Abstract
To provide efficient antioxidant capacities, proper carriers are needed to protect antioxidants against oxidative stress. Collagen mesh structure or chitosan gel was loaded with α-tocopherol and their effects were evaluated in bulk corn oil or oil-in-water (O/W) emulsion at 60 °C. Added collagen and chitosan enhanced oxidative stability in corn oil and O/W emulsions at 60 °C compared to corn oils without carriers or with addition of α-tocopherol (p < 0.05). Stability of α-tocopherol in corn oil loaded in collagen or chitosan was significantly enhanced compared to that in oils without carriers (p < 0.05). In O/W emulsions, α-tocopherol loaded collagen showed higher antioxidant properties than α-tocopherol loaded chitosan (p < 0.05). Collagen mesh structure and chitosan gel retarded the rates of lipid oxidation efficiently in both food matrices when α-tocopherol was not loaded. Collagen mesh structure and chitosan gel can be useful carriers for α-tocopherol in bulk oil or O/W emulsion.
Keywords: Collagen, Chitosan, α-tocopherol, Oxidative stability, Bulk oil, Oil-in-water emulsion
Introduction
Lipid oxidations are inevitable chemical reactions in lipid-rich foods during production and storage. The physicochemical changes in colour, aroma, and texture caused by lipid oxidation can deteriorate the sensory attributes and nutritional values of food products. Adding antioxidants is one of the practical strategies to control the rate of lipid oxidation [1]. Many factors can influence the oxidative stability of foods, including the degree of unsaturation in lipids, types and concentrations of oxygen molecules, the presence of antioxidants and pro-oxidants, and the type of food matrix [2, 3]. The interfaces of oil–water are known to play important roles as major places for lipid oxidation and antioxidant action of chemical compounds in diverse matrix such as bulk oil and oil-in-water emulsion [2, 4, 5]. To control the rates of lipid oxidation, chemical potentials (including bond dissociation enthalpy and one electron reduction potential) and stabilities of compounds against oxidative forces are critical factors in real food systems. Therefore, it is necessary to protect antioxidant compounds using carriers from oxidative stress including heat and irradiation.
Carbohydrate based carriers including calcium-alginate-inulin microbeads [6] and phytochemical grafted chitosan [7], and protein based carriers such as gelatin [8] are some examples of biopolymer-based antioxidant carriers. Collagen is a major constituent of connective tissues. It is the most abundant structural protein in skin and bones of all animals. Collagen has been widely used in biomedical applications to enhance the interaction between cultured cells and biomaterials due to its good biophysical structure and biochemical components [9]. Collagen has been developed as carriers of food additives or extracts of natural resources in foods [10].
Chitosan is a linear polysaccharide composed of glucosamine and acetyl glucosamine. It is made by deacetylation of chitin which is found in exoskeletons of crustacean shells. Chitosan has been studied in many areas, including food, agriculture, biomedical, and pharmaceutical fields due to its beneficial properties such as antimicrobial activity, antioxidant activity, biodegradability, and biocompatibility [11, 12]. The antioxidant ability of chitosan is attribute to the radical scavenging abilities including hydroxyl radicals and superoxide radicals [12, 13] and metal chelating ability due to the presence of primary amine groups [14]. It has been developed as drug carriers and food packaging film materials as an alternative to synthetic polymers [12]. Chitosan has been used as a carrier for phenolic compounds such as gallic acid through grafting method [15]. Our research groups tested the effects of β-cyclodextrin, chitosan, and collagen on the stability of α-tocopherol in heated oils [16] and collagen mesh complexed with α-tocopherol with core/shell structures protected the oxidative stability of heated oil and of α-tocopherol [17]. However, comparison studies of tocopherol loaded collagen mesh structure or chitosan gel in food matrix such as bulk oil and oil-in-water (O/W) emulsion are rare in the literature.
The objective of this study was to evaluate the antioxidant efficiency of collagen mesh structure or chitosan gel loaded with α-tocopherol in different matrix. Antioxidant carriers using proteins and carbohydrates were designed and their enhancement effect for the stability of α-tocopherol was evaluated in bulk corn oil and O/W emulsions.
Materials and methods
Materials
Standard α-, γ-, and δ-tocopherols were purchased from Sigma Aldrich (St. Louis, MO, USA). Corn oils were purchased from a local market (Suwon, Korea). Karl Fisher reagent was purchased from Fluka (Buchs, Switzerland). Rubber septa and aluminum caps were purchased from Supelco, Inc. (Bellefonte, PA, USA). Anisidine was purchased from Kanto Chemical Co. (Tokyo, Japan). Other reagent grade chemicals were purchased from Daejung Chemical Co. (Seoul, Korea). Collagen solution (type I collagen, Matrixen-PSP; Bioland Ltd., Cheonan, Korea) was prepared in 0.05 M acetic acid (pH 3.2) at a fixed concentration of 4 wt %.
Sample preparation
Polymer based carrier preparation
In this work, type-I collagen (Matrixen-PSP; SKBioland, South Korea) derived from porcine tendon was used. To obtain collagen pore structure, a low temperature (− 40 °C) printing system (DTR2–2210T; Dongbu Robot, Bucheon, South Korea) was used. The structures of collagen were produced using a nozzle with diameter of 300 µm. The nozzle moving speed was 10 mm/s, the pneumatic pressure was 150 ± 5 kPa, and the temperature was maintained at − 40 °C for the manufacturing stage. Fabricated collagen pore structure was placed in a freeze-dryer (SFDSM06; Samwon, Busan, South Korea) at − 75 °C for 3 days. For cross-linking, the dried collagen pore structure was immersed in 1–100 mM 1-ethyl-(3-3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) solution in 95% ethanol at room temperature for 24 h. After cleaning, these cross-linked structures were freeze-dried again [18].
