Abstract
Purpose
To evaluate the quality of life among survivors after sepsis in 2 years, comparing with critical patients without sepsis and the general people, analyze the changes and the predictors of quality of life among septic survivors.
Methods
This prospective case-control study screened the intensive care unit (ICU) patients in Tianjin Third Central Hospital from January 2014 to October 2017, and the Chinese general population in the previous studies was also included. According to inclusion criteria and exclusion criteria, 306 patients with sepsis were enrolled as the observation group, and another 306 patients without sepsis in ICU during the same period, whose ages, gender and Charlson Comorbidity Index matched with observation group, were enrolled as the control group. At 3 mo, 12 mo, and 24 mo after discharge, the Mos 36-item Short Form Health Survey (SF-36), the Euroqol-5 dimension (EQ-5D), and the activities of daily living (ADL) were evaluated in face-to-face for the quality of life among survivors.
Results
There were 210 (68.6%) septic patients and 236 (77.1%) non-septic critically ill patients surviving. At 3 months after discharge, the observation and control groups had the similar demographic characteristics (age: 58.8 ± 18.1years vs. 57.5 ± 17.6 years, p = 0.542; male: 52.0% vs. 51.4%, p = 0.926). However, the observation group had higher acute physiology and chronic health evaluation II (APACHEII) scores, higher sequential organ failure assessment (SOFA) scores, longer hospital stay, and longer ICU stay than the control group did (p < 0.05). There were no significant differences in the eight dimensions of the SF36 scale, the EQ-5D health utility scores, and the activities of daily life scores between septic survivors and non-septic survivors (p > 0.05). In addition, compared with the quality of life of the Chinese general population (aged 55–64 years), the quality of life of septic patients were significantly lower at 3 months after discharge (p < 0.05). Comparing the quality of life of the ill patients who had been discharged at 3 mo and 24 mo, the general health improved statistically (p = 0.000) and clinically (score improvement > 5 points). Older age (OR, 1.050; 95% CI, 1.022–1.078, p = 0.000), female (OR, 3.375; 95% CI, 1.434–7.941, p = 0.005) and longer mechanical ventilation time (OR, 3.412; 95% CI, 1.413, 8.244, p = 0.006) were the risk factors for the quality of life of septic survivors.
Conclusion
The long-term quality of life of septic survivors was similar to that of non-sepsis critically ill survivors. After discharge, the general health of sepsis improved overtime. Age, female and mechanical ventilation time (>5 days) were the predictors of the quality of life after sepsis.
Keywords: Sepsis, Survivors, Long-term quality of life, Risk factors
Introduction
Sepsis is a systemic inflammatory response syndrome caused by infection,1 which is one of the most common diseases in the intensive care unit (ICU). An epidemiological study estimated that there were 4,857,000 septic patients, 1,265,000 patients with severe sepsis and septic shock in China per year, the annualized mortality rate of sepsis was 79/100,000. According to this, there would be 4,025,000 survivors of sepsis every year.2
The long-term prognosis of the patients with sepsis is of great concern to the patients, their families, health care workers, and health care decision makers. The study showed that survivor among sepsis patient had the increasing long-term mortality, decreasing quality of life and mounting health care costs, due to the long-term neuropsychiatric damage, physical dysfunction, residual inflammatory reaction, and impaired immune function, which maked sepsis a “hidden public health problem”.3 Other studies also depicted a higher mortality rate and the impaired quality of life in a longer period after sepsis.4, 5, 6 Currently there has been only two studies on long-term quality of life of sepsis patients in China, although there were many survivors.7, 8 One study evaluated the quality of life in survivors of severe sepsis over 6 years. However, it had a small sample size.7 The other study used only one questionnaire to evaluate the quality of life by telephone interview, which may lead to the inaccurate results.8
The purpose of this study was to evaluate the quality of life of septic patients within 2 years after discharge. We used multiple questionnaires to measure the quality of life from multiple perspectives, and followed up survivors by face-to-face to improve the quality of the investigation. In addition, the study analyzed the changes and the risk factors of the quality of life, providing references for the treatment and rehabilitation of sepsis.
Methods
Study subjects
This was a prospective case-control study that screened the ICU patients of Tianjin Third Central Hospital from January 1, 2014 to October 31, 2017. The general population of China in the previous studies were also included.9, 10
Inclusion criteria
Sepsis was defined in accordance with the 2001 septic criteria issued by the Society of Critical Care Medicine (SCCM), the European Society of Intensive Care Medicine (ESICM) and the American College of Chest Physicians (ACCP). Patients with sepsis were considered as observation group; patients of non-sepsis who were similar in age, sex, and Charlson Comorbidity Index (CCI) with septic patients during the same period served as the control group. The general population included had similar ages with ICU patients.
