Abstract
Purpose
To investigate the effects of estrogen G protein-coupled receptor 30 (GPR30) agonist G1 on hippocampal neuronal apoptosis and microglial polarization in rat traumatic brain injury (TBI).
Methods
Male SD rats were randomly divided into sham group, TBI + vehicle group, TBI + G1 group. Experimental moderate TBI was induced using Feeney's weigh-drop method. G1 (100μg/kg) or vehicle was intravenously injected from femoral vein at 30 min post-injury. Rats were sacrificed at 24 h after injury for detection of neuronal apoptosis and microglia polarization. Neuronal apoptosis was assayed by immunofluorescent staining of active caspase-3. M1 type microglia markers (iNOS and IL-1β) and M2 type markers (Arg1 and IL-4) were examined by immunoblotting or ELISA. Total protein level of Akt and phosphorylated Akt were assayed by immunoblotting.
Results
G1 significantly reduced active caspase-3 positive neurons in hippocampus. Meanwhile G1 increased the ratio of Arg1/iNOS. IL-1β production was decreased but IL-4 was increased after G1 treatment. G1 treatment also increased the active form of Akt.
Conclusions
GPR30 agonist G1 inhibited neuronal apoptosis and favored microglia polarization to M2 type.
Keywords: GPR30, Traumatic brain injury, Microglia, Neuron
Introduction
Traumatic brain injury (TBI) is a major cause of human death and disability worldwide.1, 2 It is well established that TBI survivors have a higher risk of developing dementia than healthy control.3 The hippocampus plays a critical role in memory formation in both humans and rodents. TBI-induced hippocampal damage can cause or exacerbate cognitive impairment after brain injury.4, 5 Previous studies have shown that neurons in hippocampal CA1 and CA3 areas were significantly reduced at 7th day after TBI.4 Therefore, early intervention to reduce the hippocampal neuronal damage in TBI patients is a promising therapeutic strategy to improve the cognitive function and life quality.
TBI-induced brain injury can be characterized by both primary and secondary injury phases. The primary injury is the mechanical disruption of brain tissue, whereas secondary injury subsequently develops over an elongated time including excitotoxicity, oxidative stress and inflammatory response that ultimately lead to neuronal cell death.6 Neuroinflammation plays a key role in secondary injury mechanism after TBI.7, 8 Microglial activation is a key event in the process of inflammatory response after brain injury. More importantly, microglia can monitor neuronal activity and play a functionally dynamic role in synaptic plasticity that supports cognition.9 Microglia can switch to different functional states within different microenvironment. The activated microglial cell exhibits the same pattern as macrophages, namely pro-inflammatory type (M1 type) and anti-inflammatory type (M2 type).10 Therefore, promoting microglial polarization to M2 type may alleviate neuronal injury and neurological deficit.
Estrogen, a steroid hormone, plays an important role not only in female reproductive system but also in cardiovascular system, digestive system, immune system and central nervous system.11 It's widely recognized that the neuroprotective effect of estrogen is mediated by ERs (estrogen receptors), including classical ERs and non-classical ERs.12 G protein-coupled receptor 30 (GPR30) is a novel membrane ER receptor. Different from traditional estrogen nuclear receptors, GPR30 exerts its biological effects through rapid non-genomic mechanisms.13 Recent studies found that activation of GPR30 has neuroprotective effects on ischemic brain injury.14 However, whether GPR30 has similar protective effects in TBI remains to be further elucidated. The purpose of this study was to investigate the effects of GPR30 agonist G1 on neuronal apoptosis and microglial polarization in TBI rat model.
Methods
Animals
Adult male Sprague-Dawley rats (250–280 g body weight) were housed with 3 rats per cage on a 12 h light/dark cycle in a temperature-controlled room (23–25 °C) with free access to water and food. All animal use and experimental protocols were approved and carried out in compliance with the IACUC guidelines and the Animal Care and Ethics Committee of Wuhan University School of Medicine. The experimental groups were assigned by randomization. The experiments were performed by investigators blinded to group assignment. After brain injury, rats were housed in cages under the same conditions as pre-injury. A total of 50 rats (5 rats died shortly after surgical operation) were used in the study. Rats were sacrificed at 24 h post-injury for collection of brain tissues.