For the formation of chitosan gel, low molecular weight chitosan was dissolved in 1% acetic acid at the concentration of 2 mg/mL (chitosan solution). Sodium tripolyphosphate dissolved in deionized water (TPP solution) at the concentration of 1 mg/mL was mixed with the chitosan solution through drop wise addition followed by stirring for 30 min. Then 28% ammonia solution was added to the mixture to make pH 9. The ratio of chitosan solution and TPP solution was 4:2 based on volume. The mixture was centrifuged at 100,000×g and the supernatant was removed. The recovered chitosan gel was washed with deionized water and centrifuge again. This washing procedure was repeated three times. Chitosan gel was dried under nitrogen gas flow and freeze-dried in a freeze-dryer (Ilshinbiobase, Gyeonggi, Korea).
Loading α-tocopherol in collagen mesh structure and chitosan gel
First, α-tocopherol was dissolved in ethanol at concentration of 7200 ppm. Collagen mesh structure (500 mg) was then immersed in 50 mL of α-tocopherol solution in ethanol at room temperature for 120 min with gentle stirring using a magnetic stir-bar. After the treatment, collagen mesh structure was recovered and dried under nitrogen gas flow. The content of α-tocopherol was determined using HPLC.
For loading α-tocopherol to chitosan gel, 1.08 g of α-tocopherol was dissolved in 150 mL ethanol solution and added to 600 mL chitosan solution before adding TPP solution. The ratio of chitosan solution, α-tocopherol solution, and TPP solution was 4:1:2 based on volume. Procedures for chitosan gel production were the same as described above.
Preparation for bulk oil system
One gram of corn oil was mixed with 2.7 mg of collagen mesh structure and 10 mg chitosan gel with or without loading of α-tocopherol. The concentration of carrier was determined based on the loading yield of α-tocopherol in each carrier. As controls, α-tocopherol dissolved in n-hexane was directly added to corn oils at a concentration of 1436 ppm and n-hexane was removed under nitrogen gas flow. One gram of corn oil containing α-tocopherol or carriers was put in 10-mL bottles which were sealed air-tight with Teflon-coated rubber septa and aluminum caps. Sample bottles were placed in a convection oven (HYSC, Seoul, Korea) at 60 °C. Samples were prepared in triplicates at each sampling time (0, 3, 4, and 5 days) and analyzed.
Preparation for O/W emulsion
The O/W emulsions were prepared according to previous reports [19]. Briefly, deionized water was mixed with Tween-20 and then combined with corn oil with the concentrations of 2.5% (w/w) oil and 0.25% (w/w) Tween-20. A coarse emulsion was made by homogenizing the mixture for 3 min using a HB501 instrument (Tepal, Rumilly, Haute-Savoie, France). Coarse emulsion was passed three times in a high pressure homogenizer (APV, SPX Flow Technology, Crawley West Sussex, UK) at 5000 psi. Collagen mesh structure or chitosan gel loaded with α-tocopherol was added to the O/W emulsion at a concentration of 1.0 or 0.54% (w/w), respectively. The concentrations of collagen mesh structure or chitosan gel was determined based on the loading yield of α-tocopherol. To prepare samples without α-tocopherol loaded carriers, collagen mesh structure or chitosan gel was added to O/W emulsion at a concentration of 1.0 or 0.54% (w/w), respectively. Corn oil was mixed with 57,440 ppm α-tocopherol in n-hexane and the solvent was removed under nitrogen flow. Corn oil with α-tocopherol was used as dispersed phase in O/W emulsion. Because the oil content in O/W emulsion was 2.5%, final α-tocopherol concentration was 1436 ppm in O/W emulsion. Samples with α-tocopherol were used as controls. Two milliliters of each O/W emulsion was put in a 10-mL bottle with an air-tight seal. Sample bottles were stored in the convection oven (HYSC, Seoul, Korea) at 60 °C and analyzed at different time points (0, 2, 3, and 4 days). All samples were prepared in triplicates at each sampling time.
Scanning electron microscopy (SEM) analysis of carriers
The morphologies of collagen mesh structure and chitosan gel were characterized by scanning electron microscopy (SEM) (SNE-3000 M; SEC Inc., Suwon, Korea). Detailed sample treatment and measuring procedure followed the manufacturer’s instructions.
Headspace oxygen content analysis in bulk oil and emulsion system
Headspace oxygen content in sample bottles was determined by injecting 30 μL headspace gas into a gas chromatograph (7890 A, Agilent Technologies, Inc., Santa Clara, CA, USA) equipped with a thermal conductivity detector (TCD). For stationary phase, a stainless steel column (1.8 m × 0.32 cm) packed with 60/80 Molecular Sieve 13 × (Alltech Assoc., Inc. Deerfield, IL, USA) was used. For mobile phase, helium gas at flow rate of 200 mL/min was used. The temperatures of the oven, injector, and TCD were 60, 180, and 180 °C, respectively [20].
Conjugated dienoic acid (CDA) and p-anisidine value (p-AV) analysis in bulk oil
CDA and p-AV of samples were determined according to AOCS method Ti la-64 and AOCS Cd 18–90 method [21], respectively.
A modified DPPH method for antioxidant capacity in bulk oil
The 1 mL of 0.1 mM DPPH in methanol was mixed with 0.04 g of oil for one min mixing. The mixture was centrifuged at 2208×g for 3 min and then 0.75 mL DPPH in methanol was mixed with 0.25 mL of supernatant in the mixture of oil and methanol. The absorbance of the sample mixture was measured at wavelength of 517 nm after 30 min standing in the dark using a UV/VIS-spectrometer (Model Genesys 10uv, Thermo Fisher Scientific Inc., Waltham, MA, USA). If the absorbance of DPPH was below 0.2, oils were diluted further. Absorbance of DPPH was expressed as DPPH loss with the following equation [22, 23]:
Analysis of tocopherol content in bulk oils
A 0.1 g of corn oil dissolved in 1 mL of n-hexane was filtered through a polytetrafluorethylene membrane filter. Tocopherol content in oil was analyzed by HPLC with a fluorescence detector (JASCO International Co. Ltd., Tokyo, Japan) with a μ-Porasil™ column (3.9 × 150 mm, 10 μm ID, Waters, Milford, MA, USA). Mixtures of n-hexane and isopropanol at a ratio of 99.8–0.2 (v/v) were used as the mobile phase with an isocratic 0.7 mL/min. The temperature of a column oven was 35 °C and the injection volume of samples was 20 μL. Tocopherols were detected at excitation wavelength of 290 nm and emission wavelength of 330 nm. Calibration curves were constructed using standard α-, γ-, and δ-tocopherols (Sigma-Aldrich) dissolved in n-hexane [24].
