Abstract
In this study, we investigated the anti-atopic dermatitis (AD) activity of β-glucans derived from Aureobasidium pullulans SM-2001 (βGdAP). βGdAP was orally administered to AD animal models such as vasodilation, allergic pruritus and contact dermatitis. Administration of βGdAP attenuated the amount of Evans blue solution on vasodilation rat. Scratching behaviors, secretion of histamine and ear thickness were significantly (p < 0.05) attenuated in the βGdAP-treated mouse groups. Interestingly, transcriptional expression of T-bet, a transcription factor for Th1 reactions, was increased, but that of GATA-3, a transcription factor for Th2 reactions, was attenuated in the βGdAP-treated groups (p < 0.05). In addition, we found that reduced transcriptional expression of forkhead box P3 and galectin-9, regulators of regulatory T cells, was recovered in the βGdAP-treated groups (p < 0.05). Taken together, these data indicate that administration of βGdAP could effectively attenuate AD-like phenotypes via regulation of Th1/Th2 transcriptional activity and Treg activation.
Keywords: β-Glucan, Aureobasidium pullulans SM-2001, Atopic dermatitis, Th1/Th2, FOXP3, Galectin-9
Introduction
Atopic dermatitis (AD) is a chronic relapsing disease that is characterized by xeroderma, pruritus, and inflammation [1]. Although the detailed mechanisms of AD disease are still not clear, various factors such as air pollution, allergens in housing environment, and stress have been suggested as critical factors that exacerbating AD symptoms [2]. From the immunological perspective, AD is induced by an imbalance of Th1/Th2 cells that is caused by inhibition of the proliferation and differentiation of Th1 cells along with the release of diverse Th2 cytokines such as interleukin-4 (IL-4), IL-5, and IL-13 [3, 4]. In addition, regulatory T cells (Tregs) that are positive for foxhead box P3 (FOXP3) have been reported to play critical roles in immunosuppressive function [5]. FOXP3 is a well-known transcription factor that is associated with the activation of Tregs [6]. Moreover, expression of galectin-9 in intestinal epithelial cells has been reported to play an immunomodulatory role in Treg polarization [7]. Therefore, agents that activate FOXP3 and galectin-9 expression may be useful for alleviating the symptoms of AD.
AD is primarily treated with local steroids including calcineurin inhibitors, antihistamines, γ-linolenic acid, cyclosporine, and interferon gamma (IFN-γ). However, these therapies often result in relapse when discontinued, or cause side effects when used for a long time [8]. Therefore, there is growing interest in natural remedies for AD that can regulate inflammation-related cytokine expression and have minimal side effects. One such compound that has attracted attention is β-glucan, which is a biologically active polysaccharide present in the cell wall of yeasts, mushrooms, cereals, algae, and a few bacteria [9–11]. β-Glucan can activate the immune functions of normal human cellular tissues to inhibit the proliferation of cancer cells, prevent relapse of cancer, and promote the production of IFN-γ and IL, which are known to indirectly inhibit the proliferation of cancer cells [12, 13]. β-Glucan has been reported to have role in innate immune responses through activating B-lymphocytes [14]. β-Glucan has also been found to have anti-hyperlipidemic effects [15], anti-obesity effects via inhibition of the formation and accumulation of body fat [16], anti-oxidative activity [17], and arthritis-alleviating effects [18]; thus, it can be used as a health food ingredient and a food additive.
The exopolymer of Aureobasidium pullulans SM-2001 is mainly composed of β-1,3- and β-1,6-glucans and has been reported to have anti-osteoporotic effects [19] and inhibitory effects against UVB-induced skin damage [20]. However, despite there being evidence for the diverse effects of β-glucans derived from A. pullulans SM-2001 (βGdAP), there is not enough information about its immunomodulatory effects in atopic dermatitis disease models. Therefore, in the present study, several AD-like phenotypes were induced in rats and mice, and βGdAP was orally administered in order to investigate its immunomodulatory effects.
Materials and methods
Reagents
The compound 48/80 (COM), Evans blue solution, dinitrofluorobenzene (DNFB), and aluminum hydroxide gel were obtained from Sigma-Aldrich (CA, USA), and dinitrophenyl-derivatized ovalbumin (DNP-OVA) were purchased from Alpha diagnostic Intl. Inc., USA.