TBI model
Traumatic brain injury was induced using Feeney's weight-drop as previously described.15, 16 Briefly, rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (0.35 ml/100 g body weight). The rats then were fixed in a stereotactic frame to undergo a right parietal craniotomy (3.5 mm posterior and 2.5 mm lateral to bregma, diameter 5 mm). Moderate parietal contusion was produced by dropping a 20 g, flat-ended steel rod (diameter 4.5 mm) from a height of 20 cm onto a piston resting on the dura. Sham-operated animals were anesthetized and subjected to the surgical procedure without the rod drop.
G-1 (Cayman Chemical, Ann Arbor, MI, USA) was dissolved in a solution of 10% EtOH and 90% saline. G1 (100μg/kg) or vehicle was intravenously injected from femoral vein at 30 min post-injury.
Immunofluorescence analysis
Brain tissues were removed and post-fixed in 4% paraformaldehyde at 4 °C for 24 h, and then transferred into 30% sucrose solution in 0.1 mol/L phosphate buffer at 4 °C for 72 h. Thirty-μm serials sections were coronally cut by a Cryostat (Leica Micro-systems AG, Germany) and mounted onto glass slides. Sections were incubated with 0.3% Triton X for 30 min at room temperature, followed by blocking with 3% bovine serum albumin for 1 h. Then sections were incubated with the primary antibody anti-active caspase-3 (abcam, 1:100) overnight at 4 °C. After washing with phosphate buffer, sections were incubated with Alexa Fluor-tagged secondary antibody. Positive staining cells were counted under an inverted fluorescence microscope at high magnification (400 × ) (Zeiss, Hamburg, Germany). Three serial sections from the same rat were counted to provide an average value for each animal. For each brain section, three region of the ipsilateral hippocampal CA1 were counted to provide the mean value.
Western blot
The ipsilateral hippocampi were isolated and homogenized in RIPA buffer containing a protease and phosphatase inhibitor cocktail (Roche, Indianapolis, IN, USA). The tissue homogenate was centrifuged at 12,000 rpm for 20 min at 4 °C and the supernatant was stored at −20 °C. Proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel and transferred to polyvinylidene difluoride membrane (Millipore). After blocking with 5% non-fat milk in phosphate buffer, membranes were incubated with primary antibodies overnight at 4 °C and subsequently incubated with secondary antibodies for 2 h at room temperature. Protein bands were visualized by enhanced chemiluminescence. β-Actin was used as a loading control. The following primary antibodies were used: active caspase-3 (abcam, 1:200), Arg1 (cell signaling, 1:1000), iNOS (Santa Cruz, 1:500), CD11b (Bioworld, 1:1000), β-Actin (cell signaling, 1:2000), p-Akt (cell signaling, 1:1000) and Akt (cell signaling, 1:1000). The data of western blot were quantified by Image J software.
ELISA analysis
Ipsilateral hippocampi were collected and homogenized in RIPA buffer containing a protease and phosphatase inhibitor cocktail. After centrifugation at 12,000 rpm for 20 min at 4 °C, the supernatant was collected for IL-1β and IL-4 measurement, the ELISA kit (R & D Systems, Minneapolis, Minn., USA) were used according to the manufacturer's protocol for the quantification. Finally, the reaction plates were read at 450 nm on a Microplate Reader. The results were expressed as picograms per milligram of tissues.
Statistical analysis
All data are presented as mean ± SEM. Group differences were analyzed by a One-way analysis of variance (ANOVA) followed by Dunnett's post hoc test. p value less than 0.05 considered to be statistically significant.
