Abstract
Purpose
Both temozolomide (TMZ) and poly (ADP-ribose) polymerase (PARP) inhibitors are active in small-cell lung cancer (SCLC). This phase II, randomized, double-blind study evaluated whether addition of the PARP inhibitor veliparib to TMZ improves 4-month progression-free survival (PFS).
Patients and Methods
A total of 104 patients with recurrent SCLC were randomly assigned 1:1 to oral veliparib or placebo 40 mg twice daily, days 1 to 7, and oral TMZ 150 to 200 mg/m2/day, days 1 to 5, of a 28-day cycle until disease progression, unacceptable toxicity, or withdrawal of consent. Response was determined by imaging at weeks 4 and 8, and every 8 weeks thereafter. Improvement in PFS at 4 months was the primary end point. Secondary objectives included overall response rate (ORR), overall survival (OS), and safety and tolerability of veliparib with TMZ. Exploratory objectives included PARP-1 and SLFN11 immunohistochemical expression, MGMT promoter methylation, and circulating tumor cell quantification.
Results
No significant difference in 4-month PFS was noted between TMZ/veliparib (36%) and TMZ/placebo (27%; P = .19); median OS was also not improved significantly with TMZ/veliparib (8.2 months; 95% CI, 6.4 to 12.2 months; v 7.0 months; 95% CI, 5.3 to 9.5 months; P = .50). However, ORR was significantly higher in patients receiving TMZ/veliparib compared with TMZ/placebo (39% v 14%; P = .016). Grade 3/4 thrombocytopenia and neutropenia more commonly occurred with TMZ/veliparib: 50% versus 9% and 31% versus 7%, respectively. Significantly prolonged PFS (5.7 v 3.6 months; P = .009) and OS (12.2 v 7.5 months; P = .014) were observed in patients with SLFN11-positive tumors treated with TMZ/veliparib.
Conclusion
Four-month PFS and median OS did not differ between the two arms, whereas a significant improvement in ORR was observed with TMZ/veliparib. SLFN11 expression was associated with improved PFS and OS in patients receiving TMZ/veliparib, suggesting a promising biomarker of PARP-inhibitor sensitivity in SCLC.
INTRODUCTION
Therapeutic options for patients with relapsed small-cell lung cancer (SCLC) have remained unchanged for three decades. The only Food and Drug Administration–approved agent for recurrent or progressive SCLC is topotecan, on the basis of three phase III trials,1-3 which showed modest response rates of 24% in patients with platinum-sensitive disease and 2% to 6% in platinum-refractory SCLC.3-6 Median time to progression with topotecan is short, between 13 and 16 weeks,1,3 and there are no approved regimens after second-line treatment. More effective therapies in SCLC are critically needed.
SCLC is characterized by aberrant expression of several genes implicated in DNA damage repair. Proteomic profiling previously identified poly (ADP-ribose) polymerase (PARP)-1 as a candidate drug target.7 Frequent epigenetic silencing of the MGMT gene, which encodes the DNA-repair protein O6 methylguanine-DNA methyltransferase (MGMT), also has been demonstrated.8-10 As such, DNA damage response pathways represent attractive targets in SCLC.11
Temozolomide (TMZ) is an oral alkylating agent that produces O6-alkyl-guanine lesions on DNA, which are removed by MGMT. Left unrepaired, TMZ-induced lesions are cytotoxic and trigger apoptosis.9,10 We previously showed single-agent activity of TMZ in patients with relapsed SCLC,12 leading to its incorporation into treatment guidelines for this disease.13 However, the benefit provided by single-agent TMZ typically is brief, with median progression-free survival (PFS) of 3.5 months.12
One well-defined mechanism of resistance to TMZ is through the PARP-dependent base excision repair pathway.14-16 In several cancer types, the combination of veliparib (formerly ABT-888), an oral inhibitor of PARP-1 and PARP-2, and TMZ results in greater tumor growth delay or regression, relative to TMZ alone.17 Furthermore, PARP inhibitors (PARPi) have single-agent activity in SCLC models and potentiate the effect of cytotoxic agents.7,18,19 On the basis of this, PARPi trials have been initiated in SCLC.20,21 In this multi-institutional, double-blind, placebo-controlled, randomized phase II study (ClinicalTrials.gov identifier: NCT01638546), we hypothesized that adding veliparib to TMZ may overcome resistance and improve outcomes in patients with relapsed SCLC and explored candidate predictive biomarkers, including MGMT promoter methylation.
PATIENTS AND METHODS
This study was reviewed and approved by the institutional review boards of each center (Appendix Table A1, online only). Written informed consent was provided by all patients. See the Data Supplement for the trial protocol.
Eligibility Criteria
Patients had SCLC that was sensitive or refractory to platinum-based chemotherapy (Fig 1). Sensitive disease was defined as progression or relapse ≥ 60 days after completion of first-line chemotherapy.1 Refractory disease was defined as progression during initial therapy or within 60 days after completing first-line treatment. For the purposes of this study, patients receiving third-line therapy and those with refractory disease were all considered refractory. Patients were eligible if they were ≥ 18 years of age and had one or two prior chemotherapeutic regimens, Karnofsky performance status ≥ 70%, measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) 1.1,22 and adequate liver, kidney, and bone marrow function. Those with asymptomatic progression of disease in the brain were eligible. Patients were excluded if they had chemotherapy or radiation treatment within 21 days, leptomeningeal involvement, or a history of seizures.
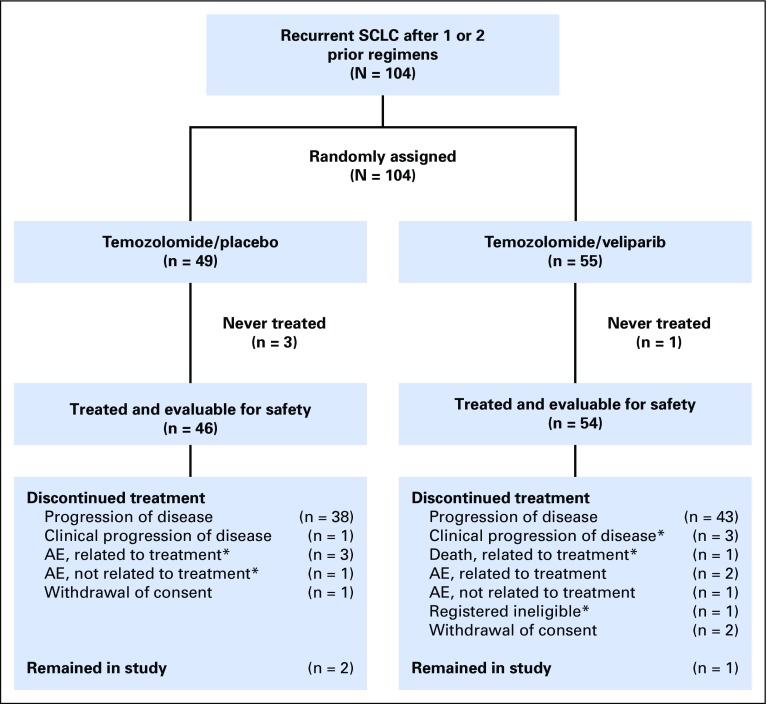
Fig 1.