Animals and treatment
Six-week-old Sprague–Dawley (SD) male rats from Samtako (Osan, Korea) and six-week-old male ddY mice from Central Lab. Animal Inc. (Seoul, Korea) were purchased for the experiment. During the acclimation and experimental periods, a room temperature of 22 ± 1°C, humidity of 60 ± 10%, and a 12-h light/12-h dark cycle were maintained. The animals were given free access to pellet-type solid feed AIN-76A (Choongang Co., Seoul, Korea) and water. After an acclimation period of 1 week, the experimental animals were divided into six groups of ten animals each: (1) Control group (C: normal diet), (2) Negative control group (N: normal diet + AD induction), (3) Positive control group (P: normal diet + 0.2 mg/kg body weight of Zyrtec + AD induction), (iv) βGdAP treatment group 1 (T1: normal diet + 1 mg/kg body weight of βGdAP + AD induction), (v) βGdAP treatment group 2 (T2: normal diet + 10 mg/kg body weight of βGdAP + AD induction), and (vi) βGdAP treatment group 3 (T3: normal diet + 20 mg/kg body weight of βGdAP + AD induction). Zyrtec (cetirizine) (UCB FARCHIM S.A., Switzerland) was used to attenuate the AD-like symptoms. Macromolecular βGdAP polymers externally secreted from the black yeast strain A. pullulans SM-2001 with an average molecular weight of 2.6 × 105 Da were obtained from Glucan Co. (Seoul, Korea) [21]. The doses of βGdAP, which used in this study, were determined by considering the guideline for adults from Glucan Co. (250 mg/60 kg body weight). The purity of the β-1,3- and β-1,6-glucan used in the present experiment was 15%. The animal experiment was performed with the approval of the Institutional Animal Care Board of Gyeongnam National University of Science and Technology (Jinju, Korea) (Approval No. 2014-04).
Histamine-induced vasodilation
The compound 48/80 (COM), which promotes histamine secretion, was used to induce the AD-like phenotype in the SD rats according to the method of Ishiguro et al. [22]. Briefly, Zyrtec and βGdAP were orally administered to the positive control (P) and βGdAP groups (T1, T2, and T3) respectively. At 1 h after Zyrtec and βGdAP administration, COM (10 μg/mL) at a dose of 50 μl was intra-dermally injected into the shaved dorsal dermis of rats once every 7 days. At 30 min after the COM injection, 200 μl of Evans blue solution was injected into the tail vein, and all the animals were sacrificed 30 min later. The dorsal dermis was cut out 30 min later to measure the diameters of the spots (in millimeters) representing extravasation of the blue dye due to vasodilation, using a digital caliper (Bluebird, NA500-150S). To measure the amount of leaked dye, the dorsal dermis of each animal was cut in a 10-mm diameter circle, and the cut pieces were kept in a 1.0 N KOH (1.0 mL) solution at 37°C for 48 h to initiate the reaction. Then, 0.6 N H3PO4 (2.5 mL) and acetone (6.5 mL) were added to the reaction mixture, which was shaken and centrifuged at 900×g for 10 min. The absorbance of the supernatant was determined at 620 nm by spectrophotometric analysis (iMAX microplate reader, Biorad co., Korea) to measure the amount of blue dye that had leaked in milligrams per site. To measure the level of serum histamine, the animals were sacrificed at 30 min after the administration of Evans blue, and blood samples taken from the vena cava were centrifuged for 15 min at 1300×g to separate the serum. The level of histamine in the separated serum was measured using Histamine EIA Kits (LDN, Germany).
Mouse model of allergic pruritus
The histamine secretion promoter COM (3 mg/kg body weight) was subcutaneously injected into the shaved dorsal dermis of ddY mice to induce the AD-like phenotype. Zyrtec and βGdAP were orally administered once a day for 7 days. At 30 min after COM administration, the number of reactions (expressed in the form of pruritus) in the entire body was counted for 20 min. When the animals were sacrificed 30 min after Evans blue administration, blood samples taken from the vena cava were centrifuged for 15 min at 1300×g to separate the serum. The level of histamine in the separated serum was measured using Histamine EIA Kits (LDN, Germany).
Mouse model of contact dermatitis
To induce contact dermatitis, which is an AD-like phenotype, the ddY mice were sensitized by intraperitoneal injection of 0.2 mL of saline solution containing 10 μg DNP-OVA and 1 mg of aluminum hydroxide gel. Seven days after sensitization, 10 μl of 0.1% 2,4-DNFB dissolved in ethanol was applied to the ear and paw regions. Ear thickness was measured before DNFB application and 1 h and 24 h after DNFB application using a digital caliper (Bluebird, NA500-150S). Immediately after the DNFB application, the number of reactions (in the form of pruritus) on the entire body within a span of 1 h was counted according to a previously reported method [23].