Results
GPR30 agonist G1 reduces hippocampal neuronal apoptosis in TBI rats
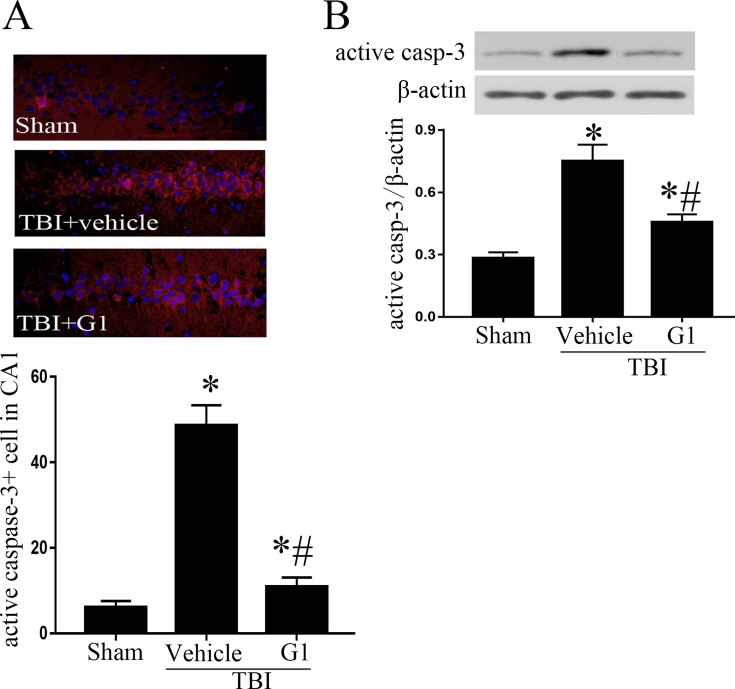
Previous study has shown the neuron loss and neuronal apoptosis in CA1 and CA3 area in TBI rats.4 Active caspase-3 is an important terminal cleavage enzyme in apoptosis pathway. Immunofluorescent staining of active caspase-3 showed that positive staining cells were predominately located in CA1 area in TBI rats. The positive staining cells in CA1 region in TBI + vehicle group was significantly higher than that in sham group, while the positive cells in TBI + G1 group was lower than TBI + vehicle group (Fig. 1A). Similar results were observed in Western blot analysis. An increased protein level of active caspase-3 was observed in TBI + vehicle group in comparison with sham group, whereas G1 treatment reduced active caspase-3 in TBI rats (Fig. 1B). The results suggest that G1 reduces the neuronal apoptosis in hippocampus in TBI rats.
Fig. 1.
Active caspase-3 expression in ipsilateral hippocampus. A: Immunofluorescent staining of active caspase-3 in hippocampal CA1 area. B: Western blots analysis of active caspase-3 in hippocampus. (*p < 0.05, vs sham group, # p < 0.05, G1 group vs vehicle group, n = 5).
GPR30 agonist G1 promotes microglial polarization to M2 type
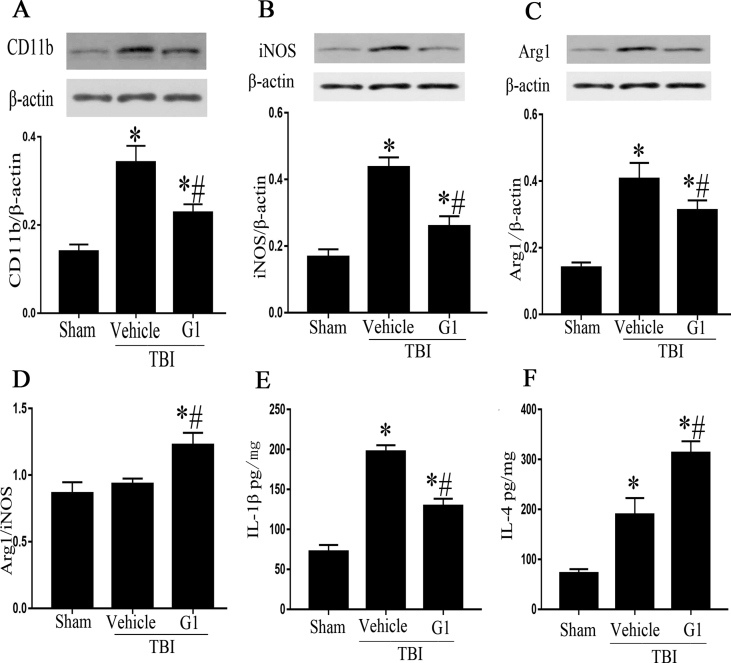
Next, we examined whether G1 could attenuate microglia-mediate inflammatory response. CD11b is a specific marker of microglia, which indirectly reflects the number of microglia. Compare to sham group, the protein level of CD11b in TBI + vehicle group was significantly increased, while CD11b protein level in TBI + G1 group was significantly lower than TBI + vehicle group (Fig. 2A).
Fig. 2.
G1 promotes microglial polarization to M2 type. A: Western blot analysis of CD11b in ipsilateral hippocampus. B, C: Western blot analysis of iNOS and Arg1 in ipsilateral hippocampus. D: Comparison of Arg1/iNOS ratios. E, F: ELISA analysis of IL-1β and IL-4 in ipsilateral hippocampus. (*p < 0.05, vs sham group, # p < 0.05, G1 group vs vehicle group, n = 5).