Moisture content analysis in bulk oil
The moisture content in corn oil was analyzed by a coulometric KF titrator (C20, Mettler-Toledo Intl., Columbus, OH, USA) according to the manufacturer’s instructions.
Lipid hydroperoxide analysis for O/W emulsion
Lipid hydroperoxides were determined using a method of Yi et al. [19]. Briefly, oil in emulsion samples (0.2 mL) were recovered 1.5 mL of isooctane/2-propanol (3:1, v/v), with centrifugation at 2000×g for 2 min. Recovered oil was mixed with 2.8 mL of methanol/1-butanol (2:1, v/v) and then 30 μL of thiocyanate/Fe2+ solution was added to the mixture. Absorbance of the mixture was measured at wavelength of 510 nm using a UV/VIS-spectrometer (Jenesis 10UV, Thermo, Waltham, MA, USA) after incubation at room temperature for 20 min. The concentration of lipid hydroperoxide was calculated using a cumene hydroperoxide standard curve.
Headspace volatile compound analysis for O/W emulsion
Headspace volatiles in samples were determined using a Hewlett-Packard 6890 gas chromatograph (GC) equipped with a 5971A mass-selective detector (Agilent Technologies) and a MultiPurpose Sampler (MPS) (Gerstel, Mülheim, Germany). The solid phase was a 50/30 μm Divinylbenzene/Carboxen/Polydimethylsiloxane solid phase microextraction (SPME) fiber. Sample bottles were incubated in the MPS agitator at 30 °C for 30 min to and SPME fiber was exposed at 30 °C for 10 min to extract volatiles. Volatiles in the SPME fiber were desorbed in a GC injection port for 2 min at 250 °C. Stationary phase was a DB-5 ms column (30 m × 0.25 mm i.d., 0.25 μm film thickness) with gradient temperatures. Oven temperature started at 40 °C for 2 min, increased at the rate of 5.5 °C/min to 160 °C and at 10 °C/min to 220 °C, and stayed at 220 °C for 1 min. The flow rate of helium carrier gas was 1.0 mL/min. The GC system was operated in splitless mode [25]. All mass spectra were obtained at 70 eV and ion source temperature of 220 °C. Identification of compounds was made by a combination of NIST Mass Spectra and Kovat index.
Statistical analysis
All data were analyzed statistically by analysis of variance (ANOVA) and Duncan’s multiple range test using SPSS software program version 19 (SPSS Inc., Chicago, IL, USA). A p < 0.05 was considered statistically significant. Headspace oxygen content, CDA, p-AV, DPPH loss, tocopherol content, and moisture content were evaluated in bulk oil system while headspace oxygen content, lipid hydroperoxides, and headspace volatiles were determined in O/W emulsion system.
Results and discussion
Preparation of carriers
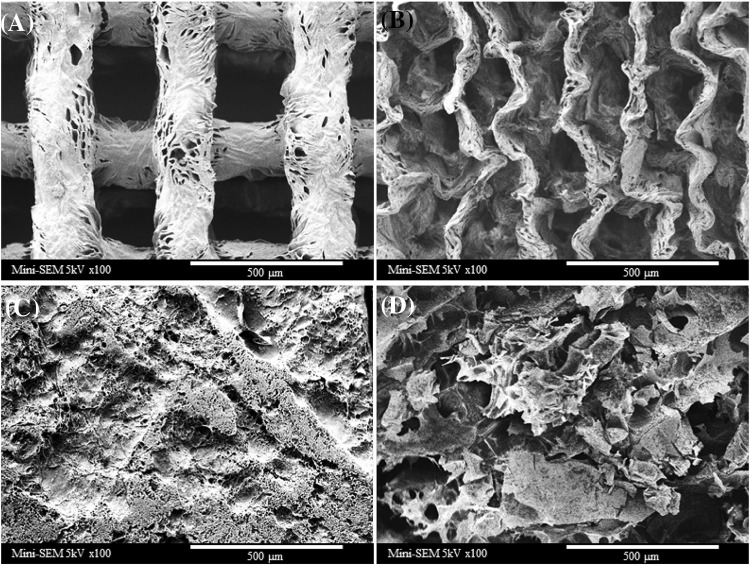
SEM images of collagen mesh structure (A), collagen mesh structure with α-tocopherol (B), chitosan gel (C), and chitosan gel with α-tocopherol (D) are shown in Fig. 1, Clear mesh structures (average pore size: 353 ± 27 µm; average structure diameter: 307 ± 16 µm) of collagen are shown in Fig. 1(A). After the loading procedure of α-tocopherol, the mesh structure of collagen did not maintain its original form. The collagen mesh structure was shrunken and narrowed with irregular pattern. This change in structure of collagen mesh structure could be due to the absorption of α-tocopherol and/or ethanol effects [Fig. 1(B)]. In case of chitosan gel, porous, rough, and dense structure on the surface of the gel was achieved [Fig. 1(C)]. After the loading procedure of α-tocopherol, the surface structure of chitosan gel was relatively sparse and coarse and crumbled into pieces [Fig. 1(D)].
Fig. 1.
SEM of collagen mesh structure (A), collagen mesh structure loaded with α-tocopherol (B), chitosan gel (C), and chitosan gel loaded with α-tocopherol (D)
Oxidative stability in bulk corn oil
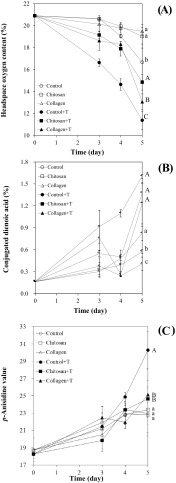
Changes in headspace oxygen content (A), CDA (B), and p-AV (C) in corn oil for chitosan and collagen with or without loading of α-tocopherol at 60 °C are shown in Fig. 2. As storage time was increased to 5 days, headspace oxygen contents over control samples were decreased from 20.9 to 17.0%. The samples containing collagen mesh or chitosan gel showed significantly higher headspace oxygen content than the controls (p < 0.05), indicating that the added carriers retarded the consumption of headspace oxygen molecules by unsaturated lipids.