Exclusion criteria
Age≤18 years old; ICU stay≤24 h; patients from other places except Tianjin; patients with other diseases that significantly affect the quality of life, such as head injury with sequelae, Alzheimer's disease, severe fractures, etc.
Ethics
This study was approved by the Ethics Committees of Tianjin Third Centre Hospital. Written informed consent was obtained from all patients included in the study.
Questionnaires
Three scales were used in this study: the Mos 36-item Short Form Health Survey (SF-36), the Euroqol-5 dimension (EQ-5D), the Activities of Daily Living (ADL). The SF-36 scale includes 8 dimensions, each dimension scores from 0 to 100 points. The higher score, the better quality of life is, and a 5-point or more difference in the SF-36 score was assessed as clinically meaningful.11
Follow-up and data collection
At 3 months, 12 months, and 24 months after discharge, we contacted the survivors included in the follow-up to appoint home or hospital visits. We recorded the number of patients who died after discharge; we considered those who did not answer the phone for five times on different days and had wrong phone number as out of touch; we regarded those who did not accept the visits for three times on different days as rejection. Reviewed the medical records of patients and collected the following information: age, gender, CCI (Charlson Comorbidity Index), the number of comorbidities (the number of diseases in the Charlson Comorbidity Index), acute physiology and chronic health evaluation II (APACHE II) score within 24 h of admission to the ICU, sequential organ failure assessment (SOFA) score and organs with acute dysfunction (defined as SOFA≥2), hypoxemia (defined as blood oxygen saturation <90%) and hypotension (defined as mean arterial pressure<60 mmHg), treatment of mechanical ventilation and continuous venous-venous hemofiltration. Two trained researchers completed all the above issues.
Statistical analysis
The continuous data were presented as mean ± standard deviation or median with the 25th and 75th percentile. Comparisons between the two groups were performed using the Student t-test (normal distribution) or the Mann-Whitney U test (non-normal distribution). Comparisons of quality of life between two time points used paired t-test (normal distribution) or Wilcoxon (W) test (non-normal distribution). For categorical variables, comparisons between groups used chi-square test (with large samples) or Fisher exact test (with small samples). The binary logistic regression analysis was used to analyze the risk factors of quality of life. We assumed statistical significance at P < 0.05. Statistical analyses were conducted in SPSS 21.0.
Results
Demographic and clinical characteristics
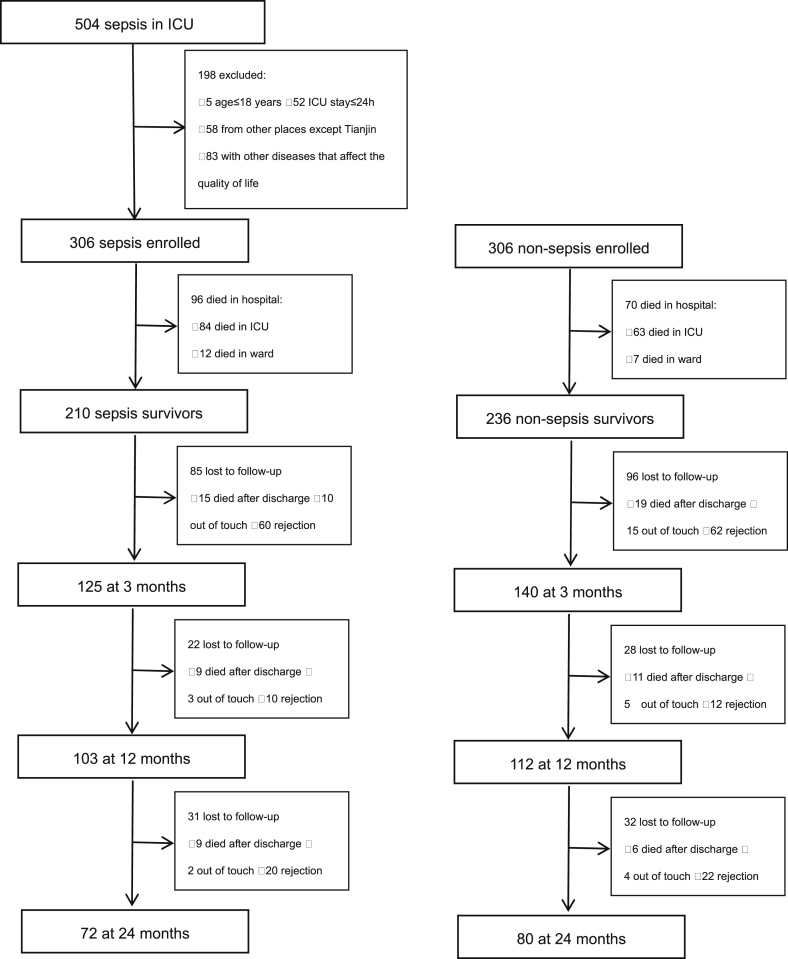
As shown in Fig. 1, there were 210 (68.6%) of sepsis and 236 (77.1%) of non-sepsis critically ill patients surviving at discharge respectively, the in-hospital mortality rate of sepsis was significantly higher than that of the non-sepsis was (p = 0.018). At 3 months after discharge, the observation and control group had the similar demographic characteristics (age: 58.8 ± 18.1years vs. 57.5 ± 17.6 years old, p = 0.542, male: 52.0% vs. 51.4%, p = 0.926) and some clinical characteristics (p > 0.05). However, the observation group had higher APACHEII scores (p = 0.000), higher SOFA scores (p = 0.000), longer hospital stay (p = 0.000), and longer ICU stay (p = 0.047) than the control group did (Table 1).