CONSORT diagram. A total of 104 patients were randomly assigned in a 1:1 fashion, stratified by sensitive disease or refractory disease and center. Four patients were not treated: three in the temozolomide (TMZ)/placebo arm (one each: withdrawal of consent, complications of disease, and concomitant therapy prohibitive to initiate study medication), and one in the TMZ/veliparib arm (complications of disease). Forty-six and 54 patients were evaluable for safety in the TMZ/placebo and TMZ/veliparib arms, respectively. In the TMZ/placebo arm, 44 patients were evaluable for response because two patients were removed for toxicity during the first cycle and before undergoing imaging, indicated by (*). In the TMZ/veliparib arm, five patients were removed from the study, indicated by (*), during the first cycle: registered ineligible (n = 1), clinical progression of disease (n = 3), and death due to treatment toxicity (n = 1). As such, 49 patients were evaluable for response. AE, adverse event; SCLC, small-cell lung cancer.
Treatment
Veliparib and was provided by the Cancer Therapy Evaluation Program at the National Cancer Institute. TMZ was obtained commercially. After randomization, treatment was started within 7 days. Patients received oral veliparib or placebo 40 mg twice daily on days 1 to 7 and oral TMZ 200 mg/m2/day on days 1 to 5 of a 28-day cycle, on the basis of a phase II study of the combination and our prior experience.23,24 See the Data Supplement for additional details.
Study Evaluation
Patients were assessed every 2 weeks during the first two cycles and every 4 weeks thereafter. At each visit, a history, physical examination, toxicity assessment, CBC, and comprehensive metabolic panel were performed. At cycle 3 and beyond, patients were required to have a CBC on day 15. Toxicities were graded using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0. Tumor assessments are described in the Data Supplement.
Immunohistochemistry, Promoter Methylation, Mutational Analysis, and Circulating Tumor Cell Enumeration
Details are included in the Data Supplement.
Statistical Analysis
The primary end point was improvement in PFS at 4 months in patients receiving TMZ/veliparib compared with TMZ/placebo. Patients were stratified according to sensitive disease versus refractory disease and center. In the phase II study of TMZ in SCLC, which enrolled sensitive and refractory patients in a proportion of 4:1, PFS at 4 months was 18% for the combined groups.12 On the basis of these findings, the expected PFS at 4 months in the control group was 15%. With 50 patients per arm, the study had 85% power to detect an improvement in 4-month PFS from 15% to 35% (one-sided type I error, 0.15). All randomly assigned patients were included in the intent-to-treat analysis. PFS was calculated as the proportion of patients alive and without disease progression at 4 months after randomization and compared across the two arms using a χ2 test. A patient who discontinued therapy before 4 months but was alive without documented progression at 4 months was not considered a failure for this end point. See the Data Supplement for additional details regarding secondary and exploratory objectives.
RESULTS
Patient Characteristics
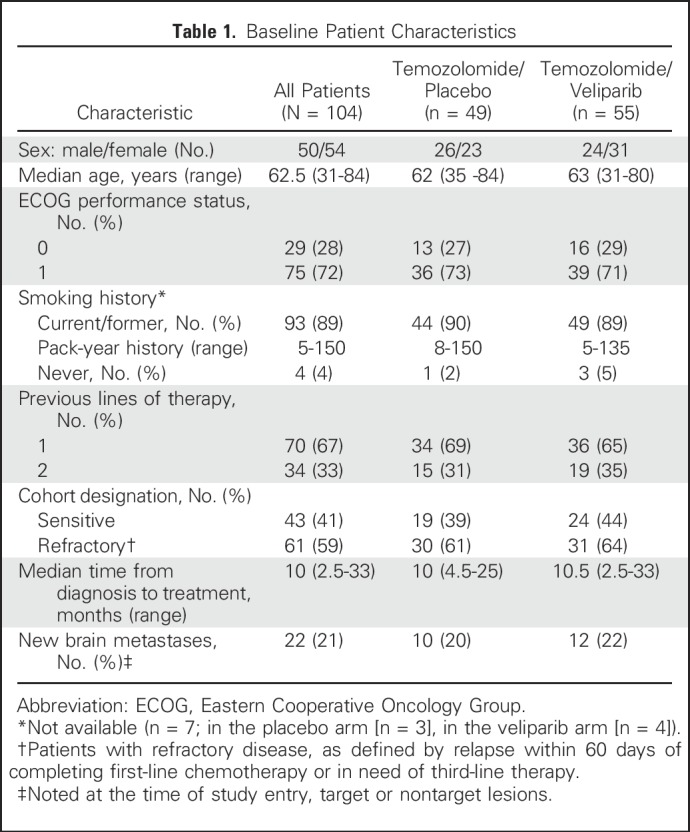
Between August 2012 and February 2015, 104 patients from seven centers in the United States were randomly assigned to receive veliparib or placebo with TMZ (Fig 1). Baseline characteristics were balanced between treatment arms (Table 1). All 104 randomly assigned patients were included in the intent-to-treat analysis for PFS and overall survival (OS). Those with diagnostic imaging at least once beyond baseline were evaluated for response (n = 93). Safety was assessed in patients who initiated one cycle of study treatment (n = 100; Fig 1).
Table 1.
Baseline Patient Characteristics
Efficacy
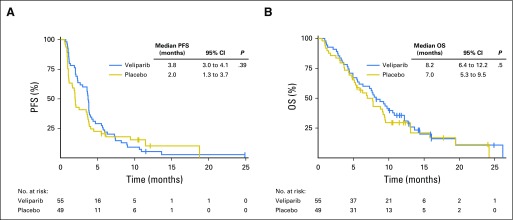
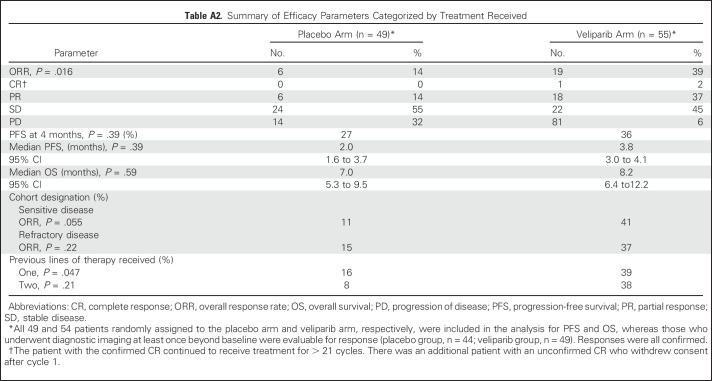
At the final analysis, no significant difference in 4-month PFS was demonstrated between TMZ/veliparib (20 of 55; 36%) and TMZ/placebo (13 of 49; 27%; P = .19). Median PFS was 3.8 months and 2.0 months in the TMZ/veliparib and TMZ/placebo arms, respectively (log-rank P = .39; hazard ratio, 0.84; 95% CI, 0.56 to 1.25; Fig 2A; Appendix Table A2, online only). The median duration of response was 4.61 months (95% CI, 2.86 to 9.9 months) and 3.68 months (95% CI, 2.76 months to not achieved) in the TMZ/veliparib (n = 19) and TMZ/placebo (n = 6) arms, respectively (log rank P = .507). At the time of data cutoff, 19 patients (18%) remained alive (TMZ/veliparib, n = 9; TMZ/placebo, n = 10). Median OS was similar between TMZ/veliparib and TMZ/placebo: 8.2 months (95% CI, 6.4 to 12.2 months) versus 7.0 months (95% CI, 5.3 to 9.5 months; P = .50), respectively (Fig 2B; Appendix Table A2). One- and 2-year survival rates were 35% and 10% for TMZ/veliparib versus 30% and 11% for TMZ/placebo, respectively.