To further investigate the effect of G1 on microglial activation and function, the expression of microglial marker iNOS (M1 type) and Arg1 (M2 type) was detected by Western blotting. TBI induced a significant increase in both iNOS and Arg1, demonstrating by elevated protein level of iNOS and Arg1 in TBI + vehicle group compared to sham group. However, G1 treatment attenuated the increase of iNOS and Arg1 in TBI rats (Fig. 2B and C). Further analysis showed that Arg1/iNOS ratio was comparable between sham and TBI + vehicle groups, but the ratio was significantly elevated in TBI + G1 group (Fig. 2D). The production of cytokine IL-1β (M1 type marker) and IL-4 (M2 type marker) in ipsilateral hippocampi was assayed by ELISA. G1 treatment decreased IL-1β production but increased IL-4 (Fig. 2E and F). The data indicate that G1 treatment favors microglia polarization to M2 type.
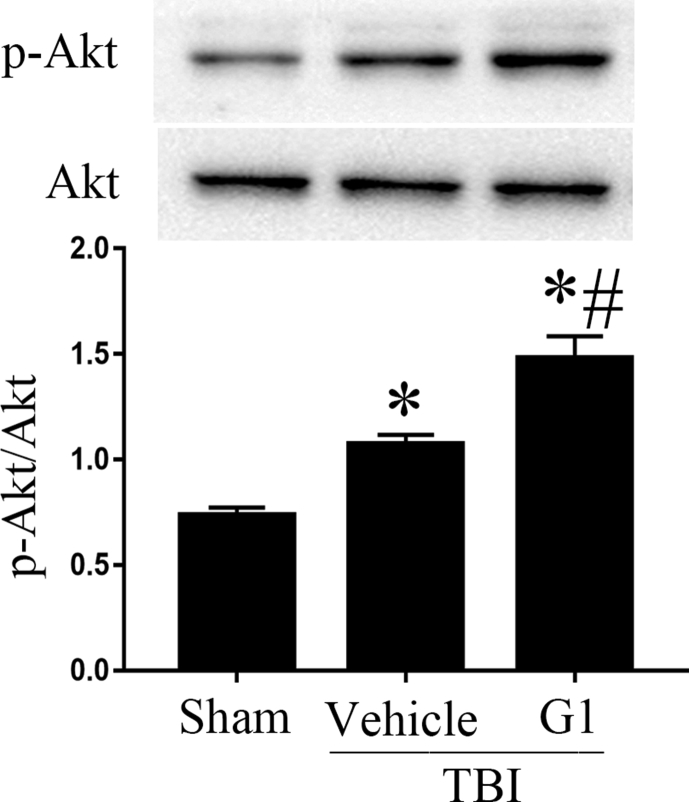
GPR30 agonist G1 increases the level of p-Akt
GPR30 can mediate PI3K/Akt signaling pathway.17 Akt activation was quantified by measuring Akt phosphorylation (p-Akt) at Ser473. Compare to sham group, the level of p-Akt in TBI + vehicle group was increased. G1 treatment led to an additional increase in p-Akt, indicating that G1 enhanced Akt signaling pathway (Fig. 3).
Fig. 3.
p-Akt expression in ipslateral hippcocampus. (*p < 0.05, vs sham group, # p < 0.05, G1 group vs vehicle group, n = 5).
Discussion
Cognitive impairment is a common complication of TBI. In addition to primary neuronal damage directly caused by brain contusion, secondary neuronal damage caused by inflammatory response also induces or aggravate neuronal death. Microglia plays a critical role not only in the inflammatory response after brain injury but also in synaptic plasticity and cognition.9, 18 Microglia are classified into M1 type and M2 type according to their role in the inflammatory response.19 M1 microglia secretes inflammatory cytokines (NO, IL-1, IL-6, TNF-α, etc.) and aggravate inflammation and neuronal death.20 M2 microglia secretes neurotropic factors and anti-inflammatory factors (IL-4, IL-10, etc.), thus reducing neuronal damage.21 Therefore, the early therapeutic interventions to eliminate neuronal death and switch microglia polarization to M2 type are helpful to improve cognitive function after brain injury.