RNA isolation and analysis
To analyze the immunomodulatory effects of βGdAP, rats with vasodilation were sacrificed, and their mesenteric lymph nodes were resected. The separated mesenteric lymph nodes were placed in Trizol® Reagent (Ambion, CA, USA) and homogenized using SilentCrusher M (Heiodlph, Schwabach, Germany). RNA was isolated using the total RNA isolation kit (Intronbio Co., Sungnam, Korea) and stored at − 20°C until use for cDNA synthesis (TaKaRa Co., Tokyo, Japan). Quantitation was performed using a CFX connect real-time system (Bio-Rad Co., CA, USA) with the SYBR Green real-time master mix (Toyobo Co., Tokyo, Japan). Relative expression of each gene was determined by using the comparative Ct method and normalized to the expression of GAPDH. The sequences of the T-bet primers are 5′-CCACCCAGAAGACTGTGGAT-3′ (forward) and 5′-CACATTGGGGGTAGGAACAC-3′ (reverse); GATA-3 primers, 5′-CATTACCACCTATCCGCCCTATG-3′ (forward) and 5′-CACACACTCCCTGCCTTCTGT-3′ (reverse); FOXP3 primers, 5′-CCCATCCCCAGGAGTCTTG-3′ (forward) and 5′-CCATGACTAGGGGCACTGTA-3′ (reverse); galectin-9 primers, 5′-GAGAGGAAGACACACATGCCTTTC-3′ (forward) and 5′-GACCACAGCATTCTCATCAAAACG-3′ (reverse); GAPDH primers, 5′-CCACCCAGAAGACTGTGGAT-3′ (forward) and 5′-CACATTGGGGGTAGGAACAC-3′ (reverse).
Statistical analysis
The variance of the data obtained through repeated experiments was analyzed using SPSS 12.0 (SPSS Inc., IL, USA), and the results are represented as the mean ± standard deviation values. The significance was determined by analysis of variance followed by Duncan’s multiple range test at the level of p < 0.05.
Results and discussion
Effects of βGdAP on COM-induced vasodilation
To analyze the effects of βGdAP on vasodilation, an AD-like phenotype, rats in which vasodilation was induced were orally administered βGdAP (one group of control rats was not administered βGdAP). The degree of vasodilation was determined by measuring the size of the spots containing leaked Evans blue. As shown in Fig. 1(A), there was no dye leakage in the control group (C), and the largest blue-dye spots (largest diameter, 5.31 mm) were observed in the COM-treated groups (N). The diameter of the spots significantly (p < 0.05) decreased on Zyrtec administration in the positive control groups. Among the βGdAP groups, T1 had the smallest blue-dye spot diameter of 3.81 mm, which indicates the lowest degree of vasodilation and therefore the greatest degree of inhibition against vasodilation. To determine the amount of Evans blue dye that had leaked, the dorsal dermis of the rats was resected and dissolved in an alkaline solution, and the absorbance of the supernatant was determined through spectrophotometric analysis. The positive control group (P) had the lowest amount of dye (0.024 mg/site), while the negative control groups (N) had the highest amount of dye (0.248 mg/site) and exhibited remarkable AD-like symptoms [Fig. 1(B)]. Histamine, which is mainly secreted by mast cells and basophilic cells, is the main factor that causes vasodilation in AD. To further investigate the effects of βGdAP on vasodilation, the concentration of serum histamine was measured in each group. As shown in Fig. 1(C), the COM-treated negative control group (N) showed the highest level of serum histamine (28.09 ng/mL), and the positive control (P) and βGdAP groups (T1, T2, and T3) showed lower levels of serum histamine than the N group (p < 0.05). In an experiment conducted using type I dermatitis models with passive cutaneous anaphylaxis, it was reported that remarkable extravasation of the blue dye due to vasodilation occurred 30 min after exposure to the antigen [24]. The significant decreases in the diameter of the leaked dye spots and the amount of leaked blue dye shown in the present experiment can be considered as direct evidence of the effects of βGdAP on the inhibition of vasodilation, which represents the acute inflammatory changes caused by AD. Taken together, these data indicate that administration of βGdAP was effective for attenuating vasodilation, an AD-like phenotype.
Fig. 1.