Fig 2.
Kaplan Meier curves for outcomes. (A) Progression-free (PFS) and (B) overall survival (OS) for the 104 patients with sensitive or refractory small-cell lung cancer in need of second- or third line-therapy.
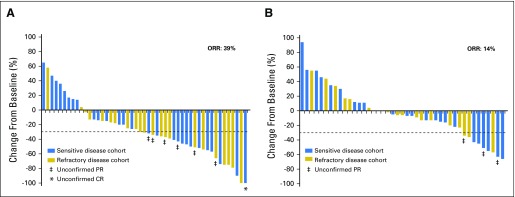
In 93 evaluable patients (Appendix Table A2; Figs 3A and 3B; Appendix Fig A1, online only), a significantly higher objective response rate (ORR) was observed in patients receiving TMZ/veliparib (ORR, 39%; 95% CI, 25% to 54%) versus TMZ/placebo (ORR, 14%; 95% CI, 5% to 27%; P = .016). Two patients who received veliparib had a complete response, including one with sensitive disease who continued to receive treatment, with continued response for over 2 years.
Fig 3.
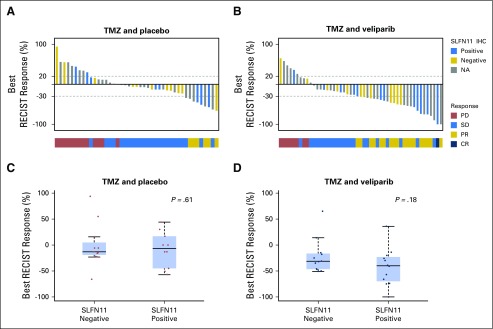
Tumor response. The best calculated percentage change in tumor size on the basis of measurable lesions for (A) 49 evaluable patients in the temozolomide (TMZ)/veliparib arm and (B) 44 evaluable patients in the TMZ/placebo arm. In the TMZ/veliparib arm, five patients were removed from the study during the first cycle and were not evaluable for response: registered ineligible (n = 1), clinical progression of disease (n = 3), and death due to treatment toxicity (n = 1). In the TMZ/placebo arm, two patients were removed for toxicity during the first cycle and before undergoing imaging; thus, they were not evaluable for response; one other patient’s tumor measurements were not available, although the patient developed progression of disease on the basis of the appearance of new nontarget lesions. CR, complete response; ORR, .overall response rate; PR, partial response.
A preplanned subgroup analysis found that responses were higher with TMZ/veliparib in both platinum-sensitive and platinum-refractory patients. In sensitive patients, the ORR for TMZ/veliparib was 41% (9 of 22) versus 11% (2 of 18) for TMZ/placebo (P = .055); in refractory patients, the ORR for TMZ/veliparib was 37% (10 of 27) versus 15% (4 of 26) for TMZ/placebo (P = .22). Furthermore, the improvement in ORR for TMZ/veliparib compared with TMZ/placebo was similar for second- and third-line patients. In patients with one previous line of therapy, the ORR for TMZ/veliparib was 39% (13 of 33) versus 16% (5 of 31) for TMZ/placebo (P = .047), whereas patients with two prior lines of therapy had an ORR with TMZ/veliparib of 38% (6 of 16) versus 8% (1 of 13) with TMZ/placebo (P = .21).
Treatment Exposure
One hundred of 104 patients enrolled and randomly assigned received at least one cycle of treatment. Twelve of the 54 treated patients (22%) in the TMZ/veliparib arm received five or more cycles of therapy (median, 3; range, 1 to 21), compared with six of the 46 patients who were treated (13%) in the TMZ/placebo arm (median, 2; range, 1 to 19). Reasons for discontinuation of study treatment were disease progression (81%), unacceptable toxicity related or unrelated to treatment (6%), intercurrent illness/symptomatic deterioration (4%), withdrawal of consent (3%), more than a 3-week delay in treatment administration due to thrombocytopenia (2%), and death (1%).
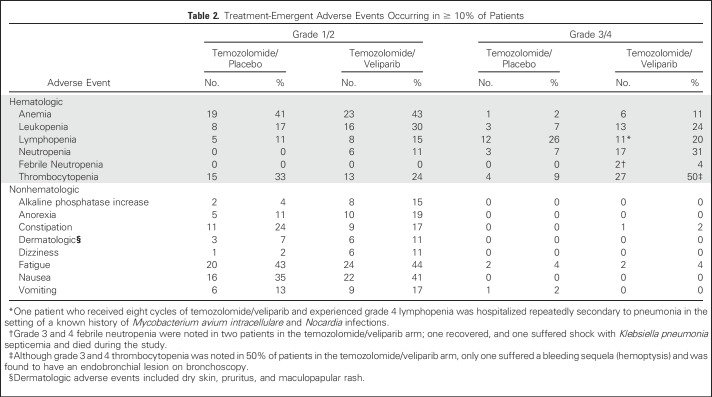
Toxicity
Table 2 lists the most common treatment-related toxicities. Hematologic toxicities were the most common adverse effects in both study arms. After the first 24 patients were accrued and evaluated for at least one cycle, it was noted that 14 incurred the following adverse events: grade 3/4 neutropenia (TMZ/veliparib, n = 7; TMZ/placebo, n = 2); grade 3/4 thrombocytopenia (TMZ/veliparib, n = 10; TMZ/placebo, n = 3); and grade 4 febrile neutropenia (TMZ/veliparib, n = 1; leading to sepsis and death). Four of these patients had their second cycle of treatment held and subsequently were found to have disease progression at week 8 (TMZ/veliparib, n = 3; TMZ/placebo, n = 1). Therefore, the protocol was amended in October 2013 to reduce the starting dose of TMZ to 150 mg/m2/day to avoid myelosuppression and dose delays. At the lower dose of TMZ, only three patients treated with TMZ/veliparib and one treated with TMZ/placebo experienced multiple dosing delays. Prolonged thrombocytopenia led to treatment termination for an additional two patients, one in each arm.
Table 2.