Fig. 1.
Flow chart of screening and follow-up.
Table 1.
Characteristics of study patients at 3 months after discharge.
| Variable | Sepsis (n = 125) |
Non-sepsis (n = 140) |
Statistics | p |
|---|---|---|---|---|
| Age, years | 58.82 ± 18.07 | 57.48 ± 17.56 | 0.611 | 0.542 |
| Male sex, n (%) | 65 (52.0) | 72 (51.4) | 0.009 | 0.926 |
| CCI | 2.0 (1.0–3.5) | 2.0 (1.0–3.8) | −0.187 | 0.852 |
| With comorbidities, n (%) | 1.924 | 0.860 | ||
| 0 | 21 (16.8) | 29 (20.7) | ||
| 1 | 41 (32.8) | 39 (27.9) | ||
| 2 | 27 (21.6) | 35 (25.0) | ||
| 3 | 22 (17.6) | 20 (14.3) | ||
| 4 | 11 (8.8) | 13 (9.3) | ||
| 5 | 3 (2.4) | 4 (2.9) | ||
| APACHEⅡ | 19.01 ± 7.33 | 14.75 ± 6.15 | 5.089 | 0.000 |
| SOFA | 7.58 ± 3.18 | 5.43 ± 3.40 | 5.307 | 0.000 |
| Type of organ dysfunctions, n (%) | ||||
| respiration | 112 (89.6) | 111 (79.3) | 5.268 | 0.022 |
| coagulation | 31 (24.8) | 29 (20.7) | 0.629 | 0.428 |
| liver | 22 (17.6) | 15 (10.7) | 2.606 | 0.106 |
| cardiovascular | 59 (47.2) | 18 (12.9) | 37.784 | 0.000 |
| central nervous system | 25 (20.0) | 19 (13.6) | 1.971 | 0.160 |
| renal | 30 (24.0) | 23 (16.4) | 2.366 | 0.124 |
| With organ dysfunctions, n (%) | 31.228 | 0.000 | ||
| 0 | 2 (1.6) | 17 (12.1) | ||
| 1 | 31 (24.8) | 55 (39.3) | ||
| 2 | 46 (36.8) | 50 (35.7) | ||
| 3 | 33 (26.4) | 12 (8.6) | ||
| 4 | 11 (8.8) | 6 (4.3) | ||
| 6 | 2 (1.6) | 0 (0.0) | ||
| Hypoxemia, n (%) | 36 (28.8) | 29 (20.7) | 2.332 | 0.127 |
| Hypotension, n (%) | 28 (22.4) | 22 (15.7) | 1.928 | 0.165 |
| CVVH, n (%) | 57 (45.6) | 25 (17.9) | 23.786 | 0.000 |
| Hospital stay, days | 25.0 (16.0–35.0) | 18.0 (12.0–25.8) | −4.382 | 0.000 |
| ICU stay, days | 10.0 (5.5–16.0) | 7.0 (5.0–12.0) | −1.990 | 0.047 |
| Duration of MV, days | 5.0 (1.5–9.0) | 4.0 (1.1–7.0) | −1.639 | 0.101 |
CCI = Charlson comorbidity index, APACHE Ⅱ = acute physiology and chronic health evaluation II, SOFA = sequential organ failure assessment, CVVH = continuous venous-venous hemofiltration, MV = mechanical ventilation.
Therapies according to guidelines were given to patients with sepsis and other different non-sepsis diseases, although survivors in the control group were admitted for different reasons, with most frequent being postoperative surveillance, acute heart failure, gastrointestinal bleeding, acute kidney injury, obstetric critical illness, trauma.