Effects of βGdAP on vasodilation induced by COM. Administration of βGdAP significantly attenuated vasodilation induced by COM, as evident from (A) the diameter of the blue-dye spots and (B) the amount of leaked dye in mice from the different groups. (C) Serum histamine level in rats with vasodilation induced by COM. C Control, N Negative control (COM-induced AD), P Positive control (COM-induced AD + Zyrtec), T1 COM-induced AD + 1 mg/kg body weight of βGdAP, T2 COM-induced AD + 10 mg/kg body weight of βGdAP, T3 COM-induced AD + 20 mg/kg body weight of βGdAP. a–dSignificant difference at p < 0.05. Data represent the mean ± SD values from five replicates
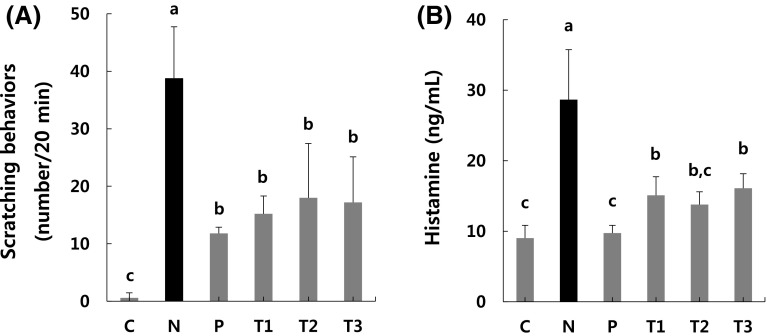
Effects of βGdAP on allergic pruritus
To further confirm the effects of βGdAP on AD-like phenotypes, scratching behaviors were examined using a mouse model of allergic pruritus. As shown in Fig. 2(A), the COM-treated mouse group (N) showed the most frequent scratching behaviors, while the other mouse groups (P, T1, T2, and T3) showed significantly (p < 0.05) less frequent scratching behaviors than the COM-treated mouse group (N). Figure 2(B) shows the levels of serum histamine, which is a factor that causes allergic pruritus, in the different groups. The N group showed significantly higher levels of histamine than the other groups (p < 0.05). In the case of AD, mast cells sensitized by IgE secrete histamine, and this leads to the development of symptoms such as edema, erythema, and pruritus [25]. In particular, histamine secretion induced by COM leads to edematous changes [26]. In the present experiment, the significant decrease observed in the serum histamine levels in the treatment and P groups compared to the N group was direct evidence of the effects of βGdAP on the inhibition of the series of reactions caused by AD. These findings indicate that βGdAP alleviates inflammation. COM is known to promote histamine secretion from mast cells. In addition, COM promotes histamine secretion to induce scratching behaviors in mice [27]. The decrease in scratching behaviors in AD mice treated with βGdAP indicates that βGdAP has a positive effect on allergic pruritus [28]. In the present experiment, scratching behaviors significantly decreased in the AD group in which βGdAP was orally administered compared to the AD group that was not treated with βGdAP. Therefore, βGdAP appears to have inhibitory effects on COM-induced pruritus. These results indicate that administration of βGdAP can attenuate allergic pruritus, which is an AD-like phenotype.
Fig. 2.
Effects of βGdAP on allergic pruritus induced by COM. Administration of βGdAP significantly attenuated acute pruritus on mice induced by COM, as evident from (A) the frequency of scratching behaviors and (B) the serum histamine level. C Control, N Negative control (COM-induced AD), P Positive control (COM-induced AD + Zyrtec), T1 COM-induced AD + 1 mg/kg body weight of βGdAP, T2 COM-induced AD + 10 mg/kg body weight of βGdAP, T3 COM-induced AD + 20 mg/kg body weight of βGdAP. a–cSignificant differences between means within the same row at p < 0.05. Data represent the mean ± SD values from five replicates
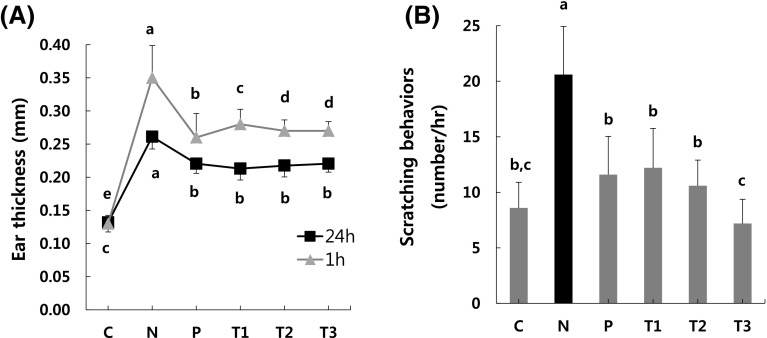
Effects of βGdAP on contact dermatitis
Finally, we tested the effects of βGdAP on AD-like phenotypes, using the DNP-OVA- and DNFB-induced mouse model of contact dermatitis. Ear thicknesses was measured twice, at 1 h and 24 h after DNFB treatment. The ear thicknesses of the DNP-OVA- and DNFB-treated mouse group (N) dramatically increased compared to the control mouse group (C) (p < 0.05). However, it was significantly decreased in the P and βGdAP-treated mouse groups (T1, T2, and T3) (p < 0.05) [Fig. 3(A)]. To investigate pruritus associated with contact dermatitis induced by DNP-OVA and DNFB, signs of pruritus were observed for 1 h after DNFB treatment. In the P and βGdAP-treated groups (T1, T2, and T3), pruritus was significantly (p < 0.05) alleviated compared to the DNFB-treated mouse group (N) [Fig. 3(B)]. When DNFB was repeatedly applied to the ears of the mice, the increase in the level of serum IgE induced an increase in the thickness of the ears. Similarly, it has been reported that repeated DNFB application induces epidermal thickening, scab formation, and inflammatory cell infiltration [29]. DNFB-induced contact dermatitis models are useful for the evaluation of type I allergic dermatitis. When DNFB is applied to the ears of mice sensitized with DNP-OVA, two types of edema are induced: the edema that occurs 1 h after DNFB application is called the immediate-phase response (IPR), and the edema that occurs 24 h after DNFB application is called the late-phase response (LPR) [30]. After DNFB application, scratching behaviors are observed during IPR, which is an inflammatory reaction caused by the chemical mediators released from mast cells. In the present experiment, βGdAP inhibited edema in both IPR and LPR and significantly inhibited scratching behaviors in IPR. Therefore, the present data indicate that administration of βGdAP can alleviate the DNFB-induced contact dermatitis phenotype.