GPR30, a seven transmembrane G protein-coupled receptor, is a novel estrogen membrane receptor. Unlike estrogen nuclear receptors, and activation of GPR30 by binding with estrogen causes rapid non-genetic effects.13 GPR30 is expressed in neurons and glial cells,22 indicating its possible involvement in regulating the activities and functions of neuron and microglia. It is well known that estrogen has neuroprotective effects. The incidence of stroke in pre-menopausal women is lower than that in men, but the incidence in post-menopausal women is the same as in men.23 In addition, neurological rehabilitation after acute brain injury is better in women than in men. However, the clinical use of estrogen therapy may increase the risk of breast cancer and cardiovascular disease in postmenopausal women. These side effects are predominately related to the activation of estrogen nuclear receptors ERα and ERβ. The non-genetic action of GPR30 may avoid such side effects. GPR30 agonist G1 improved the memory function in female rats, and its antagonist G15 inhibits memory function in female rats,24 suggesting the involvement of GPR30 in cognitive function.
It has been demonstrated that activation of GPR30 improved spatial cognitive impairment in TBI rats,25 In this study, neuronal apoptosis and microglia polarization in the hippocampus were examined to elucidate the mechanism of cognitive improvement following GPR30 activation. The active caspase-3 positive neurons were predominately observed in CA1 area after brain injury. G1 treatment reduced the number of active caspase-3 positive neurons in CA1 area and attenuated the protein level of active caspase-3 in ipsilateral hippocampus. These results suggest that G1 could reduce hippocampal neuronal apoptosis. We further examined the state of microglia polarization following G1 treatment. G1 administration reduced the expression of CD11b, a specific marker for microglia, in TBI rats, which indicated that G1 inhibited injury-induced microglia accumulation. A switch of microglia polarization to anti-inflammatory M2 type following G1 treatment was indicated by increased Arg1/iNOS ratio and IL-4 as well as decreased IL-1β. iNOS and Arg1 regulate the synthesis of inflammatory cytokine NO by competitively combing with the substrate arginine. Increased Arg1/iNOS ratio results in less production of NO. Therefore, G1 treatment not only reduced neuronal apoptosis but also favored microglia polarization to M2 type.
Next, we investigated the signaling pathway underlying the action of GPR30 activation. It has been demonstrated that GPR30 activation can mediate PI3K/AKT pathway.26, 27 PI3K/Akt signaling pathway plays an important role in neuronal survival, proliferation and growth. Additionally, PI3K/Akt pathway can regulate microglial polarization and p-Akt contributes to microglia polarization to M2 type.28 In our study, the level of p-Akt was increased following G1 administration. Thus, G1 may promote neuron survival and microglia polarization to M2 type by enhanced Akt pathway.
Acknowledgments
This work was supported by Natural Science Foundation of China (NSFC; 81470599).
Footnotes
Peer review under responsibility of Daping Hospital and the Research Institute of Surgery of the Third Military Medical University.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.cjtee.2018.04.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Langlois J.A., Rutland-Brown W., Wald M.M. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 2.León-Carrión J., Domínguez-Morales Mdel R., Barroso y Martín J.M. Epidemiology of traumatic brain injury and subarachnoid hemorrhage. Pituitary. 2005;8:197–202. doi: 10.1007/s11102-006-6041-5. [DOI] [PubMed] [Google Scholar]
- 3.Djordjevic J., Sabbir M.G., Albensi B.C. Traumatic Brain injury as a risk factor for Alzheimer's disease: is inflammatory signaling a key player? Curr Alzheimer Res. 2016;13:730–738. doi: 10.2174/1567205013666160222110320. [DOI] [PubMed] [Google Scholar]
- 4.Yang L.Y., Greig N.H., Huang Y.N. Post-traumatic administration of the p53 inactivator pifithrin-α oxygen analogue reduces hippocampal neuronal loss and improves cognitive deficits after experimental traumatic brain injury. Neurobiol Dis. 2016;96:216–226. doi: 10.1016/j.nbd.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ezaki J., Shimada R., Shibuya M. Hippocampal neuronal degeneration in the traumatic brain injury mouse: non-trivial effect of scalp incision. Neurol Res. 2016;38:994–1002. doi: 10.1080/01616412.2016.1228746. [DOI] [PubMed] [Google Scholar]
