KA, Sundaram HS, Suhaib A, Jiang S, Cook KE. Multimodal, Biomaterial-Focused Anticoagulation via Superlow Fouling Zwitterionic Functional Groups Coupled with Anti-Platelet Nitric Oxide Release. Adv Mater Interfaces. 2016;3:1500646. [Google Scholar]
- 42.Gupta S, Amoako KA, Suhaib A, Cook KE. Multi-Modal, Surface-Focused Anticoagulation Using Poly-2-methoxyethylacrylate Polymer Grafts and Surface Nitric Oxide Release. Adv Mater Interfaces. 2014;1:1400012. [Google Scholar]
- 43.Sundaram HS, Han X, Nowinski AK, Brault ND, Li Y, Ella-Menye J-R, Amoaka KA, Cook KE, Marek P, Senecal K, Jiang S. Achieving One-Step Surface Coating of Highly Hydrophilic Poly(Carboxybetaine Methacrylate) Polymers on Hydrophobic and Hydrophilic Surfaces. Adv Mater Interfaces. 2014;1:1400071. doi: 10.1002/admi.201400071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y-B, Gong M, Yang S, Nakashima K, Gong Y-K. Hemocompatibility and film stability improvement of crosslinkable MPC copolymer coated polypropylene hollow fiber membrane. J Membr Sci. 2014;452:29–36. [Google Scholar]
- 45.Chen S-H, Chang Y, Lee K-R, Wei T-C, Higuchi A, Ho F-M, Tsou C-C, Ho H-T, Lai J-Y. Hemocompatible Control of Sulfobetaine-Grafted Polypropylene Fibrous Membranes in Human Whole Blood via Plasma-Induced Surface Zwitterionization. Langmuir. 2012;28:17733–42. doi: 10.1021/la3036902. [DOI] [PubMed] [Google Scholar]
- 46.Grode GA, Falb RD, Crowley JP. Biocompatible materials for use in the vascular system. J Biomed Mater Res. 1972;6:77–84. doi: 10.1002/jbm.820060407. [DOI] [PubMed] [Google Scholar]
- 47.Inauen W, Baumgartner HR, Bombeli T, Haeberli A, Straub PW. Dose-and shear rate-dependent effects of heparin on thrombogenesis induced by rabbit aorta subendothelium exposed to flowing human blood. Arterioscler Thromb Vasc Biol. 1990;10:607–615. doi: 10.1161/01.atv.10.4.607. [DOI] [PubMed] [Google Scholar]
- 48.Olsson P, Sanchez J, Mollnes TE, Riesenfeld J. On the blood compatibility of end-point immobilized heparin. J Biomater Sci Polym Ed. 2000;11:1261–73. doi: 10.1163/156856200744192. [DOI] [PubMed] [Google Scholar]
- 49.Yang Z, Wang J, Luo R, Maitz MF, Jing F, Sun H, Huang N. The covalent immobilization of heparin to pulsed-plasma polymeric allylamine films on 316L stainless steel and the resulting effects on hemocompatibility. Biomaterials. 2010;31:2072–83. doi: 10.1016/j.biomaterials.2009.11.091. [DOI] [PubMed] [Google Scholar]
- 50.Kang I-K, Kwon OH, Lee YM, Sung YK. Preparation and surface characterization of functional group-grafted and heparin-immobilized polyurethanes by plasma glow discharge. Biomaterials. 1996;17:841–7. doi: 10.1016/0142-9612(96)81422-0. [DOI] [PubMed] [Google Scholar]
- 51.Semsarilar M, Perrier S. “Green” reversible addition-fragmentation chain-transfer (RAFT) polymerization. Nat Chem. 2010;2:811–20. doi: 10.1038/nchem.853. [DOI] [PubMed] [Google Scholar]
- 52.Ye SH, Johnson CA, Jr, Woolley JR, Murata H, Gamble LJ, Ishihara K, Wagner WR. Simple surface modification of a titanium alloy with silanated zwitterionic phosphorylcholine or sulfobetaine modifiers to reduce thrombogenicity. Colloids Surf B Biointerfaces. 2010;79:357–64. doi: 10.1016/j.colsurfb.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlenoff JB. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir. 2014;30:9625−9636. doi: 10.1021/la500057j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu J, Zhao C, Hu R, Lin W, Wang Q, Zhao J, Bilinovich SM, Leeper TC, Li L, Cheung HM, Chen S, Zheng J. Probing the weak interaction of proteins with neutral and zwitterionic antifouling polymers. Acta Biomater. 2014;10:751–60. doi: 10.1016/j.actbio.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 55.Wu J, He C, He H, Cheng C, Zhu J, Xiao Z, Zhang H, Li X, Zheng J, Xiao J. Importance of zwitterionic incorporation into polymethacrylate-based hydrogels for simultaneously improving optical transparency, oxygen permeability, and antifouling properties. J. Mater Chem B. 2017;5:4595–606. doi: 10.1039/c7tb00757d. [DOI] [PubMed] [Google Scholar]
- 56.Mary P, Bendejacq DD, Labeau MP, Dupuis P. Reconciling low- and high-salt solution behavior of sulfobetaine polyzwitterions. J Phys Chem B. 2007;111:7767–77. doi: 10.1021/jp071995b. [DOI] [PubMed] [Google Scholar]
- 57.Chang Y, Liao SC, Higuchi A, Ruaan RC, Chu CW, Chen WY. A highly stable nonbiofouling surface with well-packed grafted zwitterionic polysulfobetaine for plasma protein repulsion. Langmuir. 2008;24:5453–8. doi: 10.1021/la800228c. [DOI] [PubMed] [Google Scholar]
- 58.Koseoglu M, Hur A, Atay A, Cuhadar S. Effects of hemolysis interference on routine biochemistry parameters. Biochem Medica Biochem Medica. 2011;21:79–85. doi: 10.11613/bm.2011.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.