Fig. 2.
Changes in headspace oxygen content (A), CDA (B), and p-AV (C) in corn oil containing chitosan or collagen loaded with α-tocopherol at 60 °C. Different capital or small letters are significantly different at 0.05 with α-tocopherol added samples or without α-tocopherol added samples, respectively. ‘Control’, ‘Chitosan’, and ‘Collagen’ were oil samples without addition of α-tocopherol nor carriers, with added chitosan gel, and with added collagen mesh structure, respectively. ‘Control + T’, ‘Chitosan + T’, and ‘Collagen + T’ were oil samples with addition of α-tocopherol, with α-tocopherol loaded chitosan gel, and with α-tocopherol loaded collagen mesh structure, respectively
Controls with α-tocopherol (Control + T) had significantly lower headspace oxygen content than controls without α-tocopherol (Control), indicating that the added α-tocopherol significantly accelerated the consumption of oxygen molecules (p < 0.05). Samples of chitosan gel loaded with α-tocopherol had higher headspace oxygen content than those of collagen loaded with α-tocopherol [Fig. 2(A)]. Therefore, oxidative stability in Chitosan + T was higher than that in Collagen + T based on the consumption of headspace oxygen content.
Results of CDA values indicating the degree of primary oxidation products showed similar trend with those of headspace oxygen contents. Control samples had significantly (p < 0.05) lower CDA values than corresponding samples containing carriers irrespective of α-tocopherol loading. However, samples containing collagen showed the lowest CDA values followed by those containing chitosan gel [Fig. 2(B)], which was slightly different from the results obtained for headspace oxygen content. p-AV (representing the contents of secondary oxidation products) in control samples with α-tocopherol was significantly higher than other samples loaded with α-tocopherol (p < 0.05). However, those samples without the addition of carriers were not significantly (p > 0.05) different from each other [Fig. 2(C)]. Depending on the types of assays, different results were observed. Lipid oxidation, especially autoxidation, has the following three steps: initiation, propagation, and termination. Each assay has limited validity due to difference in target compounds and principles. A combination of assays has been recommended to cover different degrees of oxidation in samples [1, 19, 25].
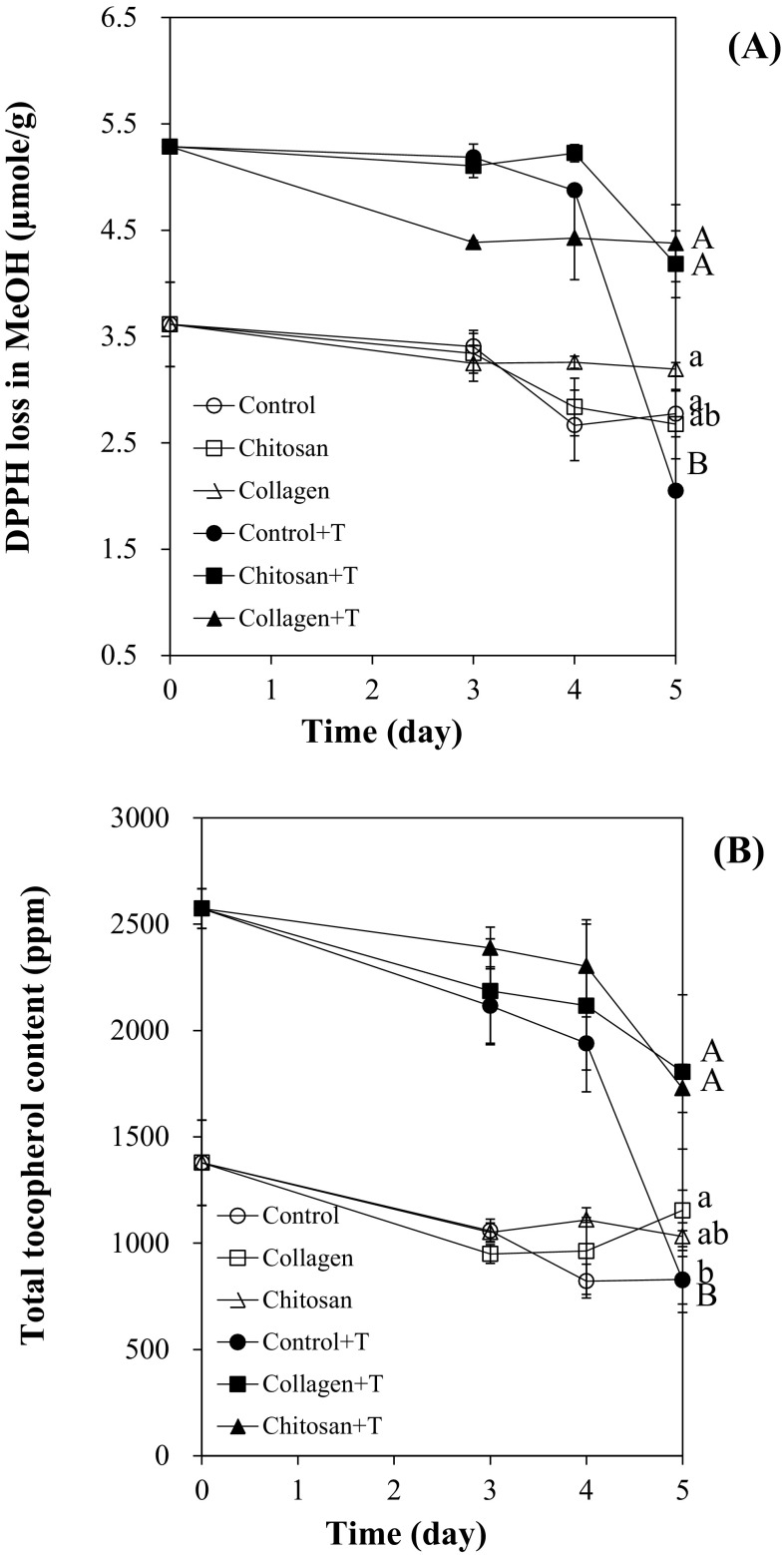
Changes in DPPH loss in methanol (A) and total tocopherol contents (B) in corn oil containing collagen mesh structure or chitosan gel with α-tocopherol at 60 °C are shown in Fig. 3. DPPH loss in oils with α-tocopherol was decreased significantly after 5 days of storage. It was lower than that in oils with α-tocopherol loaded polymers (p < 0.05). High concentration of antioxidants can react with high concentration of DPPH radicals. The results of DPPH loss indicated that the oil containing α-tocopherol loaded polymers had more remained antioxidants than α-tocopherol containing oil without polymers. The DPPH loss is a concentration of DPPH radicals reacted by antioxidants and it should increase when the concentration of antioxidant is high. Song et al. [23] reported that correlation between DPPH loss in methanol and the contents of antioxidants such as TBHQ, sesamol, and α-tocopherol in the thermally oxidized bulk oils was high.