Fig. 3.
Effects of βGdAP on contact dermatitis induced by DNP-OVA and DNFB. Administration of βGdAP significantly attenuated the contact dermatitis phenotype, as evident from (A) ear thickness and (B) the frequency of scratching behaviors in the contact dermatitis mouse model induced by DNP-OVA and DNFB. C Control, N Negative control (COM-induced AD), P Positive control (COM-induced AD + Zyrtec), T1 COM-induced AD + 1 mg/kg body weight of βGdAP, T2 COM-induced AD + 10 mg/kg body weight of βGdAP, T3 COM-induced AD + 20 mg/kg body weight of βGdAP. a–cSignificant differences between means within the same row at p < 0.05. Data represent the mean ± SD values from five replicates
Changes in transcriptional activity in βGdAP-administered rat groups
To investigate the mechanisms through which βGdAP alleviates AD-like phenotypes, the expression of T-bet and GATA-3, as transcription factors of Th1 and Th2 cells, respectively, was examined in the mesenteric lymph node tissues of rats with vasodilation. As shown in Fig. 4, T-bet, a transcription factor associated with Th1 reactions, showed significantly (p < 0.05) higher expression in the βGdAP groups (T1, T2 and T3) than in the negative control group (N). However, GATA-3, a transcription factor associated with Th2 reactions, showed significantly (p < 0.05) lower expression in the βGdAP groups (T1, T2 and T3) than in the negative control group (N). Based on the T-bet/GATA-3 expression ratio, the Th1/Th2 transcriptional ratio was found to be significantly (p < 0.05) decreased in the AD-induced negative control group (N), but it dramatically recovered in the βGdAP-treated groups (T1, T2 and T3) [Fig. 4(C)]. Cytokines are substances produced mostly by immune cells that are closely related to immune regulation. Th1 cell cytokines are known to enhance cellular immune responses and inhibit allergy-related cellular immune responses [31]. Further, Th2 cells secrete cytokines to promote the inflow and activation of allergy-related cells, such as B cells, mast cells, and eosinophils, and cause damage to tissues through fibrosis [32]. Activated immune cells release IgE, histamine, and cytotoxic substances to intensify the inflammatory responses and induce clinical signs [33]. Therefore, inhibiting Th2 cell action that is central to allergic reactions is essential for the treatment of allergic diseases. In the present study, βGdAP inhibited Th2 cell reactions that are central to allergic reactions that have immunomodulatory effects.
Fig. 4.