Treatment-Emergent Adverse Events Occurring in ≥ 10% of Patients
PARP-1 and SLFN11 Immunohistochemistry as Biomarkers
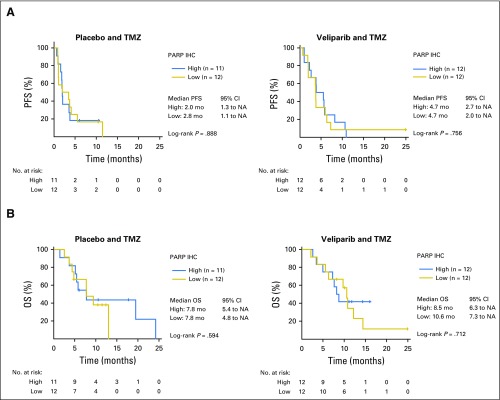
Unlike other cancer types, mutations in DNA repair genes (eg, BRCA1/2) are uncommon and do not predict PARPi response in SCLC models.25 Schlafen-11 (SLFN11) regulates response to DNA damage and replication stress,26 and was recently identified as a candidate predictive marker of sensitivity to DNA-damaging chemotherapies27 and PARPi in several cancers, including SCLC.18,25,28 Therefore, we amended our original planned biomarker analysis to investigate whether PARP-1 or SLFN11 expression levels predicted clinical benefit of TMZ/veliparib.18,25,28,29 Unstained tumor sections from original diagnostic biopsies were available from 58 patients (56%), of whom 48 and 47 had adequate tumor content for PARP-1 and SLFN11 analysis, respectively. PARP-1 expression was detected in 87% of tumors (H-score range, 0 to 219; median, 78). However, there was no association between PARP-1 expression and clinical outcomes ( Appendix Fig A2, online only).
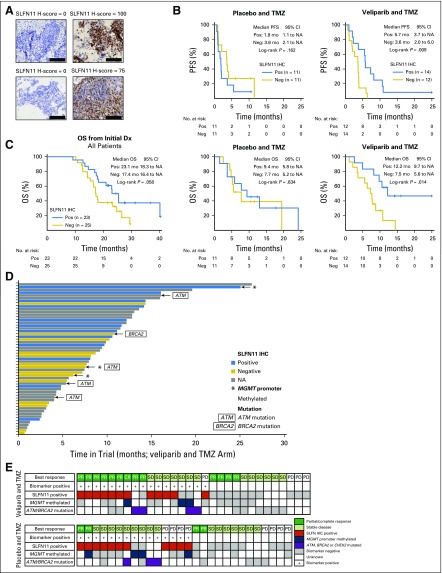
For SLFN11 biomarker analysis, we used an H-score cutoff ≥ 1 to define SLFN11-positive (n = 23) versus SLFN11-negative tumors (H-score < 1; n =25; Fig 4A). SLFN11-positive tumors were equally distributed between the treatment arms (TMZ/veliparib, n = 12; TMZ/placebo, n = 11). Clinical stage at initial diagnosis, platinum-sensitivity, and smoking history were not significantly different between the SLFN11-positive and SLFN11-negative groups.
Fig 4.
SLFN11 immunohistochemistry (IHC) predicts improved survival. (A) Example images of tumors with negative (neg) and positive (pos) SLFN11 by IHC (scale bar = 100 uM, 400× magnification). (B) Overall survival (OS) and progression-free survival (PFS) from date of randomization was improved in patients with SLFN11-positive disease in the temozolomide (TMZ)/veliparib treatment arm (PFS overall interaction log-rank P = .046; OS overall interaction log-rank P = .095). (C) OS from time of diagnosis trends toward increased survival in patients with SLFN11 positive (IHC score ≥ 1) disease. (D) Swim-plot of months on trial in the TMZ/veliparib treatment arm color coded by potential biomarker of response (time calculated from start of treatment to date of last follow-up). Blue indicates SLFN11 positive; (*)MGMT promoter methylation. (E) Summary of biomarker status (SLFN11; MGMT methylation; ATM, BRCA2, or CHEK2 mutation for patients with response data). Gray indicates biomarker assayed and not detected; white indicates no data. Best response to treatment in each treatment arm. ATM, ATM mutation; BRCA2, BRCA2 mutation; CR, complete response; Dx, diagnosis; mo, months; NA, not achieved; PD, progression of disease; PR, partial response; SD, stable disease.
Patients with SLFN11-positive tumors treated with TMZ/veliparib had significantly prolonged PFS (5.7 v 3.6 months; P = .009) and OS (12.2 v 7.5 months; P = .014) from time of randomization (Fig 4B). In contrast, no differences in PFS or OS were observed in those patients treated with TMZ/placebo on the basis of SLFN11 expression (P = .162 and .634, respectively). The interaction P value was .0092 (by Cox proportional hazards regression model), demonstrating an improved PFS in patients with SLFN11-positive disease receiving TMZ/veliparib. ORR was not significantly different on the basis of SLFN11 levels in either study arm (Appendix Fig A3, online only; TMZ/veliparib, P = .614; TMZ/placebo, P = .178). Interestingly, there also was a trend toward improved OS (from initial diagnosis) in patients with SLFN11-positive tumors (Fig 4C; P = .058) in the overall patient population. This may be due to SLFN11 also predicting sensitivity to platinum chemotherapy and topoisomerase inhibitors,25 which is associated with improved prognosis in patients with SCLC.
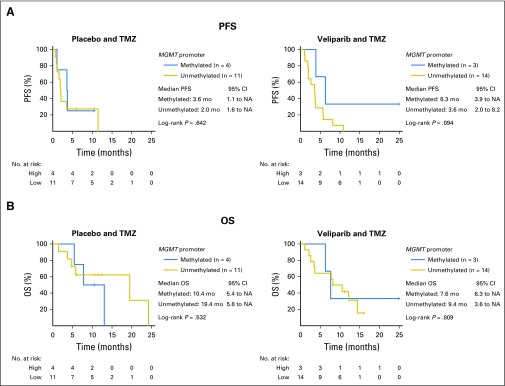
MGMT Promoter Methylation as a Biomarker
Analysis of MGMT promoter methylation30-32 was limited by the availability of adequate tissue, because sufficient DNA was present in only 32 tumor samples (TMZ/veliparib, n = 17; TMZ/placebo, n = 15). The MGMT promoter was methylated in 31% of the tumor samples tested (seven of 32) and was not associated with response to treatment among all patients treated (P = .657), or within either treatment arm (TMZ/veliparib, P = .283; TMZ/placebo, P = .882). MGMT promoter methylation also was not associated with improved PFS or OS (Appendix Fig A4, online only).