Fig. 3.
DPPH loss in methanol (A) and total tocopherol contents (B) in corn oil containing chitosan or collagen loaded with α-tocopherol during 60 °C oxidation. Different capital or small letters are significantly different at 0.05 with α-tocopherol added samples or without α-tocopherol added samples, respectively. Abbreviations were listed in the captions of Fig. 2
Total tocopherol contents in control samples without added polymers were significantly (p < 0.05) lower than those in samples with carriers after 5 days of storage [Fig. 3(B)]. This trend was clearly observed in samples with carriers loaded with α-tocopherol. Samples with α-tocopherol added underwent higher degradation of α-tocopherol than those without the addition of α-tocopherol after 5 days.
Changes in α-tocopherols (ppm) in corn oil with chitosan and collagen loaded with α-tocopherol at 60 °C are shown in Table 1. Contents of α-tocopherols in control samples were decreased gradually during 5 days of storage at 60 °C. Added chitosan gel and collagen mesh structures significantly (p < 0.05) enhanced the remained tocopherol content. There was no significant (p > 0.05) difference in the content of α-tocopherol between chitosan gel and collagen mesh structures.
Table 1.
Changes of α-tocopherol (ppm) in corn oils with chitosan and collagen loaded with α-tocopherol at 60 °C storage
| Oxidation time (day) | ||||
|---|---|---|---|---|
| 0 | 3 | 4 | 5 | |
| Controla | 222 ± 7bac | 200 ± 11a | 107 ± 11b | 67 ± 40b |
| Chitosan | 222 ± 7a | 207 ± 49a | 194 ± 5a | 138 ± 11a |
| Collagen | 222 ± 7a | 209 ± 24a | 204 ± 5a | 173 ± 13a |
| Control + T | 1747 ± 66A | 1336 ± 176A | 1117 ± 83A | 115 ± 30B |
| Chitosan + T | 1747 ± 66A | 1528 ± 145A | 1405 ± 173A | 877 ± 95A |
| Collagen + T | 1747 ± 66A | 1413 ± 266A | 1232 ± 390A | 842 ± 339A |
a‘Control’, ‘Chitosan’, and ‘Collagen’ were oil samples without addition of α-tocopherol nor carriers, with chitosan gel, and with collagen mesh structure, respectively. ‘Control + T’, ‘Chitosan + T’, and ‘Collagen + T’ were oil samples with addition of α-tocopherol, with α-tocopherol loaded chitosan gel, and with α-tocopherol loaded collagen mesh structure, respectively
bMean ± standard deviation (n = 3)
cDifferent letters indicated significant differences in the same day at 0.05 in the same column
The stability of α-tocopherol has been reported to be much lower than that of other tocopherols and it has been reported that α-tocopherol disappears sooner than other tocopherol homologs [26]. The lower bond dissociation enthalpy value and standard one electron reduction potential of α-tocopherol which are 75.8 kcal/mol and 270 mV, respectively, might explain the low stability of α-tocopherol [27]. The bond dissociation enthalpy value and standard one electron reduction potential of γ-Tocopherol are 78.2 kcal/mol and 350 mV, respectively, which are higher than those of α-tocopherol [27]. Added carriers somehow protected the loss of α-tocopherol from oxidative stress compared to control oils with α-tocopherols. Gim et al. [16] reported that addition of β-cyclodextrin and collagen could enhance the stability of tocopherols while chitosan did not show such effects. This could be due to the limitation of diffusion of molecular oxygens or other factors such as physical location.
Moisture contents in corn oils with chitosan and collagen with α-tocopherol at 60 °C are shown in Table 2. The moisture content in control was increased after 5 days of storage while that in sample containing α-tocopherol was significantly decreased (p < 0.05) after 5 days of storage. Addition of chitosan or collagen in oils did not significantly (p > 0.05) change the moisture content compared to their corresponding controls during storage. The decrease in the moisture content in oils containing carriers were also observed in heated oils containing β-cyclodextrin and chitosan [16]. Reduced moisture content in oils may affect the rates of lipid oxidation in bulk oils.
Table 2.