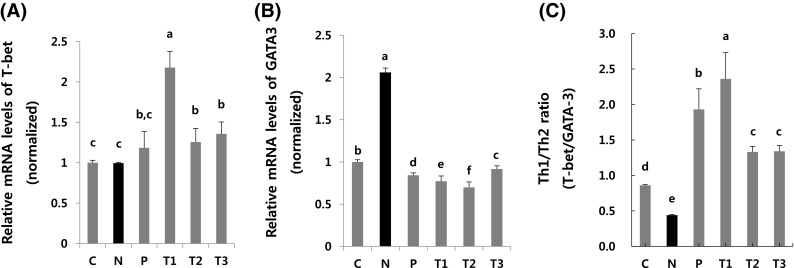
Effects of oral administration of βGdAP on the expression of Th1 and Th2. T cell polarization in mesenteric lymph node (MLN) tissues of vasodilation rats was evaluated by analyzing the expression of T-bet (Th1, A) and GATA-3 (Th2, B), and the Tbet/GATA-3 ratio (Th1/Th2, C). C Control, N Negative control (COM-induced AD), P Positive control (COM-induced AD + Zyrtec), T1 COM-induced AD + 1 mg/kg body weight of βGdAP, T2 COM-induced AD + 10 mg/kg body weight of βGdAP, T3 COM-induced AD + 20 mg/kg body weight of βGdAP. a–fSignificant differences between means at p < 0.05. Data represent the mean ± SD values from four replicates
Stimulation of FOXP3 and galectin-9 expression by administration of βGdAP
To determine the effects of βGdAP on the activation of Tregs, transcriptional changes in FOXP3, a transcription factor of Tregs, and galectin-9, which is known as a stimulator of Treg activation, were investigated in the mesenteric lymph nodes of rats with vasodilation. As shown in Fig. 5, the expression levels of FOXP3 and galectin-9 were significantly (p < 0.05) attenuated in the AD-induced group (N) compared with the control group (C). However, FOXP3 and galectin-9 expression was dramatically recovered in the βGdAP groups (T1, T2 and T3) (p < 0.05). FOXP3 is a well-known specific marker of Tregs, and FOXP3-positive Tregs have been reported to play an important role in immune tolerance [34]. Further, activation of FOXP3-positive Tregs was effective in reducing disease severity in inflammatory bowel disease and animal models of asthma [35]. Dietary synbiotics such as galacto- and fructo-oligosaccharides have been reported to have suppressive effects on allergic symptoms in mice and humans via upregulation of the expression of galectin-9 [36]. Since activation of Treg and galectin-9 expression may be useful for suppressing the immune responses in AD, our data indicate that administration of βGdAP may activate Tregs via stimulation of the transcriptional expression of FOXP3 and galectin-9, which may result in the attenuation of AD-like phenotypes.
Fig. 5.
Effects of βGdAP on Treg activation. Total RNA was isolated from the mesenteric lymph node (MLN) tissues of rats with vasodilation, and transcriptional expression of FOXP3 (A) and galectin-9 (B) was analyzed by qRT-PCR. C Control, N Negative control (COM-induced AD), P Positive control (COM-induced AD + Zyrtec), T1 COM-induced AD + 1 mg/kg body weight of βGdAP, T2 COM-induced AD + 10 mg/kg body weight of βGdAP, T3 COM-induced AD + 20 mg/kg body weight of βGdAP. a–cSignificant differences between means at p < 0.05. Data represent the mean ± SD values from four replicates
Overall, in the present study, we investigated the effects of βGdAP against AD-like phenotypes by using three AD animal models. Oral administration of βGdAP in a COM-induced AD rat model significantly reduced vasodilation, which is an acute inflammatory change caused by AD, and allergic pruritus. We found that contact dermatitis was also significantly attenuated with βGdAP treatment. Taken together, we believe that these results, to the best of our knowledge, are the first to demonstrate the anti-AD effects of βGdAP via regulation of the expression of Th1/Th2 and activators of Tregs.
Acknowledgements
This research was undertaken with the aid of the Industry Core Technology Development Project (Nos. 10049026 and 10063302), Ministry of Trade, Industry, and Energy, Korea.
Compliance with ethical standards
Conflict of interest
We certify that there is no conflict of interest in the manuscript.
References
- 1.Leung DYM, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J. Clin. Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sim TK, Ko DK, Kim HC, Baek YJ, Lee JS, Yoo HS. Effects of Amyda sinensis on allergic inflammation mechanism related atopy dermatitis. Daejeon University Oriental Medicine. 2011;20:69–83. [Google Scholar]
- 3.Meagher LJ, Wines NY, Cooper AJ. Atopic dermatitis; review of immunopathogenesis and advances in immunosuppressive therapy. Aust. J. Dermatol. 2002;43:247–254. doi: 10.1046/j.1440-0960.2002.00610.x. [DOI] [PubMed] [Google Scholar]
- 4.Blaser K, Ring J, Capron. T cell regulation in allergy, asthma and atopic skin diseases. S. Karger Pub. 121-137 (2008).