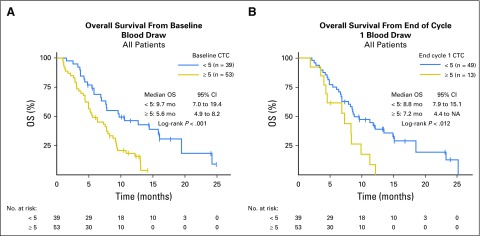
Circulating Tumor Cells
Baseline circulating tumor cells (CTCs) were evaluated on 94 patients at baseline and ranged from 0 to 262 per 7.5mL. In univariable analysis, elevated baseline CTCs ≥ 5, which had been validated in other tumor types,33-35 seemed to be associated with worse OS: median OS, 5.6 versus 9.7 months; (P < .001; Appendix Fig A5A, online only). CTCs after one cycle of treatment were evaluated in 64 patients. A persistently elevated CTC number ≥ 5 at cycle 2, day 1, also was associated with worse OS in univariable analysis: median OS, 7.2 versus 8.8 months (P = .012; Appendix Fig A5B).
Analysis of Mutations in DNA Damage Response Genes
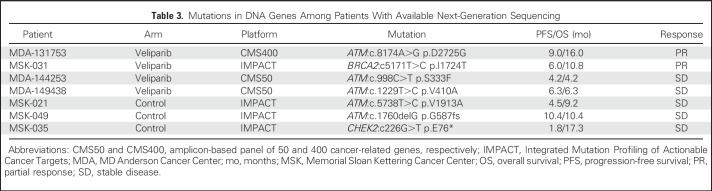
Targeted sequencing was performed on tumors from few patients (n = 22) at their respective treating institutions and revealed mutations in the following DNA repair genes previously implicated in PARPi response in other disease types: ATM (n = 5), BRCA2 (n = 1), and CHEK2 (n = 1; Table 3).36 Although none of these seven mutations previously have been described as deleterious to gene/protein function, two (CHEK2 p.E76* and ATM p.G587fs) may confer functional homologous repair deficiency.
Table 3.
Mutations in DNA Genes Among Patients With Available Next-Generation Sequencing
Three patients with DNA repair gene mutations (ATM, n = 2; CHEK2, n = 1) received TMZ/placebo and had a median OS of 10.4 months, compared with 6.2 months for all patients treated with TMZ/placebo. In the TMZ/veliparib arm, the four patients with mutations (ATM, n = 3; BRCA2, n = 1) had a median OS of 8.6 months, compared with 8.1 months for others in this cohort (Fig 4D). Interestingly, two of the four partial responses with sequencing data observed in the TMZ/veliparib arm had DNA repair gene mutations (Fig 4E).
DISCUSSION
This randomized phase II study assessed the efficacy of veliparib, a PARPi, with TMZ compared with TMZ monotherapy in patients with relapsed SCLC. Although 4-month PFS did not differ significantly between veliparib- and placebo-treated patients, we observed significant improvement in ORR with the addition of veliparib. Furthermore, we demonstrated for the first time in a clinical trial that SLFN11—a promising biomarker of PARPi sensitivity—may identify patients who benefit from PARPi therapy.
In our prior phase II study of single-agent TMZ, 4-month PFS was 18%,12 which we hoped to improve significantly by adding veliparib. However, we found no significant difference in 4-month PFS between patients in the TMZ/veliparib arm (36%) and those in the TMZ/placebo arm (27%; P = .19). Although median PFS and OS in patients receiving TMZ/veliparib were improved numerically by 1.8 months and 1.2 months, respectively, neither reached statistical significance. However, the substantially higher ORR and depth of response observed in patients receiving TMZ/veliparib (ORR, 39%; 95% CI, 25% to 54%) versus TMZ/placebo (ORR, 14%; 95% CI, 5% to 27%; P = .016) was statistically significant and is encouraging.
Several reasons may account for the high response rates found with the combination not translating into an improvement in PFS or OS. These include more frequent myelosuppression, treatment delays, dose reductions in patients receiving TMZ/veliparib, and a higher-than-expected number of platinum-resistant patients enrolled in the trial. Whereas we anticipated that approximately 20% of the study population would have platinum-refractory disease, in actuality, this highly resistant patient population represented the majority of study participants (59%), although well balanced between the two arms. A recent retrospective study challenged the premise that platinum sensitivity is associated with outcomes,37 yet data consistently have shown that those with platinum-resistant disease treated with cytotoxic agents have worse PFS and OS, which may have affected the observed study outcomes.38,39
Preclinical data show that the dose levels chosen for the two agents in combination is important, with recent data suggesting that optimal synergy may result from near-maximal dosing of a PARPi, with substantially submaximal dose exposure of TMZ.23,24,40,41 Here, in contrast, we used a recommended monotherapy treatment dose and schedule of TMZ (per prior SCLC study24) and a low dose of veliparib (per a phase II breast cancer study23). This may have compromised the effectiveness of the combination, especially because veliparib is relatively less potent compared with other PARPi that produce greater PARP-DNA trapping, a secondary mechanism by which these agents function.42-44 Furthermore, hematologic toxicities were greater with TMZ/veliparib versus TMZ/placebo, including grade 3/4 thrombocytopenia, neutropenia, and anemia, which often were incidental laboratory findings and not clinically significant. Cytopenias with TMZ/veliparib were often observed early, leading to treatment delays and, potentially, loss of response. After such toxicities occurred in 14 of the first 24 patients, the protocol was amended to start at a lower dose of TMZ. After this change, fewer patients required treatment delays.
In breast, ovarian, and prostate cancers, mutations in BRCA1/2, ATM, and other homologous repair genes predict PARPi response.36,45-47 However, in preclinical models of SCLC, neither mutations in DNA repair genes nor homologous repair deficiency scores predict PARPi sensitivity.18,25 In this trial, we tested, for the first time, SLFN11 expression by immunohistochemistry as a predictive biomarker of clinical response to PARPi therapy on the basis of preclinical data from SCLC and other cancers.18,25,27-29 In addition to SLFN11, biomarker analysis included PARP-1 expression7,25 and MGMT promoter hypermethylation,12,30-32 although these were not associated with differences in response or survival.
In contrast, patients with SLFN11-positive tumors (H-score ≥ 1) who received TMZ/veliparib had significantly better PFS and OS than those treated with TMZ/placebo. This finding is consistent with several recent preclinical studies in SCLC18,25,28,29 and other cancer types,29 which have shown greater activity of multiple PARPi, including veliparib in models expressing relatively high levels of SLFN11. However, our groups recently have also found that SLFN11 decreases in many models after exposure to chemotherapy,18,25,48 suggesting that a repeat biopsy to assess SLFN11 levels at the time of study entry may be important to optimize its predictive power, as opposed to using pretreatment samples from diagnosis.
To our knowledge, this is the first demonstration of SFLN11 as a predictive biomarker in a randomized, double-blind clinical trial. SLFN11 warrants further investigation in other trials of PARPi combinations for SCLC. Should this result be substantiated, high SLFN11 expression could represent a biomarker in select patients with SCLC for treatment with PARPi.
In conclusion, despite not achieving the primary end point of an improvement in 4-month PFS, we did observe significantly higher ORR in patients with relapsed SCLC treated with TMZ/veliparib, supporting additional studies of this regimen. Hematologic toxicities were noted with the combination of veliparib and TMZ, most of which did not lead to untoward clinical events and were less frequent after adjusting the starting dose of TMZ. Importantly, we demonstrated that high SLFN11 expression, a promising candidate biomarker of PARPi sensitivity, predicts longer survival in patients treated with TMZ/veliparib, substantiating our preclinical findings. Careful patient selection, application of SLFN11 as a biomarker, and optimization of the dosing schedule have the potential to further improve outcomes of the combination of PARPi and TMZ in SCLC.