Moisture contents (ppm) in corn oils with chitosan and collagen loaded with α-tocopherol at 60 °C
| Samples | Oxidation time (day) | |||
|---|---|---|---|---|
| 0 | 3 | 4 | 5 | |
| Controla | 446 ± 3bac | 601 ± 46abc | 521 ± 68c | 801 ± 66a |
| Chitosan | 446 ± 3a | 575 ± 14bc | 694 ± 94ab | 771 ± 136ab |
| Collagen | 446 ± 3a | 675 ± 16ab | 766 ± 43a | 843 ± 59a |
| Control + T | 446 ± 3a | 495 ± 98c | 560 ± 72bc | 556 ± 50c |
| Chitosan + T | 446 ± 3a | 587 ± 60bc | 660 ± 72ab | 659 ± 34bc |
| Collagen + T | 446 ± 3a | 707 ± 71a | 690 ± 72ab | 561 ± 69c |
a‘Control’, ‘Chitosan’, and ‘Collagen’ were oil samples without addition of α-tocopherol nor carriers, with chitosan gel, and with collagen mesh structure, respectively. ‘Control + T’, ‘Chitosan + T’, and ‘Collagen + T’ were oil samples with addition of α-tocopherol, with α-tocopherol loaded chitosan gel, and with α-tocopherol loaded collagen mesh structure, respectively
bMean ± standard deviation (n = 3)
cDifferent letters indicated significant differences in the same day at 0.05 in the same column
Oxidative stability in O/W emulsions
Changes in headspace oxygen content (A), lipid hydroperoxides (B), and volatiles (C) in O/W emulsions containing chitosan or collagen with loaded α-tocopherol at 60 °C are shown in Fig. 4. Headspace oxygen contents in control O/W emulsions were significantly (p < 0.05) lower than those in samples containing carriers irrespective of α-tocopherol loading after 4 days of storage. Interestingly, O/W emulsions containing α-tocopherol had significantly higher headspace oxygen contents than those without α-tocopherol, which was different compared to bulk corn oil results. Samples with collagen had the highest headspace oxygen content, followed by samples with chitosan [Fig. 4(A)]. Also, oils with α-tocopherol loaded collagen had significantly higher headspace oxygen content than oils with α-tocopherol loaded chitosan.
Fig. 4.
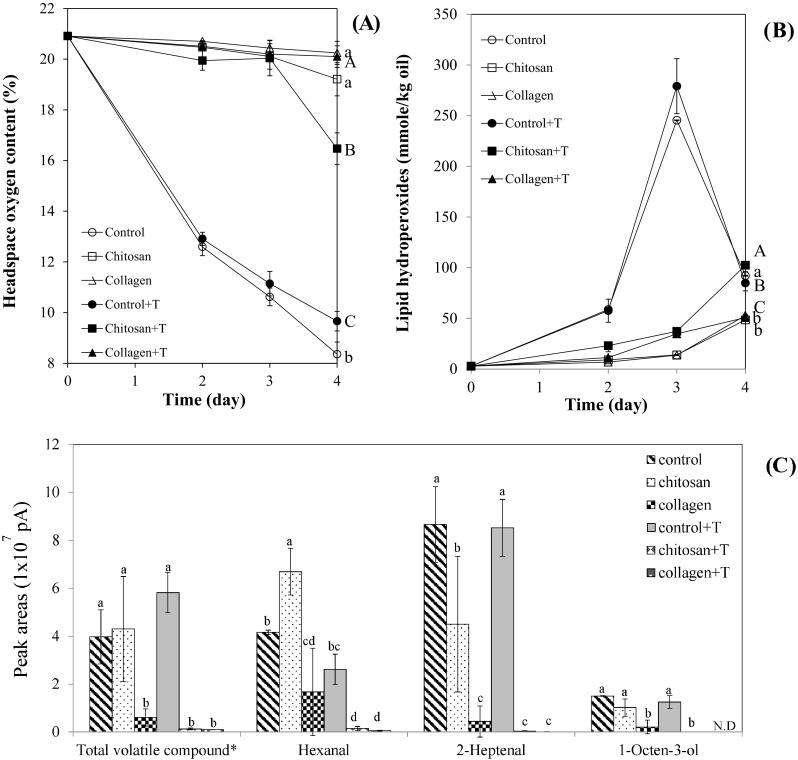
Changes in headspace oxygen content (A), lipid hydroperoxides (B), and volatiles (C) in oil-in-water emulsion containing chitosan or collagen loaded with α-tocopherol at 60 °C treatment. Different capital or small letters are significantly different at 0.05 with α-tocopherol added samples or without α-tocopherol added samples, respectively. Abbreviations were listed in the captions of Fig. 2. *The unit of total volatile compound is 1 × 108 pA
In the results of lipid hydroperoxides, the addition of collagen and chitosan showed strong antioxidant properties. The sample containing collagen had lower lipid hydroperoxide value than the sample containing chitosan [Fig. 4(B)].
Volatiles are secondary oxidation products from lipid oxidation. They are useful indicators for determining the degree of oxidation in O/W emulsion [25, 28]. Addition of α-tocopherol in controls did not show significant (p > 0.05) decrease in total volatiles in O/W emulsion controls. Total volatiles in oils with collagen were significantly (p < 0.05) lower than those of controls and samples with chitosan. However, total volatiles in oils with α-tocopherol loaded collagen or α-tocopherol loaded chitosan were significantly (p < 0.05) lower than those of controls with α-tocopherol. This trend can be observed in the changes of selected volatiles including 1-octen-3-ol and 2-heptetenal which are commonly detected in the oxidation of linoleic acid [25]. Hexanal, a typical oxidation volatile from linoleic acid oxidation, showed a little different pattern in samples without α-tocopherol loading. However, in samples with α-tocopherol added, emulsion containing carriers showed significantly (p < 0.05) lower hexanal content than controls. Overall, the addition of collagen significantly reduced the rates of lipid oxidation in O/W emulsion. Especially, α-tocopherol loaded chitosan or collagen greatly reduced the formation of volatiles in O/W emulsion at 60 °C storage condition. Antioxidant properties of chitosan has been utilized coating materials in liposome [29].
In bulk oil systems, carriers itself and carriers loaded with α-tocopherol had higher protection ability against headspace oxygen consumption. They inhibited the formation of primary and secondary oxidation products. It is known that α-tocopherol can efficiently donate its hydrogen atom to the peroxyl radical (LOO·), resulting in the formation of more lipid hydroperoxides [1]. Interestingly, adding α-tocopherol accelerated the rates of lipid oxidation. This could be due to the over production of radicals from tocopherols. It is well-known that tocopherol has optimum concentration. Once over such concentration, tocopherol can act as a prooxidant [30].