- 5.Tao JH, Cheng M, Tang JP, Liu Q, Pan F, Li XP. Foxp3, Regulatory T cell, and autoimmune diseases. Inflammation. 2017;40:328–339. doi: 10.1007/s10753-016-0470-8. [DOI] [PubMed] [Google Scholar]
- 6.Kanamori M, Nakatsukasa H, Okada M, Lu Q, Yoshimura A. Induced regulatory T cells: their development, stability, and applications. Trends Immunol. 2016;37:803–811. doi: 10.1016/j.it.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 7.de Kivit S, Kraneveld DA, Knippels LMJ, van Kooyk Y, Garssen J, Willemsen LEM. Intestinal epithelium-derived galectin-9 is involved in the immunomodulating effects of nondigestible oligosaccharides. J. Innate Immun. 2013;5:625–638. doi: 10.1159/000350515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Terada Y, Tsubota M, Sugo H, Wakitani K, Sekiguchi F, Wada K, Takada M, Oita A, Kawabata A. Tacrolimus triggers transient receptor potential vanilloid-1-dependent relapse of pancreatitis-related pain in mice. Pharmacology. 2017;99:281–285. doi: 10.1159/000454816. [DOI] [PubMed] [Google Scholar]
- 9.Delaney B, Nicolosi RJ, Wilson TA. Beta-glucan fractions from barley and oats are similarly antiatherogenic in hypercholesterolemic Syrian golden hamsters. J. Nutr. 2003;133:468–475. doi: 10.1093/jn/133.2.468. [DOI] [PubMed] [Google Scholar]
- 10.Babicek K, Cechova I, Simon RR. Toxicological assessment of a particulate yeast (1, 3/1,6)-beta-D-glucan in rats. Food Chem. Toxicol. 2007;45:1719–1730. doi: 10.1016/j.fct.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Mclntosh M, Stone BA, Stanisich VA. Curdlan and other bacterial (1 → 3)-beta-D-glucans. Appl. Microbiol. Biot. 2005;68:163–173. doi: 10.1007/s00253-005-1959-5. [DOI] [PubMed] [Google Scholar]
- 12.Sandula J, Kogan G, Kacurakova M, Machova E. Microbial (1 → 3)-β-D-glucans, their preparation, physico-chemical characterization and immunomodulatory activity. Carbohyd. Polym. 1999;38:247–253. doi: 10.1016/S0144-8617(98)00099-X. [DOI] [Google Scholar]
- 13.Ohno N, Miura T, Miura NN, Adachi Y, Yadomae T. Structure and biological activities of hypochlorite oxidizes zymosan. Carbohyd. Polym. 2001;44:339–349. doi: 10.1016/S0144-8617(00)00250-2. [DOI] [Google Scholar]
- 14.Ali MF, Driscoll CB, Walters PR, Limper AH, Carmona EM. β-Glucan-activated human B lymphocytes participate in innate immune responses by releasing proinflammatory cytokines and stimulating neutrophil chemotaxis. J. Immunol. 2015;1:5318–5326. doi: 10.4049/jimmunol.1500559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YH, Han KI, Jeon M, Hwang SG, Jung EG, Kwon HJ, Han MD. Anti hyperlipidemic activities of a chemically engineered sulfated mushroom β-glucan on high fat dietary-induced hyperlipidemia in Sprague-Dawley rats. J. Life Sci. 2014;24:1209–1216. doi: 10.5352/JLS.2014.24.11.1209. [DOI] [Google Scholar]
- 16.Ingeborg B, Yokoyama W, Davis P, Hudson C, Backus R, Richter D, Knuckles B, Schneeman BO. Phstprandial lipid, glucose, insulin, and cholecystokinin responses in men fed barley pasta enriched with beta-glucan. Am. J. Clin. Nutr. 1999;69:55–63. doi: 10.1093/ajcn/69.1.55. [DOI] [PubMed] [Google Scholar]
- 17.Hui YF, Den ES, Chi TH. Antioxidant and free radical scavenging activities of edible mushrooms. J. Food Lipids. 2002;9:35–43. doi: 10.1111/j.1745-4522.2002.tb00206.x. [DOI] [Google Scholar]
- 18.Kim JW, Cho HR, Kim KY, Ku SK, Lee HS. Effect of beta-glucan on the collagen-induced rheumatoid arthritis. J. Vet. Clin. 2010;27:315–324. [Google Scholar]
- 19.Jung MY, Kim JW, Kim KY, Choi SH, Ku SK. Polycan, a β-glucan from Aureobasidium pullulans SM-2001 mitigates ovariectomy-induced osteoporosis in rats. Exp. Ther. Med. 2016;12:1251–1262. doi: 10.3892/etm.2016.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KH, Park SJ, Lee YJ, Lee JE, Song CH, Choi SH, Ku SK, Kang SJ. Inhibition of UVB-induced skin damage by exopolymers from Aureobasidium pullulans SM-2001 in hairless mice. Basic Clin. Pharmacol. 2015;116:73–86. doi: 10.1111/bcpt.12288. [DOI] [PubMed] [Google Scholar]