ACKNOWLEDGMENT
We thank Andy Ni, Amy Quinterio, Isabella Bergagnini, Gianna McArthur, Patrick Nolan, Lakeisha Lubin, Sarah Riva, and Hazem Karabeber for the collection and organization of data.
Appendix
Fig A1.
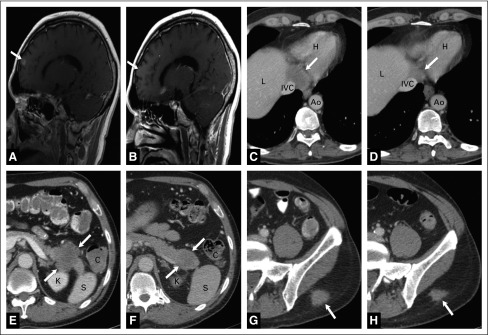
Tumor response in a patient treated with veliparib and temozolomide. A 57-year-old man with small-cell lung cancer metastatic to the brain, pancreatic tail, juxtaphrenic nerve, and subcutaneous tissue treated with the temozolomide (TMZ)/veliparib arm. (A) Sagittal T1-weighted magnetic resonance imaging scan with contrast shows a 1-cm metastasis (arrow) in the right frontal lobe at the time of enrollment in the study. (B) Sagittal T1-weighted magnetic resonance imaging scan with contrast demonstrates complete resolution of the brain lesion (arrow shows previous location) after therapy with TMZ/veliparib. (C) Axial computed tomography scan (CT) with contrast in narrow windows illustrates a 5-cm juxtaphrenic nodal mass (arrow) at the time that therapy was commenced. (D) Axial CT with contrast after therapy with TMZ/veliparib shows significant decrease in the lesion (arrow) compatible with response to therapy. (E) Axial abdominal CT with contrast shows a 5-cm heterogeneously enhancing lesion in the pancreatic tail (arrows). (F) Axial abdominal CT with contrast after therapy with TMZ/veliparib shows interval decrease in the pancreatic lesion to 3.5 cm. (G) Axial CT with contrast in narrow windows shows a 2.5-cm soft tissue implant (arrow) in the subcutaneous fat overlying the left gluteal muscles. Axial CT with contrast after treatment with the combination of veliparib and TMZ demonstrates a decrease in the size of the lesion (arrow). Importantly, there was significant pain associated with the lesion, which improved with temozolomide/veliparib therapy. Ao, aorta; C, colon; IVC, inferior vena cava; H, heart; K, kidney; L, liver; S, spleen.
Fig A2.
Poly (ADP-ribose) polymerase (PARP)-1 expression does not predict improved survival. (A) Progression-free survival (PFS) and (B) overall survival (OS) from date of randomization was not improved in patients whose tumors expressed PARP-1 by immunohistochemistry in the temozolomide (TMZ)/veliparib arm compared with the TMZ/placebo arm. IHC, immunohistochemistry; mo, months; NA, not achieved.
Fig A3.
SLFN11 expression does not predict improved response to treatment. Waterfall plots of best Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 response (%) in each treatment arm color coded by SLFN-11 immunohistochemistry (IHC) status (positive, negative, or unknown): (A) temozolomide (TMZ)/placebo and (B) TMZ/veliparib. Boxplot of RECIST 1.1 responses in each treatment arm by SLFN-11 IHC: (C) TMZ/placebo and (D) TMZ/veliparib; trend toward deeper responses among patients with SLFN11-positive disease receiving veliparib and TMZ combination. CR, complete response; NA, not available; PD, progression of disease; PR, partial response; SD, stable disease.
Fig A4.
MGMT promoter methylation did not predict improved survival. (A) Progression-free survival (PFS) and (B) overall survival (OS) from the date of randomization in patients with known MGMT promoter methylation status. mo, months; NA, not achieved; TMZ, temozolomide.
Fig A5.
Low circulating tumor cell (CTC) numbers were associated with improved outcomes. CTCs < 5 in 7.5 mL were associated with improved survival. (A) At baseline and (B) at the end of cycle 1, CTCs < 5 in 7.5 mL were associated with improved survival. mo, months; OS, overall survival.
Table A1.
Accrual by Site
Table A2.
Summary of Efficacy Parameters Categorized by Treatment Received
Footnotes
Supported by the Cancer Therapy Evaluation Program at the National Cancer Institute (NCI; Grant No. UM1CA186691); National Institutes of Health (NIH)/NCI Grants No. CCSG P30-CA008748 and NIH/NCI CCSG P30-CA016672; University of Texas-Southwestern and MD Anderson Cancer Center Lung SPORE (Grant No. 5 P50 CA070907); through generous philanthropic contributions to The University of Texas MD Anderson Lung Cancer Moon Shot Program (J.W., J.V.H., L.A.B.); MD Anderson Cancer Center Physician Scientist Award (L.A.B.); The LUNGevity Foundation (L.A.B.); Lee Clark Fellowship of The University of Texas MD Anderson Cancer Center, supported by the Jeane F. Shelby Scholarship Fund (L.A.B.); NIH/NCI award No. 1-R01-CA207295 (L.A.B.); an NCI Cancer Clinical Investigator Team Leadership Award (No. P30-CA016672; L.A.B.); The Rexanna Foundation (J.V.H, L.A.B.); The Sidney Kimmel Foundation for Cancer Research (L.A.B.); and The Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer Therapy (L.A.B.).
C.M.R. and L.A.B. are cosenior authors of this manuscript.
Clinical trial information: NCT01638546.