Contents of moisture could play pivotal roles in the rates of lipid oxidation because interfaces between oil and moisture are believed to be major places for lipid oxidation and action of antioxidants. Moisture may incorporate with diverse amphiphilic compounds (including free fatty acids, monoacylglycerols, diacylglycerols, phospholipids, and moisture) to form association colloids or supramolecular structures [2, 31]. Also, moisture content in bulk oils regulated by relative humidity from saturated salt solution can greatly influence the stability of antioxidant compounds, including lipophilic tocopherols [24] and hydrophilic ascorbic acid [32]. To act as efficient antioxidants, it is necessary to position the chemical compounds near these interfaces of oil and water. In addition, the reactivity of transition metals should be considered to understand the antioxidant role of tocopherol homologs [33, 34]. The concentration of cupric ion is related to the decomposition of α-tocopherol [33]. It is known that α-tocopherol can reduce ferric ion (Fe3+) into ferrous ion (Fe2+) and ferrous ion can accelerate the decomposition of lipid hydroperoxides rapidly [35]. Addition of carriers with shapes like mesh structures may hinder the migration of metal ions and limit the prooxidative action of transition metals, which will make more tocopherol contents in oils. On the other hand, adding α-tocopherol without polymers may not be able to protect against oxidative stress such as attacks of transition metals and increased decomposition rates of α-tocopherol. Other possibilities of low stability of α-tocopherol in oils without carriers might come from the involvement of α-tocopherol in supramolecular structures with moisture based on the decrease in moisture content compared to control oils (Table 2). However, further studies are needed to confirm this possibility.
In conclusion, collagen mesh structures and chitosan gel were designed to deliver α-tocopherol and their antioxidant properties were tested in bulk corn oil and O/W emulsion at 60 °C. Collagen mesh structures and chitosan gel loaded with α-tocopherol provided greatly enhanced the oxidative stability. Also, carriers showed antioxidant properties in both food matrices when α-tocopherol was not loaded. In this study, the applicability of collagen mesh structures and chitosan gel as carriers for liphophilic antioxidants was confirmed in both bulk oil and O/W emulsion systems. Current study can help to extend the oxidative stability in foods with diverse matrix using carriers as antioxidant carriers.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF-2014R1A2A1A11050047) and (NRF-2017R1A2B4002613) funded by the Ministry of Education, Science and Technology.
References
- 1.Decker EA, Warner K, Richards MP, Shahidi E. Measuring antioxidant effectiveness in food. J. Agr. Food Chem. 2005;53:4303–4310. doi: 10.1021/jf058012x. [DOI] [PubMed] [Google Scholar]
- 2.Chaiyasit W, Elias RJ, McClements DJ, Decker EA. Role of physical structures in bulk oils on lipid oxidation. Crit. Rev. Food Sci. 2007;47:299–317. doi: 10.1080/10408390600754248. [DOI] [PubMed] [Google Scholar]
- 3.Choe E, Min DB. Chemistry of deep-fat frying oils. J. Food Sci. 2007;72:77–86. doi: 10.1111/j.1750-3841.2007.00352.x. [DOI] [PubMed] [Google Scholar]
- 4.McClements DJ, Decker EA. Lipid oxidation in oil-in water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000;65:1270–1282. doi: 10.1111/j.1365-2621.2000.tb10596.x. [DOI] [Google Scholar]
- 5.Laguerre M, Bayrasy C, Panya A, Weiss J, McClements DJ, Lecomte J, Decker EA, Villeneuve P. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit. Rev. Food Sci. 2015;55:183–201. doi: 10.1080/10408398.2011.650335. [DOI] [PubMed] [Google Scholar]
- 6.Balanč B, Kalušević A, Drvenica I, Coelho MT, Djordjević V, Alves VD, Sousa I, Moldão-Martins M, Rakić V, Nedović V, Bugarski B. Calcium-alginate-inulin microbeads as carriers for aqueous carqueja extract. J. Food Sci. 2016;81:E65–E75. doi: 10.1111/1750-3841.13167. [DOI] [PubMed] [Google Scholar]
- 7.Xie M, Hu B, Wang Y, Zeng X. Grafting of gallic acid onto chitosan enhances antioxidant activities and alters rheological properties of the copolymer. J Agr. Food Chem. 2014;62:9128–9136. doi: 10.1021/jf503207s. [DOI] [PubMed] [Google Scholar]
- 8.Gómez-Mascaraque LG, López-Rubio A. Protein-based emulsion electrosprayed micro- and submicroparticles for the encapsulation and stabilization of thermosensitive hydrophobic bioactives. J. Colloid Interf. Sci. 2016;465:259–270. doi: 10.1016/j.jcis.2015.11.061. [DOI] [PubMed] [Google Scholar]
- 9.Pal GK, Suresh PV. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. 2016;37:201–215. doi: 10.1016/j.ifset.2016.03.015. [DOI] [Google Scholar]
- 10.Waszkowiak K, Dolata W. The application of collagen preparations as carriers of rosemary extract in the production of processed meat. Meat Sci. 2007;75:178–183. doi: 10.1016/j.meatsci.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Koide SS. Chitin-chitosna:Properites, benefits and risks. Nutr. Res. 1998;18:1091–1101. doi: 10.1016/S0271-5317(98)00091-8. [DOI] [Google Scholar]
- 12.Yen MT, Yang JH, Mau JL. Antioxidant properties of chitosan from crab shells. Carbohyd. Polym. 2008;74:840–844. doi: 10.1016/j.carbpol.2008.05.003. [DOI] [Google Scholar]
- 13.Wan A, Xu Q, Sun Y, Li H. Antioxidant activity of high molecular weight chitosan and N. O-quaternized chitosans. J. Agr. Food Chem. 2013;61:6921–6928. doi: 10.1021/jf402242e. [DOI] [PubMed] [Google Scholar]