Quality of life
Compared to the control group and the general population
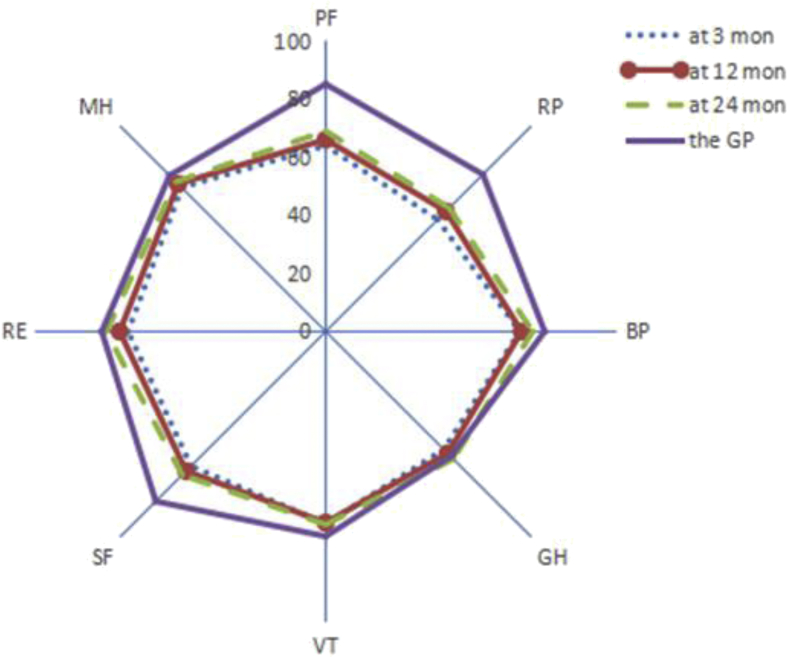
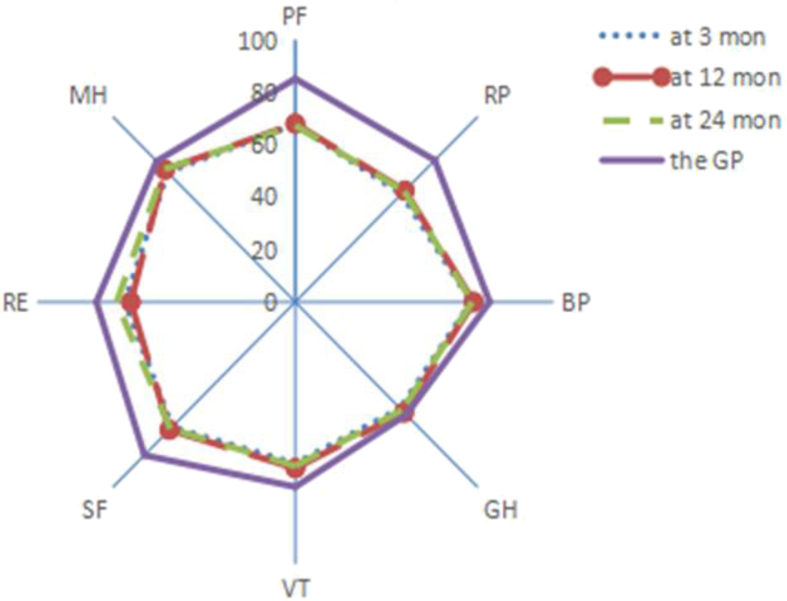
The quality of life of the follow-up survivors is shown in Table 2, Table 3, Table 4. Within 2 years after discharge, there were no statistically significant differences in the scores of SF-36 except vitality (VT) and in the scores of EQ-5D between the observation and control group (p > 0.05). In the 24 months after discharge, the observation group had better daily activities (p = 0.016) than the control group did. Compared with the quality of life of the Chinese general population (aged 55–64 years),9, 10 the quality of life of sepsis and non-sepsis survivors were significantly lower at 3 months after discharge (p < 0.05). At 24 months, sepsis and non-sepsis survivors still had a lower scores of physical functioning (PF), role physical (RP), and social functioning (SF) than the general population did (p < 0.05), in addition, non-sepsis survivors had a lower scores of bodily pain (BP), vitality (VT), mental health (MH), and the health utility scores (Fig. 2, Fig. 3).
Table 2.
Comparison of quality of life between sepsis and non-sepsis at 3 months.
| Quality of life | Sepsis (n = 125) | Non-sepsis (n = 140) | Statistics | p |
|---|---|---|---|---|
| Physical functioning | 63.88 ± 24.54 | 67.71 ± 23.88 | −1.288 | 0.199 |
| Role physical | 54.40 ± 32.54 | 58.21 ± 32.91 | 0.918 | 0.359 |
| Bodily pain | 66.71 ± 20.69 | 68.14 ± 19.92 | 0.727 | 0.468 |
| General health | 57.40 ± 17.28 | 57.43 ± 17.96 | −0.012 | 0.991 |
| Vitality | 66.12 ± 17.98 | 61.61 ± 18.66 | 2.000 | 0.047 |
| Social functioning | 65.46 ± 22.57 | 67.50 ± 24.03 | 0.912 | 0.362 |
| Role emotional | 68.00 ± 31.22 | 65.95 ± 31.35 | −0.627 | 0.530 |
| Mental health | 70.03 ± 16.52 | 70.06 ± 14.45 | −0.013 | 0.989 |
| Health utility scores | 0.89 (0.78, 1.00) | 1.00 (0.78, 1.00) | 0.562 | 0.574 |
| Activities of daily living | 100.0(90.0–100.0) | 100.0(90.0–100.0) | −0.31 | 0.757 |
Physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, mental health are 8 dimensions of the SF-36 scale, each dimension scores were presented as mean ± standard deviation. Health utility score was the score of EQ-5D scale and activities of daily living was the score of ADL scale, which were presented as median with the 25th and 75th percentile.