AUTHOR CONTRIBUTIONS
Conception and design: M. Catherine Pietanza, Lee M. Krug, Alice Chen, John V. Heymach, Mark G. Kris, Charles M. Rudin, Lauren Averett Byers
Financial support: John V. Heymach, Mark G. Kris, Lauren Averett Byers
Administrative support: M. Catherine Pietanza, Afshin Dowlati, John V. Heymach, Lauren Averett Byers
Provision of study materials or patients: M. Catherine Pietanza, Lee M. Krug, Afshin Dowlati, John V. Heymach, Lauren Averett Byers
Collection and assembly of data: M. Catherine Pietanza, Saiama N. Waqar, Lee M. Krug, Afshin Dowlati, Christine L. Hann, Alberto Chiappori, Taofeek K. Owonikoko, Robert J. Cardnell, Junya Fujimoto, Lihong Long, Yevgeniva Bensman, Brenda Hurtado, Patricia de Groot, Erik P. Sulman, Ignacio I. Wistuba, Martin Fleisher, John V. Heymach, Mark G. Kris, Charles M. Rudin, Lauren Averett Byers
Data analysis and interpretation: M. Catherine Pietanza, Lee M. Krug, Taofeek K. Owonikoko, Kaitlin M. Woo, Robert J. Cardnell, Junya Fujimoto, Lihong Long, Lixia Diao, Jing Wang, Patricia de Groot, Erik P. Sulman, Ignacio I. Wistuba, John V. Heymach, Lauren Averett Byers
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized, Double-Blind, Phase II Study of Temozolomide in Combination With Either Veliparib or Placebo in Patients With Relapsed-Sensitive or Refractory Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
M. Catherine Pietanza
Employment: Merck
Stock or Other Ownership: Merck
Saiama N. Waqar
Research Funding: Spectrum Pharmaceuticals (Inst), Eli Lilly (Inst), Pfizer (Inst), Genentech/Roche (Inst), Daiichi Sankyo (Inst), Newlink Genetics (Inst), EMD Serono (Inst), Puma Biotechnology (Inst), Novartis (Inst), Xcovery (Inst), Synermore biologics (Inst), Celgene (Inst), Vertex (Inst), Bristol-Myers Squibb (Inst), Stemcentrix (Inst), Hengrui Therapeutics (Inst), Checkpoint Therapeutics (Inst), Ignyta (Inst), AstraZeneca (Inst)
Lee M. Krug
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Afshin Dowlati
Consulting or Advisory Role: AbbVie, ARIAD
Research Funding: Endocyte, Merck Serono, Helix, Loxo Oncology, AstraZeneca,Mirati Therapeutics, AbbVie Stemcentrx, Vertex
Christine L. Hann
Consulting or Advisory Role: AbbVie, Genentech/Roche, Bristol-Myers Squibb
Research Funding: GlaxoSmithKline (Inst), AbbVie (Inst), Bristol-Myers Squibb (Inst), Merrimack
Alberto Chiappori
Honoraria: Genentech, Celgene, Takeda, Novartis, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Merck, AbbVie, AstraZeneca
Consulting or Advisory Role: Genentech, Bristol-Myers Squibb, Novartis, AbbVie, AstraZeneca
Speakers' Bureau: Boehringer Ingelheim, Merck, Genentcech, Takeda, Celgene, Novartis, Pfizer
Research Funding: Novartis, Bristol-Myers Squibb
Taofeek K. Owonikoko
Consulting or Advisory Role: Celgene, Eli Lilly, Sandoz, AbbVie, Eisai, G1 Therapeutics, Takeda, Seattle Genetics, Bristol-Myers Squibb, MedImmune
Research Funding: Novartis (Inst), Astellas Pharma (Inst), Celgene (Inst), Bayer (Inst), Stemcentrix (Inst), Regeneron (Inst), AstraZeneca/MedImmune (Inst), AbbVie (Inst), G1 Therapeutics (Inst), Bristol-Myers Squibb
Patents, Royalties, Other Intellectual Property: Overcoming acquired resistance to chemotherapy treatments through suppression of STAT3 (Inst), selective chemotherapy treatments and diagnostic methods related thereto (Inst)
Kaitlin M. Woo
No relationship to disclose
Robert J. Cardnell
No relationship to disclose
Junya Fujimoto
Research Funding: Astellas Pharma
Lihong Long
No relationship to disclose
Lixia Diao
No relationship to disclose
Jing Wang
No relationship to disclose
Yevgeniva Bensman
No relationship to disclose
Brenda Hurtado
No relationship to disclose
Patricia de Groot
No relationship to disclose
Erik P. Sulman
Honoraria: Merck Sharp & Dohme, Novocure
Consulting or Advisory Role: Merck Sharp & Dohme, Novocure
Research Funding: AbbVie (Inst), Novocure (Inst)
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Novocure
Ignacio I. Wistuba
Consulting or Advisory Role: Genentech/Roche, Eli Lilly, Bristol-Myers Squibb, HTG Molecular Diagnostics, Asuragen, Pfizer, AstraZeneca/MedImmune, GlaxoSmithKline, Bayer
Speakers' Bureau: Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol-Myers Squibb, Genentech/Roche, AstraZeneca/MedImmune
Research Funding: Genentech, Merck, HTG Molecular Diagnostics, Silicon Biosytems, Adaptimmune, EMD Serono, Pfizer, MedImmune, Amgen, Takeda, Karus Therapeutics, Adaptive Biotechnologies
Alice Chen
No relationship to disclose
Martin Fleisher
No relationship to disclose
John V. Heymach
Consulting or Advisory Role: AstraZeneca, AbbVie, Boehringer Ingelheim, Bristol-Myers Squibb, Medivation, ARIAD, AstraZeneca, Synta, Oncomed, Novartis, Genentech, Calithera Biosciences
Research Funding: AstraZeneca (Inst)
Mark G. Kris
Consulting or Advisory Role: AstraZeneca
Charles M. Rudin
Consulting or Advisory Role: Bristol-Myers Squibb, AbbVie, Seattle Genetics, Harpoon Therapeutics, Genentech/Roche, AstraZeneca
Lauren Averett Byers
Consulting or Advisory Role: AbbVie, AstraZeneca
Research Funding: AbbVie, AstraZeneca, Tolero Pharmaceuticals
REFERENCES
- 1.von Pawel J, Schiller JH, Shepherd FA, et al. : Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 17:658-667, 1999 [DOI] [PubMed] [Google Scholar]
- 2.O’Brien ME, Ciuleanu TE, Tsekov H, et al. : Phase III trial comparing supportive care alone with supportive care with oral topotecan in patients with relapsed small-cell lung cancer. J Clin Oncol 24:5441-5447, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Eckardt JR, von Pawel J, Pujol JL, et al. : Phase III study of oral compared with intravenous topotecan as second-line therapy in small-cell lung cancer. J Clin Oncol 25:2086-2092, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Eckardt J, Gralla R, Palmer MC, et al: Topotecan as second-line therapy in patients with small cell lung cancer: A phase II study. Ann Oncol 7:107, 1996 (abstr 513) [Google Scholar]
- 5.Ardizzoni A, Hansen H, Dombernowsky P, et al. : Topotecan, a new active drug in the second-line treatment of small-cell lung cancer: A phase II study in patients with refractory and sensitive disease. The European Organization for Research and Treatment of Cancer Early Clinical Studies Group and New Drug Development Office, and the Lung Cancer Cooperative Group. J Clin Oncol 15:2090-2096, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Depierre A, Von Pawel J, Hans K, et al: Evaluation of topotecan (hycamtin) in relapsed small-cell lung cancer (SCLC): A multicenter phase II study. Lung Cancer 18:35, 1997, (abstr 126) [Google Scholar]
- 7.Byers LA, Wang J, Nilsson MB, et al. : Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov 2:798-811, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toyooka S, Toyooka KO, Maruyama R, et al. : DNA methylation profiles of lung tumors. Mol Cancer Ther 1:61-67, 2001 [PubMed] [Google Scholar]
- 9.Gerson SL: Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol 20:2388-2399, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Esteller M, Herman JG: Generating mutations but providing chemosensitivity: The role of O6-methylguanine DNA methyltransferase in human cancer. Oncogene 23:1-8, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Pietanza MC, Byers LA, Minna JD, et al. : Small cell lung cancer: Will recent progress lead to improved outcomes? Clin Cancer Res 21:2244-2255, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pietanza MC, Kadota K, Huberman K, et al. : Phase II trial of temozolomide in patients with relapsed sensitive or refractory small cell lung cancer, with assessment of methylguanine-DNA methyltransferase as a potential biomarker. Clin Cancer Res 18:1138-1145, 2012 [DOI] [PubMed] [Google Scholar]
- 13. Kalemkerian GP, Loo BW, Akerley W, et al: NCCN clinical practice guidelines in oncology: Small cell lung cancer. https://www.nccn.org/professionals/physician_gls/default.aspx.