- 6.McConeghy K.W., Hatton J., Hughes L. A review of neuroprotection pharmacology and therapies in patients with acute traumatic brain injury. CNS Drugs. 2012;26:613–636. doi: 10.2165/11634020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A., Loane D.J. Neuroinflammation after traumatic brain injury: opportunities for therapeutic intervention. Brain Behav Immun. 2012;26:1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Woodcock T., Morganti-Kossmann M.C. The role of markers of inflammation in traumatic brain injury. Front Neurol. 2013;4:18. doi: 10.3389/fneur.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris G.P., Clark I.A., Zinn R. Microglia: a new frontier for synaptic plasticity, learning and memory, and neurodegenerative disease research. Neurobiol Learn Mem. 2013;105:40–53. doi: 10.1016/j.nlm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Chen Z., Trapp B.D. Microglia and neuroprotection. J Neurochem. 2016;136(suppl 1):10–17. doi: 10.1111/jnc.13062. [DOI] [PubMed] [Google Scholar]
- 11.Couse J.F., Lindzey J., Grandien K. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997;138:4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- 12.Arevalo M.A., Azcoitia I., Garcia-Segura L.M. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16:17–29. doi: 10.1038/nrn3856. [DOI] [PubMed] [Google Scholar]
- 13.Prossnitz E.R., Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kosaka Y., Quillinan N., Bond C. GPER1/GPR30 activation improves neuronal survival following global cerebral ischemia induced by cardiac arrest in mice. Transl Stroke Res. 2012;3:500–507. doi: 10.1007/s12975-012-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S., Zhang L., Wu Q. Chemokine CCL2 induces apoptosis in cortex following traumatic brain injury. J Mol Neurosci. 2013;51:1021–1029. doi: 10.1007/s12031-013-0091-8. [DOI] [PubMed] [Google Scholar]
- 16.Feeney D.M., Boyeson M.G., Linn R.T. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211:67–77. doi: 10.1016/0006-8993(81)90067-6. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J., Yang Y., Zhang Z. Gankyrin plays an essential role in estrogen-driven and GPR30-mediated endometrial carcinoma cell proliferation via the PTEN/PI3K/AKT signaling pathway. Cancer Lett. 2013;339:279–287. doi: 10.1016/j.canlet.2012.10.037. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Ontiveros D.G., Tajiri N., Acosta S. Microglia activation as a biomarker for traumatic brain injury. Front Neurol. 2013;4:30. doi: 10.3389/fneur.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A., Alvarez-Croda D.M., Stoica B.A. Microglial/Macrophage polarization dynamics following traumatic brain injury. J Neurotrauma. 2016;33:1732–1750. doi: 10.1089/neu.2015.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loane D.J., Byrnes K.R. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durafourt B.A., Moore C.S., Zammit D.A. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60:717–727. doi: 10.1002/glia.22298. [DOI] [PubMed] [Google Scholar]
- 22.Fuente-Martin E., Garcia-Caceres C., Morselli E. Estrogen, astrocytes and the neuroendocrine control of metabolism. Rev Endocr Metab Disord. 2013;14:331–338. doi: 10.1007/s11154-013-9263-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reeves M.J., Bushnell C.D., Howard G. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C.L., Herndon C. New roles for neuronal estrogen receptors. Neuro Gastroenterol Motil. 2017;29 doi: 10.1111/nmo.13121. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z.F., Pan Z.Y., Xu C.S. Activation of G-protein coupled estrogen receptor 1 improves early-onset cognitive impairment via PI3K/Akt pathway in rats with traumatic brain injury. Biochem Biophys Res Commun. 2017;482:948–953. doi: 10.1016/j.bbrc.2016.11.138. [DOI] [PubMed] [Google Scholar]
- 26.Yang K., Zhang H., Luo Y. Gypenoside XVII prevents atherosclerosis by attenuating endothelial apoptosis and oxidative stress: insight into the ERalpha-Mediated PI3K/Akt pathway. Int J Mol Sci. 2017;18(2) doi: 10.3390/ijms18020077. pii: E77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L., Zhao Y., Guo L. 17beta-estradiol protects INS-1 insulinoma cells from mitophagy via G protein-coupled estrogen receptors and the PI3K/Akt signaling pathway. Int J Mol Med. 2018;41:2839–2846. doi: 10.3892/ijmm.2018.3470. [DOI] [PubMed] [Google Scholar]
- 28.Wang G., Shi Y., Jiang X. HDAC inhibition prevents white matter injury by modulating microglia/macrophage polarization through the GSK3beta/PTEN/Akt axis. Proc Natl Acad Sci USA. 2015;112:2853–2858. doi: 10.1073/pnas.1501441112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.