- 14.Qin Y. The chelating properties of chitosan fibers. J. Appl. Polym. Sci. 1993;49:727–731. doi: 10.1002/app.1993.070490418. [DOI] [Google Scholar]
- 15.Cho YS, Kim SK, Ahn CB, Je JY. Preparation, characterization, and antioxidant properties of gallic acid-grafted-chitosans. Carbohyd. Polym. 2011;83:1617–1622. doi: 10.1016/j.carbpol.2010.10.019. [DOI] [Google Scholar]
- 16.Gim SY, Kwon YJ, Kim MJ, Kim GH, Lee JH. Addition of β-cyclodextrin, chitosan, and collagen on the enhancement of tocopherol stability in heated oil at frying temperature. Eur. J. Lipid Sci. Tech. 10.1002/ejlt.201700124 (2017)
- 17.Gim SY, Hong SM, Kim JS, Kwon YJ, Kim MJ, Lee JH. Enhancing oxidative stability in oils using core/shell structures of collagen and α-tocopherol complex. Food Chem. 2017;235:160–166. doi: 10.1016/j.foodchem.2017.05.057. [DOI] [PubMed] [Google Scholar]
- 18.Ahn SH, Koh YH, Kim GH. A three-dimensional hierarchical collagen scaffold fabricated by a combined solid freeform fabrication (SFF) and electrospinning process to enhance mesenchymal stem cell (MSC) proliferation. J. Micromech. Microeng. 2010;20:065015. doi: 10.1088/0960-1317/20/6/065015. [DOI] [Google Scholar]
- 19.Yi BR, Ka HJ, Kim MJ, Lee JH. Effects of curcumin on the oxidative stability of oils depending on type of matrix, photosensitizers, and temperature. J. Am. Oil Chem. Soc. 2015;92:685–691. doi: 10.1007/s11746-015-2639-y. [DOI] [Google Scholar]
- 20.Kim JY, Yi BR, Kim MJ, Lee JH. Oxidative stability of solid fats containing ethylcellulose determined based on the headspace oxygen content. Food Sci. Biotechnol. 2014;23:1779–1784. doi: 10.1007/s10068-014-0243-9. [DOI] [Google Scholar]
- 21.AOCS. Official methods and recommended practices of the American Oil Chemists’ Society. 4th ed. Urbana, Ill: American Oil Chemists’ Society (2006)
- 22.Ha DO, Yeo JD, Kang ST, Kim MJ, Lee JH. Sodium azide and metal chelator effects on 2,2-diphenyl-1-picrylhydrazyl(DPPH) radical scavenging compounds from methylene blue photosensitized. Eur. J. Lipid Sci. Tech. 2012;114:780–786. doi: 10.1002/ejlt.201100329. [DOI] [Google Scholar]
- 23.Song J, Jang EY, Kim MJ, Lee JH. Development of a spectroscopic method to determine the content of free radical scavenging compounds and oxidation products in thermally oxidized oils. Int. J. Food Sci. Tech. 2016;51:2424–2432. doi: 10.1111/ijfs.13223. [DOI] [Google Scholar]
- 24.Kim JY, Kim MJ, Yi BR, Oh SM, Lee JH. Effects of relative humidity on the antioxidant properties of α-tocopherol in stripped corn oil. Food Chem. 2015;167:191–196. doi: 10.1016/j.foodchem.2014.06.108. [DOI] [PubMed] [Google Scholar]
- 25.Kim TS, Decker EA, Lee JH. Antioxidant capacities of α-tocopherol, trolox, ascorbic acid, and ascorbyl palmitate in riboflavin photosensitized oil-in-water emulsions. Food Chem. 2012;133:68–75. doi: 10.1016/j.foodchem.2011.12.069. [DOI] [Google Scholar]
- 26.Jung JY, Gim SY, Lee CK, Kim MJ, Lee JH. Effects of moisture content and presence of γ-tocopherol on the stability of α-tocopherol in stripped corn oils. Eur. J. Lipid Sci. Tech. 2016;118:1926–1934. doi: 10.1002/ejlt.201500554. [DOI] [Google Scholar]
- 27.Kim HJ, Lee HO, Min DB. Effects and prooxidant mechanisms of oxidized α-tocopherol on the oxidative stability of soybean oil. J. Food Sci. 2007;72:C223–C230. doi: 10.1111/j.1750-3841.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Decker EA. Effects of metal chelators, sodium azide, and superoxide dismutase (SOD) on the oxidative stability in riboflavin photosensitized O/W emulsion systems. J. Agr. Food Chem. 2011;59:6271–6276. doi: 10.1021/jf2001537. [DOI] [PubMed] [Google Scholar]
- 29.Panya A, Laguerre M, Lecomte J, Villeneuve P, Weiss J, McClements JD, Decker EA. Effects of chitosan and rosmarinate esters on the physical and oxidative stability of liposomes. J. Agr. Food Chem. 2010;58:5679–5684. doi: 10.1021/jf100133b. [DOI] [PubMed] [Google Scholar]
- 30.Cillard J, Cillard P, Cormier M. Effect of experimental factors on the prooxidant behavior of e-tocopherol. J. Am. Oil Chem. Soc. 1980;57:255–261. doi: 10.1007/BF02668255. [DOI] [Google Scholar]
- 31.Budilarto ES, Kamal-Eldin A. The supramolecular chemistry of lipid oxidation and antioxidation in bulk oils. Eur. J. Lipid Sci. Tech. 2015;117:1095–1137. doi: 10.1002/ejlt.201400200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JY, Kim MJ, Yi BR, Oh SM, Lee JH. Antioxidant properties of ascorbic acid in bulk oils at different relative humidity. Food Chem. 2015;176:302–307. doi: 10.1016/j.foodchem.2014.12.079. [DOI] [PubMed] [Google Scholar]
- 33.Chen B, McClements DJ, Decker EA. Minor components in food oils: a critical review of their roles on lipid oxidation chemistry in bulk oils and emulsions. Crit. Rev. Food Sci. 2011;51:901–916. doi: 10.1080/10408398.2011.606379. [DOI] [PubMed] [Google Scholar]
- 34.Chen B, Panya A, McClements DJ, Decker EA. New insights into the role of iron in the promotion of lipid oxidation in bulk oils containing reverse micelles. J. Agr. Food Chem. 2012;60:3524–3552. doi: 10.1021/jf300138h. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto K, Niki E. Interaction of alpha-tocopherol with iron: antioxidant and prooxidant effects of alpha-tocopherol in the oxidation of lipids in aqueous dispersions in the presence of iron. Biochem. Biophys. Acta. 1988;958:19–23. doi: 10.1016/0005-2760(88)90241-X. [DOI] [PubMed] [Google Scholar]