Table 3.
Comparison of quality of life between sepsis and non-sepsis at 12 months.
| Quality of life | Sepsis (n = 103) | Non-sepsis (n = 112) | Statistics | p |
|---|---|---|---|---|
| Physical functioning | 66.07 ± 23.81 | 67.99 ± 24.82 | −0.579 | 0.563 |
| Role physical | 58.50 ± 31.61 | 60.04 ± 32.96 | 0.384 | 0.701 |
| Bodily pain | 67.38 ± 21.10 | 69.12 ± 20.66 | −0.610 | 0.543 |
| General health | 59.30 ± 19.26 | 59.59 ± 18.55 | −0.111 | 0.912 |
| Vitality | 65.78 ± 18.91 | 63.39 ± 19.32 | 0.910 | 0.362 |
| Social functioning | 67.91 ± 23.78 | 68.97 ± 24.72 | 0.550 | 0.582 |
| Role emotional | 70.87 ± 31.55 | 63.69 ± 32.44 | −1.758 | 0.079 |
| Mental health | 71.79 ± 16.76 | 71.34 ± 14.74 | 0.210 | 0.835 |
| Health utility scores | 0.89 (0.78, 1.00) | 1.00 (0.78, 1.00) | 1.348 | 0.178 |
| Activities of daily living | 100.0 (90.0, 100.0) | 100.0 (90.0, 100.0) | −0.533 | 0.594 |
Physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, mental health are 8 dimensions of the SF-36 scale, each dimension scores were presented as mean ± standard deviation. Health utility score was the score of EQ-5D scale and activities of daily living was the score of ADL scale, which were presented as median with the 25th and 75th percentile.
Table 4.
Comparison of quality of life between sepsis and non-sepsis at 24 months.
| Quality of life | Sepsis (n = 72) | Non-sepsis (n = 80) | Statistics | p |
|---|---|---|---|---|
| Physical functioning | 68.96 ± 22.04 | 67.31 ± 24.98 | 0.431 | 0.667 |
| Role physical | 60.07 ± 34.77 | 59.69 ± 35.24 | −0.042 | 0.967 |
| Bodily pain | 71.15 ± 20.78 | 68.54 ± 21.33 | 0.764 | 0.446 |
| General health | 61.99 ± 20.16 | 58.45 ± 18.93 | −1.021 | 0.307 |
| Vitality | 66.46 ± 19.94 | 62.56 ± 19.39 | 1.220 | 0.224 |
| Social functioning | 69.79 ± 23.05 | 68.44 ± 24.28 | 0.053 | 0.958 |
| Role emotional | 75.47 ± 33.09 | 69.17 ± 33.03 | −1.585 | 0.113 |
| Mental health | 72.86 ± 16.15 | 71.65 ± 14.56 | 0.486 | 0.627 |
| Health utility scores | 1.00 (0.87, 1.00) | 1.00 (0.78, 1.00) | 3.095 | 0.213 |
| Activities of daily living | 100.0 (100.0, 100.0) | 100.0 (90.0, 100.0) | −2.409 | 0.016 |
Physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, mental health are 8 dimensions of the SF-36 scale, each dimension scores were presented as mean ± standard deviation. Health utility score was the score of EQ-5D scale and activities of daily living was the score of ADL scale, which were presented as median with the 25th and 75th percentile.
Fig. 2.
Comparison of quality of life between sepsis survivors and the general population. GP = the general population, PF = physical functioning, RP = role physical, BP = bodily pain, GH = general health, VT = vitality, SF = social functioning, RE = role emotional, MH = mental health.
Fig. 3.
Comparison of quality of life between non-sepsis survivors and the general population. GP = the general population, PF = physical functioning, RP = role physical, BP = bodily pain, GH = general health, VT = vitality, SF = social functioning, RE = role emotional, MH = mental health.
Changes in quality of life
Comparison with the quality of life among survivors who completed three follow-ups between 3 months and 24 months after discharge (Table 5, Table 6), the multiple domains of SF-36, health utility, and activities of daily living of sepsis survivors increased significantly (p < 0.05), only general health (GH) improved significantly in clinic (score improvement>5 points). In the control group, the scores for PF (physical functioning) (p = 0.008), BP (bodily pain) (p = 0.002), GH (general health) (p = 0.005), and VT (vitality) (p = 0.000) also increased, but there were no clinically significance (score improvement < 5 points).