- 21.Seo HP, Kim JM, Shin HD, Kim TK, Chang HJ, Park BR, Lee JW. Production of β-1,3/1,6-glucan by Aureobasidium pullulans SM-2001. Korean J. Biotechnol. Bioeng. 2002;17:376–380. [Google Scholar]
- 22.Ishiguro K, Oku H, Suitani A, Yamamoto Y. Effects of conjugated linoleic acid on anaphylaxis and allergic pruritus. Biol. Pharm. Bull. 2002;25:1655–1657. doi: 10.1248/bpb.25.1655. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda H, Tomohiro N, Ido Y, Kubo M. Anti-allergic effects of cnidii monnieri fructus (dried fruits of Cnidium monnieri) and its major component, osthol. Biol. Pharm. Bull. 2002;25:809–812. doi: 10.1248/bpb.25.809. [DOI] [PubMed] [Google Scholar]
- 24.Ryu JC, Lee JG, Ku SK, Jee SY. Effect of sopung-san extracts on the passive cutaneous anaphylaxis of wistar rats (type I allergic dermatitis) J. Korean Med. Ophthalmol. Otolaryngol. Dermatol. 2009;22:1–10. [Google Scholar]
- 25.Byun DG, Kim JW. Influence of cytokines on the development of T cell subsets. Pediat. Allergy Respir. Dis. 1996;6:20–32. [Google Scholar]
- 26.Bunker CB, Foreman JC, Dowd PM. Vascular responses to histamine at low temperatures in normal digital skin and Raynaud’s phenomenon. Agents Actions. 1991;33:197–199. doi: 10.1007/BF01993166. [DOI] [PubMed] [Google Scholar]
- 27.Sugimoto Y, Umakoshi K, Nojiri N, Kamei C. Effects of histamine H1 receptor antagonists on compound 48/80-induced scratching behavior in mice. Eur. J. Pharmacol. 1998;351:1–5. doi: 10.1016/S0014-2999(98)00288-X. [DOI] [PubMed] [Google Scholar]
- 28.Shinmei Y, Hossen MA, Okihara K, Sugimoto H, Yamada H, Kamei C. Effect of Brazilian propolis on scratching behavior induced by compound 48/80 and histamine in mice. Int. Immunopharmacol. 2004;4:1431–1436. doi: 10.1016/j.intimp.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Ueda Y, Sone T, Inagaki N, Nagai H. Effects of prednisolone on the cutaneous reaction and skin barrier function in mice treated with a hapten. Biol. Pharm. Bull. 2003;26:618–621. doi: 10.1248/bpb.26.618. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe C, Satoh T, Tahara E, Murakami K, Hayashi K, Hase K, Andoh T, Kuraishi Y, Kadota Y, Nagai H, Saiki I. Inhibitory mechanisms of glycoprotein fraction derived from Miscanthus sinensis for the immediate phase response of an IgE-mediated cutaneous reaction. Biol. Pharm. Bull. 1999;22:26–30. doi: 10.1248/bpb.22.26. [DOI] [PubMed] [Google Scholar]
- 31.Hatanaka H, Abe Y, Kamiya T, Morino F, Nagata J, Tokunaga T, Oshika Y, Suemizu H, Kijima H, Tsuchida T, Yamazaki H, Inoue H, Nakamura M, Ueyama Y. Clinical implications of interleukin (IL)-10 induced by non-small-cell lung cancer. Ann. Oncol. 2000;11:815–819. doi: 10.1023/A:1008375208574. [DOI] [PubMed] [Google Scholar]
- 32.Romagnani S. The role of lymphocytes in allergic disease. J. Allergy Clin. Immun. 2000;105:399–408. doi: 10.1067/mai.2000.104575. [DOI] [PubMed] [Google Scholar]
- 33.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J. Allergy Clin. Immun. 2010;125:73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohki H, Martin C, Corbel C, Coltey M, Le Douarin NM. Tolerance induced by thymic epithelial grafts in birds. Science. 1987;237:1032–1035. doi: 10.1126/science.3616623. [DOI] [PubMed] [Google Scholar]
- 35.Suri-Payer E, Fritzsching B. Regulatory T cells in experimental autoimmune disease. Springer Semi. Immun. 2006;28:3–16. doi: 10.1007/s00281-006-0021-8. [DOI] [PubMed] [Google Scholar]
- 36.de Kivit S, Saeland E, Kraneveld AD, van de Kant HJ, Schouten B, van Esch BC, Knol J, Sprikkelman AB, van der Aa LB, Knippels LM, Garssen J, van Kooyk Y, Willemsen LE. Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy. 2012;67:343–352. doi: 10.1111/j.1398-9995.2011.02771.x. [DOI] [PubMed] [Google Scholar]