- 14.Memisoglu A, Samson L: Base excision repair in yeast and mammals. Mutat Res 451:39-51, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Schreiber V, Amé JC, Dollé P, et al. : Poly(ADP-ribose) polymerase-2 (PARP-2) is required for efficient base excision DNA repair in association with PARP-1 and XRCC1. J Biol Chem 277:23028-23036, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Tentori L, Graziani G: Chemopotentiation by PARP inhibitors in cancer therapy. Pharmacol Res 52:25-33, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Palma JP, Wang YC, Rodriguez LE, et al. : ABT-888 confers broad in vivo activity in combination with temozolomide in diverse tumors. Clin Cancer Res 15:7277-7290, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Lok BH, Gardner EE, Schneeberger VE, et al. : PARP inhibitor activity correlates with SLFN11 expression and demonstrates synergy with temozolomide in small cell lung cancer. Clin Cancer Res 23:523-535, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardnell RJ, Feng Y, Diao L, et al. : Proteomic markers of DNA repair and PI3K pathway activation predict response to the PARP inhibitor BMN 673 in small cell lung cancer. Clin Cancer Res 19:6322-6328, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Bono J, Ramanathan RK, Mina L, et al. : Phase I, dose-escalation, two-part trial of the parp inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov 7:620-629, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Owonikoko TK, Dahlberg SE, Khan SA, et al. : A phase 1 safety study of veliparib combined with cisplatin and etoposide in extensive stage small cell lung cancer: A trial of the ECOG-ACRIN Cancer Research Group (E2511). Lung Cancer 89:66-70, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Isakoff S, Overmoyer B, Tung N, et al. : A phase II trial of the PARP inhibitor veliparib (ABT888) and temozolomide for metastatic breast cancer. J Clin Oncol 28, 2010. (15, suppl) [Google Scholar]
- 24.Zauderer MG, Drilon A, Kadota K, et al. : Trial of a 5-day dosing regimen of temozolomide in patients with relapsed small cell lung cancers with assessment of methylguanine-DNA methyltransferase. Lung Cancer 86:237-240, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allison Stewart C, Tong P, Cardnell RJ, et al. : Dynamic variations in epithelial-to-mesenchymal transition (EMT), ATM, and SLFN11 govern response to PARP inhibitors and cisplatin in small cell lung cancer. Oncotarget 8:28575-28587, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. doi: 10.1016/j.molcel.2018.01.012. Murai J, Tang SW, Leo E, et al: SLFN11 blocks stressed replication forks independently of ATR. Mol Cell 69:371-384 e6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoppoli G, Regairaz M, Leo E, et al. : Putative DNA/RNA helicase Schlafen-11 (SLFN11) sensitizes cancer cells to DNA-damaging agents. Proc Natl Acad Sci USA 109:15030-15035, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polley E, Kunkel M, Evans D, et al. : Small cell lung cancer screen of oncology drugs, investigational agents, and gene and microRNA expression. J Natl Cancer Inst 108:djw122, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murai J, Feng Y, Yu GK, et al. : Resistance to PARP inhibitors by SLFN11 inactivation can be overcome by ATR inhibition. Oncotarget 7:76534-76550, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hattermann K, Mehdorn HM, Mentlein R, et al. : A methylation-specific and SYBR-green-based quantitative polymerase chain reaction technique for O6-methylguanine DNA methyltransferase promoter methylation analysis. Anal Biochem 377:62-71, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Esteller M, Toyota M, Sanchez-Cespedes M, et al. : Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is associated with G to A mutations in K-ras in colorectal tumorigenesis. Cancer Res 60:2368-2371, 2000 [PubMed] [Google Scholar]
- 32.Esteller M, Hamilton SR, Burger PC, et al. : Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res 59:793-797, 1999 [PubMed] [Google Scholar]
- 33.Cristofanilli M, Budd GT, Ellis MJ, et al. : Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351:781-791, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Danila DC, Heller G, Gignac GA, et al. : Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 13:7053-7058, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Krebs MG, Sloane R, Priest L, et al. : Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 29:1556-1563, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Mateo J, Carreira S, Sandhu S, et al. : DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med 373:1697-1708, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lara PN, Jr, Moon J, Redman MW, et al. : Relevance of platinum-sensitivity status in relapsed/refractory extensive-stage small-cell lung cancer in the modern era: A patient-level analysis of Southwest Oncology Group trials. J Thorac Oncol 10:110-115, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ardizzoni A, Tiseo M, Boni L: Validation of standard definition of sensitive versus refractory relapsed small cell lung cancer: A pooled analysis of topotecan second-line trials. Eur J Cancer 50:2211-2218, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Owonikoko TK, Behera M, Chen Z, et al. : A systematic analysis of efficacy of second-line chemotherapy in sensitive and refractory small-cell lung cancer. J Thorac Oncol 7:866-872, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta SK, Mladek AC, Carlson BL, et al. : Discordant in vitro and in vivo chemopotentiating effects of the PARP inhibitor veliparib in temozolomide-sensitive versus -resistant glioblastoma multiforme xenografts. Clin Cancer Res 20:3730-3741, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith MA, Reynolds CP, Kang MH, et al. : Synergistic activity of PARP inhibition by talazoparib (BMN 673) with temozolomide in pediatric cancer models in the pediatric preclinical testing program. Clin Cancer Res 21:819-832, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopkins TA, Shi Y, Rodriguez LE, et al. : Mechanistic dissection of PARP1 trapping and the impact on in vivo tolerability and efficacy of PARP inhibitors. Mol Cancer Res 13:1465-1477, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Shen Y, Aoyagi-Scharber M, Wang B: Trapping poly(ADP-ribose) polymerase. J Pharmacol Exp Ther 353:446-457, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Lord CJ, Ashworth A: PARP inhibitors: Synthetic lethality in the clinic. Science 355:1152-1158, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farmer H, McCabe N, Lord CJ, et al. : Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434:917-921, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Fong PC, Boss DS, Yap TA, et al. : Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361:123-134, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Kaufman B, Shapira-Frommer R, Schmutzler RK, et al. : Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 33:244-250, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gardner EE, Lok BH, Schneeberger VE, et al. : Chemosensitive relapse in small cell lung cancer proceeds through an EZH2-SLFN11 axis. Cancer Cell 31:286-299, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]