Table 5.
Changes in quality of life of sepsis patients who finished three follow-ups.
| Sepsis | At 3 mon (n = 72) | At 24 mon (n = 72) | Statistics | p |
|---|---|---|---|---|
| Physical functioning | 65.42 ± 23.52 | 68.96 ± 22.04 | 3.197 | 0.002 |
| Role physical | 59.03 ± 32.85 | 60.07 ± 34.77 | −0.561 | 0.575 |
| Bodily pain | 66.85 ± 20.51 | 71.15 ± 20.78 | 3.780 | 0.000 |
| General health | 55.86 ± 18.03 | 61.99 ± 20.16 | −5.367 | 0.000 |
| Vitality | 63.82 ± 18.53 | 66.46 ± 19.94 | 2.753 | 0.008 |
| Social functioning | 65.56 ± 21.35 | 69.79 ± 23.05 | −2.142 | 0.032 |
| Role emotional | 73.15 ± 28.33 | 75.47 ± 33.09 | −0.172 | 0.863 |
| Mental health | 71.39 ± 16.64 | 72.86 ± 16.15 | 1.480 | 0.143 |
| Health utility scores | 0.89 (0.86, 1.00) | 1.00 (0.87, 1.00) | −2.553 | 0.011 |
| Activities of daily living | 100.0 (95.0, 100.0) | 100.0 (100.0, 100.0) | −2.346 | 0.019 |
Table 6.
Changes in quality of life of non-sepsis patients who finished three follow-ups.
| Non-sepsis | At 3 mon (n = 80) | At 24 mon (n = 80) | Statistics | p |
|---|---|---|---|---|
| Physical functioning | 65.13 ± 25.63 | 67.31 ± 24.98 | 2.718 | 0.008 |
| Role physical | 56.56 ± 34.39 | 59.69 ± 35.24 | −1.733 | 0.083 |
| Bodily pain | 66.46 ± 20.72 | 68.54 ± 21.33 | 3.199 | 0.002 |
| General health | 56.93 ± 19.43 | 58.45 ± 18.93 | −2.797 | 0.005 |
| Vitality | 60.38 ± 19.89 | 62.56 ± 19.39 | 3.946 | 0.000 |
| Social functioning | 68.13 ± 25.38 | 68.44 ± 24.28 | −0.224 | 0.823 |
| Role emotional | 67.50 ± 32.69 | 73.75 ± 32.13 | −2.239 | 0.025 |
| Mental health | 69.90 ± 14.33 | 71.65 ± 14.56 | 2.902 | 0.005 |
| Health utility scores | 1.00 (0.72,1.00) | 1.00 (0.78,1.00) | −2.168 | 0.030 |
| Activities of daily living | 100.0(90.0,100.0) | 100.0(90.0,100.0) | −1.384 | 0.166 |
Risk factors
In order to determine the predictors of the quality of life after sepsis, we defined the quality of life as poor when the EQ-5D health utility scores was less than the median of 0.89 at 3 months after discharge. Univariate and multivariate analysis were performed in turn using binary logistic regression. The results are shown in Table 7: older age (OR, 1.050; 95% CI, 1.022–1.078, p = 0.000), female (OR, 3.375; 95% CI, 1.434–7.941, p = 0.005) and prolonged mechanical ventilation time (>5 days) (OR, 3.412; 95% CI, 1.413–8.244, p = 0.006) were independent risk factors for the quality of life of septic survivors.
Table 7.
Logistic regression analyses of predictors for quality of life of sepsis survivors.
| Variables | References | Measures | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |||
| Age (yrs) | 1.044 | (1.021,1.068) | 0.000 | 1.050 | (1.022,1.078) | 0.000 | ||
| Gender | Male | Female | 2.614 | (1.267,5.394) | 0.009 | 3.375 | (1.434,7.941) | 0.005 |
| CCI | 1.360 | (1.094,1.692) | 0.006 | – | – | – | ||
| With comorbidities (n) | 0 | ≥2 | 3.230 | (1.128,9.251) | 0.029 | – | – | – |
| Duration of MV (d) | <5.0 | ≥5.0 | 2.074 | (1.013,4.248) | 0.046 | 3.412 | (1.413,8.244) | 0.006 |
CCI = Charlson comorbidity index, MV = mechanical ventilation, OR = odds ratio, CI = confidence interval.
Discussion
This study mainly assessed the quality of life of septic survivors within two years after discharge, and analyzed the changes and risk factors of quality of life. Firstly, the study found that the sepsis survivors whose age, sex, CCI -matched non-sepsis critically ill survivors during the same period had the similar quality of life, although the sepsis had a more serious condition (with higher APACHEII scores and SOFA scores), longer hospital and ICU stay, and higher in-hospital mortality rate. This suggested that the patients with sepsis recovered as well as or better than the patients with other critical illness did. It may attribute to the irreversible factors affecting quality of life in non-sepsis critically ill patients, which needs further research to confirm. However, the quality of life among survivors suffered from sepsis and non-sepsis critical ill decreased compared with the general population with similar age. This is a common manifestation of “post intensive care syndrome” (PICS), or the result of their own pathophysiological response, or both of the above reasons, which also needs further research to confirm.
Granja and coworkers12 drew a similar conclusion using the EQ-5D to assess the long-term quality of life after severe sepsis and septic shock. Zhang and colleagues7 used the SF36 to conduct a multicenter study in China. The study showed that severe sepsis survivors performed as well as the non-sepsis survivors did, worse than the general population in PF (p = 0.016), VT (p = 0.037), MH (p = 0.038), role emotional (RE, p = 0.043) did. However, the number of people who completed questionnaires in the study was relatively small.
Secondly, the study found the significant increase in general health (GH) at 24 months after discharge. It suggested that the quality of life of sepsis survivors may improve over time after discharge. Hence, early and aggressive treatment, comprehensive care should be given to the patients with sepsis to get a better recovery.
The study also demonstrated that older age, female and longer mechanical ventilation time (>5 days) were the risk factors for the quality of life of sepsis survivors. This result was in accord with the previous study.4 It was interesting that Brown and colleagues13, 14 also depicted that older age and female were associated with worse quality of life in patients with acute respiratory distress syndrome (ARDS).The older age of our study population (average age>50 years) may lead to the poor quality of life for women. The absence of protection from sex hormones deteriorated immune function, meanwhile unfavorable inflammatory mediator regulation in older female may contribute to the worse prognosis.15 Additionally, we observed that women were more vulnerable compared to men after the critically illness, which may cause women prone to the worse quality of life. However, these hypotheses need to be confirmed in large, prospective studies. Mechanical ventilation is one of the most important supportive treatments in the ICU, but prolonged mechanical ventilation time was associated with worse quality of life. This may be related to increasing sedation or analgesic use, muscle weakness, and ventilator-associated lung injury resulting in prolonged treatment of mechanical ventilation. It suggests that the appropriate time of mechanical ventilation may improve the long-term quality of life of sepsis patients. To date, the predictors for the long-term quality of life after sepsis were not identified clearly, it may be associated with the underlying disease of the patients before hospitalization, during hospitalization and after discharge. However, our study did not analyze the situation after discharge, it requires further investigation.
The SF36 and the EQ-5D were recommended in the 2002 Brussels Roundtable16 and they were also used in many previous studies. The SF-36 scale is the most widely used assessment tool for quality of life in the world, its reliability and validity have been validated in critically ill patients.17, 18 Moreover, the reliability and validity of the Chinese version have also been verified.19 The EQ-5D scale is a general assessment tool for health status,20 and it was used in multiple studies about sepsis.4, 5, 8 This study used the Chinese version of the questionnaire and the Chinese utility values.21 We also used the ADL scale, which is a reliable scale for assessing the independence and functional status of daily activities.22, 23 The decline in daily activity may lead to a worse quality of life,24 therefore, this scale can help us further understand the subjects' health status. Using these three scales at the same time can not only help us understand the quality of life of patients from many aspects, but also the results can be mutually verified, increasing the reliability of the study. This study has some limitations. Firstly, we did not obtain the quality of life before admission. Because of the urgent conditions at admission, this was also a problem that most studies encountered. Secondly, most patients who participated in the follow-ups had better physical and mental state, so this study may overestimate the quality of life of sepsis. In the future, we can interview patients by face-to-face and telephone, to increase the subjects for more comprehensive information.
In summary, the quality of life of sepsis survivors is fair within 2 years after discharge, which is similar to the quality of life of non-sepsis survivors, although it is still worse than the quality of life of the general population in China. After discharge, the general health of sepsis patients improved significantly over time. Older age, female, and longer time of mechanical ventilation (>5 days) are the risk factors for the quality of life after sepsis. This study provides a reference for further treatment, rehabilitation and long-term prognosis of sepsis.
Fund
Health and Family Planning Commission Technology Fund of Tianjin (20152D-GG-xulei).
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
References
- 1.Levy M.M., Fink M.P., Marshall J.C. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 2.Zhou J., Tian H., Du X. Population-based epidemiology of sepsis in a subdistrict of Beijing. Crit Care Med. 2017;45:1168–1176. doi: 10.1097/CCM.0000000000002414. [DOI] [PubMed] [Google Scholar]
- 3.Maley J.H., Mikkelsen M.E. Short-term gains with long-term consequences: the evolving story of sepsis survivorship. Clin Chest Med. 2016;37:367–380. doi: 10.1016/j.ccm.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Yende S., Austin S., Rhodes A. Long-term quality of life among survivors of severe sepsis: analyses of two international trials. Crit Care Med. 2016;44:1461–1467. doi: 10.1097/CCM.0000000000001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honselmann K.C., Buthut F., Heuwer B. Long-term mortality and quality of life in intensive care patients treated for pneumonia and/or sepsis: predictors of mortality and quality of life in patients with sepsis/pneumonia. J Crit Care. 2015;30:721–726. doi: 10.1016/j.jcrc.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Cuthbertson B.H., Elders A., Hall S. Mortality and quality of life in the five years after severe sepsis. Crit Care. 2013;17:R70. doi: 10.1186/cc12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang K., Mao X., Fang Q. Impaired long-term quality of life in survivors of severe sepsis : Chinese multicenter study over 6 years. Anaesthesist. 2013;62:995–1002. doi: 10.1007/s00101-013-2257-8. [DOI] [PubMed] [Google Scholar]
- 8.He X.L., Liao X.L., Xie Z.C. Pulmonary infection is an independent risk factor for long-term mortality and quality of life for sepsis patients. BioMed Res Int. 2016;2016:4213712. doi: 10.1155/2016/4213712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu Y.B., Wang Q,Chen K.F., Luo X.X., Tang F. Predictors of health-related quality of life in the general population. Chin J Behav Brain Sci. 2009;18(3):254–259. [Google Scholar]
- 10.Guan H.J., Liu G.E. Comparison analysis on health related quality of life among urban and rural residents in 4 cities of China. Chin Health Economics. 2015;34:5–12. [Google Scholar]
- 11.Ware J., Snow K.K., Kosinski M.A. The Health Institute, New England Medical Center; Boston: 1993. SF 36 health survey: manual and interpretation guide. [Google Scholar]
- 12.Granja C., Dias C., Costa-Pereira A. Quality of life of survivors from severe sepsis and septic shock may be similar to that of others who survive critical illness. Crit Care. 2004;8:R91. doi: 10.1186/cc2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown S.M., Wilson E., Presson A.P. Predictors of 6-month health utility outcomes in survivors of acute respiratory distress syndrome. Thorax. 2017;72:311–317. doi: 10.1136/thoraxjnl-2016-208560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown S.M., Wilson E.L., Presson A.P. Understanding patient outcomes after acute respiratory distress syndrome: identifying subtypes of physical, cognitive and mental health outcomes. Thorax. 2017;72:1094–1103. doi: 10.1136/thoraxjnl-2017-210337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papathanassoglou E., Middleton N., Benbenishty J. Systematic review of sex-dependant outcomes in sepsis. Nurs Crit Care. 2017;22:284–292. doi: 10.1111/nicc.12280. [DOI] [PubMed] [Google Scholar]
- 16.Angus D.C., Carlet J. Surviving intensive care: a report from the 2002 Brussels Roundtable. Intensive Care Med. 2003;29:368–377. doi: 10.1007/s00134-002-1624-8. [DOI] [PubMed] [Google Scholar]
- 17.Heyland D.K., Hopman W., Coo H. Long-term health-related quality of life in survivors of sepsis. Short Form 36: a valid and reliable measure of health-related quality of life. Crit Care Med. 2000;28:3599–3605. doi: 10.1097/00003246-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Chrispin P.S., Scotton H., Rogers J. Short Form 36 in the intensive care unit: assessment of acceptability, reliability and validity of the questionnaire. Anaesthesia. 1997;52:15–23. doi: 10.1111/j.1365-2044.1997.015-az014.x. [DOI] [PubMed] [Google Scholar]
- 19.Li L., Wang H., Shen Y. Development and psychometric tests of a Chinese version of the SF-36 health survey scales. Zhonghua Yufang Yixue Zazhi. 2002;36:109–113. [PubMed] [Google Scholar]
- 20.EuroQol Group EuroQol –a new facility for the measurement of health-related quality of life. Health Pol. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 21.Liu G.G., Wu H., Li M. Chinese time trade-off values for EQ-5D health states. Value Health. 2014;17:597–604. doi: 10.1016/j.jval.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Mahoney F.I., Barthel D.W. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 23.Collin C., Wade D.T., Davies S. The Barthel ADL Index: a reliability study. Int Disabil Stud. 1988;10:61–63. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 24.Winters B.D., Eberlein M., Leung J. Long-term mortality and quality of life in sepsis: a systematic review. Crit Care Med. 2010;38:1276–1283. doi: 10.1097/CCM.0b013e3181d8cc1d. [DOI] [PubMed] [Google Scholar]